Abstract
New psychoactive substances (NPSs) are emerging narcotics or psychotropics that pose a public health risk. The most commonly reported NPSs are synthetic cannabinoids and synthetic cathinones. Synthetic cannabinoids mimic the effects of Δ9-tetrahydrocannabinol (Δ9-THC), often with greater potency, while synthetic cathinones act as stimulants, frequently serving as cheaper alternatives to amphetamines, 3,4-methylenedioxymethamphetamine (MDMA) and cocaine. While some synthetic cannabinoids exhibit chirality depending on their synthesis precursors, synthetic cathinones are intrinsically chiral. Biotargets can recognize and differentiate between enantiomers, leading to distinct biological responses (enantioselectivity). Understanding these differences is crucial; therefore, the development of enantioresolution methods to assess the biological and toxicological effects of enantiomer is necessary. This work systematically compiles enantioselectivity studies and enantioresolution methods of synthetic cannabinoids and synthetic cathinones, following PRISMA guidelines. The main aim of this review is to explore the impact of chirality on these NPSs, improving our understanding of their toxicological behavior and evaluating advances in analytical techniques for their enantioseparation. Key examples from both groups are presented. This review highlights the importance of continuing research in this field, as demonstrated by the differing properties of synthetic cannabinoid and synthetic cathinone enantiomers, which are closely linked to variations in biological and toxicological outcomes.
1. Introduction
The European Union Drugs Agency (EUDA) defines a new psychoactive substance (NPS) as “a new narcotic or psychotropic in pure form or in preparation, that is not controlled by the United Nations drug conventions, but which may pose a public health threat comparable to that posed by substances listed in these conventions” [1]. NPSs are commonly known as synthetic or designer drugs. They represent an extensive and diverse group of substances, often analogs of already legally controlled drugs [2]. NPSs are generally marketed as “bath salts” or “plant food”, accompanied by the disclaimer “not for human consumption” to bypass the country’s drug legislation [3].
As of 2021, the EUDA was monitoring 880 NPSs, including 370 first reported in 2020 [4]. By the end of 2023, this number had risen to approximately 950, with 26 newly reported in Europe that year [5]. The rapid emergence of these new substances is unprecedented and alarming, with estimates suggesting that, at its peak, a new NPS appeared every week [2]. Figure 1 illustrates the number and categories of NPSs first reported between 2005 and 2023 [5].
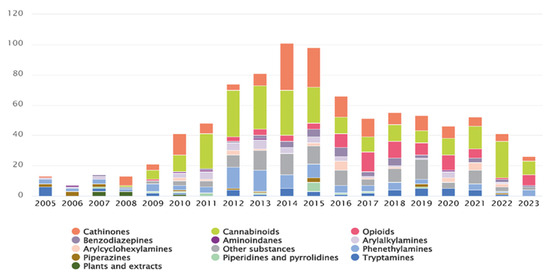
Figure 1.
Number and categories of new psychoactive substances reported for the first time from 2005 to 2023 [5].
The popularity of these compounds has grown mainly due to their psychoactive effects, namely their stimulatory, hallucinogenic and depressant effects, and because they are easily available online. However, many NPSs remain unstudied, and data on their toxicological and pharmacological properties is limited. Additionally, the composition of NPSs purchased online may differ from what is stated on the packaging, leading users to unknowingly consume unlisted substances. These factors emphasize the significant public health risks associated with NPS use [3,6]. According to EUDA, NPSs are classified into many categories, based on their chemical structure (Figure 1) [5]. Among these, synthetic cannabinoids and synthetic cathinones are the most commonly reported, accounting for more than two-thirds of all available NPSs [7].
Synthetic cannabinoids first emerged in the 1960s and the 1970s when researchers began studying the endocannabinoid system’s role in biological functions [8,9,10]. They are a chemically and structurally diverse group of NPSs designed to target the endocannabinoid system. These substances exhibit a higher affinity for cannabinoid receptors (CBRs) than Δ9-tetrahydrocannabinol (Δ9-THC, Figure 2a), the main psychoactive compound in cannabis, resulting in Δ9-THC-like effects that can be more potent and vary in duration (either longer or shorter depending on the specific substance) [10,11]. To classify these substances, the EUDA proposed a model that identifies the names of the key structural components of each molecule: a “core”, a “linker”, a “linked group” and a “tail” (Figure 2b) [12]. Depending on the chemical nature of the linked group, some synthetic cannabinoids can be chiral, meaning they exist as two enantiomers [13]. Since the detection of JWH-018 (Figure 2c) in the drug market in 2008 [8,14], synthetic cannabinoids have dominated the NPS market, particularly between 2008 and 2013, as well as in recent years (2020–2023) [15].
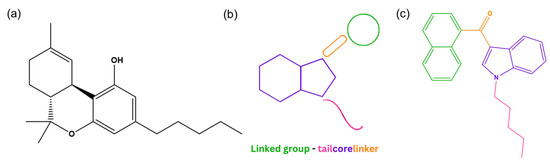
Figure 2.
Structure of Δ9-THC (a), structural model of synthetic cannabinoids (b), and structure of JWH-018 (c). (ChemDraw® Professional 18.0).
On the other hand, synthetic cathinones are a group of compounds derived from cathinone (Figure 3a), a natural alkaloid found in Catha edulis leaves, commonly known as khat. Structurally, cathinone resembles amphetamine (Figure 3b) [7], with the key difference being the presence of a ketone group at the β-position of the amino alkyl chain attached to the phenyl ring [16].
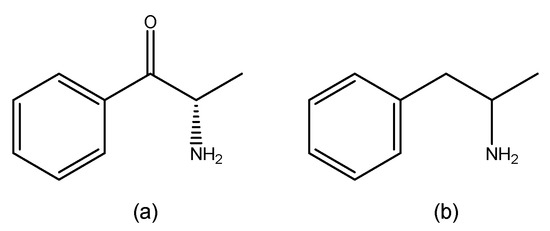
Figure 3.
Structures of cathinone (a) and amphetamine (b) (ChemDraw® Professional 18.0).
This chemically diverse group of NPSs is widely abused for its psychostimulant effects, similar to those of cocaine or 3,4-methylenedioxymethamphetamine (MDMA) [17,18]. Their simple molecular structure and high variability facilitate the development of new analogs with unknown pharmacological and toxicological properties. This allows them to evade legal restrictions, making them more dangerous, as reports of fatal intoxications continue to rise [19]. All synthetic cathinones are chiral molecules. Since biotargets are intrinsically chiral, they can recognize and differentiate between enantiomers, leading to distinct biological responses (enantioselectivity). As a result, each enantiomer may exhibit different biological and/or toxicological properties [20]; therefore, enantioresolution methods are essential for assessing their individual impacts [21].
The relationship between chirality and biological activity has gained increasing importance since Louis Pasteur’s [22] first observation of biological enantioselectivity and the tragic thalidomide incident [23,24]. Chirality can now be considered one of the major topics in many research fields, including the medical and toxicological sciences [20,25,26]. Furthermore, in the past few decades, numerous analytical and preparative methods for enantioresolution have been developed to facilitate the analysis and isolation of single enantiomers [27,28]. This work compiles enantioselectivity and enantioseparation studies on both synthetic cannabinoids and synthetic cathinones. To the best of our knowledge, no other review exists for synthetic cannabinoids. On the other hand, reviews on synthetic cathinones (published in 2018 and 2022) indicate a growing research focus in this area [7,29]. Therefore, an update on recent developments, particularly from the past three years, is necessary to track this progression. This review aims to explore the impact of chirality in both groups of NPSs by examining reports on the differing effects of their enantiomers across various biological systems and evaluating advances in analytical techniques for their enantioseparation.
The key novelty of this review lies in it being the first to analyze the impact of chirality on synthetic cannabinoids, thereby enhancing our understanding of their toxicological behavior, as stereochemical features are often closely linked to variations in biological and toxicological outcomes.
2. Literature Research Methodology (PRISMA Guidelines)
This research followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [30]. Research on the literature was conducted in the PubMed and SCOPUS databases to identify studies on enantioselectivity and enantioseparation methods for synthetic cannabinoids. The search used the following keywords: “Synthetic cannabinoids AND chiral OR enantioresolution OR enantioseparation OR enantioselectivity OR enantiomer OR stereochemical OR stereochemistry”. Study selection took place in February 2025, with the earliest identified study dating back to 1989. Only English-language papers were considered. A total of 156 records were identified and screened for duplicates. In the first screening phase, 78 records were assessed by title and abstract to exclude irrelevant studies and review papers. In the second phase, a full-text review of 65 records was conducted, further eliminating non-relevant studies. Additionally, studies identified through other methods, such as citation searching, were included. The final selected records are categorized into enantioselectivity studies, enantioseparation studies or both.
Similarly, to identify relevant studies on enantioselectivity and enantioseparation methods for synthetic cathinones, a literature search was conducted in the PubMed and SCOPUS databases using the following keywords: “Synthetic cathinones AND chiral OR enantioresolution OR enantioseparation OR enantioselectivity OR enantiomer OR stereochemical OR stereochemistry”. The study selection took place in February 2025. Since this research is an update of previous review papers [7,29], only studies published between 2022 and February 2025 were included. Only English-language papers were considered. A total of 94 records were identified and screened for duplicates. In the first screening phase, 57 records were assessed by title and abstract to exclude irrelevant studies and review papers. In the second phase, a full-text review of 48 records was conducted, with further exclusions of non-relevant studies. Additionally, studies identified through other methods, such as citation searching, were included. The final selected records were categorized into enantioselectivity studies, enantioseparation studies, or both.
Screening at both the title/abstract and full-text stages and data collection were initially performed by two reviewers. All screening and data collection outcomes were subsequently discussed and reviewed by additional reviewers. No automation tools were applied. The collected data were critically and impartially analyzed in accordance with PRISMA guidelines. Most of the authors have previous experience in PRISMA analysis [31,32,33,34], along with topics of research such as NPSs [35,36,37], enantioselectivity studies [38,39,40] and chiral separations [17,41,42].
This review focused on enantioselectivity and enantioseparation studies of synthetic cathinones and synthetic cannabinoids. Therefore, the inclusion criteria were original research articles on enantioselectivity studies (biological/toxicological assays) and enantioseparation methods using synthetic cannabinoids or synthetic cathinones. The exclusion criteria included reviews, theoretical/computational studies, articles focusing on non-chiral molecules and studies lacking either enantioselectivity or enantioresolutiona. For enantioselectivity studies, the primary outcomes were the distinct effects of enantiomers in various biological systems. For enantioseparation methods, relevant experimental conditions such as analytes, samples, methods, and analytical conditions were noted. If studies reported inconsistent outcomes or incomplete outcomes, data were selected based on their relevance to the review’s objective and consistency with other results.
Given the heterogeneity in study designs and outcome metrics, we performed a descriptive synthesis. To summarize the enantioselectivity results for both NPS groups, the main findings from each study were highlighted. For synthetic cannabinoids, studies on the same class of compounds were grouped together. Additionally, the structure of some compounds was generated using ChemDraw 18.0.
For the enantioseparation studies, a table was created to present the analytes, samples, methods, and analytical conditions relevant for each approach. Bar charts illustrating the distribution of analytical techniques, chiral selectors, and detection methods used in the enantioseparation studies of synthetic cathinones and cannabinoids were created using GraphPad Prism 9. No statistical or meta-analysis was conducted on the data.
