Application of Biomarkers in Spinal Muscular Atrophy
Abstract
1. Introduction to Spinal Muscular Atrophy
2. Molecular Biomarkers for Spinal Muscular Atrophy
2.1. Survival Motor Neuron
2.2. Neurofilaments
2.3. Combinatory microRNAs
2.4. Cytokines
2.5. Chitotriosidase 1 and Chitinase-3-like Protein 1
2.6. Serum and Urinary Creatinine
2.7. Creatine Kinase
2.8. Glial Fibrillary Acidic Protein
2.9. Amyloid-β 40 and 42
2.10. Tau Protein
2.11. S100 Calcium-Binding Protein B
2.12. Conventional Cerebrospinal Fluid Parameters
2.13. Gemin Proteins
3. Non-Molecular Biomarkers for Spinal Muscular Atrophy
3.1. Electrophysiological Biomarkers for Spinal Muscular Atrophy
3.2. Imaging Technologies for Spinal Muscular Atrophy
4. Emerging Technologies in Biomarker Discovery for Spinal Muscle Atrophy
5. Discussion and Conclusions
6. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6MWT | 6-Minute Walk Test |
| AAV | Adeno-associated virus |
| ACTB | Actin beta |
| AD | Alzheimer’s disease |
| ADM | Abductor digiti minimi |
| Aldh4a1 | Aldehyde dehydrogenase 4 family, member A1 |
| ADMA | Asymmetric dimethylarginine |
| ALS | Amyotrophic lateral sclerosis |
| ALSFRS-R | Amyotrophic Lateral Sclerosis Functional Rating Scale—Revised |
| APP | Amyloid precursor protein |
| ASC-EVs | Exosomes derived from adipose-derived stem cells |
| ASOs | Antisense oligonucleotides |
| Aβ | Amyloid-β |
| BACE1 | β-site APP cleaving enzyme-1 |
| BBB | Blood–brain barrier |
| Bcl2 | B-cell lymphoma 2 |
| BI | Barthe index |
| BMI | Body mass index |
| Bmp4 | Barthel Index |
| CCL5 | C-C motif chemokine ligand 5 |
| CCR | Creatinine-to-cystatin C ratio |
| ceRNA | Competing endogenous RNA |
| CHD4 | Chromodomain helicase DNA binding protein 4 |
| CHI3L1 | Chitinase-3-like protein 1 |
| CHIT1 | Chitotriosidase 1 |
| Chl1 | Cell adhesion molecule L1-like protein |
| CHOP INTEND | Children’s Hospital of Philadelphia Infant test of Neuromuscular Disorders |
| CK | Creatine kinase |
| CMAP | Compound muscle action potential |
| CNS | Central nervous system |
| Cplx2 | Complexin 2 |
| Crn | Creatinine |
| CSF | Cerebrospinal fluid |
| DYNC1H1 | Dynein cytoplasmic 1 heavy chain 1 |
| EI | Muscle echogenicity |
| EIM | Electrical impedance myography |
| ELISA | Enzyme-linked immunosorbent assay |
| EMG | Electromyographic |
| Gata6 | GATA binding protein 6 |
| GDF15 | Growth differentiation factor 15 |
| GFAP | Glial fibrillary acidic protein |
| GSEA | Gene set enrichment analysis |
| HDL | High-density lipoprotein |
| HFMSE | Hammersmith functional motor scale–expanded |
| HINE-2 | Section 2 of the Hammersmith Infant Neurological Examination |
| HNRNPA1 | Heterogeneous nuclear ribonucleoprotein A1 |
| HSPA7 | Heat shock protein family A member 7 |
| IBA1 | Ionized calcium binding adaptor molecule 1 |
| IL | Interleukin |
| IL-1ra | Interleukin-1 receptor antagonist |
| INF-γ | Interferon-γ |
| ISL1 | ISL LIM homeobox 1 |
| JUND | JunD Proto-Oncogene |
| LDA | Linear discriminant analysis |
| Lif | Leukemia inhibitory factor |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MFM | Motor Function Measurement |
| MIP-1β | Macrophage inflammatory protein-1β |
| miRNAs | MicroRNAs |
| MMA/SDMA | Monomethylarginine/symmetric dimethylarginine |
| MOST | Multispectral optoacoustic tomography |
| MT | Muscle thickness |
| MUNE | Motor unit number estimation |
| MUNIX | Motor unit number index |
| myomiRs | Muscle-specific miRNAs |
| NCOR2 | Nuclear receptor corepressor 2 |
| NCX2 | Sodium-calcium exchanger 2 |
| NfH | Neurofilament heavy chain |
| NfL | Neurofilament light chain |
| NfM | Neurofilament medium chain |
| NFs | Neurofilaments |
| NF-κB | Nuclear factor kappa B |
| NPV | Negative predictive value |
| Pak4 | P21-activated kinase 4 |
| PCR | Polymerase chain reaction. |
| PLS3 | Plastin 3 |
| PME | Progressive myoclonic epilepsy |
| pNfH | Phosphorylated neurofilament heavy chain |
| PRMT | Protein arginine methyltransferase |
| Ptgs2 | Prostaglandin-endoperoxide synthase 2 |
| QAlb | Albumin quotient |
| RHOA | Ras homolog family member A |
| ROCK1 | Rho-associated coiled-coil containing protein kinase 1 |
| RULM | Revised upperlimb module |
| S100B | S100 calcium-binding protein B |
| sAPP | Soluble amyloid precursor protein |
| SD | Standard deviation |
| SIMOA | Sensitive single-molecule array |
| SLC23A2 | Solute carrier family 23 member 2 |
| SMA | Spinal muscular atrophy |
| SMA-PME | SMA with progressive myoclonic epilepsy |
| SMN | Survival motor neuron |
| snRNP | Small nuclear ribonucleoprotein |
| Syt | Synaptotagmin |
| SOX2 | SRY-box transcription factor 2 |
| TBXT | T-box transcription factor T |
| TNF-α | Tumor necrosis factor-alpha |
| TRADD | TNF Receptor-Associated Death Domain |
| WGCNA | Weighted correlation network analysis |
References
- Monani, U.R. Spinal muscular atrophy: A deficiency in a ubiquitous protein; a motor neuron-specific disease. Neuron 2005, 48, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.C.; Taylor, J.L.; Hannon, H.; Howell, R. Newborn screening for spinal muscular atrophy: Anticipating an imminent need. Semin. Perinatol. 2015, 39, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Wilson, R.B. Genetic testing and risk assessment for spinal muscular atrophy (SMA). Hum. Genet. 2002, 111, 477–500. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.B.; Chen, X.; Zhang, C.; Wang, Y.P.; Ma, P.P.; Hao, S.J.; Hui, L.; Bai, Y.F. Analysis of spinal muscular atrophy carrier screening results in 32,416 pregnant women and 7231 prepregnant women. Front. Neurol. 2024, 15, 1357476. [Google Scholar] [CrossRef]
- Chan, S.H.S.; Wong, C.K.H.; Wu, T.; Wong, W.; Yu, M.K.L.; Au, I.C.H.; Chan, G.C.F. Significant healthcare burden and life cost of spinal muscular atrophy: Real-world data. Eur. J. Health Econ. 2023, 24, 1373–1382. [Google Scholar] [CrossRef]
- López-Bastida, J.; Peña-Longobardo, L.M.; Aranda-Reneo, I.; Tizzano, E.; Sefton, M.; Oliva-Moreno, J. Social/economic costs and health-related quality of life in patients with spinal muscular atrophy (SMA) in Spain. Orphanet J. Rare Dis. 2017, 12, 141. [Google Scholar] [CrossRef]
- Younger, D.S.; Mendell, J.R. Chapter 2—Childhood spinal muscular atrophy. In Handbook of Clinical Neurology; Younger, D.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 43–58. [Google Scholar]
- Bürglen, L.; Lefebvre, S.; Clermont, O.; Burlet, P.; Viollet, L.; Cruaud, C.; Munnich, A.; Melki, J. Structure and organization of the human survival motor neurone (SMN) gene. Genomics 1996, 32, 479–482. [Google Scholar] [CrossRef]
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef]
- Keinath, M.C.; Prior, D.E.; Prior, T.W. Spinal Muscular Atrophy: Mutations, Testing, and Clinical Relevance. Appl. Clin. Genet. 2021, 14, 11–25. [Google Scholar] [CrossRef]
- Wirth, B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum. Mutat. 2000, 15, 228–237. [Google Scholar] [CrossRef]
