Characteristics of Staphylococcus saprophyticus Isolated from Humans and Animals
Abstract
1. Introduction
2. Results
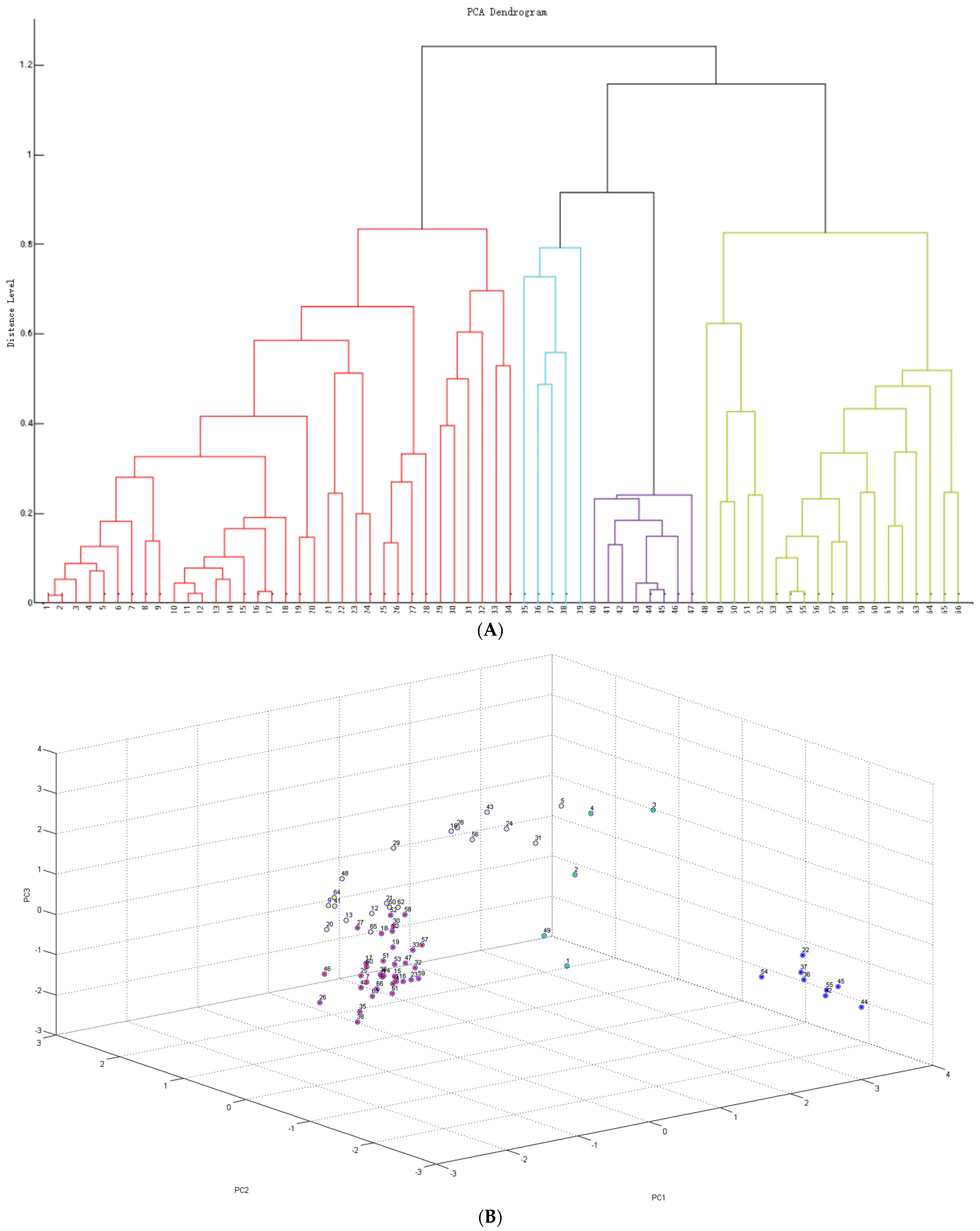
2.1. Identification and Cluster Analysis
2.2. Antimicrobial Phenotyping Testing
2.2.1. Disk Diffusion Method
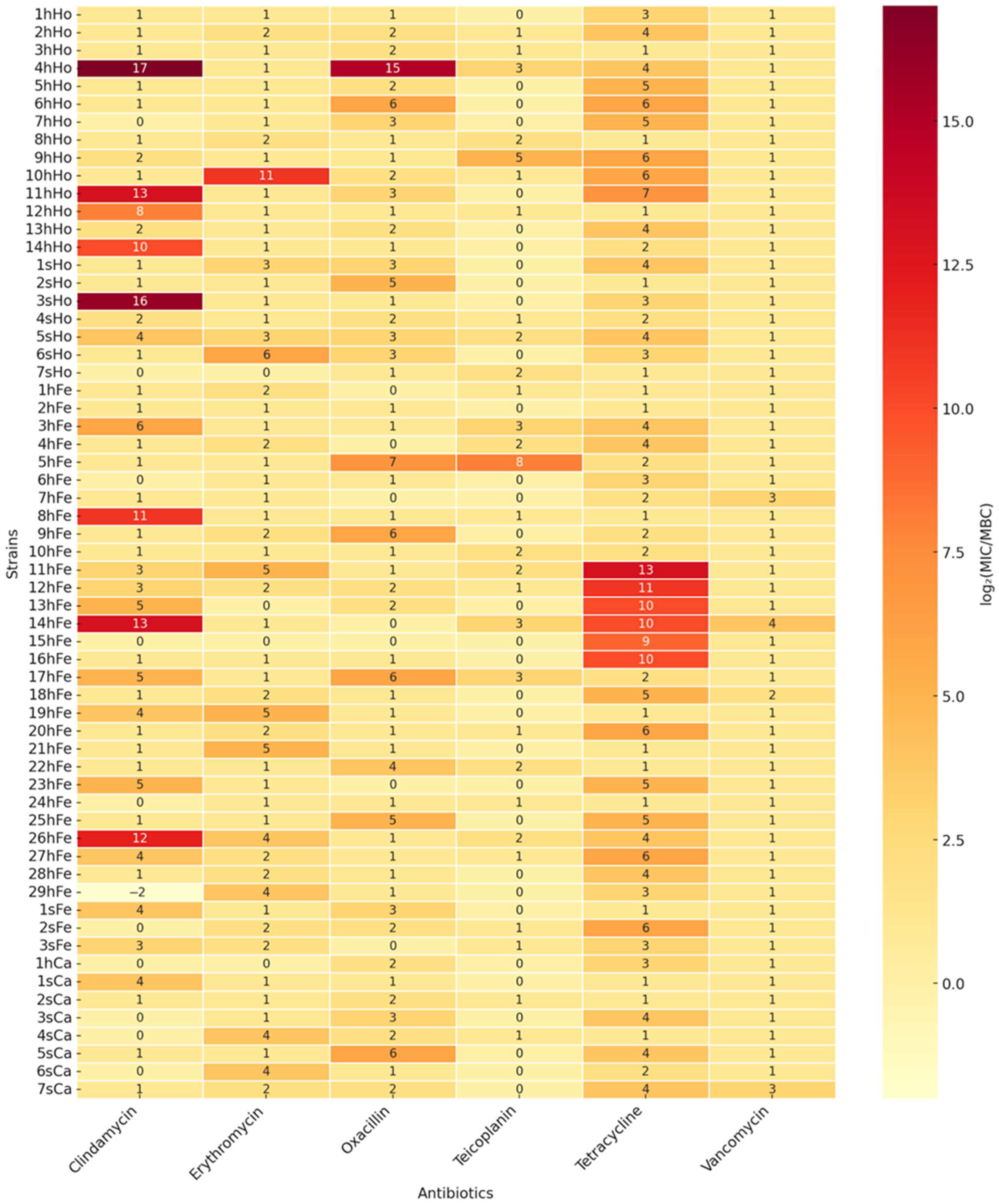
2.2.2. Minimal Inhibitory Concentration and Minimal Bactericidal Concentration for Selected Antimicrobials
2.3. Detection of Resistance Genes Using PCR-Based Genotypic Analysis
2.4. Analysis of Bacterial Growth Curves and Biofilm Formation
2.5. Concordance Between Phenotypic and Genotypic Resistance
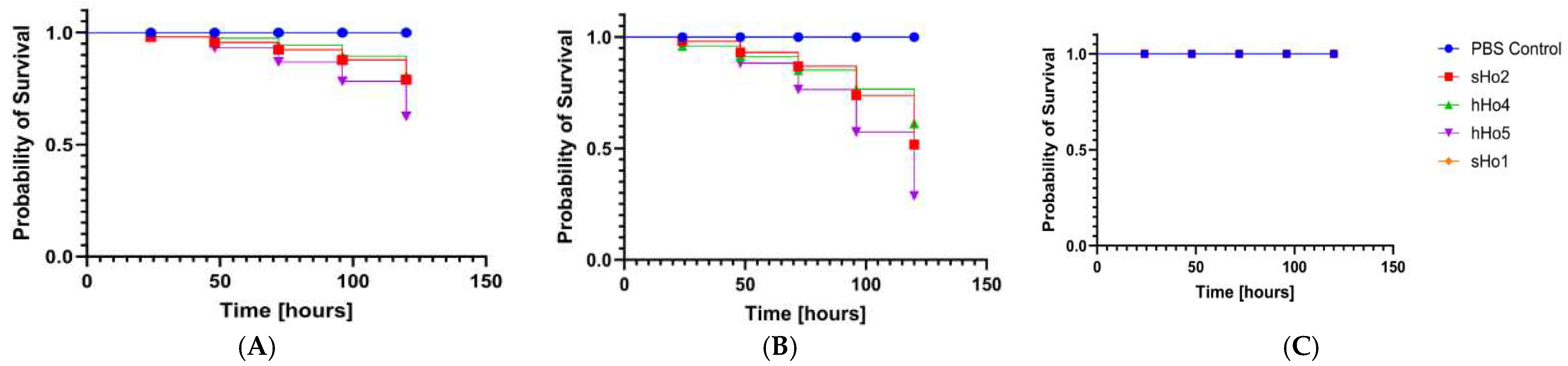
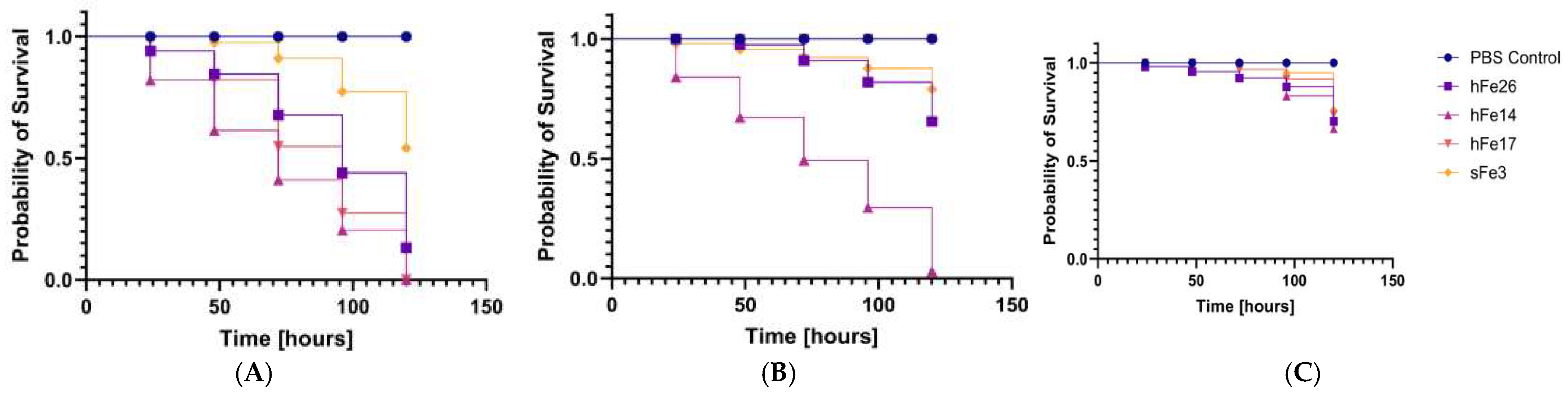
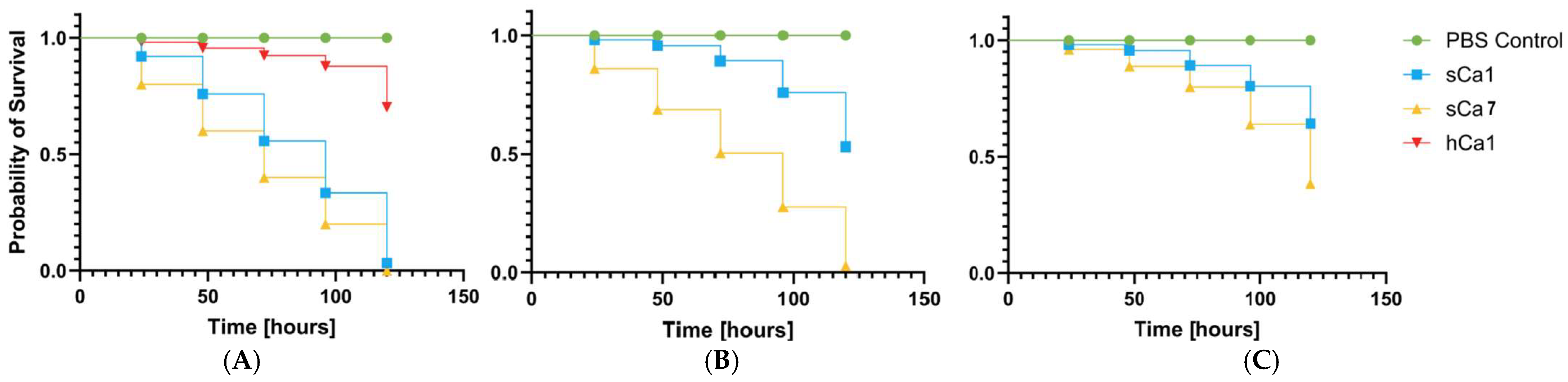
2.6. Analysis of Pathogenicity Tests on Galleria mellonella Larvae Model
3. Discussion
4. Materials and Methods
4.1. S. saprophyticus Strains Collection
4.2. Identification of S. saprophyticus
4.3. Detection of hrcA and Resistance Genes Using PCR-Based Genotypic Analysis
4.4. Antimicrobial Phenotypic Testing
4.5. Bacterial Growth Curves and Biofilm Formation
4.6. Pathogenicity Tests on Galleria mellonella Larvae Model
4.7. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [PubMed]
- Podkowik, M.; Bania, J.; Schubert, J.; Bystroń, J. Gronkowce koagulazo-ujemne: Nowe zagrożenie dla zdrowia publicznego? Życie Weter. 2014, 89, 60–66. [Google Scholar]
- Huebner, J.; Goldmann, D.A. Coagulase-negative staphylococci: Role as pathogens. Annu. Rev. Med. 1999, 50, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.; Ziebuhr, W.; Becker, K. Are coagulase-negative staphylococci virulent? Clin. Microbiol. Infect. 2019, 25, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Virulence factors of coagulase-negative staphylococci. Front. Biosci. 2004, 9, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Raz, R.; Colodner, R.; Kunin, C.M. Who are you—Staphylococcus saprophyticus? Clin. Infect. Dis. 2005, 40, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.E.; Soper, D.E.; Archer, G.L. Colonization of the female genital tract with Staphylococcus saprophyticus. J. Clin. Microbiol. 1992, 30, 2975–2979. [Google Scholar] [CrossRef] [PubMed]
- Białek, B.; Tyski, S.; Hryniewicz, W.; Kasprowicz, A.; Heczko, P.B. Role of Staphylococcus saprophyticus in human infection. Acta Microbiol. Pol. 1990, 39, 129–135. [Google Scholar] [PubMed]
- Latham, R.H.; Running, K.; Stamm, W.E. Urinary tract infections in young adult women caused by Staphylococcus saprophyticus. JAMA 1983, 250, 3063–3066. [Google Scholar] [CrossRef] [PubMed]
- Hovelius, B.; Mårdh, P.A. Staphylococcus saprophyticus as a common cause of urinary tract infections. Rev. Infect. Dis. 1984, 6, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.F.; Riley, T.V. Staphylococcus saprophyticus urinary tract infections: Epidemiological data from Western Australia. Eur. J. Epidemiol. 1996, 12, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Jordan, P.A.; Iravani, A.; Richard, G.A.; Baer, H. Urinary tract infection caused by Staphylococcus saprophyticus. J. Infect. Dis. 1980, 142, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Garduño, E.; Márquez, I.; Beteta, A.; Said, I.; Blanco, J.; Pineda, T. Staphylococcus saprophyticus causing native valve endocarditis. Scand. J. Infect. Dis. 2005, 37, 690–691. [Google Scholar] [CrossRef] [PubMed]