The main findings of the enantioseparation methods were analyzed and discussed according to various topics: technique, chiral selector, detection method, and sample type. For synthetic cannabinoids, the most prevalent classes of cannabinoids in the studies were also discussed. For synthetic cathinones, a comparison with previous reviews was made to provide a more comprehensive analysis of enantioseparation methods. Additionally, a comparison between the enantioseparation methods for synthetic cannabinoids and synthetic cathinones was conducted, with representative examples from the selected studies described. No meta-analysis was performed.
Although a formal risk of bias assessment was not conducted, we acknowledge the potential for bias in the reviewed studies due to variations in study design, sample selection, data collection methods, and statistical analyses. Furthermore, the possibility of publication bias, where studies with significant or positive results are more likely to be published, is also recognized as a limitation.
To minimize potential biases, several steps were taken. The literature search was conducted using multiple databases (PubMed and SCOPUS), and citation searches were included to ensure comprehensive coverage of the relevant studies. Only original research articles were considered, while review articles were excluded to avoid duplication of findings.
3. Results and Discussion
3.1. Synthetic Cannabinoids
3.1.1. PRISMA Search
The PRISMA approach was conducted according to the flowchart presented in Figure 4. In the screening phase, some articles that seemed to meet the inclusion criteria had to be excluded since they were not relevant for the aims of this review. For instance, Huffman et al. [43] performed a synthesis of both enantiomers of nabilone. This article appeared in our search through the keywords “synthetic cannabinoids AND enantiomer”; however, it was out of the scope of this review since it was not an enantioselectivity or enantioseparation study. Moreover, Feinstein et al. [44] evaluated the effects of vitamin K1 treatment on plasma concentrations after poisoning with long-acting anticoagulant rodenticide enantiomers contaminated with synthetic cannabinoids. Although this is an enantioselectivity study and related to synthetic cannabinoids, the focus of the study is on the enantiomers of the long-acting anticoagulant rodenticides and not the enantiomers of synthetic cannabinoids; therefore, this article was excluded from the research. In the end, 48 scientific articles were selected for further discussion, 36 corresponding to enantioselectivity studies, 9 to enantioseparation studies and 3 including both enantioselectivity and enantioseparation studies.
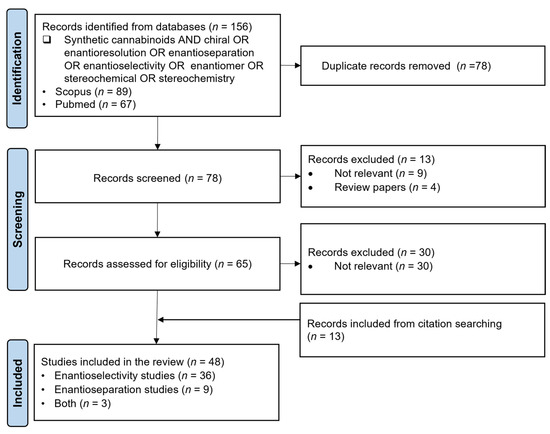
Figure 4.
Flow diagram of the literature search based on PRISMA guidelines (n = number of scientific articles; time frame: 1989–February 2025; database: SCOPUS and Pubmed).
3.1.2. Enantioseparation
Although minor variations in the chiral profiles of synthetic cannabinoids have been reported, the limited number of tested samples has hindered the identification of discriminatory differences between synthesis supplies and/or final products. The rapid emergence of new CBR agonists underscore the need for quick adaptation of enantioseparation methods. Understanding enantioseparation mechanisms enables this adaptation. Reliable separation methods allow for better pharmacological and analytical assessment [13]. Therefore, chiral profiling and enantioseparation are crucial for assessing enantioselectivity [7,45] and more accurately evaluating risks to develop harm reduction strategies [13].
To our knowledge, no review has been published on the techniques developed over the years for the chiral separation of synthetic cannabinoids. This information is compiled in Table 1.

Table 1.
Analytical methods for the enantioresolution of synthetic cannabinoids.
Liquid chromatography (LC), particularly high-performance liquid chromatography (HPLC), has been the predominant method for the enantioseparation of synthetic cannabinoids (Figure 5a). LC is now a standard technique for enantiomeric separations [57], offering a wide variety of chiral stationary phases (CSPs) [58] and compatibility with various detection methods, including fluorescence, ultra-violet (UV), and mass spectrometry (MS) [57].
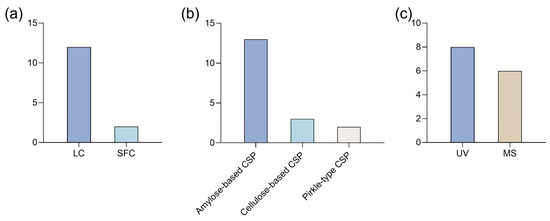
Figure 5.
Bar charts regarding (a) type of technique, (b) chiral stationary phases (CSPs) and (c) detection methods used for enantioseparation of synthetic cannabinoids. LC: liquid chromatography; MS: mass spectrometry; SFC: super-critical fluid chromatography; UV: ultra-violet.
Beyond LC techniques, two studies have explored super critical fluid chromatography (SFC), a hybrid of gas chromatography (GC) and LC that enables faster separations. However, its complexity and high cost present limitations [57]. For instance, Breitenback et al. [51] investigated the potential of an ultra-high performance SFC (UHPSFC) method for analyzing synthetic cannabinoids in samples of seized drugs. This technique demonstrated high precision and strong resolving power in separating positional isomers and diastereomers of synthetic cannabinoids [51].
According to our research, other techniques that are often applied in enantioseparation studies such as GC and capillary electrophoresis (CE) were not selected for the enantioseparation of synthetic cannabinoids.
All the studies used CSPs, indicating a preference for direct methods. The success of efficient enantioseparation largely depends on the chiral discriminative ability of CSP [59]. Polysaccharide-based CSPs were the most used, with amylose derivatives being the predominant one (Figure 5b). Indeed, polysaccharide-based CSPs are widely regarded as the most effective and commonly used CSPs for enantioseparation, both in analytical and preparative contexts [60]. This widespread use can be attributed to several factors, including the strong chiral recognition capabilities of polysaccharide derivatives and their natural abundance. For example, Antonides et al. [13] performed enantioseparation of four indazole-3-carboxamide synthetic cannabinoids using polysaccharide-based CSPs. After testing several columns, they found that Lux® Amylose-1 provided the highest selectivity for compounds with a terminal methyl ester moiety, while Lux® i-Cellulose-5 was more effective for those with a terminal amide moiety [13].
Only one study employed a different class of CSPs, specifically Pirkle-type CSPs. This class of CSPs, in which the chiral selector is a small molecule covalently bound to the chromatographic support via a spacer, is also broadly applied for enantioseparations [61]. Varfaj et al. [46] used the (R,R)-Whelk-O® 1 and (S,S)-Whelk-O® 1 columns to achieve the separation of enantiomers of synthetic cannabinoids found in seized samples.
Another key factor in the enantioseparation process is the mobile phase, which significantly affects the retention enantiomers, enantioselectivity, and resolution [62]. Both Pirkle-type and polysaccharide-based CSPs can be employed in normal-phase, reversed-phase, polar organic, and polar ionic conditions due to their compatibility with a wide range of solvents [58]. Although these CSPs support various elution modes, as shown in Table 1, normal-phase and reversed-phase conditions were the most commonly used for the enantioseparation of this class of NPSs. In particular, for normal-phase separations, mixtures of hexane and 2-propanol proved to be the most effective in achieving enantioseparation. For the reversed-phase mode, mobile phases typically consisted of water and acetonitrile, with or without an ionization suppressor such as formic acid.
Detection techniques for synthetic cannabinoids vary, with MS and UV detection being the most commonly used (Figure 5c). MS detection offers higher sensitivity and specificity [63], which makes it especially useful to identify enantiomers in complex samples, such as herbal material [13,49,52] and biological matrices like urine [53]. In some cases, both detection methods have been combined. For example, Antonides et al. [13] initially applied an HPLC method with a photodiode array (PDA) detector to test the enantioseparation of several synthetic cannabinoids using polysaccharide-based CSPs. Then, they conducted further analysis using HPLC coupled with PDA and quadrupole time of flight MS (HPLC-PDA-MS/MS) to determine the enantiopurity of the samples. The results revealed that the (S)-enantiomer predominated in all samples [13].
The carboxamide moiety and the JWH family of compounds were the most commonly studied cannabinoids for enantioseparation. While most analyzed samples consisted of herbal material or powders, Patton et al. [53] developed a chiral approach using LC-MS/MS to investigate the chiral metabolites of JWH-018 and AM2201 in human urine. Their aim was to evaluate enantiospecific excretion patterns and gain a better understanding of the pharmacokinetic properties of these two synthetic cannabinoids.
Although this review focuses on the enantioseparation of racemates, referred to as the “racemic approach”, to obtain individual enantiomers, an alternative method, known as the “chiral approach”, can also be used. This approach involves enantioselective synthesis to directly produce the enantiomer [64,65] and has been reported in some enantioselectivity studies with synthetic cannabinoids [50,66,67]. Nonetheless, even after enantioselective synthesis, enantioseparation methods remain valuable for assessing the enantiomeric purity of the compounds. For example, Doi et al. [50] synthesized enantiomers of several carboxamide-type synthetic cannabinoids to evaluate their activities as CB1R/CB2Rr agonists and evaluated their enantiomeric purity using an LC-MS method.
3.1.3. Enantioselectivity Studies
Synthetic cannabinoids are widely studied [8,10], and several reports on enantioselectivity for specific groups can be found.
Aminoalkylindoles are the most prevalent class of synthetic cannabinoids found in herbal products [68]. Enantioselectivity studies are crucial for understanding the duration of effects caused by this widely consumed class of substances. The aminoalkylindole derivative WIN 55,212-2 is a potent CBR agonist [69]. Numerous in vivo and in vitro studies have been conducted considering its enantioselective effects on biological activity [70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87]. The chemical structures of R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3 are represented in Figure 6, summarizing and emphasizing the main enantioselective effects observed in rats in in vivo studies [70,71,74,75,76,81,82,83,84,85].
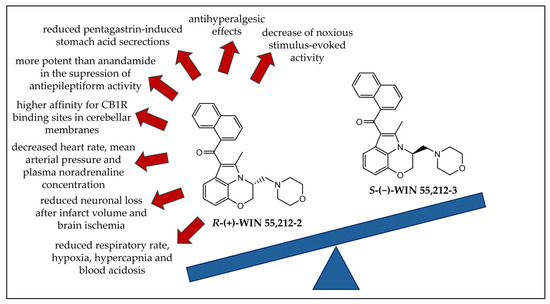
Figure 6.
Chemical structures of R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3 and their enantioselective effects observed in rats. CB1R: cannabinoid receptor 1.
All the effects reported were observed only for R-(+)-WIN 55,212-2, while the enantiomer S-(−)-WIN 55,212-3 showed little or no effect. Since S-(−)-WIN 55,212-3 displays no pharmacological activity, these results suggest that some effects might be associated with CBRs.
Apart from these studies using rats as the in vivo model, Rawls et al. [86] performed an in vivo study using planarian to compare the effects of R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3. The results showed that planarians display abstinence-induced withdrawal after exposure to R-(+)-WIN 55,212-2, but not after exposure to S-(−)-WIN 55,212-3.