- Burghes, A.H. When is a deletion not a deletion? When it is converted. Am. J. Hum. Genet. 1997, 61, 9–15. [Google Scholar] [CrossRef]
- Calucho, M.; Bernal, S.; Alías, L.; March, F.; Venceslá, A.; Rodríguez-Álvarez, F.J.; Aller, E.; Fernández, R.M.; Borrego, S.; Millán, J.M.; et al. Correlation between SMA type and SMN2 copy number revisited: An analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul. Disord. 2018, 28, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Alías, L.; Bernal, S.; Fuentes-Prior, P.; Barceló, M.J.; Also, E.; Martínez-Hernández, R.; Rodríguez-Alvarez, F.J.; Martín, Y.; Aller, E.; Grau, E.; et al. Mutation update of spinal muscular atrophy in Spain: Molecular characterization of 745 unrelated patients and identification of four novel mutations in the SMN1 gene. Hum. Genet. 2009, 125, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.K.; Mishra, A. Spinal Muscular Atrophy (SMA): Treatment strategies, challenges and future prospects. Pharmacol. Res.-Rep. 2025, 3, 100031. [Google Scholar] [CrossRef]
- Holzwarth, D.; Calaminus, G.; Friese, J.; Sejersen, T.; Büning, H.; John-Neek, P.; Bastone, A.L.; Rothe, M.; Mansfield, K.; Libertini, S.; et al. Pilocytic astrocytoma in a child with spinal muscular atrophy treated with onasemnogene abeparvovec. Mol. Ther. 2025, 33, 2842–2850. [Google Scholar] [CrossRef]
- Magelssen, M.; Rasmussen, M.; Wallace, S.; Førde, R. Priority setting at the clinical level: The case of nusinersen and the Norwegian national expert group. BMC Med. Ethics 2021, 22, 54. [Google Scholar] [CrossRef]
- Zhong, Z.J.; Zheng, P.M.; Dou, H.H.; Wang, J.G. Adverse events in the treatment of spinal muscular atrophy in children and adolescents with nusinersen: A systematic review and meta-analysis. Front. Pediatr. 2023, 11, 1152318. [Google Scholar] [CrossRef]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018, 28, 103–115. [Google Scholar] [CrossRef]
- Tiziano, F.D.; Pinto, A.M.; Fiori, S.; Lomastro, R.; Messina, S.; Bruno, C.; Pini, A.; Pane, M.; D’Amico, A.; Ghezzo, A.; et al. SMN transcript levels in leukocytes of SMA patients determined by absolute real-time PCR. Eur. J. Hum. Genet. 2010, 18, 52–58. [Google Scholar] [CrossRef]
- Butchbach, M.E. Copy Number Variations in the Survival Motor Neuron Genes: Implications for Spinal Muscular Atrophy and Other Neurodegenerative Diseases. Front. Mol. Biosci. 2016, 3, 7. [Google Scholar] [CrossRef]
- Liguori, M.; Bianco, A.; Introna, A.; Consiglio, A.; Milella, G.; Abbatangelo, E.; D’Errico, E.; Licciulli, F.; Grillo, G.; Simone, I.L. An early Transcriptomic Investigation in Adult Patients with Spinal Muscular Atrophy Under Treatment with Nusinersen. J. Mol. Neurosci. 2024, 74, 89. [Google Scholar] [CrossRef]
- Jedrzejowska, M.; Borkowska, J.; Zimowski, J.; Kostera-Pruszczyk, A.; Milewski, M.; Jurek, M.; Sielska, D.; Kostyk, E.; Nyka, W.; Zaremba, J.; et al. Unaffected patients with a homozygous absence of the SMN1 gene. Eur. J. Hum. Genet. 2008, 16, 930–934. [Google Scholar] [CrossRef][Green Version]
- Ricci, M.; Cicala, G.; Capasso, A.; Coratti, G.; Fiori, S.; Cutrona, C.; D’Amico, A.; Sansone, V.A.; Bruno, C.; Messina, S.; et al. Clinical Phenotype of Pediatric and Adult Patients with Spinal Muscular Atrophy with Four SMN2 Copies: Are They Really All Stable? Ann. Neurol. 2023, 94, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Prior, T.W.; Swoboda, K.J.; Scott, H.D.; Hejmanowski, A.Q. Homozygous SMN1 deletions in unaffected family members and modification of the phenotype by SMN2. Am. J. Med. Genet. A 2004, 130a, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Monani, U.R.; Sendtner, M.; Coovert, D.D.; Parsons, D.W.; Andreassi, C.; Le, T.T.; Jablonka, S.; Schrank, B.; Rossoll, W.; Prior, T.W.; et al. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn−/− mice and results in a mouse with spinal muscular atrophy. Hum. Mol. Genet. 2000, 9, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Hsieh-Li, H.M.; Chang, J.G.; Jong, Y.J.; Wu, M.H.; Wang, N.M.; Tsai, C.H.; Li, H. A mouse model for spinal muscular atrophy. Nat. Genet. 2000, 24, 66–70. [Google Scholar] [CrossRef]
- Pane, M.; Coratti, G.; Sansone, V.A.; Messina, S.; Bruno, C.; Catteruccia, M.; Sframeli, M.; Albamonte, E.; Pedemonte, M.; D’Amico, A.; et al. Nusinersen in type 1 spinal muscular atrophy: Twelve-month real-world data. Ann. Neurol. 2019, 86, 443–451. [Google Scholar] [CrossRef]
- Audic, F.; Dubois, S.M.; Durigneux, J.; Barnerias, C.; Isapof, A.; Nougues, M.C.; Davion, J.B.; Richelme, C.; Vuillerot, C.; Legoff, L.; et al. Effect of nusinersen after 3 years of treatment in 57 young children with SMA in terms of SMN2 copy number or type. Arch. Pediatr. 2024, 31, 117–123. [Google Scholar] [CrossRef]
- Xing, X.; Liu, X.; Li, X.; Li, M.; Wu, X.; Huang, X.; Xu, A.; Liu, Y.; Zhang, J. Insights into spinal muscular atrophy from molecular biomarkers. Neural Regen. Res. 2025, 20, 1849–1863. [Google Scholar] [CrossRef]
- Czech, C.; Tang, W.; Bugawan, T.; Mano, C.; Horn, C.; Iglesias, V.A.; Fröhner, S.; Zaworski, P.G.; Paushkin, S.; Chen, K.; et al. Biomarker for Spinal Muscular Atrophy: Expression of SMN in Peripheral Blood of SMA Patients and Healthy Controls. PLoS ONE 2015, 10, e0139950. [Google Scholar] [CrossRef]
- Wadman, R.I.; Stam, M.; Jansen, M.D.; van der Weegen, Y.; Wijngaarde, C.A.; Harschnitz, O.; Sodaar, P.; Braun, K.P.; Dooijes, D.; Lemmink, H.H.; et al. A Comparative Study of SMN Protein and mRNA in Blood and Fibroblasts in Patients with Spinal Muscular Atrophy and Healthy Controls. PLoS ONE 2016, 11, e0167087. [Google Scholar] [CrossRef]
- Zaworski, P.; von Herrmann, K.M.; Taylor, S.; Sunshine, S.S.; McCarthy, K.; Risher, N.; Newcomb, T.; Weetall, M.; Prior, T.W.; Swoboda, K.J.; et al. SMN Protein Can Be Reliably Measured in Whole Blood with an Electrochemiluminescence (ECL) Immunoassay: Implications for Clinical Trials. PLoS ONE 2016, 11, e0150640. [Google Scholar] [CrossRef]
- Alves, C.R.R.; Zhang, R.; Johnstone, A.J.; Garner, R.; Eichelberger, E.J.; Lepez, S.; Yi, V.; Stevens, V.; Poxson, R.; Schwartz, R.; et al. Whole blood survival motor neuron protein levels correlate with severity of denervation in spinal muscular atrophy. Muscle Nerve 2020, 62, 351–357. [Google Scholar] [CrossRef]
- Mercuri, E.; Sumner, C.J.; Muntoni, F.; Darras, B.T.; Finkel, R.S. Spinal muscular atrophy. Nat. Rev. Dis. Primers 2022, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Iyer, C.C.; Wang, X.; Renusch, S.R.; Duque, S.I.; Wehr, A.M.; Mo, X.M.; McGovern, V.L.; Arnold, W.D.; Burghes, A.H.; Kolb, S.J. SMN Blood Levels in a Porcine Model of Spinal Muscular Atrophy. J. Neuromuscul. Dis. 2017, 4, 59–66. [Google Scholar] [CrossRef]
