The Study of Approaches to Modeling Oxidative Stress in Male Wistar Rats: The Comparative Analysis of Diet-Induced, Chemically Induced, and Physiologically Induced Models
Abstract
1. Introduction
2. Results
2.1. Fatty Acid Composition
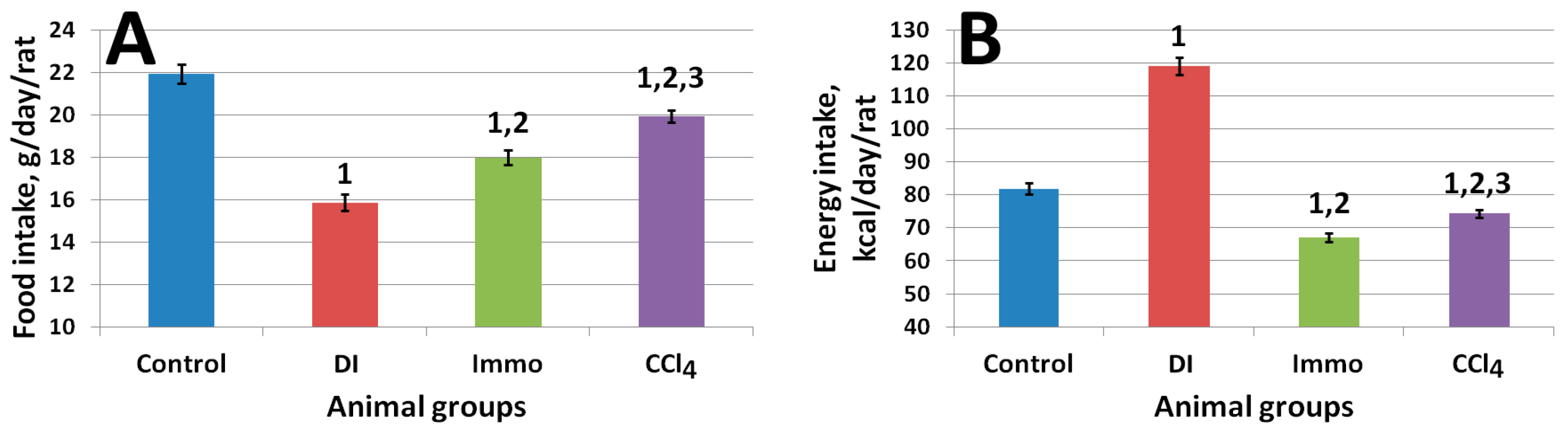
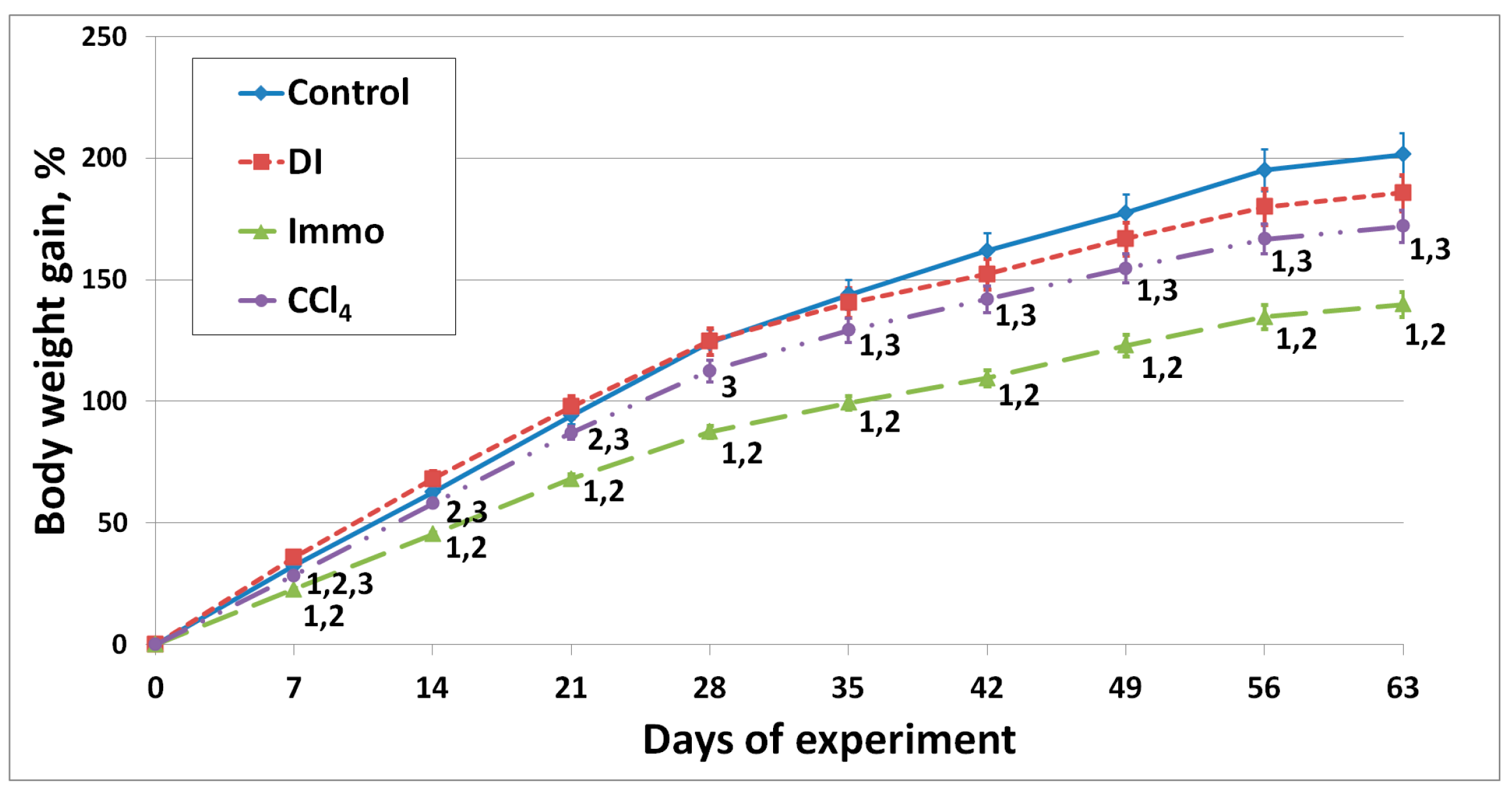
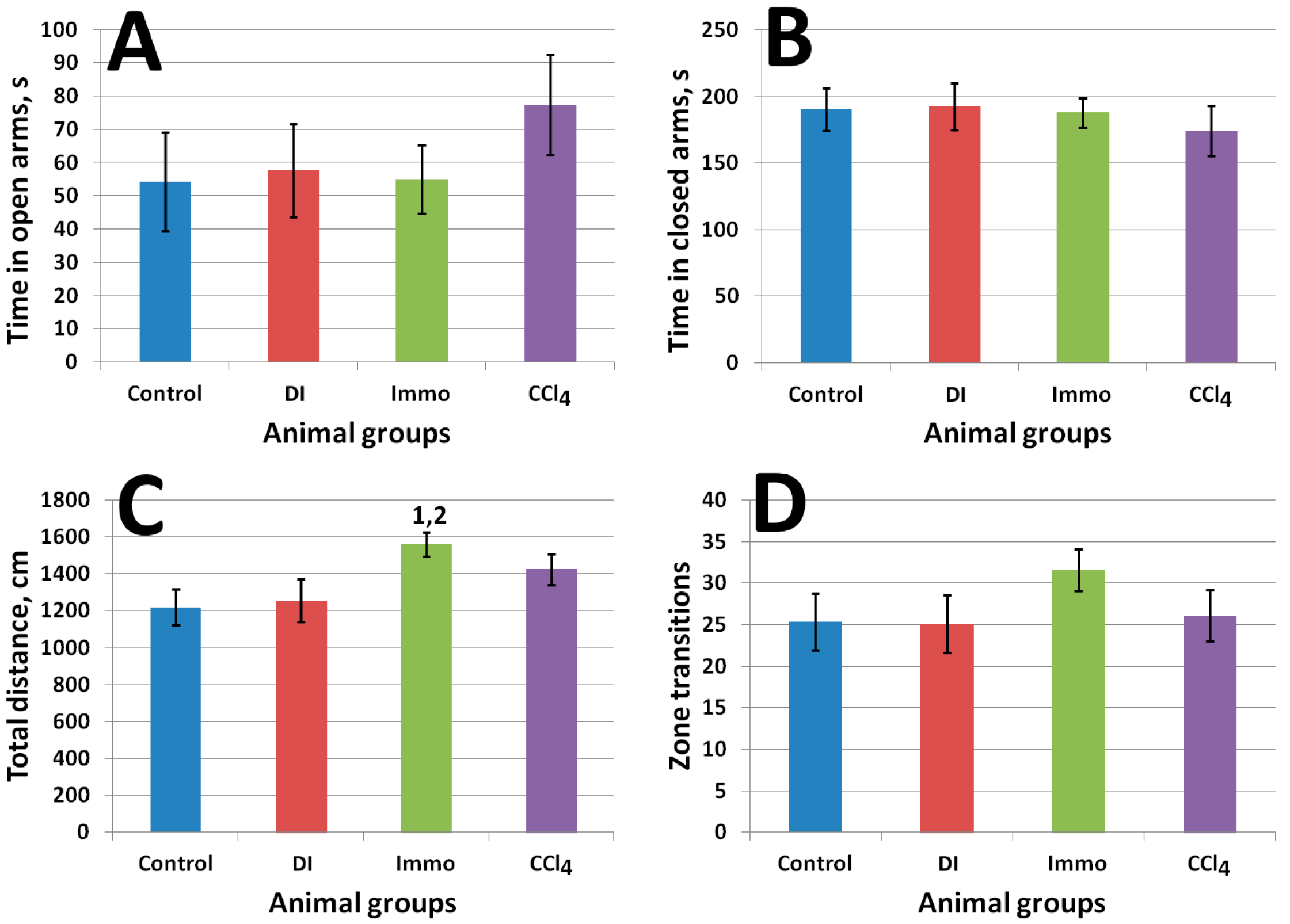
2.2. In Vivo Study Results
3. Discussion
Limitations of the Study
4. Materials and Methods
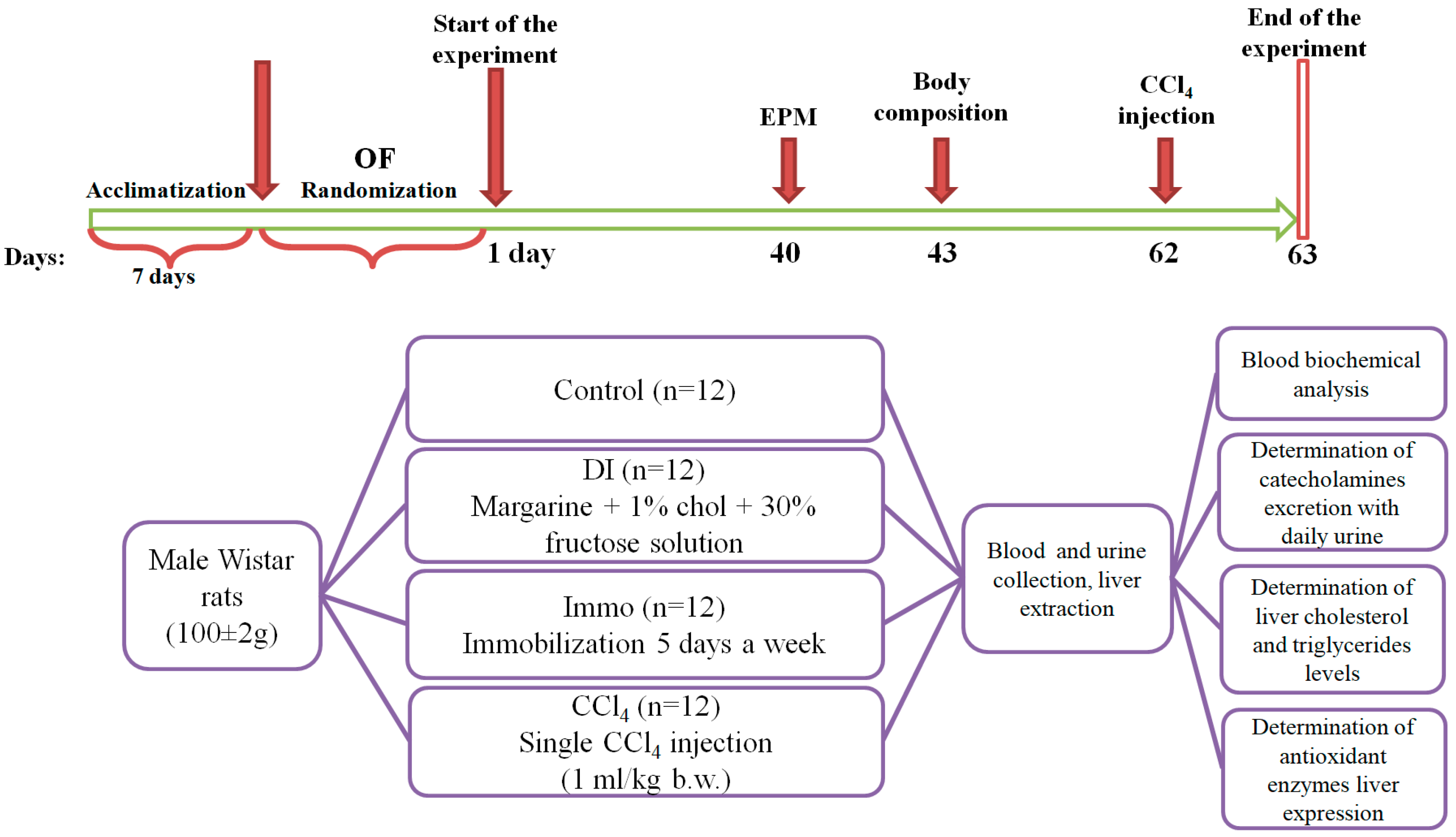
4.1. Experimental Animals
4.2. Determination of Fatty Acid Composition of Oil Samples (Sunflower and Soybean)
Gas Chromatography Conditions with Flame Ionization Detector
4.3. Experimental Design
4.4. Antioxidant System Gene Expression
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| ALP | Alkaline phosphatase |
| AST | Aspartate aminotransferase |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| MDA | Malon dialdehyde |
| OF | Open Field |
| PCR | Polymerase chain reaction |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
References
- Wilson, O.W.A.; Garra, S.; Bopp, M.; Bopp, C.M. Incorporating the American College of Cardiology/American Heart Association Hypertension Diagnostic Criteria into Metabolic Syndrome Criteria Will Significantly Increase the Prevalence of Metabolic Syndrome among College Students. J. Hum. Hypertens. 2021, 35, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sairam, M.R. Sex Hormone Imbalances and Adipose Tissue Dysfunction Impacting on Metabolic Syndrome; a Paradigm for the Discovery of Novel Adipokines. Horm. Mol. Biol. Clin. Investig. 2014, 17, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [PubMed]
- Doulberis, M.; Papaefthymiou, A.; Polyzos, S.A.; Katsinelos, P.; Grigoriadis, N.; Srivastava, D.S.; Kountouras, J. Rodent Models of Obesity. Minerva Endocrinol. 2020, 45, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Gao, Y.; Xue, Y.; Zhang, C.; Qiu, J.; Qian, Y.; Fang, M.; Zhang, X.; Sun, X.; Kong, X.; et al. Shenge Formula Attenuates High-Fat Diet-Induced Obesity and Fatty Liver via Inhibiting ACOX1. Phytomedicine 2024, 123, 155183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yuan, Y.; Hu, L.; Tang, J.; Meng, Z.; Dai, L.; Gao, Y.; Ma, S.; Wang, X.; Yuan, Y.; et al. ANGPTL8 Accelerates Liver Fibrosis Mediated by HFD-Induced Inflammatory Activity via LILRB2/ERK Signaling Pathways. J. Adv. Res. 2023, 47, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Malakul, W.; Seenak, P.; Jumroon, N.; Arikit, S.; Kumphune, S.; Nernpermpisooth, N. Novel Coconut Vinegar Attenuates Hepatic and Vascular Oxidative Stress in Rats Fed a High-Cholesterol Diet. Front. Nutr. 2022, 9, 835278. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, F.; Kucukler, S.; Eldutar, E.; Caglayan, C.; Gülçin, İ. Chrysin Protects Rat Kidney from Paracetamol-Induced Oxidative Stress, Inflammation, Apoptosis, and Autophagy: A Multi-Biomarker Approach. Sci. Pharm. 2017, 85, 4. [Google Scholar] [CrossRef] [PubMed]
