Structural Characterization of Micronized Lignocellulose Date Pits as Affected by Water Sonication Followed by Alcoholic Fractionations
Abstract
1. Introduction
2. Result and Discussion
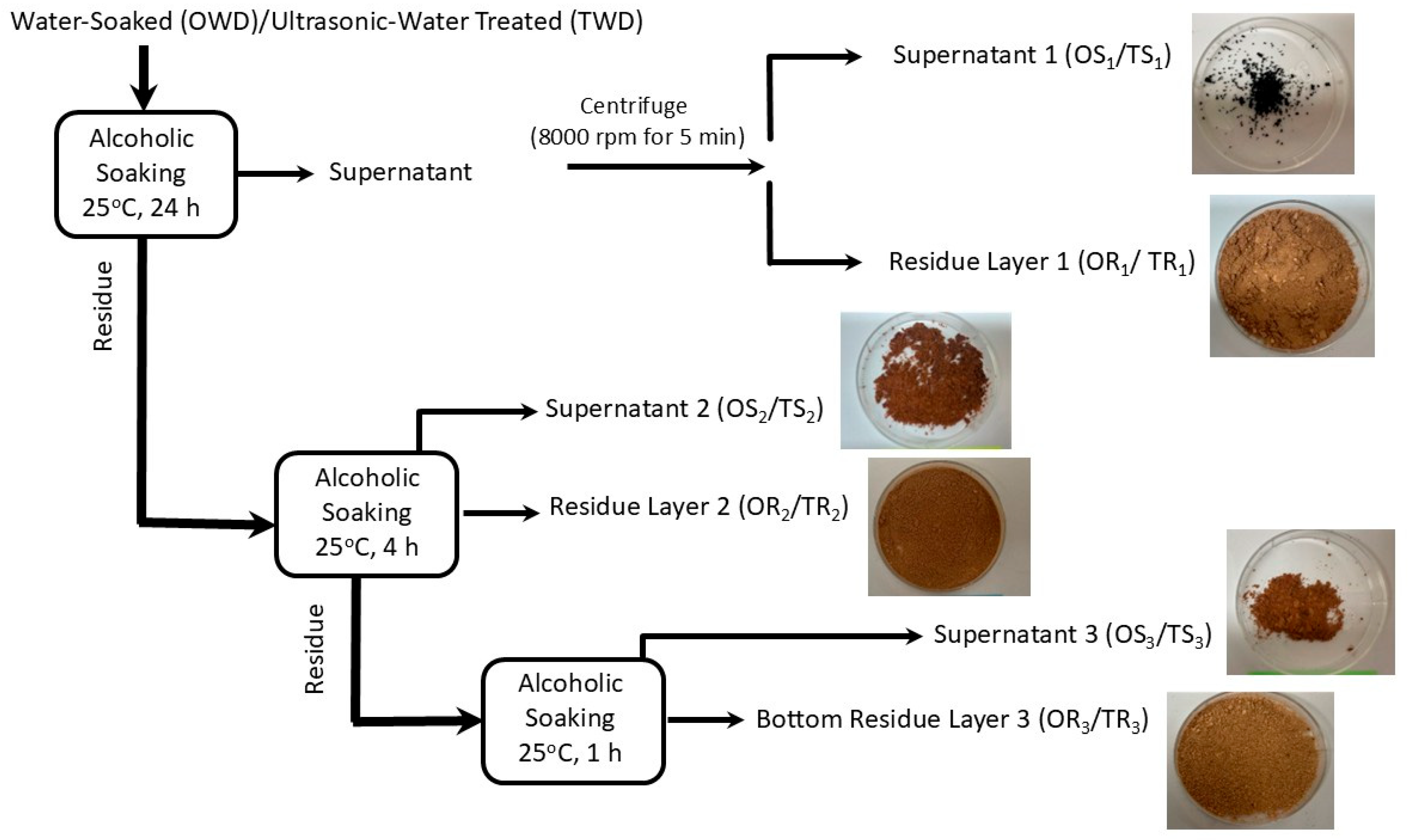
2.1. Fractionation Mechanisms
2.2. Particle Size Distribution
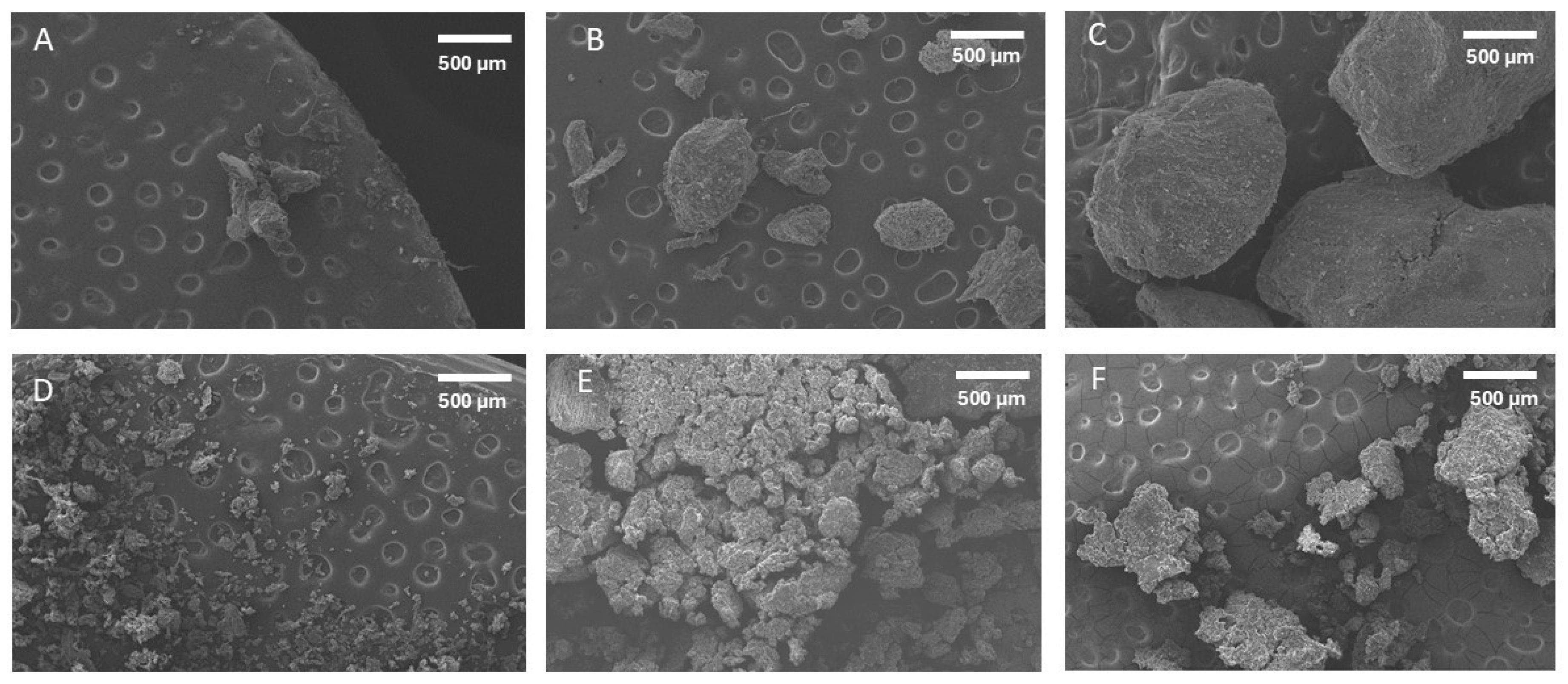
2.3. Scanning Electron Microscopy (SEM)
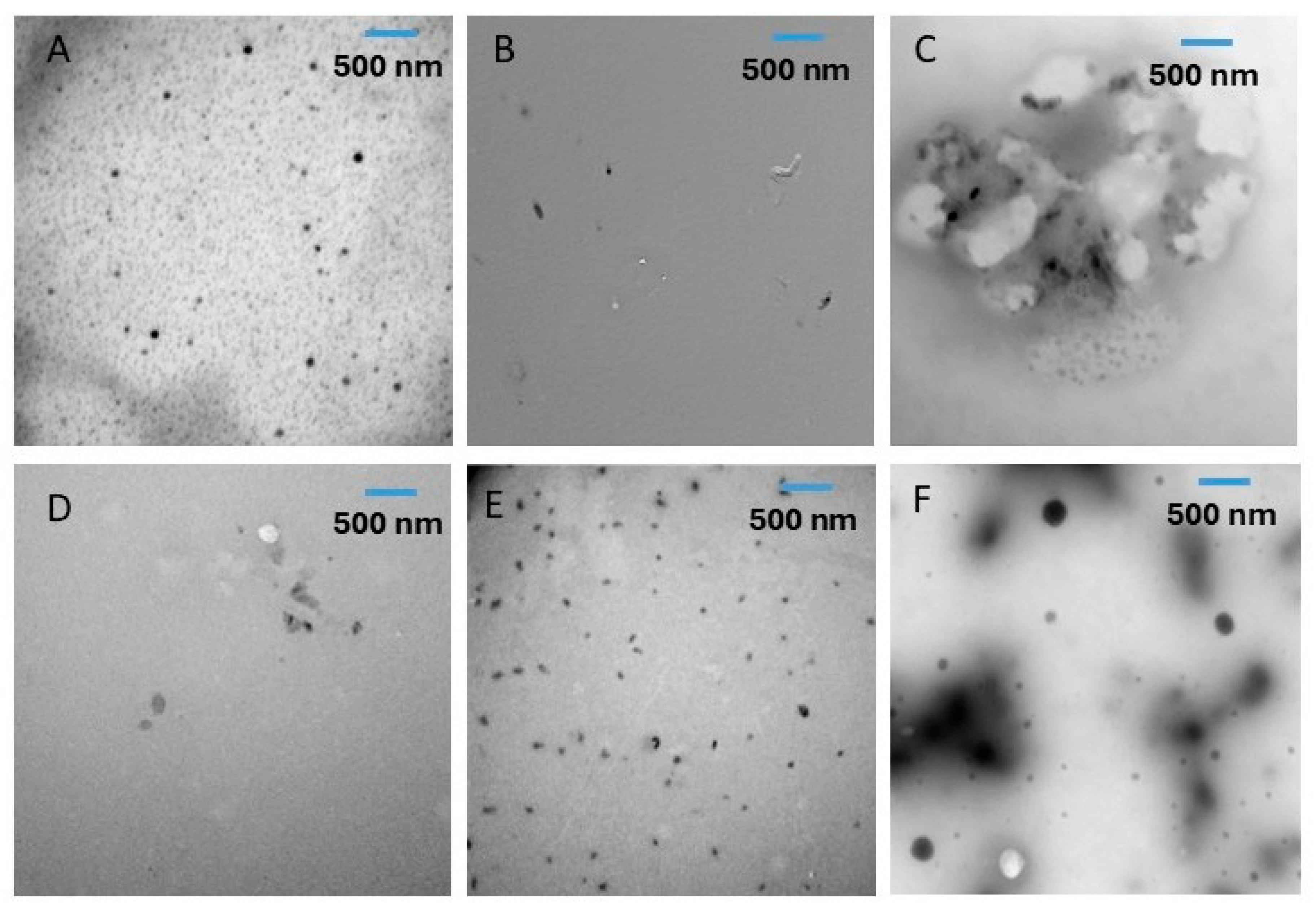
2.4. Transmission Electron Microscopy (TEM)
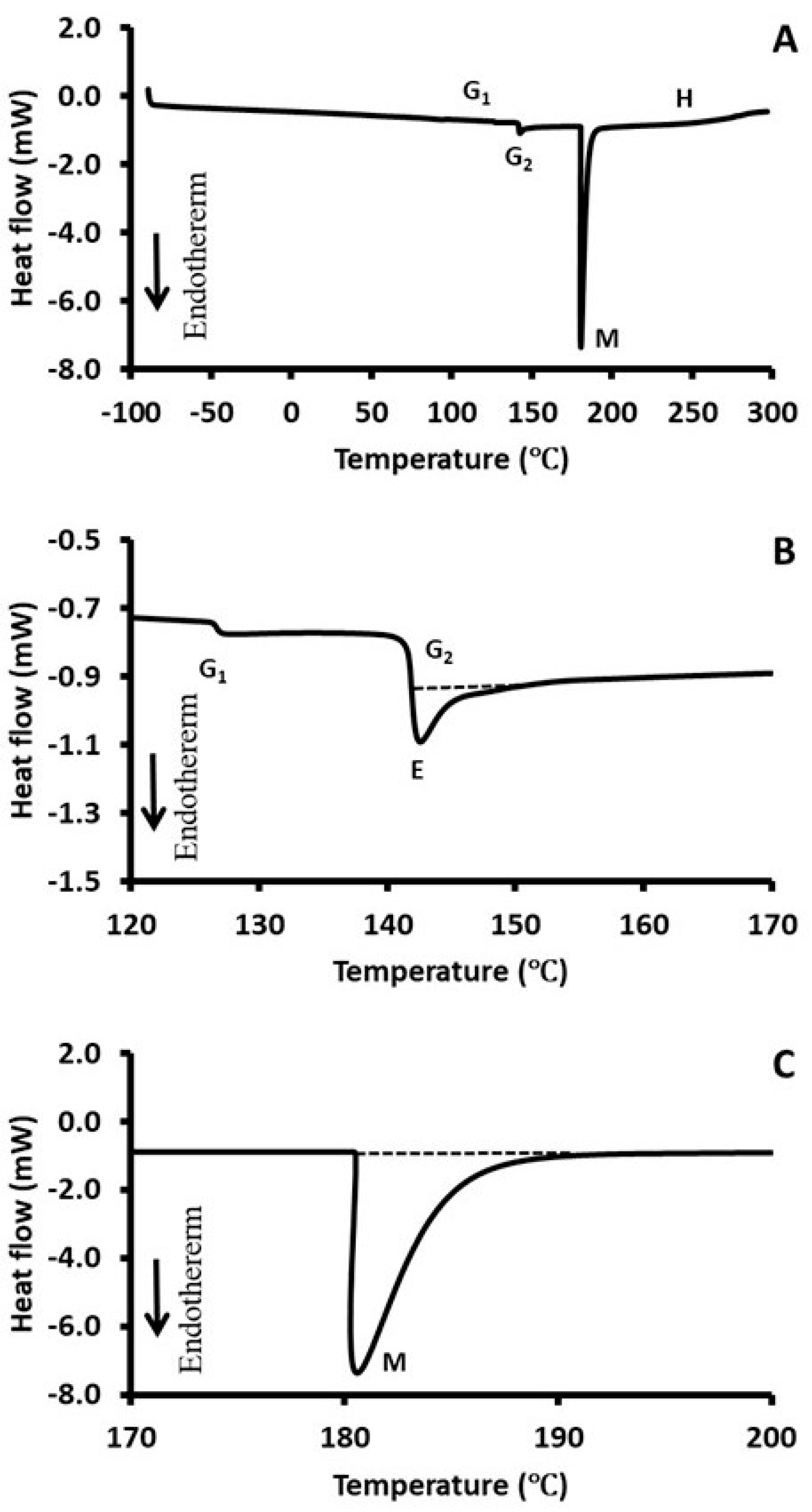
2.5. Differential Scanning Calorimetry (DSC)
2.6. Fourier Transform Infrared (FTIR) Analysis
3. Materials and Methods
3.1. Raw Materials
3.2. Extraction of Different Fractions
3.3. Measurement of Particle Size Distribution
3.4. Morphological Analysis by Scanning Electron Microscopy (SEM)
3.5. Morphological Analysis by Transmission Electron Microscopy (TEM)
3.6. Thermal Analysis by Differential Scanning Calorimetry (DSC)
3.7. Functional Group Measurement by Fourier Transform Infrared (FTIR) Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abushal, S.A.; Elhendy, H.A.; Abd, E.L.; Maged, E.M.; Darwish, A.M. Impact of ground Ajwa (Phoenix dactylifera L.) seeds fortification on physical and nutritional properties of functional cookies and chocolate sauce. Cereal Chem. 2021, 98, 958–967. [Google Scholar] [CrossRef]
- Al-Khalili, M.; Al-Habsi, N.; Rahman, M.S. Applications of date pits in foods to enhance their functionality and quality: A review. Front. Sustain. Food Syst. 2023, 6, 1101043. [Google Scholar] [CrossRef]
- Salem, E.M.; Almohmadi, N.; Al-Khataby, N.F. Utilization of Date seeds powder as antioxidant activities components in preparation of some baking products. J. Food Dairy Sci. 2011, 2, 399–409. [Google Scholar] [CrossRef]
- Zamzam, S.; Nafiea, E.R.; Al-Hadhromi, H.A.; Ali, F.A. Utilization of date-pits in the production of functional chocolates. In Proceedings of the Qatar Foundation Annual Research Conference Proceedings, Doha, Qatar, 19–20 March 2018. [Google Scholar]
