Integrated MicroRNA–mRNA Sequencing Analysis Identifies Regulators and Networks Involved in Feline Hypertrophic Cardiomyopathy
Abstract
1. Introduction
2. Results
2.1. Differentially Expressed Myocardial mRNAs and microRNAs in Feline HCM and Health
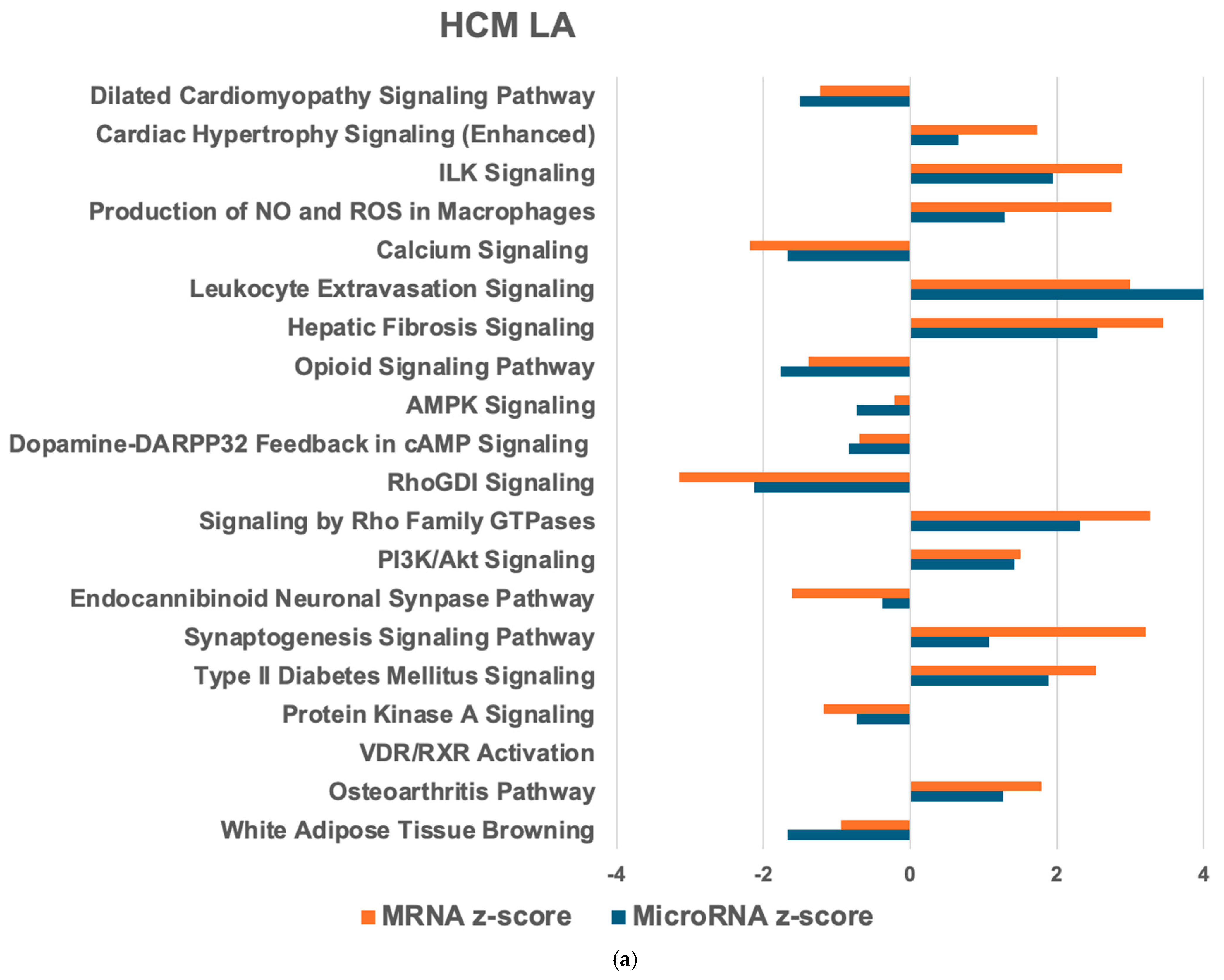
2.2. MicroRNA–mRNA Interactions and Associated Pathways in the Heart from Cats with HCM
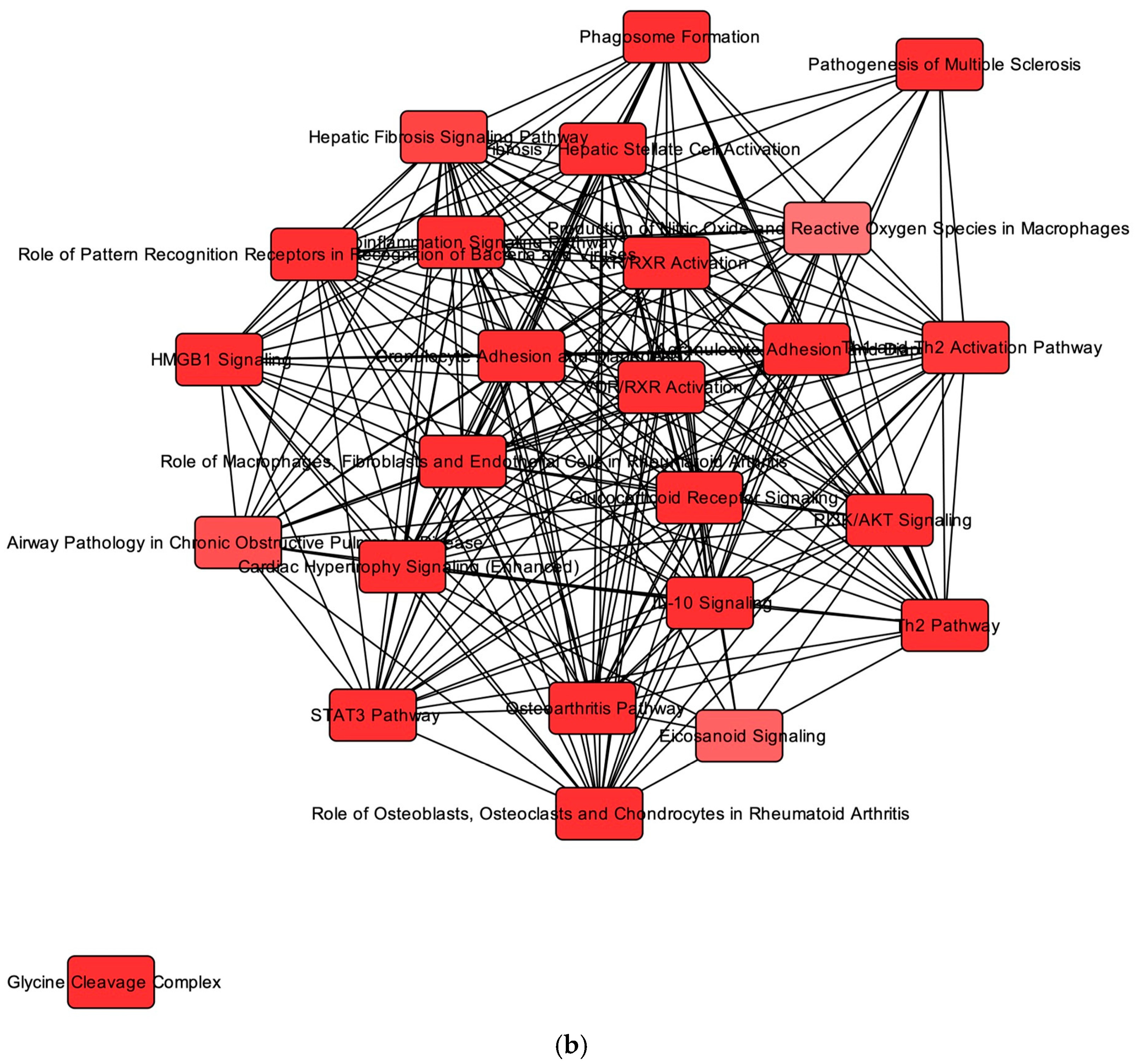
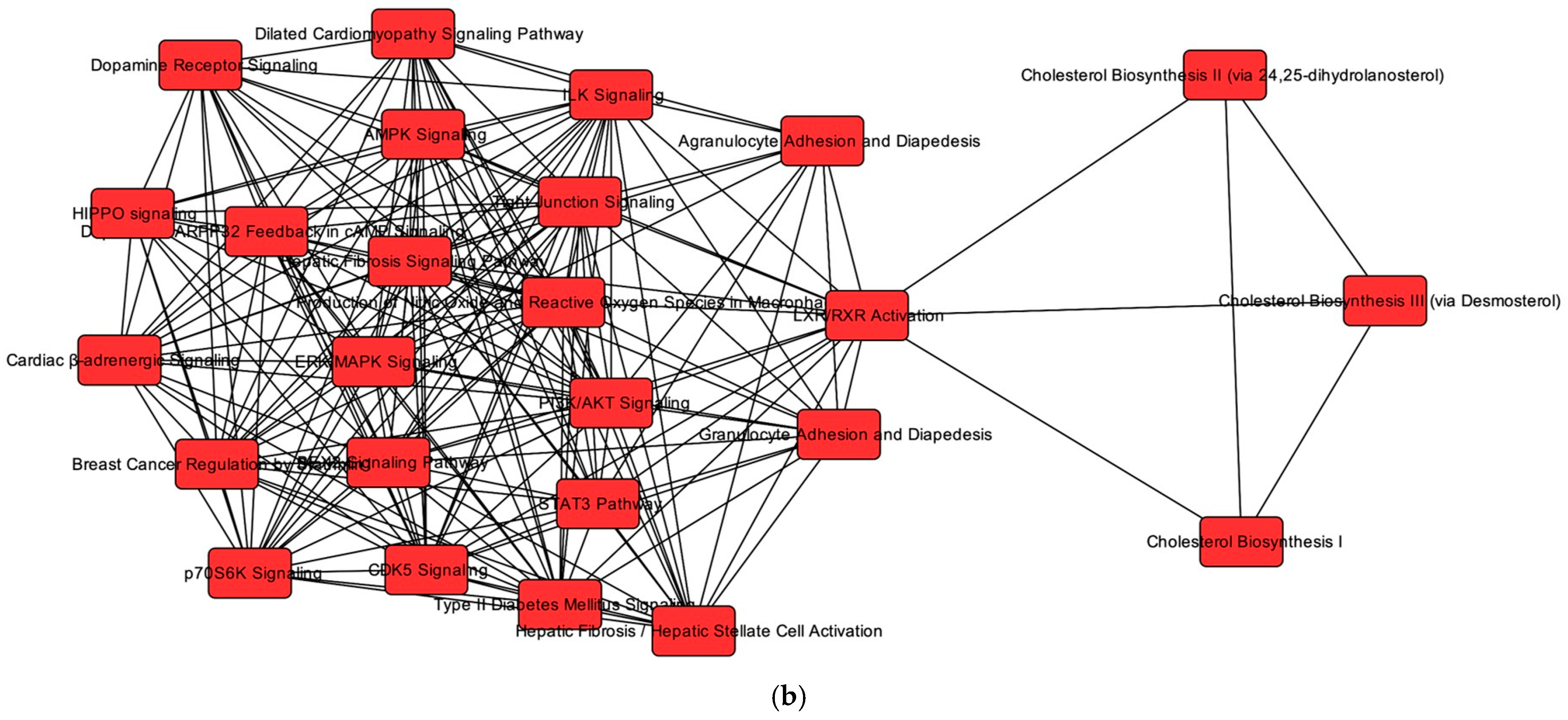
2.3. Overlapping mRNA and microRNA Pathways and Networks in the Heart of Cats with HCM
2.4. Regulators of Networks and Target Molecules in the Heart from Cats with HCM
2.5. MicroRNA–mRNA Interactions and Associated Pathways in the Healthy Feline Heart
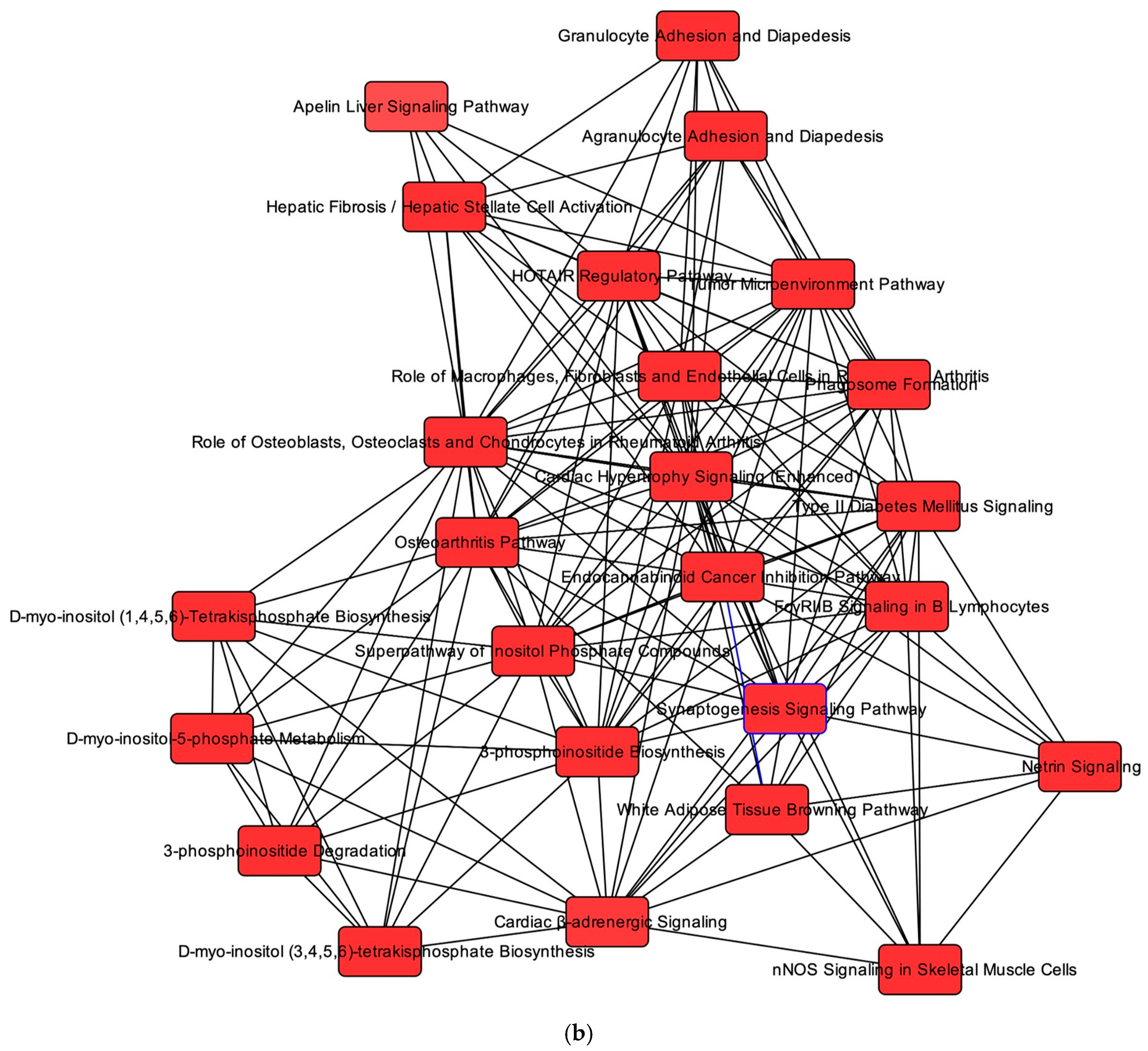
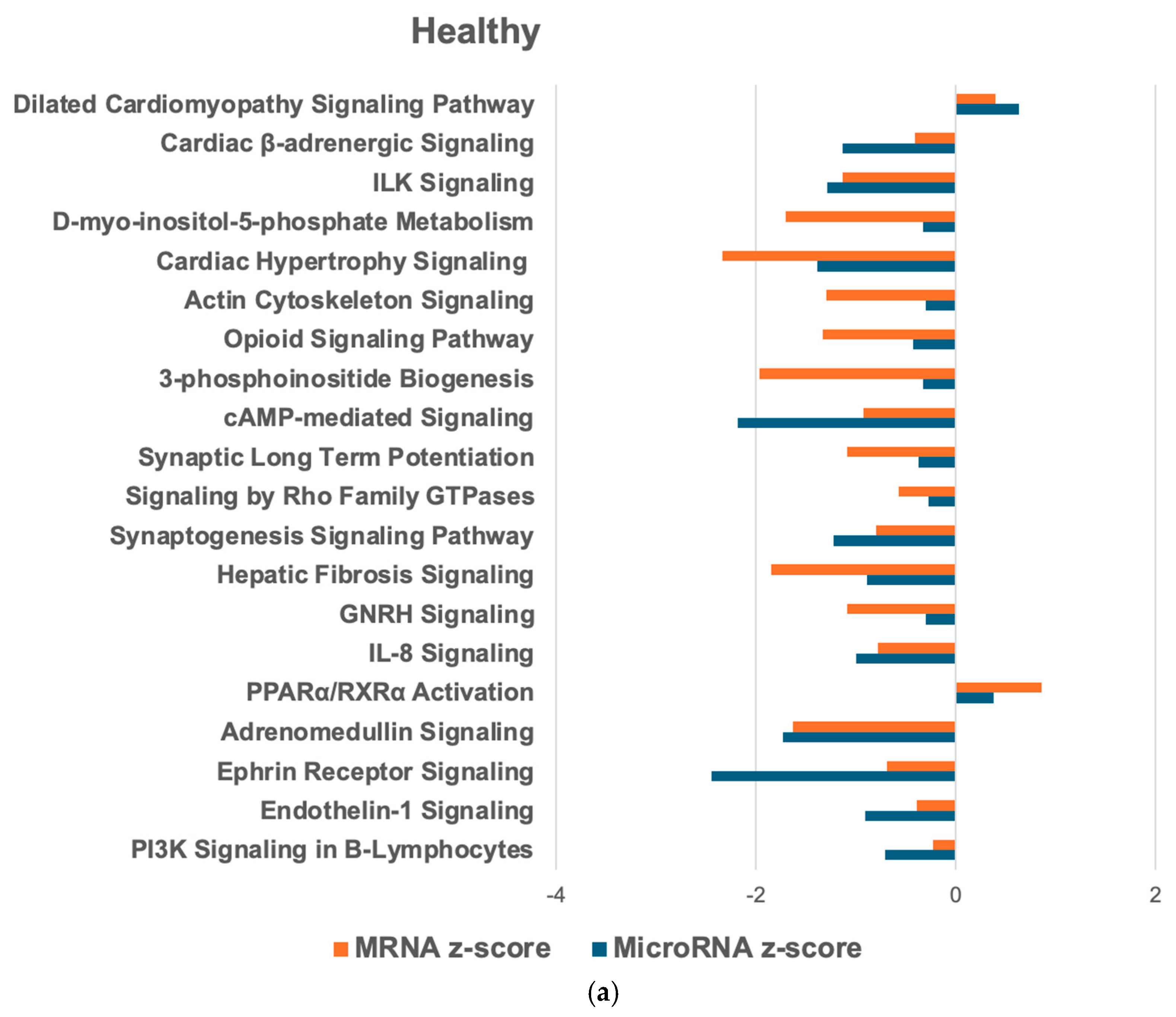
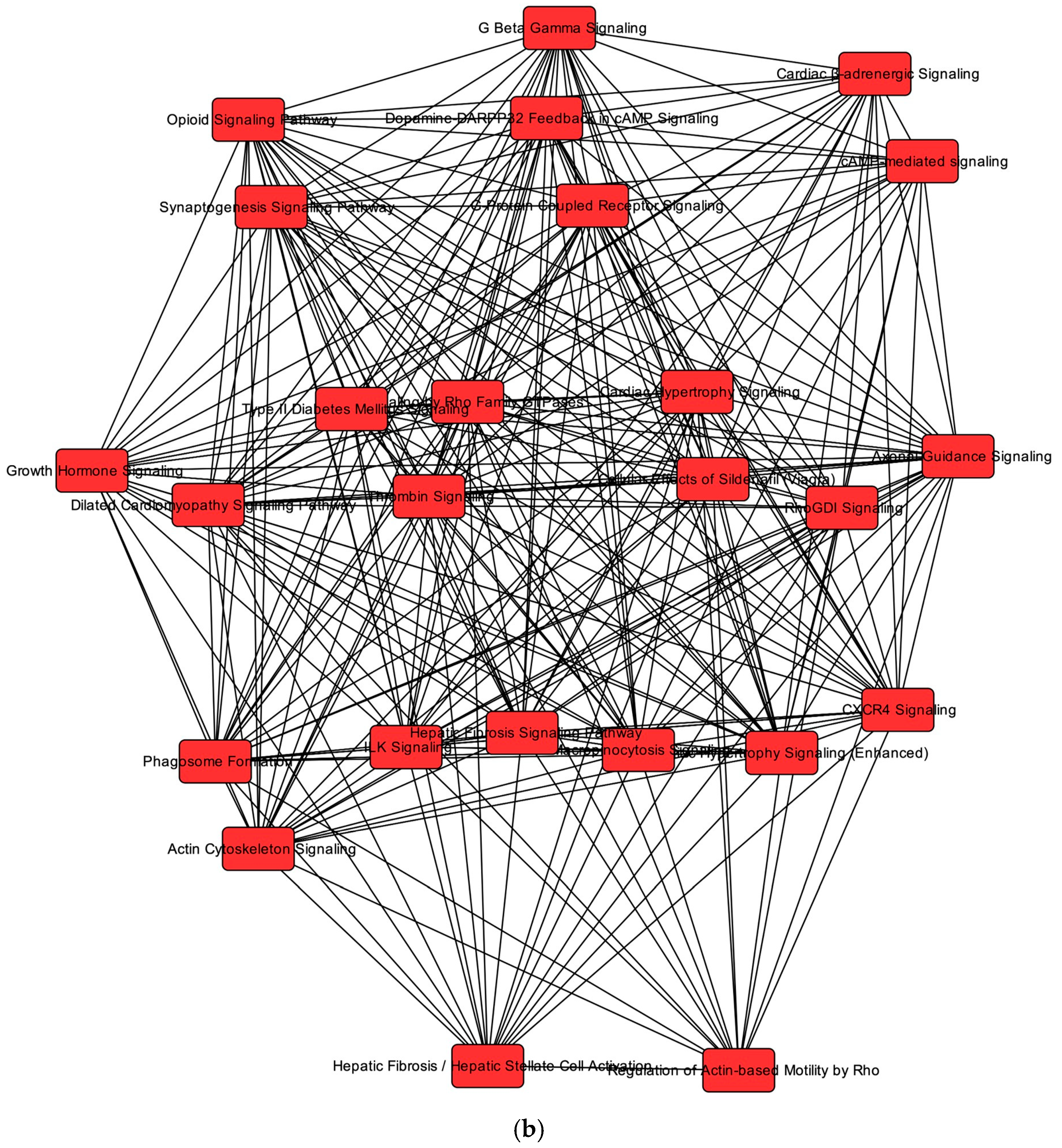
2.6. Overlapping mRNA and microRNA Pathways and Networks in the Healthy Feline Heart
2.7. Regulators of Networks and Target Molecules in the Healthy Feline Heart
3. Discussion
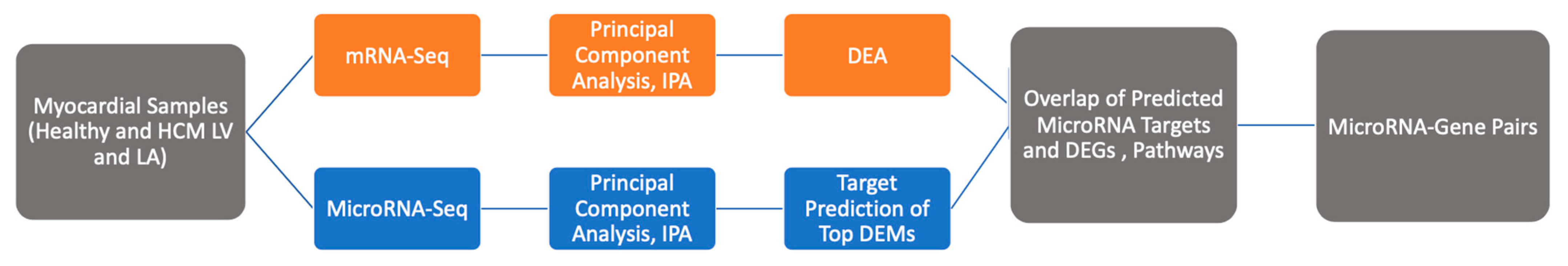
4. Materials and Methods
4.1. Data of Gene Expression and microRNA Profiles
4.2. Differentially Expressed mRNAs and microRNAs
4.3. Pathway and Network Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DEG | Differentially expressed genes |
| DEM | Differentially expressed microRNAs |
| HCM | Hypertrophic cardiomyopathy |
| hLA | Healthy left atrium |
| hLV | Healthy left ventricle |
| LA | Left atrium |
| LV | Left ventricle |
| mReg | Master regulator |
| PW | Pathway |
| upReg | Upstream regulator |
References
- Payne, J.R.; Brodbelt, D.C.; Luis Fuentes, V. Cardiomyopathy Prevalence in 780 Apparently Healthy Cats in Rehoming Centres (the CatScan Study). J. Vet. Cardiol. 2015, 17, S244–S257. [Google Scholar] [CrossRef]
- Novo Matos, J.; Payne, J.R.; Seo, J.; Luis Fuentes, V. Natural History of Hypertrophic Cardiomyopathy in Cats from Rehoming Centers: The CatScan II Study. Vet. Intern. Med. 2022, 36, 1900–1912. [Google Scholar] [CrossRef]
- Fox, P.R.; Schober, K.E. Management of Asymptomatic (Occult) Feline Cardiomyopathy: Challenges and Realities. J. Vet. Cardiol. 2015, 17, S150–S158. [Google Scholar] [CrossRef]