- Kadiiska, M.B.; Peddada, S.; Herbert, R.A.; Basu, S.; Hensley, K.; Jones, D.P.; Hatch, G.E.; Mason, R.P. Biomarkers of Oxidative Stress Study VI. Endogenous Plasma Antioxidants Fail as Useful Biomarkers of Endotoxin-Induced Oxidative Stress. Free Radic. Biol. Med. 2015, 81, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, W.; Cheng, N.; Zhu, Y.; Li, H.; Zhang, S.; Guo, W.; Ge, G. Pectolinarigenin Ameliorates Acetaminophen-Induced Acute Liver Injury via Attenuating Oxidative Stress and Inflammatory Response in Nrf2 and PPARa Dependent Manners. Phytomedicine 2023, 113, 154726. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Mohammad Pour Kargar, H.; Beheshti, F.; Anaeigoudari, A.; Vaezi, G.; Hosseini, M. The Effects of Carvacrol on Oxidative Stress, Inflammation, and Liver Function Indicators in a Systemic Inflammation Model Induced by Lipopolysaccharide in Rats. Int. J. Vitam. Nutr. Res. 2023, 93, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Khan, A.; Baig, M.W.; Ullah, N.; Ahmed, N.; Tipu, M.K.; Ali, H.; Khan, S. Poncirin Attenuates CCL4-Induced Liver Injury through Inhibition of Oxidative Stress and Inflammatory Cytokines in Mice. BMC Complement. Med. Ther. 2020, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Huang, C.; Wu, Q.; Su, Y.; Wang, X.; Xuan, Z.; Wang, Y.; Xu, F.; Ge, C. Sini San Ameliorates CCl4-Induced Liver Fibrosis in Mice by Inhibiting AKT-Mediated Hepatocyte Apoptosis. J. Ethnopharmacol. 2023, 303, 115965. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, S.-S.; An, M.; Xia, Y.; Fan, M.-S.; Sun, Z.; Zhang, L.-J.; Zhao, Y.; Sheng, J.; Wang, X.-J. Neferine Ameliorates Nonalcoholic Steatohepatitis through Regulating AMPK Pathway. Phytomedicine 2023, 114, 154798. [Google Scholar] [CrossRef] [PubMed]
- Fareed, M.M.; Khalid, H.; Khalid, S.; Shityakov, S. Deciphering Molecular Mechanisms of Carbon Tetrachloride- Induced Hepatotoxicity: A Brief Systematic Review. Curr. Mol. Med. 2024, 24, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Unsal, V.; Cicek, M.; Sabancilar, İ. Toxicity of Carbon Tetrachloride, Free Radicals and Role of Antioxidants. Rev. Environ. Health 2021, 36, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of Lipid Peroxidation by Measuring Malondialdehyde (MDA) and Relatives in Biological Samples: Analytical and Biological Challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.W.D.; Boll, M.; Stampfl, A. Hepatotoxicity and Mechanism of Action of Haloalkanes: Carbon Tetrachloride as a Toxicological Model. Crit. Rev. Toxicol. 2003, 33, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Karna, K.; Soni, K.; You, J.; Choi, N.; Kim, H.; Kim, C.; Lee, S.; Shin, Y.; Park, J. MOTILIPERM Ameliorates Immobilization Stress-Induced Testicular Dysfunction via Inhibition of Oxidative Stress and Modulation of the Nrf2/HO-1 Pathway in SD Rats. Int. J. Mol. Sci. 2020, 21, 4750. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, L.I.; Kolesnikov, S.I.; Korytov, L.I.; Suslikova, M.I.; Darenskaya, M.A.; Grebenkina, L.A.; Kolesnikova, L.R. Oxidative Stress as a Mechanisms of Reduced Glucose Absorption under Conditions of Immobilization Stress. Bull. Exp. Biol. Med. 2017, 164, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T.; Samini, F. Anti-Oxidative Effects of Curcumin on Immobilization-Induced Oxidative Stress in Rat Brain, Liver and Kidney. Biomed. Pharmacother. 