Calprotectin as a Biomarker for Infectious Diseases: A Comparative Review with Conventional Inflammatory Markers
Abstract
1. Introduction
Aims of the Review
2. Bacterial Infections—An Overview
3. Inflammation and Chronic Inflammatory States
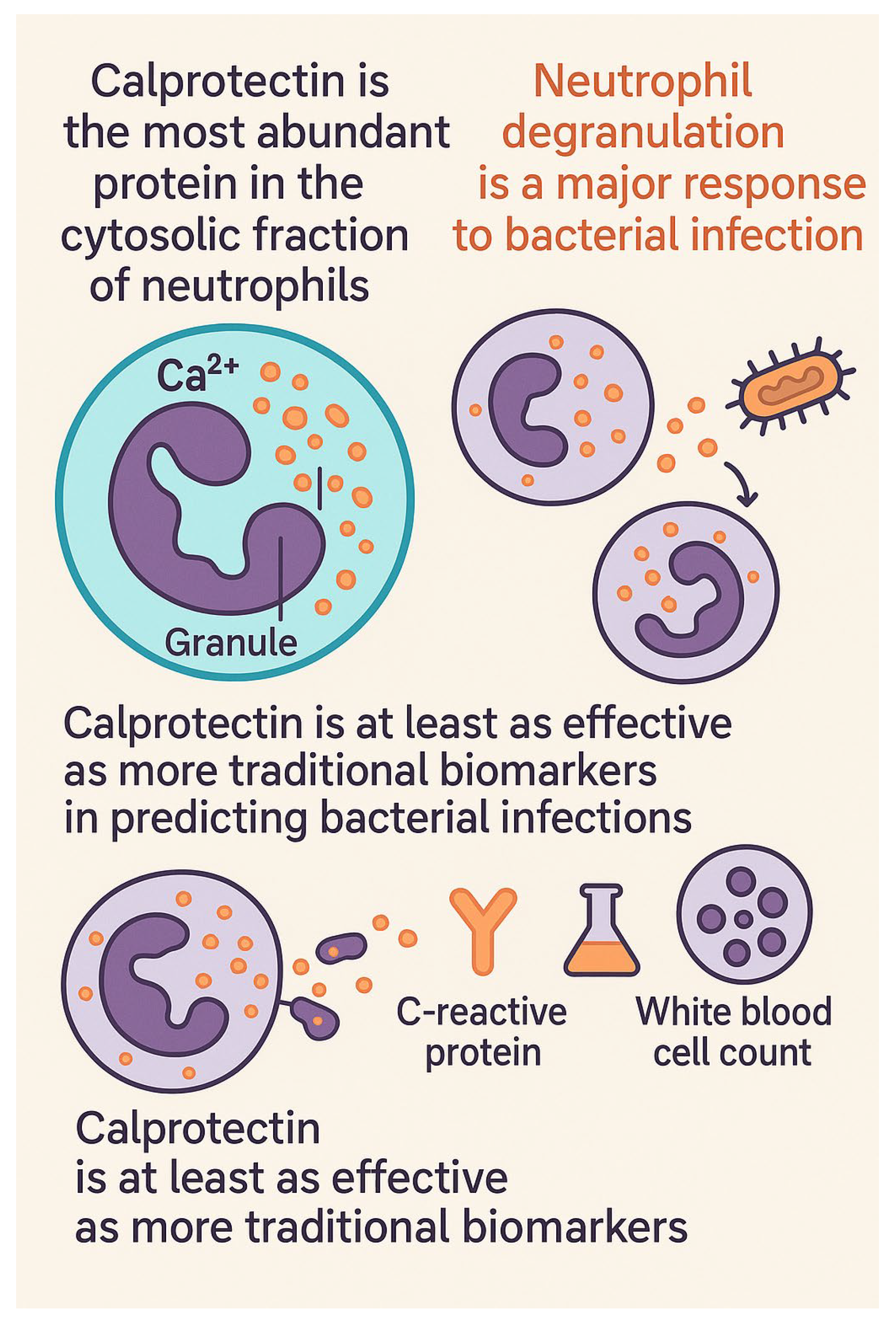
4. Physicochemical Properties and Pathophysiological Data of Calprotectin
4.1. Calprotectin’s Role in Infection Diagnosis
4.2. Method for Calprotectin Analysis: Particle-Enhanced Turbidimetric Immunoassay (PETIA)
5. White Blood Cell (WBC) Count
6. Neutrophil Count
7. C-Reactive Protein (CRP)
8. Procalcitonin (PCT)
9. Interleukin-6 (IL-6)
10. Neutrophil Gelatinase-Associated Lipocalin (NGAL)/Lipocalin-2 (LCN2)
11. Heparin-Binding Protein (HBP)
12. Neutrophil-Derived Cytokines
13. Fecal Calprotectin as a Biomarker in Acute Intestinal Infections
14. Schematic Presentations of Inflammatory Biomarkers
15. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Fagerhol, M.K.; Dale, I.; Anderson, T. Release and quantitation of a leucocyte derived protein (L1). Scand. J. Haematol. 1980, 24, 393–398. [Google Scholar] [CrossRef]
- Sander, J.; Fagerhol, M.K.; Bakken, J.S.; Dale, I. Plasma levels of the leucocyte L1 protein in febrile conditions: Relation to aetiology, number of leucocytes in blood, blood sedimentation reaction and C-reactive protein. Scand. J. Clin. Lab. Investig. 1984, 44, 357–362. [Google Scholar] [CrossRef]
- Roth, J.; Goebeler, M.; Sorg, C. S100A8 and S100A9 in inflammatory diseases. Lancet 2001, 357, 1041. [Google Scholar] [CrossRef]
- Srikrishna, G.; Panneerselvam, K.; Westphal, V.; Abraham, V.; Varki, A.; Freeze, H.H. Two proteins modulating transendothelial migration of leukocytes recognize novel carboxylated glycans on endothelial cells. J. Immunol. 2001, 166, 4678–4688. [Google Scholar] [CrossRef]
- Sampson, B.; Fagerhol, M.K.; Sunderkötter, C.; Golden, B.E.; Richmond, P.; Klein, N.; Kovar, I.Z.; Beattie, J.H.; Wolska-Kusnierz, B.; Saito, Y.; et al. Hyperzincaemia and hypercalprotectinaemia: A new disorder of zinc metabolism. Lancet 2002, 360, 1742–1745. [Google Scholar] [CrossRef]
- Goebeler, M.; Roth, J.; van den Bos, C.; Ader, G.; Sorg, C. Increase of calcium levels in epithelial cells induces translocation of calcium-binding proteins migration inhibitory factor-related protein 8 (MRP8) and MRP14 to keratin intermediate filaments. Biochem. J. 1995, 309 Pt 2, 419–424. [Google Scholar] [CrossRef]
- Edgeworth, J.; Gorman, M.; Bennett, R.; Freemont, P.; Hogg, N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J. Biol. Chem. 1991, 266, 7706–7713. [Google Scholar] [CrossRef]
- Chai, Q.; Zhang, Y.; Liu, C.H. Mycobacterium tuberculosis: An Adaptable Pathogen Associated with Multiple Human Diseases. Front. Cell Infect. Microbiol. 2018, 8, 158. [Google Scholar] [CrossRef]
- Marchesan, J.T.; Girnary, M.S.; Moss, K.; Monaghan, E.T.; Egnatz, G.J.; Jiao, Y.; Zhang, S.; Beck, J.; Swanson, K.V. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontology 2000 2020, 82, 93–114. [Google Scholar] [CrossRef]
- Wilson, D.J.; Gabriel, E.; Leatherbarrow, A.J.; Cheesbrough, J.; Gee, S.; Bolton, E.; Fox, A.; Fearnhead, P.; Hart, C.A.; Diggle, P.J. Tracing the source of campylobacteriosis. PLoS Genet. 2008, 4, e1000203. [Google Scholar] [CrossRef]
- Pen, D.L.; Yan, G.F.; He, L.Y.; Yan, W.L.; Chen, W.M.; Liu, J.; Ying, J.Y.; Wang, C.Q.; Lu, G.P. The role of bacterial colonization of ventilator circuit in development of ventilator-associated pneumonia: A prospective observational cohort study. Clin. Microbiol. Infect. 2021, 27, e461–e467. [Google Scholar] [CrossRef]
- Orsini, J.; Frawley, B.J.; Gawlak, H.; Gooch, R.; Escovar, J. Severe Sepsis with Septic Shock as a Consequence of a Severe Community-Acquired Pneumonia Resulting from a Combined Legionella pneumophila and Streptococcus pneumoniae Infection. Cureus 2020, 12, e10966. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Dane, E.L.; O’Toole, G.A.; Grinstaff, M.W. Exploiting dendrimer multivalency to combat emerging and re-emerging infectious diseases. Mol. Pharm. 2012, 9, 342–354. [Google Scholar] [CrossRef]
- Ning, X.; Lee, S.; Wang, Z.; Kim, D.; Stubblefield, B.; Gilbert, E.; Murthy, N. Maltodextrin-based imaging probes detect bacteria in vivo with high sensitivity and specificity. Nat. Mater. 2011, 10, 602–607. [Google Scholar] [CrossRef]
- Heemskerk, A.D.; Bang, N.D.; Mai, N.T.; Chau, T.T.; Phu, N.H.; Loc, P.P.; Chau, N.V.; Hien, T.T.; Dung, N.H.; Lan, N.T.; et al. Intensified Antituberculosis Therapy in Adults with Tuberculous Meningitis. N. Engl. J. Med. 2016, 374, 124–134. [Google Scholar] [CrossRef]
- Broz, P.; Monack, D.M. Newly described pattern recognition receptors team up against intracellular pathogens. Nat. Rev. Immunol. 2013, 13, 551–565. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Roers, A.; Hiller, B.; Hornung, V. Recognition of Endogenous Nucleic Acids by the Innate Immune System. Immunity 2016, 44, 739–754. [Google Scholar] [CrossRef]
- West, A.P.; Shadel, G.S. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 2017, 17, 363–375. [Google Scholar] [CrossRef]
- Hopfner, K.P.; Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Reinhart, K.; Daniels, R.; Kissoon, N.; Machado, F.R.; Schachter, R.D.; Finfer, S. Recognizing Sepsis as a Global Health Priority—A WHO Resolution. N. Engl. J. Med. 2017, 377, 414–417. [Google Scholar] [CrossRef]
- Perner, A.; Gordon, A.C.; De Backer, D.; Dimopoulos, G.; Russell, J.A.; Lipman, J.; Jensen, J.U.; Myburgh, J.; Singer, M.; Bellomo, R.; et al. Sepsis: Frontiers in diagnosis, resuscitation and antibiotic therapy. Intensive Care Med. 2016, 42, 1958–1969. [Google Scholar] [CrossRef]
- Sakr, Y.; Jaschinski, U.; Wittebole, X.; Szakmany, T.; Lipman, J.; Ñamendys-Silva, S.A.; Martin-Loeches, I.; Leone, M.; Lupu, M.N.; Vincent, J.L. Sepsis in Intensive Care Unit Patients: Worldwide Data From the Intensive Care over Nations Audit. Open Forum Infect. Dis. 2018, 5, ofy313. [Google Scholar] [CrossRef]
- Bennett, S.R. Sepsis in the intensive care unit. Surgery 2015, 33, 565–571. [Google Scholar] [CrossRef]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial Resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef]
- Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [CrossRef]
- Xu, S.; Venge, P. Lipocalins as biochemical markers of disease. Biochim. Biophys. Acta 2000, 1482, 298–307. [Google Scholar] [CrossRef]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef]
- Sehgal, M.; Ladd, H.J.; Totapally, B. Trends in Epidemiology and Microbiology of Severe Sepsis and Septic Shock in Children. Hosp. Pediatr. 2020, 10, 1021–1030. [Google Scholar] [CrossRef]
- Kaukonen, K.M.; Bailey, M.; Pilcher, D.; Cooper, D.J.; Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 2015, 372, 1629–1638. [Google Scholar] [CrossRef]
- Joynes, E. More challenges around sepsis: Definitions and diagnosis. J. Thorac. Dis. 2016, 8, E1467–E1469. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Tang, F.; Yuan, H.; Li, X.; Qiao, L. Effect of delayed antibiotic use on mortality outcomes in patients with sepsis or septic shock: A systematic review and meta-analysis. Int. Immunopharmacol. 2024, 129, 111616. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Ferreira, F.L.; Bota, D.P.; Bross, A.; Mélot, C.; Vincent, J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001, 286, 1754–1758. [Google Scholar] [CrossRef]
