Thermal Cycling-Hyperthermia Attenuates Rotenone-Induced Cell Injury in SH-SY5Y Cells Through Heat-Activated Mechanisms
Abstract
1. Introduction
2. Results
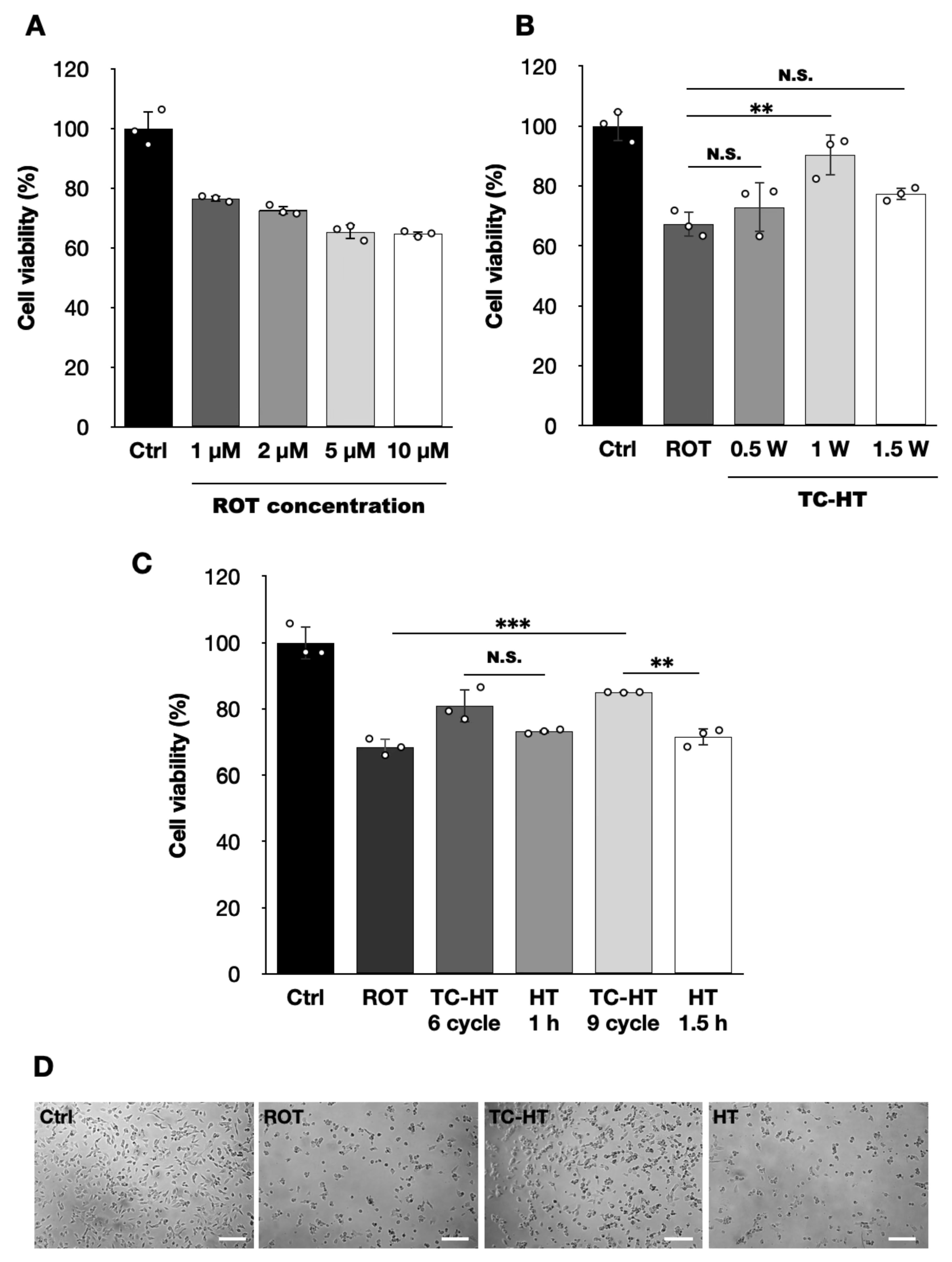
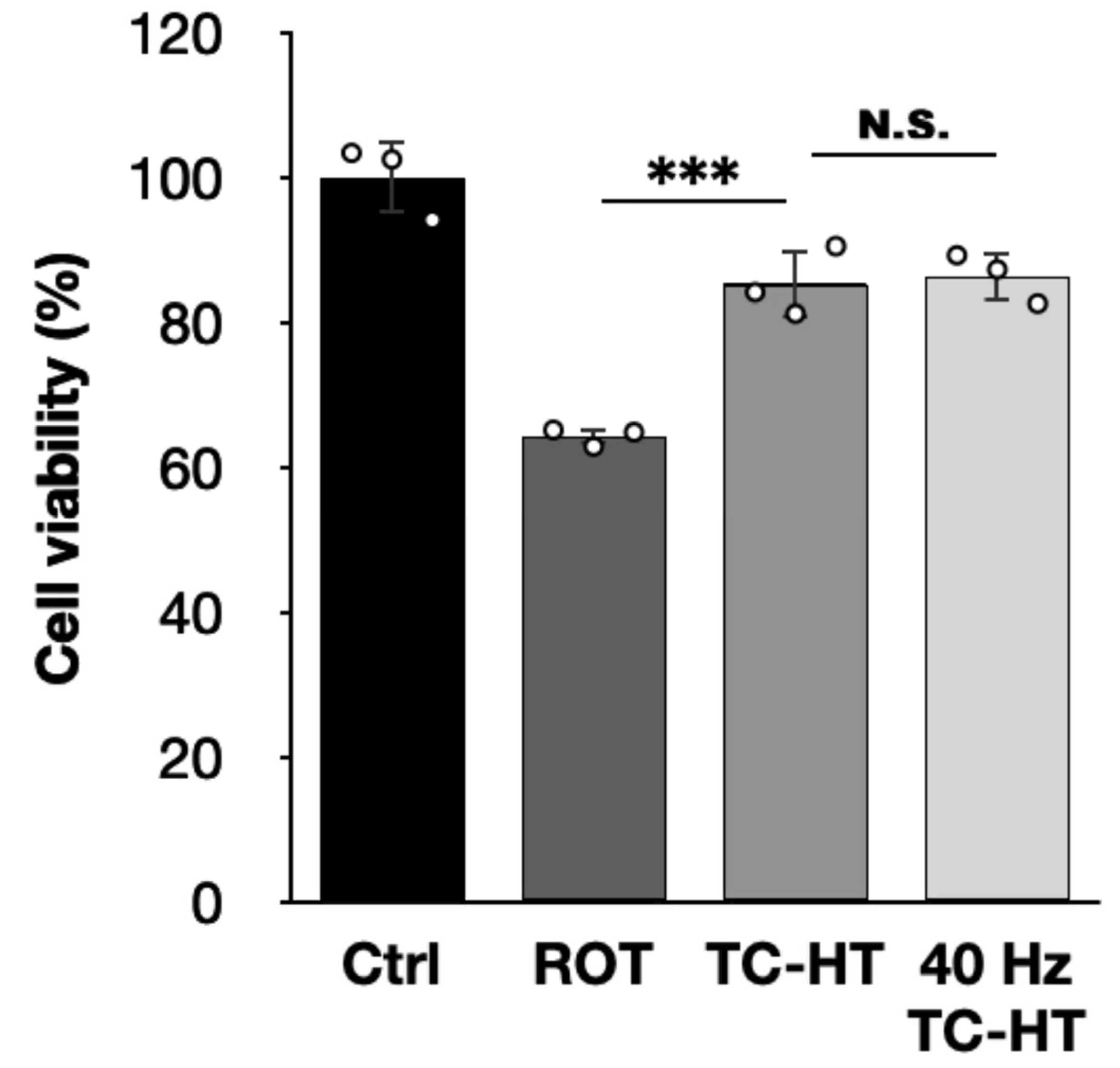
2.1. TC-HT Restores the ROT-Impaired Viability of SH-SY5Y Cells
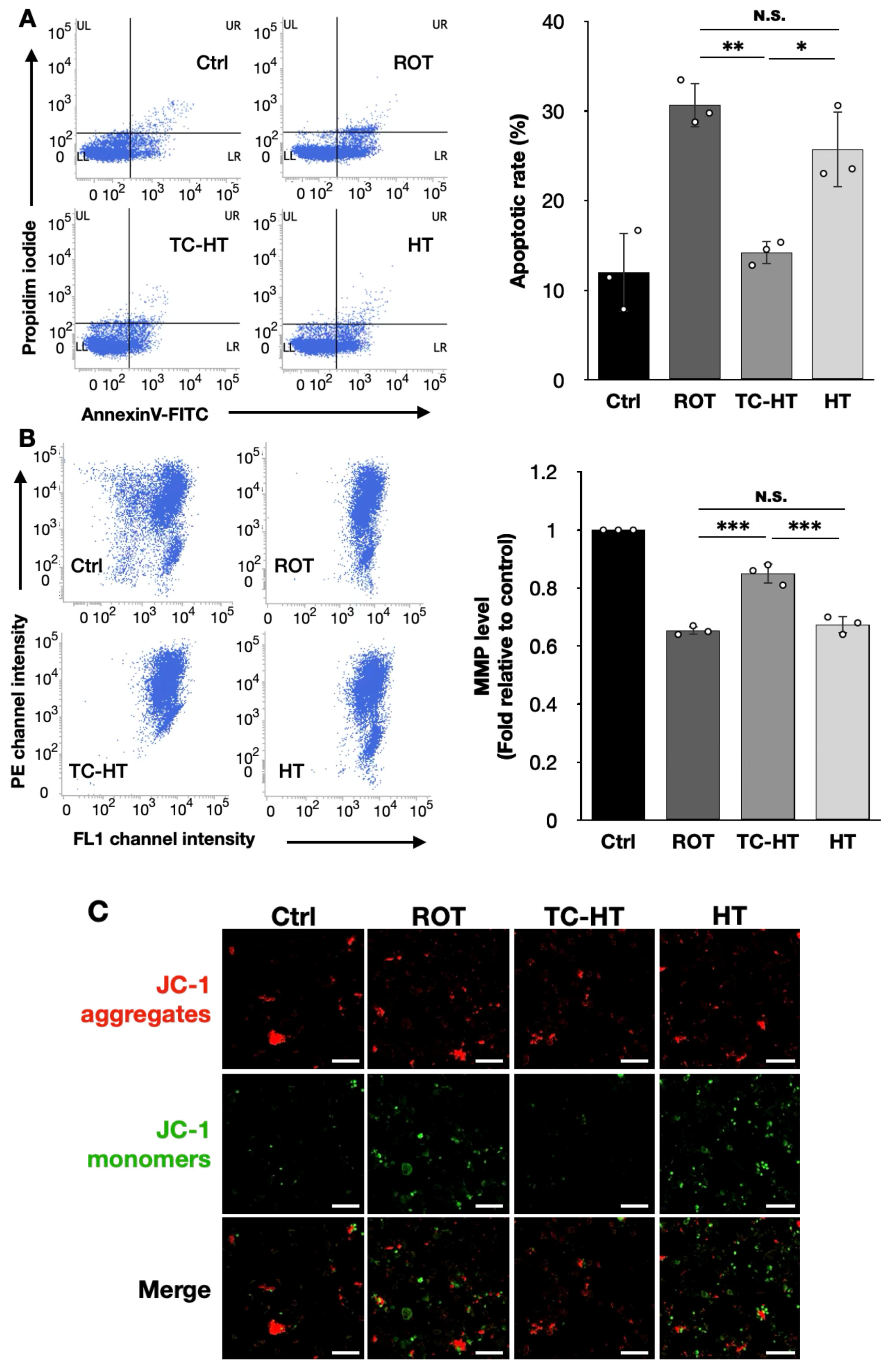
2.2. TC-HT Attenuates ROT-Induced Mitochondrial Apoptosis
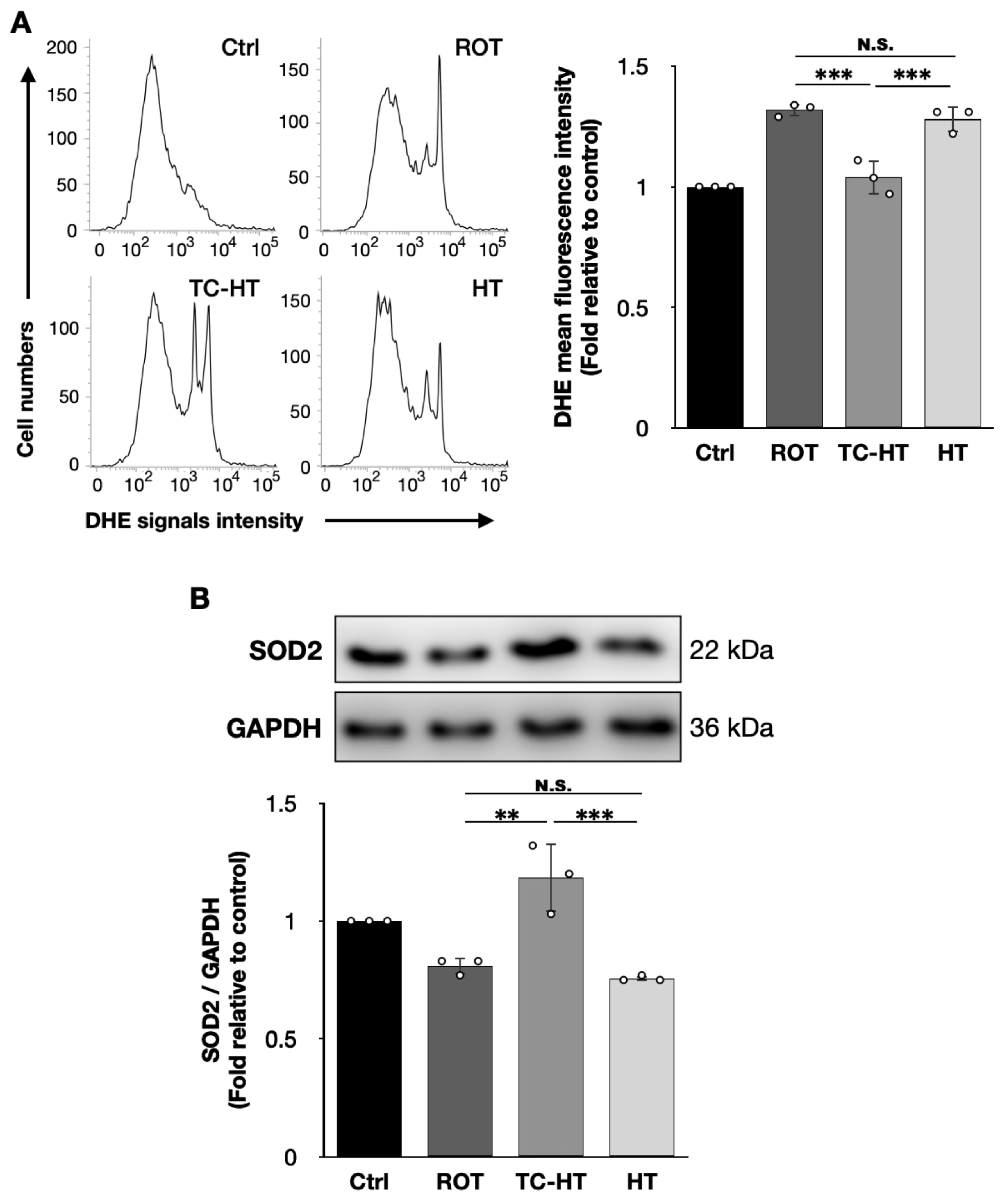
2.3. TC-HT Reduces ROT-Induced Intracellular ROS and Promotes Antioxidative Protein Expression
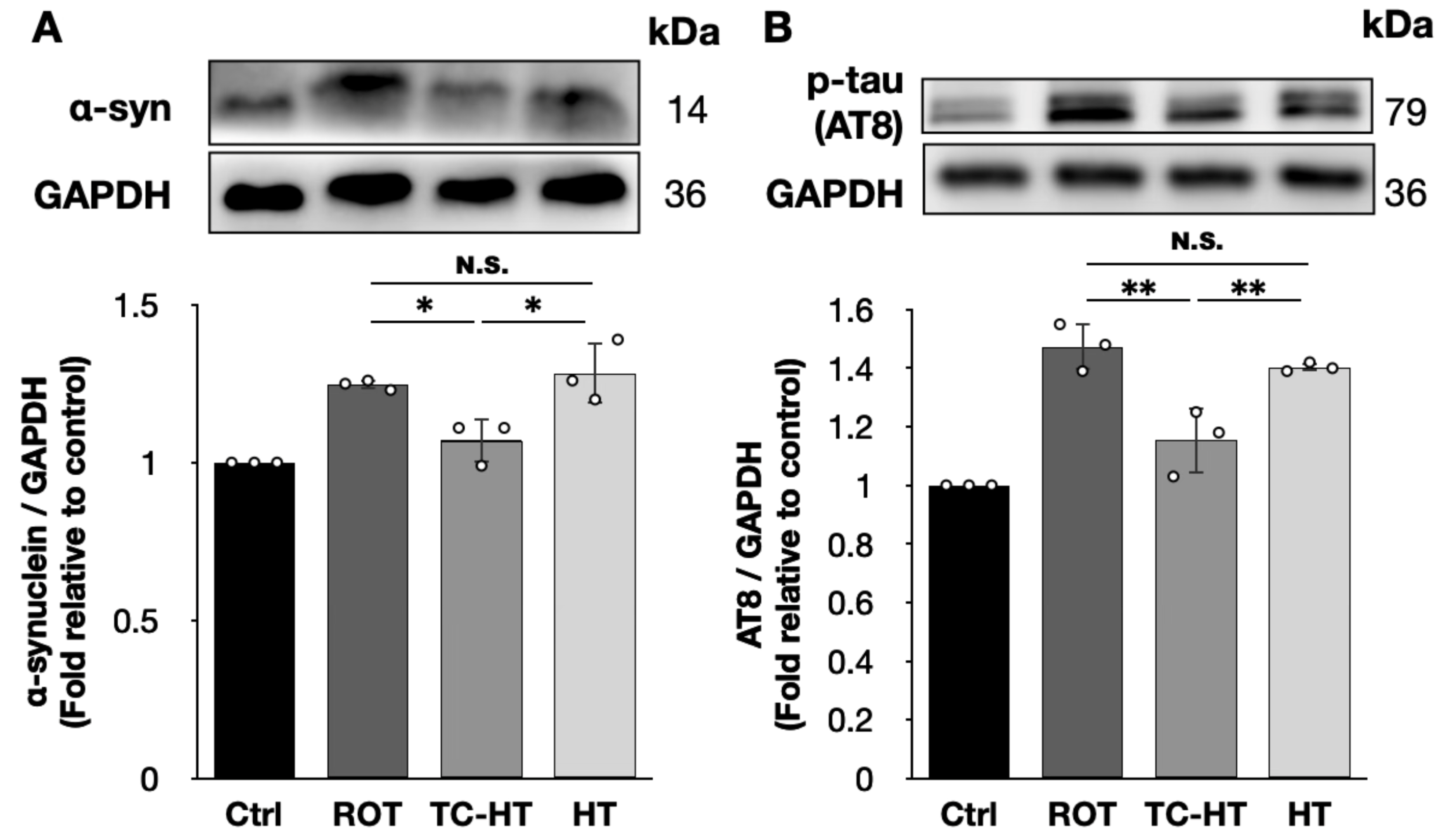
2.4. TC-HT Down-Regulates the Expression of ROT-Induced PD Marker Proteins
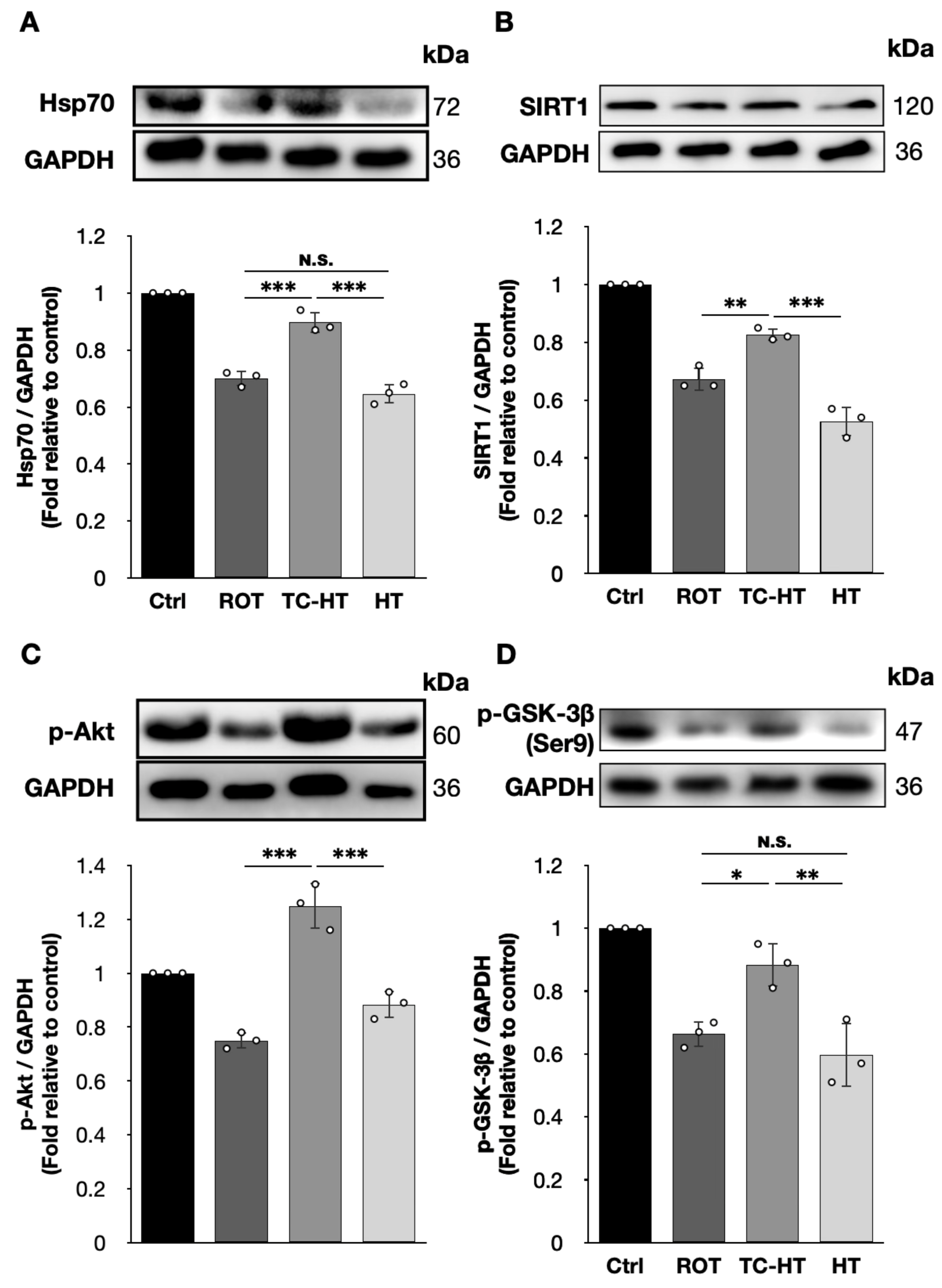
2.5. TC-HT Attenuates ROT-Induced PD In Vitro via Heat-Activated Mechanisms
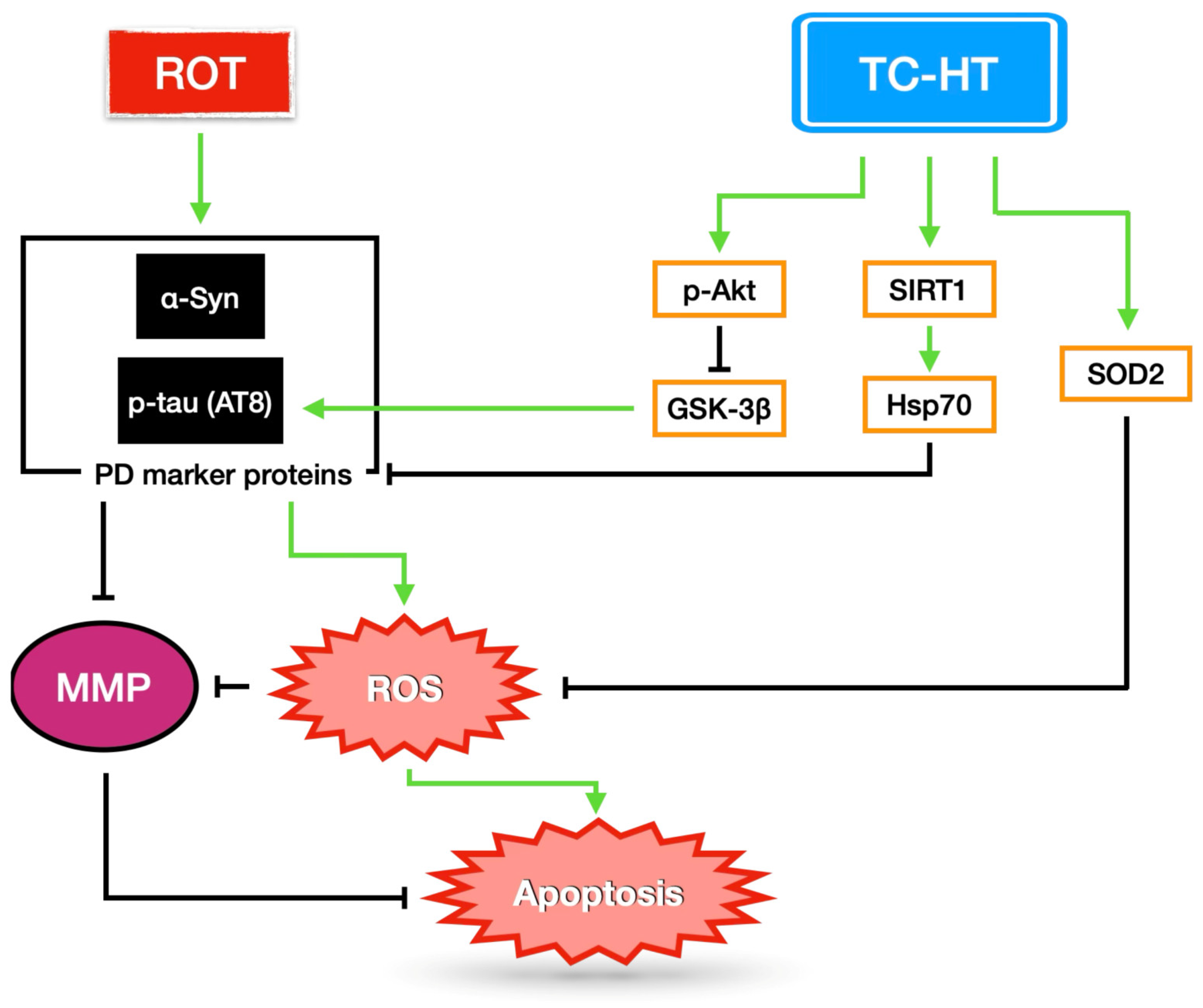
3. Discussion
4. Materials and Methods
4.1. Cell Culture
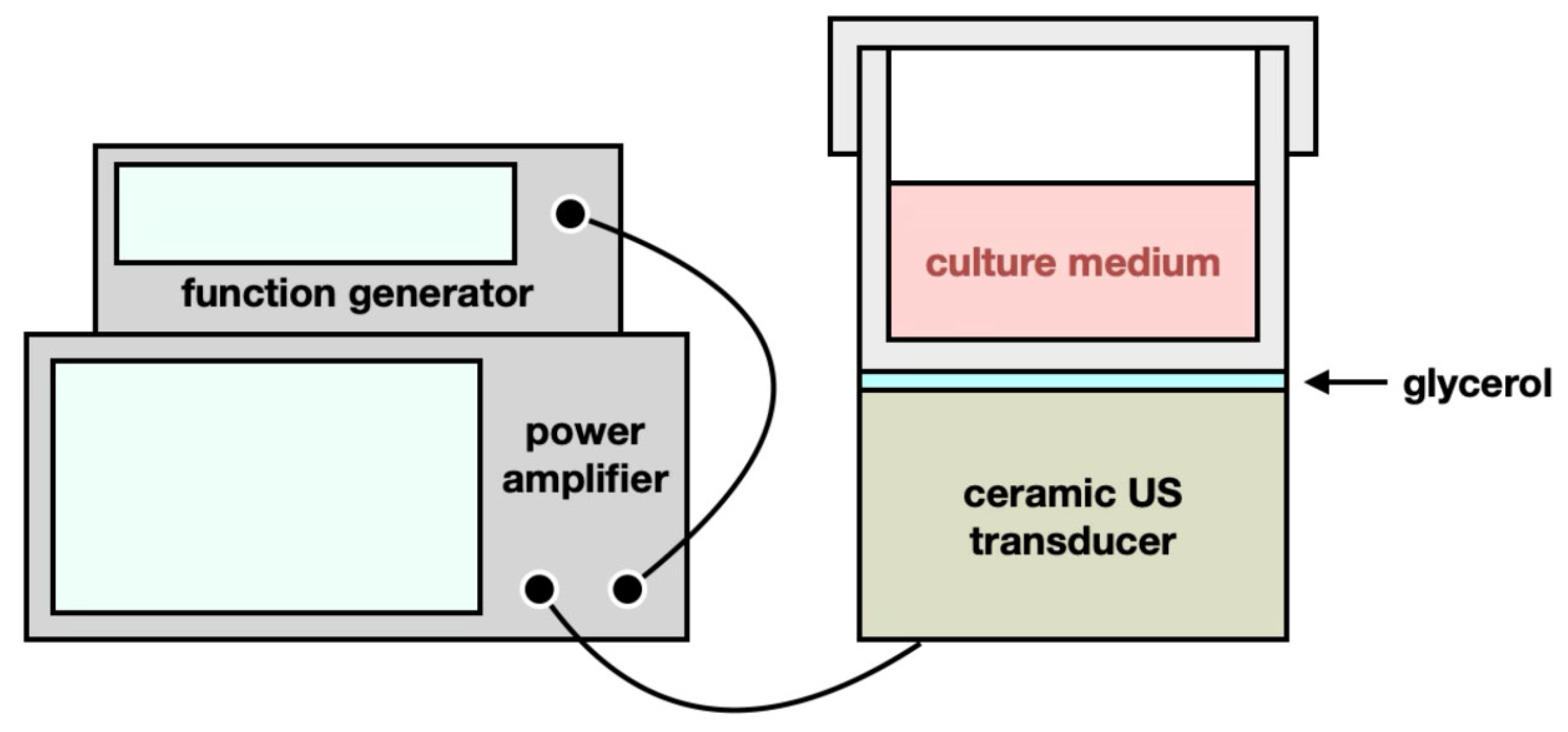
4.2. Ultrasound (US) Exposure
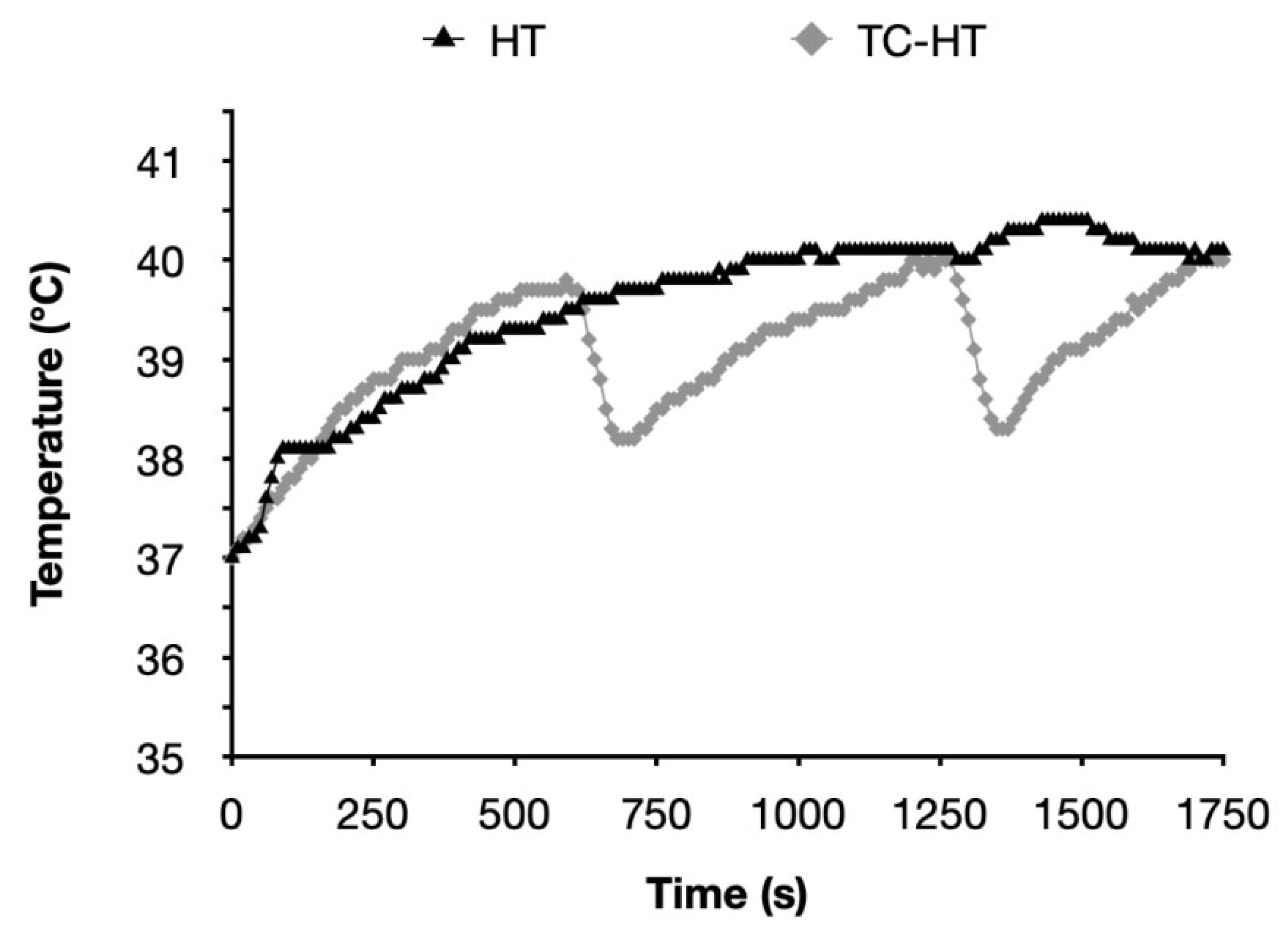
4.3. TC-HT Treatment
4.4. Rotenone Treatment
4.5. Cell Viability Assessment
4.6. Morphological Observation of SH-SY5Y Cells Using Light Microscopy
4.7. Apoptotic Analysis via Flow Cytometry
4.8. Detection of ROS Levels via Flow Cytometry
4.9. MMP Analysis via Flow Cytometry
4.10. JC-1 Fluorescence Detection by Laser Scanning Confocal Microscopy
4.11. Western Blot Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| NDD | neurodegenerative disease |
| ROT | rotenone |
| ROS | reactive oxygen species |
| α-syn | α-synuclein |
| p-tau | phosphorylated-tau protein |
| HT | hyperthermia |
| Hsp70 | heat-shock protein 70 |
| p-Akt | phosphorylated Akt |
| GSK-3β | glycogen synthase kinase-3β |
| FUS | focused ultrasound |
| US | ultrasound |
| TC-HT | thermal cycling-hyperthermia |
| SIRT1 | sirtuin-1 |
| Nrf2 | nuclear factor E2-related factor 2 |
| ARE | antioxidant response element |
| CCK-8 | cell counting kit-8 |
| O2•− | superoxide anion |
| DHE | dihydroethidium |
| MMP | mitochondrial membrane potential |
