Syringin and Phillygenin—Natural Compounds with a Potential Role in Preventing Lipid Deposition in Macrophages in the Context of Human Atherosclerotic Plaque
Abstract
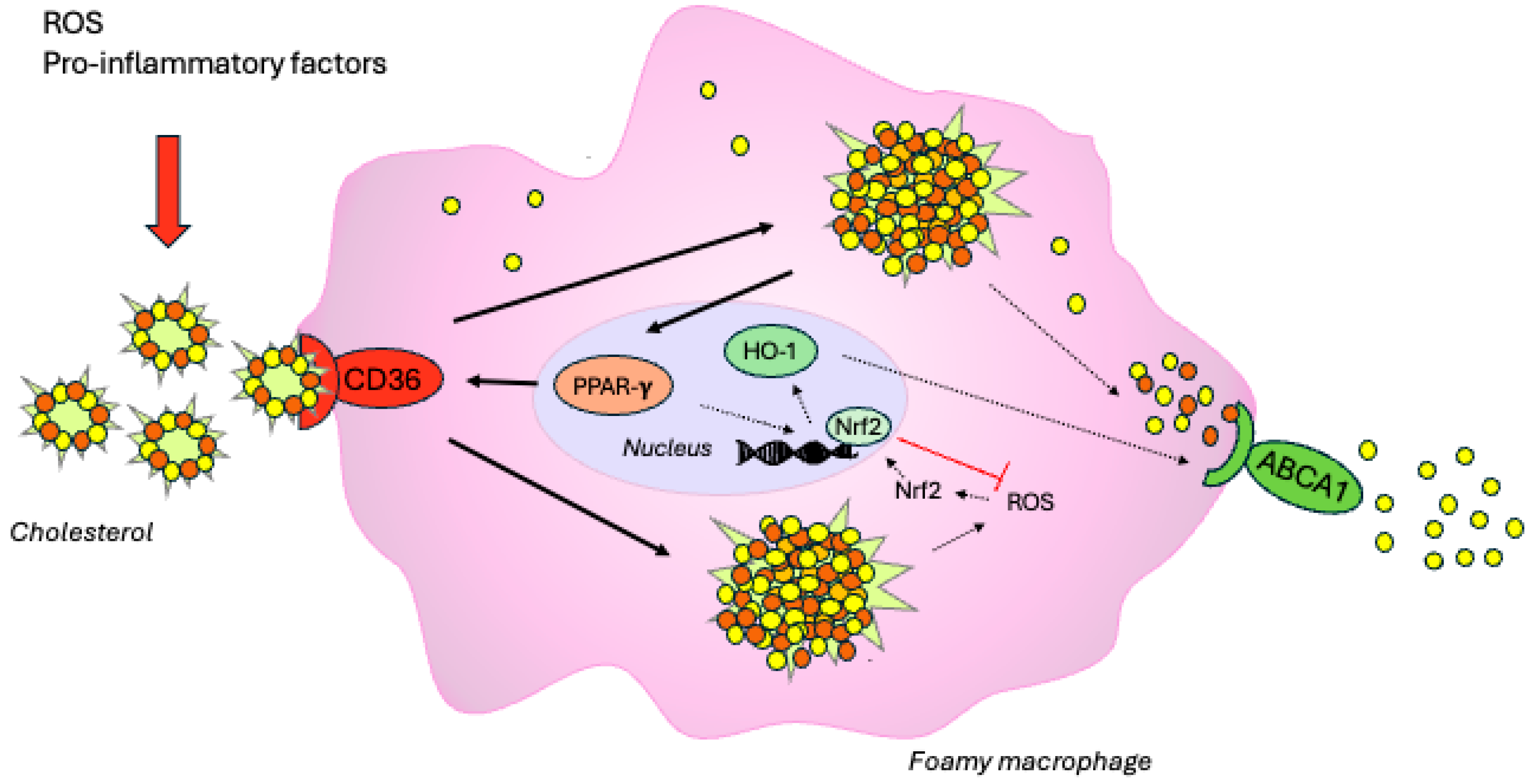
1. Introduction
2. Results
2.1. Effect on Cytotoxicity
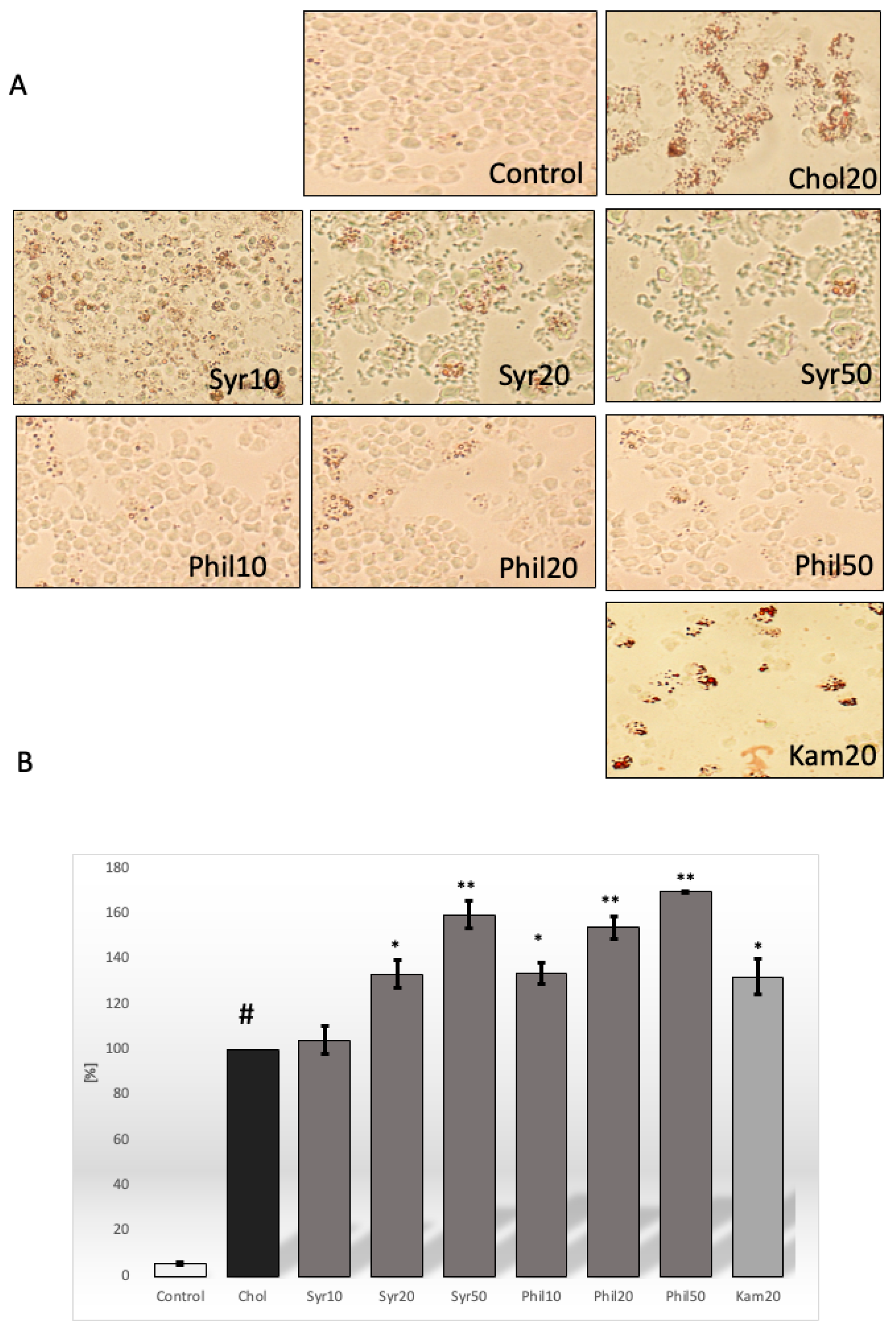
2.2. Effect of Syringin and Phillygenin on Cholesterol Deposits in Macrophages
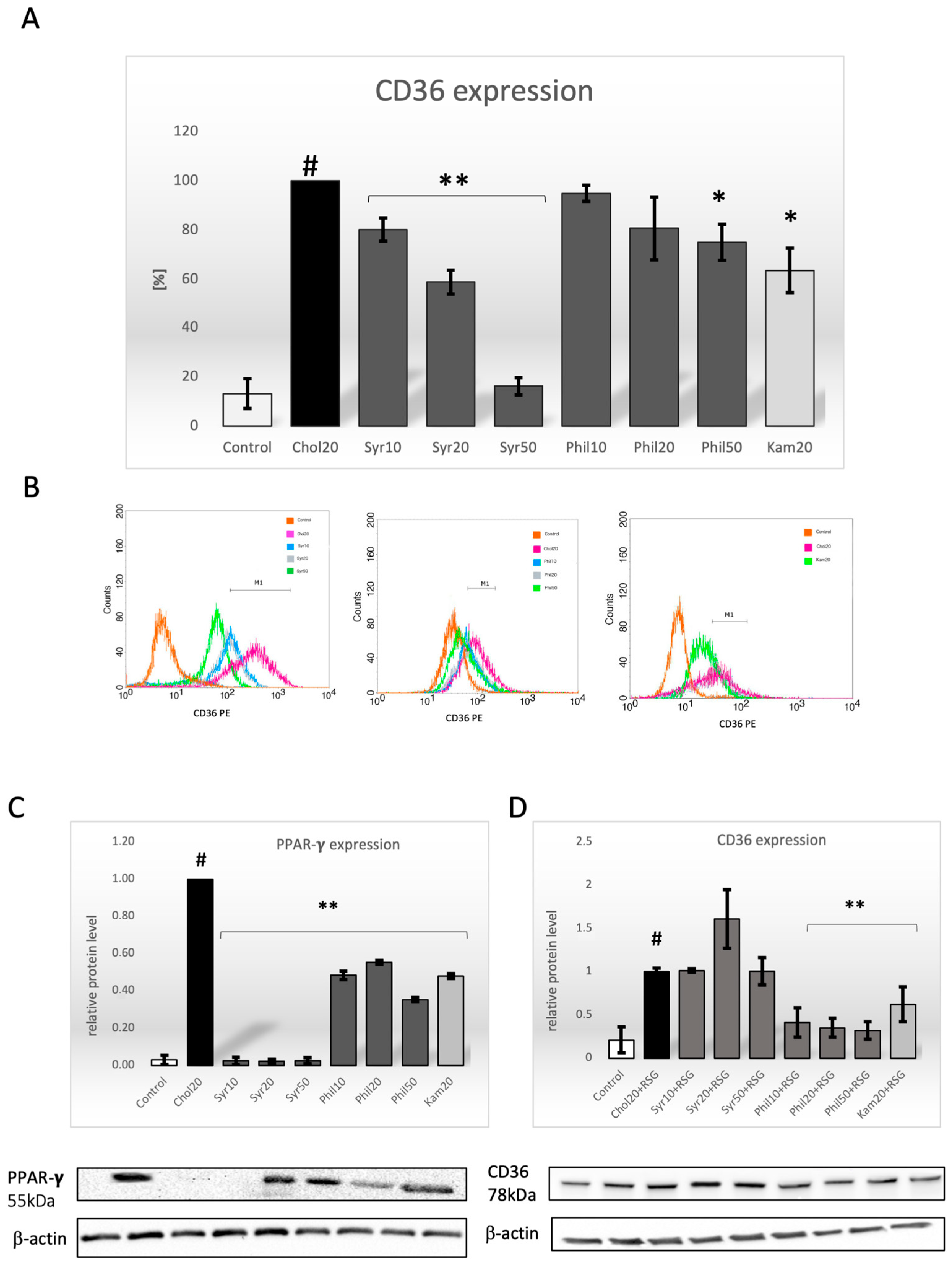
2.3. Effect of Syringin and Phillygenin on CD36 Expression
2.4. Effect of Syringin and Phillygenin on Expression of the PPAR-γ Protein
2.5. Effect of Syringin and Phillygenin on CD36 Protein Expression After Preincubation with Rosiglitazone (RSG)
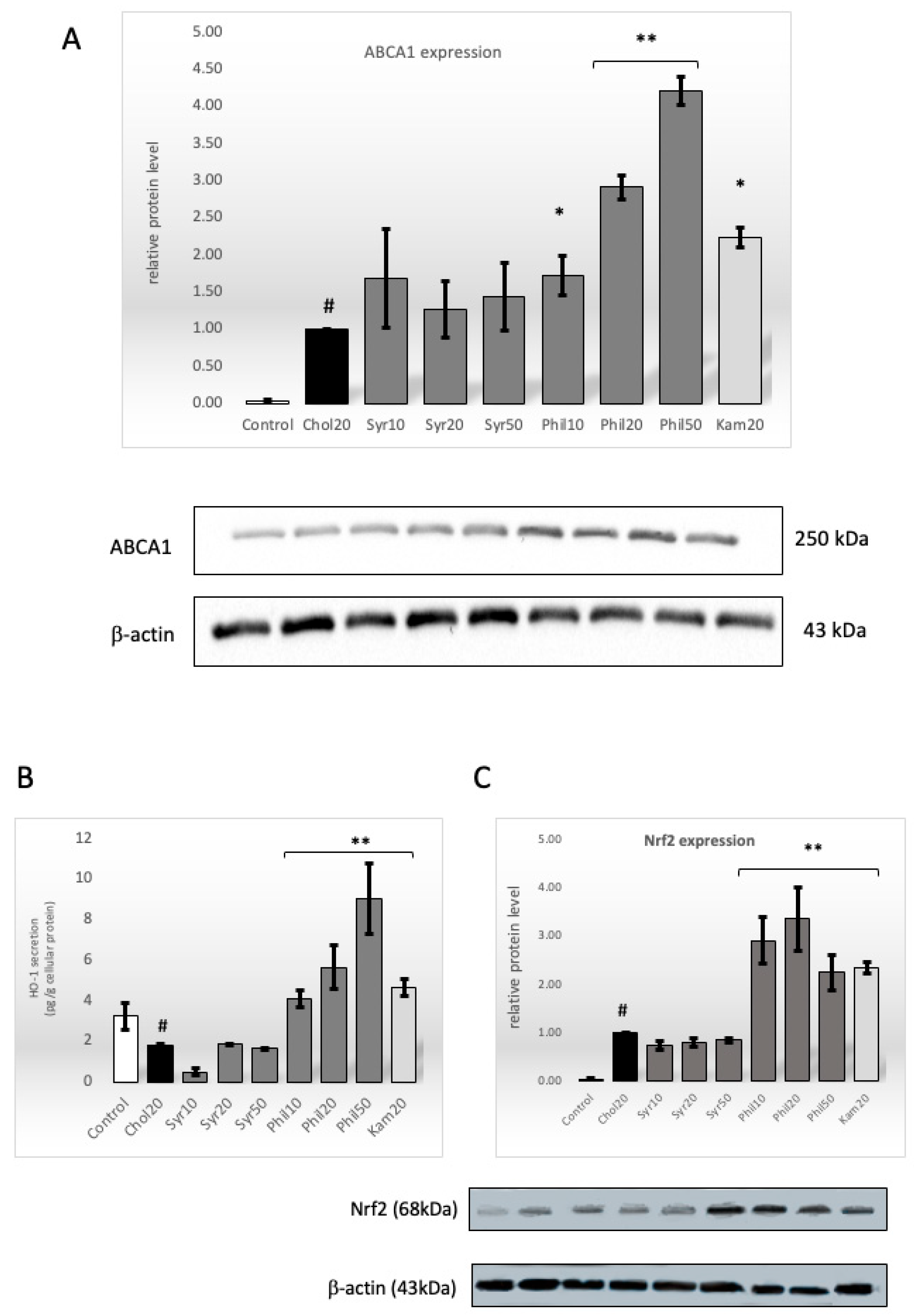
2.6. Effect of Syringin and Phillygenin on Protein Level of ABCA1 Transporter
2.7. Effect of Syringin and Phillygenin on HO-1 Intracellular Secretion
2.8. Effect of Syringin and Phillygenin on Nrf2 Protein Expression
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
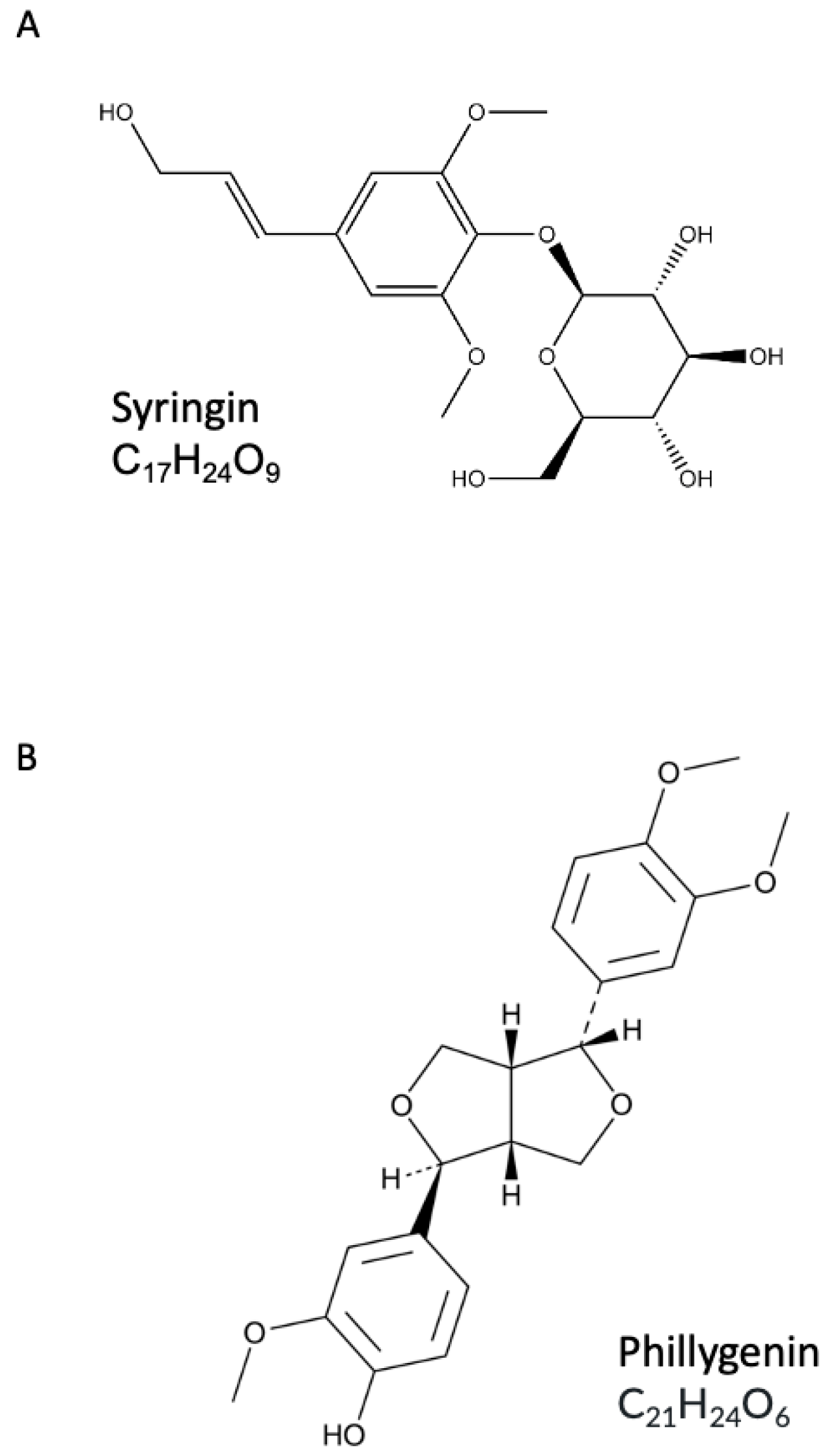
4.2. Syringin and Phillygenin
4.3. Cell Culture
4.4. Oil Red O Staining
4.5. Flow Cytometry Analysis
4.6. Western Blot Analysis
4.7. Immunofluorescence Assay
4.8. Positive Control and Agonist PPAR-γ
4.9. Cytotoxicity Assay
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations and Acronyms
References