- Freeman, L.M.; Rush, J.E.; Stern, J.A.; Huggins, G.S.; Maron, M.S. Feline Hypertrophic Cardiomyopathy: A Spontaneous Large Animal Model of Human HCM. Cardiol. Res. 2017, 8, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Meurs, K.M.; Williams, B.G.; DeProspero, D.; Friedenberg, S.G.; Malarkey, D.E.; Ezzell, J.A.; Keene, B.W.; Adin, D.B.; DeFrancesco, T.C.; Tou, S. A Deleterious Mutation in the ALMS1 Gene in a Naturally Occurring Model of Hypertrophic Cardiomyopathy in the Sphynx Cat. Orphanet J. Rare Dis. 2021, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Schipper, T.; Van Poucke, M.; Sonck, L.; Smets, P.; Ducatelle, R.; Broeckx, B.J.G.; Peelman, L.J. A Feline Orthologue of the Human MYH7 c.5647G>A (p.(Glu1883Lys)) Variant Causes Hypertrophic Cardiomyopathy in a Domestic Shorthair Cat. Eur. J. Hum. Genet. 2019, 27, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Meurs, K.M.; Sanchez, X.; David, R.M.; Bowles, N.E.; Towbin, J.A.; Reiser, P.J.; Kittleson, J.A.; Munro, M.J.; Dryburgh, K.; MacDonald, K.A.; et al. A Cardiac Myosin Binding Protein C Mutation in the Maine Coon Cat with Familial Hypertrophic Cardiomyopathy. Hum. Mol. Genet. 2005, 14, 3587–3593. [Google Scholar] [CrossRef]
- Joshua, J.; Caswell, J.; O’Sullivan, M.L.; Wood, G.; Fonfara, S. Feline Myocardial Transcriptome in Health and in Hypertrophic Cardiomyopathy—A Translational Animal Model for Human Disease. PLoS ONE 2023, 18, e0283244. [Google Scholar] [CrossRef]
- Joshua, J.; Caswell, J.L.; Monné Rodriguez, J.M.; Kipar, A.; O’Sullivan, M.L.; Wood, G.; Fonfara, S. MicroRNA Profiling of the Feline Left Heart Identifies Chamber-Specific Expression Signatures in Health and in Advanced Hypertrophic Cardiomyopathy. J. Mol. Cell. Cardiol. Plus 2023, 4, 100037. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Raposo, L.R.; Fernandes, A.R. MicroRNAs Based Therapy of Hypertrophic Cardiomyopathy: The Road Traveled So Far. BioMed Res. Int. 2015, 2015, 983290. [Google Scholar] [CrossRef]
- Fernández-Hernando, C.; Ramírez, C.M.; Goedeke, L.; Suárez, Y. MicroRNAs in Metabolic Disease. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A Mammalian microRNA Expression Atlas Based on Small RNA Library Sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef]
- Thum, T.; Galuppo, P.; Wolf, C.; Fiedler, J.; Kneitz, S.; van Laake, L.W.; Doevendans, P.A.; Mummery, C.L.; Borlak, J.; Haverich, A.; et al. MicroRNAs in the Human Heart: A Clue to Fetal Gene Reprogramming in Heart Failure. Circulation 2007, 116, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Romaine, S.P.R.; Tomaszewski, M.; Condorelli, G.; Samani, N.J. MicroRNAs in Cardiovascular Disease: An Introduction for Clinicians. Heart 2015, 101, 921–928. [Google Scholar] [CrossRef]
