Growth Hormone Therapy in Recurrent Implantation Failure: Stratification by FSH Receptor Polymorphism (Asn680Ser) Reveals Genotype-Specific Benefits
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Overall Impact of GH
2.3. Outcomes by FSHR Genotype
2.4. Frozen Embryotransfer (FET) Outcomes
3. Discussion
4. Materials and Methods
4.1. Study Design and Ethical Approval
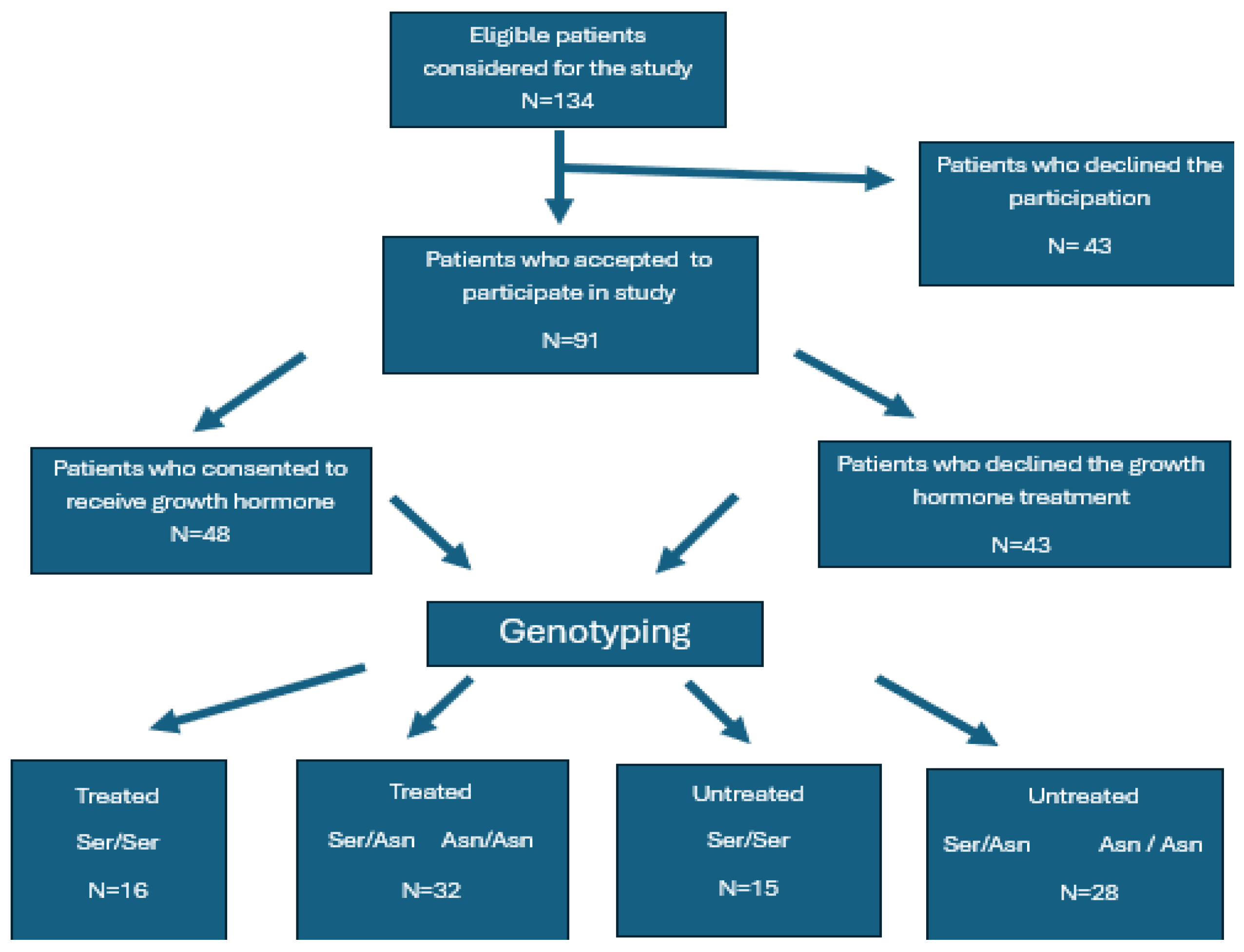
4.2. Patient Selection
4.3. Genetic Testing
4.4. Study Group and FSHR Stratification
- Group Ser/Ser treated.
