The Effects of Psychotherapy on Single and Repeated Ketamine Infusion(s) Therapy for Treatment-Resistant Depression: The Convergence of Molecular and Psychological Treatment
Abstract
1. Introduction
1.1. Treatment Resistant Depression
1.2. Ketamine
1.2.1. Pharmacology of Ketamine
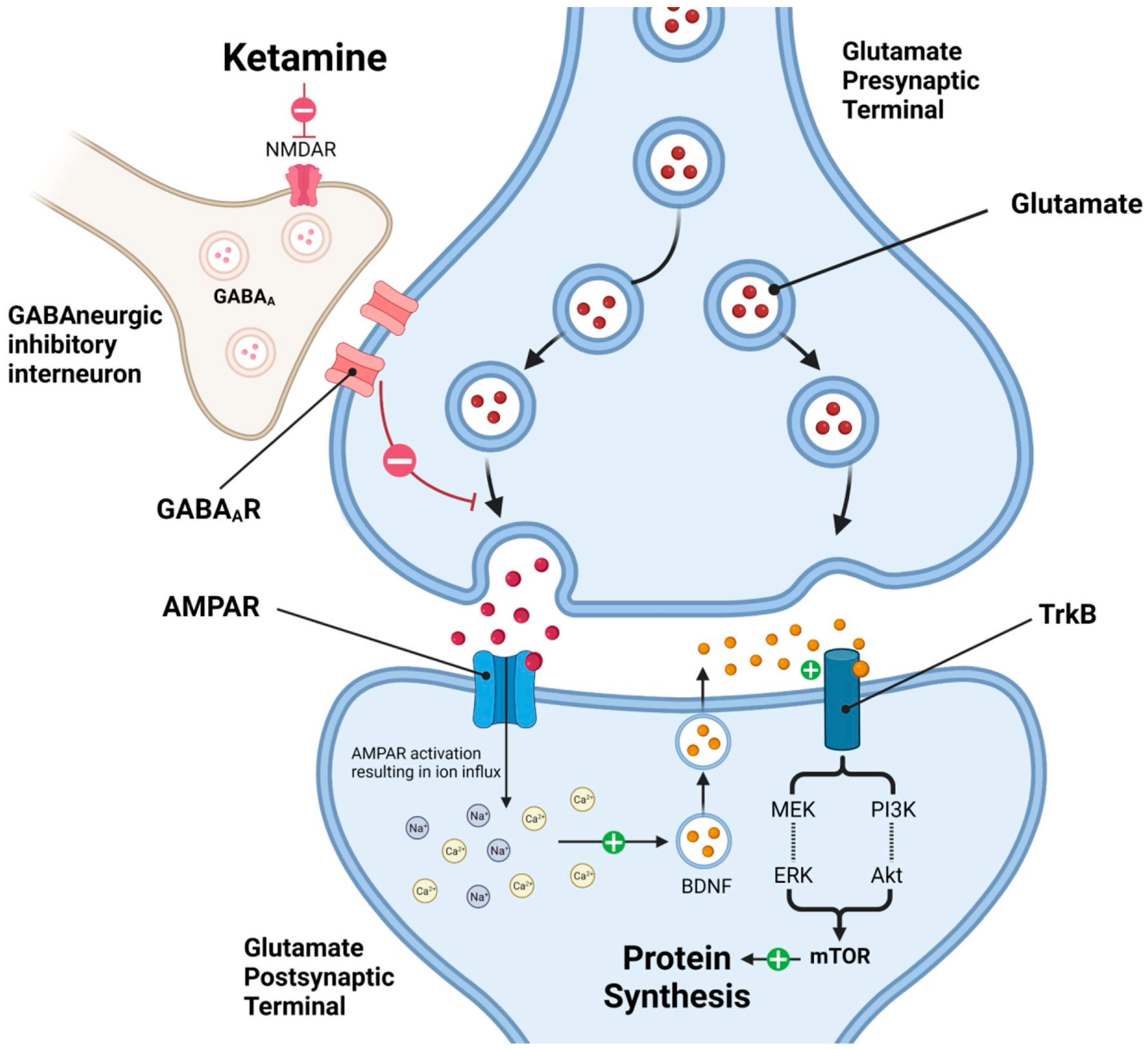
1.2.2. Ketamine Mechanism of Action
1.2.3. Molecular Pathways Underlying Ketamine’s Rapid Antidepressant Effects
1.2.4. Psychotherapy and Molecular Correlates
1.2.5. Molecular Synergy in Ketamine-Assisted Psychotherapy (KAP)
1.2.6. Epigenetic Mechanisms of Ketamine
1.2.7. Non-Ketamine Psychotherapy and Comparative Molecular Profiles
1.3. Ketamine Safety and Tolerability
Ketamine-Induced Bladder Toxicity
1.4. Ketamine for Treatment Resistant Depression
1.4.1. Dissociation
1.4.2. Rapid Onset
1.4.3. Antidepressant Effects of Ketamine on TRD
2. Results
2.1. Clinical Characteristics
2.2. The Effects of Psychotherapy on Single and Repeated Ketamine Infusion(s) Therapy for TRD
2.3. Depression Categorizations Pre- and Post-Infusion(s)
2.4. Tolerability and Safety
3. Discussion
3.1. Limitations
3.2. Directions for Future Research
4. Materials and Methods
4.1. Participants
4.2. Measures
4.2.1. Beck Depression Inventory
4.2.2. Antidepressant Treatment History Form
4.2.3. Inclusion Criteria
4.2.4. Exclusion Criteria
4.3. Study Design
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TRD | Treatment-resistant depression |
| MDD | Major depressive disorder |
| SSRIs | Selective serotonin reuptake inhibitors |
| SNRIs | Serotonin–norepinephrine reuptake inhibitors |
| MAOIs | Monoamine oxidase inhibitors |
| TCAs | Tricyclic antidepressants |
| IV | Intravenous |
| IM | Intramuscular |
| NMDA | N-methyl-D-aspartate |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| BDNF | Brain-derived neurotrophic factor |
| mTOR | Rapamycin |
| CBT | Cognitive Behavioral Therapy |
| HPA | Hypothalamic–pituitary–adrenal |
| IL-6 | Interleukin-6 (IL-6) |
| TNF-α | Tumor necrosis factor-alpha |
| PTSD | Post-traumatic stress disorder |
| KAP | Ketamine-assisted psychotherapy |
| KIC | Ketamine-induced cystitis |
| GAG | Glycosaminoglycan |
| ANCOVA | Analysis of covariance |
References
- Liu, J.; Liu, Y.; Ma, W.; Tong, Y.; Zheng, J. Temporal and Spatial Trend Analysis of All-Cause Depression Burden Based on Global Burden of Disease (GBD) 2019 Study. Sci. Rep. 2024, 14, 12346. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M. Depression: The Disorder and the Burden. Indian J. Psychol. Med. 2010, 32, 1–2. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Alsuwaidan, M.; Baune, B.T.; Berk, M.; Demyttenaere, K.; Goldberg, J.F.; Gorwood, P.; Ho, R.; Kasper, S.; Kennedy, S.H.; et al. Treatment-Resistant Depression: Definition, Prevalence, Detection, Management, and Investigational Interventions. World Psychiatry 2023, 22, 394–412. [Google Scholar] [CrossRef]
- Zhdanava, M.; Pilon, D.; Ghelerter, I.; Chow, W.; Joshi, K.; Lefebvre, P.; Sheehan, J.J. The Prevalence and National Burden of Treatment-Resistant Depression and Major Depressive Disorder in the United States. J. Clin. Psychiatry 2021, 82, 29169. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of Depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- Hashimoto, K. Emerging Role of Glutamate in the Pathophysiology of Major Depressive Disorder. Brain Res. Rev. 2009, 61, 105–123. [Google Scholar] [CrossRef]