- Riedel, S. Predicting Bacterial Versus Viral Infection, or None of the Above: Current and Future Prospects of Biomarkers. Clin. Lab. Med. 2019, 39, 453–472. [Google Scholar] [CrossRef]
- Kapasi, A.J.; Dittrich, S.; González, I.J.; Rodwell, T.C. Host Biomarkers for Distinguishing Bacterial from Non-Bacterial Causes of Acute Febrile Illness: A Comprehensive Review. PLoS ONE 2016, 11, e0160278. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Pecchi, E.; Dallaporta, M.; Jean, A.; Thirion, S.; Troadec, J.D. Prostaglandins and sickness behavior: Old story, new insights. Physiol. Behav. 2009, 97, 279–292. [Google Scholar] [CrossRef]
- Eaves-Pyles, T.; Allen, C.A.; Taormina, J.; Swidsinski, A.; Tutt, C.B.; Jezek, G.E.; Islas-Islas, M.; Torres, A.G. Escherichia coli isolated from a Crohn’s disease patient adheres, invades, and induces inflammatory responses in polarized intestinal epithelial cells. Int. J. Med. Microbiol. 2008, 298, 397–409. [Google Scholar] [CrossRef]
- Ferguson, L.R. Chronic inflammation and mutagenesis. Mutat. Res. 2010, 690, 3–11. [Google Scholar] [CrossRef]
- Odink, K.; Cerletti, N.; Brüggen, J.; Clerc, R.G.; Tarcsay, L.; Zwadlo, G.; Gerhards, G.; Schlegel, R.; Sorg, C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature 1987, 330, 80–82. [Google Scholar] [CrossRef]
- Fagerhol, M.K.; Dale, I.; Andersson, T. A radioimmunoassay for a granulocyte protein as a marker in studies on the turnover of such cells. Bull. Eur. Physiopathol. Respir. 1980, 16, 273–282. [Google Scholar] [CrossRef]
- Dale, I.; Brandtzaeg, P.; Fagerhol, M.K.; Scott, H. Distribution of a new myelomonocytic antigen (L1) in human peripheral blood leukocytes. Immunofluorescence and immunoperoxidase staining features in comparison with lysozyme and lactoferrin. Am. J. Clin. Pathol. 1985, 84, 24–34. [Google Scholar] [CrossRef]
- Calprotectin-4GGF.Png. Available online: https://creativecommons.org/licenses/by-sa/4.0/deed.en (accessed on 14 May 2025).
- Schenten, V.; Bréchard, S.; Plançon, S.; Melchior, C.; Frippiat, J.P.; Tschirhart, E.J. iPLA2, a novel determinant in Ca2+- and phosphorylation-dependent S100A8/A9 regulated NOX2 activity. Biochim. Biophys. Acta 2010, 1803, 840–847. [Google Scholar] [CrossRef]
- Roth, J.; Burwinkel, F.; van den Bos, C.; Goebeler, M.; Vollmer, E.; Sorg, C. MRP8 and MRP14, S-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner. Blood 1993, 82, 1875–1883. [Google Scholar] [CrossRef][Green Version]
- Vogl, T.; Ludwig, S.; Goebeler, M.; Strey, A.; Thorey, I.S.; Reichelt, R.; Foell, D.; Gerke, V.; Manitz, M.P.; Nacken, W.; et al. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood 2004, 104, 4260–4268. [Google Scholar] [CrossRef]
- Foell, D.; Roth, J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004, 50, 3762–3771. [Google Scholar] [CrossRef]
- Korndörfer, I.P.; Brueckner, F.; Skerra, A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J. Mol. Biol. 2007, 370, 887–898. [Google Scholar] [CrossRef]
- Vogl, T.; Tenbrock, K.; Ludwig, S.; Leukert, N.; Ehrhardt, C.; van Zoelen, M.A.; Nacken, W.; Foell, D.; van der Poll, T.; Sorg, C.; et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 2007, 13, 1042–1049. [Google Scholar] [CrossRef]
- Yen, T.; Harrison, C.A.; Devery, J.M.; Leong, S.; Iismaa, S.E.; Yoshimura, T.; Geczy, C.L. Induction of the S100 chemotactic protein, CP-10, in murine microvascular endothelial cells by proinflammatory stimuli. Blood 1997, 90, 4812–4821. [Google Scholar] [CrossRef]
- Nukui, T.; Ehama, R.; Sakaguchi, M.; Sonegawa, H.; Katagiri, C.; Hibino, T.; Huh, N.H. S100A8/A9, a key mediator for positive feedback growth stimulation of normal human keratinocytes. J. Cell Biochem. 2008, 104, 453–464. [Google Scholar] [CrossRef]
- Van Lent, P.L.; Grevers, L.C.; Blom, A.B.; Arntz, O.J.; van de Loo, F.A.; van der Kraan, P.; Abdollahi-Roodsaz, S.; Srikrishna, G.; Freeze, H.; Sloetjes, A.; et al. Stimulation of chondrocyte-mediated cartilage destruction by S100A8 in experimental murine arthritis. Arthritis Rheum. 2008, 58, 3776–3787. [Google Scholar] [CrossRef]
- Youssef, P.; Roth, J.; Frosch, M.; Costello, P.; Fitzgerald, O.; Sorg, C.; Bresnihan, B. Expression of myeloid related proteins (MRP) 8 and 14 and the MRP8/14 heterodimer in rheumatoid arthritis synovial membrane. J. Rheumatol. 1999, 26, 2523–2528. [Google Scholar]
- Van Lent, P.L.; Grevers, L.; Blom, A.B.; Sloetjes, A.; Mort, J.S.; Vogl, T.; Nacken, W.; van den Berg, W.B.; Roth, J. Myeloid-related proteins S100A8/S100A9 regulate joint inflammation and cartilage destruction during antigen-induced arthritis. Ann. Rheum. Dis. 2008, 67, 1750–1758. [Google Scholar] [CrossRef]
- Ehrchen, J.M.; Sunderkötter, C.; Foell, D.; Vogl, T.; Roth, J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009, 86, 557–566. [Google Scholar] [CrossRef]
- Kharbanda, A.B.; Rai, A.J.; Cosme, Y.; Liu, K.; Dayan, P.S. Novel serum and urine markers for pediatric appendicitis. Acad. Emerg. Med. 2012, 19, 56–62. [Google Scholar] [CrossRef]
- Sohnle, P.G.; Hunter, M.J.; Hahn, B.; Chazin, W.J. Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14). J. Infect. Dis. 2000, 182, 1272–1275. [Google Scholar] [CrossRef]
- Loser, K.; Vogl, T.; Voskort, M.; Lueken, A.; Kupas, V.; Nacken, W.; Klenner, L.; Kuhn, A.; Foell, D.; Sorokin, L.; et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat. Med. 2010, 16, 713–717. [Google Scholar] [CrossRef]
- Jonsson, N.; Nilsen, T.; Gille-Johnson, P.; Bell, M.; Martling, C.R.; Larsson, A.; Mårtensson, J. Calprotectin as an early biomarker of bacterial infections in critically ill patients: An exploratory cohort assessment. Crit. Care Resusc. 2017, 19, 205–213. [Google Scholar] [CrossRef]
- Havelka, A.; Larsson, A.O.; Mårtensson, J.; Bell, M.; Hultström, M.; Lipcsey, M.; Eriksson, M. Analysis of Calprotectin as an Early Marker of Infections Is Economically Advantageous in Intensive Care-Treated Patients. Biomedicines 2023, 11, 2156. [Google Scholar] [CrossRef]
- Larsson, A.; Tydén, J.; Johansson, J.; Lipcsey, M.; Bergquist, M.; Kultima, K.; Mandic-Havelka, A. Calprotectin is superior to procalcitonin as a sepsis marker and predictor of 30-day mortality in intensive care patients. Scand. J. Clin. Lab. Investig. 2020, 80, 156–161. [Google Scholar] [CrossRef]
- Sejersen, K.; Havelka, A.; Sanchez Salas, P.; Larsson, A. Early kinetics of calprotectin in plasma following inguinal hernia surgery. Innate Immun. 2022, 28, 49–54. [Google Scholar] [CrossRef]
- Havelka, A.; Sejersen, K.; Venge, P.; Pauksens, K.; Larsson, A. Calprotectin, a new biomarker for diagnosis of acute respiratory infections. Sci. Rep. 2020, 10, 4208. [Google Scholar] [CrossRef]
- Wirtz, T.H.; Buendgens, L.; Weiskirchen, R.; Loosen, S.H.; Haehnsen, N.; Puengel, T.; Abu Jhaisha, S.; Brozat, J.F.; Hohlstein, P.; Koek, G.; et al. Association of Serum Calprotectin Concentrations with Mortality in Critically Ill and Septic Patients. Diagnostics 2020, 10, 990. [Google Scholar] [CrossRef]
