Influence of Methyl Jasmonate and Short-Term Water Deficit on Growth, Redox System, Proline and Wheat Germ Agglutinin Contents of Roots of Wheat Seedlings
Abstract
1. Introduction
2. Results
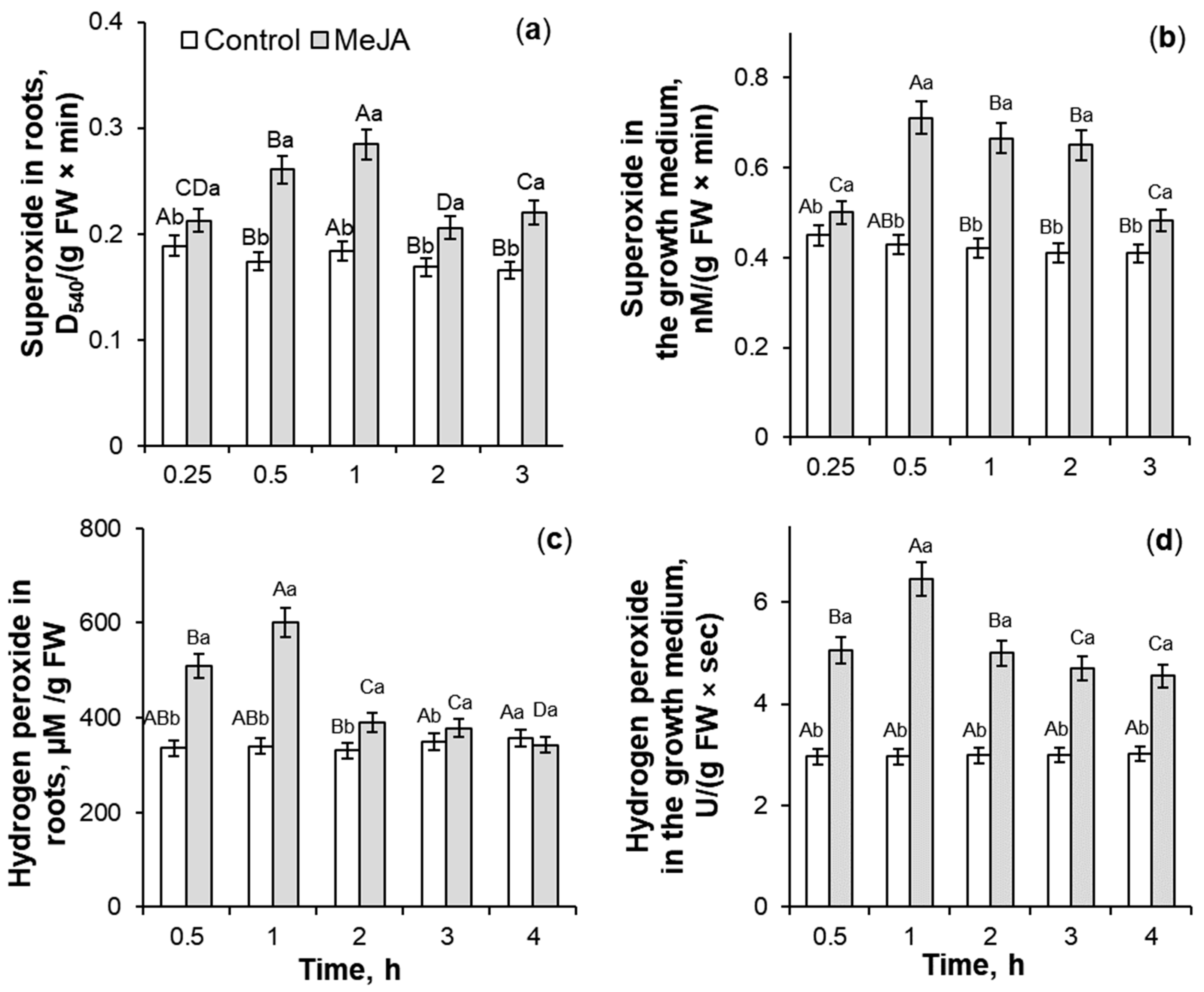
2.1. The Influence of 10−7 M MeJA Application on ROS Generation
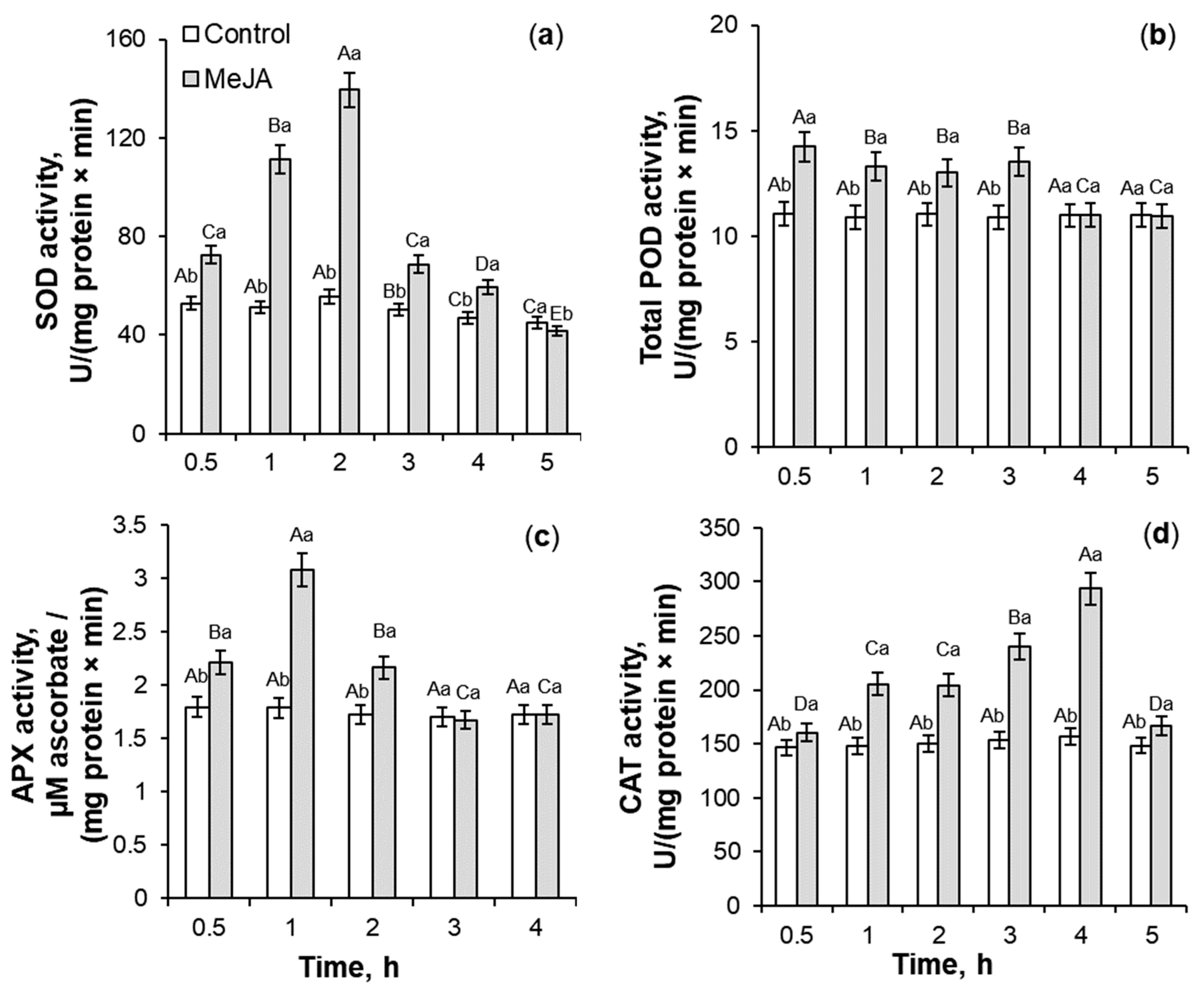
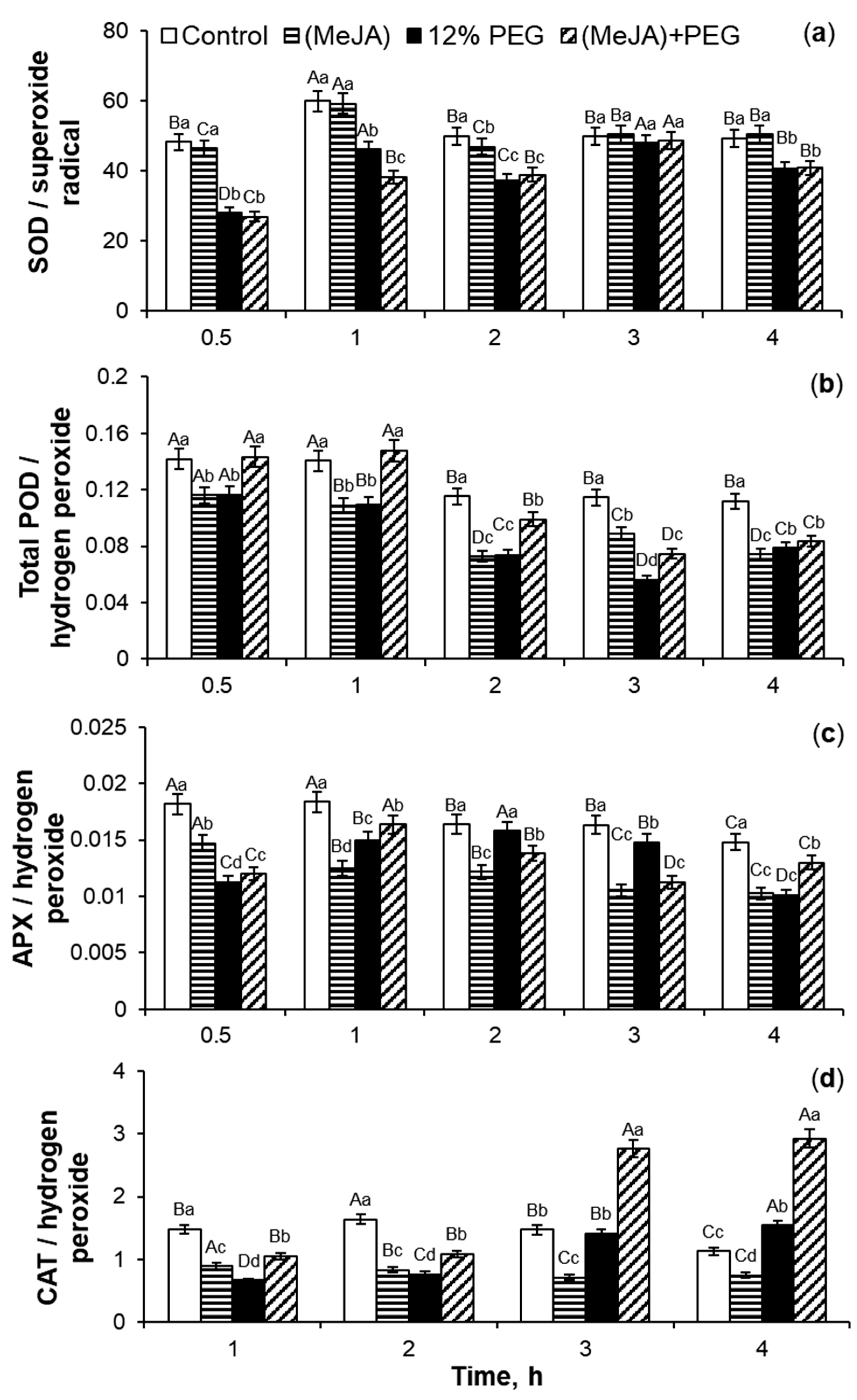
2.2. Antioxidant Enzymes Activity Under 10−7 M MeJA Application
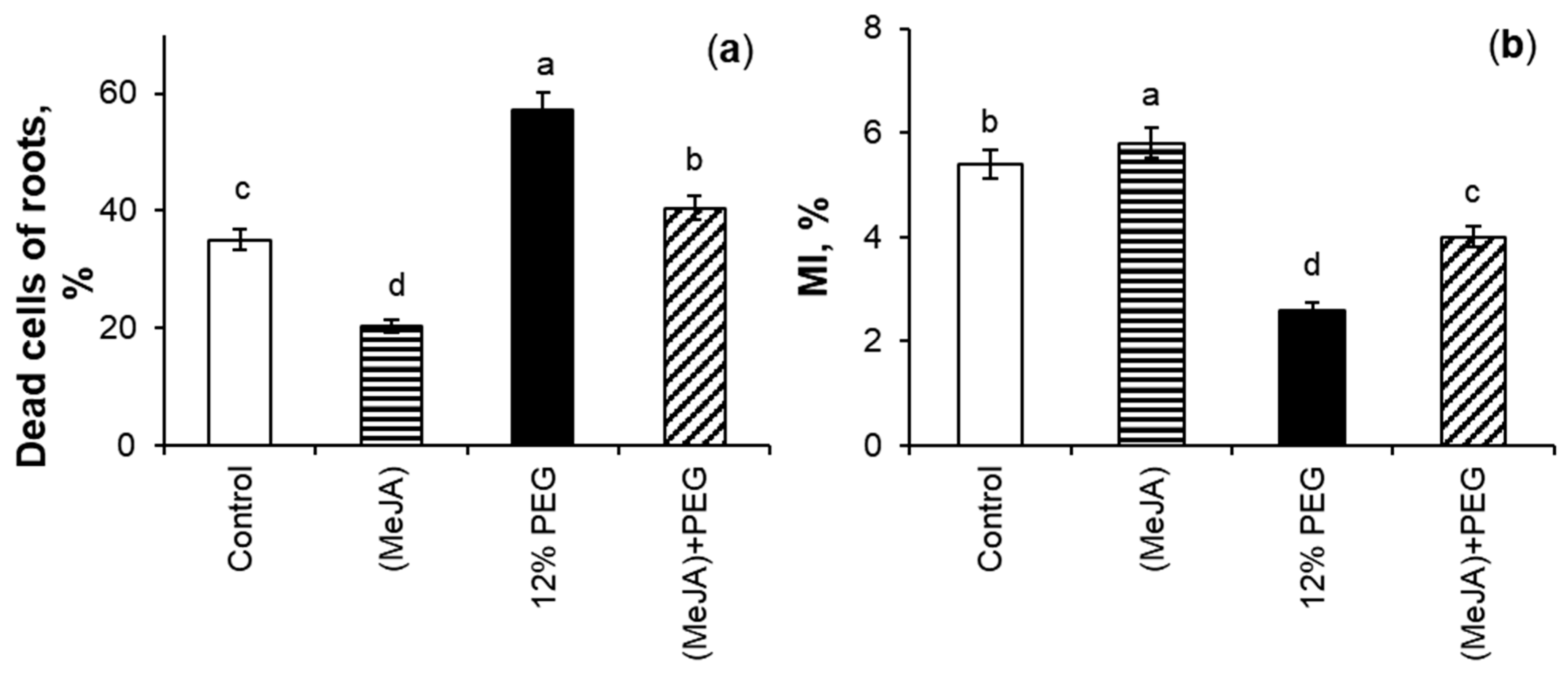
2.3. The Effect of MeJA Pretreatment on Mitotic Index and Percent of Dead Cells Under 12% PEG Exposure
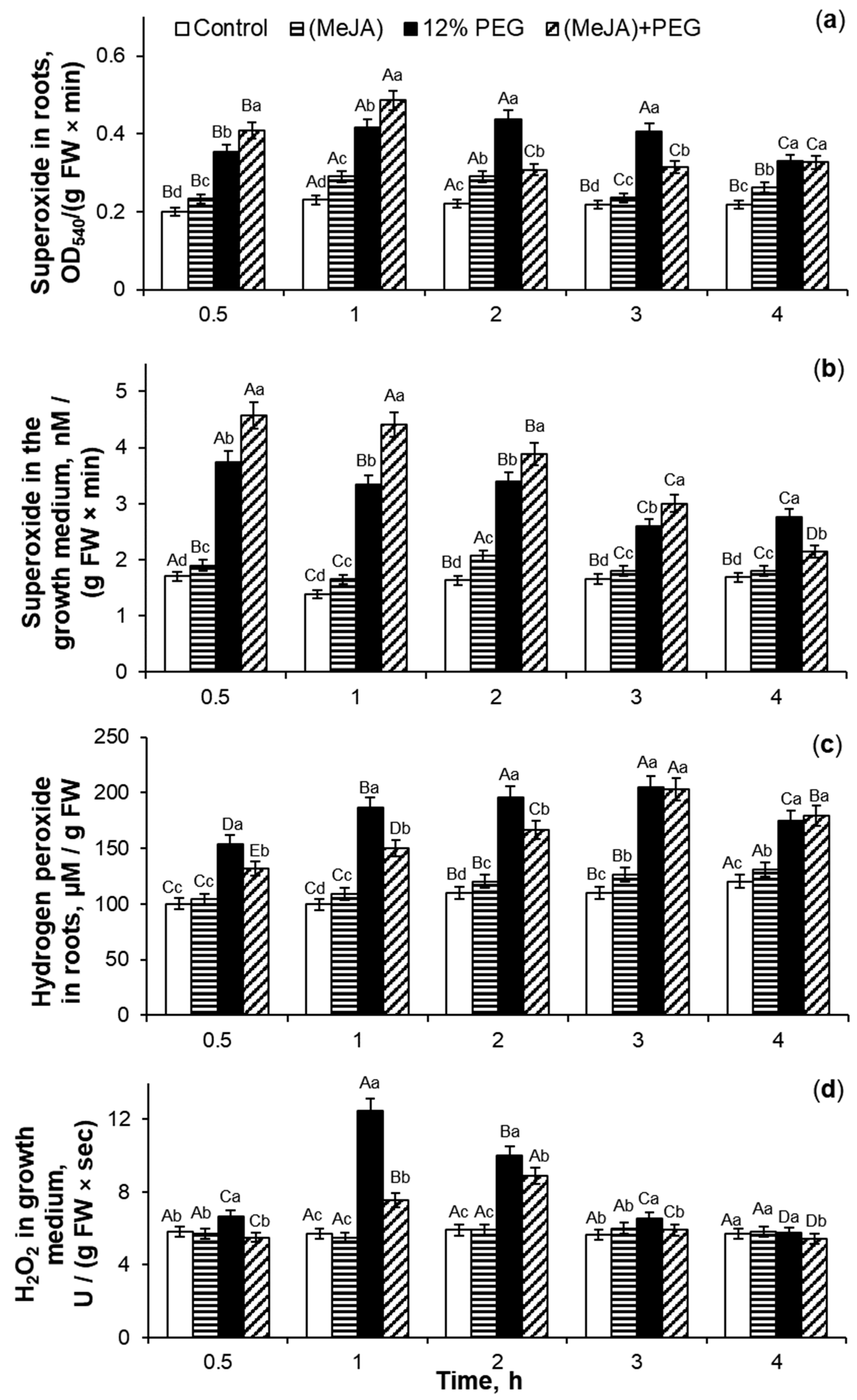
2.4. The Effect of MeJA Pretreatment on ROS Generation in Wheat Roots Under 12% PEG Exposure
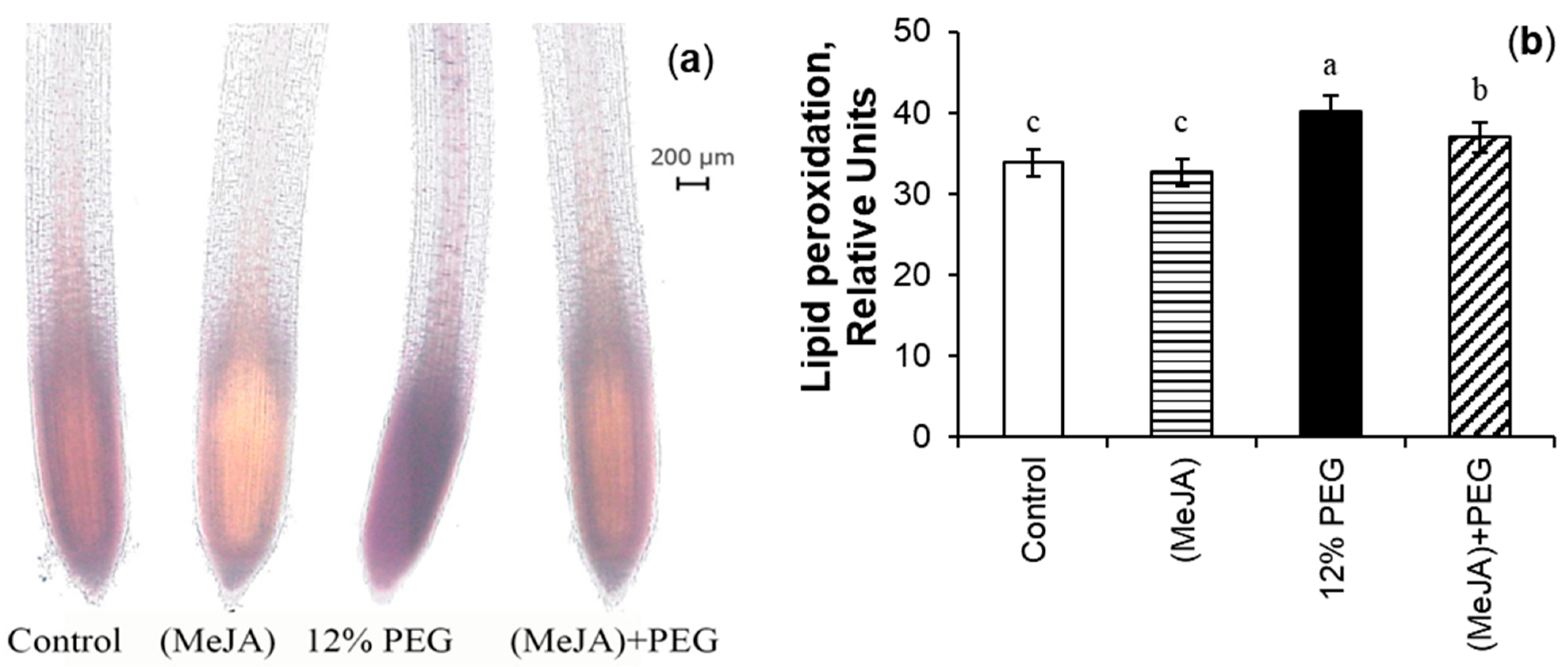
2.5. MeJA-Induced Regulation of Activity of Antioxidant Enzymes During Drought Stress
2.6. MeJA-Induced Regulation of WGA Accumulation Under 12% PEG Treatment
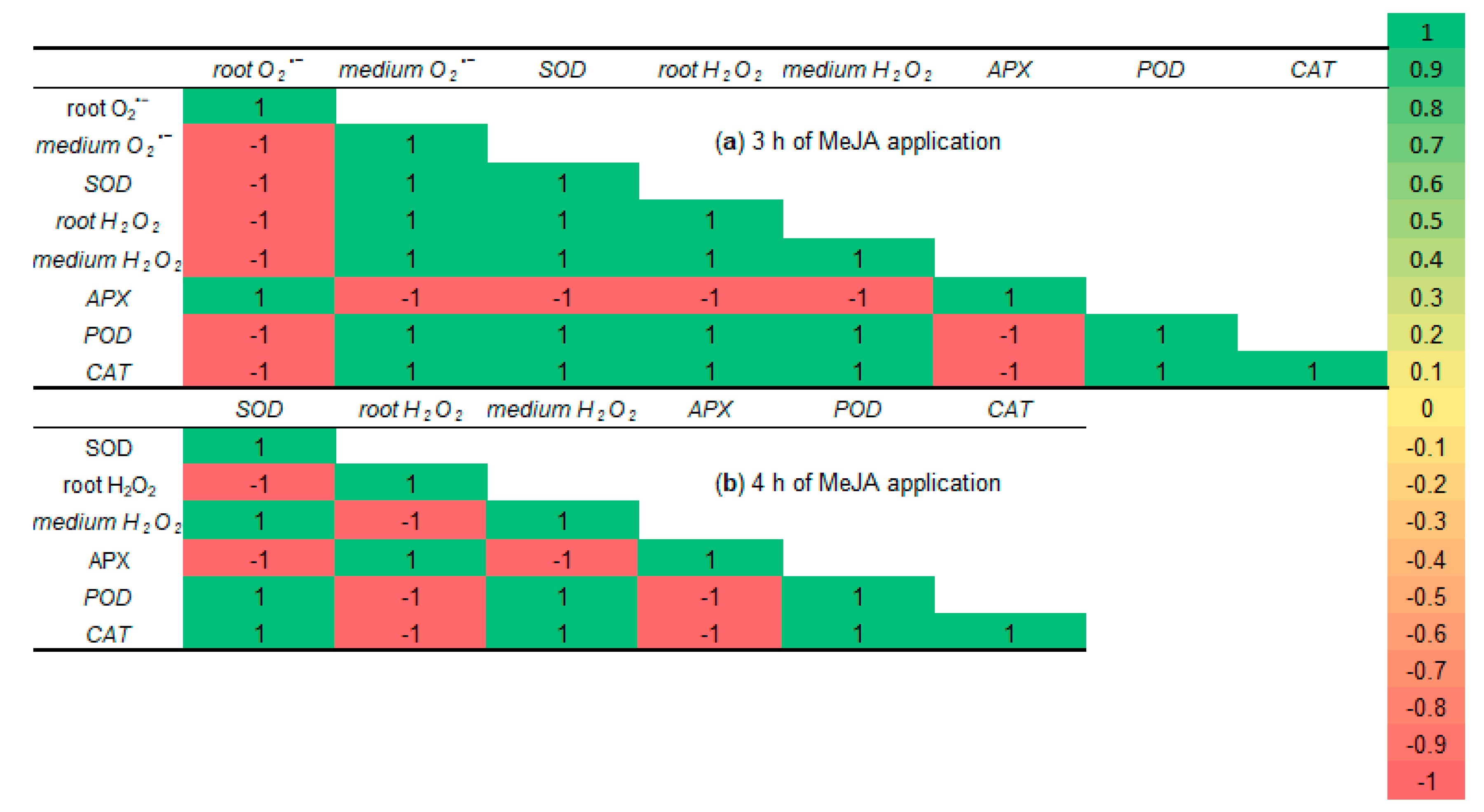
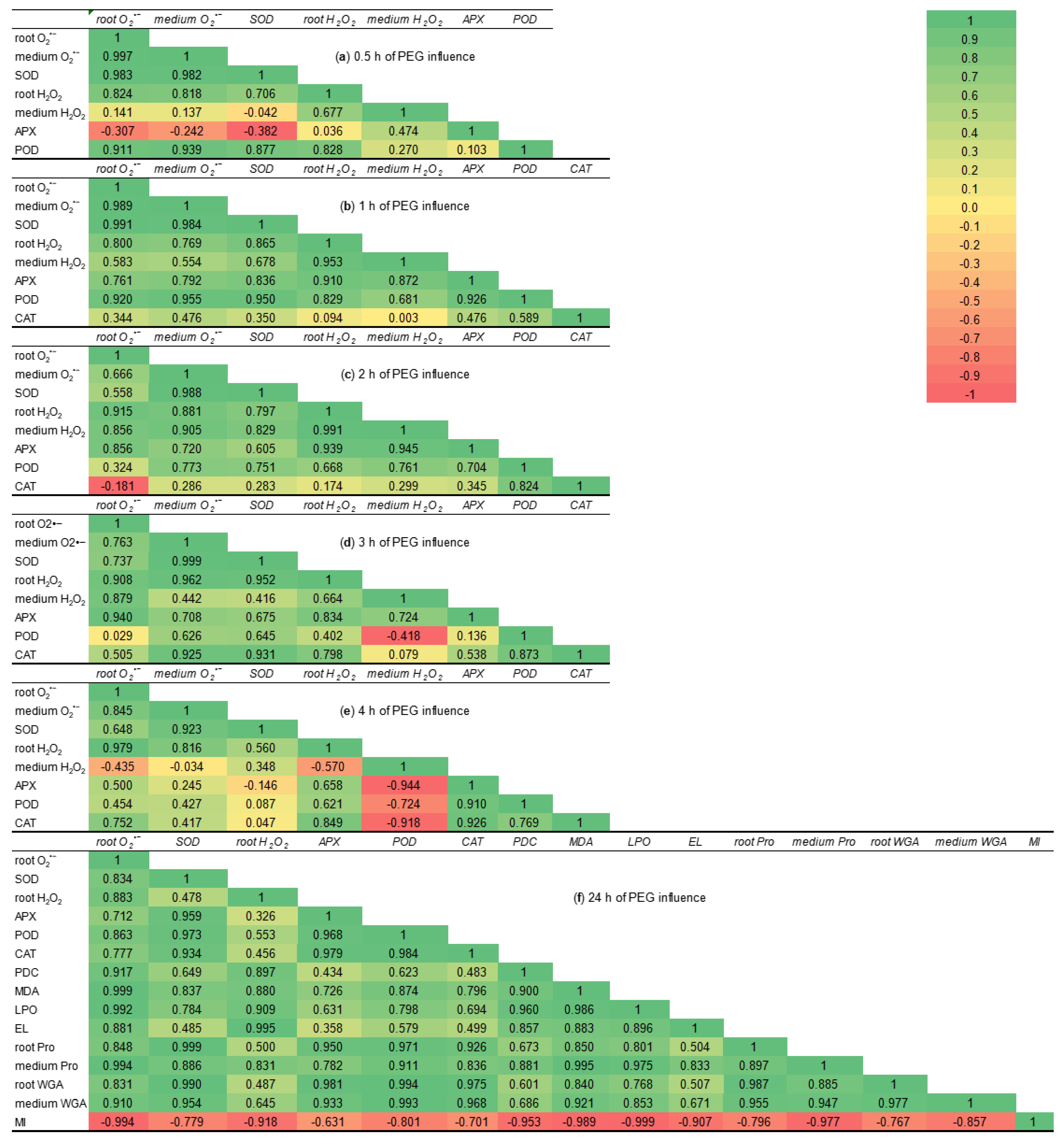
2.7. Correlation Matrices
3. Discussion
3.1. ROS Generation Under 10−7 M MeJA Application
3.2. SOD, Total POD, APX, and CAT Activities During MeJA Treatment
3.3. The Effect of MeJA Pretreatment on MI and PDC Under 12% PEG Exposure
3.4. The Effect of MeJA Pretreatment on ROS Generation in Roots Under 12% PEG Exposure
3.5. MeJA-Induced Regulation of Activity of Antioxidant Enzymes Under Water Deficit
3.6. MeJA-Induced Mitigation of MDA During Drought Stress
3.7. MeJA-Induced Regulation of Pro and WGA Accumulation Under Short-Time Water Deficit Stress
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Assay of Antioxidant Enzymes
4.3. Determination of Superoxide Anion Radical in the Plant Growth Medium
4.4. Estimation of O2•− Content in Roots of Wheat Seedlings
4.5. Extracellular H2O2 Content
4.6. Estimation of H2O2 Content in Roots of Wheat Seedlings
4.7. Malondialdehyde (MDA) Accumulation in Roots of Wheat Seedlings
4.8. Estimation of Electrolyte Leakage (EL)
4.9. Proline (Pro) Accumulation in Roots and in the Plant Growth Medium
4.10. Analysis of Percentage of Death Cells (PDC) of Wheat Roots
4.11. Histochemical Detection of Lipid Peroxidation
4.12. Mitotic Index (MI) Analysis
4.13. Indirect Immunohistochemical Localization of Wheat Germ Agglutinin (WGA) Using Confocal Microscopy
4.14. Estimation of WGA Accumulation in Roots and in the Nutrition Medium
4.15. Statistical Analysis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakovljević, D.; Stanković, M. Adaptive Strategies of Plants Under Adverse Environment: Mitigating Effects of Antioxidant System. In Plant Ecophysiology and Adaptation Under Climate Change: Mechanisms and Perspectives II: Mechanisms of Adaptation and Stress Amelioration; Hasanuzzaman, M., Ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 163–186. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Bapela, T.; Shimelis, H.; Tsilo, T.J.; Mathew, I. Genetic Improvement of Wheat for Drought Tolerance: Progress, Challenges and Opportunities. Plants 2022, 11, 1331. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, D.; Budhlakoti, N.; Singh, A.K.; Bansal, R.; Kumari, J.; Chaudhary, N.; Padaria, J.C.; Sareen, S.; Kumar, S. Drought Tolerance in Triticum aestivum L. Genotypes Associated with Enhanced Antioxidative Protection and Declined Lipid Peroxidation. 