| JC-1 | 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine chloride |
| PI | propidium iodide |
| SOD2 | superoxide dismutase |
| p-GSK-3β (Ser9) | phosphorylated GSK-3β (serine 9) |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| ANOVA | analysis of variance |
References
- Prajjwal, P.; Flores Sanga, H.S.; Acharya, K.; Tango, T.; John, J.; Rodriguez, R.S.C.; Dheyaa Marsool Marsool, M.; Sulaimanov, M.; Ahmed, A.; Hussin, O.A. Parkinson’s disease updates: Addressing the pathophysiology, risk factors, genetics, diagnosis, along with the medical and surgical treatment. Ann. Med. Surg. 2023, 85, 4887–4902. [Google Scholar] [CrossRef]
- Soilemezi, D.; Palmar-Santos, A.; Navarta-Sánchez, M.V.; Roberts, H.C.; Pedraz-Marcos, A.; Haahr, A.; Sørensen, D.; Bragstad, L.K.; Hjelle, E.G.; Haavaag, S.B.; et al. Understanding support systems for Parkinson’s disease management in community settings: A cross-national qualitative study. Health Expect. 2023, 26, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.; Pari, G.; Rossiter, J.P. Approach to diagnosis of Parkinson disease. Can. Fam. Physician 2006, 52, 862–868. [Google Scholar] [PubMed]
- Han, X.; Han, B.; Zhao, Y.; Li, G.; Wang, T.; He, J.; Du, W.; Cao, X.; Gan, J.; Wang, Z.; et al. Rosmarinic acid attenuates rotenone-induced neurotoxicity in SH-SY5Y Parkinson’s disease cell model through Abl inhibition. Nutrients 2022, 14, 3508. [Google Scholar] [CrossRef]
- Kamal, R.E.; Menze, E.; Albohy, A.; Ahmed, H.I.; Azab, S.S. Neuroprotective repositioning and anti-tau effect of carvedilol on rotenone induced neurotoxicity in rats: Insights from an insilico & in vivo anti-Parkinson’s disease study. Eur. J. Pharmacol. 2022, 932, 175204. [Google Scholar] [CrossRef]
- Lee, J.; Huang, M.S.; Yang, I.C.; Lai, T.C.; Wang, J.L.; Pang, V.F.; Hsiao, M.; Kuo, M.Y. Essential roles of caspases and their upstream regulators in rotenone-induced apoptosis. Biochem. Biophys. Res. Commun. 2008, 371, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Watabe, M.; Nakaki, T. Rotenone induces apoptosis via activation of bad in human dopaminergic SH-SY5Y cells. J. Pharmacol. Exp. Ther. 2004, 311, 948–953. [Google Scholar] [CrossRef]