- Silvin, A.; Chapuis, N.; Dunsmore, G.; Goubet, A.G.; Dubuisson, A.; Derosa, L.; Almire, C.; Hénon, C.; Kosmider, O.; Droin, N.; et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell 2020, 182, 1401–1418.e1418. [Google Scholar] [CrossRef]
- Carnazzo, V.; Redi, S.; Basile, V.; Natali, P.; Gulli, F.; Equitani, F.; Marino, M.; Basile, U. Calprotectin: Two sides of the same coin. Rheumatology 2024, 63, 26–33. [Google Scholar] [CrossRef]
- Waldecker-Gall, S.; Waldecker, C.B.; Babel, N.; Baraliakos, X.; Seibert, F.; Westhoff, T.H. Urinary calprotectin as a diagnostic tool for detecting significant bacteriuria. Sci. Rep. 2024, 14, 12230. [Google Scholar] [CrossRef]
- Hantouly, A.T.; Salameh, M.; Toubasi, A.A.; Salman, L.A.; Alzobi, O.; Ahmed, A.F.; Hameed, S.; Zikria, B.; Ahmed, G. Synovial fluid calprotectin in diagnosing periprosthetic joint infection: A meta-analysis. Int. Orthop. 2022, 46, 971–981. [Google Scholar] [CrossRef]
- Suren, C.; Lazic, I.; Haller, B.; Pohlig, F.; von Eisenhart-Rothe, R.; Prodinger, P. The synovial fluid calprotectin lateral flow test for the diagnosis of chronic prosthetic joint infection in failed primary and revision total hip and knee arthroplasty. Int. Orthop. 2023, 47, 929–944. [Google Scholar] [CrossRef]
- Lazic, I.; Burdach, A.; Pohlig, F.; von Eisenhart-Rothe, R.; Suren, C. Utility of synovial calprotectin lateral flow test to exclude chronic prosthetic joint infection in periprosthetic fractures: A prospective cohort study. Sci. Rep. 2022, 12, 18385. [Google Scholar] [CrossRef]
- Lin, Q.; Huang, E.; Fan, K.; Zhang, Z.; Shangguan, H.; Zhang, W.; Fang, W.; Ou, Q.; Liu, X. Cerebrospinal Fluid Neutrophil Gelatinase-Associated Lipocalin as a Novel Biomarker for Postneurosurgical Bacterial Meningitis: A Prospective Observational Cohort Study. Neurosurgery 2024, 95, 1418–1428. [Google Scholar] [CrossRef]
- Dastych, M.; Gottwaldová, J.; Čermáková, Z. Calprotectin and lactoferrin in the cerebrospinal fluid; biomarkers utilisable for differential diagnostics of bacterial and aseptic meningitis? Clin. Chem. Lab. Med. 2015, 53, 599–603. [Google Scholar] [CrossRef]
- Sánchez-Otero, N.; Blanco-Prieto, S.; Páez de la Cadena, M.; Vázquez-Iglesias, L.; Fernández-Villar, A.; Botana-Rial, M.I.; Rodríguez-Berrocal, F.J. Calprotectin: A novel biomarker for the diagnosis of pleural effusion. Br. J. Cancer 2012, 107, 1876–1882. [Google Scholar] [CrossRef]
- Hatemi, I.; Hatemi, G.; Çelik, A.F. Systemic vasculitis and the gut. Curr. Opin. Rheumatol. 2017, 29, 33–38. [Google Scholar] [CrossRef]
- Åsberg, A.; Løfblad, L.; Felic, A.; Hov, G.G. Measuring calprotectin in plasma and blood with a fully automated turbidimetric assay. Scand. J. Clin. Lab. Investig. 2019, 79, 50–57. [Google Scholar] [CrossRef]
- Larsson, A.; Sjöquist, J. Chicken antibodies: A tool to avoid false positive results by rheumatoid factor in latex fixation tests. J. Immunol. Methods 1988, 108, 205–208. [Google Scholar] [CrossRef]
- Larsson, A.; Mellstedt, H. Chicken antibodies: A tool to avoid interference by human anti-mouse antibodies in ELISA after in vivo treatment with murine monoclonal antibodies. Hybridoma 1992, 11, 33–39. [Google Scholar] [CrossRef]
- Hoefer, I.E.; Grundmann, S.; van Royen, N.; Voskuil, M.; Schirmer, S.H.; Ulusans, S.; Bode, C.; Buschmann, I.R.; Piek, J.J. Leukocyte subpopulations and arteriogenesis: Specific role of monocytes, lymphocytes and granulocytes. Atherosclerosis 2005, 181, 285–293. [Google Scholar] [CrossRef]
- Aloulou, M.; Fazilleau, N. Regulation of B cell responses by distinct populations of CD4 T cells. Biomed. J. 2019, 42, 243–251. [Google Scholar] [CrossRef]
- Foy, B.H.; Sundt, T.M.; Carlson, J.C.T.; Aguirre, A.D.; Higgins, J.M. Human acute inflammatory recovery is defined by co-regulatory dynamics of white blood cell and platelet populations. Nat. Commun. 2022, 13, 4705. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2020, 108, 377–396. [Google Scholar] [CrossRef]
- Hidalgo, A.; Chilvers, E.R.; Summers, C.; Koenderman, L. The Neutrophil Life Cycle. Trends Immunol. 2019, 40, 584–597. [Google Scholar] [CrossRef]
- Greenlee-Wacker, M.C. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 2016, 273, 357–370. [Google Scholar] [CrossRef]
- Natoli, G.; Ostuni, R. Adaptation and memory in immune responses. Nat. Immunol. 2019, 20, 783–792. [Google Scholar] [CrossRef]
- Monga, I.; Kaur, K.; Dhanda, S.K. Revisiting hematopoiesis: Applications of the bulk and single-cell transcriptomics dissecting transcriptional heterogeneity in hematopoietic stem cells. Brief. Funct. Genom. 2022, 21, 159–176. [Google Scholar] [CrossRef]
- Chabot-Richards, D.S.; George, T.I. Leukocytosis. Int. J. Lab. Hematol. 2014, 36, 279–288. [Google Scholar] [CrossRef]
- Riley, L.K.; Rupert, J. Evaluation of Patients with Leukocytosis. Am. Fam. Physician 2015, 92, 1004–1011. [Google Scholar]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Gibson, C.; Berliner, N. How we evaluate and treat neutropenia in adults. Blood 2014, 124, 1251–1258; quiz 1378. [Google Scholar] [CrossRef]
- Schatzman, A.; Vandenheuvel, J.; Villalobos, T.; Rooney, K. Transient leukopenia, thrombocytopenia, and severe neutropenia associated with acute SARS-CoV-2 infection. Pediatr. Blood Cancer 2021, 68, e29105. [Google Scholar] [CrossRef]
- Christen, D.; Brümmendorf, T.H.; Panse, J. Leukopenia—A Diagnostic Guideline for the Clinical Routine. Dtsch. Med. Wochenschr. 2017, 142, 1744–1749. [Google Scholar] [CrossRef]
- Bhatt, V.; Saleem, A. Review: Drug-induced neutropenia—Pathophysiology, clinical features, and management. Ann. Clin. Lab. Sci. 2004, 34, 131–137. [Google Scholar]
- Boccia, R.; Glaspy, J.; Crawford, J.; Aapro, M. Chemotherapy-Induced Neutropenia and Febrile Neutropenia in the US: A Beast of Burden That Needs to Be Tamed? Oncologist 2022, 27, 625–636. [Google Scholar] [CrossRef]
- Weycker, D.; Barron, R.; Kartashov, A.; Legg, J.; Lyman, G.H. Incidence, treatment, and consequences of chemotherapy-induced febrile neutropenia in the inpatient and outpatient settings. J. Oncol. Pharm. Pract. 2014, 20, 190–198. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
- Horne, B.D.; Anderson, J.L.; John, J.M.; Weaver, A.; Bair, T.L.; Jensen, K.R.; Renlund, D.G.; Muhlestein, J.B. Which white blood cell subtypes predict increased cardiovascular risk? J. Am. Coll. Cardiol. 2005, 45, 1638–1643. [Google Scholar] [CrossRef]
- Gao, S.; Deng, Y.; Wu, J.; Zhang, L.; Deng, F.; Zhou, J.; Yuan, Z.; Wang, L. Eosinophils count in peripheral circulation is associated with coronary artery disease. Atherosclerosis 2019, 286, 128–134. [Google Scholar] [CrossRef]
- Van der Wal, A.C.; Becker, A.E.; van der Loos, C.M.; Das, P.K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994, 89, 36–44. [Google Scholar] [CrossRef]
- Parke, Å.; Unge, C.; Yu, D.; Sundén-Cullberg, J.; Strålin, K. Plasma calprotectin as an indicator of need of transfer to intensive care in patients with suspected sepsis at the emergency department. BMC Emerg. Med. 2023, 23, 16. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, L.; Xu, M.; Chen, L.; Lu, W.; Wang, W. Serum calprotectin as a prognostic predictor in severe traumatic brain injury. Clin. Chim. Acta 2021, 520, 101–107. [Google Scholar] [CrossRef]