- Group Ser/Ser untreated.
- Group Ser/Asn + Asn/Asn treated.
- Group Ser/Asn + Asn/Asn untreated.
4.5. Ovarian Stimulation Protocols and Embryo Transfer
4.6. Biological Sample Collection and Analysis
4.6.1. Endometrial LIF Assessment
4.6.2. Serum Progesterone Measurements
4.7. Outcomes and Definitions
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RIF | recurrent implantation failure |
| GH | growth hormone |
| FSHR | follicle-stimulating hormone receptor |
| LHR | luteinizing hormone receptor |
| BMPR1B | bone morphogenetic protein receptor 1B |
| LIF | leukemia inhibiting factor |
| VEGF | vascular endothelial growth factor |
| FSH | follicle-stimulating hormone |
| LH | luteinizing hormone |
| IGF-1 | insulin-like growth factor 1 |
| SNPs | single-nucleotide polymorphisms |
| FORT | Follicular Output Rate |
| FOI | Follicular Output Index |
| LBR | live births rate |
| CLBR | cumulative live births rate |
| FET | frozen embryo transfer |
| MAPK | mitogen-activated protein kinase |
| RCT | randomized controlled trials |
| JAK/STAT3 | Janus kinase/signal transducer and activator of transcription |
| SOCS1 | Suppressor of Cytokine Signaling 1 |
| IVF | in vitro fertilization |
| SD | standard deviation |
| IQR | interquartile range |
References
- Cimadomo, D.; Craciunas, L.; Vermeulen, N.; Vomstein, K.; Toth, B. Definition, diagnostic and therapeutic options in recurrent implantation failure: An international survey of clinicians and embryologists. Hum. Reprod. 2021, 36, 305–317. [Google Scholar] [CrossRef]
- Somigliana, E.; Vigano, P.; Busnelli, A.; Paffoni, A.; Vegetti, W.; Vercellini, P. Repeated implantation failure at the crossroad between statistics, clinics and over-diagnosis. Reprod. Biomed. Online 2018, 36, 32–38. [Google Scholar] [CrossRef]
- (The Writing Group) for the Participants to the 2022 Lugano RIF Workshop; Pirtea, P.; Cedars, M.I.; Devine, K.; Ata, B.; Franasiak, J.; Racowsky, C.; Toner, J.; Scott, R.T.; de Ziegler, D.; et al. Recurrent implantation failure: Reality or a statistical mirage?: Consensus statement from the July 1, 2022 Lugano Workshop on recurrent implantation failure. Fertil. Steril. 2023, 120, 45–59. [Google Scholar] [CrossRef]
- Hill, M.J. Recurrent implantation failure: Sapereaude. Fertil. Steril. 2021, 116, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Alecsandru, D.; Forman, E.J.; Gemmell, L.C.; Goldberg, J.M.; Llarena, N.; Margolis, C.; Laven, J.; Schoenmakers, S.; Seli, E. A review of the pathophysiology of recurrent implantation failure. Fertil. Steril. 2021, 116, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Lédée, N.; Munaut, C.; Aubert, J.; Sérazin, V.; Rahmati, M.; Chaouat, G.; Sandra, O.; Foidart, J.M. Specific and extensive endometrial deregulation is present before conception in IVF/ICSI repeated implantation failures (IF) or recurrent miscarriages. J. Pathol. 2011, 225, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Cimadomo, D.; de los Santos, M.J.; Griesinger, G.; Lainas, G.; Le Clef, N.; McLernon, D.J.; Montjean, D.; Toth, B.; Vermeulen Macklon, N.; ESHRE Working Group on Recurrent Implantation Failure. ESHRE good practice recommendations on recurrent implantation failure. Hum. Reprod. Open 2023, 2023, hoad023. [Google Scholar] [CrossRef]