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam Cells in Atherosclerosis: Novel Insights into Its Origins, Consequences, and Molecular Mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Seyed-Alireza Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Yu, M.; Jiang, M.; Chen, Y.; Zhang, S.; Zhang, W.; Yang, X.; Li, X.; Li, Y.; Duan, S.; Han, J.; et al. Inhibition of Macrophage CD36 Expression and Cellular Oxidized Low Density Lipoprotein (oxLDL) Accumulation by Tamoxifen: A Peroxisome Proliferator-Activated Receptor (ppar)γ-Dependent Mechanism. J. Biol. Chem. 2016, 291, 16977–16989. [Google Scholar] [CrossRef]
- Zhong, Y.; Feng, J.; Fan, Z.; Li, J. Curcumin increases cholesterol efflux via heme oxygenase-1-mediated ABCA1 and SR-BI expression in macrophages. Mol. Med. Rep. 2018, 17, 6138–6143. [Google Scholar] [CrossRef] [PubMed]
- Uitz, E.; Bahadori, B.; McCarty, M.; Moghadasian, M.H. Practical strategies for modulating foam cell formation and behavior. World J. Clin. Cases. 2014, 2, 497–506. [Google Scholar] [CrossRef]
- Filipek, A.; Czerwińska, M.E.; Kiss, A.K.; Wrzosek, M.; Naruszewicz, M. Oleacein enhances anti-inflammatory activity of human macrophages by increasing CD163 receptor expression. Phytomedicine 2015, 22, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Galindo, C.L.; Khan, S.; Zhang, X.; Yeh, Y.S.; Liu, Z.; Razani, B. Lipid-laden foam cells in the pathology of atherosclerosis: Shedding light on new therapeutic targets. Expert Opin. Ther. Targets 2023, 27, 1231–1245. [Google Scholar] [CrossRef]