- Tamura, D.; Yamane, H.; Tabakodani, H.; Yamagishi, H.; Nakazato, E.; Kimura, Y.; Shinjoh, M.; Yamagata, T. Clinical impact of bacteremia due to Staphylococcus saprophyticus. Adv. Infect. Dis. 2021, 11, 6–12. [Google Scholar] [CrossRef]
- Hur, J.; Lee, A.; Hong, J.; Jo, W.Y.; Cho, O.H.; Kim, S.; Bae, I.G. Staphylococcus saprophyticus bacteremia originating from urinary tract infections: A case report and literature review. Infect. Chemother. 2016, 48, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.D.; Clarke, A.M.; Anderson, M.E.; Isaac-Renton, J.L.; McLoughlin, M.G. Urinary tract infections due to Staphylococcus saprophyticus biotype 3. CMAJ 1981, 124, 415–418. [Google Scholar]
- Golledge, C.L. Staphylococcus saprophyticus bacteremia. J. Infect. Dis. 1988, 157, 215. [Google Scholar] [CrossRef] [PubMed]
- Kline, K.A.; Kau, A.L.; Chen, S.L.; Lim, A.; Pinkner, J.S.; Rosch, J.; Nallapareddy, S.R.; Murray, B.E.; Henriques-Normark, B.; Beatty, W.; et al. Characterization of a novel murine model of Staphylococcus saprophyticus urinary tract infection reveals roles for Ssp and SdrI in virulence. Infect. Immun. 2010, 78, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Hedman, P.; Ringertz, O.; Lindström, M.; Olsson, K. The origin of Staphylococcus saprophyticus from cattle and pigs. Scand. J. Infect. Dis. 1993, 25, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Hedman, P.; Ringertz, O.; Eriksson, B.; Kvarnfors, P.; Andersson, M.; Bengtsson, L.; Olsson, K. Staphylococcus saprophyticus found to be a common contaminant of food. J. Infect. 1990, 21, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Penna, B.; Varges, R.; Martins, R.; Martins, G.; Lilenbaum, W. In vitro antimicrobial resistance of staphylococci isolated from canine urinary tract infection. Can. Vet. J. 2010, 51, 738–742. [Google Scholar] [PubMed]
- Hauschild, T.; Wójcik, A. Species distribution and properties of staphylococci from canine dermatitis. Res. Vet. Sci. 2007, 82, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mossakowski, P.; Lew-Kojrys, S. Nietypowy rodzaj kamieni moczowych u psa—Węglan apatytu. Mag. Wet. 2023, 11. Available online: https://magwet.pl/39622,nietypowy-rodzaj-kamieni-moczowych-u-psa-weglan-apatytu?page=2&srsltid=AfmBOoqy7mINfe1_aG_E86b7c1SQb289iagQZ0h_GMph-lL_k7zILTPG (accessed on 15 June 2025).
- Guo, C.; Sun, W.; Cheng, W.; Chen, N.; Lv, Y. Isolation and characterization of Staphylococcus saprophyticus responsible for the death of two six-band-banded armadillos (Euphractus sexcinctus). Vet. Rec. Case Rep. 2024, 12, e878. [Google Scholar] [CrossRef]
- Miszczak, M.; Korzeniowska-Kowal, A.; Wzorek, A.; Gamian, A.; Rypuła, K.; Bierowiec, K. Colonization of methicillin-resistant Staphylococcus species in healthy and sick pets: Prevalence and risk factors. BMC Vet. Res. 2023, 19, 85. [Google Scholar] [CrossRef] [PubMed]