- Lundin, K.; Bentzen, J.G.; Bozdag, G.; Ebner, T.; Harper, J.; Le Clef, N.; Moffett, A.; Norcross, S.; Polyzos, N.P.; ESHRE Add-Ons Working Group; et al. ESHRE Add-ons working group; Good practice recommendations on add-ons in reproductive medicine. Hum. Reprod. 2023, 38, 2062–2104. [Google Scholar] [CrossRef]
- Hart, R.J. Use of growth hormone in the IVF treatment of women with poor ovarian reserve. Front. Endocrinol. 2019, 10, 500. [Google Scholar] [CrossRef]
- Abir, R.; Garor, R.; Felz, C.; Nitke, S.; Krissi, H.; Fisch, B. Growth hormone and its receptor in human ovaries from fetuses and adults. Fertil. Steril. 2008, 90, 1333–1339. [Google Scholar] [CrossRef]
- Silva, J.R.; Figueiredo, J.R.; van den Hurk, R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 2009, 71, 1193–1208. [Google Scholar] [CrossRef] [PubMed]
- Altmäe, S.; Aghajanova, L. Growth Hormone and Endometrial Receptivity. Front. Endocrinol. 2019, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Childs, G.V. Growth hormone cells as co-gonadotropes: Partners in the regulation of the reproductive system. Trends Endocrinol. Metab. 2000, 11, 168–175. [Google Scholar] [CrossRef]
- Juengel, J.L.; Nett, T.M.; Anthony, R.V.; Niswender, G.D. Effects of luteotrophic and luteolytic hormones on expression of mRNA encoding insulin-like growth factor I and growth hormone receptor in the ovine corpus luteum. J. Reprod. Fertil. 1997, 110, 291–298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takeda, M.; Otsuka, F.; Takahashi, H.; Inagaki, K.; Miyoshi, T.; Tsukamoto, N.; Makino, H.; Lawson, M.A. Interaction between gonadotropin-releasing hormone and bone morphogenetic protein-6 and−7 signaling in LßT2 gonadotrope cells. Mol. Cell. Endocrinol. 2012, 348, 147–154. [Google Scholar] [CrossRef][Green Version]
- Devesa, J.; Caicedo, D. The Role of Growth Hormone on Ovarian Functioning and Ovarian Angiogenesis. Front. Endocrinol. 2019, 10, 450. [Google Scholar] [CrossRef]
- Rosario, G.X.; Stewart, C.L. The Multifaceted Actions of Leukaemia Inhibitory Factor in Mediating Uterine Receptivity and Embryo Implantation. Am. J. Reprod. Immunol. 2016, 75, 246–255. [Google Scholar] [CrossRef]
- Jain, M.; Samokhodskaya, L.; Mladova, E.; Panina, O. Mucosal biomarkers for endometrial receptivity: A promising yet underexplored aspect of reproductive medicine. Syst. Biol. Reprod. Med. 2022, 68, 13–24. [Google Scholar] [CrossRef]
- Ali, R.; Iqbal, M.U.N.; Rehman, R.; Khan, T.A. Interplay of "leukemia inhibitory factor receptor gene" (rs3099124) polymorphism, leukemia inhibitory factor and ovarian steroids with unexplained infertility. Nucleosides Nucleotides Nucleic Acids 2023, 42, 718–730. [Google Scholar] [CrossRef]