- Wirth, B. Spinal Muscular Atrophy: In the Challenge Lies a Solution. Trends Neurosci. 2021, 44, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Chiriboga, C.A.; Swoboda, K.J.; Darras, B.T.; Iannaccone, S.T.; Montes, J.; De Vivo, D.C.; Norris, D.A.; Bennett, C.F.; Bishop, K.M. Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology 2016, 86, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Tiziano, F.D.; Lomastro, R.; Abiusi, E.; Pasanisi, M.B.; Di Pietro, L.; Fiori, S.; Baranello, G.; Angelini, C.; Sorarù, G.; Gaiani, A.; et al. Longitudinal evaluation of SMN levels as biomarker for spinal muscular atrophy: Results of a phase IIb double-blind study of salbutamol. J. Med. Genet. 2019, 56, 293–300. [Google Scholar] [CrossRef]
- Yeo, C.J.J.; Tizzano, E.F.; Darras, B.T. Challenges and opportunities in spinal muscular atrophy therapeutics. Lancet Neurol. 2024, 23, 205–218. [Google Scholar] [CrossRef]
- Lapp, H.S.; Freigang, M.; Hagenacker, T.; Weiler, M.; Wurster, C.D.; Günther, R. Biomarkers in 5q-associated spinal muscular atrophy-a narrative review. J. Neurol. 2023, 270, 4157–4178. [Google Scholar] [CrossRef]
- Liem, R.K.; Yen, S.H.; Salomon, G.D.; Shelanski, M.L. Intermediate filaments in nervous tissues. J. Cell Biol. 1978, 79, 637–645. [Google Scholar] [CrossRef]
- Spicer, C.; Lu, C.H.; Catapano, F.; Scoto, M.; Zaharieva, I.; Malaspina, A.; Morgan, J.E.; Greensmith, L.; Muntoni, F.; Zhou, H. The altered expression of neurofilament in mouse models and patients with spinal muscular atrophy. Ann. Clin. Transl. Neurol. 2021, 8, 866–876. [Google Scholar] [CrossRef]
- Nitz, E.; Smitka, M.; Schallner, J.; Akgün, K.; Ziemssen, T.; von der Hagen, M.; Tüngler, V. Serum neurofilament light chain in pediatric spinal muscular atrophy patients and healthy children. Ann. Clin. Transl. Neurol. 2021, 8, 2013–2024. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef]
- Bayoumy, S.; Verberk, I.M.W.; Vermunt, L.; Willemse, E.; den Dulk, B.; van der Ploeg, A.T.; Pajkrt, D.; Nitz, E.; van den Hout, J.M.P.; van der Post, J.; et al. Neurofilament light protein as a biomarker for spinal muscular atrophy: A review and reference ranges. Clin. Chem. Lab. Med. 2024, 62, 1252–1265. [Google Scholar] [CrossRef]
- Darras, B.T.; Crawford, T.O.; Finkel, R.S.; Mercuri, E.; De Vivo, D.C.; Oskoui, M.; Tizzano, E.F.; Ryan, M.M.; Muntoni, F.; Zhao, G.; et al. Neurofilament as a potential biomarker for spinal muscular atrophy. Ann. Clin. Transl. Neurol. 2019, 6, 932–944. [Google Scholar] [CrossRef]
- Olsson, B.; Alberg, L.; Cullen, N.C.; Michael, E.; Wahlgren, L.; Kroksmark, A.K.; Rostasy, K.; Blennow, K.; Zetterberg, H.; Tulinius, M. NFL is a marker of treatment response in children with SMA treated with nusinersen. J. Neurol. 2019, 266, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; Ryan, M.M.; et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 2019, 29, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Wurster, C.D.; Steinacker, P.; Günther, R.; Koch, J.C.; Lingor, P.; Uzelac, Z.; Witzel, S.; Wollinsky, K.; Winter, B.; Osmanovic, A.; et al. Neurofilament light chain in serum of adolescent and adult SMA patients under treatment with nusinersen. J. Neurol. 2020, 267, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Freigang, M.; Steinacker, P.; Wurster, C.D.; Schreiber-Katz, O.; Osmanovic, A.; Petri, S.; Koch, J.C.; Rostásy, K.; Huss, A.; Tumani, H.; et al. Glial fibrillary acidic protein in cerebrospinal fluid of patients with spinal muscular atrophy. Ann. Clin. Transl. Neurol. 2022, 9, 1437–1448. [Google Scholar] [CrossRef]
- Rich, K.A.; Fox, A.; Yalvac, M.; Heintzman, S.; Tellez, M.; Bartlett, A.; Severyn, S.; Linsenmayer, M.; Kelly, K.; Reynolds, J.; et al. Neurofilament Levels in CSF and Serum in an Adult SMA Cohort Treated with Nusinersen. J. Neuromuscul. Dis. 2022, 9, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Verberk, I.M.W.; Thijssen, E.H.; Vermunt, L.; Hansson, O.; Zetterberg, H.; van der Flier, W.M.; Mielke, M.M.; del Campo, M. Blood-based biomarkers for Alzheimer’s disease: Towards clinical implementation. Lancet Neurol. 2022, 21, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Moussa-Pacha, N.M.; Abdin, S.M.; Omar, H.A.; Alniss, H.; Al-Tel, T.H. BACE1 inhibitors: Current status and future directions in treating Alzheimer’s disease. Med. Res. Rev. 2020, 40, 339–384. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, J.; Weiss, D.; Daubmann, A.; Schmitz, L.; Denecke, J. Evaluation of putative CSF biomarkers in paediatric spinal muscular atrophy (SMA) patients before and during treatment with nusinersen. J. Cell Mol. Med. 2021, 25, 8419–8431. [Google Scholar] [CrossRef]
- Verma, S.; Perry, K.; Razdan, R.; Howell, J.C.; Dawson, A.L.; Hu, W.T. CSF IL-8 Associated with Response to Gene Therapy in a Case Series of Spinal Muscular Atrophy. Neurotherapeutics 2023, 20, 245–253. [Google Scholar] [CrossRef]
- Introna, A.; Milella, G.; D’Errico, E.; Fraddosio, A.; Scaglione, G.; Ucci, M.; Ruggieri, M.; Simone, I.L. Is cerebrospinal fluid amyloid-β42 a promising biomarker of response to nusinersen in adult spinal muscular atrophy patients? Muscle Nerve 2021, 63, 905–909. [Google Scholar] [CrossRef]
- Walter, M.C.; Wenninger, S.; Thiele, S.; Stauber, J.; Hiebeler, M.; Greckl, E.; Stahl, K.; Pechmann, A.; Lochmüller, H.; Kirschner, J.; et al. Safety and Treatment Effects of Nusinersen in Longstanding Adult 5q-SMA Type 3—A Prospective Observational Study. J. Neuromuscul. Dis. 2019, 6, 453–465. [Google Scholar] [CrossRef]
- Totzeck, A.; Stolte, B.; Kizina, K.; Bolz, S.; Schlag, M.; Thimm, A.; Kleinschnitz, C.; Hagenacker, T. Neurofilament Heavy Chain and Tau Protein Are Not Elevated in Cerebrospinal Fluid of Adult Patients with Spinal Muscular Atrophy during Loading with Nusinersen. Int. J. Mol. Sci. 2019, 20, 5397. [Google Scholar] [CrossRef]
- Abyadeh, M.; Gupta, V.; Paulo, J.A.; Mahmoudabad, A.G.; Shadfar, S.; Mirshahvaladi, S.; Gupta, V.; Nguyen, C.T.O.; Finkelstein, D.I.; You, Y.; et al. Amyloid-beta and tau protein beyond Alzheimer’s disease. Neural Regen. Res. 2024, 19, 1262–1276. [Google Scholar] [CrossRef]
- Sinsky, J.; Pichlerova, K.; Hanes, J. Tau Protein Interaction Partners and Their Roles in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2021, 22, 9207. [Google Scholar] [CrossRef]
- Šimić, G.; Vukić, V.; Babić, M.; Banović, M.; Berečić, I.; Španić, E.; Zubčić, K.; Golubić, A.T.; Barišić Kutija, M.; Merkler Šorgić, A.; et al. Total tau in cerebrospinal fluid detects treatment responders among spinal muscular atrophy types 1–3 patients treated with nusinersen. CNS Neurosci. Ther. 2024, 30, e14051. [Google Scholar] [CrossRef]
- Milella, G.; Introna, A.; D’Errico, E.; Fraddosio, A.; Scaglione, G.; Morea, A.; Ucci, M.; Ruggieri, M.; Mastrapasqua, M.; Megna, M.; et al. Cerebrospinal Fluid and Clinical Profiles in Adult Type 2–3 Spinal Muscular Atrophy Patients Treated with Nusinersen: An 18-Month Single-Centre Experience. Clin. Drug Investig. 2021, 41, 775–784. [Google Scholar] [CrossRef]