- Kloos, W.E.; Bannerman, T.L. Update on clinical significance of coagulase-negative staphylococci. Clin. Microbiol. Rev. 1994, 7, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Schulin, T.; Voss, A. Coagulase-negative staphylococci as a cause of infections related to intravascular prosthetic devices: Limitations of present therapy. Clin. Microbiol. Infect. 2001, 7, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giormezis, N.; Kolonitsiou, F.; Foka, A.; Drougka, E.; Liakopoulos, A.; Makri, A.; Papanastasiou, A.D.; Vogiatzi, A.; Dimitriou, G.; Marangos, M.; et al. Coagulase-negative staphylococcal bloodstream and prosthetic-device-associated infections: The role of biofilm formation and distribution of adhesion and toxin genes. J. Med. Microbiol. 2014, 63, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- May, L.; Klein, E.Y.; Rothman, R.E.; Laxminarayan, R. Trends in antibiotic resistance in coagulase-negative staphylococci in the United States 1999 to 2012. Antimicrob. Agents Chemother. 2014, 58, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Marincola, G.; Liong, O.; Schoen, C.; Abouelfetouh, A.; Hamdy, A.; Wencker, F.D.R.; Marciniak, T.; Becker, K.; Köck, R.; Ziebuhr, W. Antimicrobial resistance profiles of coagulase-negative staphylococci in community-based healthy individuals in Germany. Front. Public Health 2021, 9, 684456. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Coagulase-negative staphylococci as reservoirs of genes facilitating MRSA infection. Bioessays 2012, 35, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Lai, L.C.; Chen, Y.A.; Lin, K.Y.; Chou, Y.H.; Chen, H.C.; Wang, S.S.; Wang, J.T.; Chang, S.C. Colonization with multidrug-resistant organism among healthy adults in the community setting: Prevalence, risk factors, and composition of gut microbiome. Front. Microbiol. 2020, 11, 1402. [Google Scholar] [CrossRef] [PubMed]
- Diop, M.; Bassoum, O.; Ndong, A.; Wone, F.; Tamouh, A.G.; Ndoye, M.; Youbong, T.; Daffé, S.M.M.; Radji, R.O.; Gueye, M.W.; et al. Prevalence of multidrug-resistant bacteria in healthcare and community settings in West Africa: Systematic review and meta-analysis. BMC Infect. Dis. 2025, 25, 292. [Google Scholar] [CrossRef] [PubMed]
- Cristino, J.A.; Pereira, A.T.; Andrade, L.G. Diversity of plasmids in Staphylococcus saprophyticus isolated from urinary tract infections in women. Epidemiol. Infect. 1989, 102, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.A.; Chopra, I.; O’Neill, A.J. Intrinsic novobiocin resistance in Staphylococcus saprophyticus. Antimicrob. Agents Chemother. 2007, 51, 4484–4485. [Google Scholar] [CrossRef] [PubMed]
- De Pavia-Santos, W.; Barros, E.M.; de Sousa, V.S.; Silva Laport, M.; Giambiagi-deMarval, M. Identification of coagulase-negative Staphylococcus saprophyticus by PCR based on the heat-shock repressor encoding hrcA gene. Diagn. Microbiol. Infect. Dis. 2016, 86, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Method for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically, Approved Standard, 10th ed.; CLSI Document M07-Ed10; CLSI: Wayne, PA, USA, 2024. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Suseptibility Test for Bacteria Isolated from Animals, 7th ed.; CLSI Supplement VET01S; CLSI: Wayne, PA, USA, 2024. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI Supplement M100; CLSI: Wayne, PA, USA, 2024. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 14.0; EUCAST: Växjö, Sweden, 2024. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Belas, A.; Franco, A.; Aboim, C.; Telo Gama, L.; Pomba, C. Increase in antimicrobial resistance and emergence of major international high-risk clonal lineages in dogs and cats with urinary tract infection: 16-year retrospective study. BMC Vet. Res. 2022, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Widerström, M.; Wiström, J.; Sjöstedt, A.; Monsen, T. Coagulase-negative staphylococci: Update on the molecular epidemiology and clinical presentation, with a focus on Staphylococcus epidermidis and Staphylococcus saprophyticus. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.L.R.S.; Sinzato, Y.K.; Silveira, L.V.A. Comparison of methods for the identification of coagulase-negative staphylococci. Mem. Inst. Oswaldo Cruz 2004, 99, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Kleeman, K.T.; Bannerman, T.L. Evaluation of the Vitek System Gram-positive identification card for species identification of coagulase-negative staphylococci. J. Clin. Microbiol. 1993, 31, 1322–1325. [Google Scholar] [CrossRef]
- Mlaga, K.D.; Dubourg, G.; Abat, C.; Chaudet, H.; Lotte, L.; Diene, S.M.; Raoult, D.; Ruimy, R.; Rolain, J.M. Using MALDI-TOF MS typing method to decipher outbreak: The case of Staphylococcus saprophyticus causing urinary tract infections (UTIs) in Marseille, France. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Dubois, D.; Leyssene, D.; Chacornac, J.P.; Kostrzewa, M.; Schmit, P.O.; Talon, R.; Bonnet, R.; Delmas, J. Identification of a variety of Staphylococcus species by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 2010, 48, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Potter, R.F.; Marino, J.; Muenks, C.E.; Lammers, M.G.; Dien Bard, J.; Dingle, T.C.; Humphries, R.; Westblade, L.F.; Burnham, C.A.D.; et al. Comparative genomics reveals the correlations of stress response genes and bacteriophages in developing antibiotic resistance of Staphylococcus saprophyticus. mSystems 2023, 8, e00697–23. [Google Scholar] [CrossRef] [PubMed]
- Youngblom, M.A.; Imhoff, M.R.; Smyth, L.M.; Mohamed, M.A.; Pepperell, C.S. Portrait of a generalist bacterium: Pathoadaptation, metabolic specialization and extreme environments shape diversity of Staphylococcus saprophyticus. BioRxiv 2023. [Google Scholar] [CrossRef]
- Lawal, O.U.; Fraqueza, M.J.; Bouchami, O.; Worning, P.; Bartels, M.D.; Gonçalves, M.L.; Paixão, P.; Gonçalves, E.; Toscano, C.; Empel, J.; et al. Foodborne origin and local and global spread of Staphylococcus saprophyticus causing human urinary tract infections. Emerg. Infect. Dis. 2021, 27, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.S.; Rabello, R.F.; Dias, R.C.S.; Martins, I.S.; Santos, L.B.G.S.; Alves, E.M.; Riley, L.W.; Moreira, B.M. Time-based distribution of Staphylococcus saprophyticus pulsed-field gel-electrophoresis clusters in community-acquired urinary tract infections. Mem. Inst. Oswaldo Cruz 2013, 108, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, M.; Tabarraei, A.; Yazdi, M.; Mohebbi, A.; Ghaemi, E.A. Antimicrobial resistance patterns of Staphylococcus saprophyticus isolates causing urinary tract infections in Gorgan, North of Iran. Med. Lab. J. 2023, 17, 33–38. [Google Scholar] [CrossRef]
- Khan, F.; Haadi, S.; Khan, F.A.; Shakir, J.; Shafiq, M.; Tariq, S.; Ahmad, J.; Afzal, Q.; Khan, A.A.; Afridi, P. Antibiotic susceptibility profile of Staphylococcus saprophyticus isolated from clinical samples in Peshawar, Pakistan. Sciencetech 2023, 4, 75–80. [Google Scholar]