- Pathare, A.D.S.; Zaveri, K.; Hinduja, I. Downregulation of genes related to immune and inflammatory response in IVF implantation failure cases under controlled ovarian stimulation. Am. J. Reprod. Immunol. 2017, 78, e12679. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, P.; Zhou, W.; Xia, M.; Li, J.; Lu, J.; Ma, J.L.; Chen, Z.J.; Yan, J. Growth hormone supplementation ameliorates blastocyst euploidy rates and improves pregnancy outcomes in women undergoing preimplantation genetic testing for aneuploidy cycles. Front. Endocrinol. 2023, 14, 1117706. [Google Scholar] [CrossRef]
- Skillern, A.; Leonard, W.; Pike, J.; Mak, W. Growth hormone supplementation during ovarian stimulation improves oocyte and embryo outcomes in IVF/PGT-A cycles of women who are not poor responders. J. Assist. Reprod. Genet. 2021, 38, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Recchia, K.; Jorge, A.S.; Pessôa, L.V.F.; Botigelli, R.C.; Zugaib, V.C.; de Souza, A.F.; Martins, D.D.S.; Ambrósio, C.E.; Bressan, F.F.; Pieri, N.C.G. Actions and Roles of FSH in Germinative Cells. Int. J. Mol. Sci. 2021, 22, 10110. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, D.; Patel, H. An overview of FSH-FSHR biology and explaining the existing conundrums. J. Ovarian Res. 2021, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.A.; Joseph, S.; Mahale, S.D. From cell surface to signalling and back: The life of the mammalian FSH receptor. FEBS J. 2021, 288, 2673–2696. [Google Scholar] [CrossRef]
- Ghaderian, S.M.H.; Akbarzadeh, R.; Salehpour, S. Involvement of single nucleotide polymorphisms in ovarian poor response. J. Assist. Reprod. Genet. 2021, 38, 2405–2413. [Google Scholar] [CrossRef]
- Boudjenah, R.; Molina-Gomes, D.; Torre, A.; Bergere, M.; Bailly, M.; Boitrelle, F.; Taieb, S.; Wainer, R.; Benahmed, M.; de Mazancourt, P.; et al. Genetic polymorphisms influence the ovarian response to rFSH stimulation in patients undergoing in vitro fertilization programs with ICSI. PLoS ONE 2012, 7, e38700. [Google Scholar] [CrossRef]
- Prodromidou, A.; Dimitroulia, E.; Mavrogianni, D.; Kathopoulis, N.; Pappa, K.I.; Loutradis, D. The Effect of the Allelics of Ser680Asn Polymorphisms of Follicle-Stimulating Hormone Receptor Gene in IVF/ICSI Cycles: A Systematic Review and Meta-analysis. Reprod. Sci. 2023, 30, 428–441. [Google Scholar] [CrossRef]
- Pabalan, N.; Trevisan, C.M.; Peluso, C.; Jarjanazi, H.; Christofolini, D.M.; Barbosa, C.P.; Bianco, B. Evaluating influence of the genotypes in the follicle-stimulating hormone receptor (FSHR) Ser680Asn (rs6166) polymorphism on poor and hyper-responders to ovarian stimulation: A meta-analysis. J. Ovarian Res. 2014, 7, 285. [Google Scholar] [CrossRef]
- Lindgren, I.; Bååth, M.; Uvebrant, K.; Dejmek, A.; Kjaer, L.; Henic, E.; Bungum, M.; Bungum, L.; Cilio, C.; Leijonhufvud, I.; et al. Combined assessment of polymorphisms in the LHCGR and FSHR genes predict chance of pregnancy after in vitro fertilization. Hum. Reprod. 2016, 31, 672–683. [Google Scholar] [CrossRef]
