MH002, a Novel Butyrate-Producing Consortium of Six Commensal Bacterial Strains Has Immune-Modulatory and Mucosal-Healing Properties
Abstract
1. Introduction
2. Results
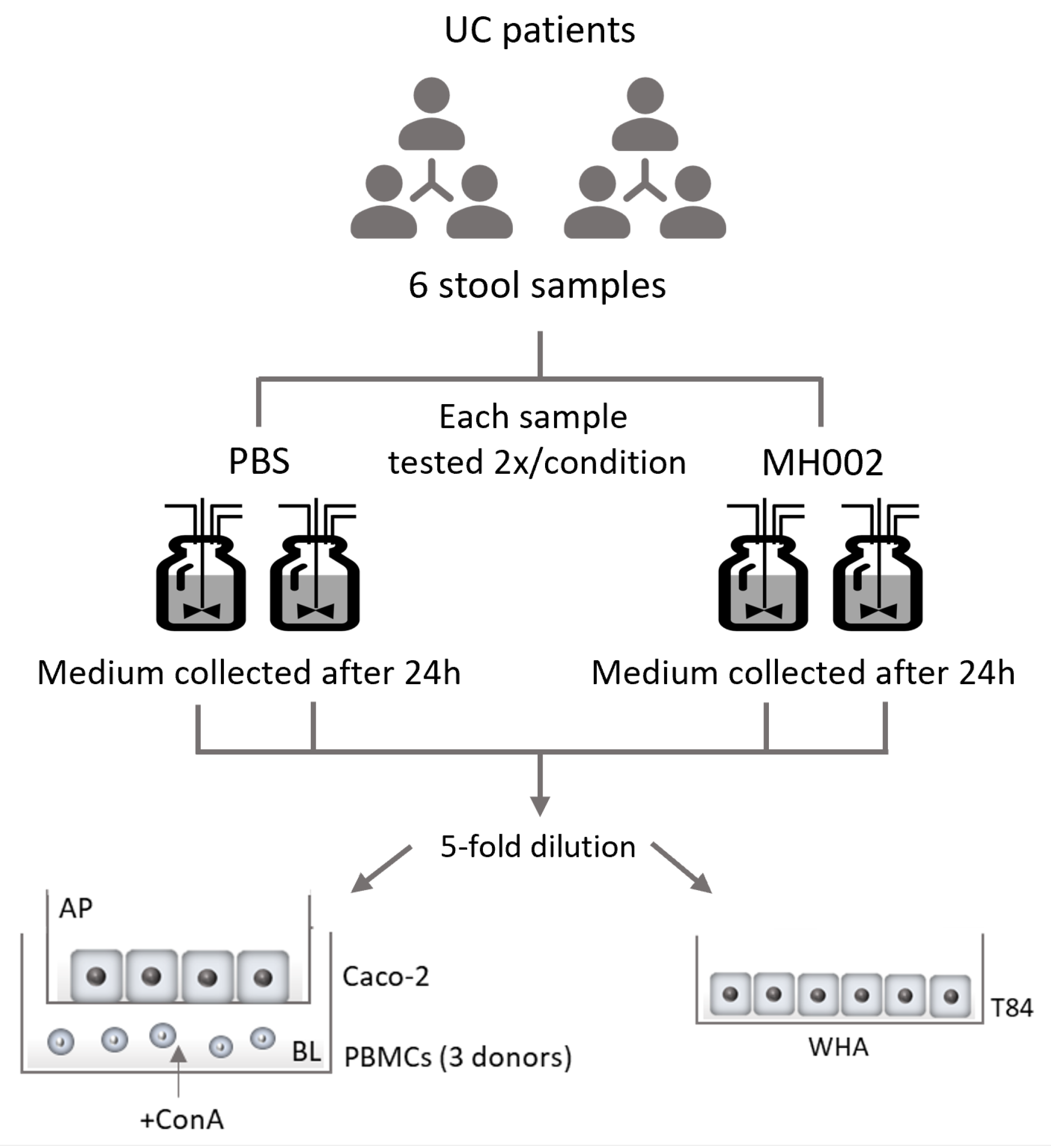
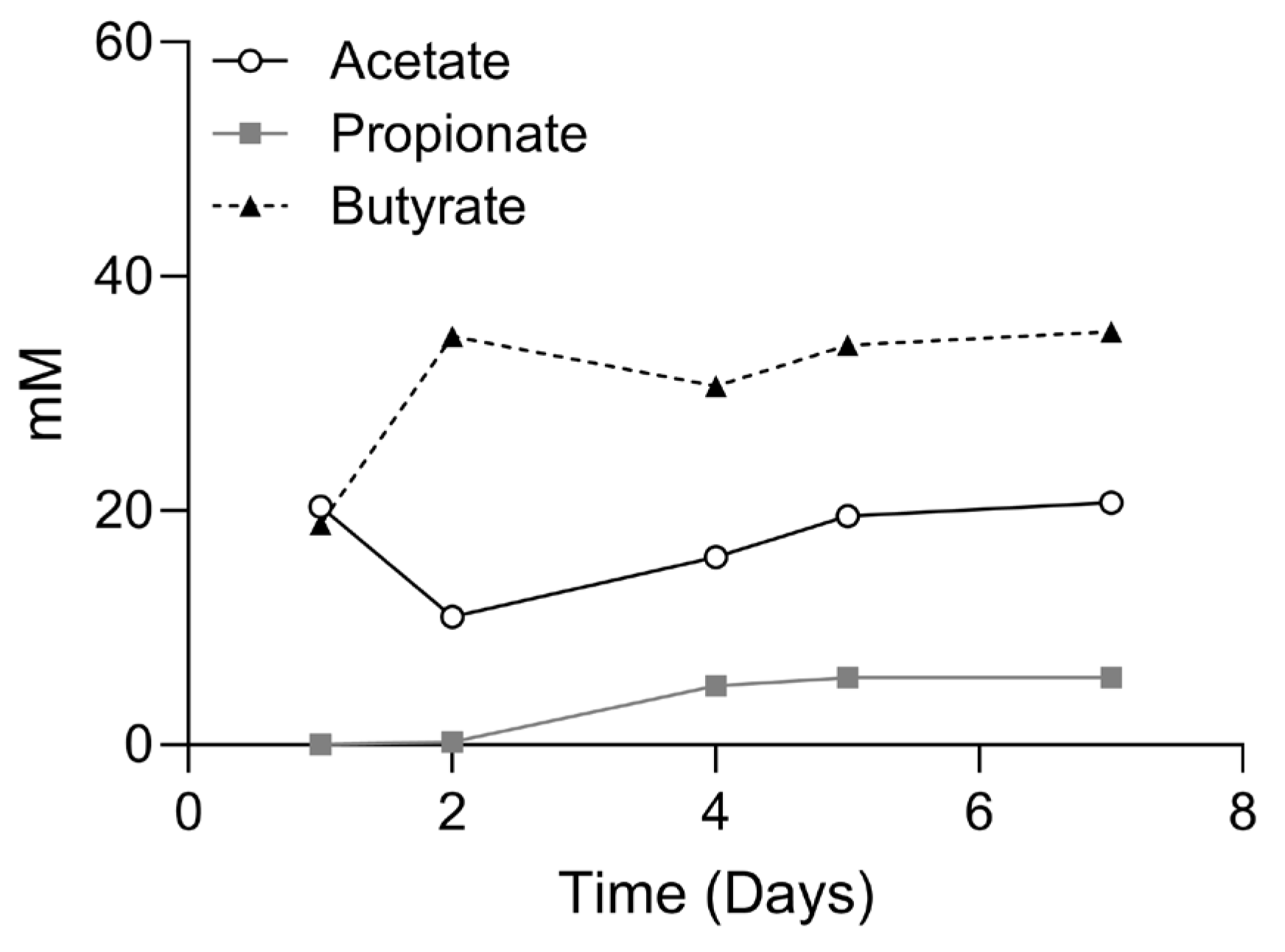
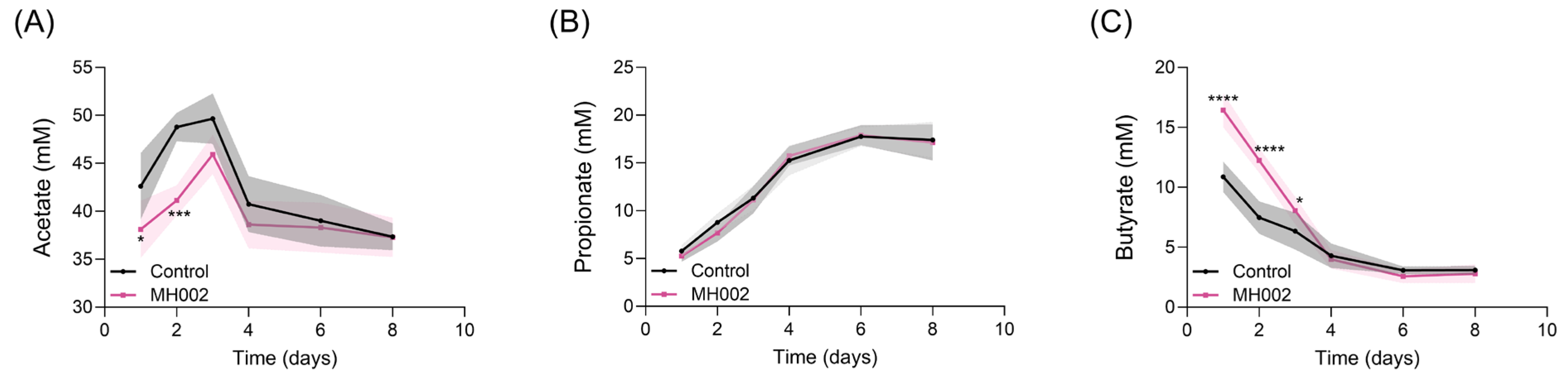
2.1. SCFA Production in the Presence and Absence of IBD Fecal Microbiome
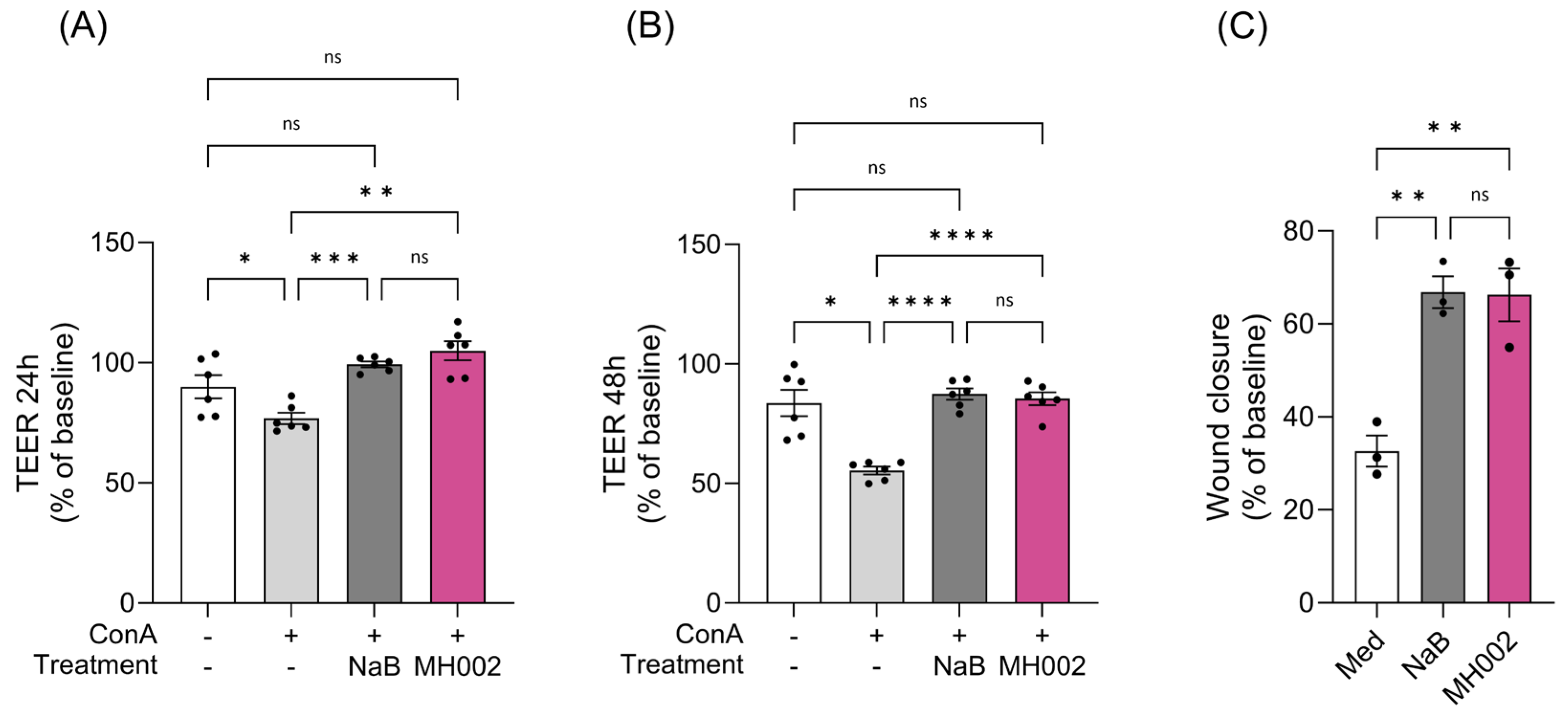
2.2. Effect of MH002 on Intestinal Barrier Integrity and Cytokine Release In Vitro
2.3. Effect of MH002 on In Vitro Wound Repair
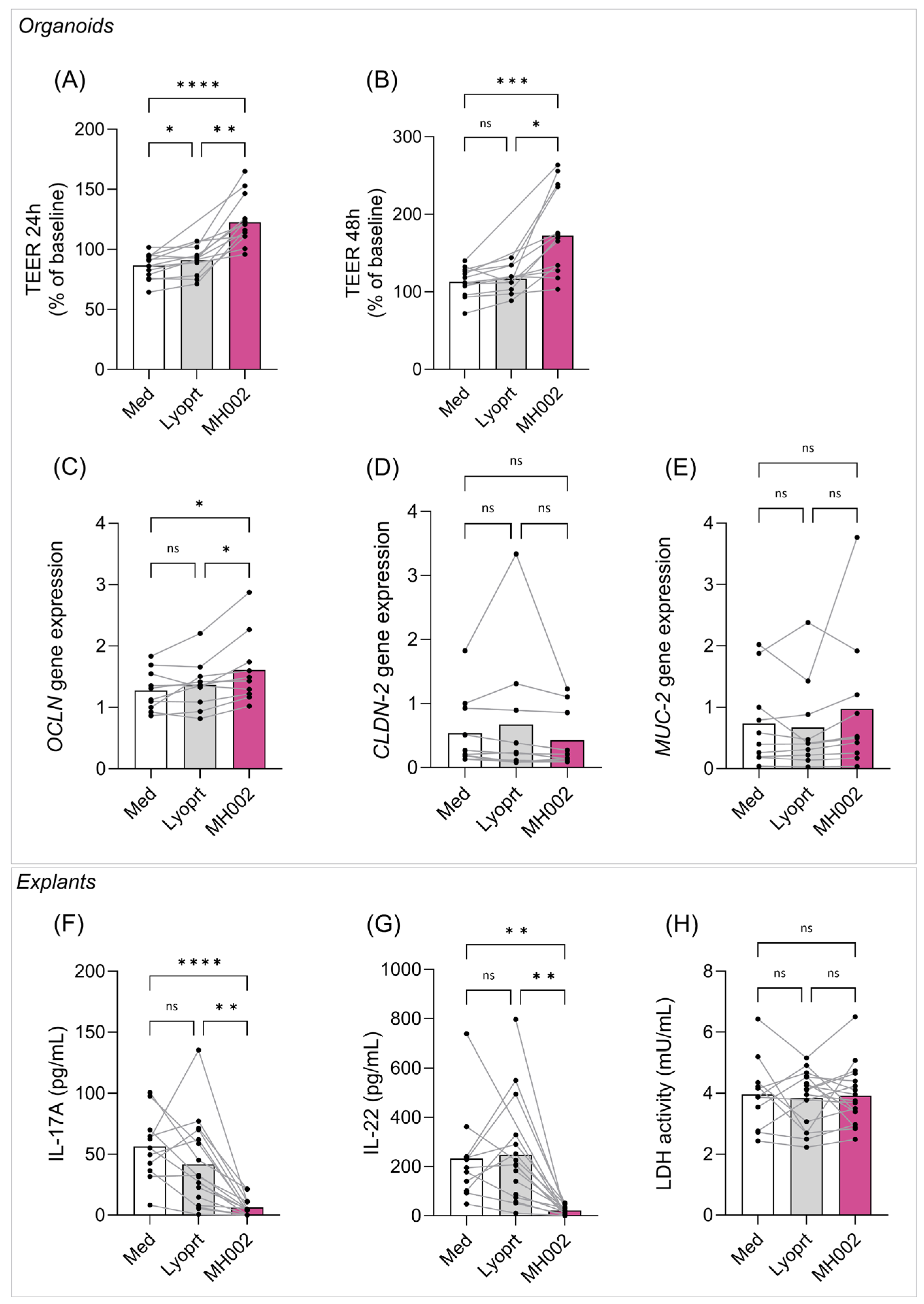
2.4. Effect of MH002 on Biopsy-Derived Intestinal Organoids and Colonic Explants
2.4.1. Effect of MH002 on IBD Patients-Derived Organoids
2.4.2. Effect of MH002 on IBD Patients-Derived Colonic Explants
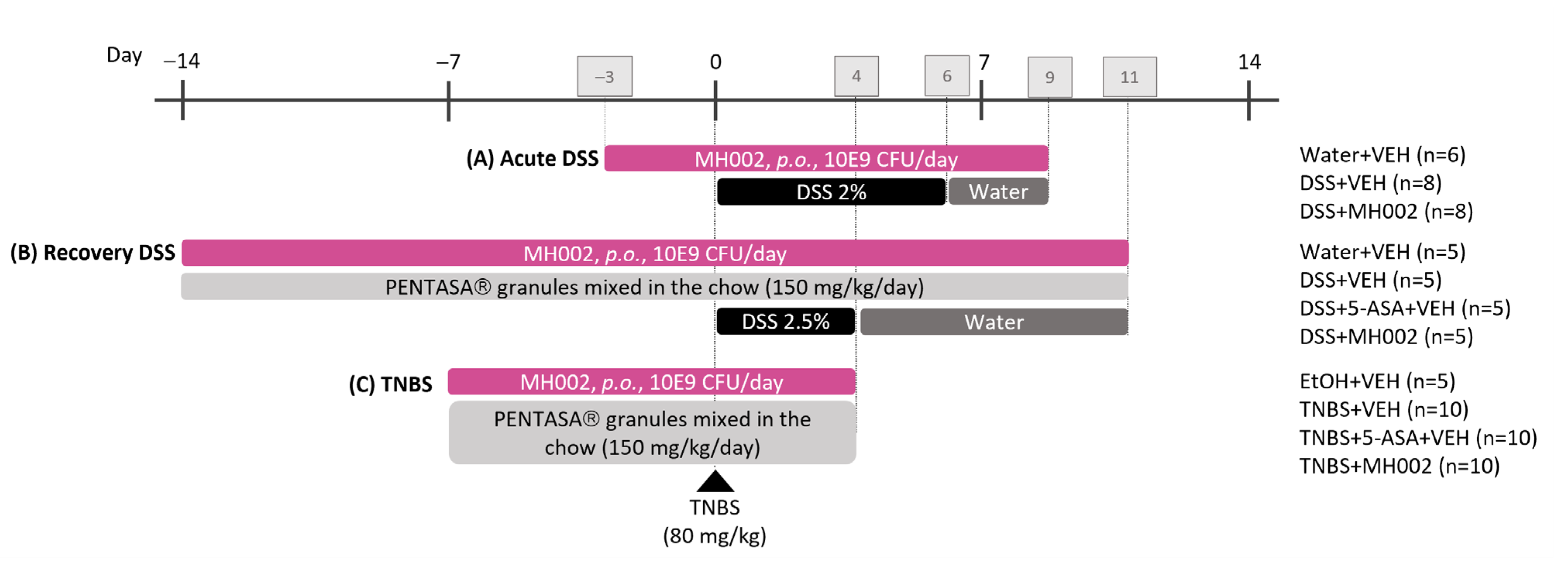
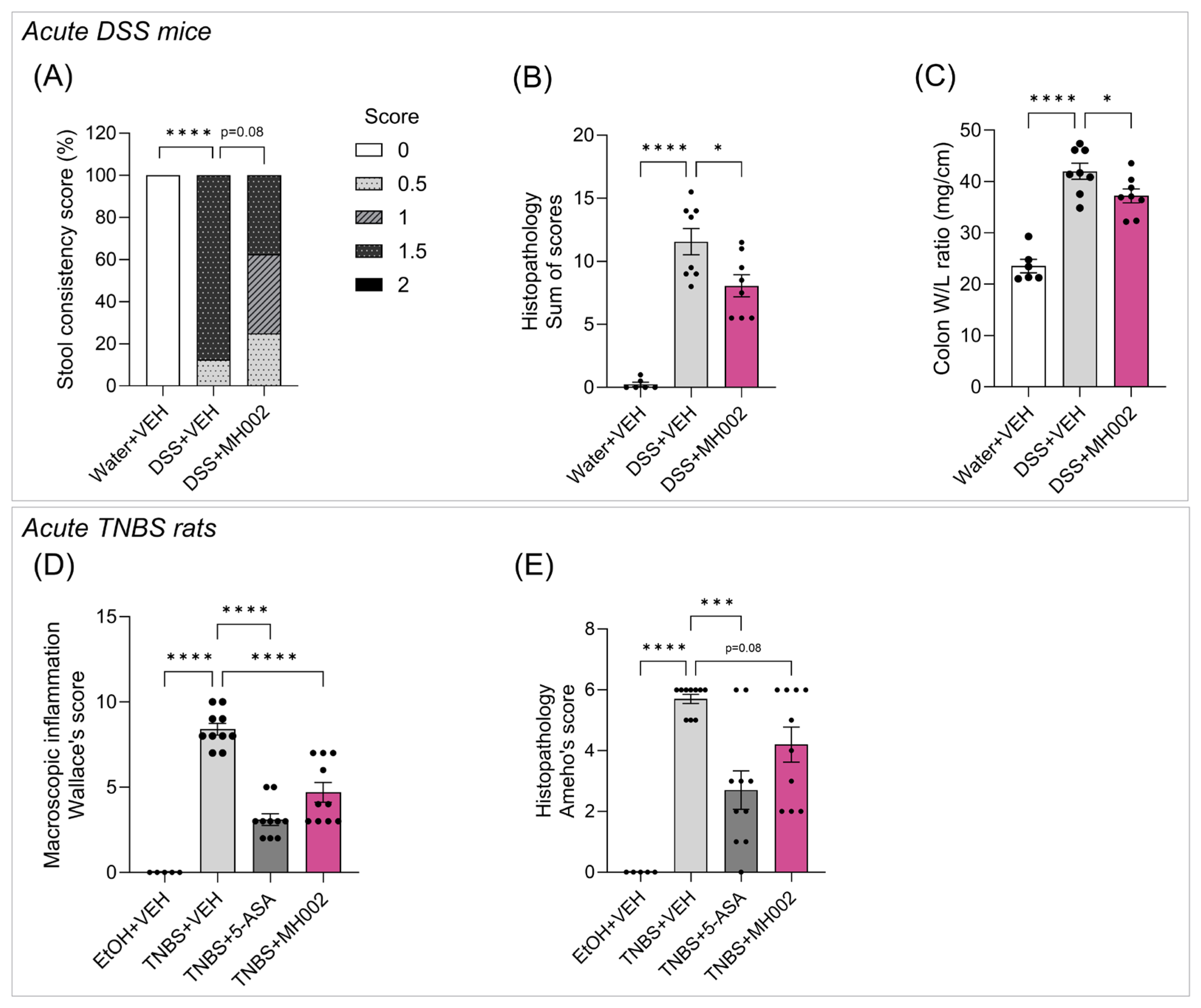
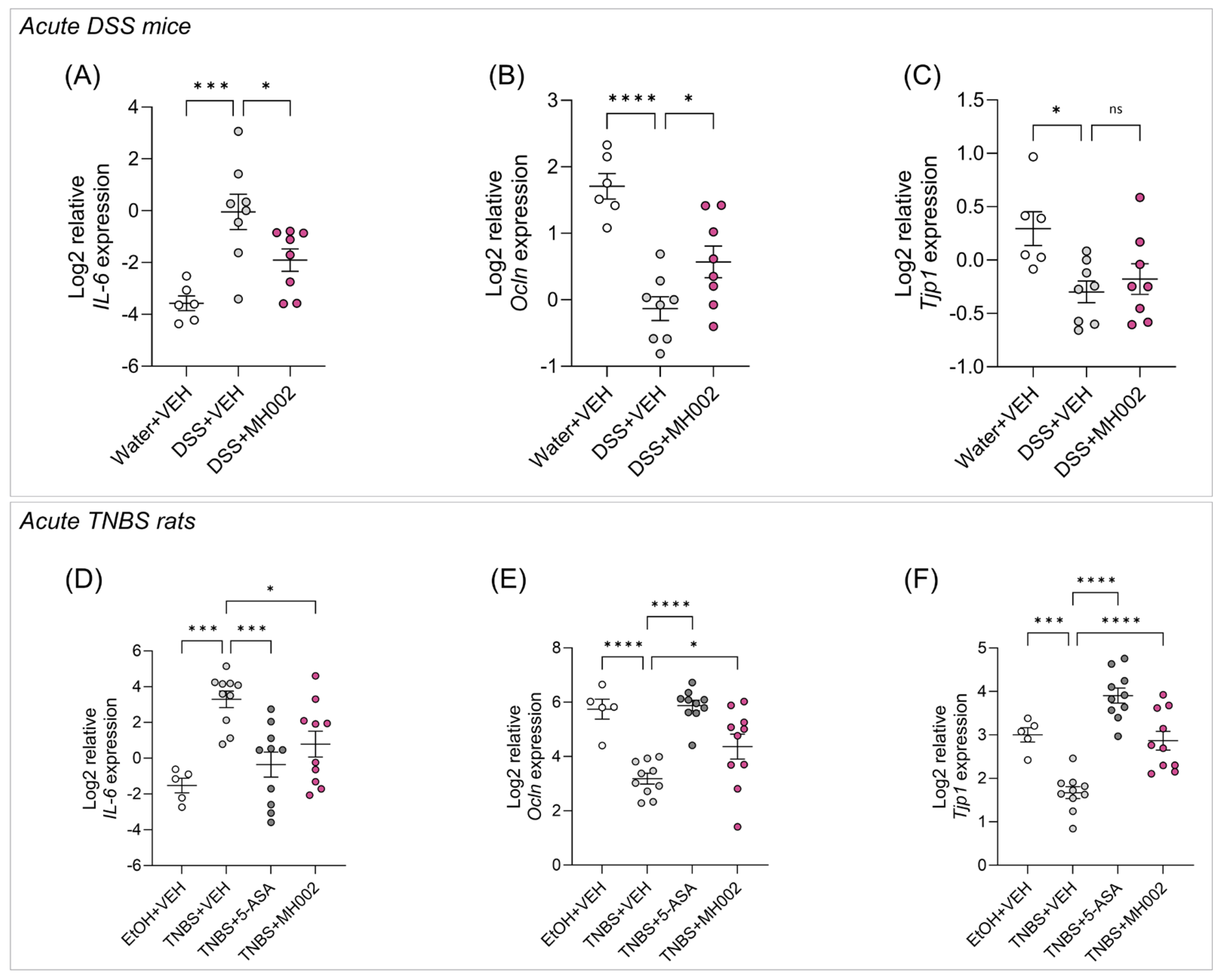
2.5. Effect of MH002 in Chemical-Induced Acute Colitis in Rats and Mice
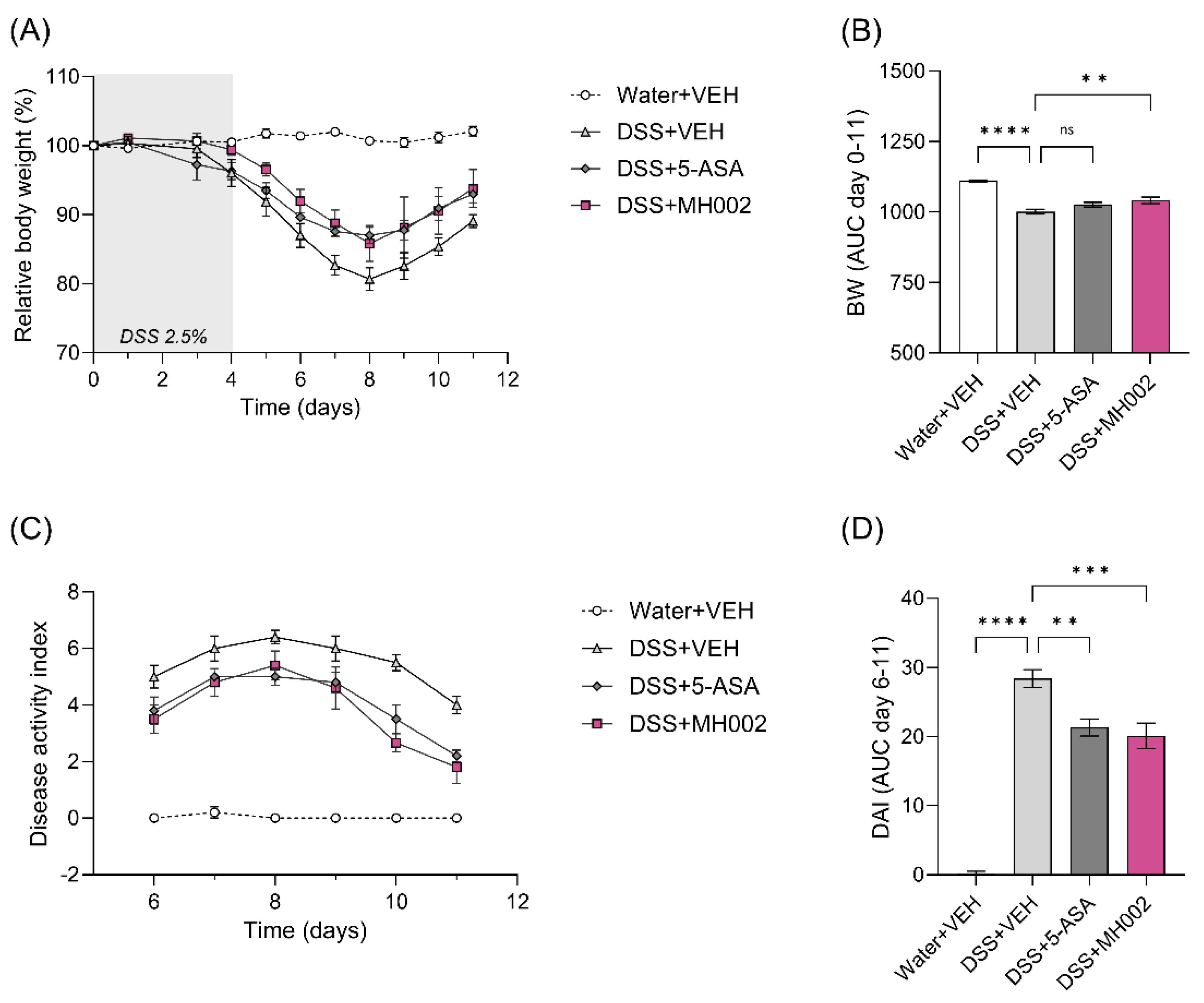
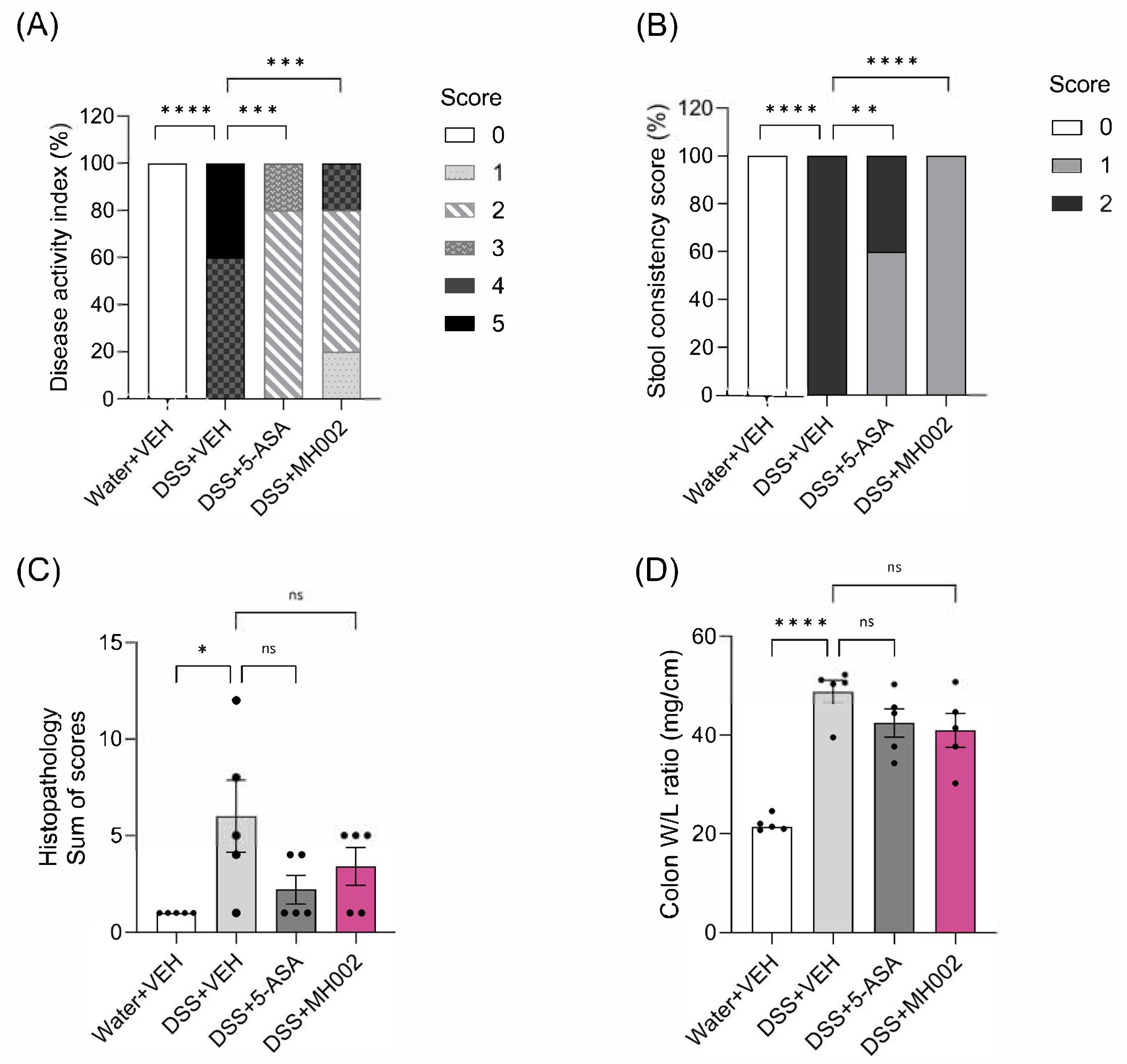
2.6. Effect of MH002 on the Recovery from Acute DSS-Induced Colitis in Mice
3. Discussion
4. Materials and Methods
4.1. MH002 Test Materials
4.1.1. Fed-Batch Cultures
4.1.2. Formulations for In Vivo Studies
4.2. Caco-2/PBMCs Co-Cultures
4.3. Wound Healing Assay (WHA)
4.4. Simulator of the Human Intestinal Microbial Ecosystem (SHIME®)