- Winter, B.; Guenther, R.; Ludolph, A.C.; Hermann, A.; Otto, M.; Wurster, C.D. Neurofilaments and tau in CSF in an infant with SMA type 1 treated with nusinersen. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Benito, P.; Vázquez-Costa, J.F.; Ñungo Garzón, N.C.; Colomina, M.J.; Marco, C.; González, L.; Terrafeta, C.; Domínguez, R.; Ferrer, I.; Povedano, M. Neurodegeneration Biomarkers in Adult Spinal Muscular Atrophy (SMA) Patients Treated with Nusinersen. Int. J. Mol. Sci. 2024, 25, 3810. [Google Scholar] [CrossRef] [PubMed]
- Gingele, S.; Hümmert, M.W.; Alvermann, S.; Jendretzky, K.F.; Bönig, L.; Brieskorn, M.; Schwenkenbecher, P.; Sühs, K.W.; Müschen, L.H.; Osmanovic, A.; et al. Routine Cerebrospinal Fluid Cytology Reveals Unique Inclusions in Macrophages During Treatment With Nusinersen. Front. Neurol. 2019, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Kessler, T.; Latzer, P.; Schmid, D.; Warnken, U.; Saffari, A.; Ziegler, A.; Kollmer, J.; Möhlenbruch, M.; Ulfert, C.; Herweh, C.; et al. Cerebrospinal fluid proteomic profiling in nusinersen-treated patients with spinal muscular atrophy. J. Neurochem. 2020, 153, 650–661. [Google Scholar] [CrossRef]
- Hegen, H.; Auer, M.; Zeileis, A.; Deisenhammer, F. Upper reference limits for cerebrospinal fluid total protein and albumin quotient based on a large cohort of control patients: Implications for increased clinical specificity. Clin. Chem. Lab. Med. 2016, 54, 285–292. [Google Scholar] [CrossRef]
- McCudden, C.R.; Brooks, J.; Figurado, P.; Bourque, P.R. Cerebrospinal Fluid Total Protein Reference Intervals Derived from 20 Years of Patient Data. Clin. Chem. 2017, 63, 1856–1865. [Google Scholar] [CrossRef]
- Freigang, M.; Steinacker, P.; Wurster, C.D.; Schreiber-Katz, O.; Osmanovic, A.; Petri, S.; Koch, J.C.; Rostásy, K.; Falkenburger, B.; Ludolph, A.C.; et al. Increased chitotriosidase 1 concentration following nusinersen treatment in spinal muscular atrophy. Orphanet J. Rare Dis. 2021, 16, 330. [Google Scholar] [CrossRef]
- Müschen, L.H.; Osmanovic, A.; Binz, C.; Jendretzky, K.F.; Ranxha, G.; Bronzlik, P.; Abu-Fares, O.; Wiehler, F.; Möhn, N.; Hümmert, M.W.; et al. Cerebrospinal Fluid Parameters in Antisense Oligonucleotide-Treated Adult 5q-Spinal Muscular Atrophy Patients. Brain Sci. 2021, 11, 296. [Google Scholar] [CrossRef]
- Stolte, B.; Nonnemacher, M.; Kizina, K.; Bolz, S.; Totzeck, A.; Thimm, A.; Wagner, B.; Deuschl, C.; Kleinschnitz, C.; Hagenacker, T. Nusinersen treatment in adult patients with spinal muscular atrophy: A safety analysis of laboratory parameters. J. Neurol. 2021, 268, 4667–4679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hong, Y.; Brusa, C.; Scoto, M.; Cornell, N.; Patel, P.; Baranello, G.; Muntoni, F.; Zhou, H. Profiling neuroinflammatory markers and response to nusinersen in paediatric spinal muscular atrophy. Sci. Rep. 2024, 14, 23491. [Google Scholar] [CrossRef] [PubMed]
- Rybova, J.; Sundararajan, T.; Kuchar, L.; Dlugi, T.A.; Ruzicka, P.; McKillop, W.M.; Medin, J.A. Hematopoietic stem cell transplantation leads to biochemical and functional correction in two mouse models of acid ceramidase deficiency. Mol. Ther. 2024, 32, 3402–3421. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.L.; Mangione, C.C.; Suneja, M.; Gawrys, J.; Melvin, B.M.; Belous, N.; LaCroix, M.; Harmelink, M.; Burnett, B.G.; Ebert, A.D. IL-1ra and CCL5, but not IL-10, are promising targets for treating SMA astrocyte-driven pathology. Mol. Ther. 2025, 33, 734–751. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Fu, J.; Wei, Q.; Ou, R.; Lai, X.; Chen, X.; Shang, H. Serum creatinine to cystatin C ratio as monitoring biomarker in Chinese adult spinal muscular atrophy: A prospective cohort study. Orphanet J. Rare Dis. 2025, 20, 209. [Google Scholar] [CrossRef]
- Kim, E.J.; Wierzbicki, A.S. Investigating raised creatine kinase. BMJ 2021, 373, n1486. [Google Scholar] [CrossRef]
- Rudnik-Schöneborn, S.; Lützenrath, S.; Borkowska, J.; Karwanska, A.; Hausmanowa-Petrusewicz, I.; Zerres, K. Analysis of creatine kinase activity in 504 patients with proximal spinal muscular atrophy types I-III from the point of view of progression and severity. Eur. Neurol. 1998, 39, 154–162. [Google Scholar] [CrossRef]
- Freigang, M.; Wurster, C.D.; Hagenacker, T.; Stolte, B.; Weiler, M.; Kamm, C.; Schreiber-Katz, O.; Osmanovic, A.; Petri, S.; Kowski, A.; et al. Serum creatine kinase and creatinine in adult spinal muscular atrophy under nusinersen treatment. Ann. Clin. Transl. Neurol. 2021, 8, 1049–1063. [Google Scholar] [CrossRef]
- Bahadır Şenol, H.; Yıldız, G.; Polat, A.; Aydın, A.; Hız, A.S.; Soylu, A.; Yiş, U. Safety and Efficacy of Nusinersen Focusing on Renal and Hematological Parameters in Spinal Muscular Atrophy. Brain Behav. 2025, 15, e70221. [Google Scholar] [CrossRef]
- Zhao, X.; Gong, Z.; Luo, H.; Li, Z.; Gao, R.; Yang, K.; Deng, W.; Peng, S.; Ba, L.; Liu, Y.; et al. A cross-sectional and longitudinal evaluation of serum creatinine as a biomarker in spinal muscular atrophy. Orphanet J. Rare Dis. 2024, 19, 489. [Google Scholar] [CrossRef]
- Mazur, M.; Zielińska, A.; Grzybowski, M.M.; Olczak, J.; Fichna, J. Chitinases and Chitinase-Like Proteins as Therapeutic Targets in Inflammatory Diseases, with a Special Focus on Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6966. [Google Scholar] [CrossRef]
- De Wel, B.; De Schaepdryver, M.; Poesen, K.; Claeys, K.G. Biochemical and clinical biomarkers in adult SMA 3-4 patients treated with nusinersen for 22 months. Ann. Clin. Transl. Neurol. 2022, 9, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Ishikawa, N.; Tateishi, Y.; Izumo, H.; Eto, S.; Eguchi, Y.; Okada, S. Evaluation of cerebrospinal fluid biomarkers in pediatric patients with spinal muscular atrophy. Brain Dev. 2023, 45, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, J.; Hol, E.M. GFAP in health and disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Zhang, S.; Shi, S.; Lu, Y.; Leng, Y.; Li, C. Biomarkers for neuromyelitis optica: A visual analysis of emerging research trends. Neural Regen. Res. 2024, 19, 2735–2749. [Google Scholar] [CrossRef]
- Michetti, F.; D’Ambrosi, N.; Toesca, A.; Puglisi, M.A.; Serrano, A.; Marchese, E.; Corvino, V.; Geloso, M.C. The S100B story: From biomarker to active factor in neural injury. J. Neurochem. 2019, 148, 168–187. [Google Scholar] [CrossRef]
- Michetti, F.; Clementi, M.E.; Di Liddo, R.; Valeriani, F.; Ria, F.; Rende, M.; Di Sante, G.; Romano Spica, V. The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker. Int. J. Mol. Sci. 2023, 24, 9605. [Google Scholar] [CrossRef]
- Chen, T.H.; Chen, J.A. Multifaceted roles of microRNAs: From motor neuron generation in embryos to degeneration in spinal muscular atrophy. eLife 2019, 8, e50848. [Google Scholar] [CrossRef]
- Chen, T.H. Circulating microRNAs as potential biomarkers and therapeutic targets in spinal muscular atrophy. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420979954. [Google Scholar] [CrossRef]