- Ke, J.; Wang, X.; Gao, X.; Zhou, Y.; Wei, D.; Ma, Y.; Li, C.; Liu, Y.; Chen, Z. Ball milling improves physicochemical, functionality, and emulsification characteristics of insoluble dietary fiber from polygonatum sibiricum. Foods 2024, 13, 2323. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Zhang, X.; Devahastin, S. Bioactive dietary Fiber powder from asparagus leaf by-product: Effect of nlow-temperature ball milling on physico-chemical, functional and microstructural characteristics. Powder Technol. 2020, 366, 275–282. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Ramachandra Swamy, S.R.; Rastogi, N.K.; Raghavarao, K.S.M.S.; Kumar, S.; Tharanathan, R.N. Grinding characteristics and hydration properties of coconut residue: A source of dietary fibre. J. Food Eng. 2006, 72, 281–286. [Google Scholar] [CrossRef]
- Al-Khalili, M.; Al-Habsi, N.; Al-Alawi, A.; Al-Subhi, L.; Myint, M.T.Z.; Al-Abri, M.; Waly, M.I.; Al-Harthi, S.; Al-Mamun, A.; Rahman, M.S. Structural characteristics of alkaline treated fibers from date-pits: Residual and precipitated fibers at different pH. Bioact. Carbohydr. Diet. Fibre 2021, 25, 100251. [Google Scholar] [CrossRef]
- Al-Khalili, M.; Al-Habsi, N.; Al-Kindi, M.; Rahman, M.S. Characteristics of crystalline and amorphous fractions of date-pits as treated by alcohol-water pressure cooking. Bioact. Carbohydr. Diet. Fibre 2022, 28, 100331. [Google Scholar] [CrossRef]
- Campagnol, P.C.B.; dos Santos, B.A.; Wagner, R.; Terra, N.N.; Pollonio, M.A.R. Amorphous cellulose gel as a fat substitute in fermented sausages. Meat Sci. 2012, 90, 36–42. [Google Scholar] [CrossRef]
- El-Salhy, M.; Ystad, S.O.; Mazzawi, T.; Gundersen, D. Dietary fiber in irritable bowel syndrome. Int. J. Mol. Med. 2017, 40, 607–613. [Google Scholar] [CrossRef]
