Unveiling Synergistic Antioxidant Effects of Green Tea and Peppermint: Role of Polyphenol Interactions and Blend Preparation
Abstract
1. Introduction
2. Results
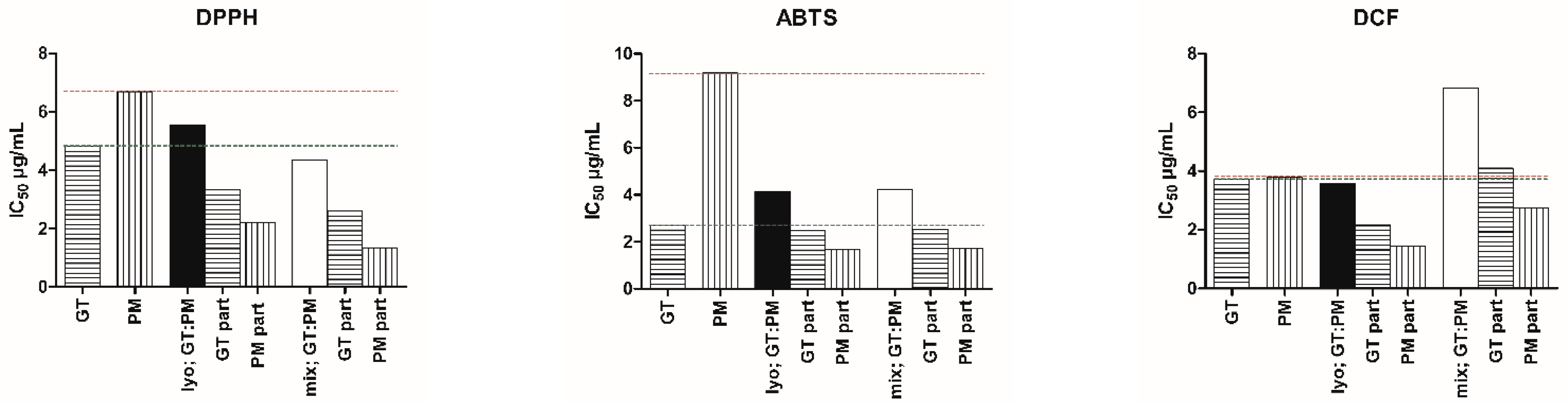
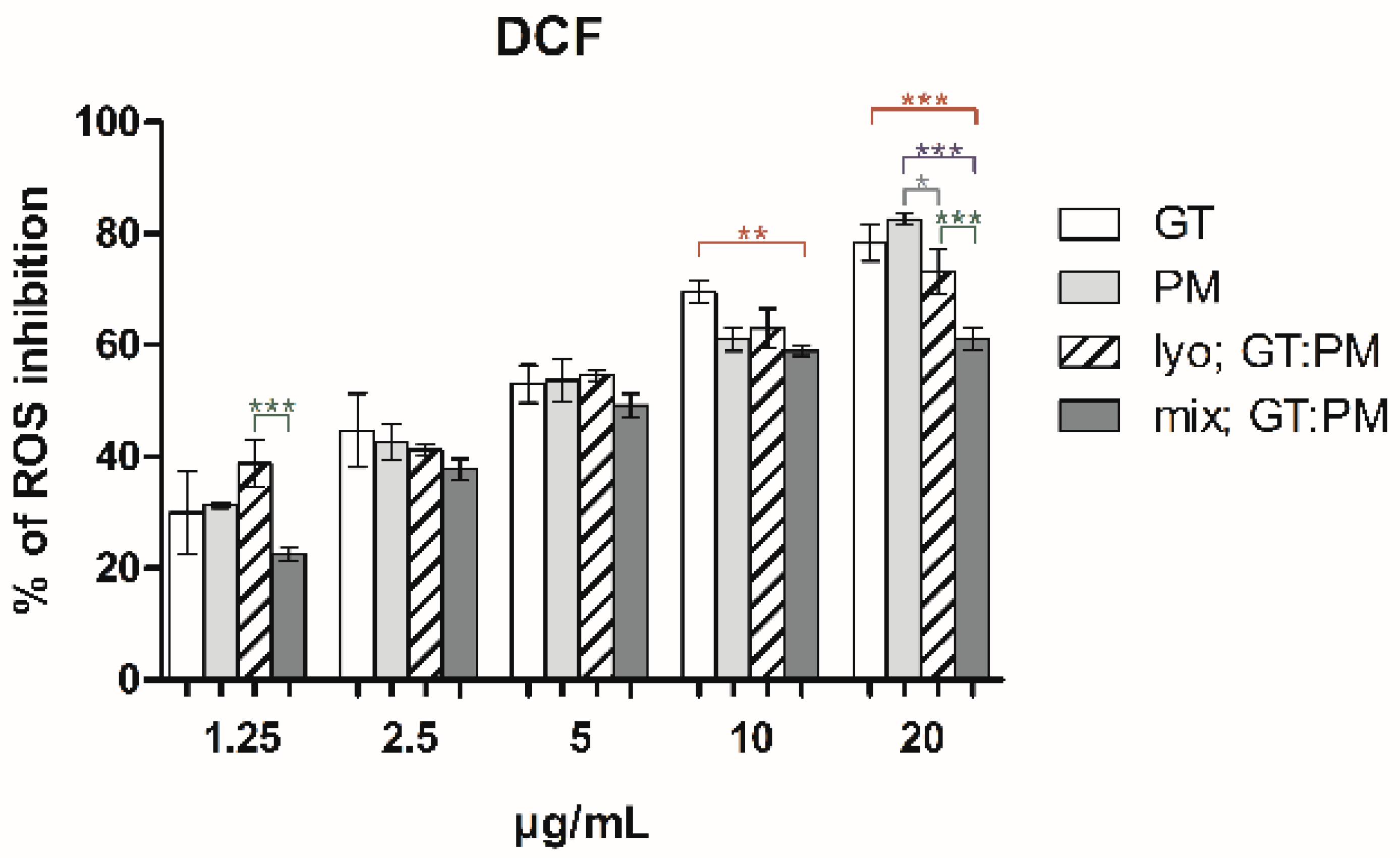
2.1. Antioxidant Activity and the Impact of Preparation Method on GT and PM Extracts and Mixtures
2.2. Interaction Analysis of GT and PM Extracts and Mixtures
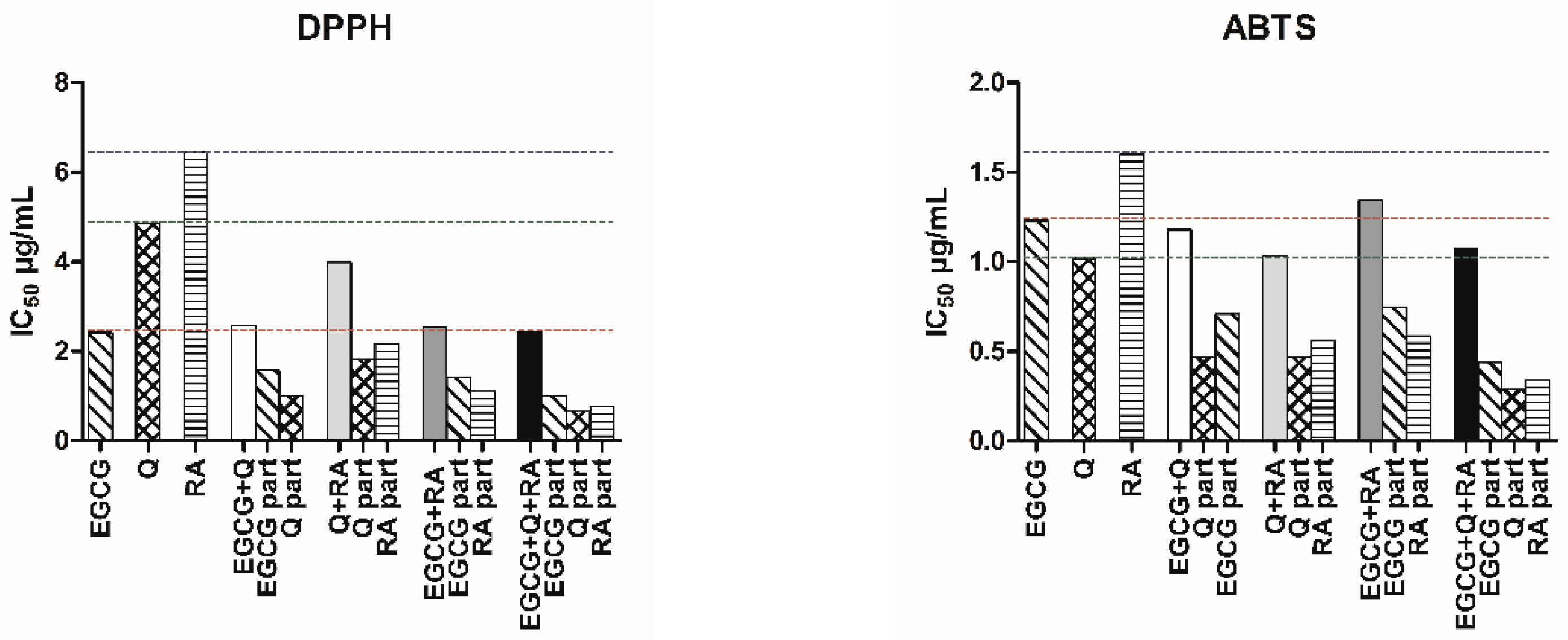
2.3. Antioxidant Activity of Polyphenols and Their Combinations
2.4. Interaction Analysis of Polyphenol Combinations
2.5. Phytochemical Profile of Infusions
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Infusions and Lyophilizates
4.3. DPPH Scavenging Assay
4.4. ABTS Scavenging Assay
4.5. Intracellular Detection of Oxidative Stress Using the DCF Assay
4.6. Interaction Analysis/Synergy Evaluation
4.7. Phytochemical Analysis
4.7.1. LC-MS/MS-DAD Analysis
4.7.2. Spectrophotometric Analysis
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| CI | combination index |
| DCF | dichlorofluorescein |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DRI | dose reduction index |
| EGCG | (-)-epigallocatechin gallate |
| GT | green tea |
| lyo; GT:PM | lyophilizate prepared from a co-macerated mixture of GT and PM |
| mix; GT:PM | mixture of separately lyophilized extracts GT and PM |
| NIH/3T3 | mouse embryo fibroblast cell line clone 3T3 |
| PM | peppermint |
| Q | quercetin |
| RA | rosmarinic acid |
| SDA | sequential deletion analysis |
| TR | retention time |
References
- Kopjar, M.; Tadić, M.; Piližota, V. Phenol Content and Antioxidant Activity of Green, Yellow and Black Tea Leaves. Chem. Biol. Technol. Agric. 2015, 2, 1. [Google Scholar] [CrossRef]
- Zeljković, S.Ć.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic Compounds and Biological Activity of Selected Mentha Species. Plants 2021, 10, 550. [Google Scholar] [CrossRef]
- Forester, S.C.; Lambert, J.D. The Role of Antioxidant versus Pro-Oxidant Effects of Green Tea Polyphenols in Cancer Prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.S.; Kim, S.H.; Kim, Y.B.; Kim, Y.C. Quantitative Analysis of Major Constituents in Green Tea with Different Plucking Periods and Their Antioxidant Activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, M.; Kiani, M.; Nikpey, A. Antioxidant and Scolicidal Activities of Four Iranian Mentha Species (Lamiaceae) in Relation to Phenolic Elements. J. HerbMed Pharmacol. 2020, 9, 200–208. [Google Scholar] [CrossRef]