- Abdallah, C.G.; Sanacora, G.; Duman, R.S.; Krystal, J.H. Ketamine and Rapid-Acting Antidepressants: A Window into a New Neurobiology for Mood Disorder Therapeutics. Annu. Rev. Med. 2015, 66, 509–523. [Google Scholar] [CrossRef]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The Stressed Synapse: The Impact of Stress and Glucocorticoids on Glutamate Transmission. Nat. Rev. Neurosci. 2011, 13, 22–37. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic Plasticity and Depression: New Insights from Stress and Rapid-Acting Antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, C.; Öngür, D. Magnetic Resonance Spectroscopy Studies of Glutamate-Related Abnormalities in Mood Disorders. Biol. Psychiatry 2010, 68, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant Effects of Ketamine in Depressed Patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Daly, E.J.; Trivedi, M.H.; Janik, A.; Li, H.; Zhang, Y.; Li, X.; Lane, R.; Lim, P.; Duca, A.R.; Hough, D.; et al. Efficacy of Esketamine Nasal Spray plus Oral Antidepressant Treatment for Relapse Prevention in Patients with Treatment-Resistant Depression. JAMA Psychiatry 2019, 76, 893–903. [Google Scholar] [CrossRef]
- Al-harbi, K.S. Treatment-Resistant Depression: Therapeutic Trends, Challenges, and Future Directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef]
- Jaffe, D.H.; Rive, B.; Denee, T.R. The Humanistic and Economic Burden of Treatment-Resistant Depression in Europe: A Cross-Sectional Study. BMC Psychiatry 2019, 19, 247. [Google Scholar] [CrossRef]
- Kolovos, S.; van Tulder, M.W.; Cuijpers, P.; Prigent, A.; Chevreul, K.; Riper, H.; Bosmans, J.E. The Effect of Treatment as Usual on Major Depressive Disorder: A Meta-Analysis. J. Affect. Disord. 2017, 210, 72–81. [Google Scholar] [CrossRef]
- Akil, H.; Gordon, J.; Hen, R.; Javitch, J.; Mayberg, H.; McEwen, B.; Meaney, M.J.; Nestler, E.J. Treatment Resistant Depression: A Multi-Scale, Systems Biology Approach. Neurosci. Biobehav. Rev. 2018, 84, 272–288. [Google Scholar] [CrossRef]
- Conway, C.R.; George, M.S.; Sackeim, H.A. Toward an Evidence-Based, Operational Definition of Treatment-Resistant Depression. JAMA Psychiatry 2017, 74, 9–10. [Google Scholar] [CrossRef]
- Ignácio, Z.M.; Réus, G.Z.; Arent, C.O.; Abelaira, H.M.; Pitcher, M.R.; Quevedo, J. New Perspectives on the Involvement of MTOR in Depression as Well as in the Action of Antidepressant Drugs. Br. J. Clin. Pharmacol. 2016, 82, 1280–1290. [Google Scholar] [CrossRef]
- Li, L.; Vlisides, P.E. Ketamine: 50 Years of Modulating the Mind. Front. Hum. Neurosci. 2016, 10, 612. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, S.B.; Gupta, V.; Patel, P.; Palacios, J.L. Ketamine. Nih.gov. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK470357/ (accessed on 13 June 2024).
- Drug Enforcement Administration. Ketamine. 2020. Available online: https://www.dea.gov/sites/default/files/2020-06/Ketamine-2020.pdf (accessed on 10 January 2023).
- Matveychuk, D.; Thomas, R.K.; Swainson, J.; Khullar, A.; MacKay, M.-A.; Baker, G.B.; Dursun, S.M. Ketamine as an Antidepressant: Overview of Its Mechanisms of Action and Potential Predictive Biomarkers. Ther. Adv. Psychopharmacol. 2020, 10, 204512532091665. [Google Scholar] [CrossRef]
- Zarate, C.A.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A Randomized Trial of an N-Methyl-D-Aspartate Antagonist in Treatment-Resistant Major Depression. Arch. Gen. Psychiatry 2006, 63, 856. [Google Scholar] [CrossRef]