- Li, N.; Ragheb, K.; Lawler, G.; Sturgis, J.; Rajwa, B.; Melendez, J.A.; Robinson, J.P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003, 278, 8516–8525. [Google Scholar] [CrossRef]
- Kempster, P.; Ma, A. Parkinson’s disease, dopaminergic drugs and the plant world. Front. Pharmacol. 2022, 13, 970714. [Google Scholar] [CrossRef]
- Zahoor, I.; Shafi, A.; Haq, E. Pharmacological treatment of Parkinson’s disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects [Internet]; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018; pp. 129–144. ISBN 978-0-9944381-6-4. [Google Scholar]
- Cheng, G.; Liu, Y.; Ma, R.; Cheng, G.; Guan, Y.; Chen, X.; Wu, Z.; Chen, T. Anti-parkinsonian therapy: Strategies for crossing the blood-brain barrier and nano-biological effects of nanomaterials. Nanomicro. Lett. 2022, 14, 105. [Google Scholar] [CrossRef]
- Srinivasan, E.; Chandrasekhar, G.; Chandrasekar, P.; Anbarasu, K.; Vickram, A.S.; Karunakaran, R.; Rajasekaran, R.; Srikumar, P.S. Alpha-synuclein aggregation in Parkinson’s disease. Front. Med. 2021, 8, 736978. [Google Scholar] [CrossRef]
- Pan, L.; Meng, L.; He, M.; Zhang, Z. Tau in the pathophysiology of Parkinson’s disease. J. Mol. Neurosci. 2021, 71, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
- Habash, R.W.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal therapy, part 1: An introduction to thermal therapy. Crit. Rev. Biomed. Eng. 2006, 34, 459–489. [Google Scholar] [CrossRef]
- Banerjee Mustafi, S.; Chakraborty, P.K.; Dey, R.S.; Raha, S. Heat stress upregulates chaperone heat shock protein 70 and antioxidant manganese superoxide dismutase through reactive oxygen species (ROS), p38MAPK, and Akt. Cell Stress Chaperones 2009, 14, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Tundo, G.R.; Sbardella, D.; Ciaccio, C.; Bianculli, A.; Orlandi, A.; Desimio, M.G.; Arcuri, G.; Coletta, M.; Marini, S. Insulin-degrading enzyme (IDE): A novel heat shock-like protein. J. Biol. Chem. 2013, 288, 2281–2289. [Google Scholar] [CrossRef]