Several in vitro studies were also reported for this compound, the results of which are summarized in Table 2 [72,73,77,78,79,80,87].

Table 2.
Summary of the results from in vitro studies using R-(+)-WIN 55,212-2 and S-(−)-WIN 55,212-3.
Similarly to in vivo studies, in vitro studies showed stereospecificity for R-(+)-WIN 55,212-2. Nonetheless, Price et al. [78] and Scholl et al. [80] reported in vitro studies where no enantioselectivity was observed for the enantiomers.
There are other notable examples of enantioselectivity involving the aminoalkylindole class of synthetic cannabinoids. For instance, Willis et al. [88] synthesized a novel series of aminoalkylindoles, and, through a structure–activity study, identified the racemic ligand with highest affinity for the CB1R, which was then labeled with [18F] (Figure 7a,b). They demonstrated that one enantiomer specifically binds to the CBR, while the inactive enantiomer may be useful for assessing the non-displaceable binding of the active enantiomer.
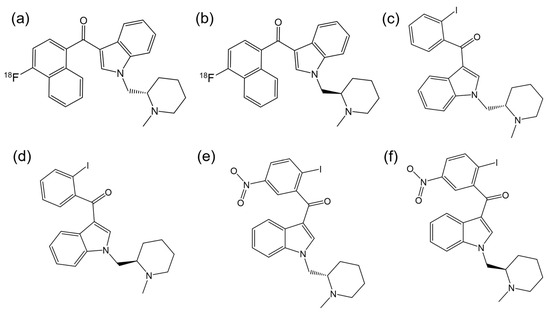
Figure 7.
Chemical structures of (a) S-enantiomer of 3-(4-fluoronaphthoyl)-1-(N-methylpiperidin-2-ylmeth-yl)indole; (b) R-enantiomer of 3-(4-fluoronaphthoyl)-1-(N-methylpiperidin-2-ylmeth-yl)indole; (c) S-AM2233; (d) R-AM2233; (e) S-AM1241; (f) R-AM1241. (ChemDraw® Professional 18.0.).
Deng et al. [89] synthesized a CB1Ragonist, AM2233 (Figure 7c,d). Experiments with radio-iodinated versions of the enantiomers and mouse hippocampal membranes showed that R-enantiomer had the highest affinity for CB1R. Little specific binding with the S-enantiomer and no specific binding with the R-enantiomer were observed in mouse brains lacking CB1R.
Bingham et al. [90] conducted cAMP inhibition assays to assess the antinociceptive efficacy of AM1241 (Figure 7e,f). This compound acts as an agonist for the human CB2R, with the R-enantiomer exhibiting greater affinity than the S-enantiomer. However, in pain models, the S-enantiomer was more effective as analgesic than either R-AM1241 or the racemate, with its in vivo effect mediated by CB2R. Rahn et al. [91] evaluated both racemic and enantiomeric forms of AM1241 for CB2R mediated antinociceptive effects. While R-AM1241 produced stronger antinociception at both higher and lower doses compared to S-AM1241 and racemic AM1241, it was found that these effects were not dependent on opioid receptors.
Some enantioselectivity studies have also been performed with HU-210 [92,93,94,95,96] (Figure 8a) and CP-55,940 [54,93,97] (Figure 8b).
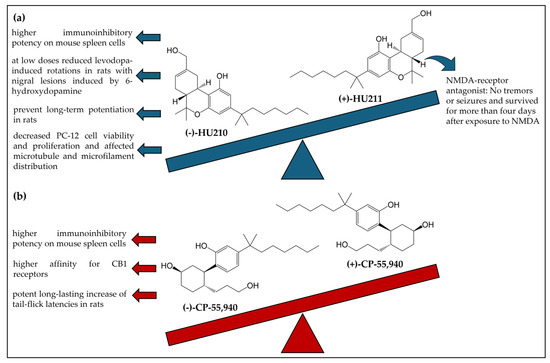
Figure 8.
Chemical structures and enantiospecific effects of the enantiomers HU-210 (a) and CP-55,940 (b). CB1R: cannabinoid receptor 1; NMDA: N-methyl-D-aspartate.
Generally, the studies showed that the (−)-enantiomer of both compounds displayed most of the effects while the (+)-enantiomers were less active or inactive. In only one study, the (+)-enantiomer of HU-210, designed in the literature as HU-211, displayed stronger effects. Feigenaum et al. [92] investigated the activity of HU-211. Mice treated with HU-211 showed no tremors or seizures and survived for more than four days after exposure to N-methyl-D-aspartate (NMDA). In contrast, HU-210 produced a sedative effect in mice but no significant impact on NMDA actions [92].
In recent years, carboxamide-type compounds have become more prevalent [13]. These substances were first developed by Pfizer and patented as potential analgesics; however, the patent only includes S-enantiomers due to the precursors (L-leucinamide, L-tert-leucine methyl ester and L-valinamide) [13,50,98]. The pharmacological activity of the R-enantiomers remains unknown [99]. Several studies evaluated the effect of chirality on the affinity and potency of various carboxamide-type synthetic cannabinoids to CB1R and CB2R [13,50,66,100,101,102,103,104,105]. The enantioselective results from these studies are summarized in Table 3.

Table 3.
Summary of enantioselective results from studies related to the interaction of carboxamide-type synthetic cannabinoids with cannabinoid receptors (CB1R and CB2R).
The results showed that S-enantiomers exhibited greater potency and higher affinity for CB1R compared to R-enantiomers [13,50,66,101,102] with the exception of one study where the R-enantiomer showed greater activity [104]. In contrast, the effects on CB2R were more variable across studies. In studies by Antonides et al. [13] and Ametovsky et al. [101], S-enantiomers were also found to be more potent. Conversely, Baraldi et al. [100] and Stern et al. [104] reported stronger effects for the R-enantiomers. In a study by Doi et al. [50], three R-enantiomers and two S-enantiomers showed greater potency for CB2R.
Apart from the studies related to the interaction of carboxamide-type synthetic cannabinoids with CBRs, Brandon et al. [106] studied the structure–metabolism relationship and physicochemical parameters of twelve indazole and indole-3-carboxamide-type synthetic cannabinoids (AB-CHMINACA, AB-FUBINACA, AMB-FUBINACA, 5F-AMB-PINACA, AMB-CHMICA, AMB-4en-PICA, MDMB-4en-PINACA, 5F-MDMB-PINACA, 5F-MDMB-PICA, MDMB-4en-PICA, 4F-MDMB-BINACA, and MDMB-FUBINACA). In both cell models used, pooled cryopreserved human hepatocytes (pHHeps) and pooled human liver microsomes (pHLM), R-enantiomers were cleared at a slower rate than S-enantiomers. An exception was observed in the cell line pHLM for compounds containing an alkene tail group, including MDMB-4en-PICA, MDMB-4en-PINACA and AMB-4en-PICA.
3.2. Synthetic Cathinones
3.2.1. PRISMA Search
The PRISMA approach was conducted according to the flowchart presented in Figure 9. In the screening phase, some articles that seemed to meet the inclusion criteria had to be excluded since they were not relevant for the aims of this review. For instance, Lawtrakul and Toochinda [107] evaluated the potential of methylated β-cyclodextrin derivatives for the enantiorecognition of butylone. Moreover, Holowinski and Dybowski [108] studied the interactions of 3- and 4-chloromethcathinone with plasma proteins. In this report, the binding modes of the enantiomers of both synthetic cathinones in binding sites of human serum albumin (HSA) were analyzed using molecular docking and molecular dynamics studies [108]. Although both articles appear to be relevant for this research, they were excluded since they are theoretical analyses and therefore out of the scope of this review. Nonetheless, they highlight the potential of molecular docking as a predictive tool for both enantioselectivity and enantioseparation studies with synthetic cathinones. In total, 26 scientific articles were selected for further discussion, 1 focused on enantioselectivity study, 20 focused on enantioseparation and 1 addressing both enantioselectivity and enantioseparation.
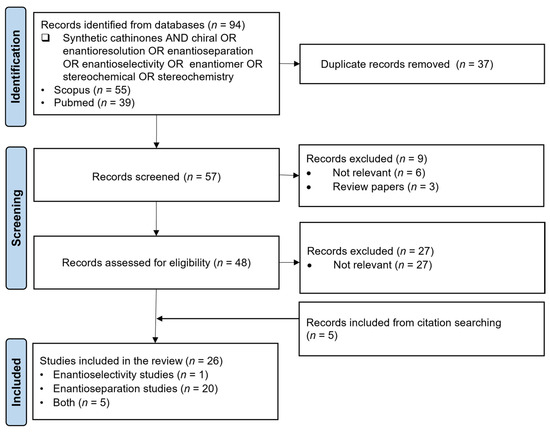
Figure 9.
Flow diagram of the literature search based on PRISMA guidelines (n = number of scientific articles; time frame: 2022–February 2025; database: SCOPUS and Pubmed).
3.2.2. Enantioseparation (Update 2021–2025)
Enantioseparation methods for synthetic cathinones were previously reviewed by Silva et al. [29] up until 2018 and later updated by Almeida et al. [7], covering publications through to 2022. Table 4 provides an updated review of enantioseparation methods for this class of NPSs, spanning from 2022 to 2025.

Table 4.
Analytical methods for enantioseparation of synthetic cathinones.
A wide variety of methods can be used for enantioseparation, and they are generally classified into direct and indirect methods. In indirect methods, enantiomers are derivatized to form diastereomers, which are then separated using conventional separation techniques. In contrast, direct methods perform the separation in a chiral environment, using a chiral selector [65,129,130].
In this updated compilation, all studies, except for two that used an indirect method with GC (Figure 10a), employed direct enantioseparation methods based on LC, CE, or SFC techniques. In GC, indirect methods are more common. Despite its high efficiency and sensitivity, GC requires derivatization, and its high operating temperatures can lead to racemization or even decomposition of the analytes [57].
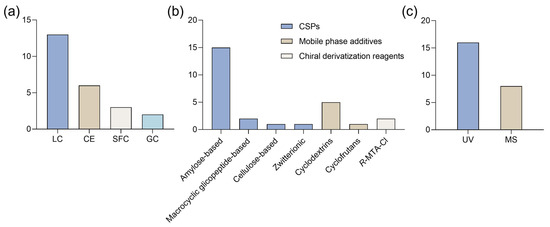
Figure 10.
Bar charts regarding (a) type of technique, (b) chiral selectors/resolution agents, and (c) detection methods used for enantioseparation of synthetic cathinones. CE: capillary electrophoresis; CSPs: chiral stationary phases; GC: gas chromatography; LC: liquid chromatography; MS: mass spectrometry; R-MTPA-Cl: R-(−)-α-Methoxy-α-(trifluoromethyl)phenylacetyl chloride; SFC: super-critical fluid chromatography; UV: ultra-violet.
HPLC was the most frequently used technique for the enantioseparation of synthetic cathinones, showing a clear preference over other methods (Figure 10a). For example, Seibert et al. [119] tested an HPLC method for the enantioseparation of cathinone derivatives. Under the initial normal-phase conditions, 75 out of 80 compounds were successfully separated, while the remaining compounds achieved partial separation after optimization. Furthermore, using polar organic conditions, 63 compounds were enantioseparated with short retention times [119].