- Larsson, A.; Lipcsey, M.; Sjölin, J.; Hansson, L.O.; Eriksson, M.B. Slight increase of serum S-100B during porcine endotoxemic shock may indicate blood-brain barrier damage. Anesth. Analg. 2005, 101, 1465–1469. [Google Scholar] [CrossRef]
- Bohn, M.K.; Havelka, A.; Eriksson, M.; Adeli, K. Validation of Serum Calprotectin Relative to Other Biomarkers of Infection in Febrile Infants Presenting to the Emergency Department. Antibiotics 2024, 13, 425. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef]
- Borregaard, N. Neutrophils, from marrow to microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef]
- Lu, T.; Kobayashi, S.D.; Quinn, M.T.; Deleo, F.R. A NET Outcome. Front. Immunol. 2012, 3, 365. [Google Scholar] [CrossRef]
- Duffin, R.; Leitch, A.E.; Fox, S.; Haslett, C.; Rossi, A.G. Targeting granulocyte apoptosis: Mechanisms, models, and therapies. Immunol. Rev. 2010, 236, 28–40. [Google Scholar] [CrossRef]
- Schenten, V.; Plançon, S.; Jung, N.; Hann, J.; Bueb, J.L.; Bréchard, S.; Tschirhart, E.J.; Tolle, F. Secretion of the Phosphorylated Form of S100A9 from Neutrophils Is Essential for the Proinflammatory Functions of Extracellular S100A8/A9. Front. Immunol. 2018, 9, 447. [Google Scholar] [CrossRef]
- Chan, J.K.; Roth, J.; Oppenheim, J.J.; Tracey, K.J.; Vogl, T.; Feldmann, M.; Horwood, N.; Nanchahal, J. Alarmins: Awaiting a clinical response. J. Clin. Investig. 2012, 122, 2711–2719. [Google Scholar] [CrossRef]
- Rørvig, S.; Østergaard, O.; Heegaard, N.H.; Borregaard, N. Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: Correlation with transcriptome profiling of neutrophil precursors. J. Leukoc. Biol. 2013, 94, 711–721. [Google Scholar] [CrossRef]
- Cassatella, M.A.; Östberg, N.K.; Tamassia, N.; Soehnlein, O. Biological Roles of Neutrophil-Derived Granule Proteins and Cytokines. Trends Immunol. 2019, 40, 648–664. [Google Scholar] [CrossRef]
- Nauseef, W.M.; Borregaard, N. Neutrophils at work. Nat. Immunol. 2014, 15, 602–611. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017, 17, 248–261. [Google Scholar] [CrossRef]
- Ortega-Gomez, A.; Salvermoser, M.; Rossaint, J.; Pick, R.; Brauner, J.; Lemnitzer, P.; Tilgner, J.; de Jong, R.J.; Megens, R.T.; Jamasbi, J.; et al. Cathepsin G Controls Arterial But Not Venular Myeloid Cell Recruitment. Circulation 2016, 134, 1176–1188. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef]
- Zenaro, E.; Pietronigro, E.; Della Bianca, V.; Piacentino, G.; Marongiu, L.; Budui, S.; Turano, E.; Rossi, B.; Angiari, S.; Dusi, S.; et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 2015, 21, 880–886. [Google Scholar] [CrossRef]
- Scapini, P.; Cassatella, M.A. Social networking of human neutrophils within the immune system. Blood 2014, 124, 710–719. [Google Scholar] [CrossRef]
- Caielli, S.; Banchereau, J.; Pascual, V. Neutrophils come of age in chronic inflammation. Curr. Opin. Immunol. 2012, 24, 671–677. [Google Scholar] [CrossRef]
- Soehnlein, O.; Wantha, S.; Simsekyilmaz, S.; Döring, Y.; Megens, R.T.; Mause, S.F.; Drechsler, M.; Smeets, R.; Weinandy, S.; Schreiber, F.; et al. Neutrophil-derived cathelicidin protects from neointimal hyperplasia. Sci. Transl. Med. 2011, 3, 103ra198. [Google Scholar] [CrossRef]
- Jones, H.R.; Robb, C.T.; Perretti, M.; Rossi, A.G. The role of neutrophils in inflammation resolution. Semin. Immunol. 2016, 28, 137–145. [Google Scholar] [CrossRef]
- Ferraro, B.; Leoni, G.; Hinkel, R.; Ormanns, S.; Paulin, N.; Ortega-Gomez, A.; Viola, J.R.; de Jong, R.; Bongiovanni, D.; Bozoglu, T.; et al. Pro-Angiogenic Macrophage Phenotype to Promote Myocardial Repair. J. Am. Coll. Cardiol. 2019, 73, 2990–3002. [Google Scholar] [CrossRef]
- Butin-Israeli, V.; Bui, T.M.; Wiesolek, H.L.; Mascarenhas, L.; Lee, J.J.; Mehl, L.C.; Knutson, K.R.; Adam, S.A.; Goldman, R.D.; Beyder, A.; et al. Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. J. Clin. Investig. 2019, 129, 712–726. [Google Scholar] [CrossRef]
- Lipcsey, M.; Hanslin, K.; Stålberg, J.; Smekal, D.; Larsson, A. The time course of calprotectin liberation from human neutrophil granulocytes after Escherichia coli and endotoxin challenge. Innate Immun. 2019, 25, 369–373. [Google Scholar] [CrossRef]
- Marsh, J.C.; Boggs, D.R.; Cartwright, G.E.; Wintrobe, M.M. Neutrophil kinetics in acute infection. J. Clin. Investig. 1967, 46, 1943–1953. [Google Scholar] [CrossRef]
- Ishimine, N.; Honda, T.; Yoshizawa, A.; Kawasaki, K.; Sugano, M.; Kobayashi, Y.; Matsumoto, T. Combination of white blood cell count and left shift level real-timely reflects a course of bacterial infection. J. Clin. Lab. Anal. 2013, 27, 407–411. [Google Scholar] [CrossRef]
- Menaker, J.; Martin, I.B.; Hirshon, J.M. Marked elevation of cerebrospinal fluid white blood cell count: An unusual case of Streptococcus pneumoniae meningitis, differential diagnosis, and a brief review of current epidemiology and treatment recommendations. J. Emerg. Med. 2005, 29, 37–41. [Google Scholar] [CrossRef]
- Honda, T.; Uehara, T.; Matsumoto, G.; Arai, S.; Sugano, M. Neutrophil left shift and white blood cell count as markers of bacterial infection. Clin. Chim. Acta 2016, 457, 46–53. [Google Scholar] [CrossRef]
- Czaikoski, P.G.; Mota, J.M.; Nascimento, D.C.; Sônego, F.; Castanheira, F.V.; Melo, P.H.; Scortegagna, G.T.; Silva, R.L.; Barroso-Sousa, R.; Souto, F.O.; et al. Neutrophil Extracellular Traps Induce Organ Damage during Experimental and Clinical Sepsis. PLoS ONE 2016, 11, e0148142. [Google Scholar] [CrossRef]
- Mao, J.Y.; Zhang, J.H.; Cheng, W.; Chen, J.W.; Cui, N. Effects of Neutrophil Extracellular Traps in Patients with Septic Coagulopathy and Their Interaction with Autophagy. Front. Immunol. 2021, 12, 757041. [Google Scholar] [CrossRef]
- Tillett, W.S.; Francis, T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J. Exp. Med. 1930, 52, 561–571. [Google Scholar] [CrossRef]
- Shrive, A.K.; Cheetham, G.M.; Holden, D.; Myles, D.A.; Turnell, W.G.; Volanakis, J.E.; Pepys, M.B.; Bloomer, A.C.; Greenhough, T.J. Three dimensional structure of human C-reactive protein. Nat. Struct. Biol. 1996, 3, 346–354. [Google Scholar] [CrossRef]
- Thompson, D.; Pepys, M.B.; Wood, S.P. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure 1999, 7, 169–177. [Google Scholar] [CrossRef]
- Xia, D.; Samols, D. Transgenic mice expressing rabbit C-reactive protein are resistant to endotoxemia. Proc. Natl. Acad. Sci. USA 1997, 94, 2575–2580. [Google Scholar] [CrossRef]
- Gershov, D.; Kim, S.; Brot, N.; Elkon, K.B. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: Implications for systemic autoimmunity. J. Exp. Med. 2000, 192, 1353–1364. [Google Scholar] [CrossRef]
- Markanday, A. Acute Phase Reactants in Infections: Evidence-Based Review and a Guide for Clinicians. Open Forum Infect. Dis. 2015, 2, ofv098. [Google Scholar] [CrossRef]
- Costenbader, K.H.; Chibnik, L.B.; Schur, P.H. Discordance between erythrocyte sedimentation rate and C-reactive protein measurements: Clinical significance. Clin. Exp. Rheumatol. 2007, 25, 746–749. [Google Scholar]