- Bonanno, S.; Marcuzzo, S.; Malacarne, C.; Giagnorio, E.; Masson, R.; Zanin, R.; Arnoldi, M.T.; Andreetta, F.; Simoncini, O.; Venerando, A.; et al. Circulating MyomiRs as Potential Biomarkers to Monitor Response to Nusinersen in Pediatric SMA Patients. Biomedicines 2020, 8, 21. [Google Scholar] [CrossRef]
- Abiusi, E.; Infante, P.; Cagnoli, C.; Lospinoso Severini, L.; Pane, M.; Coratti, G.; Pera, M.C.; D’Amico, A.; Diano, F.; Novelli, A.; et al. SMA-miRs (miR-181a-5p, -324-5p, and -451a) are overexpressed in spinal muscular atrophy skeletal muscle and serum samples. eLife 2021, 10, e68054. [Google Scholar] [CrossRef]
- Zaharieva, I.T.; Scoto, M.; Aragon-Gawinska, K.; Ridout, D.; Doreste, B.; Servais, L.; Muntoni, F.; Zhou, H. Response of plasma microRNAs to nusinersen treatment in patients with SMA. Ann. Clin. Transl. Neurol. 2022, 9, 1011–1026. [Google Scholar] [CrossRef]
- Barbo, M.; Glavač, D.; Jezernik, G.; Ravnik-Glavač, M. MicroRNAs as Biomarkers in Spinal Muscular Atrophy. Biomedicines 2024, 12, 2428. [Google Scholar] [CrossRef]
- Welby, E.; Rehborg, R.J.; Harmelink, M.; Ebert, A.D. Assessment of cerebral spinal fluid biomarkers and microRNA-mediated disease mechanisms in spinal muscular atrophy patient samples. Hum. Mol. Genet. 2022, 31, 1830–1843. [Google Scholar] [CrossRef]
- Magen, I.; Aharoni, S.; Yacovzada, N.S.; Tokatly Latzer, I.; Alves, C.R.R.; Sagi, L.; Fattal-Valevski, A.; Swoboda, K.J.; Katz, J.; Bruckheimer, E.; et al. Muscle microRNAs in the cerebrospinal fluid predict clinical response to nusinersen therapy in type II and type III spinal muscular atrophy patients. Eur. J. Neurol. 2022, 29, 2420–2430. [Google Scholar] [CrossRef] [PubMed]
- D’Silva, A.M.; Kariyawasam, D.; Venkat, P.; Mayoh, C.; Farrar, M.A. Identification of Novel CSF-Derived miRNAs in Treated Paediatric Onset Spinal Muscular Atrophy: An Exploratory Study. Pharmaceutics 2023, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Chang, S.H.; Wu, Y.F.; Yen, Y.P.; Hsu, F.Y.; Chen, Y.C.; Ming, Y.; Hsu, H.C.; Su, Y.C.; Wong, S.T.; et al. MiR34 contributes to spinal muscular atrophy and AAV9-mediated delivery of MiR34a ameliorates the motor deficits in SMA mice. Mol. Ther. Nucleic Acids 2023, 32, 144–160. [Google Scholar] [CrossRef]
- Catapano, F.; Zaharieva, I.; Scoto, M.; Marrosu, E.; Morgan, J.; Muntoni, F.; Zhou, H. Altered Levels of MicroRNA-9, -206, and -132 in Spinal Muscular Atrophy and Their Response to Antisense Oligonucleotide Therapy. Mol. Ther. Nucleic Acids 2016, 5, e331. [Google Scholar] [CrossRef]
- Malacarne, C.; Galbiati, M.; Giagnorio, E.; Cavalcante, P.; Salerno, F.; Andreetta, F.; Cagnoli, C.; Taiana, M.; Nizzardo, M.; Corti, S.; et al. Dysregulation of Muscle-Specific MicroRNAs as Common Pathogenic Feature Associated with Muscle Atrophy in ALS, SMA and SBMA: Evidence from Animal Models and Human Patients. Int. J. Mol. Sci. 2021, 22, 5673. [Google Scholar] [CrossRef]
- Sison, S.L.; Patitucci, T.N.; Seminary, E.R.; Villalon, E.; Lorson, C.L.; Ebert, A.D. Astrocyte-produced miR-146a as a mediator of motor neuron loss in spinal muscular atrophy. Hum. Mol. Genet. 2017, 26, 3409–3420. [Google Scholar] [CrossRef]
- Xu, C.; Ishikawa, H.; Izumikawa, K.; Li, L.; He, H.; Nobe, Y.; Yamauchi, Y.; Shahjee, H.M.; Wu, X.H.; Yu, Y.T.; et al. Structural insights into Gemin5-guided selection of pre-snRNAs for snRNP assembly. Genes. Dev. 2016, 30, 2376–2390. [Google Scholar] [CrossRef]
- Grass, T.; Dokuzluoglu, Z.; Buchner, F.; Rosignol, I.; Thomas, J.; Caldarelli, A.; Dalinskaya, A.; Becker, J.; Rost, F.; Marass, M.; et al. Isogenic patient-derived organoids reveal early neurodevelopmental defects in spinal muscular atrophy initiation. Cell Rep. Med. 2024, 5, 101659. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Mu, L.; Shen, C.; Kong, X.; Wang, Y.; Hou, Y.; Zhang, R. Negative cooperativity between Gemin2 and RNA provides insights into RNA selection and the SMN complex’s release in snRNP assembly. Nucleic Acids Res. 2020, 48, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Salas, E.; Francisco-Velilla, R. GEMIN5 and neurodevelopmental diseases: From functional insights to disease perception. Neural Regen. Res. 2025, 21, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; So, B.R.; Li, P.; Yong, J.; Glisovic, T.; Wan, L.; Dreyfuss, G. Structure of a key intermediate of the SMN complex reveals Gemin2’s crucial function in snRNP assembly. Cell 2011, 146, 384–395. [Google Scholar] [CrossRef]
- Borg, R.M.; Fenech Salerno, B.; Vassallo, N.; Bordonne, R.; Cauchi, R.J. Disruption of snRNP biogenesis factors Tgs1 and pICln induces phenotypes that mirror aspects of SMN-Gemins complex perturbation in Drosophila, providing new insights into spinal muscular atrophy. Neurobiol. Dis. 2016, 94, 245–258. [Google Scholar] [CrossRef]
- Henderson, R.D.; McCombe, P.A. Assessment of Motor Units in Neuromuscular Disease. Neurotherapeutics 2017, 14, 69–77. [Google Scholar] [CrossRef]
- Kariyawasam, D.S.T.; D’Silva, A.; Lin, C.; Ryan, M.M.; Farrar, M.A. Biomarkers and the Development of a Personalized Medicine Approach in Spinal Muscular Atrophy. Front. Neurol. 2019, 10, 898. [Google Scholar] [CrossRef]
- Pino, M.G.; Rich, K.A.; Kolb, S.J. Update on Biomarkers in Spinal Muscular Atrophy. Biomark. Insights 2021, 16, 11772719211035643. [Google Scholar] [CrossRef]
- Gavriilaki, M.; Moschou, M.; Papaliagkas, V.; Notas, K.; Chatzikyriakou, E.; Zafeiridou, G.; Papagiannopoulos, S.; Arnaoutoglou, M.; Kimiskidis, V.K. Biomarkers of disease progression in adolescents and adults with 5q spinal muscular atrophy: A systematic review and meta-analysis. Neuromuscul. Disord. 2022, 32, 185–194. [Google Scholar] [CrossRef]
- Glascock, J.; Darras, B.T.; Crawford, T.O.; Sumner, C.J.; Kolb, S.J.; DiDonato, C.; Elsheikh, B.; Howell, K.; Farwell, W.; Valente, M.; et al. Identifying Biomarkers of Spinal Muscular Atrophy for Further Development. J. Neuromuscul. Dis. 2023, 10, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Geisbush, T.R.; Arnold, W.D.; Rosen, G.D.; Zaworski, P.G.; Rutkove, S.B. A comparison of three electrophysiological methods for the assessment of disease status in a mild spinal muscular atrophy mouse model. PLoS ONE 2014, 9, e111428. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.; McGovern, V.L.; Sanchez, B.; Li, J.; Corlett, K.M.; Kolb, S.J.; Rutkove, S.B.; Burghes, A.H. The neuromuscular impact of symptomatic SMN restoration in a mouse model of spinal muscular atrophy. Neurobiol. Dis. 2016, 87, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Sonbas Cobb, B.; Kolb, S.J.; Rutkove, S.B. Machine learning-enhanced electrical impedance myography to diagnose and track spinal muscular atrophy progression. Physiol. Meas. 2024, 45, 095002. [Google Scholar] [CrossRef]
- Mah, J.K.; van Alfen, N. Neuromuscular Ultrasound: Clinical Applications and Diagnostic Values. Can. J. Neurol. Sci. 2018, 45, 605–619. [Google Scholar] [CrossRef]