4.5. SCFA Measurement
4.6. Biopsy-Derived Intestinal Organoids and Colonic Explants from IBD Patients
4.6.1. Biopsy Collection
4.6.2. Preparation of Intestinal Organoids
4.6.3. Preparation of Colonic Explants
4.7. Chemical-Induced Colitis in Rats and Mice
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loddo, I.; Romano, C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front Immunol. 2015, 6, 551. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Ng, S.C. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology 2017, 152, 313–321.e2. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.P.; Ratliff, H.; Abdelwahab, A.; Vohra, M.H.; Kuang, A.; Shatila, M.; Khan, M.A.; Shafi, M.A.; Thomas, A.S.; Philpott, J.; et al. The Safety of Immunosuppressants Used in the Treatment of Immune-Related Adverse Events due to Immune Checkpoint Inhibitors: A Systematic Review. J. Cancer 2023, 14, 2956–2963. [Google Scholar] [CrossRef]
- Fumery, M.; Singh, S.; Dulai, P.S.; Gower-Rousseau, C.; Peyrin-Biroulet, L.; Sandborn, W.J. Natural History of Adult Ulcerative Colitis in Population-based Cohorts: A Systematic Review. Clin. Gastroenterol. Hepatol. 2018, 16, 343–356.e3. [Google Scholar] [CrossRef]
- Afzali, A.; Lukanova, R.; Hennessy, F.; Kakehi, S.; Knight, H.; Milligan, G.; Gupte-Singh, K. Unmet Needs in Real-World Advanced Therapy-Naïve and -Experienced Patients with Moderately to Severely Active Ulcerative Colitis in the United States. Adv. Ther. 2023, 40, 4321–4338. [Google Scholar] [CrossRef]