- Dowton, S.B.; Colten, H.R. Acute phase reactants in inflammation and infection. Semin. Hematol. 1988, 25, 84–90. [Google Scholar]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Keenan, R.T.; Swearingen, C.J.; Yazici, Y. Erythrocyte sedimentation rate and C-reactive protein levels are poorly correlated with clinical measures of disease activity in rheumatoid arthritis, systemic lupus erythematosus and osteoarthritis patients. Clin. Exp. Rheumatol. 2008, 26, 814–819. [Google Scholar]
- Litao, M.K.; Kamat, D. Erythrocyte sedimentation rate and C-reactive protein: How best to use them in clinical practice. Pediatr. Ann. 2014, 43, 417–420. [Google Scholar] [CrossRef]
- Benitz, W.E.; Han, M.Y.; Madan, A.; Ramachandra, P. Serial serum C-reactive protein levels in the diagnosis of neonatal infection. Pediatrics 1998, 102, E41. [Google Scholar] [CrossRef]
- Mustard, R.A., Jr.; Bohnen, J.M.; Haseeb, S.; Kasina, R. C-reactive protein levels predict postoperative septic complications. Arch. Surg. 1987, 122, 69–73. [Google Scholar] [CrossRef]
- Verma, S.; Yeh, E.T. C-reactive protein and atherothrombosis—Beyond a biomarker: An actual partaker of lesion formation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R1253–R1256; discussion R1257–R1258. [Google Scholar] [CrossRef]
- Black, S.; Kushner, I.; Samols, D. C-reactive Protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef]
- Kushner, I.; Rzewnicki, D.; Samols, D. What does minor elevation of C-reactive protein signify? Am. J. Med. 2006, 119, e117–e128. [Google Scholar] [CrossRef]
- Meisner, M.; Tschaikowsky, K.; Palmaers, T.; Schmidt, J. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit. Care 1999, 3, 45–50. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, H.; Kang, Y. Comparison of diagnostic accuracy among procalcitonin, C-reactive protein, and interleukin 6 for blood culture positivity in general ICU patients. Crit. Care 2018, 22, 339. [Google Scholar] [CrossRef]
- Lee, E.H.; Lee, K.H.; Song, Y.G.; Han, S.H. Discrepancy of C-Reactive Protein, Procalcitonin and Interleukin-6 at Hospitalization: Infection in Patients with Normal C-Reactive Protein, Procalcitonin and High Interleukin-6 Values. J. Clin. Med. 2022, 11, 7324. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Adams, J.E., 3rd; Sicard, G.A.; Allen, B.T.; Bridwell, K.H.; Lenke, L.G.; Dávila-Román, V.G.; Bodor, G.S.; Ladenson, J.H.; Jaffe, A.S. Diagnosis of perioperative myocardial infarction with measurement of cardiac troponin I. N. Engl. J. Med. 1994, 330, 670–674. [Google Scholar] [CrossRef]
- Hoeboer, S.H.; van der Geest, P.J.; Nieboer, D.; Groeneveld, A.B. The diagnostic accuracy of procalcitonin for bacteraemia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2015, 21, 474–481. [Google Scholar] [CrossRef]
- Assicot, M.; Gendrel, D.; Carsin, H.; Raymond, J.; Guilbaud, J.; Bohuon, C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993, 341, 515–518. [Google Scholar] [CrossRef]
- Dandona, P.; Nix, D.; Wilson, M.F.; Aljada, A.; Love, J.; Assicot, M.; Bohuon, C. Procalcitonin increase after endotoxin injection in normal subjects. J. Clin. Endocrinol. Metab. 1994, 79, 1605–1608. [Google Scholar] [CrossRef]
- Brunkhorst, F.M.; Heinz, U.; Forycki, Z.F. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998, 24, 888–889. [Google Scholar] [CrossRef]
- Müller, B.; Becker, K.L.; Schächinger, H.; Rickenbacher, P.R.; Huber, P.R.; Zimmerli, W.; Ritz, R. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit. Care Med. 2000, 28, 977–983. [Google Scholar] [CrossRef]
- Nylen, E.S.; Whang, K.T.; Snider, R.H., Jr.; Steinwald, P.M.; White, J.C.; Becker, K.L. Mortality is increased by procalcitonin and decreased by an antiserum reactive to procalcitonin in experimental sepsis. Crit. Care Med. 1998, 26, 1001–1006. [Google Scholar] [CrossRef]
- Wacker, C.; Prkno, A.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 426–435. [Google Scholar] [CrossRef]
- Facy, O.; Paquette, B.; Orry, D.; Binquet, C.; Masson, D.; Bouvier, A.; Fournel, I.; Charles, P.E.; Rat, P.; Ortega-Deballon, P. Diagnostic Accuracy of Inflammatory Markers As Early Predictors of Infection After Elective Colorectal Surgery: Results From the IMACORS Study. Ann. Surg. 2016, 263, 961–966. [Google Scholar] [CrossRef]
- El-Solh, A.A.; Vora, H.; Knight, P.R., 3rd; Porhomayon, J. Diagnostic use of serum procalcitonin levels in pulmonary aspiration syndromes. Crit. Care Med. 2011, 39, 1251–1256. [Google Scholar] [CrossRef]
- Liu, D.; Su, L.; Han, G.; Yan, P.; Xie, L. Prognostic Value of Procalcitonin in Adult Patients with Sepsis: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0129450. [Google Scholar] [CrossRef]
- Hong, D.Y.; Park, S.O.; Kim, J.W.; Lee, K.R.; Baek, K.J.; Na, J.U.; Choi, P.C.; Lee, Y.H. Serum Procalcitonin: An Independent Predictor of Clinical Outcome in Health Care-Associated Pneumonia. Respiration 2016, 92, 241–251. [Google Scholar] [CrossRef]
- Julián-Jiménez, A.; Timón Zapata, J.; Laserna Mendieta, E.J.; Sicilia-Bravo, I.; Palomo-de Los Reyes, M.J.; Cabezas-Martínez, A.; Laín-Terés, N.; Estebaran-Martín, J.; Lozano-Ancín, A.; Cuena-Boy, R. Diagnostic and prognostic power of biomarkers to improve the management of community acquired pneumonia in the emergency department. Enferm. Infecc. Microbiol. Clin. 2014, 32, 225–235. [Google Scholar] [CrossRef]
- Azevedo, J.R.; Torres, O.J.; Czeczko, N.G.; Tuon, F.F.; Nassif, P.A.; Souza, G.D. Procalcitonin as a prognostic biomarker of severe sepsis and septic shock. Rev. Col. Bras. Cir. 2012, 39, 456–461. [Google Scholar] [CrossRef]
- De Azevedo, J.R.; Torres, O.J.; Beraldi, R.A.; Ribas, C.A.; Malafaia, O. Prognostic evaluation of severe sepsis and septic shock: Procalcitonin clearance vs. Δ Sequential Organ Failure Assessment. J. Crit. Care 2015, 30, 219.e9–219.e12. [Google Scholar] [CrossRef]
- Jain, S.; Sinha, S.; Sharma, S.K.; Samantaray, J.C.; Aggrawal, P.; Vikram, N.K.; Biswas, A.; Sood, S.; Goel, M.; Das, M.; et al. Procalcitonin as a prognostic marker for sepsis: A prospective observational study. BMC Res. Notes 2014, 7, 458. [Google Scholar] [CrossRef]
- Cornelissen, C.G.; Frechen, D.A.; Schreiner, K.; Marx, N.; Krüger, S. Inflammatory parameters and prediction of prognosis in infective endocarditis. BMC Infect. Dis. 2013, 13, 272. [Google Scholar] [CrossRef]
- Bloos, F.; Marshall, J.C.; Dellinger, R.P.; Vincent, J.L.; Gutierrez, G.; Rivers, E.; Balk, R.A.; Laterre, P.F.; Angus, D.C.; Reinhart, K.; et al. Multinational, observational study of procalcitonin in ICU patients with pneumonia requiring mechanical ventilation: A multicenter observational study. Crit. Care 2011, 15, R88. [Google Scholar] [CrossRef]
- Guo, S.; Mao, X.; Liang, M. The moderate predictive value of serial serum CRP and PCT levels for the prognosis of hospitalized community-acquired pneumonia. Respir. Res. 2018, 19, 193. [Google Scholar] [CrossRef]
- Huang, D.T.; Yealy, D.M.; Filbin, M.R.; Brown, A.M.; Chang, C.H.; Doi, Y.; Donnino, M.W.; Fine, J.; Fine, M.J.; Fischer, M.A.; et al. Procalcitonin-Guided Use of Antibiotics for Lower Respiratory Tract Infection. N. Engl. J. Med. 2018, 379, 236–249. [Google Scholar] [CrossRef]