- Marepalli, N.R.; Nadipelli, A.R.; Jain, M.K.; Parnam, L.S.; Vashyani, A. Patterns of Antibiotic Resistance in Urinary Tract Infections: A Retrospective Observational Study. Cureus 2024, 16, e62771. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Bonesso, M.F.; Mondelli, A.L.; Camargo, C.H.; Cunha, M.L.R.S. Oxacillin resistance and antimicrobial susceptibility profile of Staphylococcus saprophyticus. Chemotherapy 2002, 48, 267–270. [Google Scholar] [CrossRef]
- Chambers, H.F. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 2001, 7, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Jeong, D.W.; Lee, J.H. Genetic diversity and antibiotic resistance of Staphylococcus saprophyticus isolates from fermented foods and clinical samples. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 659–668. [Google Scholar] [CrossRef]
- Chua, K.Y.L.; Yang, M.; Wong, L.; Knox, J.; Lee, L.Y. Antimicrobial resistance and its detection in Staphylococcus saprophyticus urinary isolates. Pathology 2023, 55, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, F.J.; Theis, S.; Fluit, A.C.; Verhoef, J.; Heinz, H.P.; Jones, M.E. Antimicrobial susceptibility of coagulase-negative staphylococci isolated between 1991 and 1996 from a German university hospital. Clin. Microbiol. Infect. 1999, 5, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Wang, X.; Zhang, H.; Liu, J. Antimicrobial resistance and virulence profiles of staphylococci from clinical bovine mastitis in Ningxia Hui Autonomous Region of China. Front. Microbiol. 2023, 14, 1190790. [Google Scholar] [CrossRef]
- Amiri, R.; Alipour, M.; Engasi, A.K.; Amiri, A.R.; Mofarrah, R. Monitoring and investigation of resistance genes gyrA, parC, blaZ, ermA, ermB and ermC in Staphylococcus saprophyticus isolated from urinary tract infections in Mazandaran Province, Iran. Infect. Epidemiol. Microbiol. 2023, 9, 117–125. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, X.; Cai, S.; Hu, N.; Yuan, Y.; Wu, Y.; Wang, Y.; Mi, J.; Liao, X. Pet cats may shape the antibiotic resistome of their owner’s gut and living environment. Microbiome 2023, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Guardabassi, L.; Schwarz, S.; Lloyd, D.H. Pet animals are reservoirs of antimicrobial resistant bacteria: Review. J. Antimicrob. Chemother. 2004, 54, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Yazdankhah, S.P.; Asli, A.W.; Sorum, H.; Oppegaard, H.; Sunde, M. Fusidic acid resistance, mediated by fusB in bovine coagulase-negative staphylococci. J. Antimicrob. Chemother. 2006, 58, 1254–1256. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.B.; Gorwitz, R.J.; Jernigan, J.A. Mupirocin resistance. Clin. Infect. Dis. 2009, 49, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.H. Reservoirs of antimicrobial resistance in pet animals. Clin. Infect. Dis. 2007, 45 (Suppl. S2), S148–S152. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, K.; Katayama, Y.; Matsuo, M.; Sasaki, T.; Morimoto, Y.; Sekiguchi, A.; Baba, T. Multi-drug-resistant Staphylococcus aureus and future chemotherapy. J. Infect. Chemother. 2014, 20, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. Updated recommendations: International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Blondeau, J.; Boothe, D.; Breitschwerdt, E.; Guardabassi, L.; Hillier, A.; Lloyd, D.H.; Papich, M.G.; Rankin, S.; Turnidge, J.; et al. ISCAID guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. J. Vet. Intern. Med. 2019, 33, 1–13. [Google Scholar] [CrossRef]