2017, 87, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Naoe, S.; Fujimoto, Y.; Murakami, K.; Yukimine, R.; Tanaka, A.; Yamaoka, K.; Kataoka, T. Effects of Low-Dose/High-Dose-Rate X-Irradiation on Oxidative Stress in Organs Following Forced Swim Test and Its Combined Effects on Alcohol-Induced Liver Damage in Mice. J. Radiat. Res. 2023, 64, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Al-Sowayan, N.S. Possible Modulation of Nervous Tension-Induced Oxidative Stress by Vitamin E. Saudi J. Biol. Sci. 2020, 27, 2563–2566. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, C.; Wu, J.; Wang, S.; Zhao, R.; Ma, J. Xiaoyaosan Decoction Regulates Food Intake and Body Weight via the Leptin-Leptin Receptor-Proopiomelanocortin Pathway in Rats with Chronic Immobilization Stress. J. Tradit. Chin. Med. Chung I Tsa Chih Ying Wen Pan 2019, 39, 34–44. [Google Scholar] [PubMed]
- Molina, P.; Andero, R.; Armario, A. Restraint or Immobilization: A Comparison of Methodologies for Restricting Free Movement in Rodents and Their Potential Impact on Physiology and Behavior. Neurosci. Biobehav. Rev. 2023, 151, 105224. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-Z.; Liang, Y.-D.; Ma, Q.-Y.; Hao, W.-Z.; Li, X.-J.; Wu, M.-S.; Deng, L.-J.; Li, Y.-M.; Chen, J.-X. Xiaoyaosan Improves Depressive-like Behavior in Rats with Chronic Immobilization Stress through Modulation of the Gut Microbiota. Biomed. Pharmacother. 2019, 112, 108621. [Google Scholar] [CrossRef] [PubMed]
- Michajlovskij, N.; Lichardus, B.; Kvetnanský, R.; Ponec, J. Effect of Acute and Repeated Immobilization Stress on Food and Water Intake, Urine Output and Vasopressin Changes in Rats. Endocrinol. Exp. 1988, 22, 143–157. [Google Scholar] [PubMed]
- Kizilaslan, N.; Zekiye Erdem, N.; Katar, M.; Gevrek, F. The Effects of Probiotics and Omega-3 Fatty Acids in Liver Steatosis Induced in Rats by High-Fructose Corn Syrup. Int. J. Clin. Pract. 2022, 2022, 7172492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Q.-Y.; Zeng, L.-T.; Wang, J.-J.; Liu, Z.; Fan, G.-Q.; Li, J.; Cai, J.-P. Long-Term High-Fat High-Fructose Diet Induces Type 2 Diabetes in Rats through Oxidative Stress. Nutrients 2022, 14, 2181. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Gutiérrez, E.; Fuentes-Venado, C.E.; Rodríguez-Páez, L.; Guerra-Araiza, C.; Larqué, C.; Martínez-Herrera, E.; Ocharan-Hernández, M.E.; Lomelí, J.; Loza-Mejía, M.A.; Salazar, J.R.; et al. High Fructose and High Fat Diet Impair Different Types of Memory through Oxidative Stress in a Sex- and Hormone-Dependent Manner. Metabolites 2022, 12, 341. [Google Scholar] [CrossRef] [PubMed]
- Miles, T.K.; Odle, A.K.; Byrum, S.D.; Lagasse, A.; Haney, A.; Ortega, V.G.; Bolen, C.R.; Banik, J.; Reddick, M.M.; Herdman, A.; et al. Anterior Pituitary Transcriptomics Following a High-Fat Diet: Impact of Oxidative Stress on Cell Metabolism. Endocrinology 2023, 165, bqad191. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Ko, M.; Ahn, M.; Shin, T. Hepatoprotective Effects of Norgalanthamine on Carbon Tetrachloride Induced-Hepatotoxicity in Mice. Drug Chem. Toxicol. 2023, 46, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Krähenbühl, S.; Reichen, J. Decreased Hepatic Glucose Production in Rats with Carbon Tetrachloride-Induced Cirrhosis. J. Hepatol. 1993, 19, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, L.; Bounous, D.I.; Elmore, S.A. Hematopoietic System. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1863–1933. [Google Scholar] [CrossRef]