3 Biotech 2020, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Dashtaki, M.; Bihamta, M.R.; Majidi, E.; Nejad, R.A. Differential Responses of Wheat Genotypes to Irrigation Regimes Through Antioxidant Defense System, Grain Yield, Gene Expression, and Grain Fatty Acid Profile. Cereal Res. Commun. 2023, 51, 879–890. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2020. Overcoming Water Challenges in Agriculture; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global Food Demand and the Sustainable Intensification of Agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Suekawa, M.; Fujikawa, Y.; Esaka, M. Exogenous Proline has Favorable Effects on Growth and Browning Suppression in Rice but not in Tobacco. Plant Physiol. Biochem. 2019, 142, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Outoukarte, I.; El Keroumi, A.; Dihazi, A.; Naamani, K. Use of Morpho-Physiological Parameters and Biochemical Markers to Select Drought Tolerant Genotypes of Durum Wheat. Plant Stress Physiol. 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Vives–Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root Exudates: From Plant to Rhizosphere and Beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef] [PubMed]
- El-Hassouni, K.; Alahmad, S.; Belkadi, B.; Filali-Maltouf, A.; Hickey, L.T.; Bassi, F.M. Root System Architecture and Its Association with Yield Under Different Water Regimes in Durum Wheat. Crop Sci. 2018, 58, 2331–2346. [Google Scholar] [CrossRef]
- Ávila-Juárez, L.; Miranda-Rodríguez, H. Variations in Bioactive Content in Different Tomato Trusses due to Elicitor Effects. J. Chem. 2018, 2018, 2736070. [Google Scholar] [CrossRef]
- Cadena-Zamudio, J.D.; Monribot-Villanueva, J.L.; Pérez-Torres, C.A.; Alatorre-Cobos, F.; Guerrero-Analco, J.A.; Ibarra-Laclette, E. Non-Targeted Metabolomic Analysis of Arabidopsis thaliana (L.) Heynh: Metabolic Adaptive Responses to Stress Caused by N Starvation. Metabolites 2023, 13, 1021. [Google Scholar] [CrossRef] [PubMed]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Methyl Jasmonate Effects on Table Grape Ripening, Vine Yield, Berry Quality and Bioactive Compounds Depend on Applied Concentration. Sci. Hortic. 2019, 247, 380–389. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, R.; Liu, S.; Li, R.; Wang, Y.; Chen, Y.; Hou, H.; Dai, Q. Methyl Jasmonate Alleviates the Deleterious Effects of Salinity Stress by Augmenting Antioxidant Enzyme Activity and Ion Homeostasis in Rice (Oryza sativa L.). Agronomy 2022, 12, 2343. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on Crops: Their Impact under Abiotic Stress Conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Iqbal, S.; Wang, X.; Mubeen, I.; Kamran, M.; Kanwal, I.; Díaz, G.A.; Abbas, A.; Parveen, A.; Atiq, M.N.; Alshaya, H.; et al. Phytohormones Trigger Drought Tolerance in Crop Plants: Outlook and Future Perspectives. Front. Plant Sci. 2022, 12, 799318. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.K.; Jez, J.M. Structural Biology of Jasmonic Acid Metabolism and Responses in Plants. In Plant Structural Biology: Hormonal Regulations; Hejátko, J., Hakoshima, T., Eds.; Springer Nature Singapore Publishers: Cham, Switzerland, 2018; pp. 67–82. [Google Scholar] [CrossRef]
- Ho, T.T.; Murthy, H.N.; Park, S.Y. Methyl Jasmonate Induced Oxidative Stress and Accumulation of Secondary Metabolites in Plant Cell and Organ Cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef] [PubMed]