- Bittner Fialová, S.; Kurin, E.; Trajčíková, E.; Jánošová, L.; Šušaníková, I.; Tekeľová, D.; Nagy, M.; Mučaji, P. Mentha Rhizomes as an Alternative Source of Natural Antioxidants. Molecules 2020, 25, 200. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, Z.; Zhao, J. Identification of Green Tea’s (Camellia sinensis (L.)) Quality Level According to Measurement of Main Catechins and Caffeine Contents by HPLC and Support Vector Classification Pattern Recognition. J. Pharm. Biomed. Anal. 2008, 48, 1321–1325. [Google Scholar] [CrossRef]
- Jakabová, S.; Árvay, J.; Šnirc, M.; Lakatošová, J.; Ondejčíková, A.; Golian, J. HPLC-DAD Method for Simultaneous Determination of Gallic Acid, Catechins, and Methylxanthines and Its Application in Quantitative Analysis and Origin Identification of Green Tea. Heliyon 2024, 10, e35819. [Google Scholar] [CrossRef]
- Nain, C.W.; Mignolet, E.; Herent, M.F.; Quetin-Leclercq, J.; Debier, C.; Page, M.M.; Larondelle, Y. The Catechins Profile of Green Tea Extracts Affects the Antioxidant Activity and Degradation of Catechins in DHA-Rich Oil. Antioxidants 2022, 11, 1844. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S. Phenolic Compounds of Green Tea: Health Benefits and Technological Application in Food. Asian Pac. J. Trop. Biomed. 2016, 6, 709–719. [Google Scholar] [CrossRef]
- Bittner Fialová, S.; Harris, C.; Ordsmith, V.; Nagy, M.; Jonáš, F.; Mučaji, P. Polar Phenolic Compounds in Peppermint Rhizomes and Leaves. Eur. Pharm. J. 2019, 66, 28–31. [Google Scholar] [CrossRef]
- Hudz, N.; Kobylinska, L.; Pokajewicz, K.; Horčinová Sedláčková, V.; Fedin, R.; Voloshyn, M.; Myskiv, I.; Brindza, J.; Wieczorek, P.P.; Lipok, J. Mentha Piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products. Molecules 2023, 28, 7444. [Google Scholar] [CrossRef] [PubMed]