- Santos, R.D.; Shapiro, M.D.; Ballantyne, C.M. Glycated Hemoglobin to Detect Subclinical Atherosclerosis in People Without Diabetes. J. Am. Coll. Cardiol. 2021, 77, 2792–2795. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Al Mamun, M.A.; Faruk, M.; Ul Islam, M.T.; Rahman, M.M.; Alam, M.; Rahman, A.F.M.T.; Reza, H.; Alam, M.A. Astaxanthin Ameliorates Hepatic Damage and Oxidative Stress in Carbon Tetrachloride-Administered Rats. Pharmacogn. Res. 2017, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Ilderbayev, O.; Okassova, A.; Rakhyzhanova, S.; Ilderbayeva, G.; Zhazykbayeva, L. The Levels of Oxidative Stress in a Combination of Stress Factors. J. Med. Life 2022, 15, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Pengnet, S.; Prommaouan, S.; Sumarithum, P.; Malakul, W. Naringin Reverses High-Cholesterol Diet-Induced Vascular Dysfunction and Oxidative Stress in Rats via Regulating LOX-1 and NADPH Oxidase Subunit Expression. Biomed Res. Int. 2019, 2019, 3708497. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, Y.; Jin, L.; Qian, C.; Zuo, W.; Lin, J.; Xie, L.; Jin, B.; Zhao, Y.; Huang, L.; et al. Alantolactone Attenuates High-Fat Diet-Induced Inflammation and Oxidative Stress in Non-Alcoholic Fatty Liver Disease. Nutr. Diabetes 2024, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-T.; Hsu, T.-H.; Chen, W.-L.; Yang, C.-K.; Lo, H.-C. Polysaccharides of Grifola Frondosa Ameliorate Oxidative Stress and Hypercholesterolaemia in Hamsters Fed a High-Fat, High-Cholesterol Diet. J. Pharm. Pharmacol. 2022, 74, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Salehi, I.; Mohammadi, M.; Mirzaei, F.; Soufi, F.G. Amlodipine Attenuates Oxidative Stress in the Heart and Blood of High-Cholesterol Diet Rabbits. Cardiovasc. J. Afr. 2012, 23, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Cederbaum, A.I. Inactivation and Degradation of Human Cytochrome P4502E1 by CCl4 in a Transfected HepG2 Cell Line. J. Pharmacol. Exp. Ther. 1995, 275, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Bevan, C.; Gollapudi, B.; Klaunig, J.E. Evaluation of the Carcinogenicity of Carbon Tetrachloride. J. Toxicol. Environ. Health Part B 2023, 26, 342–370. [Google Scholar] [CrossRef] [PubMed]
- Amini, R.; Asle-Rousta, M.; Aghazadeh, S. Hepatoprotective Effect of Limonene against Chronic Immobilization Induced Liver Damage in Rats. Naunyn Schmiedebergs. Arch. Pharmacol. 2020, 393, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Diduk, R.; Galano, A. Adrenaline and Noradrenaline: Protectors against Oxidative Stress or Molecular Targets? J. Phys. Chem. B 2015, 119, 3479–3491. [Google Scholar] [CrossRef] [PubMed]