- Smith, C.E.; Tucker, K.L. Health benefits of cereal fibre: A review of clinical trials. Nutr. Res. Rev. 2011, 24, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.; Roberton, A.; Watson, M.; Triggs, C.; Harris, P. The effects of soluble-fibre polysaccharide on the adsorption of carcinogens to insoluble dietary. Chem.-Biol. Interact. 1995, 95, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Kruer-Zerhusen, N.; Cantero-Tubilla, B.; Wilson, D.B. Characterization of cellulose crystallinity after enzymatic treatment using Fourier transform infrared spectroscopy (FTIR). Cellulose 2018, 25, 37–48. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Chen, M.; Goff, H.D.; Zhong, F.; Sharif, H.R.; Li, Y. Functionality and nutritional aspects of microcrystalline cellulose in food. Carbohydr. Polym. 2017, 172, 159–174. [Google Scholar] [CrossRef] [PubMed]
- De Maesschalck, C.; Eeckhaut, V.; Maertens, L.; De Lange, L.; Marchal, L.; Daube, G.; Dewulf, J.; Haesebrouck, F.; Ducatelle, R.; Taminau, B.; et al. Amorphous cellulose feed supplement alters the broiler caecal microbiome. Poult. Sci. 2019, 98, 3811–3817. [Google Scholar] [CrossRef]
- Hu, R.; Li, S.; Diao, H.; Huang, C.; Yan, J.; Wei, X.; Zhou, M.; He, P.; Wang, T.; Fu, H.; et al. The interaction between dietary fiber and gut microbiota, and its effect on pig intestinal health. Front. Immunol. 2023, 14, 1095740. [Google Scholar] [CrossRef]
- Sakr, H.; Ammar, A.; Zaki, H.; Salama, M.A.; Ali, M. Impact of ball milling on physicochemical structural functional properties of Moringa oleifera, L. leaf powders. J. Food Meas. Charact. 2024, 18, 320–330. [Google Scholar] [CrossRef]
- Al-Mawali, M.; Al-Habsi, N.; Rahman, M.S. Thermal characteristics and proton mobility of date-pits and their alkaline treated fibers. Food Eng. Rev. 2020, 13, 236–246. [Google Scholar] [CrossRef]
- Nabili, A.; Fattoum, A.; Passas, R.; Elaloui, E. Extraction and characterization of cellulose from date palm seeds (Phoenix dactylifera L.). Cellul. Chem. Technol. 2016, 50, 1015–1023. [Google Scholar]