- Carè, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.-L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 Controls Cardiac Hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef]
- Scolari, F.L.; Faganello, L.S.; Garbin, H.I.; Piva e Mattos, B.; Biolo, A. A Systematic Review of microRNAs in Patients with Hypertrophic Cardiomyopathy. Int. J. Cardiol. 2021, 327, 146–154. [Google Scholar] [CrossRef]
- Kuster, D.W.D.; Mulders, J.; ten Cate, F.J.; Michels, M.; dos Remedios, C.G.; da Costa Martins, P.A.; van der Velden, J.; Oudejans, C.B.M. MicroRNA Transcriptome Profiling in Cardiac Tissue of Hypertrophic Cardiomyopathy Patients with MYBPC3 Mutations. J. Mol. Cell. Cardiol. 2013, 65, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Osmak, G.; Baulina, N.; Kiselev, I.; Favorova, O. MiRNA-Regulated Pathways for Hypertrophic Cardiomyopathy: Network-Based Approach to Insight into Pathogenesis. Genes 2021, 12, 2016. [Google Scholar] [CrossRef]
- Wang, L.; Lu, F.; Xu, J. Identification of Potential miRNA-mRNA Regulatory Network Contributing to Hypertrophic Cardiomyopathy (HCM). Front. Cardiovasc. Med. 2021, 8, 660372. [Google Scholar] [CrossRef]
- Chen, J.; Xie, Z.; Dai, J.; Han, J.; Wang, K.; Lu, L.; Jin, J.; Xue, S. Reconstruction and Analysis of Potential Biomarkers for Hypertrophic Cardiomyopathy Based on a Competing Endogenous RNA Network. BMC Cardiovasc. Disord. 2022, 22, 422. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.; Zheng, D.; Sun, Y.; Wang, S.; Yan, Y. Bioinformatics Analysis of the Regulatory lncRNA-miRNA-mRNA Network and Drug Prediction in Patients with Hypertrophic Cardiomyopathy. Mol. Med. Rep. 2019, 20, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Garmany, R.; Bos, J.M.; Dasari, S.; Tester, D.; dos Remedios, C.; Maleszewski, J.; Ommen, S.; Dearani, J.A.; Giudicessi, J.R.; Ackerman, M.J. PO-02-142 Mirna Down-Regulate Hypertrophy and Inflammatory Pathways in the Transcriptome of Hypertrophic Cardiomyopathy Myectomy Tissue. Heart Rhythm. 2023, 20, S262. [Google Scholar] [CrossRef]
- Chaffin, M.; Papangeli, I.; Simonson, B.; Akkad, A.-D.; Hill, M.C.; Arduini, A.; Fleming, S.J.; Melanson, M.; Hayat, S.; Kost-Alimova, M.; et al. Single-Nucleus Profiling of Human Dilated and Hypertrophic Cardiomyopathy. Nature 2022, 608, 174–180. [Google Scholar] [CrossRef]