- Labban, R.S.M.; Alfawaz, H.; Almnaizel, A.T.; Hassan, W.M.; Bhat, R.S.; Moubayed, N.M.; Bjørklund, G.; El-Ansary, A. High-Fat Diet-Induced Obesity and Impairment of Brain Neurotransmitter Pool. Transl. Neurosci. 2020, 11, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Chainy, G.B.N.; Sahoo, D.K. Hormones and Oxidative Stress: An Overview. Free Radic. Res. 2020, 54, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jun, C.; Lu, Y.; Jiangmei, S. Combination of Metformin and Luteolin Synergistically Protects Carbon Tetrachloride-induced Hepatotoxicity: Mechanism Involves Antioxidant, Anti-inflammatory, Antiapoptotic, and Nrf2/HO-1 Signaling Pathway. BioFactors 2019, 45, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Ojeaburu, S.; Oriakhi, K. Hepatoprotective, Antioxidant and, Anti-Inflammatory Potentials of Gallic Acid in Carbon Tetrachloride-Induced Hepatic Damage in Wistar Rats. Toxicol. Rep. 2021, 8, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, H.; Song, E.; Xu, X.; Liu, L.; Song, Y. Nrf2/ARE Pathway Activation, HO-1 and NQO1 Induction by Polychlorinated Biphenyl Quinone Is Associated with Reactive Oxygen Species and PI3K/AKT Signaling. Chem. Biol. Interact. 2014, 209, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Sun, T.; Xv, G.; Wang, S.; Gu, J.; Liu, C. Berberine Ameliorates CCl4-induced Liver Injury in Rats through Regulation of the Nrf2-Keap1-ARE and P53 Signaling Pathways. Mol. Med. Rep. 2019, 20, 3095–3102. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, Q.; Wei, L.; Zhou, X.; Wang, R.; Zhang, H. Asiatic Acid Ameliorates CC L4-Induced Liver Fibrosis in Rats: Involvement of Nrf2/ARE, NF-ΚB/IκBα, and JAK1/STAT3 Signaling Pathways. Drug Des. Devel. Ther. 2018, 12, 3595–3605. [Google Scholar] [CrossRef] [PubMed]
- Bona, S.; Rodrigues, G.; Moreira, A.J.; Di Naso, F.C.; Dias, A.S.; Da Silveira, T.R.; Marroni, C.A.; Marroni, N.P. Antifibrogenic Effect of Melatonin in Rats with Experimental Liver Cirrhosis Induced by Carbon Tetrachloride. JGH Open 2018, 2, 117–123. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr. 1997, 127 (Suppl. S5), 838S–841S. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Almatroudi, A.; Allemailem, K.S.; Alharbi, H.O.A.; Babiker, A.Y.; Althwab, S.A.; Alsuhaymi, N.; Alsugoor, M.H.; Khan, A.A.; Al-Megrin, W.A.I. Oleuropein, a Phenolic Component of Olea europaea L. Ameliorates CCl4-Induced Liver Injury in Rats through the Regulation of Oxidative Stress and Inflammation. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Demagny, H.; Faure, A.; Pontanari, F.; Jalil, A.; Bresciani, N.; Yildiz, E.; Korbelius, M.; Perino, A.; Schoonjans, K. Asparagine Protects Pericentral Hepatocytes during Acute Liver Injury. J. Clin. Investig. 2023, 133, e163508. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Z.; Cui, H.; Wang, T.; Xu, Y.; Zhao, J. Urantide Prevents CCl4-induced Acute Liver Injury in Rats by Regulating the MAPK Signalling Pathway. Mol. Med. Rep. 2021, 24, 688. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Savir, S.; Gruenbaum, B.F.; Melamed, I.; Grinshpun, J.; Kuts, R.; Knyazer, B.; Zlotnik, A.; Vinokur, M.; Boyko, M. Inducing Acute Liver Injury in Rats via Carbon Tetrachloride (CCl4) Exposure Through an Orogastric Tube. J. Vis. Exp. 2020, 158, e60695. [Google Scholar] [CrossRef] [PubMed]