- Sato, K.; Saito, H.; Matsuki, N. HSP70 is essential to the neuroprotective effect of heat-shock. Brain Res. 1996, 740, 117–123. [Google Scholar] [CrossRef]
- Cao, L.; Cao, D.X.; Su, X.W.; Chen, L.J.; Liu, A.L.; Jian, W.J.; Wu, Y.P.; Qiu, P.X.; Yan, G.M. Activation of PI3-K/Akt pathway for thermal preconditioning to protect cultured cerebellar granule neurons against low potassium-induced apoptosis. Acta Pharmacol. Sin. 2007, 28, 173–179. [Google Scholar] [CrossRef]
- Sousa, L.; Guarda, M.; Meneses, M.J.; Macedo, M.P.; Vicente Miranda, H. Insulin-degrading enzyme: An ally against metabolic and neurodegenerative diseases. J. Pathol. 2021, 255, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Noel, A.; Barrier, L.; Ingrand, S. The Tyr216 phosphorylated form of GSK3β contributes to tau phosphorylation at PHF-1 epitope in response to Aβ in the nucleus of SH-SY5Y cells. Life Sci. 2016, 158, 14–21. [Google Scholar] [CrossRef]
- Partanen, A.; Tillander, M.; Yarmolenko, P.S.; Wood, B.J.; Dreher, M.R.; Kohler, M.O. Reduction of peak acoustic pressure and shaping of heated region by use of multifoci sonications in MR-guided high-intensity focused ultrasound mediated mild hyperthermia. Med. Phys. 2013, 40, 013301. [Google Scholar] [CrossRef]
- Chaplin, V.; Caskey, C.F. Multi-focal HIFU reduces cavitation in mild-hyperthermia. J. Ther. Ultrasound. 2017, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Choi, J.C.; Kang, S.Y.; Kang, J.H.; Park, J.K. Heat stroke: Increased signal intensity in the bilateral cerebellar dentate nuclei and splenium on diffusion-weighted MR imaging. AJNR. Am. J. Neuroradiol. 2009, 30, E58. [Google Scholar] [CrossRef]