- EMA/HMPC/283630/2012; Community Herbal Monograph on Camellia sinensis (L.) Kuntze, Non Fermentatum Folium. European Medicines Agency: Amsterdam, The Netherlands, 2012. Available online: https://www.ema.europa.eu/en/medicines/herbal/camelliae-sinensis-non-fermentatum-folium (accessed on 1 May 2025).
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2019, 11, 39. [Google Scholar] [CrossRef]
- EMA/HMPC/572705/2014; European Union Herbal Monograph on Mentha x piperita L., folium. European Medicines Agency: Amsterdam, The Netherlands, 2014. Available online: https://www.ema.europa.eu/en/medicines/herbal/menthae-piperitae-folium (accessed on 1 May 2025).
- Romeo-Arroyo, E.; Mora, M.; Noguera-Artiaga, L.; Vázquez-Araújo, L. Tea Pairings: Impact of Aromatic Congruence on Acceptance and Sweetness Perception. Curr. Res. Food Sci. 2023, 6, 100432. [Google Scholar] [CrossRef] [PubMed]
- Mair, V.H.; Hoh, E. The True History of Tea, 1st ed.; Thames & Hudson, Limited: London, UK, 2009. [Google Scholar]
- El Hassani, F.Z. Characterization, Activities, and Ethnobotanical Uses of Mentha Species in Morocco. Heliyon 2020, 6, e05480. [Google Scholar] [CrossRef]
- Malongane, F.; McGaw, L.J.; Mudau, F.N. The Synergistic Potential of Various Teas, Herbs and Therapeutic Drugs in Health Improvement: A Review. J. Sci. Food Agric. 2017, 97, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Simmonds, M.S.J. Molecules of Interest Rosmarinic Acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Tang, L.; Yi, Q. Salvianolic Acids: Potential Source of Natural Drugs for the Treatment of Fibrosis Disease and Cancer. Front. Pharmacol. 2019, 10, 97. [Google Scholar] [CrossRef]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Basile, A.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin Gallate (EGCG): Pharmacological Properties, Biological Activities and Therapeutic Potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, G.; Olayemi Aluko, O.; Mo, Z.; Mao, J.; Zhang, H.; Liu, X.; Ma, M.; Wang, Q.; Liu, H. Bitter and Astringent Substances in Green Tea: Composition, Human Perception Mechanisms, Evaluation Methods and Factors Influencing Their Formation. Food Res. Int. 2022, 157, 111262. [Google Scholar] [CrossRef]