Beyond HPLC, three studies used SFC, while six used CE, a direct method based on capillary electromigration techniques that rely on electrophoretic phenomena to move the analyte [130]. In CE, a chiral selector, such as cyclodextrins, is typically added to the running buffer, forming a “pseudophase” that facilitates separation [131].
Most studies used polysaccharide-based CSPs, particularly amylose derivatives (Figure 10b). Additionally, one study applied an LC method with two macrocyclic glycopeptides-based CSPs, while another study used an SFC method with a zwitterionic CSP.
For five CE methods, cyclodextrin derivatives were added as chiral selectors in the mobile phase, while the remaining study used a cyclofructan derivative. In the two GC-MS methods, (R)-(−)-α-methoxy-α-(trifluoromethyl) phenylacetyl chloride (R-MTPA-Cl) was selected as the chiral derivatization reagent. Regarding detection methods, UV and MS detection were the most commonly used, with UV being preferred over MS (Figure 10c). However, both detection methods were also applied complementarily. For instance, in developing an enantioselective LC method to monitor MDPV in ecotoxicity assays, Pérez-Pereira et al. [115] tested amylose- and cellulose-based CSP, as well as various mobile phases, using ultra-fast liquid chromatography (UFLC) with a UV detector. The optimized conditions, including reverse-phase mode with an amylose CSP, were then applied to an LC-MS/MS system [115].
Regarding the type of samples analyzed, most of the synthetic cathinones were analyzed in powder form as hydrochloride salts. Nevertheless, Pérez-Alcaraz et al. [126] developed a CE-MS method for the enantiodetermination of MDPV in urine samples, while Langa et al. [125] applied a GC-MS method to analyze several chiral psychoactive substances, including synthetic cathinones, in effluents and river surface water samples. Additionally, Pérez-Pereira et al. [115] developed an LC-MS method to monitor the racemization of MDPV enantiomers in culture media from Daphnia magna ecotoxicity assays. These studies underscore the importance of enantioresolution methods for determining enantiomeric ratios in biological samples. Such analyses are crucial for assessing the prevalence of each enantiomer, enabling more accurate risk assessments, and detecting potential racemization to ensure the reliability of enantioselective results.
Additionally, it is important to infer that sample preparation is a critical step in analytical studies, particularly when working with chiral compounds, as improper handling can lead to racemization and degradation, ultimately compromising analytical results. In most of the studies, NPSs were analyzed in powder form and required no prior treatment beyond dissolution in a suitable solvent. However, some studies reported the need for additional sample preparation steps before analysis. Several extraction techniques such as liquid–liquid extraction, solid-phase extraction and microextraction are commonly applied. After extraction, samples are concentrated and reconstituted in an appropriate solvent that ensures compatibility with the analytical technique employed. It is crucial that these steps are optimized to maintain sample integrity and improve detection and quantification limits [132].
Compared to our previous reviews [7,29], which summarized enantioseparation studies on synthetic cathinones, the current findings confirm that the trend of using LC methods and polysaccharide-based CSPs remains strong.
When compared to the studies on synthetic cannabinoids, the literature on the enantioseparation of synthetic cathinones is significantly more advanced, with a greater number of studies available. This discrepancy can be attributed to several factors, the most obvious being the number of chiral compounds in each class of NPSs. While all synthetic cathinones are chiral, many groups of synthetic cannabinoids lack chirality, resulting in a lower number of chiral compounds available for study. Additionally, synthetic cathinones are small molecules with similar structures within each other. On the other hand, synthetic cannabinoids are bigger and more complex molecules that are divided into distinct groups with structural variabilities. Thus, while one enantioseparation method could be easily applied and adapted to several synthetic cathinones, the development of techniques for synthetic cannabinoids is more complex.
Some enantioselectivity studies on synthetic cannabinoids chose the “chiral approach” to synthesize enantiomerically pure compounds, rather than enantioseparating the racemates [50,66,67]. This may be due to the good availability of chiral precursors, such as amino acid derivatives, in their enantiomerically pure form. Nonetheless, these precursors are not always enantiomerically pure, which can result in enantiomeric mixtures with minor percentages of the opposite enantiomer. In such cases, as previously mentioned, enantioseparation methods could also be helpful for determining the enantiomeric purity of compounds.
3.2.3. Enantioselectivity Studies (Update 2021-February of 2025)
Similarly to synthetic cannabinoids, synthetic cathinones have been extensively studied; however, research on enantioselectivity remains limited. Previously, our group reviewed studies on the enantioselective biological and toxicological activity of this NPS group up until 2018 [29]. We later published an updated review focusing on enantioselectivity research in synthetic cathinones [7]. The following section provides a literature review of enantioselectivity studies on synthetic cathinones conducted over the past three years (2021 to February of 2025).
Carvalho et al. [120] assessed the ecotoxicity of the enantiomers of butylone (Figure 11a) on Daphnia magna. In terms of body size, heart size, and area, R-butylone caused an increase in body size in juveniles at concentrations of 0.10 and 1 µg/L. In contrast, S-butylone led to a decrease in heart size and area in juveniles at 1 µg/L, suggesting that both enantiomers can selectively affect critical life stages of Daphnia in an enantioselective manner. Regarding swimming behavior, S-butylone increased the total swimming distance at 0.10 µg/L while R-butylone showed no effect [120].
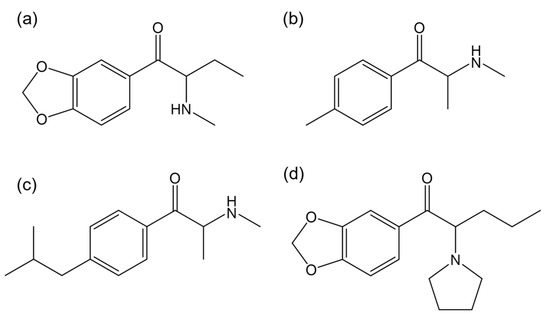
Figure 11.
Chemical structures of the synthetic cathinones: (a) butylone; (b) mephedrone; (c) 4-isobutylmethcathinone; and (d) 3,4-methylenedioxypyrovalerone (MDPV). (ChemDraw® Professional 18.0.).
A study by Czerwinska et al. [127] demonstrated that both R-(+)- and S-(−)-mephedrone presented similar kinetics; however, the R-(+)-enantiomer had a longer half-life, a higher maximum concentration, and a greater area under the curve. Therefore, the chiral nature and its associated enantiomeric purity must be considered when interpreting toxicological results. The chemical structure of mephedrone is shown in Figure 11b.
Paškan et al. [123] synthesized a new synthetic cathinone, 4-isobutylmethcathinone (Figure 11c). Subsequent in vitro studies in human neuroblastoma cells (SH-SY5Y), human microglial clone-3 cells (HMC-3), human liver cancer cells (hHep G2) and human cells derived from the urinary bladder (5637) cell lines showed no enantioselectivity in toxicity mechanisms. No enantioselectivity was observed in receptor binding studies either.
Pérez-Pereira et al. [115] evaluated the potential racemization of MDPV (Figure 11d) enantiomers in ecotoxicity assays. The results showed partial racemization of both enantiomers in chronic assays with Daphnia magna, with a higher extent of racemization for S-MDPV.
Almeida et al. [38] conducted an in vitro study with MDPV using the human colorectal adenocarcinoma (Caco-2) cell line. It was found that both enantiomers were highly permeable across the monolayer, and enantioselectivity in passage velocity was observed from the basolateral to apical direction. In another study by the same group [17], MDPV enantiomers exhibited cytotoxicity in a concentration-dependent manner. Additionally, no effects were observed on the expression of brain-derived neurotrophic factor and cyclin-dependent kinase 5 proteins in the SH-SY5Y cell line.
In the past three years, most studies on this topic have focused on the enantioseparation of synthetic cathinones. Although considerably less research has addressed the enantioselectivity of their biological and toxicological effects, it is important to highlight that this area of research is critically important. Given the prominence of synthetic cathinones among NPSs and their high abuse potential, failure to account for such differences between enantiomers can lead to underestimating the risk posed by specific enantiomers when assessing health hazards. Enantioselective toxicological data are crucial for accurately evaluating the potential harm associated with the use of these substances. This is particularly relevant given the prevalence of synthetic cathinones in recreational drug markets and their association with severe adverse effects, including neurotoxicity, cardiotoxicity, and acute behavioral disturbances [16]. Additionally, enantioselective data is critical for regulatory authorities aiming to implement effective control measures. Current regulations generally address racemic mixtures or the most common enantiomer; the lack of detailed toxicity data on individual enantiomers can lead to less accurate regulatory outcomes. Furthermore, the emergence of synthetic cathinones often involves minor structural modifications, including stereochemistry alterations, to evade legal restrictions [133]. Comprehensive enantioselective toxicological profiles would therefore support more robust and anticipatory regulatory frameworks.
In summary, while advances in analytical methods for enantioseparation are crucial, they must be complemented by studies on the enantioselective toxicology of synthetic cathinones to ensure a holistic understanding of their health risks and to inform evidence-based regulatory responses. Figure 12 provides a summary of the key points covered in this review.
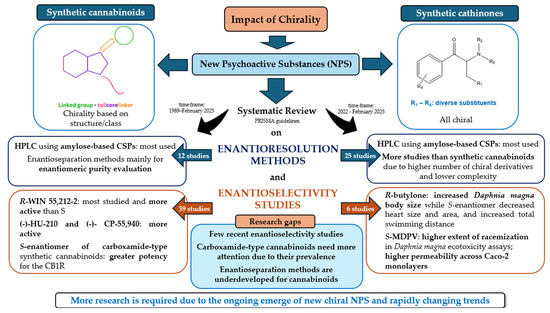
Figure 12.
Diagram to illustrate the key points addressed in this review.
4. Conclusions
Although several enantioselectivity studies on synthetic cannabinoids have been conducted over time, the number of recent publications remains limited. Nonetheless, some studies have demonstrated that enantiomers can exhibit different properties. Continuing this line of research is crucial, as these differences in properties are closely linked to variations in pharmacological and toxicological outcomes. In particular, further investigation into the enantioselectivity of carboxamide-type synthetic cannabinoids is necessary due to their high prevalence on the NPS market. Additionally, our review found that enantioseparation studies involving synthetic cannabinoids are still relatively scarce.
Synthetic cathinones, on the other hand, have received more extensive recent attention regarding enantioselectivity and the development of enantioresolution methods compared to synthetic cannabinoids. Consequently, continued literature reviews in this area are essential to support future research and regulatory efforts.
The development of enantioresolution methods is closely linked to enantioselectivity studies, as it allows for the isolation of single enantiomers with high enantiomeric purity for enantioselective studies. Additionally, it enables the determination of whether a substance being sold is a racemate, a mixture of enantiomers, or a single enantiomer. Moreover, enantioselective analytical methods are highly valuable for the simultaneous analysis of both enantiomers in biological activity assays, allowing for the evaluation of enantioselective effects.
HPLC using CSPs, coupled with various detection methods, is the preferred technique for the enantioseparation of both substance groups. This systematic review, while following PRISMA guidelines, has limitations: it lacks a formal assessment of bias and may suffer from publication bias, potentially skewing the findings. Also, variations in study methods, samples, and conditions make direct comparisons difficult. Furthermore, the rapid emergence of NPSs means that the results might quickly become outdated.