- Hillier, A.; Lloyd, D.H.; Weese, J.S.; Blondeau, J.M.; Boothe, D.; Breitschwerdt, E.; Guardabassi, L.; Papich, M.G.; Rankin, S.; Turnidge, J.D.; et al. Guidelines for the diagnosis and antimicrobial therapy of canine superficial bacterial folliculitis (Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases). Vet. Dermatol. 2014, 25, 163–e43. [Google Scholar] [CrossRef] [PubMed]
- Archer, N.K.; Mazaitis, M.J.; Costerton, J.W.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus aureus biofilms: Properties, regulation, and roles in human disease. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.Y.; Loh, J.M.S.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Koksal, F.; Yasar, H.; Samasti, M. Antibiotic resistance patterns of coagulase-negative Staphylococcus strains isolated from blood cultures of septicemic patients in Turkey. Microbiol. Res. 2009, 164, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Coote, P.J. Wax moth larva (Galleria mellonella): An in vivo model for assessing the efficacy of antistaphylococcal agents. J. Antimicrob. Chemother. 2011, 66, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Champion, O.L.; Wagley, S.; Titball, R.W. Galleria mellonella as a model host for microbiological and toxin research. Virulence 2016, 7, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Bierowiec, K.; Korzeniowska-Kowal, A.; Wzorek, A.; Rypuła, K.; Gamian, A. Prevalence of Staphylococcus species colonization in healthy and sick cats. Biomed. Res. Int. 2019, 2019, 4360525. [Google Scholar] [CrossRef] [PubMed]
- Samad, R.A.; Al Disi, Z.; Ashfaq, M.Y.M.; Wahib, S.M.; Zouari, N. The use of principle component analysis and MALDI-TOF MS for the differentiation of mineral forming Virgibacillus and Bacillus species isolated from sabkhas. RSC Adv. 2020, 10, 14606–14616. [Google Scholar] [CrossRef] [PubMed]
- Amini, R.; As, A.; Chung, C.; Jahanshiri, F.; Wong, C.B.; Poyling, B.; Hematian, A.; Sekawi, Z.; Zargar, M.; Jalilian, F.A. Circulation and transmission of methicillin-resistant Staphylococcus aureus among college students in Malaysia (cell phones as reservoir). Asian Biomed. 2012, 6, 659–673. [Google Scholar]
- Ullah, F.; Malik, S.A.; Ahmed, J.; Ullah, F.; Shah, S.M.; Ayaz, M.; Hussain, S.; Khatoon, L. Investigation of the genetic basis of tetracycline resistance in Staphylococcus aureus from Pakistan. Trop. J. Pharm. Res. 2012, 11, 925–931. [Google Scholar] [CrossRef][Green Version]
- Saadat, S.; Solhjoo, K.; Norooz-Nejad, M.J.; Kazemi, A. VanA and VanB positive vancomycin resistant Staphylococcus aureus among clinical isolates in Shiraz, South Iran. Oman Med. J. 2014, 29, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Ciesielczuk, H.; Xenophontos, M.; Lambourne, J. Methicillin-resistant Staphylococcus aureus harboring mecC still eludes us in East London, United Kingdom. J. Clin. Microbiol. 2019, 57, e00020–19. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.; Chopra, I. Molecular basis of fusB-mediated resistance to fusidic acid in Staphylococcus aureus. Mol. Microbiol. 