- Dimpfel, W.; Kler, A.; Kriesl, E.; Lehnfeld, R. Theogallin and L-Theanine as Active Ingredients in Decaffeinated Green Tea Extract: I. Electrophysiological Characterization in the Rat Hippocampus In-Vitro. J. Pharm. Pharmacol. 2010, 59, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Kurin, E.; Mučaji, P.; Nagy, M. In Vitro Antioxidant Activities of Three Red Wine Polyphenols and Their Mixtures: An Interaction Study. Molecules 2012, 17, 14336–14348. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial Effects of Green Tea—A Review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Atoui, A.K.; Mansouri, A.; Boskou, G.; Kefalas, P. Tea and Herbal Infusions: Their Antioxidant Activity and Phenolic Profile. Food Chem. 2005, 89, 27–36. [Google Scholar] [CrossRef]
- Frei, B.; Higdon, J.V. Antioxidant Activity of Tea Polyphenols In Vivo: Evidence from Animal Studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef] [PubMed]
- Mckay, D.L.; Blumberg, J.B. A Review of the Bioactivity and Potential Health Benefits of Peppermint Tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef]
- Oh, J.; Jo, H.; Cho, A.R.; Kim, S.J.; Han, J. Antioxidant and Antimicrobial Activities of Various Leafy Herbal Teas. Food Control 2013, 31, 403–409. [Google Scholar] [CrossRef]
- Farooq, S.; Sehgal, A. Synergistic Antioxidant Interactions between Green Tea and Ocimum Gratissimum. Asian Pac. J. Trop. Biomed. 2019, 9, 333–338. [Google Scholar] [CrossRef]
- Uduwana, S.; Abeynayake, N.; Wickramasinghe, I. Synergistic, Antagonistic, and Additive Effects on the Resultant Antioxidant Activity in Infusions of Green Tea with Bee Honey and Citrus Limonum Extract as Additives. J. Agric. Food Res. 2023, 12, 100571. [Google Scholar] [CrossRef]
- Shevchenko, Y.; Helfert, J.; Hemmerich, I.; Smetanska, I. Influence of Stevia: Additives on Antioxidant Properties of Different Green Teas Influence of Stevia Additives on Antioxidant Properties of Different Green Teas. Agro FOOD Ind. Hi Tech. 2013, 24, 22–26. [Google Scholar]