This review highlights the clinical importance of understanding enantioselectivity in chiral narcotics or psychotropics, more specifically synthetic cannabinoids and synthetic cathinones. By presenting examples where one enantiomer exhibits markedly different toxicological profiles compared to its counterpart, the article emphasizes the need for enantiomer-specific research, monitoring, and harm reduction strategies. Recognizing these differences can support more accurate risk assessments, inform public health policies, and ultimately contribute to the better protection of individuals vulnerable to the toxic effects of these NPSs.
Future research should focus on the biological activity of individual enantiomers of NPSs to improve risk assessment and regulation, as their composition affects toxicity. Potential racemization could complicate results, highlighting the need for better methods to separate and identify NPS enantiomers in biological samples. Thus, more research and continuous literature review are necessary to better understand these evolving chiral NPSs.
Author Contributions
Conceptualization: C.F. and F.R.; data collection and analysis: R.M.G.S. and A.S.A.; writing—original draft preparation: R.M.G.S. and A.S.A.; writing—reviewing and editing: F.R., P.G.d.P. and C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by national funds by FCT (UIDB/04423/2020, UIDB/MULTI/04378/2020, UIDP/04423/2020, LA/P/0140/2020 projects). A.S.A. acknowledges her PhD grant provided by FCT (project reference 2023.00262.BD and DOI identifier https://doi.org/10.54499/2023.00262.BD). P.G.d.P. is supported by a research contract (under Scientific Employment Stimulus) CEECINST/00108/2021/CP2794/CT0001.
Institutional Review Board Statement
This review was not registered. No review protocol was prepared.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- New Psychoactive Substances (NPS). www.emcdda.europa.eu. Available online: https://www.emcdda.europa.eu/topics/nps_en#library (accessed on 10 June 2023).
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New Psychoactive Substances: A Review and Updates. Ther. Adv. Psychopharmacol. 2020, 10, 204512532096719. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Andrzejczak, D. Next Generation of Novel Psychoactive Substances on the Horizon—A Complex Problem to Face. Drug Alcohol Depend. 2015, 157, 1–17. [Google Scholar] [CrossRef]
- European Drug Report: Trends and Developments. 2022. Available online: https://www.euda.europa.eu/publications/edr/trends-developments/2022_en (accessed on 10 June 2023).
- EMCDDA New Psychoactive Substances—The Current Situation in Europe (European Drug Report 2024). Available online: https://www.euda.europa.eu/publications/european-drug-report/2024/new-psychoactive-substances_en (accessed on 11 June 2024).
- Coppola, M.; Mondola, R.; Oliva, F.; Picci, R.L.; Ascheri, D.; Trivelli, F. Treating the Phenomenon of New Psychoactive Substances. In Neuropathology of Drug Addictions and Substance Misuse; Elsevier: Amsterdam, The Netherlands, 2016; pp. 679–686. ISBN 978-0-12-800213-1. [Google Scholar]
- Almeida, A.S.; Silva, B.; de Pinho, P.G.; Remião, F.; Fernandes, C. Synthetic Cathinones: Recent Developments, Enantioselectivity Studies and Enantioseparation Methods. Molecules 2022, 27, 2057. [Google Scholar] [CrossRef]
- Alves, V.L.; Gonçalves, J.L.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. The Synthetic Cannabinoids Phenomenon: From Structure to Toxicological Properties. A Review. Crit. Rev. Toxicol. 2020, 50, 359–382. [Google Scholar] [CrossRef]
- Le Boisselier, R.; Alexandre, J.; Lelong-Boulouard, V.; Debruyne, D. Focus on Cannabinoids and Synthetic Cannabinoids. Clin. Pharmacol. Ther. 2017, 101, 220–229. [Google Scholar] [CrossRef]
- Roque-Bravo, R.; Silva, R.S.; Malheiro, R.F.; Carmo, H.; Carvalho, F.; Da Silva, D.D.; Silva, J.P. Synthetic Cannabinoids: A Pharmacological and Toxicological Overview. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 187–209. [Google Scholar] [CrossRef]
- Silva, J.P.; Carvalho, F. The Therapeutic Use of Cannabis and Cannabinoids. Rev. Esp. Drogodepend 2022, 47, 123–141. [Google Scholar] [CrossRef]
- Pulver, B.; Fischmann, S.; Gallegos, A.; Christie, R. EMCDDA Framework and Practical Guidance for Naming Synthetic Cannabinoids. Drug Test. Anal. 2023, 15, 255–276. [Google Scholar] [CrossRef]
- Antonides, L.H.; Cannaert, A.; Norman, C.; Vives, L.; Harrison, A.; Costello, A.; Nic Daeid, N.; Stove, C.P.; Sutcliffe, O.B.; McKenzie, C. Enantiospecific Synthesis, Chiral Separation, and Biological Activity of Four Indazole-3-Carboxamide-Type Synthetic Cannabinoid Receptor Agonists and Their Detection in Seized Drug Samples. Front. Chem. 2019, 7, 321. [Google Scholar] [CrossRef]
- Synthetic Cannabinoids in Europe (Perspectives on Drugs). www.emcdda.europa.eu. Available online: https://www.emcdda.europa.eu/publications/pods/synthetic-cannabinoids_en (accessed on 26 September 2023).
- New Psychoactive Substances—The Current Situation in Europe (European Drug Report 2023). Available online: https://www.emcdda.europa.eu/publications/european-drug-report/2023/new-psychoactive-substances_en (accessed on 30 August 2023).
- Valente, M.J.; Guedes de Pinho, P.; de Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and Synthetic Cathinones: A Review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef]
- Almeida, A.S.; Silva, B.; Silva, J.P.; Pereira, J.A.; Remião, F.; Fernandes, C. Semi-Preparative Separation, Absolute Configuration, Stereochemical Stability and Effects on Human Neuronal Cells of MDPV Enantiomers. Molecules 2023, 28, 2121. [Google Scholar] [CrossRef]
- Baumann, M.H.; Partilla, J.S.; Lehner, K.R.; Thorndike, E.B.; Hoffman, A.F.; Holy, M.; Rothman, R.B.; Goldberg, S.R.; Lupica, C.R.; Sitte, H.H.; et al. Powerful Cocaine-Like Actions of 3,4-Methylenedioxypyrovalerone (MDPV), a Principal Constituent of Psychoactive ‘Bath Salts’ Products. Neuropsychopharmacology 2013, 38, 552–562. [Google Scholar] [CrossRef]
- Kuropka, P.; Zawadzki, M.; Szpot, P. A Review of Synthetic Cathinones Emerging in Recent Years (2019–2022). Forensic Toxicol. 2023, 41, 25–46. [Google Scholar] [CrossRef]
- Coelho, M.M.; Fernandes, C.; Remião, F.; Tiritan, M.E. Enantioselectivity in Drug Pharmacokinetics and Toxicity: Pharmacological Relevance and Analytical Methods. Molecules 2021, 26, 3113. [Google Scholar] [CrossRef]
- Roberts, J.B.; Colyer, C.L. Enantioselective Separation of Synthetic Cathinones by Capillary Electrophoresis with Ionic Liquid and Cyclodextrin Buffer Co-Additives. Separations 2023, 10, 417. [Google Scholar] [CrossRef]
- Pasteur, L. Memoires Sur La Relation Qui Peut Exister Entre La Forme Crystalline et la Composition Chimique, et Sur La Cause de La Polarization Rotatoire. Compt. Rend. 1848, 26, 535–538. [Google Scholar]
- Blaschke, G.; Kraft, H.P.; Fickentscher, K.; Köhler, F. Chromatographic separation of racemic thalidomide and teratogenic activity of its enantiomers (author’s transl). Arzneimittelforschung 1979, 29, 1640–1642. [Google Scholar]
- Franks, M.E.; Macpherson, G.R.; Figg, W.D. Thalidomide. Lancet 2004, 363, 1802–1811. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, L.; Kang, S.; Liew, R.K.; Lichtfouse, E. Toxicity and Environmental Fate of the Less Toxic Chiral Neonicotinoid Pesticides: A Review. Environ. Chem. Lett. 2025, 23, 733–750. [Google Scholar] [CrossRef]
- Senkuttuvan, N.; Komarasamy, B.; Krishnamoorthy, R.; Sarkar, S.; Dhanasekaran, S.; Anaikutti, P. The Significance of Chirality in Contemporary Drug Discovery-a Mini Review. RSC Adv. 2024, 14, 33429–33448. [Google Scholar] [CrossRef]
- Pinto, M.M.M.; Fernandes, C.; Tiritan, M.E. Chiral Separations in Preparative Scale: A Medicinal Chemistry Point of View. Molecules 2020, 25, 1931. [Google Scholar] [CrossRef]
- Ribeiro, A.R.L.; Maia, A.S.; Ribeiro, C.; Tiritan, M.E. Analysis of Chiral Drugs in Environmental Matrices: Current Knowledge and Trends in Environmental, Biodegradation and Forensic Fields. TrAC Trends Anal. Chem. 2020, 124, 115783. [Google Scholar] [CrossRef]
- Silva, B.; Fernandes, C.; Guedes De Pinho, P.; Remião, F. Chiral Resolution and Enantioselectivity of Synthetic Cathinones: A Brief Review. J. Anal. Toxicol. 2018, 42, 17–24. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Almeida, A.S.; Pinho, P.G.; Remião, F.; Fernandes, C. Uncovering the Metabolic Footprint of New Psychoactive Substances by Metabolomics: A Systematic Review. Molecules 2025, 30, 290. [Google Scholar] [CrossRef]
- Ochoa-Leite, C.; Rodrigues, S.; Ramos, A.S.; Ribeiro, F.; Barbosa, J.; Jerónimo, C.; de Pinho, P.G.; Dinis-Oliveira, R.J.; Costa, J.T. Metabolomics and Proteomics in Occupational Medicine: A Comprehensive Systematic Review. J. Occup. Med. Toxicol. 2024, 19, 38. [Google Scholar] [CrossRef]
- Amaro, F.; Carvalho, M.; de Lourdes Bastos, M.; Guedes de Pinho, P.; Pinto, J. Metabolic Signature Biomarkers for Predicting the Recurrence of Urological Cancers. Clin. Chim. Acta 2023, 549, 117553. [Google Scholar] [CrossRef]