- Al-Mamari, A.; Al-Habsi, N.; Al-Khalili, M.; Rahman, M.S. Extraction and characterization of residue fibers from defatted date-pits after alkaline-acid digestion: Effects of different pretreatments. J. Therm. Anal. Calorim. 2022, 147, 9405–9416. [Google Scholar] [CrossRef]
- Yang, G.; Lin, W.; Lai, H.; Tong, J.; Lei, J.; Yuan, M.; Zhang, Y.; Cui, C. Understanding the relationship between particle size and ultrasonic treatment during the synthesis of metal nanoparticles. Ultrason. Sonochemistry 2021, 73, 105497. [Google Scholar] [CrossRef] [PubMed]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Mostafa, H.; Airouyuwa, J.O.; Maqsood, S. A novel strategy for producing nano-particles from date seeds and enhancing their phenolic content and antioxidant properties using ultrasound-assisted extraction: A multivariate based optimization study. Ultrason. Sonochemistry 2022, 87, 106017. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry- A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Aliyu, M.; Hepher, M.J. Effects of ultrasound energy on degradation of cellulose material. Ultrason. Sonochemistry 2000, 7, 265–268. [Google Scholar] [CrossRef]
- Velasco, M.I.; Correa, E.; Paris, F. Interaction between fibres in the transverse damage in composites. Eng. Fract. Mech. 2020, 239, 107273. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Panovic, I.; Deuss, P.J.; Barta, K.; Westwood, N.J. Pre-treatment of lignocellulosic feedstocks using biorenewable alcohols: Towards complete biomass valorisation. Green Chem. 2017, 19, 202–214. [Google Scholar] [CrossRef]
- Zijlstra, D.S.; Lahive, C.W.; Analbers, C.A.; Figueireedo, M.B.; Wang, Z.; Lancefield, C.S.; Deuss, P.J. Mild organosolv lignin extraction with alcohols: The importance of benzylic alkoxylation. ACS Sustain. Chem. Eng. 2020, 8, 5119–5131. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Paniwnyk, L.; Herceg, Z. Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J. Food Eng. 2014, 121, 15–23. [Google Scholar] [CrossRef]