- Krystal, J.H.; Abdallah, C.G.; Sanacora, G.; Charney, D.S.; Duman, R.S. Ketamine: A Paradigm Shift for Depression Research and Treatment. Neuron 2019, 101, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Fond, G.; Loundou, A.; Rabu, C.; Macgregor, A.; Lançon, C.; Brittner, M.; Micoulaud-Franchi, J.-A.; Richieri, R.; Courtet, P.; Abbar, M.; et al. Ketamine Administration in Depressive Disorders: A Systematic Review and Meta-Analysis. Psychopharmacology 2014, 231, 3663–3676. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Ballard, E.D.; Bloch, M.H.; Mathew, S.J.; Murrough, J.W.; Feder, A.; Sos, P.; Wang, G.; Zarate, C.A.; Sanacora, G. The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data Meta-Analysis. Am. J. Psychiatry 2018, 175, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. Ketamine for Depression, 3: Does Chirality Matter? J. Clin. Psychiatry 2017, 78, e674–e677. [Google Scholar] [CrossRef]
- Caddy, C.; Amit, B.H.; McCloud, T.L.; Rendell, J.M.; Furukawa, T.A.; McShane, R.; Hawton, K.; Cipriani, A. Ketamine and Other Glutamate Receptor Modulators for Depression in Adults. Cochrane Database Syst. Rev. 2015, CD011612. [Google Scholar] [CrossRef]
- Singh, J.B.; Fedgchin, M.; Daly, E.; Xi, L.; Melman, C.; De Bruecker, G.; Tadic, A.; Sienaert, P.; Wiegand, F.; Manji, H.; et al. Intravenous Esketamine in Adult Treatment-Resistant Depression: A Double-Blind, Double-Randomization, Placebo-Controlled Study. Biol. Psychiatry 2016, 80, 424–431. [Google Scholar] [CrossRef]
- Feder, A.; Parides, M.K.; Murrough, J.W.; Perez, A.M.; Morgan, J.E.; Saxena, S.; Kirkwood, K.; aan het Rot, M.; Lapidus, K.A.B.; Wan, L.-B.; et al. Efficacy of Intravenous Ketamine for Treatment of Chronic Posttraumatic Stress Disorder. JAMA Psychiatry 2014, 71, 681–688. [Google Scholar] [CrossRef]
- Rodriguez, C.I.; Kegeles, L.S.; Levinson, A.; Feng, T.; Marcus, S.M.; Vermes, D.; Flood, P.; Simpson, H.B. Randomized Controlled Crossover Trial of Ketamine in Obsessive-Compulsive Disorder: Proof-of-Concept. Neuropsychopharmacology 2013, 38, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Israel, J.E.; St Pierre, S.; Ellis, E.; Hanukaai, J.S.; Noor, N.; Varrassi, G.; Wells, M.; Kaye, A.D. Ketamine for the Treatment of Chronic Pain: A Comprehensive Review. Health Psychol. Res. 2021, 9, 25535. [Google Scholar] [CrossRef] [PubMed]
- Corriger, A.; Pickering, G. Ketamine and Depression: A Narrative Review. Drug Des. Dev. Ther. 2019, 13, 3051–3067. [Google Scholar] [CrossRef] [PubMed]
- Curran, H.V.; Monaghan, L. In and out of the K-Hole: A Comparison of the Acute and Residual Effects of Ketamine in Frequent and Infrequent Ketamine Users. Addiction 2001, 96, 749–760. [Google Scholar] [CrossRef]
- Lener, M.S.; Niciu, M.J.; Ballard, E.D.; Park, M.; Park, L.T.; Nugent, A.C.; Zarate, C.A. Glutamate and Gamma-Aminobutyric Acid Systems in the Pathophysiology of Major Depression and Antidepressant Response to Ketamine. Biol. Psychiatry 2017, 81, 886–897. [Google Scholar] [CrossRef]
- Smith-Apeldoorn, S.Y.; Veraart, J.K.; Spijker, J.; Kamphuis, J.; Schoevers, R.A. Maintenance Ketamine Treatment for Depression: A Systematic Review of Efficacy, Safety, and Tolerability. Lancet Psychiatry 2022, 9, 907–921. [Google Scholar] [CrossRef]