- Almeida, A.S.; Guedes de Pinho, P.; Remião, F.; Fernandes, C. Metabolomics as a Tool for Unraveling the Impact of Enantioselectivity in Cellular Metabolism. Crit. Rev. Anal. Chem. 2025, 1–21. [Google Scholar] [CrossRef]
- Santos, R.M.G.; Lima, R.; Cravo, S.; Fernandes, P.A.; Remião, F.; Fernandes, C. Binding Affinity of Synthetic Cannabinoids to Human Serum Albumin: Site Characterization and Interaction Insights. Pharmaceuticals 2025, 18, 581. [Google Scholar] [CrossRef]
- Sofia Almeida, A.; Cardoso, T.; Cravo, S.; Elizabeth Tiritan, M.; Remião, F.; Fernandes, C. Binding Studies of Synthetic Cathinones to Human Serum Albumin by High-Performance Affinity Chromatography. J. Chromatogr. B 2023, 1227, 123836. [Google Scholar] [CrossRef]
- Araújo, A.M.; Carvalho, M.; Bastos, M.L.; Carvalho, F.; de Pinho, P.G. Metabolic Signature of Methylone in Primary Mouse Hepatocytes, at Subtoxic Concentrations. Arch. Toxicol. 2019, 93, 3277–3290. [Google Scholar] [CrossRef]
- Almeida, A.S.; Silva, B.; Remião, F.; Fernandes, C. Assessment of the Permeability of 3,4-Methylenedioxypyrovalerone (MDPV) across the Caco-2 Monolayer for Estimation of Intestinal Absorption and Enantioselectivity. Int. J. Mol. Sci. 2023, 24, 2680. [Google Scholar] [CrossRef]
- Coelho, M.M.; Silva, B.; Fernandes, C.; Remião, F.; Tiritan, M.E. Enantiomeric Profile of Promethazine in Metabolic Studies in Liver Microsomes. J. Chromatogr. Open 2024, 6, 100145. [Google Scholar] [CrossRef]
- Silva, B.; Palmeira, A.; Silva, R.; Fernandes, C.; Guedes de Pinho, P.; Remião, F. S-(+)-Pentedrone and R-(+)-Methylone as the Most Oxidative and Cytotoxic Enantiomers to Dopaminergic SH-SY5Y Cells: Role of MRP1 and P-Gp in Cathinones Enantioselectivity. Toxicol. Appl. Pharmacol. 2021, 416, 115442. [Google Scholar] [CrossRef]
- Fernandes, C.; Brandão, P.; Santos, A.; Tiritan, M.E.; Afonso, C.; Cass, Q.B.; Pinto, M.M. Resolution and Determination of Enantiomeric Purity of New Chiral Derivatives of Xanthones Using Polysaccharide-Based Stationary Phases. J. Chromatogr. A 2012, 1269, 143–153. [Google Scholar] [CrossRef]
- Fernandes, C.; Lima, R.; Pinto, M.M.M.; Tiritan, M.E. Chromatographic Supports for Enantioselective Liquid Chromatography: Evolution and Innovative Trends. J. Chromatogr. A 2022, 1684, 463555. [Google Scholar] [CrossRef]
- Huffman, J.W.; Joyner, H.H.; Lee, M.D.; Jordan, R.D.; Pennington, W.T. Synthesis of Both Enantiomers of Nabilone from a Common Intermediate. Enantiodivergent Synthesis of Cannabinoids. J. Org. Chem. 1991, 56, 2081–2086. [Google Scholar] [CrossRef]
- Feinstein, D.L.; Nosal, D.G.; Ramanathan, S.; Zhou, J.; Chen, L.; Hershow, R.C.; van Breemen, R.B.; Wright, E.; Hafner, J.W.; Rubinstein, I. Effects of Vitamin K1 Treatment on Plasma Concentrations of Long-Acting Anticoagulant Rodenticide Enantiomers Following Inhalation of Contaminated Synthetic Cannabinoids. Clin. Toxicol. 2020, 58, 716–724. [Google Scholar] [CrossRef]
- Schmid, M.G.; Hägele, J.S. Separation of Enantiomers and Positional Isomers of Novel Psychoactive Substances in Solid Samples by Chromatographic and Electrophoretic Techniques—A Selective Review. J. Chromatogr. A 2020, 1624, 461256. [Google Scholar] [CrossRef]
- Varfaj, I.; Protti, M.; Di Michele, A.; Gonzalez-Rodriguez, J.; Carotti, A.; Sardella, R.; Mercolini, L. Chromatographic Enantioresolution and Stereochemical Characterization of Synthetic Cannabinoid Receptor Agonists with Whelk-O®1 Chiral Stationary Phases under Mass Spectrometry Compatible Reversed-Phase Conditions: A Study Case with Seized Samples. Anal. Chim. Acta 2024, 1317, 342901. [Google Scholar] [CrossRef]
- Andernach, L.; Pusch, S.; Weber, C.; Schollmeyer, D.; Münster-Müller, S.; Pütz, M.; Opatz, T. Absolute Configuration of the Synthetic Cannabinoid MDMB-CHMICA with Its Chemical Characteristics in Illegal Products. Forensic Toxicol. 2016, 34, 344–352. [Google Scholar] [CrossRef]
- Weber, C.; Pusch, S.; Schollmeyer, D.; Münster-Müller, S.; Pütz, M.; Opatz, T. Characterization of the Synthetic Cannabinoid MDMB-CHMCZCA. Beilstein J. Org. Chem. 2016, 12, 2808–2815. [Google Scholar] [CrossRef]
- Doi, T.; Asada, A.; Takeda, A.; Tagami, T.; Katagi, M.; Kamata, H.; Sawabe, Y. Enantioseparation of the Carboxamide-Type Synthetic Cannabinoids N -(1-Amino-3-Methyl-1-Oxobutan-2-Yl)-1-(5-Fluoropentyl)-1 H -Indazole-3-Carboxamide and Methyl [1-(5-Fluoropentyl)-1 H -Indazole-3-Carbonyl]-Valinate in Illicit Herbal Products. J. Chromatogr. A 2016, 1473, 83–89. [Google Scholar] [CrossRef]
- Doi, T.; Tagami, T.; Takeda, A.; Asada, A.; Sawabe, Y. Evaluation of Carboxamide-Type Synthetic Cannabinoids as CB1/CB2 Receptor Agonists: Difference between the Enantiomers. Forensic Toxicol. 2018, 36, 51–60. [Google Scholar] [CrossRef]
- Breitenbach, S.; Rowe, W.F.; McCord, B.; Lurie, I.S. Assessment of Ultra High Performance Supercritical Fluid Chromatography as a Separation Technique for the Analysis of Seized Drugs: Applicability to Synthetic Cannabinoids. J. Chromatogr. A 2016, 1440, 201–211. [Google Scholar] [CrossRef]
- Toyo’oka, T.; Kikura-Hanajiri, R. A Reliable Method for the Separation and Detection of Synthetic Cannabinoids by Supercritical Fluid Chromatography with Mass Spectrometry, and Its Application to Plant Products. Chem. Pharm. Bull. 2015, 63, 762–769. [Google Scholar] [CrossRef]
- Patton, A.L.; Seely, K.A.; Chimalakonda, K.C.; Tran, J.P.; Trass, M.; Miranda, A.; Fantegrossi, W.E.; Kennedy, P.D.; Dobrowolski, P.; Radominska-Pandya, A.; et al. Targeted Metabolomic Approach for Assessing Human Synthetic Cannabinoid Exposure and Pharmacology. Anal. Chem. 2013, 85, 9390–9399. [Google Scholar] [CrossRef]
- Thakur, G.A.; Palmer, S.L.; Harrington, P.E.; Stergiades, I.A.; Tius, M.A.; Makriyannis, A. Enantiomeric Resolution of a Novel Chiral Cannabinoid Receptor Ligand. J. Biochem. Biophys. Methods 2002, 54, 415–422. [Google Scholar] [CrossRef]
- Stern, E.; Goossens, L.; Retailleau, P.; Kauffmann, B.; Bonte, J.-P.; Depreux, P.; Goossens, J.-F. Preparative Enantiomeric Separation of New Selective CB2 Receptor Agonists by Liquid Chromatography on Polysaccharide-Based Chiral Stationary Phases: Determination of Enantiomeric Purity and Assignment of Absolute Stereochemistry by X-Ray Structure Analysis. Chirality 2011, 23, 389–396. [Google Scholar] [CrossRef]
- Stern, E.; Goossens, L.; Vaccher, C.; Bonte, J.-P.; Depreux, P.; Henichart, J.-P.; Goossens, J.-F. Chiral Resolution of the Enantiomers of New Selective CB(2) Receptor Agonists by Liquid Chromatography on Amylose Stationary Phases. J. Pharm. Biomed. Anal. 2008, 46, 848–853. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Castro, P.M.L.; Tiritan, M.E. Chiral Pharmaceuticals in the Environment. Environ. Chem. Lett. 2012, 10, 239–253. [Google Scholar] [CrossRef]
- Teixeira, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral Stationary Phases for Liquid Chromatography: Recent Developments. Molecules 2019, 24, 865. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, S.; Yuan, L. Recent Progress in the Development of Chiral Stationary Phases for High-performance Liquid Chromatography. J. Sep. Sci. 2022, 45, 51–77. [Google Scholar] [CrossRef]
- Chen, X.; Yamamoto, C.; Okamoto, Y. Polysaccharide Derivatives as Useful Chiral Stationary Phases in High-Performance Liquid Chromatography. Pure Appl. Chem. 2007, 79, 1561–1573. [Google Scholar] [CrossRef]
- Fernandes, C.; Phyo, Y.Z.; Silva, A.S.; Tiritan, M.E.; Kijjoa, A.; Pinto, M.M.M. Chiral Stationary Phases Based on Small Molecules: An Update of the Last 17 Years. Sep. Purif. Rev. 2018, 47, 89–123. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J.; Wu, Y.; Sun, X.; Ke, Y. Enantioseparation of Cloprostenol on the Polysaccharide Chiral Stationary Phase: Influence of the Mobile Phase on Enantioselective Adsorption. J. Chromatogr. A 2021, 1653, 462413. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Xu, Z.; Dou, J. Mass Spectrometry-Based Metabolomics in Health and Medical Science: A Systematic Review. RSC Adv. 2020, 10, 3092–3104. [Google Scholar] [CrossRef]
- Francotte, E.R. Enantioselective Chromatography as a Powerful Alternative for the Preparation of Drug Enantiomers. J. Chromatogr. A 2001, 906, 379–397. [Google Scholar] [CrossRef]
- Fernandes, C.; Tiritan, M.E.; Pinto, M. Small Molecules as Chromatographic Tools for HPLC Enantiomeric Resolution: Pirkle-Type Chiral Stationary Phases Evolution. Chromatographia 2013, 76, 871–897. [Google Scholar] [CrossRef]
- Antonides, L.H.; Cannaert, A.; Norman, C.; NicDáeid, N.; Sutcliffe, O.B.; Stove, C.P.; McKenzie, C. Shape Matters: The Application of Activity-based in Vitro Bioassays and Chiral Profiling to the Pharmacological Evaluation of Synthetic Cannabinoid Receptor Agonists in Drug-infused Papers Seized in Prisons. Drug Test. Anal. 2021, 13, 628–643. [Google Scholar] [CrossRef]