- Salazar-Campos, J.; Salazar-Campos, O.; Gálvez-Ruiz, O.; Gavidia-Chávez, H.; Gavidia-Chávez, M.; Irigoin-Guevara, L.; Obregón-Domínguez, J. Functional Properties and Acceptability of Potentially Medicinal Tea Infusions Based on Equisetum Arvense, Desmodium Molliculum, and Mentha Piperita. Prev. Nutr. Food Sci. 2023, 28, 444–452. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Koşar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant Properties and Composition of Aqueous Extracts from Mentha Species, Hybrids, Varieties, and Cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- DiLabio, G.A.; Johnson, E.R. Lone Pair-π and π-π Interactions Play an Important Role in Proton-Coupled Electron Transfer Reactions. J. Am. Chem. Soc. 2007, 129, 6199–6203. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.; Zhang, H.Y. Estimation of Scavenging Activity of Phenolic Compounds Using the ABTS•+ Assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Rüweler, M.; Anker, A.; Gülden, M.; Maser, E.; Seibert, H. Inhibition of Peroxide-Induced Radical Generation by Plant Polyphenols in C6 Astroglioma Cells. Toxicol. Vitr. 2008, 22, 1377–1381. [Google Scholar] [CrossRef]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant Properties of Hydroxycinnamic Acids: A Review of Structure-Activity Relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef]
- Riachi, L.G.; De Maria, C.A.B. Peppermint Antioxidants Revisited. Food Chem. 2015, 176, 72–81. [Google Scholar] [CrossRef]
- Biskup, I.; Golonka, I.; Gamian, A.; Sroka, Z. Antioxidant activity of selected phenols estimated by ABTS and FRAP methods. Postep. Hig Med Dosw (Online) 2013, 67, 958–963. [Google Scholar] [CrossRef]
- PhBs. Pharmacopoea Bohemoslovaca, IV.; Avicenum: Praha, Czech Republic, 1987. [Google Scholar]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Rottmann, S.; Aspillaga, A.A.; Pérez, D.D.; Vasquez, L.; Martinez, A.L.F.; Leighton, F. Juice and Phenolic Fractions of the Berry Aristotelia Chilensis Inhibit LDL Oxidation in Vitro and Protect Human Endothelial Cells against Oxidative Stress. J. Agric. Food Chem. 2002, 50, 7542–7547. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Bittner Fialová, S.; Gančár, M.; Kurin, E.; Dokupilová, S.; Gažová, Z.; Nagy, M. Oregano Polyphenols Reduce Human Insulin Amyloid Aggregation. Biomed. Pharmacother. 2025, 184, 117904. [Google Scholar] [CrossRef]
- European Pharmacopoeia 11. Available online: https://pheur.edqm.eu/subhome/11-6 (accessed on 30 January 2025).