- Holman, B.W.B.; Bailes, K.L.; Meyer, R.G.; Hopkins, D.L. Effect of Modified Soxhlet (Soxtec) and Folch Extraction Method Selection on the Total Lipid Determination of Aged Beef. J. Food Sci. Technol. 2019, 56, 3957–3961. [Google Scholar] [CrossRef] [PubMed]


| Fatty Acid (FA) | FA Index | Soybean Oil | Sunflower Oil |
|---|---|---|---|
| Myristic | 14:0 | 0.10 | 0.07 |
| Pentadecanoic | 15:0 | 0.02 | 0.01 |
| Palmitic | 16:0 | 10.29 | 6.14 |
| Hexadecene | 16:1 | 0.02 | 0.02 |
| Palmitoleic | 16:1 9-cis | 0.08 | 0.04 |
| Margarine | 17:0 | 0.08 | 0.03 |
| Heptadecenoic | 17:1 | 0.04 | 0.03 |
| Stearic | 18:0 | 4.92 | 3.16 |
| Oleic | 18:1 9-cis | 24.11 | 27.31 |
| Vaccenic | 18:1 11-cis | 1.40 | 0.78 |
| Octadecene | 18:1 11-cis | 0.06 | 0.00 |
| cis, trans-linoleic | 18:2 9-cis, 12-trans | 0.05 | 0.24 |
| trans, cis-linoleic | 18:2 9-trans, 12-cis | 0.00 | 0.19 |
| Linoleic | 18:2 | 47.22 | 60.58 |
| Octadecatrienic | Total trans-isomers | 0.13 | 0.00 |
| α-linolenic | 18:3 ω-3 | 10.17 | 0.08 |
| Arachidic | 20:0 | 0.49 | 0.24 |
| gondoic (total isomers) | 20:1 | 0.24 | 0.20 |
| Behenic | 22:0 | 0.43 | 0.72 |
| Parameter | Group | Statistics | ||||
|---|---|---|---|---|---|---|
| Control | DI | Immo | CCl4 | p | η2 | |
| Fat mass, % | 13.7 (11.8–15.9) | 13.9 (9.7–15.2) | 8.1 (6.7–11.7) 1,2 | 12.3 (8.7–15.5) | 0.05 | 0.17 |
| Lean mass, % | 79.8 ± 1.0 | 80.8 ± 1.1 | 85.1 ± 1.1 1,2 | 81.9 ± 1.5 | 0.02 | 0.20 |
| Free water, % | 0.25 (0.22–0.29) | 0.20 (0.17–0.23) 1 | 0.32 (0.29–0.35) 1,2 | 0.21 (0.17–0.23) 1,3 | <0.01 | 0.39 |
| Total water, % | 67.8 ± 0.9 | 68.5 ± 0.9 | 72.3 ± 0.9 1,2 | 69.7 ± 1.2 | 0.02 | 0.21 |
| Parameter | Animal Groups | Statistics | ||||
|---|---|---|---|---|---|---|
| Control | DI | Immo | CCl4 | p | η2 | |
| Total protein, g/L | 71.9 ± 1.4 | 77.4 ± 1.8 1 | 71.5 ± 1.1 2 | 72.7 ± 1.5 2 | 0.02 | 0.20 |
| Albumin, g/L | 30.5 ± 0.3 | 31.5 ± 0.5 | 30.6 ± 0.3 | 30.3 ± 0.4 2 | 0.04 | 0.12 |
| Globulins, g/L | 41.0 [38.0–44.0] | 44.8 [43.4–49.2] 1 | 40.1 [38.9–43.3] 2 | 41.8 [39.9–43.8] | 0.04 | 0.21 |
| Cholesterol, mmol/L | 1.65 [1.37–1.79] | 2.52 [1.83–2.79] 1 | 1.51 [1.38–1.76] 2 | 1.11 [0.96–1.47] 1,2,3 | <0.01 | 0.49 |
| Triglycerides, mmol/L | 2.95 [2.29–3.86] | 2.17 [1.38–2.85] 1 | 1.36 [1.02–1.69] 1 | 0.74 [0.53–1.07] 1 | <0.01 | 0.44 |
| HDL, mmol/L | 1.28 ± 0.09 | 1.33 ± 0.09 | 1.41 ± 0.05 | 1.10 ± 0.09 2,3 | 0.05 | 0.17 |
| LDL, mmol/L | 0.10 [0.09–0.11] | 0.44 [0.33–0.70] 1 | 0.15 [0.13–0.17] 1,2 | 0.08 [0.07–0.12] 2,3 | <0.01 | 0.65 |
| Total bilirubin, μmol/L | 5.01 [4.79–5.46] | 4.51 [4.36–5.38] | 5.16 [5.00–5.43] 2 | 5.30 [5.00–5.62] 2 | 0.03 | 0.10 |
| Creatinine, μmol/L | 46.3 ± 0.8 | 46.3 ± 1.3 | 49.0 ± 0.8 | 46.3 ± 0.9 | 0.12 | 0.12 |
| Alkaline phosphatase, U/L | 159 [143–213] | 191 [178–202] | 154 [139–202] | 144 [137–171] 2 | 0.02 | 0.15 |
| ALT, U/L | 74.3 [60.9–84.4] | 91.2 [72.5–148.1] 1 | 89.5 [83.5–101.1] 1 | 102.7 [79.0–109.7] 1 | 0.02 | 0.17 |
| AST, U/L | 40.0 [34.2–172.8] | 201.7 [52.2–230.7] | 239.4 [81.7–258.7] 1 | 62.5 [42.9–271.3] | 0.03 | 0.04 |
| Urea, mmol/L | 6.33 ± 0.24 | 4.08 ± 0.29 1 | 5.59 ± 0.28 2 | 6.70 ± 0.36 2,3 | <0.01 | 0.51 |