- Tabassum, S.; Raza, N.; Shah, S.Z. Outcome of heat stroke patients referred to a tertiary hospital in Pakistan: A retrospective study. East. Mediterr. Health J. 2019, 25, 457–464. [Google Scholar] [CrossRef]
- Cremer, O.L.; Kalkman, C.J. Cerebral pathophysiology and clinical neurology of hyperthermia in humans. Prog. Brain Res. 2007, 162, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Kuo, Y.Y.; Lin, G.B.; Lu, C.H.; Hsu, H.P.; Sun, Y.K.; Chao, C.Y. Thermal cycling protects SH-SY5Y cells against hydrogen peroxide and β-amyloid-induced cell injury through stress response mechanisms involving Akt pathway. PLoS ONE 2020, 15, e0240022. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.Y.; Chen, W.T.; Lin, G.B.; Chen, Y.M.; Liu, H.H.; Chao, C.Y. Thermal cycling-hyperthermia ameliorates Aβ25-35-induced cognitive impairment in C57BL/6 mice. Neurosci. Lett. 2023, 810, 137337. [Google Scholar] [CrossRef]
- Xie, H.R.; Hu, L.S.; Li, G.Y. SH-SY5Y human neuroblastoma cell line: In vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. 2010, 123, 1086–1092. [Google Scholar] [CrossRef]
- Raynes, R.; Brunquell, J.; Westerheide, S.D. Stress inducibility of SIRT1 and its role in cytoprotection and cancer. Genes Cancer 2013, 4, 172–182. [Google Scholar] [CrossRef]
- Haque, M.M.; Murale, D.P.; Kim, Y.K.; Lee, J.S. Crosstalk between oxidative stress and tauopathy. Int. J. Mol. Sci. 2019, 20, 1959. [Google Scholar] [CrossRef]
- Ramalingam, M.; Huh, Y.J.; Lee, Y.I. The impairments of α-synuclein and mechanistic target of rapamycin in rotenone-induced SH-SY5Y cells and mice model of Parkinson’s disease. Front. Neurosci. 2019, 13, 1028. [Google Scholar] [CrossRef]
- Sur, M.; Dey, P.; Sarkar, A.; Bar, S.; Banerjee, D.; Bhat, S.; Mukherjee, P. Sarm1 induction and accompanying inflammatory response mediates age-dependent susceptibility to rotenone-induced neurotoxicity. Cell Death Discov. 2018, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Liu, L.; Hu, Y.; Cao, S.; Tan, T.; Huang, X.; Deng, Q.; Chen, J.; Guo, R.; Zhou, Q. Low-intensity pulsed ultrasound of different intensities differently affects myocardial ischemia/reperfusion injury by modulating cardiac oxidative stress and inflammatory reaction. Front. Immunol. 2023, 14, 1248056. [Google Scholar] [CrossRef]