- Danese, S.; Allez, M.; Van Bodegraven, A.A.; Dotan, I.; Gisbert, J.P.; Hart, A.; Lakatos, P.L.; Magro, F.; Peyrin-Biroulet, L.; Schreiber, S.; et al. Unmet Medical Needs in Ulcerative Colitis: An Expert Group Consensus. Dig. Dis. 2019, 37, 266–283. [Google Scholar] [CrossRef]
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Front. Cell. Infect. Microbiol. 2022, 12, 733992. [Google Scholar] [CrossRef]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- Singh, V.; Proctor, S.; Willing, B. Koch’s postulates, microbial dysbiosis and inflammatory bowel disease. Clin. Microbiol. Infect. 2016, 22, 594–599. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel. Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014, 121, 91–119. [Google Scholar]
- Zhuang, X.; Li, T.; Li, M.; Huang, S.; Qiu, Y.; Feng, R.; Zhang, S.; Chen, M.; Xiong, L.; Zeng, Z. Systematic Review and Meta-analysis: Short-Chain Fatty Acid Characterization in Patients with Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2019, 25, 1751–1763. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Aden, K.; Rehman, A.; Waschina, S.; Pan, W.H.; Walker, A.; Lucio, M.; Nunez, A.M.; Bharti, R.; Zimmerman, J.; Bethge, J.; et al. Metabolic Functions of Gut Microbes Associate With Efficacy of Tumor Necrosis Factor Antagonists in Patients With Inflammatory Bowel Diseases. Gastroenterology 2019, 157, 1279–1292.e11. [Google Scholar] [CrossRef]