- Conforti, A.; Tüttelmann, F.; Alviggi, C.; Behre, H.M.; Fischer, R.; Hu, L.; Polyzos, N.P.; Chuderland, D.; Rama Raju, G.A.; D’Hooghe, T.; et al. Effect of Genetic Variants of Gonadotropins and Their Receptors on Ovarian Stimulation Outcomes: A Delphi Consensus. Front. Endocrinol. 2022, 12, 797365. [Google Scholar] [CrossRef]
- Monge-Ochoa, B.; Montoro, L.; Gil-Arribas, E.; Montoya, J.; Ruiz-Pesini, E.; López-Pérez, M.J.; de Castro, F.; Díez-Sánchez, C. Variants Ala307Ala and Ser680Ser of 307 and 680 FSHr polymorphisms negatively influence on assisted reproductive techniques outcome and determine high probability of non-pregnancy in Caucasian patients. J. Assist. Reprod. Genet. 2021, 38, 2769–2779. [Google Scholar] [CrossRef]
- Kordowitzki, P.; Krajnik, K.; Skowronska, A.; Skowronski, M.T. Pleiotropic Effects of IGF1 on the Oocyte. Cells 2022, 11, 1610. [Google Scholar] [CrossRef]
- Carter-Su, C.; Schwartz, J.; Argetsinger, L.S. Growth hormone signaling pathways. Growth Horm. IGF Res. 2016, 28, 11–15. [Google Scholar] [CrossRef]
- Ob’edkova, K.; Kogan, I.; Krikheli, I.; Dzhemlikhanova, L.; Muller, V.; Mekina, I.; Lesik, E.; Komarova, E.; Mazilina, M.; Niauri, D.; et al. Growth hormone co-treatment in IVF/ICSI cycles in poor responders. Gynecol. Endocrinol. 2017, 33 (Suppl. 1), 15–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wu, R.; Zhang, H. The effect of growth hormone supplementation in poor ovarian responders undergoing IVF or ICSI: A meta-analysis of randomized controlled trials. Reprod. Biol. Endocrinol. 2020, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, E.H.; Abou El Serour, A.G.; Mohamed, E.A.H.; Abbasy, A.H.; Zaatar, M.; Rageh, K.A.; Shafeek, M.M.; Issak, E.R. Efficacy of growth hormone supplementation with ultrashort GnRH antagonist in IVF/ICSI for poor responders; randomized controlled trial. Taiwan. J. Obstet. Gynecol. 2021, 60, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, F.; Nong, Y.; Ruan, J.; Guo, Q.; Luo, M.; Huang, Q. Clinical efficacy and mechanism of growth hormone action in patients experiencing repeat implantation failure. Can. J. Physiol. Pharmacol. 2018, 96, 929–932. [Google Scholar] [CrossRef]
- Vera-Montoya, M.; Andrés Calvache, J.; Geber, S. Growth Hormone Administration to Improve Reproductive Outcomes in Women with Recurrent Implantation Failure (RIF): A Systematic Review. Reprod. Sci. 2023, 30, 1712–1723. [Google Scholar] [CrossRef]
- Bavan, B.; Gardner, R.M.; Zhang, W.Y.; Aghajanova, L. The Effect of Human Growth Hormone on Endometrial Growth in Controlled Ovarian Hyperstimulation Cycles. J. Pers. Med. 2022, 12, 1991. [Google Scholar] [CrossRef]