- Aimaijiang, M.; Liu, Y.; Zhang, Z.; Qin, Q.; Liu, M.; Abulikemu, P.; Liu, L.; Zhou, Y. LIPUS as a potential strategy for periodontitis treatment: A review of the mechanisms. Front. Bioeng. Biotechnol. 2023, 11, 1018012. [Google Scholar] [CrossRef]
- Grondin, M.; Chabrol, C.; Averill-Bates, D.A. Mild heat shock at 40 °C increases levels of autophagy: Role of Nrf2. Cell Stress Chaperones 2024, 29, 567–588. [Google Scholar] [CrossRef]
- Riederer, P.; Nagatsu, T.; Youdim, M.B.H.; Wulf, M.; Dijkstra, J.M.; Sian-Huelsmann, J. Lewy bodies, iron, inflammation and neuromelanin: Pathological aspects underlying Parkinson’s disease. J. Neural Transm. 2023, 130, 627–646. [Google Scholar] [CrossRef]
- Deas, E.; Cremades, N.; Angelova, P.R.; Ludtmann, M.H.; Yao, Z.; Chen, S.; Horrocks, M.H.; Banushi, B.; Little, D.; Devine, M.J.; et al. Alpha-synuclein oligomers interact with metal ions to induce oxidative stress and neuronal death in Parkinson’s disease. Antioxid. Redox Signal. 2016, 24, 376–391. [Google Scholar] [CrossRef]
- Puspita, L.; Chung, S.Y.; Shim, J.W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain 2017, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Dorszewska, J.; Kowalska, M.; Prendecki, M.; Piekut, T.; Kozłowska, J.; Kozubski, W. Oxidative stress factors in Parkinson’s disease. Neural Regen. Res. 2021, 16, 1383–1391. [Google Scholar] [CrossRef]
- Tao, J.; Berthet, A.; Citron, Y.R.; Tsiolaki, P.L.; Stanley, R.; Gestwicki, J.E.; Agard, D.A.; McConlogue, L. Hsp70 chaperone blocks α-synuclein oligomer formation via a novel engagement mechanism. J. Biol. Chem. 2021, 296, 100613. [Google Scholar] [CrossRef]