- Bussemaker, M.J.; Xu, F.; Zhang, D. Manipulation of ultrasonic effects on lignocellulose by varying the frequency, particle size, loading and stirring. Bioresour. Technol. 2013, 148, 15–23. [Google Scholar] [CrossRef]
- Che, L.M.; Li, D.; Wang, L.J.; Chen, X.D.; Mao, Z.H. Micronization and hydrophobic modification of cassava starch. Int. J. Food Prop. 2007, 10, 527–536. [Google Scholar] [CrossRef]
- Wei, C.; Ge, Y.; Liu, D.; Zhao, S.; Wei, M.; Jiliu, J.; Hu, X.; Quan, Z.; Wu, Y.; Su, Y.; et al. Effects of high-temperature, high-pressure, and ultrasonic treatment on the physicochemical properties and structure of soluble dietary fibers of millet bran. Front. Nutr. 2022, 8, 820715. [Google Scholar] [CrossRef] [PubMed]
- Salim, R.M.; Asik, J.; Sarjadi, M.S. Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Ghodake, V.; Khare, R.; Mhaske, S. An insight into formation and characterization of nanocellulose prepared from industrial cellulosic wastes. J. Polym. Environ. 2021, 30, 319–332. [Google Scholar] [CrossRef]
- Larkin, P. IR and Raman spectra-structure correlations: Characteristic group frequencies. In Infrared and Raman Spectroscopy; Elsevier Science: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Li, W.W.; Wang, C.; Yuan, G.Q. Physicochemical characterisation and α-amylase inhibitory activity of tea polysaccharides under simulated salivary, gastric and intestinal conditions. Int. J. Food Sci. Technol. 2018, 53, 423–429. [Google Scholar] [CrossRef]
- Nada, A.; Kamel, S.; El-Sahkhawy, M. Thermal behavior and infrared spectroscopy of cellulose carbamates. Polym. Degrad. Stab. 2000, 70, 347–355. [Google Scholar] [CrossRef]
- Al-Areimi, M.; Al-Habsi, N.; Al-Jufaili, S.M.; Rahman, M.S. State Diagram of Freeze-dried Sardines (Sardinella longiceps, Valenciennes). J. Food Eng. 2024, 379, 112107. [Google Scholar] [CrossRef]