- Foinquinos, A.; Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Gyöngyösi, M.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D.; et al. Preclinical Development of a miR-132 Inhibitor for Heart Failure Treatment. Nat. Commun. 2020, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Täubel, J.; Hauke, W.; Rump, S.; Viereck, J.; Batkai, S.; Poetzsch, J.; Rode, L.; Weigt, H.; Genschel, C.; Lorch, U.; et al. Novel Antisense Therapy Targeting microRNA-132 in Patients with Heart Failure: Results of a First-in-Human Phase 1b Randomized, Double-Blind, Placebo-Controlled Study. Eur. Heart J. 2021, 42, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Faul, C. Cardiac Actions of Fibroblast Growth Factor 23. Bone 2017, 100, 69–79. [Google Scholar] [CrossRef]
- Leifheit-Nestler, M.; Haffner, D. Paracrine Effects of FGF23 on the Heart. Front. Endocrinol. 2018, 9, 278. [Google Scholar] [CrossRef]
- Gurvich, O.L.; Puttonen, K.A.; Bailey, A.; Kailaanmäki, A.; Skirdenko, V.; Sivonen, M.; Pietikäinen, S.; Parker, N.R.; Ylä-Herttuala, S.; Kekarainen, T. Transcriptomics uncovers substantial variability associated with alterations in manufacturing processes of macrophage cell therapy products. Sci. Rep. 2020, 10, 14049. [Google Scholar] [CrossRef]
- Wei, Y.; Lan, Y.; Zhong, Y.; Yu, K.; Xu, W.; Zhu, R.; Sun, H.; Ding, Y.; Wang, Y.; Zeng, Q. Interleukin-38 Alleviates Cardiac Remodelling after Myocardial Infarction. J. Cell. Mol. Medi 2020, 24, 371–384. [Google Scholar] [CrossRef]
- Mora, J.; Schlemmer, A.; Wittig, I.; Richter, F.; Putyrski, M.; Frank, A.-C.; Han, Y.; Jung, M.; Ernst, A.; Weigert, A.; et al. Interleukin-38 Is Released from Apoptotic Cells to Limit Inflammatory Macrophage Responses. J. Mol. Cell Biol. 2016, 8, 426–438. [Google Scholar] [CrossRef]
- Martínez-González, J.; Cañes, L.; Alonso, J.; Ballester-Servera, C.; Rodríguez-Sinovas, A.; Corrales, I.; Rodríguez, C. NR4A3: A Key Nuclear Receptor in Vascular Biology, Cardiovascular Remodeling, and Beyond. Int. J. Mol. Sci. 2021, 22, 11371. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.V.; Ricardo, S.D. Macrophages and CSF-1: Implications for Development and Beyond. Organogenesis 2013, 9, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Leblond, A.-L.; Klinkert, K.; Martin, K.; Turner, E.C.; Kumar, A.H.; Browne, T.; Caplice, N.M. Systemic and Cardiac Depletion of M2 Macrophage through CSF-1R Signaling Inhibition Alters Cardiac Function Post Myocardial Infarction. PLoS ONE 2015, 10, e0137515. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.M.M.; Fonfara, S.; Hetzel, U.; Kipar, A. Feline Hypertrophic Cardiomyopathy: Reduced Microvascular Density and Involvement of CD34+ Interstitial Cells. Vet. Pathol. 2022, 59, 269–283. [Google Scholar] [CrossRef]