| Uric acid, mmol/L | 85.1 [81.3–92.8] | 95.7 [81.6–107.1] | 90.4 [87.1–103.5] | 77.5 [73.3–93.9] | 0.31 | 0.05 |
| Phosphor, mmol/L | 2.12 [2.04–2.74] | 2.07 [1.91–2.32] | 2.39 [2.22–2.53] 2 | 2.39 [2.35–2.54] 2 | 0.03 | 0.15 |
| Magnesium, mmol/L | 0.90 [0.78–0.99] | 0.73 [0.65–0.77] 1 | 0.85 [0.82–1.02] 2 | 0.79 [0.76–0.83] | <0.01 | 0.26 |
| Calcium, mmol/L | 2.45 [2.31–3.36] | 2.42 [2.40–2.61] | 2.38 [2.31–2.42] | 2.45 [2.38–2.50] | 0.51 | 0.04 |
| Glucose, mmol/L | 6.78 [5.91–7.19] | 7.87 [7.63–8.25] 1 | 6.10 [5.65–6.47] 2 | 5.93 [5.84–6.27] 1,2 | <0.01 | 0.43 |
| Glucose, mmol/L (whole blood) | 5.85 [5.30–6.28] | 7.30 [7.05–7.55] 1 | 5.55 [5.18–6.33] 2 | 5.95 [5.53–6.03] 2 | <0.01 | 0.53 |
| Glycated hemoglobin, % | 6.3 [5.4–6.8] | 6.7 [5.7–7.5] | 6.4 [5.9–7.8] | 5.2 [5.0–5.4] 1,2,3 | 0.03 | 0.05 |
| Parameter | Animal Groups | Statistics | ||||
|---|---|---|---|---|---|---|
| Control | DI | Immo | CCl4 | p | η2 | |
| Final body weight, g | 479 [440–505] | 449 [438–498] | 369 [360–392] 1,2 | 430 [404–448] 1,3 | <0.01 | 0.38 |
| Liver weight, relative, % | 2.72 [2.60–2.93] | 4.33 [4.08–4.69] 1 | 2.42 [2.27–2.53] 1,2 | 2.68 [2.57–2.76] 2,3 | <0.01 | 0.87 |
| Fat content, mg/g liver | 89.4 [81.4–114.2] | 189.6 [121.4–223.5] 1 | 76.3 [63.3–83.3] 1,2 | 93.9 [85.6–105.5] 2,3 | <0.01 | 0.49 |
| Cholesterol, mg/g liver | 5.79 [4.14–6.92] | 14.2 [6.1–16.9] 1 | 4.12 [3.38–5.19] 2 | 17.3 [12.8–18.5] 1,3 | <0.01 | 0.62 |
| Triglycerides, mg/g liver | 44.0 [34.7–59.2] | 64.2 [48.6–98.4] 1 | 38.2 [32.0–42.2] 2 | 33.3 [24.7–38.7] 1,2 | 0.01 | 0.31 |
| Parameters | Animal Groups | Statistics | ||||
|---|---|---|---|---|---|---|
| Control | DI | Immo | CCl4 | p | η2 | |
| Lipid peroxides, ng/mL | 362.8 [318.9–438.5] | 381.4 [345.3–544.4] | 258.6 [246.2–291.1] 1 | 482.8 [420.0–534.5] 1 | <0.01 | 0.31 |
| MDA, ng/mL | 190.4 [149.8–233.1] | 352.4 [226.0–462.2] 1 | 191.5 [146.2–295.8] | 168.3 [120.6–218.9] 2 | 0.05 | 0.13 |
| Glutathione peroxidase, pg/mL | 69.5 [55.5–82.1] | 67.7 [58.2–72.5] | 69.7 [55.6–87.2] | 60.0 [49.9–81.2] | 0.88 | 0.07 |
| Catalase, ng/mL | 1.42 [1.03–1.61] | 1.58 [1.28–1.93] | 1.03 [0.83–1.29] 2 | 2.34 [1.81–2.44] 1,3 | <0.01 | 0.20 |
| SOD, ng/mL | 2.75 [2.31–3.07] | 6.55 [3.91–9.78] 1 | 4.65 [2.20–6.46] | 2.46 [1.78–3,52] 2 | 0.037 | 0.20 |
| Gene | Animal Group | Statistics | ||||
|---|---|---|---|---|---|---|
| Control | DI | Immo | CCl4 | p | η2 | |
| Cat | 1.04 [0.90–1.12] | 0.68 [0.66–0.80] 1 | 0.96 [0.94–1.11] 2 | 0.70 [0.61–0.79] 1,3 | <0.01 | 0.30 |
| Sod1 | 0.98 [0.91–1.09] | 0.77 [0.71–0.92] | 1.13 [1.04–1.17] 2 | 1.17 [1.01–1.51] 2 | 0.02 | 0.53 |
| Gpx1 | 1.04 [0.89–1.19] | 0.83 [0.69–0.86] | 0.76 [0.70–0.91] | 0.54 [0.49–0.59] 1,3 | <0.01 | 0.44 |
| Hmox1 | 0.94 [0.82–1.11] | 1.05 [0.86–1.32] | 0.64 [0.52–0.80] | 0.80 [0.58–1.17] | 0.34 | 0.18 |
| Nqo1 | 1.36 [0.85–1.57] | 1.61 [1.14–3.21] | 1.64 [0.96–2.75] | 7.13 [5.05–9.53] 1,2,3 | <0.01 | 0.44 |
| Nrf2 | 1.06 [1.05–1.16] | 0.96 [0.82–1.16] | 1.07 [0.97–1.18] | 0.87 [0.73–1.03] | 0.43 | 0.18 |
| Nfkb1 | 1.04 [0.95–1.95] | 0.95 [0.84–1.03] | 0.94 [0.90–1.01] | 1.05 [1.00–1.12] | 0.62 | 0.14 |
| Parameter | Animal Group | |||
|---|---|---|---|---|