| DPPH | ABTS | DCF | ||||
|---|---|---|---|---|---|---|
| IC50 µg/mL | r | IC50 µg/mL | r | IC50 µg/mL | r | |
| GT | 4.81 ± 0.31 | 0.92 | 2.70 ± 0.23 | 0.98 | 3.71 ± 0.25 | 0.96 |
| PM | 6.69 ± 0.28 | 0.97 | 9.18 ± 0.65 | 0.98 | 3.80 ± 0.24 | 0.97 |
| lyo; GT:PM | 5.53 ± 0.30 (3.32 + 2.21) | 0.96 | 4.14 ± 0.32 (2.48 + 1.66) | 0.98 | 3.59 ± 0.25 (2.15 + 1.44) | 0.96 |
| mix; GT:PM | 4.34 ± 0.28 (2.60 + 1.34) | 0.95 | 4.24 ± 0.36 (2.54 + 1.70) | 0.97 | 6.83 ± 0.49 (4.10 + 2.73) | 0.96 |
| DPPH | ABTS | DCF | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combination | Interaction Effect | CI | SDA | DRI GT:MP | Interaction Effect | CI | SDA | DRI GT:MP | Interaction Effect | CI | SDA | DRI GT:MP |
| lyo; GT:PM | Nearly additive | 0.99 | ±0.01 | 2.42:1.74 | Nearly additive | 0.99 | ±0.02 | 1.30:4.44 | Nearly additive | 0.96 | ±0.02 | 1.72:2.65 |
| mix; GT:PM | Slight synergism | 0.80 | ±0.01 | 3.85:1.84 | Nearly additive | 1.02 | ±0.03 | 1.27:4.33 | Antagonism | 1.83 | ±0.04 | 0.91:1.39 |
| DPPH | ABTS | |||||
|---|---|---|---|---|---|---|
| IC50 µM | IC50 µg/mL | r | IC50 µM | IC50 µg/mL | r | |
| EGCG | 5.28 ± 0.31 | 2.42 | 0.97 | 2.68 ± 0.16 | 1.23 | 0.97 |
| Q | 16.11 ± 0.27 | 4.87 | 0.99 | 3.36 ± 0.19 | 1.02 | 0.98 |
| RA | 17.90 ± 0.34 | 6.45 | 0.99 | 4.44 ± 0.29 | 1.60 | 0.97 |
| Q + EGCG | 6.91 ± 0.38 (3.46 + 3.46) | 2.58 (1.00 + 1.58) | 0.97 | 3.09 ± 0.07 (1.55 + 1.55) | 1.18 (0.47 + 0.71) | 0.99 |
| Q + RA | 12.02 ± 0.28 (6.01 + 6.01) | 3.99 (1.82 + 2.17) | 0.99 | 3.12 ± 0.16 (1.56 + 1.56) | 1.03 (0.47 + 0.56) | 0.98 |
| RA + EGCG | 6.24 ± 0.35 (3.12 + 3.12) | 2.55 (1.12 + 1.43) | 0.98 | 3.28 ± 0.14 (1.64 + 1.64) | 1.34 (0.59 + 0.75) | 0.98 |
| Q + RA + EGCG | 6.56 ± 0.36 (2.19 + 2.19 + 2.19) | 2.44 (0.66 + 0.78 + 1.00) | 0.97 | 2.86 ± 0.08 (0.95 + 0.95 + 0.95) | 1.07 (0.29 + 0.34 + 0.44) | 0.99 |
| DPPH | ABTS | |||||||
|---|---|---|---|---|---|---|---|---|
| Combinations | Interaction Effect | CI | SDA | DRI | Interaction Effect | CI | SDA | DRI |
| Q + EGCG | Slight synergism | 0.87 | ±0.01 | 4.66:1.53 | Moderate antagonism | 1.40 | ±0.01 | 2.17:1.73 |
| Q + RA | Moderate synergism | 0.70 | ±0.003 | 2.68:2.98 | Moderate synergism | 0.82 | ±0.01 | 2.15:2.85 |
| RA + EGCG | Moderate synergism | 0.76 | ±0.01 | 5.74:1.69 | Nearly additive | 0.98 | ±0.01 | 2.71:1.64 |
| Q + RA + EGCG | Synergism | 0.67 | ±0.01 | 7.36:8.18:2.41 | Slight synergism | 0.86 | ±0.01 | 3.58:4.65:2.81 |
| Peak | TR (min) | [M-H]− (m/z) | MS2 (20 eV) (m/z) | Identified Compound | Mass Concentration ppm (μg/mL) * |