- Kolupaev, Y.E.; Yastreb, T.O.; Dmitriev, A.P. Signal Mediators in the Implementation of Jasmonic Acid’s Protective Effect on Plants under Abiotic Stresses. Plants 2023, 12, 2631. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Huang, H.; Song, S.; Xie, D. Regulation of Jasmonate-Mediated Stamen Development and Seed Production by a bHLH-MYB Complex in Arabidopsis. Plant Cell 2015, 27, 1620–1633. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Takahashi, H.; Saito, T.; Okawa, K.; Ohara, H.; Shishido, M.; Ikeura, H.; Kondo, S. Jasmonate Application Influences Endogenous Abscisic Acid, Jasmonic Acid and Aroma Volatiles in Grapes Infected by a Pathogen (Glomerella cingulata). Sci. Hortic. 2015, 192, 166–172. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Yu, R.; Wu, C.; Fan, G.; Li, T. Effects of Postharvest Application of Methyl Jasmonate on Physicochemical Characteristics and Antioxidant System of the Blueberry Fruit. Sci. Hortic. 2019, 258, 108785. [Google Scholar] [CrossRef]
- Lischweski, S.; Muchow, A.; Guthörl, D.; Hause, B. Jasmonates Act Positively in Adventitious Root Formation in Petunia Cuttings. BMC Plant Biol. 2015, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Sarabandi, M.; Farokhzad, A.; Mandoulakani, B.A.; Ghasemzadeh, R. Biochemical and Gene Expression Responses of Two Iranian Grape Cultivars to Foliar Application of Methyl Jasmonate under Boron Toxicity Conditions. Sci. Hortic. 2019, 249, 355–363. [Google Scholar] [CrossRef]
- Kalatskaja, J.N.; Baliuk, N.V.; Rybinskaya, K.I.; Herasimovich, K.M.; Yalouskaya, N.A.; Yarullina, L.G.; Tsvetkov, V.O. Increasing Potato Sustainability to PVY under Water Deficiency by Bacillus Bacteria with Salicylic Acid and Methyl Jasmonate. Int. J. Plant Biol. 2023, 14, 312–328. [Google Scholar] [CrossRef]
- Dai, Z.; Yuan, Y.; Huang, H.; Hossain, M.M.; Xiong, S.; Cao, M.; Ma, L.Q.; Tu, S. Methyl Jasmonate Mitigates High Selenium Damage of Rice via Altering Antioxidant Capacity, Selenium Transportation and Gene Expression. Sci. Total Environ. 2021, 756, 143848. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate Action in Plant Growth and Development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Bhavanam, S.; Stout, M. Seed Treatment with Jasmonic Acid and Methyl Jasmonate Induces Resistance to Insects but Reduces Plant Growth and Yield in Rice, Oryza sativa. Front. Plant Sci. 2021, 12, 691768. [Google Scholar] [CrossRef] [PubMed]
- Sirhindi, G.; Mir, M.A.; Abd-Allah, E.F.; Ahmad, P.; Gucel, S. Jasmonic Acid Modulates the Physio-Biochemical Attributes, Antioxidant Enzyme Activity, and Gene Expression in Glycine max under Nickel Toxicity. Front. Plant Sci. 2016, 7, 591. [Google Scholar] [CrossRef] [PubMed]
- Abouelsaad, I.; Renault, S. Enhanced Oxidative Stress in the Jasmonic Acid-Deficient Tomato Mutant def-1 Exposed to NaCl Stress. J. Plant Physiol. 2018, 226, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, M.; Dehnavi, M.M.; Mirzaei, G.; Aghaei, F. Modulating Mycorrhiza—Plant Relationships and Improving the Physiological Responses of Barley Under Drought Stress Conditions with the Application of Methyl Jasmonate. J. Plant Growth Regul. 2023, 42, 2585–2601. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Daly, P.; Sharma, A.; Shaghaleh, H.; Hamoud, A.Y.; El-Esawi, M.A.; Pan, R.; Wan, Q.; et al. Seed priming and foliar application with jasmonic acid enhance salinity stress tolerance of soybean (Glycine max L.) seedlings. J. Sci. Food Agric. 2021, 101, 2027–2041. [Google Scholar] [CrossRef] [PubMed]