| Control | DI | Immo | CCl4 | |
| Body weight, g | 157 ± 3 | 160 ± 3 | 160 ± 3 | 157 ± 3 |
| Open Field Test Results | ||||
| Time in center, s | 1.4 ± 0.6 | 1.1 ± 0.5 | 1.9 ± 0.5 | 1.7 ± 0.8 |
| Zone transitions | 5.8 ± 1.0 | 5.5 ± 1.3 | 6.2 ± 0.9 | 5.7 ± 1.1 |
| Total distance, cm | 1581 ± 135 | 1729 ± 109 | 1768 ± 100 | 1741 ± 119 |
| Primer | Sequence |
|---|---|
| NfkB | F CGTGGAGTACGACAACATCTC |
| R GAGGTGTCGTCCCATCGTA | |
| FAM CTGCTCCTGGAGGGTGACGC-BHQ1 | |
| Nrf2 | F CACATCCAGACAGACACCAGT |
| R GAATGTCTCTGCCAAAAGC | |
| FAM CTCCCAGGTTGCCCACATTCCC-BHQ1 | |
| Hmox1 | F CCAGCCTGAACTAGCCCA |
| R CCTTGGTGGCCTCCTTC | |
| FAM CCACAGCTCGACAGCATGTCC-BHQ1 | |
| Sod1 | F GACAATACACAAGGCTGTACCA |
| R CAGGTCTCCAACATGCCTC | |
| FAM CTCACTCTAAGAAACATGGCGGTC-BHQ1 | |
| Cat | F GTCTGGGACTTCTGGAGTCTT |
| R CATAGCCATTCATGTGCCG | |
| FAM CCATCAGGTTACTTTCTTGTTCAG-BHQ1 | |
| Gpx1 | F TCAGTTCGGACATCAGGAGA |
| R CATTCACCTCGCACTTCTCAA | |
| FAM CCCTCAAGTATGTCCGACCCG-BHQ1 | |
| Nqo1 | F GGGACATGAACGTCATTCTC |
| R CACCAGTTGAGGTTCTAAGACC | |
| FAM CAATTCAGAGTGGCATTCTGCGC-BHQ1 |
| Effect Strength | Value of η2 | Interpretation |
|---|---|---|
| Weak | 0.01–0.06 | The factor explains only a small part of the variation in the dependent variable. The effect may be statistically significant with a large sample size. |
| Average | 0.06–0.14 | The factor explains a moderate part of the variation in the dependent variable. The effect can be practically significant. |
| Strong | ≥0.14 | The factor explains a significant part of the variance in the dependent variable. The effect is likely to have practical meaning. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorova, Y.; Petrov, N.; Biryulina, N.; Sokolov, I.; Balakina, A.; Trusov, N.; Kochetkova, A. The Study of Approaches to Modeling Oxidative Stress in Male Wistar Rats: The Comparative Analysis of Diet-Induced, Chemically Induced, and Physiologically Induced Models. Int. J. Mol. Sci. 2025, 26, 6872. https://doi.org/10.3390/ijms26146872
Sidorova Y, Petrov N, Biryulina N, Sokolov I, Balakina A, Trusov N, Kochetkova A. The Study of Approaches to Modeling Oxidative Stress in Male Wistar Rats: The Comparative Analysis of Diet-Induced, Chemically Induced, and Physiologically Induced Models. International Journal of Molecular Sciences. 2025; 26(14):6872. https://doi.org/10.3390/ijms26146872
Chicago/Turabian StyleSidorova, Yuliya, Nikita Petrov, Nadezhda Biryulina, Ilya Sokolov, Anastasiya Balakina, Nikita Trusov, and Alla Kochetkova. 2025. "The Study of Approaches to Modeling Oxidative Stress in Male Wistar Rats: The Comparative Analysis of Diet-Induced, Chemically Induced, and Physiologically Induced Models" International Journal of Molecular Sciences 26, no. 14: 6872. https://doi.org/10.3390/ijms26146872
APA StyleSidorova, Y., Petrov, N., Biryulina, N., Sokolov, I., Balakina, A., Trusov, N., & Kochetkova, A. (2025). The Study of Approaches to Modeling Oxidative Stress in Male Wistar Rats: The Comparative Analysis of Diet-Induced, Chemically Induced, and Physiologically Induced Models. International Journal of Molecular Sciences, 26(14), 6872. https://doi.org/10.3390/ijms26146872