- Regensburger, A.P.; Wagner, A.L.; Danko, V.; Jüngert, J.; Federle, A.; Klett, D.; Schuessler, S.; Buehler, A.; Neurath, M.F.; Roos, A.; et al. Multispectral optoacoustic tomography for non-invasive disease phenotyping in pediatric spinal muscular atrophy patients. Photoacoustics 2022, 25, 100315. [Google Scholar] [CrossRef]
- Angilletta, I.; Ferrante, R.; Giansante, R.; Lombardi, L.; Babore, A.; Dell’Elice, A.; Alessandrelli, E.; Notarangelo, S.; Ranaudo, M.; Palmarini, C.; et al. Spinal Muscular Atrophy: An Evolving Scenario through New Perspectives in Diagnosis and Advances in Therapies. Int. J. Mol. Sci. 2023, 24, 14873. [Google Scholar] [CrossRef]
- Feldkötter, M.; Schwarzer, V.; Wirth, R.; Wienker, T.F.; Wirth, B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: Fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am. J. Hum. Genet. 2002, 70, 358–368. [Google Scholar] [CrossRef]
- Wadman, R.I.; Stam, M.; Gijzen, M.; Lemmink, H.H.; Snoeck, I.N.; Wijngaarde, C.A.; Braun, K.P.; Schoenmakers, M.A.; van den Berg, L.H.; Dooijes, D.; et al. Association of motor milestones, SMN2 copy and outcome in spinal muscular atrophy types 0–4. J. Neurol. Neurosurg. Psychiatry 2017, 88, 365–367. [Google Scholar] [CrossRef]
- Simic, G. Pathogenesis of proximal autosomal recessive spinal muscular atrophy. Acta Neuropathol. 2008, 116, 223–234. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Wirth, B.; Karakaya, M.; Kye, M.J.; Mendoza-Ferreira, N. Twenty-Five Years of Spinal Muscular Atrophy Research: From Phenotype to Genotype to Therapy, and What Comes Next. Annu. Rev. Genom. Hum. Genet. 2020, 21, 231–261. [Google Scholar] [CrossRef]
- Li, J.Y.; Dai, Y.; Sun, X.H.; Ren, H.T.; Shen, D.C.; Yang, X.Z.; Liu, M.S.; Cui, L.Y. Comparison of neurofilament light and heavy chain in spinal muscular atrophy and amyotrophic lateral sclerosis: A pilot study. Brain Behav. 2023, 13, e2997. [Google Scholar] [CrossRef]
- Cheng, X.; Li, Y.N.; Fan, Y.B.; Zhao, H.H.; Li, L.; Lu, C.; Zhu, L.H.; Niu, Q. Cytokines in cerebrospinal fluid as a prognostic predictor after treatment of nusinersen in SMA patients. Clin. Neurol. Neurosurg. 2024, 244, 108462. [Google Scholar] [CrossRef] [PubMed]
- Wurster, C.D.; Günther, R.; Steinacker, P.; Dreyhaupt, J.; Wollinsky, K.; Uzelac, Z.; Witzel, S.; Kocak, T.; Winter, B.; Koch, J.C.; et al. Neurochemical markers in CSF of adolescent and adult SMA patients undergoing nusinersen treatment. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419846058. [Google Scholar] [CrossRef] [PubMed]
- Gabanella, F.; Butchbach, M.E.; Saieva, L.; Carissimi, C.; Burghes, A.H.; Pellizzoni, L. Ribonucleoprotein assembly defects correlate with spinal muscular atrophy severity and preferentially affect a subset of spliceosomal snRNPs. PLoS ONE 2007, 2, e921. [Google Scholar] [CrossRef] [PubMed]
- Jangi, M.; Fleet, C.; Cullen, P.; Gupta, S.V.; Mekhoubad, S.; Chiao, E.; Allaire, N.; Bennett, C.F.; Rigo, F.; Krainer, A.R.; et al. SMN deficiency in severe models of spinal muscular atrophy causes widespread intron retention and DNA damage. Proc. Natl. Acad. Sci. USA 2017, 114, E2347–E2356. [Google Scholar] [CrossRef]
- Lotti, F.; Imlach, W.L.; Saieva, L.; Beck, E.S.; Hao, L.T.; Li, D.K.; Jiao, W.; Mentis, G.Z.; Beattie, C.E.; McCabe, B.D.; et al. An SMN-dependent U12 splicing event essential for motor circuit function. Cell 2012, 151, 440–454. [Google Scholar] [CrossRef]
- Ng, K.W.; Connolly, A.M.; Zaidman, C.M. Quantitative muscle ultrasound measures rapid declines over time in children with SMA type 1. J. Neurol. Sci. 2015, 358, 178–182. [Google Scholar] [CrossRef]
- Pillen, S.; Verrips, A.; van Alfen, N.; Arts, I.M.; Sie, L.T.; Zwarts, M.J. Quantitative skeletal muscle ultrasound: Diagnostic value in childhood neuromuscular disease. Neuromuscul. Disord. 2007, 17, 509–516. [Google Scholar] [CrossRef]
- Yang, C.W.; Chen, C.L.; Chou, W.C.; Lin, H.C.; Jong, Y.J.; Tsai, L.K.; Chuang, C.Y. An Integrative Transcriptomic Analysis for Identifying Novel Target Genes Corresponding to Severity Spectrum in Spinal Muscular Atrophy. PLoS ONE 2016, 11, e0157426. [Google Scholar] [CrossRef]
- Woschitz, V.; Mei, I.; Hedlund, E.; Murray, L.M. Mouse models of SMA show divergent patterns of neuronal vulnerability and resilience. Skelet. Muscle 2022, 12, 22. [Google Scholar] [CrossRef]
- Nichterwitz, S.; Nijssen, J.; Storvall, H.; Schweingruber, C.; Comley, L.H.; Allodi, I.; Lee, M.V.; Deng, Q.; Sandberg, R.; Hedlund, E. LCM-seq reveals unique transcriptional adaptation mechanisms of resistant neurons and identifies protective pathways in spinal muscular atrophy. Genome Res. 2020, 30, 1083–1096. [Google Scholar] [CrossRef]
- Barp, A.; Carraro, E.; Salmin, F.; Lizio, A.; Cheli, M.; Sansone, V. Facial nerve vulnerability in spinal muscular atrophy and motor unit number index of the orbicularis oculi muscle. Muscle Nerve 2023, 67, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Coppedè, F. Epigenetics of neuromuscular disorders. Epigenomics 2020, 12, 2125–2139. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Rabea, F.; Ramaswamy, S.; Chekroun, I.; El Naofal, M.; Jain, R.; Alfalasi, R.; Halabi, N.; Yaslam, S.; Sheikh Hassani, M.; et al. Long read sequencing enhances pathogenic and novel variation discovery in patients with rare diseases. Nat. Commun. 2025, 16, 2500. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, L.; Huang, M.; Wang, Y.; Cao, J.; Wen, F.; Xiao, W.; Liu, Q. Quantitative assessment of muscle atrophy and structural changes in children with spinal muscular atrophy using ultrasound imaging. Eur. J. Paediatr. Neurol. 2025, 57, 1–6. [Google Scholar] [CrossRef]
- Pelosi, L.; Rodrigues, M.; Zhong, C.; Patel, S.; Roxburgh, R. Quantitative muscle ultrasound in adult spinal muscular atrophy. A pilot study. Muscle Nerve 2024, 69, 349–353. [Google Scholar] [CrossRef]
- Virla, F.; Turano, E.; Scambi, I.; Schiaffino, L.; Boido, M.; Mariotti, R. Administration of adipose-derived stem cells extracellular vesicles in a murine model of spinal muscular atrophy: Effects of a new potential therapeutic strategy. Stem Cell Res. Ther. 2024, 15, 94. [Google Scholar] [CrossRef]
- Wu, L.; Sun, J.; Wang, L.; Chen, Z.; Guan, Z.; Du, L.; Qu, R.; Liu, C.; Shao, Y.; Hua, Y. Whole-transcriptome sequencing in neural and non-neural tissues of a mouse model identifies miR-34a as a key regulator in SMA pathogenesis. Mol. Ther. Nucleic Acids 2025, 36, 102490. [Google Scholar] [CrossRef]
- Schorling, D.C.; Kölbel, H.; Hentschel, A.; Pechmann, A.; Meyer, N.; Wirth, B.; Rombo, R.; SMArtCARE Consortium; Sickmann, A.; Kirschner, J.; et al. Cathepsin D as biomarker in cerebrospinal fluid of nusinersen-treated patients with spinal muscular atrophy. Eur. J. Neurol. 2022, 29, 2084–2096. [Google Scholar] [CrossRef]
- Nuzzo, T.; Russo, R.; Errico, F.; D’Amico, A.; Tewelde, A.G.; Valletta, M.; Hassan, A.; Tosi, M.; Panicucci, C.; Bruno, C.; et al. Nusinersen mitigates neuroinflammation in severe spinal muscular atrophy patients. Commun. Med. 2023, 3, 28. [Google Scholar] [CrossRef]