- O’Rourke, S.A.; Dunne, A.; Monaghan, M.G. The Role of Macrophages in the Infarcted Myocardium: Orchestrators of ECM Remodeling. Front. Cardiovasc. Med. 2019, 6, 101. [Google Scholar] [CrossRef]
- Mina-Osorio, P.; Winnicka, B.; O’Conor, C.; Grant, C.L.; Vogel, L.K.; Rodriguez-Pinto, D.; Holmes, K.V.; Ortega, E.; Shapiro, L.H. CD13 Is a Novel Mediator of Monocytic/Endothelial Cell Adhesion. J. Leukoc. Biol. 2008, 84, 448–459. [Google Scholar] [CrossRef]
- Kared, H.; Martelli, S.; Ng, T.P.; Pender, S.L.F.; Larbi, A. CD57 in Human Natural Killer Cells and T-Lymphocytes. Cancer Immunol. Immunother. 2016, 65, 441–452. [Google Scholar] [CrossRef]
- Rosenberg, S.C.; Corbett, K.D. The Multifaceted Roles of the HORMA Domain in Cellular Signaling. J. Cell Biol. 2015, 211, 745–755. [Google Scholar] [CrossRef]
- Montazeri, H.; Coto-Llerena, M.; Bianco, G.; Zangene, E.; Taha-Mehlitz, S.; Paradiso, V.; Srivatsa, S.; de Weck, A.; Roma, G.; Lanzafame, M.; et al. Systematic Identification of Novel Cancer Genes through Analysis of Deep shRNA Perturbation Screens. Nucleic Acids Res. 2021, 49, 8488–8504. [Google Scholar] [CrossRef]
- Chapman, N.A.; Dupré, D.J.; Rainey, J.K. The Apelin Receptor: Physiology, Pathology, Cell Signalling, and Ligand Modulation of a Peptide-Activated Class A GPCR. Biochem. Cell Biol. 2014, 92, 431–440. [Google Scholar] [CrossRef]
- Wysocka, M.B.; Pietraszek-Gremplewicz, K.; Nowak, D. The Role of Apelin in Cardiovascular Diseases, Obesity and Cancer. Front. Physiol. 2018, 9, 557. [Google Scholar] [CrossRef]
- Shibata, R.; Ouchi, N.; Ohashi, K.; Murohara, T. The Role of Adipokines in Cardiovascular Disease. J. Cardiol. 2017, 70, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.Z.; Ivashchenko, C.Y.; Usher, M.G.; Mortensen, R.M. PPAR- γ in the Cardiovascular System. PPAR Res. 2008, 2008, 745804. [Google Scholar] [CrossRef]
- Boularan, C.; Gales, C. Cardiac cAMP: Production, Hydrolysis, Modulation and Detection. Front. Pharmacol. 2015, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Adzika, G.K.; Hou, H.; Adekunle, A.O.; Rizvi, R.; Adzraku, S.Y.; Li, K.; Deng, Q.-M.; Mprah, R.; Ndzie Noah, M.L.; Adu-Amankwaah, J.; et al. Amlexanox and Forskolin Prevents Isoproterenol-Induced Cardiomyopathy by Subduing Cardiomyocyte Hypertrophy and Maladaptive Inflammatory Responses. Front. Cell Dev. Biol. 2021, 9, 719351. [Google Scholar] [CrossRef] [PubMed]
- Colombe, A.-S.; Pidoux, G. Cardiac cAMP-PKA Signaling Compartmentalization in Myocardial Infarction. Cells 2021, 10, 922. [Google Scholar] [CrossRef]