- Suresh, S.; Al-Habsi, N.; Guizani, N.; Rahman, M.S. Thermal characteristics and state diagram of freeze-dried broccoli: Freezing curve, maximal-freeze-concentration condition, glass line and solids-melting. Thermochimica Acta 2017, 655, 129–136. [Google Scholar] [CrossRef]
- Mittal, V.; Chaudhry, A.U.; Matsko, N.B. “True” biocomposites with biopolyesters and date seed powder: Mechanical, thermal, and degradation properties. J. Appl. Polym. Sci. 2014, 131, 40816. [Google Scholar] [CrossRef]
- Al-Harrasi, K.; Al-Habsi, N.; Myint, M.T.Z.; Rahman, M.S. Molecular and structural characterization of micronized lignocellulose from date-pits by alcoholic fractionations. Carbohydr. Polymer Technol. Applications. 2024, 7, 100451. [Google Scholar] [CrossRef]




| Particles Size in the Micro-Range (µm) | Particles Size in the Nano-Range (nm) | ||||
|---|---|---|---|---|---|
| Fiber | Fraction | Size Range | Average Size | Size Range | Average Size |
| OWD | OR1 | 15–137 | 89 ± 27 c | 44–98 | 73 ± 15 b |
| OR2 | 21–354 | 98 ± 49 c | 60–134 | 98 ± 20 b | |
| OR3 | 38–494 | 164 ± 106 b | 71–1335 | 235 ± 114 c | |
| OS1 | 5–57 | 22 ± 19 a | 48–76 | 66 ± 8 b | |
| OS2 | 7–122 | 59 ± 20 a | 55–86 | 73 ± 11 b | |
| OS3 | 28–219 | 63 ± 25 a | 78–196 | 139 ± 52 a | |
| p < 0.05 | p < 0.05 | p < 0.05 | |||
| TWD | TR1 | 8–79 | 39 ± 20 c | 13–85 | 42 ± 14 a |
| TR2 | 25–95 | 44 ± 15 c | 25–80 | 52 ± 9 d | |
| TR3 | 34–150 | 65 ± 25 b | 194–234 | 91 ± 45 c | |
| TS1 | 6–54 | 18 ± 9 a | 13–41 | 27 ± 7 a | |
| TS2 | 11–78 | 38 ± 19 c | 15–57 | 30 ± 10 a | |
| TS3 | 14–143 | 44 ± 34 c | 14–119 | 49 ± 28 d | |
| p < 0.05 | p < 0.05 | p < 0.05 | |||
| Sample | Fiber | Mass (%) |
|---|---|---|
| OWD | ≥250 | 85.58 |
| ≥125 | 13.94 | |
| ≥63 | 0.48 | |
| TWD | ≥250 | 84.95 |
| ≥125 | 13.61 | |
| ≥63 | 1.44 |