- Lubyanova, A.R.; Bezrukova, M.V.; Shakirova, F.M. Involvement of Nitric Oxide in Methyl Jasmonate-Mediated Regulation of Water Metabolism in Wheat Plants under Drought Stress. Stresses 2022, 2, 477–492. [Google Scholar] [CrossRef]
- Lubyanova, A.R.; Bezrukova, M.V.; Shakirova, F.M. Interaction Between Signal Pathways Upon Formation of Plant Defence in Response to Environmental Stress Factors. Russ. J. Plant Physiol. 2021, 68, 989–1002. [Google Scholar] [CrossRef]
- Zboińska, M.; Romero, L.C.; Gotor, C.; Kabała, K. Regulation of V-ATPase by Jasmonic Acid: Possible Role of Persulfidation. Int. J. Mol. Sci. 2023, 24, 13896. [Google Scholar] [CrossRef] [PubMed]
- Munemasa, S.; Oda, K.; Watanabe-Sugimoto, M.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. The coronatine-insensitive 1 Mutation Reveals the Hormonal Signaling Interaction between Abscisic Acid and Methyl Jasmonate in Arabidopsis Guard Cells. Specific Impairment of Ion Channel Activation and Second Messenger Production. Plant Physiol. 2007, 143, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic Acid and Jasmonic Acid are Involved in Drought Priming-Induced Tolerance to Drought in Wheat. Crop J. 2021, 9, 120–132. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Alberto, J.-V.; Alberto, D.D.; Jacobo-Velázquez, L.; Cisneros-Zevallos, L. Physiological Role of Reactive Oxygen Species, Ethylene, and Jasmonic Acid on UV Light Induced Phenolic Biosynthesis in Wounded Carrot Tissue. Postharvest Biol. Technol. 2021, 172, 111388. [Google Scholar] [CrossRef]
- Noctor, G.; Lelarge-Trouverie, C.; Mhamdi, A. The Metabolomics of Oxidative Stress. Phytochemistry 2015, 112, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Tavallali, V.; Karimi, S. Methyl Jasmonate Enhances Salt Tolerance of Almond Rootstocks by Regulating Endogenous Phytohormones, Antioxidant Activity and Gas–Exchange. J. Plant Physiol. 2019, 234–235, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Per, T.S.; Khan, N.A.; Masood, A.; Fatma, M. Methyl Jasmonate Alleviates Cadmium-Induced Photosynthetic Damages Through Increased S-assimilation and Glutathione Production in Mustard. Front. Plant Sci. 2016, 7, 233002. [Google Scholar] [CrossRef] [PubMed]
- Faghih, S.; Zarei, A.; Ghobadi, C. Positive Effects of Plant Growth Regulators on Physiology Responses of Fragaria × ananassa cv. “Camarosa” Under Salt Stress. Int. J. Fruit Sci. 2019, 19, 104–114. [Google Scholar] [CrossRef]
- Bellande, K.; Bono, J.-J.; Savelli, B.; Jamet, E.; Canut, H. Plant Lectins and Lectin Receptor-Like Kinases: How Do They Sense the Outside? Int. J. Mol. Sci. 2017, 18, 1164. [Google Scholar] [CrossRef] [PubMed]
- Peumans, W.J. Biochemistry, Cell-Biology, Physiology, Biosynthesis and Function of Gramineae Lectins. Proefschift Leuven; Katholieke Universiteit: Leuven, Belgium, 1984; p. 211. [Google Scholar]
- Petrova, L.; Yordanova, A.; Bogoeva, V. Adenine Binding Capacity of a Plant Lectin Concanavalin A. Genet. Plant Physiol. 2016, 6, 135–141. [Google Scholar]
- Lubyanova, A.R.; Bezrukova, M.V.; Shakoriva, F.M. Effect of WGA and Salinity on Growth and Hormonal Status of Roots of Barley and Rice Seedling. Agrochemistry 2019, 8, 52–59. [Google Scholar] [CrossRef]