- Liu, C.; Xu, H.; Wang, P.; Li, Y.; Yi, X.; Tu, Y. Syringin: Plant Source, Traditional Uses, Anti-Cancer, Brain Protection, and Related Pharmacological Properties. Chem. Biodivers. 2024, 22, e202402272. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, K.; Wang, J.; Shao, H. Syringin exerts anti-inflammatory and antioxidant effects by regulating SIRT1 signaling in rat and cell models of acute myocardial infarction. Immun. Inflamm. Dis. 2023, 11, e775. [Google Scholar] [CrossRef]
- Tan, J.; Luo, J.; Meng, C.; Jiang, N.; Cao, J.; Zhao, J. Syringin exerts neuroprotective effects in a rat model of cerebral ischemia through the FOXO3α/NF-κB pathway. Int. Immunopharmacol. 2021, 90, 107268. [Google Scholar] [CrossRef]
- Singh, V.K.; Thakur, D.C.; Rajak, N.; Giri, R.; Garg, N. Immunomodulatory potential of bioactive glycoside syringin: A network pharmacology and molecular modeling approach. J. Biomol. Struct. Dyn. 2024, 42, 3906–3919. [Google Scholar] [CrossRef]
- Cong, S.; Wang, N.; Pei, H.; Li, Z.; Meng, Y.; Maimaitituersun, S.; Zhao, X.; Wan, R.; Wan, Q.; Luo, L.; et al. Syringin inhibits the crosstalk between macrophages and fibroblast-like synoviocytes to treat rheumatoid arthritis via PDE. Phytomedicine. 2025, 138, 156401. [Google Scholar] [CrossRef] [PubMed]
- Filipek, A.; Wyszomierska, J.; Michalak, B.; Kiss, A.K. Syringa vulgaris bark as a source of compounds affecting the release of mediators of inflammation from human neutrophils and monocytes/macrophages. Phytochem. Lett. 2019, 30, 309–313. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Z.; Sheng, L.; Wu, H. Protective effects of syringin against lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 2017, 209, 252–257. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Q.; Hao, X. Syringin ameliorates dextran sulphate colitis via alteration oxidative stress, inflammation NF-κB signalling pathway and gut microbiota. Basic Clin. Pharmacol. Toxicol. 2025, 136, e14105. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.H.; Li, J.J.; Li, S.F.; Guo, J.; Yan, R.P.; Chen, T.G.; Shi, X.H.; Wang, J.D.; Zhang, L.W. Phillygenin Attenuated Colon Inflammation and Improved Intestinal Mucosal Barrier in DSS-induced Colitis Mice via TLR4/Src Mediated MAPK and NF-κB Signaling Pathways. Int. J. Mol. Sci. 2023, 24, 2238. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, P. Phillygenin inhibits the inflammation and apoptosis of pulmonary epithelial cells by activating PPARγ signaling via downregulation of MMP8. Mol. Med. Rep. 2021, 24, 775. [Google Scholar] [CrossRef]
- Zhang, P.; Jin, Y.; Xia, W.; Wang, X.; Zhou, Z. Phillygenin inhibits inflammation in chondrocytes via the Nrf2/NF-κB axis and ameliorates osteoarthritis in mice. J. Orthop. Translat. 2023, 41, 1–11. [Google Scholar] [CrossRef]