- Cui, N.; Li, A.M.; Luo, Z.Y.; Zhao, Z.M.; Xu, Y.M.; Zhang, J.; Yang, A.M.; Wang, L.L.; Hao, G.M.; Gao, B.L. Effects of growth hormone on pregnancy rates of patients with thin endometrium. J. Endocrinol. Investig. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Dakhly, D.M.R.; Bassiouny, Y.A.; Bayoumi, Y.A.; Hassan, M.A.; Gouda, H.M.; Hassan, A.A. The addition of growth hormone adjuvant therapy to the long down regulationprotocol in poor responders undergoing in vitro fertilization: Randomized control trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.J.; Alvino, H.; Hull, L.M.; Mol, B.W.; Hart, R.J.; Kelly, T.L.; Rombauts, L. Human growth hormone for poor responders: A randomized placebo-controlled trial provides no evidence for improved live birth rate. Reprod. BioMed. Online 2019, 38, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Mohammadshirazi, Z.; Alyasin, A.; Agha Hosseini, M.; Hazari, V. The Impact of Growth Hormone Co-Treatment Duration on Outcomes in IVF/ICSI Cycles Among Poor Ovarian Responders. J. Reprod. Infertil. 2023, 24, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Mourad, A.; Jamal, W.; Hemmings, R.; Tadevosyan, A.; Phillips, S.; Kadoch, I.J. Empirical use of growth hormone in IVF is useless: The largest randomized controlled trial. Hum. Reprod. 2025, 40, 77–84. [Google Scholar] [CrossRef]
- Baldini, G.M.; Catino, A.; Palini, S.; Sciorio, R.; Ferri, D.; Vinciguerra, M.; Baldini, D. The Polymorphism Asn680Ser on the FSH Receptor and Abnormal Ovarian Response in Patients with Normal Values of AMH and AFC. Int. J. Mol. Sci. 2023, 24, 1080. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog. Horm. Res. 2000, 55, 15–35; discussion 35–36. [Google Scholar]
- Thomsen, L.H.; Kesmodel, U.S.; Erb, K.; Bungum, L.; Pedersen, D.; Hauge, B.; Elbæk, H.O.; Povlsen, B.B.; Andersen, C.Y.; Humaidan, P. The impact of luteal serum progesterone levels on live birth rates-a prospective study of 602 IVF/ICSI cycles. Hum. Reprod. 2018, 33, 1506–1516. [Google Scholar] [CrossRef]
| Parameter | Ser/Ser GH+ | Ser/Ser GH− | Ser/Asn + Asn/Asn GH+ | Ser/Asn + Asn/Asn GH− | p-Value | Summary Type |
|---|---|---|---|---|---|---|
| Age (years) | 37 [34.5–38] | 37 [34–37] | 38 [34–38] | 37 [34–37] | p > 0.05 | median [IQR] |
| Infertility duration (years) | 5 [4–6.25] | 5 [3–7] | 5 [4–6] | 5 [4–6] | p > 0.05 | median [IQR] |
| BMI (kg/m2) | 24 [22–25] | 23 [21.5–24.5] | 24 [22–25] | 23.5 [22.75–25] | p > 0.05 | median [IQR] |
| AMH (ng/mL) | 1.58 [0.83–1.86] | 1.6 [0.63–1.88] | 1.35 [0.73–1.71] | 1.45 [0.73–1.97] | p > 0.05 | median [IQR] |
| AFC | 11.25 ± 2.62 | 11.73 ± 3.63 | 11.06 ± 4.33 | 11.9 ± 3.5 | p > 0.05 | mean ± SD |
| FSH | 9.26 ± 1.84 | 9.01 ± 2.77 | 7.12 ± 1.92 | 7.12 ± 1.55 | p = 0.03 | mean ± SD |
| LH | 6.25 [5.18–6.82] | 6.5 [6.25–6.95] | 6.05 [5.2–6.82] | 6.25 [5.3–7.1] | p > 0.05 | median [IQR] |
| Estradiol | 53.19 ± 15.95 | 52.27 ± 17.26 | 56.75 ± 17.36 | 48 [38.5–66] | p > 0.05 | median [IQR] |
| Previous IVF attempts | 3.0 [2.0–3.0] | 2.5 [2.0–3.0] | 3.0 [2.0–3.0] | 3.0 [2.0–3.0] | p > 0.05 | median [IQR] |
| Parameter | GH Treated | GH Untreated | p-Value |
|---|---|---|---|
| Stimulation days | 10.25 ± 1.19 | 10.14 ± 0.83 | p > 0.05 |
| Agonist/antagonist protocol (%) | 91.67%/8.33% | 93.02%/6.98% | p > 0.05 |