- Haichar, F.Z.; Santaella, C.; Heulin, T.; Achouak, W. Root Exudates Mediated Interactions Belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Bezrukova, M.; Kildibekova, A.; Shakirova, F. WGA Reduces the Level of Oxidative Stress in Wheat Seedlings Under Salinity. Plant Growth Regul. 2008, 54, 195–201. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, J.; Li, R.; Yan, S.; Chen, W.; Guo, L.; Qin, G.; Wang, P.; Luo, C.; Huang, W.; et al. A Reactive Oxygen Species Burst Causes Haploid Induction in Maize. Mol. Plant 2022, 15, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Angelini, R.; Tisi, A.; Rea, G.; Chen, M.M.; Botta, M.; Federico, R.; Cona, A. Involvement of Polyamine Oxidase in Wound Healing. Plant Physiol. 2008, 146, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Maruta, T.; Inoue, T.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Arabidopsis NADPH oxidases, AtrbohD and AtrbohF, are Essential for Jasmonic Acid-Induced Expression of Genes Regulated by MYC2 Transcription Factor. Plant Sci. 2011, 180, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, Y.; Yang, D.; Liu, H.; Zhang, Y.; Wang, X.; Bai, F.; Cheng, S. Foliar Methyl Jasmonate (MeJA) Application Increased 2-acetyl-1-Pyrroline (2-AP) Content and Modulated Antioxidant Attributes and Yield Formation in Fragrant rice. J. Plant Physiol. 2023, 282, 153946. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Sachabutdinova, A.R.; Ishdavletova, R.S.; Lastochkina, O.V. Effect of Pretreatment with Methyl Jasmonate on Wheat Resistance to Salt Stress. Agrochemistry 2010, 7, 26–32. [Google Scholar]
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl Jasmonate Protects the PS II System by Maintaining the Stability of Chloroplast D1 Protein and Accelerating Enzymatic Antioxidants in Heat-Stressed Wheat Plants. Antioxidants 2021, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Van Breusegem, F. Reactive Oxygen Species in Plant Development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, J.; He, J.; Qin, Y.; Hua, D.; Duan, Y.; Chen, Z.; Gong, Z. ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling PLETHORA Expression in Arabidopsis. PLoS Genet. 2014, 10, e1004791. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.Y. Mechanisms of Heat and Drought Resistance in Grain Sorghum and Methods of Measurement. In Sorghum in the Seventies; Rao, N.G.P., House, L.R., Eds.; Oxford & IBH Publishing Co.: New Delhi, India, 1971; pp. 247–284. [Google Scholar]
- Zaninotto, F.; Camera, S.L.; Polverari, A.; Delledonne, M.J.P.P. Cross Talk between Reactive Nitrogen and Oxygen Species during the Hypersensitive Disease Resistance Response. Plant Physiol. 2006, 141, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Gill, R.A.; Islam, F.; Ali, B.; Liu, H.; Xu, J.; Zhou, W. Methyl Jasmonate Regulates Antioxidant Defense and Suppresses Arsenic Uptake in Brassica napus L. Front. Plant Sci. 2016, 7, 468. [Google Scholar] [CrossRef]
- Lang, D.; Yu, X.; Jia, X.; Li, Z.; Zhang, X. Methyl Jasmonate Improves Metabolism and Growth of NaCl-stressed Glycyrrhiza uralensis Seedlings. Sci. Hortic. 2020, 266, 109287. [Google Scholar] [CrossRef]