- Michalak, B.; Filipek, A.; Chomicki, P.; Pyza, M.; Woźniak, M.; Żyżyńska-Granica, B.; Piwowarski, J.P.; Kicel, A.; Olszewska, M.A.; Kiss, A.K. Lignans From Forsythia x Intermedia Leaves and Flowers Attenuate the Pro-inflammatory Function of Leukocytes and Their Interaction With Endothelial Cells. Front. Pharmacol. 2018, 9, 401. [Google Scholar] [CrossRef]
- Tong, X.; Chen, L.; He, S.; Liu, S.; Yao, J.; Shao, Z.; Ye, Y.; Yao, S.; Lin, Z.; Zuo, J. Forsythia suspensa (Thunb.) Vahl extract ameliorates ulcerative colitis via inhibiting NLRP3 inflammasome activation through the TLR4/MyD88/NF-κB pathway. Immun. Inflamm. Dis. 2023, 11, e1069. [Google Scholar] [CrossRef]
- Filipek, A.; Mikołajczyk, T.P.; Guzik, T.J.; Naruszewicz, M. Oleacein and Foam Cell Formation in Human Monocyte-Derived Macrophages: A Potential Strategy against Early and Advanced Atherosclerotic Lesions. Pharmaceuticals 2020, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Filipek, A.; Gierlikowska, B. Oleacein may intensify the efflux of oxLDL from human macrophages by increasing the expression of the SRB1 receptor, as well as ABCA1 and ABCG1 transporters. J. Funct. Foods. 2021, 78, 104373. [Google Scholar] [CrossRef]
- Albavera, L.J.; Pérez, M.D.; Leyte, D.J.M.; Garrido, A.G.; Molina, T.V. The Role of the ATP-Binding Cassette A1 (ABCA1) in Human Disease. Int. J. Mol. Sci. 2021, 22, 1593. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, X.; Li, X.; Liu, O.; Zhou, Y.; Guo, P.; Dong, Z.; Wu, C. Phenylpropanoid glucosides from Tadehagi triquetrum inhibit oxLDL-evoked foam cell formation through modulating cholesterol homeostasis in RAW264.7 macrophages. Nat. Prod. Res. 2019, 33, 893–896. [Google Scholar] [CrossRef]