- Kitz, S.; Fonfara, S.; Hahn, S.; Hetzel, U.; Kipar, A. Feline Hypertrophic Cardiomyopathy: The Consequence of Cardiomyocyte-Initiated and Macrophage-Driven Remodeling Processes? Vet. Pathol. 2019, 56, 565–575. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, Q. Understanding the Oxygen-Sensing Pathway and Its Therapeutic Implications in Diseases. Am. J. Pathol. 2020, 190, 1584–1595. [Google Scholar] [CrossRef]
- Sassi, Y.; Avramopoulos, P.; Ramanujam, D.; Grüter, L.; Werfel, S.; Giosele, S.; Brunner, A.-D.; Esfandyari, D.; Papadopoulou, A.S.; De Strooper, B.; et al. Cardiac Myocyte miR-29 Promotes Pathological Remodeling of the Heart by Activating Wnt Signaling. Nat. Commun. 2017, 8, 1614. [Google Scholar] [CrossRef]
- Fu, W.; Wang, W.E.; Zeng, C. Wnt Signaling Pathways in Myocardial Infarction and the Therapeutic Effects of Wnt Pathway Inhibitors. Acta Pharmacol. Sin. 2019, 40, 9–12. [Google Scholar] [CrossRef]
- Jiang, X.; Guan, Y.; Zhao, Z.; Meng, F.; Wang, X.; Gao, X.; Liu, J.; Chen, Y.; Zhou, F.; Zhou, S.; et al. Potential Roles of the WNT Signaling Pathway in Amyotrophic Lateral Sclerosis. Cells 2021, 10, 839. [Google Scholar] [CrossRef]
- Widiapradja, A.; Chunduri, P.; Levick, S.P. The Role of Neuropeptides in Adverse Myocardial Remodeling and Heart Failure. Cell. Mol. Life Sci. 2017, 74, 2019–2038. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, N.; Nasser, S.; Pintus, G.; Badran, A.; Eid, A.; Baydoun, E. MicroRNAs in Cardiac Hypertrophy. Int. J. Mol. Sci. 2019, 20, 4714. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kho, C. MicroRNAs and Calcium Signaling in Heart Disease. Int. J. Mol. Sci. 2021, 22, 10582. [Google Scholar] [CrossRef] [PubMed]
- Griesenauer, B.; Paczesny, S. The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases. Front. Immunol. 2017, 8, 475. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Kim, Y.; Jacobs, J.S.; Yoon, J.; Li, Q.; Cai, A.; Shankaiahgari, H.; London, B.; Irani, K.; Vikram, A. The microRNA-204-5p Inhibits APJ Signalling and Confers Resistance to Cardiac Hypertrophy and Dysfunction. Clin. Transl. Med. 2022, 12, e693. [Google Scholar] [CrossRef]
- Song, G.; Zhu, L.; Ruan, Z.; Wang, R.; Shen, Y. MicroRNA-122 Promotes Cardiomyocyte Hypertrophy via Targeting FoxO3. Biochem. Biophys. Res. Commun. 2019, 519, 682–688. [Google Scholar] [CrossRef]
- Liu, Y.; Song, J.-W.; Lin, J.-Y.; Miao, R.; Zhong, J.-C. Roles of MicroRNA-122 in Cardiovascular Fibrosis and Related Diseases. Cardiovasc. Toxicol. 2020, 20, 463–473. [Google Scholar] [CrossRef]