- Pałucha-Poniewiera, A. The Role of Glutamatergic Modulation in the Mechanism of Action of Ketamine, a Prototype Rapid-Acting Antidepressant Drug. Pharmacol. Rep. 2018, 70, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Strong, C.E.; Kabbaj, M. On the Safety of Repeated Ketamine Infusions for the Treatment of Depression: Effects of Sex and Developmental Periods. Neurobiol. Stress 2018, 9, 166–175. [Google Scholar] [CrossRef]
- Dao, R.; Aggarwal, A. Utilization of Ketamine for Major Depression. J. Explor. Res. Pharmacol. 2023, 8, 342–347. [Google Scholar] [CrossRef]
- Li, N.; Lee, B.; Liu, R.-J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.-Y.; Aghajanian, G.; Duman, R.S. MTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shirayama, Y.; Zhang, J.-C.; Ren, Q.; Yao, W.; Ma, M.; Dong, C.; Hashimoto, K. R-Ketamine: A Rapid-Onset and Sustained Antidepressant without Psychotomimetic Side Effects. Transl. Psychiatry 2015, 5, e632. [Google Scholar] [CrossRef]
- Kopschina Feltes, P.; Doorduin, J.; Klein, H.C.; Juárez-Orozco, L.E.; Dierckx, R.A.; Moriguchi-Jeckel, C.M.; de Vries, E.F. Anti-Inflammatory Treatment for Major Depressive Disorder: Implications for Patients with an Elevated Immune Profile and Non-Responders to Standard Antidepressant Therapy. J. Psychopharmacol. 2017, 31, 1149–1165. [Google Scholar] [CrossRef]
- Nestler, E.J.; Peña, C.J.; Kundakovic, M.; Mitchell, A.; Akbarian, S. Epigenetic Basis of Mental Illness. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2016, 22, 447–463. [Google Scholar] [CrossRef]
- Fonzo, G.A.; Goodkind, M.S.; Oathes, D.J.; Zaiko, Y.V.; Harvey, M.; Peng, K.K.; Weiss, M.E.; Thompson, A.L.; Zack, S.E.; Lindley, S.E.; et al. PTSD Psychotherapy Outcome Predicted by Brain Activation during Emotional Reactivity and Regulation. Am. J. Psychiatry 2017, 174, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Lin, J.; Dhabhar, F.S.; Wolkowitz, O.M.; Puterman, E.; Karan, L.; Blackburn, E.H. Dynamics of Telomerase Activity in Response to Acute Psychological Stress. Brain Behav. Immun. 2010, 24, 531–539. [Google Scholar] [CrossRef]
- Karege, F.; Perret, G.; Bondolfi, G.; Schwald, M.; Bertschy, G.; Aubry, J.-M. Decreased Serum Brain-Derived Neurotrophic Factor Levels in Major Depressed Patients. Psychiatry Res. 2002, 109, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Dore, J.; Turnipseed, B.; Dwyer, S.; Turnipseed, A.; Andries, J.; Ascani, G.; Monnette, C.; Huidekoper, A.; Strauss, N.; Wolfson, P. Ketamine Assisted Psychotherapy (KAP): Patient Demographics, Clinical Data and Outcomes in Three Large Practices Administering Ketamine with Psychotherapy. J. Psychoact. Drugs 2019, 51, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.T.; Wright, D.; Fasula, M.K.; Fenton, L.; Griepp, M.; Ostroff, R.B.; Sanacora, G. Cognitive Behavior Therapy May Sustain Antidepressant Effects of Intravenous Ketamine in Treatment-Resistant Depression. Psychother. Psychosom. 2017, 86, 162–167. [Google Scholar] [CrossRef]
- Inserra, A.; Campanale, A.; Rezai, T.; Romualdi, P.; Rubino, T. Epigenetic Mechanisms of Rapid-Acting Antidepressants. Transl. Psychiatry 2024, 14, 359. [Google Scholar] [CrossRef]
- Wellington, N.J.; Boucas, A.P.; Schwenn, P.E.; Lagopoulos, J.; Quigley, B.L.; Kuballa, A.V. Methylome and Transcriptome Functional Analysis Identifies Key Biomarkers in Ketamine’s Sustained Therapeutic Effect on PTSD. medRxiv 2025. [Google Scholar] [CrossRef]
- Dawson, K.; May, A.; Klunder, J.; Carreras-Gallo, N.; Sehgal, R.; Megilligan, S.; Askins, B.C.; Perkins, N.; Mendez, T.L.; Smith, R.; et al. Ketamine Treatment Effects on DNA Methylation and Epigenetic Biomarkers of Aging. medRxiv 2024. [Google Scholar] [CrossRef]