- Palmela, C.; Chevarin, C.; Xu, Z.; Torres, J.; Sevrin, G.; Hirten, R.; Barnich, N.; Ng, S.C.; Colombel, J.-F. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut 2018, 67, 574–587. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Hall, A.B.; Yassour, M.; Sauk, J.; Garner, A.; Jiang, X.; Arthur, T.; Lagoudas, G.K.; Vatanen, T.; Fornelos, N.; Wilson, R.; et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017, 9, 103. [Google Scholar] [CrossRef]
- Vich Vila, A.; Imhann, F.; Collij, V.; Jankipersadsing, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F.; et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl. Med. 2018, 10, eaap8914. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, J.F.; Segal, J.P.; de Campos Braz, L.M.; Hart, A.L. The microbiome as a therapy in pouchitis and ulcerative colitis. Nutrients 2021, 13, 1780. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Li, X.; Shen, J.; Feng, Q. Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease: An Update. Front. Pharmacol. 2020, 11, 574533. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, S.; Pinheiro, I.; Verhelst, A.; Van Den Abbeele, P.; Maignien, L.; Laukens, D.; Reeves, S.G.; Robinson, L.E.; Raas, T.; Schneider, Y.-J.; et al. A dried yeast fermentate selectively modulates both the luminal and mucosal gut microbiota and protects against inflammation, as studied in an integrated in vitro approach. J. Agric. Food Chem. 2013, 61, 9380–9392. [Google Scholar] [CrossRef]
- Satsu, H.; Ishimoto, Y.; Nakano, T.; Mochizuki, T.; Iwanaga, T.; Shimizu, M. Induction by activated macrophage-like THP-1 cells of apoptotic and necrotic cell death in intestinal epithelial Caco-2 monolayers via tumor necrosis factor-alpha. Exp. Cell Res. 2006, 312, 3909–3919. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17010338 (accessed on 14 July 2015). [CrossRef]
- Katial, R.K.; Sachanandani, D.; Pinney, C.; Lieberman, M.M. Cytokine production in cell culture by peripheral blood mononuclear cells from immunocompetent hosts. Clin. Diagn. Lab. Immunol. 1998, 5, 78–81. [Google Scholar] [CrossRef]
- Devriese, S.; Van den Bossche, L.; Van Welden, S.; Holvoet, T.; Pinheiro, I.; Hindryckx, P.; De Vos, M.; Laukens, D. T84 monolayers are superior to Caco-2 as a model system of colonocytes. Histochem. Cell Biol. 2017, 148, 85–93. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Laeremans, T.; Vanhove, W.; Arnauts, K.; Ramalho, A.S.; Farre, R.; Cleynen, I.; Ferrante, M.; Vermeire, S. Butyrate Does Not Protect against Inflammation-induced Loss of Epithelial Barrier Function and Cytokine Production in Primary Cell Monolayers from Patients with Ulcerative Colitis. J. Crohn’s Colitis 2019, 13, 1351–1361. [Google Scholar] [CrossRef]