|---|---|---|---|---|---|
| PM | |||||
| 1 | 6.693 | 179.149 | 135 | Caffeic acid | 22.7 |
| 2 | 9.507 | 353.0858 | 191 | Chlorogenic acid | 20.2 |
| 3 | 9.873 | 563.0689 | 436/281/237/193 | 4′,7-Dimethoxyflavone | 35.1 |
| 4 | 15.807 | 637.1032 | 351/285 | Luteolin-7-diglucuronide | 23.6 |
| 5 | 16.380 | 459.1517 | 297 | 5-Hydroxy-4′,7-dimethoxyflavone-5-glucoside | LOQ |
| 6 | 18.668 | 537.1056 | 493/295 | Lithospermic acid | 138.7 |
| 7 | 20.280 | 595.1680 | 459/287 | Eriodictyol-7-O-rutinoside (eriocitrin) | 196.6 |
| 8 | 20.687 | 593.34 | 285.0407 | Luteolin-7-O-rutinoside | 262.4 |
| 9 | 22.867 | 717.1491 | 519/439/321/295/179/135 | Salvianolic acid L/B | 168.5 |
| 10 | 24.947 | 577.37 | 269 | Apigenin-7-O-rutinoside (isorhoifolin) | 128.6 |
| 11 | 25.267 | 359.19 | 197/161/135 | Rosmarinic acid | 326.0 |
| 12 | 28.318 | 609.33 | 301/325/284 | Hesperetin-7-O-rutinoside (hesperidin) | 97.0 |
| 13 | 28.457 | 607.36 | 299.0554/284.0334 | Diosmetin-7-O-rutinoside (diosmin) | 144.1 |
| 14 | 29.846 | 717.1480 | 537/519/493/321/295 | Salvianolic acid B/L | 215.0 |
| 15 | 36.376 | 715.1307 | 535/293 | Salvianolic acid C derivative | 62.8 |
| GT | |||||
| 1 | 4.773 | 343.0680 | 191 | Theogallin | 152.9 |
| 2 | 5.513 | 169.0145 | 125/107 | Gallic acid | 80.1 |
| 3 | 8.340 | 153.0191 | 327/125/124/123/109/108 | 2,5-Dihydroxybenzoic acid | 82.5 |
| 4 | 8.847 | 305.0677 | 611/167/125 | Gallocatechin | 24.6 |
| 5 | 9.533 | 353.0887 | 191/179/135/161 | Chlorogenic acid | 54.7 |
| 6 | 12.100 | 305.0673 | 611/165/139/137/125 | Epigallocatechin (EC) | 97.2 |
| 7 | 12.320 | 353.0889 | 707/191/161 | 5-O-Caffeoylquinic acid (derivative) | 77.9 |
| 8 | 12.927 | 353.0887 | 191/173/135 | Cryptochlorogenic acid | 109.7 |
| 9 | 13.493 | 633.0355 | 463/349/300 | Monogalloyl-HHDP-glucose | 75.1 |
| 10 | 13.980 | 471.0588 | 427/275/169 | Epigallocatechin 3-O-(3′-O-methyl)gallate | 207.6 |
| 11 | 15.573 | 337.0933 | 191/163/173/119 | p-Coumaroylquinic acid | 95.2 |
| 12 | 16.407 | 289.0729 | 245/203/151 | (+)-Catechin (cinidianol) | 108.6 |
| 13 | 16.860 | 457.0796 | 305/219/193/169 | Epigallocatechin gallate (EGCG) | 249.7 |
| 14 | 17.827 | 457.0791 | 331/305/169 | Gallocatechin gallate (GCG) | 47.1 |
| 15 | 19.053 | 479.0847 | 316/271/151 | Unidentified | 45.6 |
| 16 | 21.947 | 441.0894 | 289/245/169 | (+)-Catechin gallate (CG) | 182.8 |
| 17 | 22.213 | 609.0904 | 463/301/169 | Rutin | 70.4 |