- Patterson, K.R.; Ward, S.M.; Combs, B.; Voss, K.; Kanaan, N.M.; Morfini, G.; Brady, S.T.; Gamblin, T.C.; Binder, L.I. Heat shock protein 70 prevents both tau aggregation and the inhibitory effects of preexisting tau aggregates on fast axonal transport. Biochemistry 2011, 50, 10300–10310. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The role of sirtuins in antioxidant and redox signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Martorell, A.J.; Paulson, A.L.; Suk, H.J.; Abdurrob, F.; Drummond, G.T.; Guan, W. Multi-sensory gamma stimulation ameliorates Alzheimer’s-associated pathology and improves cognition. Cell 2019, 177, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Park, H.S.; Kim, C.J.; Kang, H.S.; Kim, D.H.; Baek, S.S.; Kim, T.W. Physical exercise during exposure to 40-Hz light flicker improves cognitive functions in the 3xTg mouse model of Alzheimer’s disease. Alzheimers Res. Ther. 2020, 12, 62. [Google Scholar] [CrossRef]
- Chan, D.; Suk, H.J.; Jackson, B.L.; Milman, N.P.; Stark, D.; Klerman, E.B.; Kitchener, E.; Fernandez Avalos, V.S.; de Weck, G.; Banerjee, A.; et al. Gamma frequency sensory stimulation in mild probable Alzheimer’s dementia patients: Results of feasibility and pilot studies. PLoS ONE 2022, 17, e0278412. [Google Scholar] [CrossRef]
- Kuo, Y.Y.; Lin, G.B.; Chen, W.T.; Chen, Y.M.; Liu, H.H.; Chao, C.Y. Thermal cycling-hyperthermia down-regulates liposaccharide-induced inflammation in human blood and helps with clearance of herpes simplex virus type 1. bioRxiv 2022. [Google Scholar] [CrossRef]
- Tanaka, R.; Kim, C.H.; Yamada, N.; Saito, Y. Radiofrequency hyperthermia for malignant brain tumors: Preliminary results of clinical trials. Neurosurgery 1987, 21, 478–483. [Google Scholar] [CrossRef]
- Saha, N.; Kuehne, A.; Millward, J.M.; Eigentler, T.W.; Starke, L.; Waiczies, S.; Niendorf, T. Advanced radio frequency applicators for thermal magnetic resonance theranostics of brain tumors. Cancers 2023, 15, 2303. [Google Scholar] [CrossRef]
- Santos, M.A.; Goertz, D.E.; Hynynen, K. Focused ultrasound hyperthermia mediated drug delivery using thermosensitive liposomes and visualized with in vivo two-photon microscopy. Theranostics 2017, 7, 2718–2731. [Google Scholar] [CrossRef]