| First Glass Transition | Second Glass Transition | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Fraction | Tgi (°C) | Tgp (°C) | Tge (°C) | (∆Cp)1 (J/kg °C) | Tgi (°C) | Tgp (°C) | Tge (°C) | (∆Cp)2 J/kg (°C) |
| OWD | OR1 | 144 ± 9 f | 144 ± 9 f | 144 ± 9 f | 105 ± 7 b | 158 ± 5 d | 158 ± 5 d | 158 ± 5 d | 636 ± 8 c |
| OR2 | 129 ± 1 bc | 129 ± 1 bc | 129 ± 1 bc | 232 ± 1 e | 146 ± 3 abc | 146 ± 3 abc | 146 ± 3 abc | 696 ± 3 d | |
| OR3 | 133 ± 1 cd | 133 ± 1 cd | 134 ± 1 cd | 205 ± 8 e | 147 ± 2 abc | 147 ± 2 abc | 147 ± 2 abc | 705 ± 1 e | |
| OS1 | 129 ± 2 bc | 129 ± 2 bc | 129 ± 2 bc | 224 ± 6 f | 144 ± 3 ab | 144 ± 3 ab | 145 ± 3 ab | 928 ± 1 i | |
| OS2 | 139 ± 2 ef | 140 ± 1 ef | 140 ± 2 ef | 113 ± 8 b | 152 ± 8 bcd | 152 ± 8 bcd | 152 ± 8 bcd | 773 ± 2 h | |
| OS3 | 139 ± 2 ef | 139 ± 2 ef | 140 ± 2 ef | 112 ± 3 b | 153 ± 4 cd | 153 ± 4 cd | 153 ± 4 cd | 477 ± 4 b | |
| TWD | TR1 | N | N | N | N | 153 ± 9 cd | 153 ± 9 cd | 153 ± 9 cd | 64 ± 1 a |
| TR2 | 130 ± 1 bc | 130 ± 1 bc | 130 ± 1 bc | 178 ± 6 c | 145 ± 1 a | 145 ± 1 a | 145 ± 1 a | 736 ± 2 f | |
| TR3 | 131 ± 1 bcd | 131 ± 1 bc | 131 ± 1 bcd | 270 ± 1 g | 145 ± 1 abc | 145 ± 1 abc | 145 ± 1 abc | 762 ± 8 g | |
| TS1 | 126 ± 1 b | 127 ± 1 b | 128 ± 1 b | 242 ± 8 f | 141 ± 1 a | 141 ± 1 a | 141 ± 1 a | 926 ± 3 i | |
| TS2 | 136 ± 1 de | 136 ± 1 de | 137 ± 1 de | 195 ± 2 d | 152 ± 2 bcd | 152 ± 2 bcd | 152 ± 2 bcd | 1018 ± 4 j | |
| TS3 | 141 ± 2 f | 141 ± 2 ef | 142 ± 2 f | 170 ± 9 c | 150 ± 3 bcd | 150 ± 3 bcd | 151 ± 4 bcd | 1109 ± 8 k | |
| Solid Melting—Decomposition | |||||
|---|---|---|---|---|---|
| Sample | Fraction | Tmi (°C) | Tmp (°C) | Tme (°C) | ∆H (J/g) |
| OWD | OR1 | 190 ± 5 abc | 192 ± 5 bc | 219 ± 9 e | 174 ± 8 h |
| OR2 | 192 ± 1 bc | 193 ± 1 bc | 201 ± 8 abc | 87 ± 4 d | |
| OR3 | 192 ± 1 bc | 193 ± 1 bc | 206 ± 7 bcd | 73 ± 1 c | |
| OS1 | 187 ± 8 abc | 188 ± 7 abc | 196 ± 6 abc | 111 ± 3 f | |