- Tayyab, N.; Naz, R.; Yasmin, H.; Nosheen, A.; Keyani, R.; Sajjad, M.; Hassan, M.N.; Roberts, T.H. Combined Seed and Foliar Pre-treatments With Exogenous Methyl Jasmonate and Salicylic Acid Mitigate Drought-Induced Stress in Maize. PLoS ONE 2020, 15, e0232269. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F.; Salama, K.H.A. Proline and Abiotic Stresses: Responses and adaptation. Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II. In Mechanisms of Adaptation and Stress Amelioration; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 357–397. [Google Scholar] [CrossRef]
- Hakeem, K.R.; Alharby, H.F.; Pirzadah, T.B. Exogenously Applied Calcium Regulates Antioxidative System and Reduces Cadmium-Uptake in Fagopyrum esculentum. Plant Physiol. Bioch. 2022, 180, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Vives-Peris, V.; Molina, L.; Segura, A.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root Exudates from Citrus Plants Subjected to Abiotic Stress Conditions Have a Positive Effect on Rhizobacteria. J. Plant Physiol. 2018, 228, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Tadayon, M.R.; Bahador, M.; Razmjoo, J. Investigation of the Proline Role in Controlling Traits Related to Sugar and Root Yield of Sugar Beet Under Water Deficit Conditions. Agric. Water Manag. 2021, 243, 106448. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.; Okuma, E.; Amako, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous Proline and Glycinebetaine Increase NaCl-Induced Ascorbate-Glutathione Cycle Enzyme Activities, and Proline Improves Salt Tolerance More Than Glycinebetaine in Tobacco Bright Yellow-2 Suspension-Cultured Cells. J. Plant Physiol. 2007, 164, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zeng, B.; Sun, Z.; Zhu, C. Relationship Between Proline and Hg2+—Induced Oxidative Stress in a Tolerant Rice Mutant. Arch. Environ. Contam. Toxicol. 2009, 56, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Rajendrakumar, C.S.V.; Suryanarayana, T.; Reddy, A.R. DNA Helix Destabilization by Proline and Betaine: Possible Role in the Salinity Tolerance Process. FEBS Lett. 1997, 410, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, V.; Sinha, S.K. Influence of Exogenously Supplied Proline on Relative Water Content in Wheat and Barley. Indian J. Exp. Biol. 1980, 18, 1523–1524. [Google Scholar]
- Mona, S.A.; Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Soliman, D.W.K.; Wirth, S.; Egamberdieva, D. Increased Resistance of Drought by Trichoderma harzianum Fungal Treatment Correlates with Increased Secondary Metabolites and Proline Content. J. Integr. Agr. 2017, 16, 1751–1757. [Google Scholar] [CrossRef]
- Lubyanova, A.R. Collaborative Impact of Wheat Germ Agglutinin and Bacillus subtilis on Growth, Water Balance, Photosynthetic Traits, Lignification and Suberinization of Wheat Seedlings. Russ. J. Plant Physiol. 2025, 72, 79. [Google Scholar] [CrossRef]
- Tian, S.; Du, K.; Yan, F.; Li, Y. Microwave-Assisted Enzymatic Hydrolysis of Wheat Germ Albumin to Prepare Polypeptides and Influence on Physical and Chemical Properties. Food Chem. 2022, 374, 131707. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen Availability Regulates Proline and Ethylene Production and Alleviates Salinity Stress in Mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Bhaglal, P.; Bhullar, S. Wheat Germ Agglutinin (WGA) Gene Expression and ABA Accumulation in the Developing Embryos of Wheat (Triticum aestivum) in Response to Drought. Plant Growth Regul. 2000, 30, 145–150. [Google Scholar] [CrossRef]