- Sparkes, E.; Markham, J.W.; Boyd, R.; Udoh, M.; Gordon, R.; Zaman, H.; Walker, K.A.; Dane, C.; Kevin, R.C.; Santiago, M.J.; et al. Synthesis and Functional Evaluation of Proteinogenic Amino Acid-Derived Synthetic Cannabinoid Receptor Agonists Related to MPP-5F-PICA, MMB-5F-PICA, and MDMB-5F-PICA. RSC Med. Chem. 2024, 15, 2063–2079. [Google Scholar] [CrossRef]
- Salomone, A. Detection of New Psychoactive Substances. In Hair Analysis in Clinical and Forensic Toxicology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 301–336. ISBN 978-0-12-801700-5. [Google Scholar]
- Felder, C.C.; Joyce, K.E.; Briley, E.M.; Mansouri, J.; Mackie, K.; Blond, O.; Lai, Y.; Ma, A.L.; Mitchell, R.L. Comparison of the Pharmacology and Signal Transduction of the Human Cannabinoid CB1 and CB2 Receptors. Mol. Pharmacol. 1995, 48, 443–450. [Google Scholar] [CrossRef]
- Miller, A.S.; Walker, J.M. Electrophysiological Effects of a Cannabinoid on Neural Activity in the Globus Pallidus. Eur. J. Pharmacol. 1996, 304, 29–35. [Google Scholar] [CrossRef]
- Coruzzi, G.; Adami, M.; Coppelli, G.; Frati, P.; Soldani, G. Inhibitory Effect of the Cannabinoid Receptor Agonist WIN 55,212-2 on Pentagastrin-Induced Gastric Acid Secretion in the Anaesthetized Rat. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1999, 360, 715–718. [Google Scholar] [CrossRef]
- Ellert-Miklaszewska, A.; Ciechomska, I.A.; Kaminska, B. Synthetic Cannabinoids Induce Autophagy and Mitochondrial Apoptotic Pathways in Human Glioblastoma Cells Independently of Deficiency in TP53 or PTEN Tumor Suppressors. Cancers 2021, 13, 419. [Google Scholar] [CrossRef]
- Facchinetti, F.; Del Giudice, E.; Furegato, S.; Passarotto, M.; Leon, A. Cannabinoids Ablate Release of TNFalpha in Rat Microglial Cells Stimulated with Lypopolysaccharide. Glia 2003, 41, 161–168. [Google Scholar] [CrossRef]
- Kuster, J.E.; Stevenson, J.I.; Ward, S.J.; D’Ambra, T.E.; Haycock, D.A. Aminoalkylindole Binding in Rat Cerebellum: Selective Displacement by Natural and Synthetic Cannabinoids. J. Pharmacol. Exp. Ther. 1993, 264, 1352–1363. [Google Scholar] [CrossRef]
- Nagayama, T.; Sinor, A.D.; Simon, R.P.; Chen, J.; Graham, S.H.; Jin, K.; Greenberg, D.A. Cannabinoids and Neuroprotection in Global and Focal Cerebral Ischemia and in Neuronal Cultures. J. Neurosci. 1999, 19, 2987–2995. [Google Scholar] [CrossRef]
- Johanek, L.M.; Simone, D.A. Activation of Peripheral Cannabinoid Receptors Attenuates Cutaneous Hyperalgesia Produced by a Heat Injury. Pain 2004, 109, 432–442. [Google Scholar] [CrossRef]
- Mackie, K.; Hille, B. Cannabinoids Inhibit N-Type Calcium Channels in Neuroblastoma-Glioma Cells. Proc. Natl. Acad. Sci. USA 1992, 89, 3825–3829. [Google Scholar] [CrossRef]
- Price, T.J.; Patwardhan, A.; Akopian, A.N.; Hargreaves, K.M.; Flores, C.M. Cannabinoid Receptor-independent Actions of the Aminoalkylindole WIN 55,212-2 on Trigeminal Sensory Neurons. Br. J. Pharmacol. 2004, 142, 257–266. [Google Scholar] [CrossRef]
- Curran, N.M.; Griffin, B.D.; O’Toole, D.; Brady, K.J.; Fitzgerald, S.N.; Moynagh, P.N. The Synthetic Cannabinoid R(+)WIN 55,212-2 Inhibits the Interleukin-1 Signaling Pathway in Human Astrocytes in a Cannabinoid Receptor-Independent Manner. J. Biol. Chem. 2005, 280, 35797–35806. [Google Scholar] [CrossRef]
- Scholl, A.; Ivanov, I.; Hinz, B. Inhibition of Interleukin-1β-Induced Endothelial Tissue Factor Expression by the Synthetic Cannabinoid WIN 55,212-2. Oncotarget 2016, 7, 61438–61457. [Google Scholar] [CrossRef]
- Schmid, K.; Niederhoffer, N.; Szabo, B. Analysis of the Respiratory Effects of Cannabinoids in Rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2003, 368, 301–308. [Google Scholar] [CrossRef]
- Ameri, A.; Wilhelm, A.; Simmet, T. Effects of the Endogeneous Cannabinoid, Anandamide, on Neuronal Activity in Rat Hippocampal Slices. Br. J. Pharmacol. 1999, 126, 1831–1839. [Google Scholar] [CrossRef]
- Hohmann, A.G.; Martin, W.J.; Tsou, K.; Walker, J.M. Inhibition of Noxious Stimulus-Evoked Activity of Spinal Cord Dorsal Horn Neurons by the Cannabinoid WIN 55,212-2. Life Sci. 1995, 56, 2111–2118. [Google Scholar] [CrossRef]
- Miller, A.S.; Walker, J.M. Effects of a Cannabinoid on Spontaneous and Evoked Neuronal Activity in the Substantia Nigra Pars Reticulata. Eur. J. Pharmacol. 1995, 279, 179–185. [Google Scholar] [CrossRef]
- Strangman, N.M.; Walker, J.M. Cannabinoid WIN 55,212-2 Inhibits the Activity-Dependent Facilitation of Spinal Nociceptive Responses. J. Neurophysiol. 1999, 82, 472–477. [Google Scholar] [CrossRef]
- Rawls, S.M.; Rodriguez, T.; Baron, D.A.; Raffa, R.B. A Nitric Oxide Synthase Inhibitor (L-NAME) Attenuates Abstinence-Induced Withdrawal from Both Cocaine and a Cannabinoid Agonist (WIN 55212-2) in Planaria. Brain Res. 2006, 1099, 82–87. [Google Scholar] [CrossRef]
- Downer, E.J.; Clifford, E.; Amu, S.; Fallon, P.G.; Moynagh, P.N. The Synthetic Cannabinoid R(+)WIN55,212-2 Augments Interferon-β Expression via Peroxisome Proliferator-Activated Receptor-α. J. Biol. Chem. 2012, 287, 25440–25453. [Google Scholar] [CrossRef]
- Willis, P.G.; Pavlova, O.A.; Chefer, S.I.; Vaupel, D.B.; Mukhin, A.G.; Horti, A.G. Synthesis and Structure−Activity Relationship of a Novel Series of Aminoalkylindoles with Potential for Imaging the Neuronal Cannabinoid Receptor by Positron Emission Tomography. J. Med. Chem. 2005, 48, 5813–5822. [Google Scholar] [CrossRef]
- Deng, H.; Gifford, A.N.; Zvonok, A.M.; Cui, G.; Li, X.; Fan, P.; Deschamps, J.R.; Flippen-Anderson, J.L.; Gatley, S.J.; Makriyannis, A. Potent Cannabinergic Indole Analogues as Radioiodinatable Brain Imaging Agents for the CB1 Cannabinoid Receptor. J. Med. Chem. 2005, 48, 6386–6392. [Google Scholar] [CrossRef]
- Bingham, B.; Jones, P.G.; Uveges, A.J.; Kotnis, S.; Lu, P.; Smith, V.A.; Sun, S.; Resnick, L.; Chlenov, M.; He, Y.; et al. Species-specific in Vitro Pharmacological Effects of the Cannabinoid Receptor 2 (CB 2) Selective Ligand AM1241 and Its Resolved Enantiomers. Br. J. Pharmacol. 2007, 151, 1061–1070. [Google Scholar] [CrossRef]
- Rahn, E.J.; Zvonok, A.M.; Makriyannis, A.; Hohmann, A.G. Antinociceptive Effects of Racemic AM1241 and Its Chirally Synthesized Enantiomers: Lack of Dependence upon Opioid Receptor Activation. AAPS J. 2010, 12, 147–157. [Google Scholar] [CrossRef]
- Feigenbaum, J.J.; Bergmann, F.; Richmond, S.A.; Mechoulam, R.; Nadler, V.; Kloog, Y.; Sokolovsky, M. Nonpsychotropic Cannabinoid Acts as a Functional N-Methyl-D-Aspartate Receptor Blocker. Proc. Natl. Acad. Sci. USA 1989, 86, 9584–9587. [Google Scholar] [CrossRef]
- Kaminski, N.E.; Abood, M.E.; Kessler, F.K.; Martin, B.R.; Schatz, A.R. Identification of a Functionally Relevant Cannabinoid Receptor on Mouse Spleen Cells That Is Involved in Cannabinoid-Mediated Immune Modulation. Mol. Pharmacol. 1992, 42, 736–742. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Mechoulam, R.; Offen, D. The CB 1 Cannabinoid Receptor Agonist, HU-210, Reduces Levodopa-Induced Rotations in 6-Hydroxydopamine-Lesioned Rats. Pharmacol. Toxicol. 2003, 93, 66–70. [Google Scholar] [CrossRef]
- Collins, D.R.; Pertwee, R.G.; Davies, S.N. The Action of Synthetic Cannabinoids on the Induction of Long-Term Potentiation in the Rat Hippocampal Slice. Eur. J. Pharmacol. 1994, 259, R7–R8. [Google Scholar] [CrossRef]
- Wilson, R.G.J.; Tahir, S.K.; Mechoulam, R.; Zimmerman, S.; Zimmerman, A.M. Cannabinoid Enantiomer Action on the Cytoarchitecture. Cell Biol. Int. 1996, 20, 147–157. [Google Scholar] [CrossRef]
- Lichtman, A.H.; Martin, B.R. Spinal and Supraspinal Components of Cannabinoid-Induced Antinociception. J. Pharmacol. Exp. Ther. 1991, 258, 517–523. [Google Scholar] [CrossRef]
- Buchler, I.P.; Hayes, M.J.; Hedge, S.G.; Hockerman, S.L.; Jones, D.; Kortum, S.W.; Rico, J.G.; TenBrink, R.E.; Wu, K.K. Indazole Derivatives as CB1 Receptor Modulators and Their Preparation and Use in the Treatment of CB1-Mediated Diseases. Eur. Pat. Appl. 2009. [Google Scholar]
- Banister, S.D.; Moir, M.; Stuart, J.; Kevin, R.C.; Wood, K.E.; Longworth, M.; Wilkinson, S.M.; Beinat, C.; Buchanan, A.S.; Glass, M.; et al. Pharmacology of Indole and Indazole Synthetic Cannabinoid Designer Drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA. ACS Chem. Neurosci. 2015, 6, 1546–1559. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Saponaro, G.; Moorman, A.R.; Romagnoli, R.; Preti, D.; Baraldi, S.; Ruggiero, E.; Varani, K.; Targa, M.; Vincenzi, F.; et al. 7-Oxo-[1,4]Oxazino [2,3,4-Ij ]Quinoline-6-Carboxamides as Selective CB 2 Cannabinoid Receptor Ligands: Structural Investigations around a Novel Class of Full Agonists. J. Med. Chem. 2012, 55, 6608–6623. [Google Scholar] [CrossRef]
- Ametovski, A.; Macdonald, C.; Manning, J.J.; Haneef, S.A.S.; Santiago, M.; Martin, L.; Sparkes, E.; Reckers, A.; Gerona, R.R.; Connor, M.; et al. Exploring Stereochemical and Conformational Requirements at Cannabinoid Receptors for Synthetic Cannabinoids Related to SDB-006, 5F-SDB-006, CUMYL-PICA, and 5F-CUMYL-PICA. ACS Chem. Neurosci. 2020, 11, 3672–3682. [Google Scholar] [CrossRef]