- Moonen, C.T.W.; Kilroy, J.P.; Klibanov, A.L. Focused ultrasound: Noninvasive image-guided therapy. Investig. Radiol. 2025, 60, 205–219. [Google Scholar] [CrossRef]
- Lipsman, N.; Mainprize, T.G.; Schwartz, M.L.; Hynynen, K.; Lozano, A.M. Intracranial applications of magnetic resonance-guided focused ultrasound. Neurotherapeutics 2014, 11, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Mattay, R.R.; Kim, K.; Shah, L.; Shah, B.; Sugrue, L.; Safoora, F.; Ozhinsky, E.; Narsinh, K.H. MR thermometry during transcranial MR imaging–guided focused ultrasound procedures: A review. Am. J. Neuroradiol. 2024, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, Y.-Y.; Lin, G.-B.; Chen, Y.-M.; Liu, H.-H.; Hsu, F.-T.; Kung, Y.; Chao, C.-Y. Thermal Cycling-Hyperthermia Attenuates Rotenone-Induced Cell Injury in SH-SY5Y Cells Through Heat-Activated Mechanisms. Int. J. Mol. Sci. 2025, 26, 6671. https://doi.org/10.3390/ijms26146671
Kuo Y-Y, Lin G-B, Chen Y-M, Liu H-H, Hsu F-T, Kung Y, Chao C-Y. Thermal Cycling-Hyperthermia Attenuates Rotenone-Induced Cell Injury in SH-SY5Y Cells Through Heat-Activated Mechanisms. International Journal of Molecular Sciences. 2025; 26(14):6671. https://doi.org/10.3390/ijms26146671
Chicago/Turabian StyleKuo, Yu-Yi, Guan-Bo Lin, You-Ming Chen, Hsu-Hsiang Liu, Fang-Tzu Hsu, Yi Kung, and Chih-Yu Chao. 2025. "Thermal Cycling-Hyperthermia Attenuates Rotenone-Induced Cell Injury in SH-SY5Y Cells Through Heat-Activated Mechanisms" International Journal of Molecular Sciences 26, no. 14: 6671. https://doi.org/10.3390/ijms26146671
APA StyleKuo, Y.-Y., Lin, G.-B., Chen, Y.-M., Liu, H.-H., Hsu, F.-T., Kung, Y., & Chao, C.-Y. (2025). Thermal Cycling-Hyperthermia Attenuates Rotenone-Induced Cell Injury in SH-SY5Y Cells Through Heat-Activated Mechanisms. International Journal of Molecular Sciences, 26(14), 6671. https://doi.org/10.3390/ijms26146671