- Arora, S.; Singh, P.; Singh, P.M.; Trikha, A. Procalcitonin Levels in Survivors and Nonsurvivors of Sepsis: Systematic Review and Meta-Analysis. Shock 2015, 43, 212–221. [Google Scholar] [CrossRef]
- Tang, B.M.; Eslick, G.D.; Craig, J.C.; McLean, A.S. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: Systematic review and meta-analysis. Lancet Infect. Dis. 2007, 7, 210–217. [Google Scholar] [CrossRef]
- Durrance, R.J.; Ullah, T.; Patel, H.; Martinez, G.; Cervellione, K.; Zafonte, V.B.; Gafoor, K.; Bagheri, F. Marked Elevation in Serum Procalcitonin Levels Do Not Correlate with Severity of Disease or Mortality in Hospitalized Patients: A Retrospective Study. Biomark. Insights 2020, 15, 1177271920917941. [Google Scholar] [CrossRef]
- Siddiqui, I.; Jafri, L.; Abbas, Q.; Raheem, A.; Haque, A.U. Relationship of Serum Procalcitonin, C-reactive Protein, and Lactic Acid to Organ Failure and Outcome in Critically Ill Pediatric Population. Indian J. Crit. Care Med. 2018, 22, 91–95. [Google Scholar] [CrossRef]
- Li, S.; Rong, H.; Guo, Q.; Chen, Y.; Zhang, G.; Yang, J. Serum procalcitonin levels distinguish Gram-negative bacterial sepsis from Gram-positive bacterial and fungal sepsis. J. Res. Med. Sci. 2016, 21, 39. [Google Scholar] [CrossRef]
- Leli, C.; Ferranti, M.; Moretti, A.; Al Dhahab, Z.S.; Cenci, E.; Mencacci, A. Procalcitonin levels in gram-positive, gram-negative, and fungal bloodstream infections. Dis. Markers 2015, 2015, 701480. [Google Scholar] [CrossRef]
- He, S.; Ma, J.; Fan, C.; Tang, C.; Chen, Y.; Xie, C. Performance of Procalcitonin to Distinguish Fungal from Bacterial Infections in Patients with Systemic Lupus Erythematosus. Infect. Drug Resist. 2021, 14, 4773–4781. [Google Scholar] [CrossRef]
- Mahittikorn, A.; Kotepui, K.U.; Mala, W.; Wilairatana, P.; Kotepui, M. Procalcitonin as a Candidate Biomarker for Malarial Infection and Severe Malaria: A Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1389. [Google Scholar] [CrossRef]
- Chiwakata, C.B.; Manegold, C.; Bönicke, L.; Waase, I.; Jülch, C.; Dietrich, M. Procalcitonin as a parameter of disease severity and risk of mortality in patients with Plasmodium falciparum malaria. J. Infect. Dis. 2001, 183, 1161–1164. [Google Scholar] [CrossRef]
- Meisner, M. Update on procalcitonin measurements. Ann. Lab. Med. 2014, 34, 263–273. [Google Scholar] [CrossRef]
- Thiab, G.; Workman, A.; Khawaja, I. An Unrecognized Cause of Elevated Procalcitonin Level. Cureus 2023, 15, e39475. [Google Scholar] [CrossRef]
- Ecesoy, V.; Ecesoy, H. Is procalcitonin elevation always an indicator of bacterial infection? Arch. Rheumatol. 2024, 39, 133–135. [Google Scholar] [CrossRef]
- Von Heimburg, D.; Stieghorst, W.; Khorram-Sefat, R.; Pallua, N. Procalcitonin—A sepsis parameter in severe burn injuries. Burns 1998, 24, 745–750. [Google Scholar] [CrossRef]
- Chiesa, C.; Panero, A.; Rossi, N.; Stegagno, M.; De Giusti, M.; Osborn, J.F.; Pacifico, L. Reliability of procalcitonin concentrations for the diagnosis of sepsis in critically ill neonates. Clin. Infect. Dis. 1998, 26, 664–672. [Google Scholar] [CrossRef]
- Smith, S.E.; Muir, J.; Kalabalik-Hoganson, J. Procalcitonin in special patient populations: Guidance for antimicrobial therapy. Am. J. Health Syst. Pharm. 2020, 77, 745–758. [Google Scholar] [CrossRef]
- Buendía, J.A.; Guerrero Patiño, D. Cost-effectiveness of procalcitonin for detection of serious bacterial infections in children presenting with fever without source. BMC Pediatr. 2022, 22, 226. [Google Scholar] [CrossRef]
- Hirano, T.; Yasukawa, K.; Harada, H.; Taga, T.; Watanabe, Y.; Matsuda, T.; Kashiwamura, S.; Nakajima, K.; Koyama, K.; Iwamatsu, A.; et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 1986, 324, 73–76. [Google Scholar] [CrossRef]
- Garbers, C.; Heink, S.; Korn, T.; Rose-John, S. Interleukin-6: Designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 2018, 17, 395–412. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef]
- Libermann, T.A.; Baltimore, D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell Biol. 1990, 10, 2327–2334. [Google Scholar] [CrossRef]
- Shimizu, H.; Mitomo, K.; Watanabe, T.; Okamoto, S.; Yamamoto, K. Involvement of a NF-kappa B-like transcription factor in the activation of the interleukin-6 gene by inflammatory lymphokines. Mol. Cell Biol. 1990, 10, 561–568. [Google Scholar] [CrossRef]
- Ray, A.; Tatter, S.B.; May, L.T.; Sehgal, P.B. Activation of the human “beta 2-interferon/hepatocyte-stimulating factor/interleukin 6” promoter by cytokines, viruses, and second messenger agonists. Proc. Natl. Acad. Sci. USA 1988, 85, 6701–6705. [Google Scholar] [CrossRef]
- Matsusaka, T.; Fujikawa, K.; Nishio, Y.; Mukaida, N.; Matsushima, K.; Kishimoto, T.; Akira, S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc. Natl. Acad. Sci. USA 1993, 90, 10193–10197. [Google Scholar] [CrossRef]
- Corcoran, S.E.; O’Neill, L.A. HIF1α and metabolic reprogramming in inflammation. J. Clin. Investig. 2016, 126, 3699–3707. [Google Scholar] [CrossRef]
- Fong, Y.; Moldawer, L.L.; Marano, M.; Wei, H.; Tatter, S.B.; Clarick, R.H.; Santhanam, U.; Sherris, D.; May, L.T.; Sehgal, P.B.; et al. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J. Immunol. 1989, 142, 2321–2324. [Google Scholar] [CrossRef]
- Palsson-McDermott, E.M.; O’Neill, L.A. The Warburg effect then and now: From cancer to inflammatory diseases. Bioessays 2013, 35, 965–973. [Google Scholar] [CrossRef]
- Kohase, M.; Henriksen-DeStefano, D.; May, L.T.; Vilcek, J.; Sehgal, P.B. Induction of beta 2-interferon by tumor necrosis factor: A homeostatic mechanism in the control of cell proliferation. Cell 1986, 45, 659–666. [Google Scholar] [CrossRef]
- Kohase, M.; May, L.T.; Tamm, I.; Vilcek, J.; Sehgal, P.B. A cytokine network in human diploid fibroblasts: Interactions of beta-interferons, tumor necrosis factor, platelet-derived growth factor, and interleukin-1. Mol. Cell Biol. 1987, 7, 273–280. [Google Scholar] [CrossRef]
- Söderberg, E.; Eriksson, M.; Larsson, A.; Lipcsey, M. The impact of hydrocortisone treatment on neutrophil gelatinase-associated lipocalin release in porcine endotoxemic shock. Intensive Care Med. Exp. 2017, 5, 4. [Google Scholar] [CrossRef]
- Sehgal, P.B.; Helfgott, D.C.; Santhanam, U.; Tatter, S.B.; Clarick, R.H.; Ghrayeb, J.; May, L.T. Regulation of the acute phase and immune responses in viral disease. Enhanced expression of the beta 2-interferon/hepatocyte-stimulating factor/interleukin 6 gene in virus-infected human fibroblasts. J. Exp. Med. 1988, 167, 1951–1956. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef]
- Narazaki, M.; Kishimoto, T. The Two-Faced Cytokine IL-6 in Host Defense and Diseases. Int. J. Mol. Sci. 2018, 19, 3528. [Google Scholar] [CrossRef]
- Qu, J.; Lü, X.; Liu, Y.; Wang, X. Evaluation of procalcitonin, C-reactive protein, interleukin-6 & serum amyloid A as diagnostic biomarkers of bacterial infection in febrile patients. Indian J. Med. Res. 2015, 141, 315–321. [Google Scholar] [CrossRef]