| OS2 | 189 ± 3 abc | 193 ± 2 bc | 215 ± 4 de | 81 ± 5 cd | |
| OS3 | 185 ± 2 ab | 186 ± 1 ab | 192 ± 1 a | 137 ± 3 g | |
| TWD | TR1 | 184 ± 9 ab | 186 ± 8 ab | 199 ± 3 abc | 50 ± 5 a |
| TR2 | 181 ± 8 a | 182 ± 8 a | 196 ± 1 ab | 100 ± 3 e | |
| TR3 | 197 ± 5 c | 197 ± 5 c | 207 ± 4 cd | 86 ± 4 d | |
| TS1 | 186 ± 1 ab | 187 ± 1 ab | 199 ± 2 abc | 105 ± 1 ef | |
| TS2 | 191 ± 6 abc | 192 ± 5 bc | 202 ± 8 abc | 64 ± 3 b | |
| TS3 | 189 ± 3 abc | 191 ± 4 abc | 198 ± 5 abc | 100 ± 9 e | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Harrasi, K.; Al-Habsi, N.; Al-Kindi, M.A.; Shi, L.; Suleria, H.A.R.; Ashokkumar, M.; Rahman, M.S. Structural Characterization of Micronized Lignocellulose Date Pits as Affected by Water Sonication Followed by Alcoholic Fractionations. Int. J. Mol. Sci. 2025, 26, 6644. https://doi.org/10.3390/ijms26146644
Al-Harrasi K, Al-Habsi N, Al-Kindi MA, Shi L, Suleria HAR, Ashokkumar M, Rahman MS. Structural Characterization of Micronized Lignocellulose Date Pits as Affected by Water Sonication Followed by Alcoholic Fractionations. International Journal of Molecular Sciences. 2025; 26(14):6644. https://doi.org/10.3390/ijms26146644
Chicago/Turabian StyleAl-Harrasi, Khalid, Nasser Al-Habsi, Mohamed A. Al-Kindi, Linghong Shi, Hafiz A. R. Suleria, Muthupandian Ashokkumar, and Mohammad Shafiur Rahman. 2025. "Structural Characterization of Micronized Lignocellulose Date Pits as Affected by Water Sonication Followed by Alcoholic Fractionations" International Journal of Molecular Sciences 26, no. 14: 6644. https://doi.org/10.3390/ijms26146644
APA StyleAl-Harrasi, K., Al-Habsi, N., Al-Kindi, M. A., Shi, L., Suleria, H. A. R., Ashokkumar, M., & Rahman, M. S. (2025). Structural Characterization of Micronized Lignocellulose Date Pits as Affected by Water Sonication Followed by Alcoholic Fractionations. International Journal of Molecular Sciences, 26(14), 6644. https://doi.org/10.3390/ijms26146644