- Luettig, J.; Rosenthal, R.; Barmeyer, C.; Schulzke, J.D. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers 2015, 3, e977176. [Google Scholar] [CrossRef]
- Vadstrup, K.; Galsgaard, E.D.; Gerwien, J.; Vester-Andersen, M.K.; Pedersen, J.S.; Rasmussen, J.; Neermark, S.; Kiszka-Kanowitz, M.; Jensen, T.; Bendtsen, F.; et al. Validation and optimization of an ex vivo assay of intestinal mucosal biopsies in Crohn’s disease: Reflects inflammation and drug effects. PLoS ONE. 2016, 11, e0155335. [Google Scholar] [CrossRef] [PubMed]
- Oka, A.; Sartor, R.B. Microbial-Based and Microbial-Targeted Therapies for Inflammatory Bowel Diseases. Dig. Dis. Sci. 2020, 65, 757–788. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Jain, D.; Tang, D.W.T.; Wong, S.H.; Lal, D. Gut microbiota in pathophysiology, diagnosis, and therapeutics of inflammatory bowel disease. Intest. Res. 2023, 22, 15–43. [Google Scholar] [CrossRef] [PubMed]
- Scheppach, W.; Sommer, H.; Kirchner, T.; Paganelli, G.-M.; Bartram, P.; Christl, S.; Richter, F.; Dusel, G.; Kasper, H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 1992, 103, 51–56. [Google Scholar] [CrossRef]