- Lubyanova, A.; Allagulova, C. Exogenous Sodium Nitroprusside Affects the Redox System of Wheat Roots Differentially Regulating the Activity of Antioxidant Enzymes under Short-Time Osmotic Stress. Plants 2024, 13, 1895. [Google Scholar] [CrossRef] [PubMed]
- Beyer, Y.; Fridovich, I. Assaying for Superoxide Dismutase Activity: Some Large Consequences of Minor Changes in Conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Verma, S.; Dubey, R.S. Lead Toxicity Induces Lipid Peroxidation and Alters the Activities of Antioxidant Enzymes in Growing Rice Plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Yusupova, Z.R.; Akhmetova, I.E.; Khairullin, R.M.; Maksimov, I.V. The Effect of Chitooligosaccharides on Hydrogen Peroxidase Production and Anionic Peroxidase Activity in Wheat Coleoptiles. Russ. J. Plant Physiol. 2005, 52, 209–213. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Minibayeva, F.V.; Gordon, L.K.; Kolesnikov, O.P.; Chasov, A.V. Role of Extracellular Peroxidase in the Superoxide Production by Wheat Root Cells. Protoplasma 2001, 217, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, K.K.; Naithani, S.C. Role of Superoxide, Lipid Peroxidation and Superoxide Dismutase in Membrane Perturbation During Loss of Viability in Seeds of Shorea robusta Gaertn.f. New Phytol. 1994, 126, 623–627. [Google Scholar] [CrossRef]
- Khairullin, R.M.; Yarullina, L.G.; Troshina, N.B.; Akhmetova, I.E. Chitooligosacharide-Induced Activation of o-Phenylenediamine Oxidation by Wheat Seedlings in the Presence of Oxalic Acid. Biochemistry 2001, 66, 286–289. [Google Scholar] [PubMed]
- Bellincampi, D.; Dipierro, N.; Salvi, G.; Cervone, F.; De Lorenzo, G. Extracellular H2O2 Induced by Oligogalacturonides is not Involved in the Inhibition of the Auxin-Regulated rolB Gene Expression in Tobacco Leaf Explants. Plant Physiol. 2000, 122, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.A.; Akther, M.S.; Reza, M.A.; Kabir, A.H. Cadmium Tolerance is Associated with the Root-Driven Coordination of Cadmium Sequestration, Iron Regulation, and ROS Scavenging in Rice. Plant Physiol. Biochem. 2019, 136, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldran, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water Stress Studies. Plant Soil 1973, 39, 205–208. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Citrus Plants Exude Proline and Phytohormones under Abiotic Stress Conditions. Plant Cell Rep. 2017, 36, 1971–1984. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Kubiś, J. Involvement of Nitric Oxide in Water Stress-Induced Responses of Cucumber Roots. Plant Sci. 2009, 177, 682–690. [Google Scholar] [CrossRef]
- Gill, M.B.; Zeng, F.; Shabala, L.; Zhang, G.; Yu, M.; Demidchik, V.; Shabala, S.; Zhou, M. Identification of QTL Related to ROS Formation Under Hypoxia and Their Association with Waterlogging and Salt tolerance in Barley. Int. J. Mol. Sci. 2019, 20, 699. [Google Scholar] [CrossRef] [PubMed]