2006, 59, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Tasara, T.; Cernela, N.; Stephan, R. Function impairing mutations in blaZ and blaR genes of penicillin susceptible Staphylococcus aureus strains isolated from bovine mastitis. Schweiz. Arch. Tierheilkd. 2013, 155, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Šeol, B. Comparative in vitro activities of enrofloxacin, ciprofloxacin and marbofloxacin against Staphylococcus intermedius isolated from dogs. Vet. Arh. 2005, 75, 189–194. [Google Scholar]
- Płoneczka-Janeczko, K.; Bierowiec, K.; Lis, P.; Rypuła, K. Identification of bap and icaA genes involved in biofilm formation in coagulase-negative staphylococci isolated from feline conjunctiva. Vet. Res. Commun. 2014, 38, 337–346. [Google Scholar] [CrossRef] [PubMed]

| Antimicrobial Agents Evaluated | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | AMP | AMC | MUP | P | FD | MAR | CIP | OX | LZD | DA | E | C | RD | CN | SXT | TGC | TET |
| Healthy humans (n = 14) | 7 (50.0%) | 2 (14.29%) | 0 (0.0%) | 5 (35.71%) | 4 (28.57%) | 1 (7.14%) | 0 (0.0%) | 6 (42.86%) | 0 (0.0%) | 1 (7.14%) | 7 (50.0%) | 2 (14.29%) | 0 (0.0%) | 1 (7.14%) | 0 (0.0%) | 1 (7.14%) | 0 (0.0%) |
| Sick humans (n = 7) | 3 (42.86%) | 0 (0.0%) | 0 (0.0%) | 1 (14.29%) | 3 (42.86%) | 0 (0.0%) | 1 (14.29%) | 3 (42.86%) | 0 (0.0%) | 0 (0.0%) | 4 (57.14%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (14.29%) |
| Healthy cats (n = 29) | 6 (20.69%) | 2 (6.9%) | 2 (6.9%) | 7 (24.14%) | 5 (17.24%) | 2 (6.9%) | 4 (13.79%) | 9 (31.03%) | 1 (3.45%) | 3 (10.34%) | 9 (31.03%) | 2 (6.9%) | 1 (3.45%) | 2 (6.9%) | 0 (0.0%) | 0 (0.0%) | 5 (17.24%) |
| Sick cats (n = 3) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (33.33%) | 0 (0.0%) | 0 (0.0%) | 2 (66.66%) | 0 (0.0%) | 1 (33.33%) | 1 (33.33%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) |
| Healthy dogs (n = 1) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (100.0%) | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Sick dogs (n = 7) | 1 (14.29%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (28.57%) | 0 (0.0%) | 0 (0.0%) | 6 (85.71%) | 0 (0.0%) | 3 (42.86%) | 7 (100.0%) | 1 (14.29%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (14.29%) |
| All (n = 61) | 17 (27.87%) | 4 (6.56%) | 2 (3.28%) | 13 (21.31%) | 15 (24.59%) | 3 (4.92%) | 5 (8.20%) | 26 (42.62%) | 1 (1.64%) | 9 (14.75%) | 29 (47.54%) | 5 (8.20%) | 1 (1.64%) | 3 (4.92%) | 0 (0.0%) | 1 (1.64%) | 8 (13.11%) |
| Antimicrobial Resistance Genes Tested | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | blaZ | mecA | mecC | Aac * | ermA | ermB | ermC | tetK | tetL | tetM | tetO | fusB | vanA | vanB | mupA |
| Healthy humans (n = 14) | 14 (100%) | 14 (100%) | 0 (0%) | 0 (0%) | 13 (92.86%) | 13 (92.86%) | 0 (0%) | 0 (0.0%) | 10 (71.43%) | 14 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7.14%) | 0 (0%) |
| Sick humans (n = 7) | 7 (100%) | 7 (100%) | 0 (0%) | 0 (0%) | 6 (85.71%) | 4 (57.14%) | 0 (0%) | 0 (0%) | 7 (100%) | 7 (100%) | 2 (28.57%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Healthy cats (n = 29) | 29 (100%) | 29 (100%) | 0 (0%) | 4 (13.79%) | 25 (86.21%) | 20 (68.97%) | 4 (13.79%) | 0 (0%) | 8 (27.59%) | 28 (96.55%) | 5 (17.24%) | 4 (13.79%) | 0 (0%) | 0 (0%) | 2 (6.9%) |