- Wan, L.-B.; Levitch, C.F.; Perez, A.M.; Brallier, J.W.; Iosifescu, D.V.; Chang, L.C.; Foulkes, A.; Mathew, S.J.; Charney, D.S.; Murrough, J.W. Ketamine Safety and Tolerability in Clinical Trials for Treatment-Resistant Depression. J. Clin. Psychiatry 2014, 76, 247–252. [Google Scholar] [CrossRef]
- Wong, G.L.-H.; Tam, Y.-H.; Ng, C.-F.; Chan, A.W.-H.; Choi, P.C.-L.; Chu, W.C.-W.; Lai, P.B.-S.; Chan, H.L.-Y.; Wong, V.W.-S. Liver Injury Is Common among Chronic Abusers of Ketamine. Clin. Gastroenterol. Hepatol. 2014, 12, 1759–1762. [Google Scholar] [CrossRef]
- Jhang, J.-F.; Hsu, Y.-H.; Kuo, H.-C. Possible Pathophysiology of Ketamine-Related Cystitis and Associated Treatment Strategies. Int. J. Urol. 2015, 22, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Short, B.; Fong, J.; Galvez, V.; Shelker, W.; Loo, C.K. Side-Effects Associated with Ketamine Use in Depression: A Systematic Review. Lancet Psychiatry 2018, 5, 65–78. [Google Scholar] [CrossRef]
- Gu, D.; Huang, J.; Yin, Y.; Shan, Z.; Zheng, S.; Wu, P. Long-Term Ketamine Abuse Induces Cystitis in Rats by Impairing the Bladder Epithelial Barrier. Mol. Biol. Rep. 2014, 41, 7313–7322. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-H.; Wang, S.-C.; Wang, S.-T.; Lin, S.-M.; Wu, J.-D.; Lin, C.-T.; Liu, Y.-W. Evaluation of Urinary Bladder Fibrogenesis in a Mouse Model of Long-Term Ketamine Injection. Mol. Med. Rep. 2016, 14, 1880–1890. [Google Scholar] [CrossRef]
- Shahani, R.; Streutker, C.; Dickson, B.; Stewart, R.J. Ketamine-Associated Ulcerative Cystitis: A New Clinical Entity. Urology 2007, 69, 810–812. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Cha, T.-L.; Lin, C.-M.; Tsao, C.-W.; Tang, S.-H.; Chuang, F.-P.; Wu, S.-T.; Sun, G.-H.; Yu, D.-S.; Chang, S.-Y. Ketamine-Associated Bladder Dysfunction. Int. J. Urol. 2009, 16, 826–829. [Google Scholar] [CrossRef]
- Chu, P.S.-K.; Ma, W.-K.; Wong, S.C.-W.; Chu, R.W.-H.; Cheng, C.-H.; Wong, S.; Tse, J.M.; Lau, F.-L.; Yiu, M.-K.; Man, C.-W. The Destruction of the Lower Urinary Tract by Ketamine Abuse: A New Syndrome? BJU Int. 2008, 102, 1616–1622. [Google Scholar] [CrossRef]
- Middela, S.; Pearce, I. Ketamine-Induced Vesicopathy: A Literature Review. Int. J. Clin. Pract. 2010, 65, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, A.Y.; Onaolapo, O.J. Glutamate and Depression: Reflecting a Deepening Knowledge of the Gut and Brain Effects of a Ubiquitous Molecule. World J. Psychiatry 2021, 11, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.Y.; Hawken, E.; Vazquez, G.H. The Mechanisms behind Rapid Antidepressant Effects of Ketamine: A Systematic Review with a Focus on Molecular Neuroplasticity. Front. Psychiatry 2022, 13, 860882. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Charney, D.S.; Oren, D.A.; Berman, R.M.; Hu, X.S.; Cappiello, A.; Krystal, J.H. Attenuation of the Neuropsychiatric Effects of Ketamine with Lamotrigine. Arch. Gen. Psychiatry 2000, 57, 270. [Google Scholar] [CrossRef]
- Luckenbaugh, D.A.; Niciu, M.J.; Ionescu, D.F.; Nolan, N.M.; Richards, E.M.; Brutsche, N.E.; Guevara, S.; Zarate, C.A. Do the Dissociative Side Effects of Ketamine Mediate Its Antidepressant Effects? J. Affect. Disord. 2014, 159, 56–61. [Google Scholar] [CrossRef]
- Marguilho, M.; Figueiredo, I.; Castro-Rodrigues, P. A Unified Model of Ketamine’s Dissociative and Psychedelic Properties. J. Psychopharmacol. 2022, 37, 14–32. [Google Scholar] [CrossRef]