- Moir, E.M.; Yoshiizumi, K.; Cairns, J.; Cowley, P.; Ferguson, M.; Jeremiah, F.; Kiyoi, T.; Morphy, R.; Tierney, J.; Wishart, G.; et al. Design, Synthesis, and Structure–Activity Relationship Study of Bicyclic Piperazine Analogs of Indole-3-Carboxamides as Novel Cannabinoid CB1 Receptor Agonists. Bioorg. Med. Chem. Lett. 2010, 20, 7327–7330. [Google Scholar] [CrossRef]
- Kiyoi, T.; York, M.; Francis, S.; Edwards, D.; Walker, G.; Houghton, A.K.; Cottney, J.E.; Baker, J.; Adam, J.M. Design, Synthesis, and Structure-Activity Relationship Study of Conformationally Constrained Analogs of Indole-3-Carboxamides as Novel CB1 Cannabinoid Receptor Agonists. Bioorg. Med. Chem. Lett. 2010, 20, 4918–4921. [Google Scholar] [CrossRef]
- Stern, E.; Muccioli, G.G.; Millet, R.; Goossens, J.-F.; Farce, A.; Chavatte, P.; Poupaert, J.H.; Lambert, D.M.; Depreux, P.; Hénichart, J.-P. Novel 4-Oxo-1,4-Dihydroquinoline-3-Carboxamide Derivatives as New CB2 Cannabinoid Receptors Agonists: Synthesis, Pharmacological Properties and Molecular Modeling. J. Med. Chem. 2006, 49, 70–79. [Google Scholar] [CrossRef]
- Stern, E.; Muccioli, G.G.; Bosier, B.; Hamtiaux, L.; Millet, R.; Poupaert, J.H.; Hénichart, J.-P.; Depreux, P.; Goossens, J.-F.; Lambert, D.M. Pharmacomodulations around the 4-Oxo-1,4-Dihydroquinoline-3-Carboxamides, a Class of Potent CB2-Selective Cannabinoid Receptor Ligands: Consequences in Receptor Affinity and Functionality. J. Med. Chem. 2007, 50, 5471–5484. [Google Scholar] [CrossRef]
- Brandon, A.M.; Antonides, L.H.; Riley, J.; Epemolu, O.; McKeown, D.A.; Read, K.D.; McKenzie, C. A Systematic Study of the In Vitro Pharmacokinetics and Estimated Human In Vivo Clearance of Indole and Indazole-3-Carboxamide Synthetic Cannabinoid Receptor Agonists Detected on the Illicit Drug Market. Molecules 2021, 26, 1396. [Google Scholar] [CrossRef]
- Lawtrakul, L.; Toochinda, P. Chiral Recognition of Butylone by Methylated β-Cyclodextrin Inclusion Complexes: Molecular Calculations and Two-Level Factorial Designs. ACS Omega 2025, 10, 2003–2011. [Google Scholar] [CrossRef]
- Holowinski, P.; Dybowski, M.P. Determination of 3- and 4-Chloromethcathinone Interactions with Plasma Proteins: Study Involving Analytical and Theoretical Methods. Forensic Toxicol. 2024, 42, 111–124. [Google Scholar] [CrossRef]
- Folprechtová, D.; Seibert, E.; Schmid, M.G.; Kalíková, K. Advantages of Dimethyl Carbonate as Organic Modifier for Enantioseparation of Novel Psychoactive Substances in Sub/Supercritical Fluid Chromatography. Anal. Chim. Acta 2024, 1332, 343380. [Google Scholar] [CrossRef]
- Ioannou, K.A.; Georgiou, M.N.; Ioannou, G.D.; Christou, A.; Stavrou, I.J.; Schmid, M.G.; Kapnissi-Christodoulou, C.P. Enantiomeric Separation of Nefopam and Cathinone Derivatives Using a Supramolecular Deep Eutectic Solvent as a Chiral Selector in Capillary Electrophoresis. Electrophoresis 2024, 45, 1721–1726. [Google Scholar] [CrossRef]
- Ioannou, K.A.; Ioannou, G.D.; Christou, A.; Stavrou, I.J.; Schmid, M.G.; Kapnissi-Christodoulou, C.P. Stereoselective Separation of Psychoactive Substances: Multivariate Optimization and Validation of a Capillary Electrophoresis Method Using Carboxymethyl-β-CD/Deep Eutectic Solvent Dual System. J. Pharm. Biomed. Anal. 2024, 239, 115897. [Google Scholar] [CrossRef]
- Folprechtová, D.; Schmid, M.G.; Armstrong, D.W.; Kalíková, K. The Enantioselective Potential of NicoShell and TeicoShell Columns for Basic Pharmaceuticals and Forensic Drugs in Sub/Supercritical Fluid Chromatography. Molecules 2023, 28, 1202. [Google Scholar] [CrossRef]
- Ioannou, K.A.; Christou, A.; Stavrou, I.J.; Schmid, M.G.; Kapnissi-Christodoulou, C.P. Evaluation of Cyclodextrin- and Cyclofructan-based Chiral Selectors for the Enantioseparation of Psychoactive Substances in Capillary Electrophoresis. Electrophoresis 2022, 43, 2392–2401. [Google Scholar] [CrossRef]
- Seibert, E.; Kunert, O.; Pferschy-Wenzig, E.-M.; Schmid, M.G. NMR-Based Structure Elucidation and Chiral Separation of N-Cyclohexylmethylone, a Novel Designer Drug. Forensic Sci. Int. 2025, 367, 112351. [Google Scholar] [CrossRef]
- Pérez-Pereira, A.; Gonçalves, V.M.F.; Ribeiro, A.R.L.; Fernandes, C.; Carrola, J.S.; Ribeiro, C.; Tiritan, M.E. Development of an Enantioselective Method by Liquid Chromatography to Monitor 3,4-Methylenedioxypyrovalerone in Culture Media from Ecotoxicity Assays. Separations 2024, 11, 248. [Google Scholar] [CrossRef]
- Paškan, M.; Dobšíková, K.; Kuchař, M.; Setnička, V.; Kohout, M. Synthesis and Absolute Configuration of Cyclic Synthetic Cathinones Derived from α-Tetralone. Chirality 2024, 36, e23646. [Google Scholar] [CrossRef]
- Langa, I.M.; Lado Ribeiro, A.R.; Ratola, N.; Gonçalves, V.M.F.; Tiritan, M.E.; Ribeiro, C. Amphetamine-like Substances and Synthetic Cathinones in Portuguese Wastewater Influents: Enantiomeric Profiling and Role of Suspended Particulate Matter. Forensic Sci. Int. 2024, 361, 112128. [Google Scholar] [CrossRef]
- Paškanová, N.; Hlavatá, P.; Jurásek, B.; Kuchař, M.; Kohout, M. Tuning Parameters of Single Quadrupole Mass Detector Hyphenated to Supercritical Fluid Chromatography for Enantioseparation of Synthetic Cathinones. J. Chromatogr. Open 2024, 5, 100139. [Google Scholar] [CrossRef]
- Seibert, E.; Götz, K.; Schmid, M.G. Exploring a Lux® i-Amylose-3 Column in Normal Phase and Polar-organic Mode for Chiral Separation of Cathinone Derivatives and Pyrovalerones Using High-performance Liquid Chromatography. Chirality 2024, 36, e23679. [Google Scholar] [CrossRef]
- Carvalho, A.R.; Morão, A.M.; Gonçalves, V.M.F.; Tiritan, M.E.; Gorito, A.M.; Pereira, M.F.; Silva, A.M.T.; Castro, B.B.; Carrola, J.S.; Amorim, M.M.; et al. Toxicity of Butylone and Its Enantiomers to Daphnia Magna and Its Degradation/Toxicity Potential Using Advanced Oxidation Technologies. Aquat. Toxicol. 2024, 271, 106906. [Google Scholar] [CrossRef]
- Dobšíková, K.; Javorská, Ž.; Paškan, M.; Spálovská, D.; Trembulaková, P.; Herciková, J.; Kuchař, M.; Kozmík, V.; Kohout, M.; Setnička, V. Enantioseparation and a Comprehensive Spectroscopic Analysis of Novel Synthetic Cathinones Laterally Substituted with a Trifluoromethyl Group. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 291, 122320. [Google Scholar] [CrossRef]
- Aldubayyan, A.A.; Castrignanò, E.; Elliott, S.; Abbate, V. Development and Validation of a Chiral LC-MS/MS Method for the Separation and Quantification of Four Synthetic Cathinones in Human Whole Blood and Its Application in Stability Analysis. Talanta 2023, 253, 123986. [Google Scholar] [CrossRef]
- Paškan, M.; Rimpelová, S.; Svobodová Pavlíčková, V.; Spálovská, D.; Setnička, V.; Kuchař, M.; Kohout, M. 4-Isobutylmethcathinone—A Novel Synthetic Cathinone with High In Vitro Cytotoxicity and Strong Receptor Binding Preference of Enantiomers. Pharmaceuticals 2022, 15, 1495. [Google Scholar] [CrossRef]
- Ujj, D.; Kalydi, E.; Malanga, M.; Varga, E.; Sohajda, T.; Béni, S.; Benkovics, G. Sugammadex Analogue Cyclodextrins as Chiral Selectors for Enantioseparation of Cathinone Derivatives by Capillary Electrophoresis. J. Chromatogr. A 2022, 1683, 463506. [Google Scholar] [CrossRef]
- Langa, I.; Tiritan, M.E.; Silva, D.; Ribeiro, C. Gas Chromatography Multiresidue Method for Enantiomeric Fraction Determination of Psychoactive Substances in Effluents and River Surface Waters. Chemosensors 2021, 9, 224. [Google Scholar] [CrossRef]
- Pérez-Alcaraz, A.; Borrull, F.; Aguilar, C.; Calull, M.; Benavente, F. Enantiodetermination of R,S-3,4-Methylenedioxypyrovalerone in Urine Samples by High Pressure in-Line Solid-Phase Extraction Capillary Electrophoresis-Mass Spectrometry. Talanta 2021, 225, 121994. [Google Scholar] [CrossRef]
- Czerwinska, J.; Parkin, M.C.; Cilibrizzi, A.; George, C.; Kicman, A.T.; Dargan, P.I.; Abbate, V. Pharmacokinetics of Mephedrone Enantiomers in Whole Blood after a Controlled Intranasal Administration to Healthy Human Volunteers. Pharmaceuticals 2020, 14, 5. [Google Scholar] [CrossRef]
- Lin, H.-R.; Kuo, F.-W. Determination of the R- and S-Enantiomers of Methylone and Ethylone in Seized Drugs by Enantioselective Liquid Chromatography Tandem Mass Spectrometry Analysis. Forensic Sci. Int. 2020, 317, 110528. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral Drugs: An Overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- Scriba, G.K.E. Chiral Recognition Mechanisms in Analytical Separation Sciences. Chromatographia 2012, 75, 815–838. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.-R.; Wang-Iverson, D.B.; Tymiak, A.A. Enantioselective Chromatography in Drug Discovery. Drug Discov. Today 2005, 10, 571–577. [Google Scholar] [CrossRef]
- Bayatloo, M.R.; Tabani, H.; Nojavan, S.; Alexovič, M.; Ozkan, S.A. Liquid-Phase Microextraction Approaches for Preconcentration and Analysis of Chiral Compounds: A Review on Current Advances. Crit. Rev. Anal. Chem. 2023, 53, 1623–1637. [Google Scholar] [CrossRef]
- Majchrzak, M.; Celiński, R.; Kuś, P.; Kowalska, T.; Sajewicz, M. The Newest Cathinone Derivatives as Designer Drugs: An Analytical and Toxicological Review. Forensic Toxicol. 2018, 36, 33–50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).