| Sick cats (n = 3) | 3 (100%) | 3 (100%) | 0 (0%) | 0 (0%) | 3 (100%) | 1 (33.3%) | 0 (0%) | 0 (0%) | 2 (66.67%) | 3 (100%) | 1 (33.33%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Healthy dogs (n = 1) | 1 (100%) | 1 (100%) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Sick dogs (n = 7) | 7 (100%) | 7 (100%) | 0 (0%) | 0 (0%) | 6 (85.71%) | 3 (42.86%) | 0 (0%) | 0 (0%) | 5 (71.43%) | 7 (100%) | 1 (14.29%) | 0 (0%) | 0 (0%) | 1 (14.29%) | 0 (0%) |
| All (n = 61) | 61 (100.0%) | 61 (100%) | 0 (0.0%) | 4 (6.56%) | 54 (88.52%) | 41 (67.21%) | 4 (6.56%) | 0 (0%) | 32 (52.46%) | 60 (98.36%) | 11 (18.03%) | 4 (6.6%) | 0 (0.0%) | 2 (3.28%) | 2 (3.28%) |
| No. | Gene/Antibiotic | Agreement Type | McNemar’s p < 0.05 | Cohen’s κ |
|---|---|---|---|---|
| 1 | blaZ/ampicillin | No agreement | + | 0.00 |
| 2 | blaZ/penicillin | No agreement | + | 0.00 |
| 3 | blaZ/amoxicillin–clavulanic acid | No agreement | + | 0.00 |
| 4 | mecA/oxacillin (DD) | No agreement | + | 0.00 |
| 5 | ermB/erythromycin (MIC) | Slight agreement | - | 0.06 |
| 6 | tetM/tetracycline (MIC) | Worse than chance agreement | + | −0.03 |
| 7 | vanB/teicoplanin | Perfect agreement | - | 1.00 |
| 8 | fusB/fusidic acid | Slight agreement | + | 0.12 |
| 9 | mupA/mupirocin | Perfect agreement | - | 1.00 |
| 10 | aac(6′)-Ie-aph(2″)-Ia/gentamicin | Slight agreement | - | 0.16 |
| Groups | No Data | Oral Cavity | Nostrils | Anus | Wound | Skin | Ear Canal | Conjunctival Sac | Total |
|---|---|---|---|---|---|---|---|---|---|
| Healthy humans | 0 | 2 | 4 | 0 | 0 | 4 | 4 | 0 | 14 |
| Sick humans | 5 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 7 |
| Healthy cats | 0 | 2 | 6 | 2 | 0 | 6 | 5 | 8 | 29 |
| Sick cats | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 |
| Healthy dogs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Sick dogs | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 3 | 7 |
| All | 5 | 4 | 15 | 2 | 1 | 11 | 11 | 12 | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prorok, P.; Bierowiec, K.; Skrok, M.; Karwańska, M.; Siedlecka, M.; Miszczak, M.; Książczyk, M.; Kapczyńska, K.; Rypuła, K. Characteristics of Staphylococcus saprophyticus Isolated from Humans and Animals. Int. J. Mol. Sci. 2025, 26, 6885. https://doi.org/10.3390/ijms26146885
Prorok P, Bierowiec K, Skrok M, Karwańska M, Siedlecka M, Miszczak M, Książczyk M, Kapczyńska K, Rypuła K. Characteristics of Staphylococcus saprophyticus Isolated from Humans and Animals. International Journal of Molecular Sciences. 2025; 26(14):6885. https://doi.org/10.3390/ijms26146885
Chicago/Turabian StyleProrok, Paulina, Karolina Bierowiec, Milena Skrok, Magdalena Karwańska, Magdalena Siedlecka, Marta Miszczak, Marta Książczyk, Katarzyna Kapczyńska, and Krzysztof Rypuła. 2025. "Characteristics of Staphylococcus saprophyticus Isolated from Humans and Animals" International Journal of Molecular Sciences 26, no. 14: 6885. https://doi.org/10.3390/ijms26146885
APA StyleProrok, P., Bierowiec, K., Skrok, M., Karwańska, M., Siedlecka, M., Miszczak, M., Książczyk, M., Kapczyńska, K., & Rypuła, K. (2025). Characteristics of Staphylococcus saprophyticus Isolated from Humans and Animals. International Journal of Molecular Sciences, 26(14), 6885. https://doi.org/10.3390/ijms26146885