- Niciu, M.J.; Shovestul, B.J.; Jaso, B.A.; Farmer, C.; Luckenbaugh, D.A.; Brutsche, N.E.; Park, L.T.; Ballard, E.D.; Zarate, C.A. Features of Dissociation Differentially Predict Antidepressant Response to Ketamine in Treatment-Resistant Depression. J. Affect. Disord. 2018, 232, 310–315. [Google Scholar] [CrossRef]
- Sarasso, P.; Billeci, M.; Ronga, I.; Raffone, F.; Martiadis, V.; Di Petta, G. Disembodiment and Affective Resonances in Esketamine Treatment of Depersonalized Depression Subtype: Two Case Studies. Psychopathology 2024, 57, 480–491. [Google Scholar] [CrossRef]
- Chen, G.; Chen, L.; Zhang, Y.; Li, X.; Lane, R.; Lim, P.; Daly, E.J.; Furey, M.L.; Fedgchin, M.; Popova, V.; et al. Relationship between Dissociation and Antidepressant Effects of Esketamine Nasal Spray in Patients with Treatment-Resistant Depression. Int. J. Neuropsychopharmacol. 2022, 25, 269–279. [Google Scholar] [CrossRef]
- Valentine, G.W.; Mason, G.F.; Gomez, R.; Fasula, M.; Watzl, J.; Pittman, B.; Krystal, J.H.; Sanacora, G. The Antidepressant Effect of Ketamine Is Not Associated with Changes in Occipital Amino Acid Neurotransmitter Content as Measured by [1H]-MRS. Psychiatry Res. Neuroimaging 2011, 191, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Murrough, J.W.; Perez, A.M.; Pillemer, S.; Stern, J.; Parides, M.K.; aan het Rot, M.; Collins, K.A.; Mathew, S.J.; Charney, D.S.; Iosifescu, D.V. Rapid and Longer-Term Antidepressant Effects of Repeated Ketamine Infusions in Treatment-Resistant Major Depression. Biol. Psychiatry 2013, 74, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.L.; Norris, S.; Talbot, J.; Hatchard, T.; Ortiz, A.; Birmingham, M.; Owoeye, O.; Batten, L.A.; Blier, P. Single and Repeated Ketamine Infusions for Reduction of Suicidal Ideation in Treatment-Resistant Depression. Neuropsychopharmacology 2019, 45, 606–612. [Google Scholar] [CrossRef]
- Krystal, J.H.; Kavalali, E.T.; Monteggia, L.M. Ketamine and Rapid Antidepressant Action: New Treatments and Novel Synaptic Signaling Mechanisms. Neuropsychopharmacology 2023, 49, 41–50. [Google Scholar] [CrossRef]
- Fava, M.; Freeman, M.P.; Flynn, M.; Judge, H.; Hoeppner, B.B.; Cusin, C.; Ionescu, D.F.; Mathew, S.J.; Chang, L.C.; Iosifescu, D.V.; et al. Double-Blind, Placebo-Controlled, Dose-Ranging Trial of Intravenous Ketamine as Adjunctive Therapy in Treatment-Resistant Depression (TRD). Mol. Psychiatry 2018, 25, 1592–1603. [Google Scholar] [CrossRef]
- Murrough, J.W.; Soleimani, L.; DeWilde, K.E.; Collins, K.A.; Lapidus, K.A.; Iacoviello, B.M.; Lener, M.; Kautz, M.; Kim, J.; Stern, J.B.; et al. Ketamine for Rapid Reduction of Suicidal Ideation: A Randomized Controlled Trial. Psychol. Med. 2015, 45, 3571–3580. [Google Scholar] [CrossRef]
- Phillips, J.L.; Norris, S.; Talbot, J.; Birmingham, M.; Hatchard, T.; Ortiz, A.; Owoeye, O.; Batten, L.A.; Blier, P. Single, Repeated, and Maintenance Ketamine Infusions for Treatment-Resistant Depression: A Randomized Controlled Trial. Am. J. Psychiatry 2019, 176, 401–409. [Google Scholar] [CrossRef]
- Shiroma, P.R.; Thuras, P.; Wels, J.; Albott, C.S.; Erbes, C.; Tye, S.; Lim, K.O. A Randomized, Double-Blind, Active Placebo-Controlled Study of Efficacy, Safety, and Durability of Repeated vs. Single Subanesthetic Ketamine for Treatment-Resistant Depression. Transl. Psychiatry 2020, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.; Chrenek, C.; Swainson, J. Maintenance Ketamine Therapy for Treatment-Resistant Depression. J. Clin. Psychopharmacol. 2018, 38, 380–384. [Google Scholar] [CrossRef]