- Kitaeva, A.B.; Demchenko, K.N.; Tikhonovich, I.A.; Timmers, A.C.; Tsyganov, V.E. Comparative Analysis of the Tubulin Cytoskeleton Organization in Nodules of Medicago truncatula and Pisum sativum: Bacterial Release and Bacteroid Positioning Correlate with Characteristic Microtubule Rearrangements. New Phytol. 2016, 210, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Avalbaev, A.M.; Bezrukova, M.V.; Gimalov, F.R. Induction of Wheat Germ Agglutinin Synthesis by Abscisic and Gibberellic Acids in Roots of Wheat Seedlings. Plant Growth Regul. 2001, 33, 111–115. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the Hormonal Status of Wheat Seedlings Induced by Salicylic Acid and Salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Bezrukova, M.V.; Shayakhmetov, I.F. Effect of Temperature Shock on the Dynamics of Abscisic Acid and Wheat Germ Agglutinin Accumulation in Wheat Cell Culture. Plant Growth Regul. 1996, 19, 85–87. [Google Scholar] [CrossRef]




| Treatment | O2•− in Roots, OD540/ (g FW × min) | H2O2 in Roots, µM/g FW | SOD Activity, U/(mg Protein × Min) | POD Activity, U/(mg Protein × Min) | APX Activity, µM Ascorbate/ (mg Protein × Min) | CAT Activity, µM H2O2/(mg Protein × Min) |
|---|---|---|---|---|---|---|
| Control | 0.30 ± 0.015 c | 153.8 ± 6.9 b | 90.6 ± 4.1 c | 11.1 ± 0.5 b | 1.8 ± 0.09 d | 121.7 ± 5.9 d |
| (MeJA) | 0.29 ± 0.015 c | 148.3 ± 7.1 b | 92.4 ± 4.2 c | 12.3 ± 0.6 b | 2.3 ± 0.11 c | 182.7 ± 8.1 c |
| 12% PEG | 0.53 ± 0.026 a | 240.0 ± 10.5 a | 121.5 ± 6.1 b | 14.9 ± 0.7 a | 2.8 ± 0.14 b | 237.0 ± 10.6 b |
| (MeJA) + PEG | 0.44 ± 0.022 b | 164.0 ± 7.8 b | 135.2 ± 6.5 a | 15.6 ± 0.7 a | 3.5 ± 0.17 a | 268.0 ± 11.4 a |
| Treatment | SOD Activity/O2•− | POD Activity/H2O2 | APX Activity/H2O2 | CAT Activity/H2O2 |
|---|---|---|---|---|
| Control | 302 ± 15 a | 0.072 ± 0.003 c | 0.012 ± 0.0006 c | 0.791 ± 0.04 d |
| (MeJA) | 319 ± 16 a | 0.083 ± 0.004 b | 0.016 ± 0.0008 b | 1.232 ± 0.06 b |
| 12% PEG | 229 ± 10 b | 0.062 ± 0.003 d | 0.012 ± 0.0006 c | 0.988 ± 0.05 c |
| (MeJA) + PEG | 307 ± 15 a | 0.095 ± 0.005 a | 0.021 ± 0.0010 a | 1.634 ± 0.08 a |
| Treatment | MDA, nM/g FW | EL, mS/g FW | Pro in Roots, µM/g FW | Pro in the Growth Medium, pM/g FW |
|---|---|---|---|---|
| Control | 22.45 ± 0.55 c | 22.97 ± 0.55 d | 0.80 ± 0.04 c | 0.30 ± 0.009 d |
| (MeJA) | 22.66 ± 0.56 c | 27.07 ± 0.65 c | 0.82 ± 0.04 c | 0.37 ± 0.010 c |
| 12% PEG | 42.72 ± 0.98 a | 92.11 ± 2.10 a | 1.80 ± 0.09 b | 17.73 ± 0.43 b |
| (MeJA) + PEG | 35.04 ± 0.86 b | 35.00 ± 0.85 b | 2.20 ± 0.10 a | 12.90 ± 0.32 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubyanova, A.R. Influence of Methyl Jasmonate and Short-Term Water Deficit on Growth, Redox System, Proline and Wheat Germ Agglutinin Contents of Roots of Wheat Seedlings. Int. J. Mol. Sci. 2025, 26, 6871. https://doi.org/10.3390/ijms26146871
Lubyanova AR. Influence of Methyl Jasmonate and Short-Term Water Deficit on Growth, Redox System, Proline and Wheat Germ Agglutinin Contents of Roots of Wheat Seedlings. International Journal of Molecular Sciences. 2025; 26(14):6871. https://doi.org/10.3390/ijms26146871
Chicago/Turabian StyleLubyanova, Alsu R. 2025. "Influence of Methyl Jasmonate and Short-Term Water Deficit on Growth, Redox System, Proline and Wheat Germ Agglutinin Contents of Roots of Wheat Seedlings" International Journal of Molecular Sciences 26, no. 14: 6871. https://doi.org/10.3390/ijms26146871
APA StyleLubyanova, A. R. (2025). Influence of Methyl Jasmonate and Short-Term Water Deficit on Growth, Redox System, Proline and Wheat Germ Agglutinin Contents of Roots of Wheat Seedlings. International Journal of Molecular Sciences, 26(14), 6871. https://doi.org/10.3390/ijms26146871