- Medina, M.V.; Sapochnik, D.; Solá, M.G.; Coso, O. Regulation of the Expression of Heme Oxygenase-1: Signal Transduction, Gene Promoter Activation, and Beyond. Antioxid. Redox Signal. 2020, 32, 1033–1044. [Google Scholar] [CrossRef]
- Xu, X.; Li, Q.; Pang, L.; Huang, G.; Huang, J.; Shi, M.; Sun, X.; Wang, Y. Arctigenin promotes cholesterol efflux from THP-1 macrophages through PPAR-γ/LXR-α signaling pathway. Biochem. Biophys. Res. Commun. 2013, 441, 321–326. [Google Scholar] [CrossRef]
- Jung, C.G.; Horike, H.; Cha, B.Y.; Uhm, K.O.; Yamauchi, R.; Yamaguchi, T.; Hosono, T.; Iida, K.; Woo, J.T.; Michikawa, M. Honokiol increases ABCA1 expression level by activating retinoid X receptor beta. Biol. Pharm. Bull. 2010, 33, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rotter, S.; Ladurner, A.; Heiss, E.H.; Oberlies, N.H.; Dirsch, V.M.; Atanasov, A.G. Silymarin Constituents Enhance ABCA1 Expression in THP-1 Macrophages. Molecules 2016, 21, 55. [Google Scholar] [CrossRef]
- Jiang, J.; Mo, Z.C.; Yin, K.; Zhao, G.J.; Lv, J.C.; Ouyang, X.P.; Jiang, Z.S.; Fu, Y.; Tang, T.K. Epigallocatechin-3-gallate prevents TNF-α-induced NF-κB activation thereby upregulating ABCA1 via the Nrf2/Keap1 pathway in macrophage foam cells. Int. J. Mol. Med. 2012, 29, 946–956. [Google Scholar]