- D’Andrea, G.; Pettorruso, M.; Di Lorenzo, G.; Rhee, T.G.; Chiappini, S.; Carullo, R.; Barlati, S.; Zanardi, R.; Rosso, G.; Di Nicola, M.; et al. The Rapid Antidepressant Effectiveness of Repeated Dose of Intravenous Ketamine and Intranasal Esketamine: A Post-Hoc Analysis of Pooled Real-World Data. J. Affect. Disord. 2024, 348, 314–322. [Google Scholar] [CrossRef]
- Sanacora, G.; Frye, M.A.; McDonald, W.; Mathew, S.J.; Turner, M.S.; Schatzberg, A.F.; Summergrad, P.; Nemeroff, C.B. A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders. JAMA Psychiatry 2017, 74, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Oliver, P.A.; Snyder, A.D.; Feinn, R.; Malov, S.; McDiarmid, G.; Arias, A.J. Clinical Effectiveness of Intravenous Racemic Ketamine Infusions in a Large Community Sample of Patients with Treatment-Resistant Depression, Suicidal Ideation, and Generalized Anxiety Symptoms: A Retrospective Chart Review. J. Clin. Psychiatry 2022, 83, 21m14336. [Google Scholar] [CrossRef]
- Marcantoni, W.S.; Akoumba, B.S.; Wassef, M.; Mayrand, J.; Lai, H.; Richard-Devantoy, S.; Beauchamp, S. A Systematic Review and Meta-Analysis of the Efficacy of Intravenous Ketamine Infusion for Treatment Resistant Depression: January 2009–January 2019. J. Affect. Disord. 2020, 277, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, D.F.; Swee, M.B.; Pavone, K.J.; Taylor, N.; Akeju, O.; Baer, L.; Nyer, M.; Cassano, P.; Mischoulon, D.; Alpert, J.E.; et al. Rapid and Sustained Reductions in Current Suicidal Ideation Following Repeated Doses of Intravenous Ketamine. J. Clin. Psychiatry 2016, 77, e719–e725. [Google Scholar] [CrossRef] [PubMed]
- Sakopoulos, S.; Hinz, L.D. Long-Term Effects of Single and Repeated Ketamine Infusions on Treatment-Resistant Depression: A Retrospective Chart Review Study. Psychoactives 2024, 3, 501–512. [Google Scholar] [CrossRef]
- Zanos, P.; Gould, T.D. Mechanisms of Ketamine Action as an Antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar] [CrossRef]
- Yeung, A.; Sapirstein, G.; Crain, L.D.; Cramer, M.A.; Forcen, F.E. Pharmacotherapy and Ketamine Assisted Psychotherapy for Treatment-Resistant Depression: A Patient with Lifelong Self-Doubt and Self-Criticism. J. Clin. Psychiatry 2023, 84, 23ct14798. [Google Scholar] [CrossRef]
- Drozdz, S.J.; Goel, A.; McGarr, M.W.; Katz, J.; Ritvo, P.; Mattina, G.F.; Bhat, V.; Diep, C.; Ladha, K.S. Ketamine Assisted Psychotherapy: A Systematic Narrative Review of the Literature. J. Pain Res. 2022, 15, 1691–1706. [Google Scholar] [CrossRef]
- McMullen, E.P.; Lee, Y.; Lipsitz, O.; Lui, L.M.W.; Vinberg, M.; Ho, R.; Rodrigues, N.B.; Rosenblat, J.D.; Cao, B.; Gill, H.; et al. Strategies to Prolong Ketamine’s Efficacy in Adults with Treatment-Resistant Depression. Adv. Ther. 2021, 38, 2795–2820. [Google Scholar] [CrossRef]
- Magnusson, K.R.; Brim, B.L.; Das, S.R. Selective Vulnerabilities of N-Methyl-D-Aspartate (NMDA) Receptors during Brain Aging. Front. Aging Neurosci. 2010, 2, 11. [Google Scholar] [CrossRef]
- Zhao, X.; Rosenke, R.; Kronemann, D.; Brim, B.; Das, S.R.; Dunah, A.W.; Magnusson, K.R. The Effects of Aging on N-Methyl-d-Aspartate Receptor Subunits in the Synaptic Membrane and Relationships to Long-Term Spatial Memory. Neuroscience 2009, 162, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Wampold, B.E. How important are the common factors in psychotherapy? An update. World Psychiatry 2015, 14, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II. PsycTESTS Dataset 1996. [Google Scholar] [CrossRef]