- Gendrel, D.; Raymond, J.; Coste, J.; Moulin, F.; Lorrot, M.; Guérin, S.; Ravilly, S.; Lefèvre, H.; Royer, C.; Lacombe, C.; et al. Comparison of procalcitonin with C-reactive protein, interleukin 6 and interferon-alpha for differentiation of bacterial vs. viral infections. Pediatr. Infect. Dis. J. 1999, 18, 875–881. [Google Scholar] [CrossRef]
- Chen, T.; Lin, Y.X.; Zha, Y.; Sun, Y.; Tian, J.; Yang, Z.; Lin, S.W.; Yu, F.; Chen, Z.S.; Kuang, B.H.; et al. A Low-Producing Haplotype of Interleukin-6 Disrupting CTCF Binding Is Protective against Severe COVID-19. mBio 2021, 12, e0137221. [Google Scholar] [CrossRef]
- Balto, K.; Sasaki, H.; Stashenko, P. Interleukin-6 deficiency increases inflammatory bone destruction. Infect. Immun. 2001, 69, 744–750. [Google Scholar] [CrossRef][Green Version]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the interface of human health and disease. Front. Immunol. 2023, 14, 1255533. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Johnsen, A.H.; Sengeløv, H.; Borregaard, N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar] [CrossRef]
- Nasioudis, D.; Witkin, S.S. Neutrophil gelatinase-associated lipocalin and innate immune responses to bacterial infections. Med. Microbiol. Immunol. 2015, 204, 471–479. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Cowland, J.B.; Borregaard, N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim. Biophys. Acta 2000, 1482, 272–283. [Google Scholar] [CrossRef]
- Liu, F.; Yang, H.; Chen, H.; Zhang, M.; Ma, Q. High expression of neutrophil gelatinase-associated lipocalin (NGAL) in the kidney proximal tubules of diabetic rats. Adv. Med. Sci. 2015, 60, 133–138. [Google Scholar] [CrossRef]
- Jaberi, S.A.; Cohen, A.; D’Souza, C.; Abdulrazzaq, Y.M.; Ojha, S.; Bastaki, S.; Adeghate, E.A. Lipocalin-2: Structure, function, distribution and role in metabolic disorders. Biomed. Pharmacother. 2021, 142, 112002. [Google Scholar] [CrossRef]
- Latouche, C.; El Moghrabi, S.; Messaoudi, S.; Nguyen Dinh Cat, A.; Hernandez-Diaz, I.; Alvarez de la Rosa, D.; Perret, C.; López Andrés, N.; Rossignol, P.; Zannad, F.; et al. Neutrophil gelatinase-associated lipocalin is a novel mineralocorticoid target in the cardiovascular system. Hypertension 2012, 59, 966–972. [Google Scholar] [CrossRef]
- Eilenberg, W.; Stojkovic, S.; Piechota-Polanczyk, A.; Kaun, C.; Rauscher, S.; Gröger, M.; Klinger, M.; Wojta, J.; Neumayer, C.; Huk, I.; et al. Neutrophil Gelatinase-Associated Lipocalin (NGAL) is Associated with Symptomatic Carotid Atherosclerosis and Drives Pro-inflammatory State In Vitro. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 623–631. [Google Scholar] [CrossRef]
- Flo, T.H.; Smith, K.D.; Sato, S.; Rodriguez, D.J.; Holmes, M.A.; Strong, R.K.; Akira, S.; Aderem, A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004, 432, 917–921. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin—An emerging troponin for kidney injury. Nephrol. Dial. Transpl. 2008, 23, 3737–3743. [Google Scholar] [CrossRef]
- Cowland, J.B.; Borregaard, N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics 1997, 45, 17–23. [Google Scholar] [CrossRef]
- Yeşil, A.; Gönen, C.; Senateş, E.; Paker, N.; Gökden, Y.; Koçhan, K.; Erdem, E.D.; Gündüz, F. Relationship between neutrophil gelatinase-associated lipocalin (NGAL) levels and inflammatory bowel disease type and activity. Dig. Dis. Sci. 2013, 58, 2587–2593. [Google Scholar] [CrossRef]
- Stallhofer, J.; Friedrich, M.; Konrad-Zerna, A.; Wetzke, M.; Lohse, P.; Glas, J.; Tillack-Schreiber, C.; Schnitzler, F.; Beigel, F.; Brand, S. Lipocalin-2 Is a Disease Activity Marker in Inflammatory Bowel Disease Regulated by IL-17A, IL-22, and TNF-α and Modulated by IL23R Genotype Status. Inflamm. Bowel Dis. 2015, 21, 2327–2340. [Google Scholar] [CrossRef]
- De Bruyn, M.; Arijs, I.; De Hertogh, G.; Ferrante, M.; Van Assche, G.; Rutgeerts, P.; Vermeire, S.; Opdenakker, G. Serum Neutrophil Gelatinase B-associated Lipocalin and Matrix Metalloproteinase-9 Complex as a Surrogate Marker for Mucosal Healing in Patients with Crohn’s Disease. J. Crohns Colitis 2015, 9, 1079–1087. [Google Scholar] [CrossRef]
- Shafer, W.M.; Martin, L.E.; Spitznagel, J.K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect. Immun. 1984, 45, 29–35. [Google Scholar] [CrossRef]
- Yang, D.; de la Rosa, G.; Tewary, P.; Oppenheim, J.J. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009, 30, 531–537. [Google Scholar] [CrossRef]
- Soehnlein, O.; Lindbom, L. Neutrophil-derived azurocidin alarms the immune system. J. Leukoc. Biol. 2009, 85, 344–351. [Google Scholar] [CrossRef]
- Honore, P.M.; De Bels, D.; Barreto Gutierrez, L.; Redant, S.; Spapen, H.D. Heparin-binding protein in sepsis: Player! predictor! positioning? Ann. Intensive Care 2019, 9, 71. [Google Scholar] [CrossRef]
- Cassatella, M.A. Neutrophil-derived proteins: Selling cytokines by the pound. Adv. Immunol. 1999, 73, 369–509. [Google Scholar] [CrossRef]
- Ley, K.; Hoffman, H.M.; Kubes, P.; Cassatella, M.A.; Zychlinsky, A.; Hedrick, C.C.; Catz, S.D. Neutrophils: New insights and open questions. Sci. Immunol. 2018, 3, aat4579. [Google Scholar] [CrossRef]
- Tamassia, N.; Bianchetto-Aguilera, F.; Arruda-Silva, F.; Gardiman, E.; Gasperini, S.; Calzetti, F.; Cassatella, M.A. Cytokine production by human neutrophils: Revisiting the “dark side of the moon”. Eur. J. Clin. Investig. 2018, 48 (Suppl. S2), e12952. [Google Scholar] [CrossRef]
- Németh, T.; Futosi, K.; Sitaru, C.; Ruland, J.; Mócsai, A. Neutrophil-specific deletion of the CARD9 gene expression regulator suppresses autoantibody-induced inflammation in vivo. Nat. Commun. 2016, 7, 11004. [Google Scholar] [CrossRef]
- Roth, S.; Agthe, M.; Eickhoff, S.; Möller, S.; Karsten, C.M.; Borregaard, N.; Solbach, W.; Laskay, T. Secondary necrotic neutrophils release interleukin-16C and macrophage migration inhibitory factor from stores in the cytosol. Cell Death Discov. 2015, 1, 15056. [Google Scholar] [CrossRef]
- Arruda-Silva, F.; Bianchetto-Aguilera, F.; Gasperini, S.; Polletti, S.; Cosentino, E.; Tamassia, N.; Cassatella, M.A. Human Neutrophils Produce CCL23 in Response to Various TLR-Agonists and TNFα. Front. Cell Infect. Microbiol. 2017, 7, 176. [Google Scholar] [CrossRef]
- Tamassia, N.; Arruda-Silva, F.; Wright, H.L.; Moots, R.J.; Gardiman, E.; Bianchetto-Aguilera, F.; Gasperini, S.; Capone, M.; Maggi, L.; Annunziato, F.; et al. Human neutrophils activated via TLR8 promote Th17 polarization through IL-23. J. Leukoc. Biol. 2019, 105, 1155–1165. [Google Scholar] [CrossRef]
- Xu, J.; Guardado, J.; Hoffman, R.; Xu, H.; Namas, R.; Vodovotz, Y.; Xu, L.; Ramadan, M.; Brown, J.; Turnquist, H.R.; et al. IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translation study from a human cohort to a mouse trauma model. PLoS Med. 2017, 14, e1002365. [Google Scholar] [CrossRef]
- Sun, B.; Zhu, L.; Tao, Y.; Sun, H.X.; Li, Y.; Wang, P.; Hou, Y.; Zhao, Y.; Zhang, X.; Zhang, L.; et al. Characterization and allergic role of IL-33-induced neutrophil polarization. Cell Mol. Immunol. 2018, 15, 782–793. [Google Scholar] [CrossRef]