- Lin, X.L.; Hu, C.J.; Liu, Y.B.; Hu, X.M.; Fan, X.J.; Zou, W.W.; Pan, J.Q.; Zhou, W.Q.; Peng, M.W.; Gu, C.H. Allicin induces the upregulation of ABCA1 expression via PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells. J. Mol. Med. 2017, 39, 1452–1460. [Google Scholar] [CrossRef]
- Huang, C.S.; Lin, A.H.; Liu, C.T.; Tsai, C.W.; Chang, I.S.; Chen, H.W.; Lii, C.K. Isothiocyanates protect against oxidized LDL-induced endothelial dysfunction by upregulating Nrf2-dependent antioxidation and suppressing NFκB activation. Mol. Nutr. Food Res. 2013, 57, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.C.; Su, K.H.; Kou, Y.R.; Shyue, S.K.; Ching, L.C.; Yu, Y.B.; Wu, Y.L.; Pan, C.C.; Lee, T.S. α-Lipoic acid ameliorates foam cell formation via liver X receptor α-dependent upregulation of ATP-binding cassette transporters A1 and G1. Free Radic. Biol. Med. 2011, 50, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, Z.; Zhou, H.; Li, Y.; Li, X.; Lin, Y.; Li, W.; Shi, Y.; Zhou, M.S. Puerarin Inhibits oxLDL-Induced Macrophage Activation and Foam Cell Formation in Human THP1 Macrophage. Biomed. Res. Int. 2015, 2015, 403616. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Padilla, E.; Redondo, S.; Gordillo-Moscoso, A.; Tejerina, T. Kaempferol inhibits apoptosis in vascular smooth muscle induced by a component of oxidized LDL. Eur. J. Pharmacol. 2006, 529, 79–83. [Google Scholar] [CrossRef]