- Piotrowski, C. The Status of the Beck Inventories (BDI, BAI) in Psychology Training and Practice: A Major Shift in Clinical Acceptance. J. Appl. Biobehav. Res. 2017, 23, e12112. [Google Scholar] [CrossRef]
- Sackeim, H.A. The definition and meaning of treatment-resistant depression. J. Clin. Psychiatry 2001, 62 (Suppl. 16), 10–17. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
| Parameter | Details |
|---|---|
| Administration Routes | |
| Dosage Ranges | |
| Treatment Protocols |
|
| Targeted Clinical Conditions |
|
| (a) | |
| Diagnosis pre-treatment | Frequency (Percentage) |
| Mild depression | 3/9 (33%) |
| Moderate depression | 5/9 (55%) |
| Severe depression | 1/9 (11%) |
| Diagnosis post-treatment | Frequency (Percentage) |
| Clinically ‘normal’ | 5/9 (55%) |
| Mild depression | 4/9 (44%) |
| Moderate depression | 0/9 (0%) |
| Severe depression | 0/9 (0%) |
| (b) | |
| Diagnosis pre-treatment | Frequency (Percentage) |
| Mild depression | 0/5 (0%) |
| Moderate depression | 0/5 (0%) |
| Severe depression | 5/5 (100%) |
| Diagnosis post-treatment | Frequency (Percentage) |
| Clinically ‘normal’ | 0/5 (0%) |
| Mild depression | 0/5 (0%) |
| Moderate depression | 3/5 (60%) |
| Severe depression | 2/5 (40%) |
| (c) | |
| Diagnosis pre-treatment | Frequency (Percentage) |
| Mild depression | 2/6 (33%) |
| Moderate depression | 2/6 (33%) |
| Severe depression | 2/6 (33%) |
| Diagnosis post-treatment | Frequency (Percentage) |
| Clinically ‘normal’ | 2/6 (33%) |
| Mild depression | 1/6 (16%) |
| Moderate depression | 2/6 (33%) |
| Severe depression | 1/6 (16%) |
| (d) | |
| Diagnosis pre-treatment | Frequency (Percentage) |
| Mild depression | 0/4 (0%) |
| Moderate depression | 2/4 (50%) |
| Severe depression | 2/4 (50%) |
| Diagnosis post-treatment | Frequency (Percentage) |
| Clinically ‘normal’ | 0/4 (0%) |
| Mild depression | 2/4 (50%) |
| Moderate depression | 0/4 (0%) |
| Severe depression | 2/4 (50%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakopoulos, S.; Todman, M. The Effects of Psychotherapy on Single and Repeated Ketamine Infusion(s) Therapy for Treatment-Resistant Depression: The Convergence of Molecular and Psychological Treatment. Int. J. Mol. Sci. 2025, 26, 6673. https://doi.org/10.3390/ijms26146673
Sakopoulos S, Todman M. The Effects of Psychotherapy on Single and Repeated Ketamine Infusion(s) Therapy for Treatment-Resistant Depression: The Convergence of Molecular and Psychological Treatment. International Journal of Molecular Sciences. 2025; 26(14):6673. https://doi.org/10.3390/ijms26146673
Chicago/Turabian StyleSakopoulos, Sofia, and McWelling Todman. 2025. "The Effects of Psychotherapy on Single and Repeated Ketamine Infusion(s) Therapy for Treatment-Resistant Depression: The Convergence of Molecular and Psychological Treatment" International Journal of Molecular Sciences 26, no. 14: 6673. https://doi.org/10.3390/ijms26146673
APA StyleSakopoulos, S., & Todman, M. (2025). The Effects of Psychotherapy on Single and Repeated Ketamine Infusion(s) Therapy for Treatment-Resistant Depression: The Convergence of Molecular and Psychological Treatment. International Journal of Molecular Sciences, 26(14), 6673. https://doi.org/10.3390/ijms26146673