| 18 | 24.267 | 593.1519 | 285/257/163 | Kaempferol-3-rutinoside | 18.6 |
| 19 | 27.067 | 447.0943 | 301/151/145 | Quercetin rhamnoside | 14.1 |
| 20 | 35.447 | 516.1395 | 301/125 | Unidentified | LOD |
| Sample | THD (%) | Total Polyphenols | Tannins | Flavonoids λ = 392 nm | Flavonoids λ = 420 nm |
|---|---|---|---|---|---|
| PM | 18.9 ± 0.2 | 23.0 ± 0.3 | 16.1 ± 0.3 | 2.8 ± 0.1 | 0.6 ± 0.02 |
| GT | 4.7 ± 0.1 | 29.8 ± 0.2 | 18.8 ± 0.3 | 0.5 ± 0.01 | 0.5 ± 0.01 |
| lyo; GT:PM | 12.6 ± 0.2 | 26.3 ± 0.3 | 18.1 ± 0.1 | 1.0 ± 0.01 | 0.4 ± 0.01 |
| mix; GT:PM | 9.5 ± 0.2 | 27.0 ± 0.3 | 18.6 ± 0.4 | 1.1 ± 0.01 | 0.5 ± 0.01 |
| Interaction | CI Scale | Interpretation |
|---|---|---|
| Synergism | <0.1 | Very strong synergism |
| 0.1–0.3 | Strong synergism | |
| 0.3–0.7 | Synergism | |
| 0.7–0.85 | Moderate synergism | |
| 0.85–0.90 | Slight synergism | |
| Additivity | 0.90–1.10 | Nearly additive |
| Antagonism | 1.10–1.20 | Slight antagonism |
| 1.20–1.45 | Moderate antagonism | |
| 1.45–3.3 | Antagonism | |
| 3.3–10 | Strong antagonism | |
| >10 | Very strong antagonism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurin, E.; Hajská, M.; Kostovčíková, E.; Dokupilová, K.; Mučaji, P.; Nagy, M.; Novotný, B.; Bittner Fialová, S. Unveiling Synergistic Antioxidant Effects of Green Tea and Peppermint: Role of Polyphenol Interactions and Blend Preparation. Int. J. Mol. Sci. 2025, 26, 6257. https://doi.org/10.3390/ijms26136257
Kurin E, Hajská M, Kostovčíková E, Dokupilová K, Mučaji P, Nagy M, Novotný B, Bittner Fialová S. Unveiling Synergistic Antioxidant Effects of Green Tea and Peppermint: Role of Polyphenol Interactions and Blend Preparation. International Journal of Molecular Sciences. 2025; 26(13):6257. https://doi.org/10.3390/ijms26136257
Chicago/Turabian StyleKurin, Elena, Marianna Hajská, Ema Kostovčíková, Kamila Dokupilová, Pavel Mučaji, Milan Nagy, Branislav Novotný, and Silvia Bittner Fialová. 2025. "Unveiling Synergistic Antioxidant Effects of Green Tea and Peppermint: Role of Polyphenol Interactions and Blend Preparation" International Journal of Molecular Sciences 26, no. 13: 6257. https://doi.org/10.3390/ijms26136257
APA StyleKurin, E., Hajská, M., Kostovčíková, E., Dokupilová, K., Mučaji, P., Nagy, M., Novotný, B., & Bittner Fialová, S. (2025). Unveiling Synergistic Antioxidant Effects of Green Tea and Peppermint: Role of Polyphenol Interactions and Blend Preparation. International Journal of Molecular Sciences, 26(13), 6257. https://doi.org/10.3390/ijms26136257