- Hirakata, M.; Tozawa, R.; Imura, Y.; Sugiyama, Y. Comparison of the effects of pioglitazone and rosiglitazone on macrophage foam cell formation. Biochem. Biophys. Res. Commun. 2004, 323, 782–788. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipek, A.; Sadowska, A.; Skłodowska, M.; Muskała, M.; Czepielewska, E. Syringin and Phillygenin—Natural Compounds with a Potential Role in Preventing Lipid Deposition in Macrophages in the Context of Human Atherosclerotic Plaque. Int. J. Mol. Sci. 2025, 26, 6444. https://doi.org/10.3390/ijms26136444
Filipek A, Sadowska A, Skłodowska M, Muskała M, Czepielewska E. Syringin and Phillygenin—Natural Compounds with a Potential Role in Preventing Lipid Deposition in Macrophages in the Context of Human Atherosclerotic Plaque. International Journal of Molecular Sciences. 2025; 26(13):6444. https://doi.org/10.3390/ijms26136444
Chicago/Turabian StyleFilipek, Agnieszka, Agnieszka Sadowska, Monika Skłodowska, Maja Muskała, and Edyta Czepielewska. 2025. "Syringin and Phillygenin—Natural Compounds with a Potential Role in Preventing Lipid Deposition in Macrophages in the Context of Human Atherosclerotic Plaque" International Journal of Molecular Sciences 26, no. 13: 6444. https://doi.org/10.3390/ijms26136444
APA StyleFilipek, A., Sadowska, A., Skłodowska, M., Muskała, M., & Czepielewska, E. (2025). Syringin and Phillygenin—Natural Compounds with a Potential Role in Preventing Lipid Deposition in Macrophages in the Context of Human Atherosclerotic Plaque. International Journal of Molecular Sciences, 26(13), 6444. https://doi.org/10.3390/ijms26136444