- Oh, J.E.; Jung, C.; Yoon, Y. Human Induced Pluripotent Stem Cell-Derived Vascular Cells: Recent Progress and Future Directions. J. Cardiovasc. Dev. Dis. 2021, 8, 148. [Google Scholar] [CrossRef]
- Nistri, S.; Sassoli, C.; Bani, D. Notch Signaling in Ischemic Damage and Fibrosis: Evidence and Clues from the Heart. Front. Pharmacol. 2017, 8, 187. [Google Scholar] [CrossRef]
- Kilian, L.S.; Voran, J.; Frank, D.; Rangrez, A.Y. RhoA: A Dubious Molecule in Cardiac Pathophysiology. J. Biomed. Sci. 2021, 28, 33. [Google Scholar] [CrossRef]
- Ahn, J.; Wu, H.; Lee, K. Integrative Analysis Revealing Human Heart-Specific Genes and Consolidating Heart-Related Phenotypes. Front. Genet. 2020, 11, 777. [Google Scholar] [CrossRef] [PubMed]
- van Almen, G.C.; Verhesen, W.; van Leeuwen, R.E.W.; van de Vrie, M.; Eurlings, C.; Schellings, M.W.M.; Swinnen, M.; Cleutjens, J.P.M.; van Zandvoort, M.A.M.J.; Heymans, S.; et al. MicroRNA-18 and microRNA-19 Regulate CTGF and TSP-1 Expression in Age-Related Heart Failure: MicroRNAs in Cardiac Aging. Aging Cell 2011, 10, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Feng, D.; Xu, J. Cardioprotective Effects of microRNA-18a on Acute Myocardial Infarction by Promoting Cardiomyocyte Autophagy and Suppressing Cellular Senescence via Brain Derived Neurotrophic Factor. Cell Biosci. 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.L.; Wee, A.S.Y.; Lim, J.Y.; Ng, J.Y.X.; Chong, J.P.C.; Liew, O.W.; Lilyanna, S.; Martinez, E.C.; Ackers-Johnson, M.A.; Vardy, L.A.; et al. Natriuretic Peptide Receptor 3 (NPR3) Is Regulated by microRNA-100. J. Mol. Cell. Cardiol. 2015, 82, 13–21. [Google Scholar] [CrossRef]
- Sundararajan, V.; Burk, U.C.; Bajdak-Rusinek, K. Revisiting the miR-200 Family: A Clan of Five Siblings with Essential Roles in Development and Disease. Biomolecules 2022, 12, 781. [Google Scholar] [CrossRef]
- Dehlin, H.M.; Levick, S.P. Substance P in Heart Failure: The Good and the Bad. Int. J. Cardiol. 2014, 170, 270–277. [Google Scholar] [CrossRef]
- Zybura-Broda, K.; Wolder-Gontarek, M.; Ambrozek-Latecka, M.; Choros, A.; Bogusz, A.; Wilemska-Dziaduszycka, J.; Rylski, M. HuR (Elavl1) and HuB (Elavl2) Stabilize Matrix Metalloproteinase-9 mRNA During Seizure-Induced Mmp-9 Expression in Neurons. Front. Neurosci. 2018, 12, 224. [Google Scholar] [CrossRef]