- Steinhart, A.H.; Brzezinski, A.; Baker, J.P. Treatment of refractory ulcerative proctosigmoiditis with butyrate enemas. Am. J. Gastroenterol. 1994, 89, 179–183. [Google Scholar]
- Tanaka, J.S.; Young, R.R.; Heston, S.M.; Jenkins, K.; Spees, L.P.; Sung, A.D.; Corbet, K.; Thompson, J.C.; Bohannon, L.; Martin, P.L.; et al. Anaerobic Antibiotics and the Risk of Graft-versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2020, 26, 2053–2060. [Google Scholar] [CrossRef]
- Romick-Rosendale, L.E.; Haslam, D.B.; Lane, A.; Denson, L.; Lake, K.; Wilkey, A.; Watanabe, M.; Bauer, S.; Litts, B.; Luebbering, N.; et al. Antibiotic Exposure and Reduced Short Chain Fatty Acid Production after Hematopoietic Stem Cell Transplant. Biol. Blood Marrow Transplant. 2018, 24, 2418–2424. [Google Scholar] [CrossRef]
- Borges-Canha, M.; Portela-Cidade, J.P.; Dinis-Ribeiro, M.; Leite-Moreira, A.F.; Pimentel-Nunes, P. Role of colonic microbiota in colorectal carcinogenesis: A systematic review. Rev. Esp. Enferm. Dig. 2015, 107, 659–671. [Google Scholar] [CrossRef]
- Ma, X.; Fan, P.X.; Li, L.S.; Qiao, S.Y.; Zhang, G.L.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012, 90, 266–268. [Google Scholar] [CrossRef]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef]