| Total gonadotropin dose (IU) | 2402.08 ± 444.46 | 2581.4 ± 366.78 | p > 0.05 |
| Mature follicles | 8.71 ± 3.75 | 8.77 ± 2.77 | p > 0.05 |
| MII oocytes | 7.06 ± 3.45 | 7.16 ± 2.42 | p > 0.05 |
| FORT | 0.76 ± 0.13 | 0.72 ± 0.13 | p > 0.05 |
| FOI (metaphase II oocyte/mature follicle) | 0.6 ± 0.16 | 0.61 ± 0.14 | p > 0.05 |
| Fertilization rate | 0.79 ± 0.12 | 0.77 ± 0.1 | p > 0.05 |
| Good-quality embryos | 3.00 [1.00–3.00] | 2.00 [1.00–2.00] | p = 0.05 |
| Blastulation rate | 0.40 [0.33–0.50] | 0.33 [0.25–0.40] | p = 0.05 |
| Implantation rate (fresh) | 29% | 21% | p > 0.05 |
| Clinical pregnancy (fresh) | 33% | 23% | p > 0.05 |
| LIF (pg/mL) | 27.0 [18.75–39.00] | 20.0 [12.50–30.00] | p = 0.02 |
| Progesterone (ng/mL) | 79.23 ± 27.34 | 74.74 ± 27.57 | p > 0.05 |
| Fresh transfer miscarriage | 0 [0–0] | 0 [0–0] | p > 0.05 |
| Live birth rate (fresh) | 25% | 16% | p > 0.05 |
| Frozen embryos available | 1.0 [0.0–2.0] | 1.0 [0.0–1.0] | p = 0.04 |
| FET implantation rate | 16% | 22% | p > 0.05 |
| FET live birth rate | 15% | 12% | p > 0.05 |
| Cumulative birth rate | 44% | 21% | p = 0.04 |
| Outcome Parameter | Ser/Ser GH+ | Ser/Ser GH− | Ser/Asn + Asn/Asn GH+ | Ser/Asn + Asn/Asn GH− | p-Value (Resistant) | p-Value Resistant (Bonferroni) | p-Value (Sensitive) |
|---|---|---|---|---|---|---|---|
| Stimulation days | 10.50 ± 1.21 | 10.33 ± 0.72 | 10.13 ± 1.18 | 10.04 ± 0.88 | p > 0.05 | p > 0.05 | p > 0.05 |
| Total gonadotropin (IU) | 2418.08 ± 390.67 | 2663.63 ± 338.5 | 2393.75 ± 474.70 | 2537.5 ± 379.4 | p > 0.05 | p > 0.05 | p > 0.05 |
| Mature follicles | 8.31 ± 3.75 | 8.20 ± 2.76 | 8.91 ± 4.05 | 9.07 ± 2.46 | p > 0.05 | p > 0.05 | p > 0.05 |
| MII oocytes | 7.13 ± 3.45 | 6.53 ± 2.72 | 7.03 ± 3.78 | 7.50 ± 2.22 | p > 0.05 | p > 0.05 | p > 0.05 |
| I Fort | 0.73 ± 0.14 | 0.63 ± 0.13 | 0.78 ± 0.10 | 0.77 ± 0.11 | p > 0.05 | p > 0.05 | p > 0.05 |
| FOI (methtapahase II oocyte/mature follicle) | 0.62 ± 0.16 | 0.50 ± 0.11 | 0.59 ± 0.14 | 0.64 ± 0.13 | p > 0.05 | p > 0.05 | p > 0.05 |
| Fertilization rate | 0.80 ± 0.10 | 0.80 ± 0.10 | 0.78 ± 0.12 | 0.76 ± 0.17 | p > 0.05 | p > 0.05 | p > 0.05 |
| Blastulation rate | 0.50 [0.33–0.58] | 0.33 [0.23–0.33] | 0.39 [0.33–0.5] | 0.39 [0.27–0.5] | p = 0.003 | p = 0.006 | p > 0.05 |
| Good-quality embryos | 2.88 ± 1.66 | 1.53 ± 0.51 | 2.22 ± 1.49 | 1.96 ± 0.59 | p = 0.02 | p = 0.04 | p > 0.05 |
| Implantation rate (%) | 41% | 13% | 29% | 25% | p = 0.07 | p > 0.05 | p > 0.05 |
| Clinical pregnancy rate (%) | 40% | 20% | 31% | 32% | p > 0.05 | p > 0.05 | p > 0.05 |
| Early miscarriage rate (%) | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–0] | p > 0.05 | p > 0.05 | p > 0.05 |
| Live birth rate (%) | 31% | 13% | 25% | 17% | p > 0.05 | p > 0.05 | p > 0.05 |
| Parameter | Ser/Ser GH+ | Ser/Ser GH− | Ser/Asn + Asn/Asn GH+ | Ser/Asn + Asn/Asn GH− | p-Value (Resistant) | p-Value (Sensitive) |
|---|---|---|---|---|---|---|