- Cheung, D.S.; Sigua, J.A.; Simpson, P.M.; Yan, K.; Hussain, S.A.; Santoro, J.L.; Buell, E.J.; Hunter, D.A.; Rohlfing, M.; Patadia, D.; et al. Cysteinyl leukotriene receptor 1 expression identifies a subset of neutrophils during the antiviral response that contributes to postviral atopic airway disease. J. Allergy Clin. Immunol. 2018, 142, 1206–1217.e1205. [Google Scholar] [CrossRef]
- Wang, Y.; Bugatti, M.; Ulland, T.K.; Vermi, W.; Gilfillan, S.; Colonna, M. Nonredundant roles of keratinocyte-derived IL-34 and neutrophil-derived CSF1 in Langerhans cell renewal in the steady state and during inflammation. Eur. J. Immunol. 2016, 46, 552–559. [Google Scholar] [CrossRef]
- Bozoyan, L.; Dumas, A.; Patenaude, A.; Vallières, L. Interleukin-36γ is expressed by neutrophils and can activate microglia, but has no role in experimental autoimmune encephalomyelitis. J. Neuroinflam. 2015, 12, 173. [Google Scholar] [CrossRef]
- Chen, C.C.; Huang, J.L.; Chang, C.J.; Kong, M.S. Fecal calprotectin as a correlative marker in clinical severity of infectious diarrhea and usefulness in evaluating bacterial or viral pathogens in children. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 541–547. [Google Scholar] [CrossRef]
- Duman, M.; Gencpinar, P.; Biçmen, M.; Arslan, N.; Özden, Ö.; Üzüm, Ö.; Çelik, D.; Sayıner, A.A.; Gülay, Z. Fecal calprotectin: Can be used to distinguish between bacterial and viral gastroenteritis in children? Am. J. Emerg. Med. 2015, 33, 1436–1439. [Google Scholar] [CrossRef]
- Lué, A.; Hijos, G.; Sostres, C.; Perales, A.; Navarro, M.; Barra, M.V.; Mascialino, B.; Andalucia, C.; Puente, J.J.; Lanas, Á.; et al. The combination of quantitative faecal occult blood test and faecal calprotectin is a cost-effective strategy to avoid colonoscopies in symptomatic patients without relevant pathology. Ther. Adv. Gastroenterol. 2020, 13, 1756284820920786. [Google Scholar] [CrossRef]
- Zeng, J.; Yu, W.; Gao, X.; Yu, Y.; Zhou, Y.; Pan, X. Establishing reference values for age-related fecal calprotectin in healthy children aged 0–4 years: A systematic review and meta-analysis. PeerJ 2025, 13, e19572. [Google Scholar] [CrossRef]
- Roca, M.; Rodriguez Varela, A.; Carvajal, E.; Donat, E.; Cano, F.; Armisen, A.; Vaya, M.J.; Ekoff, H.; Hervas, D.; Rydell, N.; et al. Fecal calprotectin in healthy children aged 4–16 years. Sci. Rep. 2020, 10, 20565. [Google Scholar] [CrossRef]
- Røseth, A.G.; Fagerhol, M.K.; Aadland, E.; Schjønsby, H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand. J. Gastroenterol. 1992, 27, 793–798. [Google Scholar] [CrossRef]
- Bjarnason, I.; MacPherson, A.; Hollander, D. Intestinal permeability: An overview. Gastroenterology 1995, 108, 1566–1581. [Google Scholar] [CrossRef]

| Biomarker | Primary Source | Kinetics (Response Time) | Half-Life | Key Clinical Applications | Diagnostic Strengths | Limitations |
|---|---|---|---|---|---|---|
| WBC count | Bone marrow | 4–24 h | 6–10 h | General infection screening | Widely available, low cost | Non-specific (stress, steroids) |
| Neutrophil count | Bone marrow | 4–6 h | 6–10 h | Acute bacterial infection detection | Rapid, correlates with bacterial burden | Affected by non-infectious inflammation |
| CRP | Hepatocytes | 6–12 h | 19 h | General infection/inflammation monitoring | Low cost, widely available | Non-specific, slow rise |
| PCT | Thyroid/other tissues | 3–6 h | 24–30 h | Bacterial infection confirmation, sepsis diagnosis, antibiotic stewardship | High specificity for bacterial infections | Cost, delayed rise in localized infections |
| IL-6 | Macrophages, T cells | 1–2 h | 5 min–15.5 h * | Early sepsis, COVID-19 cytokine storm monitoring | Fastest-rising clinically available cytokine | Short half-life, no standardized cut-offs |
| NGAL | Neutrophils, epithelia | 2–4 h | ~10–20 min | Acute kidney injury, bacterial infections | Rapid response to tubular damage | Elevated in CKD, non-infectious inflammation |
| HBP | Neutrophils | Minutes–hours | ~1 h | Sepsis severity, endothelial dysfunction | Predicts organ failure | Limited clinical validation |
| Circulating calprotectin | Neutrophils, monocytes | 2 h | ~5 h | Bacterial vs. viral differentiation, sepsis, PJI/UTI diagnosis | Early and sensitive marker, superior for bacterial detection | Elevated in autoimmune/inflammatory conditions |
| IL-16 | Neutrophils | Delayed (necrosis) | Unknown | Inflammation, autoimmunity | Marker of neutrophil death/clearance | Not infection-specific, research use only |
| CCL23 | Neutrophils | Unknown | Unknown | Monocyte/T-cell recruitment | Chemoattractant, immune cell recruitment | Limited clinical validation |
| IL-23 | Dendritic cells, macrophages | Early (hours) | Unknown | Neutrophil activation, bacterial defense | Induces IL-17/IL-22, critical for neutrophil-mediated clearance | Not neutrophil-derived, research use |
| Biomarker | Common Method(s) | Approx. Cost (USD) | Turnaround Time | Routine Availability | Main Clinical Impact |
|---|---|---|---|---|---|
| Circulating calprotectin | PETIA/ELISA | USD 15–25 | 10–90 min | Specialized labs | Rapid PJI/UTI diagnosis *, bacterial–viral differentiation |
| WBC count | Automated hematology | USD 5–10 | 15–30 min | All labs | Initial infection screening |
| Neutrophil count | Automated differential | Included in CBC | 15–30 min | All labs | Bacterial infection suspicion |
| CRP | Immunoturbidimetry | USD 5–10 | 30–60 min | All labs | General inflammation monitoring |
| PCT | Chemiluminescent immunoassay | USD 20–40 | 60–120 min | Major hospitals | Antibiotic stewardship in LRTI/sepsis |
| IL-6 | ELISA | USD 30–50 | 10–90 min | Major hospitals | Early sepsis triage, COVID-19 severity assessment |
| NGAL | ELISA/Immunoassay | USD 20–40 | 1–2 h | Specialized labs | AKI risk stratification |
| HBP | ELISA | USD 20–40 | 1–2 h | Research settings | Sepsis severity prediction |
| IL-16 | ELISA | USD 30–50 | 2–4 h | Research settings | Research marker for inflammation pathways |
| CCL23 | ELISA | USD 30–50 | 2–4 h | Research settings | Immune cell recruitment studies |
| IL-23 | ELISA | USD 40–60 | 2–4 h | Research settings | Neutrophil activation research in infections/autoimmunity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sejersen, K.; Eriksson, M.B.; Larsson, A.O. Calprotectin as a Biomarker for Infectious Diseases: A Comparative Review with Conventional Inflammatory Markers. Int. J. Mol. Sci. 2025, 26, 6476. https://doi.org/10.3390/ijms26136476
Sejersen K, Eriksson MB, Larsson AO. Calprotectin as a Biomarker for Infectious Diseases: A Comparative Review with Conventional Inflammatory Markers. International Journal of Molecular Sciences. 2025; 26(13):6476. https://doi.org/10.3390/ijms26136476
Chicago/Turabian StyleSejersen, Kristina, Mats B. Eriksson, and Anders O. Larsson. 2025. "Calprotectin as a Biomarker for Infectious Diseases: A Comparative Review with Conventional Inflammatory Markers" International Journal of Molecular Sciences 26, no. 13: 6476. https://doi.org/10.3390/ijms26136476
APA StyleSejersen, K., Eriksson, M. B., & Larsson, A. O. (2025). Calprotectin as a Biomarker for Infectious Diseases: A Comparative Review with Conventional Inflammatory Markers. International Journal of Molecular Sciences, 26(13), 6476. https://doi.org/10.3390/ijms26136476