- McMillan, H.J.; Baranello, G.; Farrar, M.A.; Zaidman, C.M.; Moreno, T.; De Waele, L.; Jong, Y.J.; Laugel, V.; Quijano-Roy, S.; Mercuri, E.; et al. Safety and Efficacy of IV Onasemnogene Abeparvovec for Pediatric Patients With Spinal Muscular Atrophy: The Phase 3b SMART Study. Neurology 2025, 104, e210268. [Google Scholar] [CrossRef]
- Lu, I.N.; Cheung, P.F.Y.; Heming, M.; Thomas, C.; Giglio, G.; Leo, M.; Erdemir, M.; Wirth, T.; König, S.; Dambietz, C.A.; et al. Cell-mediated cytotoxicity within CSF and brain parenchyma in spinal muscular atrophy unaltered by nusinersen treatment. Nat. Commun. 2024, 15, 4120. [Google Scholar] [CrossRef] [PubMed]
- Weiß, C.; Becker, L.L.; Friese, J.; Blaschek, A.; Hahn, A.; Illsinger, S.; Schwartz, O.; Bernert, G.; Hagen, M.V.; Husain, R.A.; et al. Efficacy and safety of gene therapy with onasemnogene abeparvovec in children with spinal muscular atrophy in the D-A-CH-region: A population-based observational study. Lancet Reg. Health Eur. 2024, 47, 101092. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.; Kang, J.S.; Jo, Y.W.; Yoo, K.; Kim, Y.L.; Hann, S.H.; Kim, Y.E.; Kim, H.; Kim, J.H.; Kong, Y.Y. Improved therapeutic approach for spinal muscular atrophy via ubiquitination-resistant survival motor neuron variant. J. Cachexia Sarcopenia Muscle 2024, 15, 1404–1417. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, O.; Vill, K.; Pfaffenlehner, M.; Behrens, M.; Weiß, C.; Johannsen, J.; Friese, J.; Hahn, A.; Ziegler, A.; Illsinger, S.; et al. Clinical Effectiveness of Newborn Screening for Spinal Muscular Atrophy: A Nonrandomized Controlled Trial. JAMA Pediatr. 2024, 178, 540–547. [Google Scholar] [CrossRef]
- Strathmann, E.A.; Hölker, I.; Tschernoster, N.; Hosseinibarkooie, S.; Come, J.; Martinat, C.; Altmüller, J.; Wirth, B. Epigenetic regulation of plastin 3 expression by the macrosatellite DXZ4 and the transcriptional regulator CHD4. Am. J. Hum. Genet. 2023, 110, 442–459. [Google Scholar] [CrossRef]
- Valsecchi, V.; Anzilotti, S.; Serani, A.; Laudati, G.; Brancaccio, P.; Guida, N.; Cuomo, O.; Pignataro, G.; Annunziato, L. miR-206 Reduces the Severity of Motor Neuron Degeneration in the Facial Nuclei of the Brainstem in a Mouse Model of SMA. Mol. Ther. 2020, 28, 1154–1166. [Google Scholar] [CrossRef]
- Tizzano, E.F.; Lindner, G.; Chilcott, E.; Finkel, R.S.; Yáñez-Muñoz, R.J. In utero therapy for spinal muscular atrophy: Closer to clinical translation. Brain 2025, awaf123. [Google Scholar] [CrossRef]
- Zandl-Lang, M.; Züllig, T.; Holzer, M.; Eichmann, T.O.; Darnhofer, B.; Schwerin-Nagel, A.; Zobel, J.; Haidl, H.; Biebl, A.; Köfeler, H.; et al. Multi-omics profiling in spinal muscular atrophy (SMA): Investigating lipid and metabolic alterations through longitudinal CSF analysis of Nusinersen-treated patients. J. Neurol. 2025, 272, 1–18. [Google Scholar] [CrossRef]
- de Albuquerque, A.L.A.; Chadanowicz, J.K.; Giudicelli, G.C.; Staub, A.L.P.; Weber, A.C.; Silva, J.M.D.S.; Becker, M.M.; Kowalski, T.W.; Siebert, M.; Saute, J.A.M. Serum myostatin as a candidate disease severity and progression biomarker of spinal muscular atrophy. Brain Commun. 2024, 6, fcae062. [Google Scholar] [CrossRef]
- Sargeant, J.M.; O’Connor, A.M. Scoping Reviews, Systematic Reviews, and Meta-Analysis: Applications in Veterinary Medicine. Front. Veter- Sci. 2020, 7, 11. [Google Scholar] [CrossRef]
- Elliott, J.H.; Synnot, A.; Turner, T.; Simmonds, M.; Akl, E.A.; McDonald, S.; Salanti, G.; Meerpohl, J.; MacLehose, H.; Hilton, J.; et al. Living systematic review: 1. Introduction—the why, what, when, and how. J. Clin. Epidemiol. 2017, 91, 23–30. [Google Scholar] [CrossRef]
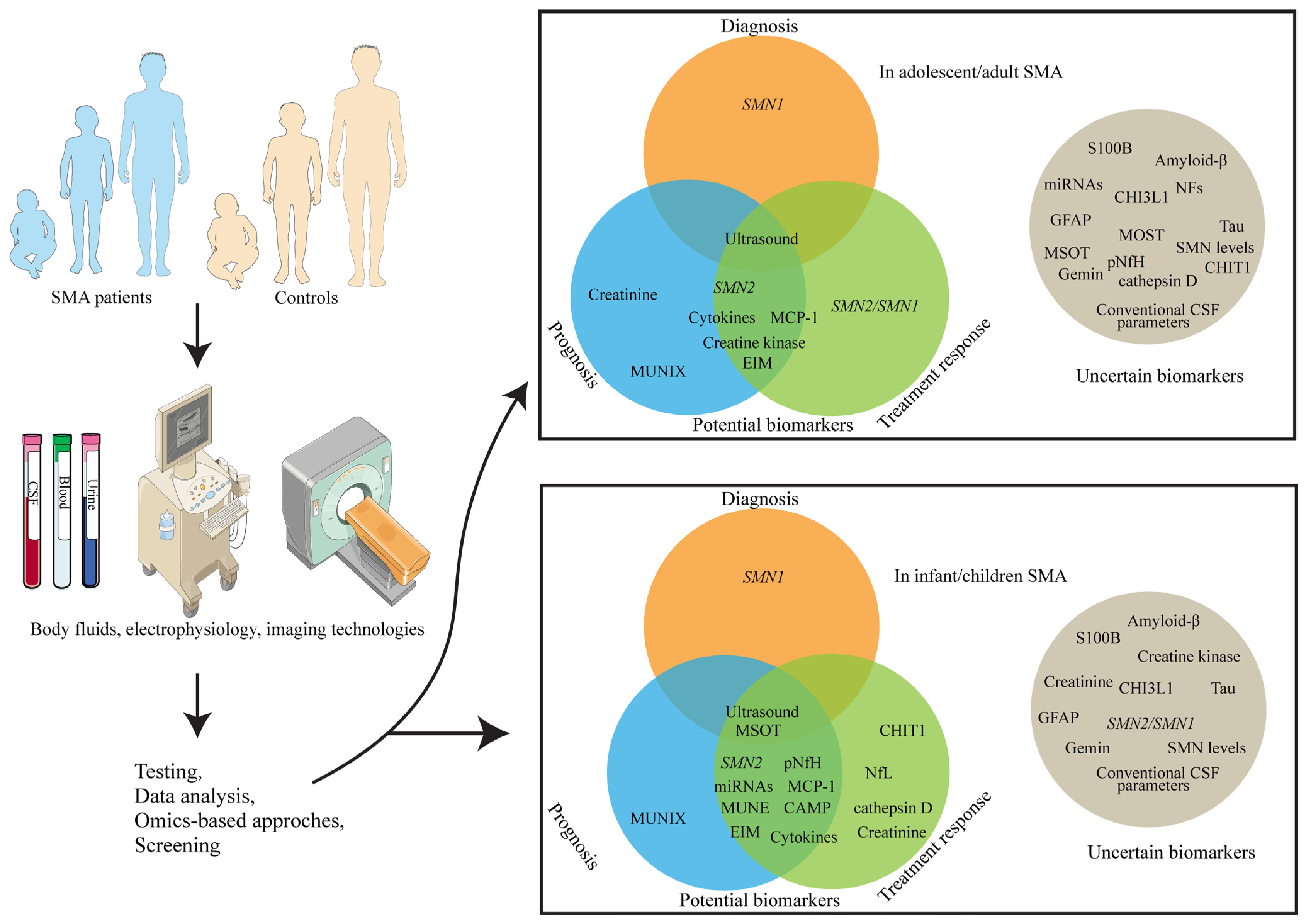
| Biomarkers | Disease Models | Functions | Application of Biomarkers | Reference |
|---|---|---|---|---|
| SMN1 gene | SMA patients | Encodes full-length, functional SMN protein; loss or mutation causes SMA | Deletion or mutation of the SMN1 gene constitutes a diagnostic biomarker, particularly when interpreted alongside clinical features. | [1,10,19] |
| SMN2 gene | SMA patients | Produces ~10% full-length SMN protein and ~90% truncated form. | SMN2 copy number is considered a neuroprotective modifier, inversely correlated with disease severity, and a predictor of treatment response in SMA | [20,21] |
| SMN2/SMN1 expression ratio | SMA patients | The SMN1 gene produces full-length, functional SMN protein, whereas the SMN2 gene generates predominantly truncated protein, with only ~10% being full-length. | Patients receiving nusinersen who showed clinical improvement exhibited an increased SMN2/SMN1 expression ratio, whereas those with stable disease had a decreased ratio. | [22] |
| SMN protein or mRNA | SMA mice; SMA patients | Normal SMN protein | The advantage lies in its high specificity, which can directly reflect the functional status of the SMN1 gene. However, the drawback is that its levels in peripheral blood may be influenced by multiple factors, and it may not fully reflect the changes in the central nervous system. | [20,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] |
| Neurofilaments (NFs): neurofilament light chain (NfL), neurofilament medium chain (NfM), neurofilament heavy chain (NfH) | SMA mice; SMA patients | Markers of neuronal injury, whose transcript and protein level changes can reflect disease progression and the degree of neuronal injury. | In SMA infants, it can serve as a reliable biomarker to assess prognosis and treatment response to nusinersen. However, its value as a biomarker in adult-onset SMA or during disease progression stages across all SMA types remains unclear. | [30,41,42,43,44,45,46,47,48,49,50,51,52] |
| Amyloid-β (Aβ40, Aβ42) | Alzheimer’s disease (AD) mice; SMA mice | Aβ is a key constituent of Alzheimer’s plaques, is neurotoxic due to its abnormal aggregation and deposition in the extracellular matrix. | Aβ protein levels (Aβ40, Aβ42, soluble amyloid precursor protein [sAPP]) correlate with motor scores in young SMA patients post-nusinersen treatment but require further validation as biomarkers for treatment response or progression. | [30,53,54,55,56,57,58] |
| Tau protein | SMA mice SMA patients | Tau, a microtubule-associated protein, plays a key role in facilitating microtubule polymerization and preserving cytoskeletal integrity. | Tau protein shows potential for monitoring nusinersen response in non-adult patients, though inconsistent in adults, requiring further validation in larger multicenter studies. NfL outperforms Tau as a biomarker for monitoring nusinersen treatment response in SMA. | [30,48,55,58,59,60,61,62,63,64,65] |
| Cerebrospinal fluid (CSF) parameters | SMA patients | Monitors inflammatory status via CSF cell counts/protein/albumin; detects nusinersen-associated “nusinophages” for treatment response insights. | Monitor nonspecific treatment-related inflammation (e.g., post-nusinersen injection) and explore “nusinophages” as potential biomarkers, requiring further clinical validation. | [30,50,63,66,67,68,69,70,71,72] |