| No of frozen embryo | 1.5 [0.0–3.0] | 0.0 [0.0–1.0] | 0.0 [0.0–2.0] | 0.0 [0.0–1.0] | p = 0.01 | p > 0.05 |
| Frozen implantation rate | 19% | 28% | 16% | 20% | p > 0.05 | p > 0.05 |
| Frozen clinical pregnancy rate (%) | 31% | 13% | 19% | 18% | p > 0.05 | p > 0.05 |
| Frozen miscarriage rate | 0.0 [0.0–1.0] | 0.0 [0.0–1.0] | 0.0 [0.0–1.0] | 0.0 [0.0–1.0] | p > 0.05 | p > 0.05 |
| Frozen live birth/cycle | 19% | 7% | 13% | 14% | p > 0.05 | p > 0.05 |
| Total clinical live birth | 50% | 13% | 41% | 25% | p = 0.05 | p > 0.05 |
| Parameter | Ser/Ser GH+ | Ser/Ser GH− | Ser/Asn + Asn/Asn GH+ | Ser/Asn + Asn/Asn GH− | p-Value (Resistant) | p-Value Resistant (Bonferroni) | p-Value (Sensitive) |
|---|---|---|---|---|---|---|---|
| LIF (pg/mL) | 27.31 ± 6.8 | 15.87 ± 5.4 | 29.13 ± 12.7 | 25.68 ± 11.5 | p = 0.009 | p = 0.01 | p > 0.05 |
| Progesterone (ng/mL) | 87.67 ± 28.43 | 64.40 ± 34.28 | 75.28 ± 26.34 | 73.25 ± 24.71 | p = 0.05 | p > 0.05 | p > 0.05 |
| Endometrial thickness (mm) | 8.99 ± 1.64 | 8.10 ± 1.32 | 9.89 ± 2.14 | 9.43 ± 1.79 | p > 0.05 | p > 0.05 | p > 0.05 |
| LIF | Progesterone | Endometrial Thickness | Implantation Rate | |
|---|---|---|---|---|
| LIF | — | 0.353 | 0.308 | 0.464 |
| Progesterone | 0.353 | — | 0.249 | 0.154 |
| Endometrial Thickness | 0.308 | 0.249 | — | 0.238 |
| Implantation Rate | 0.464 | 0.154 | 0.238 | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surcel, M.; Nemeti, G.; Goidescu, I.G.; Micu, R.; Zlatescu-Marton, C.; Cordos, A.A.; Caracostea, G.; Rotar, I.C.; Muresan, D.; Boitor-Borza, D. Growth Hormone Therapy in Recurrent Implantation Failure: Stratification by FSH Receptor Polymorphism (Asn680Ser) Reveals Genotype-Specific Benefits. Int. J. Mol. Sci. 2025, 26, 7367. https://doi.org/10.3390/ijms26157367
Surcel M, Nemeti G, Goidescu IG, Micu R, Zlatescu-Marton C, Cordos AA, Caracostea G, Rotar IC, Muresan D, Boitor-Borza D. Growth Hormone Therapy in Recurrent Implantation Failure: Stratification by FSH Receptor Polymorphism (Asn680Ser) Reveals Genotype-Specific Benefits. International Journal of Molecular Sciences. 2025; 26(15):7367. https://doi.org/10.3390/ijms26157367
Chicago/Turabian StyleSurcel, Mihai, Georgiana Nemeti, Iulian Gabriel Goidescu, Romeo Micu, Cristina Zlatescu-Marton, Ariana Anamaria Cordos, Gabriela Caracostea, Ioana Cristina Rotar, Daniel Muresan, and Dan Boitor-Borza. 2025. "Growth Hormone Therapy in Recurrent Implantation Failure: Stratification by FSH Receptor Polymorphism (Asn680Ser) Reveals Genotype-Specific Benefits" International Journal of Molecular Sciences 26, no. 15: 7367. https://doi.org/10.3390/ijms26157367
APA StyleSurcel, M., Nemeti, G., Goidescu, I. G., Micu, R., Zlatescu-Marton, C., Cordos, A. A., Caracostea, G., Rotar, I. C., Muresan, D., & Boitor-Borza, D. (2025). Growth Hormone Therapy in Recurrent Implantation Failure: Stratification by FSH Receptor Polymorphism (Asn680Ser) Reveals Genotype-Specific Benefits. International Journal of Molecular Sciences, 26(15), 7367. https://doi.org/10.3390/ijms26157367