- Kato, Y.; Iwamori, T.; Ninomiya, Y.; Kohda, T.; Miyashita, J.; Sato, M.; Saga, Y. ELAVL2-directed RNA Regulatory Network Drives the Formation of Quiescent Primordial Follicles. EMBO Rep. 2019, 20, e48251. [Google Scholar] [CrossRef]
- van den Berg, J.H.; Gomez-Eerland, R.; van de Wiel, B.; Hulshoff, L.; van den Broek, D.; Bins, A.; Tan, H.L.; Harper, J.V.; Hassan, N.J.; Jakobsen, B.K.; et al. Case Report of a Fatal Serious Adverse Event Upon Administration of T Cells Transduced With a MART-1-Specific T-Cell Receptor. Mol. Ther. 2015, 23, 1541–1550. [Google Scholar] [CrossRef]
- Abplanalp, W.T.; Merten, M.; Dimmeler, S. Straight to the Heart: T Cells That Specifically Target Cardiac Tissue. Circulation 2022, 146, 1946–1949. [Google Scholar] [CrossRef] [PubMed]


| Groups | Differentially Expressed Genes | Differentially Expressed microRNAs |
|---|---|---|
| HCM LV vs. Healthy LV | 412 (290 UP, 122 DOWN) | 80 (66 UP, 14 DOWN) |
| HCM LA vs. Healthy LA | 1707 (724 UP, 983 DOWN) | 37 (22 UP, 15 DOWN) |
| HCM LV vs. HCM LA | 1513 (545 UP, 968 DOWN) | 77 (52 UP, 25 DOWN) |
| Healthy LV vs. Healthy LA | 1423 (507 UP, 916 DOWN) | 37 (9 UP, 28 DOWN) |
| HCM Overlap (HCM vs. Healthy) | 215 (162 UP, 53 DOWN) | 15 (11 UP, 4 DOWN) |
| Regional Overlap (LV vs. LA) | 714 (219 UP, 495 DOWN) | 18 (6 UP, 12 DOWN) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshua, J.; Caswell, J.L.; Kipar, A.; O’Sullivan, M.L.; Wood, G.; Fonfara, S. Integrated MicroRNA–mRNA Sequencing Analysis Identifies Regulators and Networks Involved in Feline Hypertrophic Cardiomyopathy. Int. J. Mol. Sci. 2025, 26, 6764. https://doi.org/10.3390/ijms26146764
Joshua J, Caswell JL, Kipar A, O’Sullivan ML, Wood G, Fonfara S. Integrated MicroRNA–mRNA Sequencing Analysis Identifies Regulators and Networks Involved in Feline Hypertrophic Cardiomyopathy. International Journal of Molecular Sciences. 2025; 26(14):6764. https://doi.org/10.3390/ijms26146764
Chicago/Turabian StyleJoshua, Jessica, Jeff L. Caswell, Anja Kipar, M. Lynne O’Sullivan, Geoffrey Wood, and Sonja Fonfara. 2025. "Integrated MicroRNA–mRNA Sequencing Analysis Identifies Regulators and Networks Involved in Feline Hypertrophic Cardiomyopathy" International Journal of Molecular Sciences 26, no. 14: 6764. https://doi.org/10.3390/ijms26146764
APA StyleJoshua, J., Caswell, J. L., Kipar, A., O’Sullivan, M. L., Wood, G., & Fonfara, S. (2025). Integrated MicroRNA–mRNA Sequencing Analysis Identifies Regulators and Networks Involved in Feline Hypertrophic Cardiomyopathy. International Journal of Molecular Sciences, 26(14), 6764. https://doi.org/10.3390/ijms26146764