| Cytokines (e.g., interleukin [IL]-8, monocyte chemoattractant protein-1 [MCP-1]) | SMA patients | Neuroinflammation-associated cytokines (e.g., IL-8, MCP-1) correlate with SMA severity baseline; track nusinersen treatment response/motor improvements; monitor SMA with progressive myoclonic epilepsy (SMA-PME) progression via plasma MCP-1 changes; suggest biomarker potential for disease regulation. | Cytokines (e.g., IL-8, MCP-1) assess SMA-PME disease status/treatment efficacy; confirm neuroinflammation pathophysiology via dysregulation; screen subtype-specific cytokine panels (SMA1, SMA-PME, adult SMA) for precision diagnosis/mechanistic research. | [30,73,74,75] |
| Creatine kinase (CK) | SMA patients | Muscle injury and rhabdomyolysis | The advantage is that it is readily measurable and directly correlated with muscle injury. It is related to disease severity and treatment response in adult SMA. However, its evaluation in pediatric SMA requires further investigation. | [30,76,77,78,79] |
| Creatinine (Crn) | SMA patients | Serum Crn is the end product of creatine metabolism in skeletal muscle, and its levels can reflect the functional status and energy metabolism of skeletal muscle. | Serum Crn shows promise as a biomarker for disease severity in adult and adolescent SMA patients, while urinary Crn shows promise as a biomarker for monitoring treatment response in SMA Type 1. However, more research is needed to validate serum Crn in pediatric populations, particularly infants and young children. | [30,76,80,81] |
| Chitotriosidase 1 (CHIT1) and CHI3L1/YKL-40 | SMA patients | Reflects the proliferation of microglia and astrocytes. | The advantage lies in its role as a biomarker of neuroinflammation, potentially reflecting the disease’s inflammatory status. CHIT1 appears to be a promising CSF biomarker for tracking treatment response in pediatric SMA, while CHI3L1 shows inconsistent changes across studies and no significant alterations in slowly progressing adult SMA patients treated with nusinersen. | [30,65,70,82,83,84] |
| Glial fibrillary acidic protein (GFAP) | SMA patients | Glial cell activation and neuroinflammation- | The advantage lies in its role as a biomarker of glial cell activation, potentially reflecting the disease’s inflammatory status. However, its levels may be influenced by multiple factors. | [30,48,51,85,86] |
| S100 calcium-binding protein B (S100B) | SMA patients | Predominantly localized in astrocytes of the nervous system, it serves as a reliable biomarker reflecting neural injury. | The advantage lies in its role as a biomarker of neural injury, potentially reflecting disease progression. However, its performance may vary across studies. | [30,59,62,87,88] |
| MicroRNAs (miRNAs) | SMA cell-based experiment; SMA patients | Regulate gene expression and disease progression | miRNAs show strong potential as biomarkers for tracking SMA progression and treatment response, particularly in pediatric and infant populations. However, more research is needed to establish their utility in adult SMA patients due to limited supporting data. | [30,89,90,91,92,93,94,95,96,97,98,99,100,101] |
| Gemin proteins | SMA zebrafish, Drosophila, and mice, human induced pluripotent stem cell-derived motor neurons | Serve as crucial chaperones for the SMN protein to regulate key steps in small nuclear ribonucleoprotein (snRNP) assembly precisely | The stability of the Gemin complex, particularly spinal Gemin2 levels, and its efficiency in snRNP assembly are linked to splicing dysfunction in SMA patients. However, Gemin proteins have limitations as SMA biomarkers due to low specificity, detection challenges, and insufficient clinical correlation. | [102,103,104,105,106,107] |
| Compound muscle action potential (CMAP) and MUNE (motor units number estimation) | SMA patients | Motor unit number/function assessment is critical for SMA diagnosis, disease monitoring, treatment evaluation, and clinical decision-making. | CMAP amplitude measures motor unit function to assess SMA severity and treatment response; MUNE methods (motor unit number index [MUNIX], multipoint) explore SMA applications but face limitations from proximal muscle weakness. | [41,108,109,110,111,112,113,114] |
| Electrical impedance myography (EIM) | SMA patients | EIM is a bioimpedance-based technology that is sensitive to muscle changes. | Due to its rapid, non-invasive, quantitative, and painless characteristics, this technology is highly suitable for tracking pediatric neuromuscular diseases. Additionally, it has previously been studied in older children with SMA. | [115] |
| Ultrasound | SMA patients | Quantifies muscle structural changes (e.g., increased echogenicity [EI], reduced muscle thickness [MT], moth-eaten heterogeneity) for disease assessment. | Muscle ultrasound identifies SMA patients (e.g., type 1) via EI deviations, quadriceps fat thickening, and mixed atrophy/hypertrophy; monitors progression in type 1 infants through parameter decline; evaluates treatment response (e.g., nusinersen) non-invasively. It also shows promise as a biomarker for diagnosing, monitoring disease progression, and assessing treatment response in adult SMA. However, larger validation studies are required to establish its clinical utility. | [116] |
| Multispectral optoacoustic tomography (MSOT) | SMA patients | Enables multispectral tissue imaging and quantitative analysis via photoacoustic ultrasonic signals generated from near-infrared laser absorption variations. | Photoacoustic signals assess SMA severity via disrupted muscle patterns and dispersed/diminished signals in ambulatory patients. MSOT holds promise for assessing pediatric SMA, but its utility in adult patients remains uncertain and requires further validation across age groups and disease stages. | [117] |
| Clinical Type | SMN2 Copy Number | Clinical Features |
| SMA Type 0 | 1 |
|
| SMA Type 1 | 1 or 2 |
|
| SMA Type 2 | 3 |
|
| SMA Type 3 | 3 or 4 |
|
| SMA Type 4 | 4 or more |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Zhan, Y.; Chen, H.; Deng, C. Application of Biomarkers in Spinal Muscular Atrophy. Int. J. Mol. Sci. 2025, 26, 6887. https://doi.org/10.3390/ijms26146887
Gao C, Zhan Y, Chen H, Deng C. Application of Biomarkers in Spinal Muscular Atrophy. International Journal of Molecular Sciences. 2025; 26(14):6887. https://doi.org/10.3390/ijms26146887
Chicago/Turabian StyleGao, Changyi, Yanqiang Zhan, Hong Chen, and Chunchu Deng. 2025. "Application of Biomarkers in Spinal Muscular Atrophy" International Journal of Molecular Sciences 26, no. 14: 6887. https://doi.org/10.3390/ijms26146887
APA StyleGao, C., Zhan, Y., Chen, H., & Deng, C. (2025). Application of Biomarkers in Spinal Muscular Atrophy. International Journal of Molecular Sciences, 26(14), 6887. https://doi.org/10.3390/ijms26146887