- Yu, A.S.L.; McCarthy, K.M.; Francis, S.A.; McCormack, J.M.; Lai, J.; Rogers, R.A.; Lynch, R.D.; Schneeberger, E.E. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am. J. Physiol. Cell Physiol. 2005, 288, C1231–C1241. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, M.M.; Shen, L.; Rajapakse, H.; Raleigh, D.R.; Wang, Y.; Wang, Y.; Lingaraju, A.; Zha, J.; Abbott, E.; McAuley, E.M.; et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol. Biol. Cell 2013, 24, 3056–3068. [Google Scholar] [CrossRef]
- Balda, M.S.; Whitney, J.A.; Flores, C.; González, S.; Cereijido, M.; Matter, K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 1996, 134, 1031–1049. [Google Scholar] [CrossRef]
- Poritz, L.S.; Harris LR3rd Kelly, A.A.; Koltun, W.A. Increase in the tight junction protein claudin-1 in intestinal inflammation. Dig. Dis. Sci. 2011, 56, 2802–2809. [Google Scholar] [CrossRef]
- Zeissig, S.; Bürgel, N.; Günzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.-D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007, 56, 61–72. [Google Scholar] [CrossRef]
- Kucharzik, T.; Walsh, S.V.; Chen, J.; Parkos, C.A.; Nusrat, A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am. J. Pathol. 2001, 159, 2001–2009. [Google Scholar] [CrossRef]
- Rosenthal, R.; Milatz, S.; Krug, S.M.; Oelrich, B.; Schulzke, J.-D.; Amasheh, S.; Günzel, D.; Fromm, M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J. Cell Sci. 2010, 123, 1913–1921. [Google Scholar] [CrossRef]
- Turksen, K.; Troy, T.-C. Barriers built on claudins. J. Cell Sci. 2004, 117, 2435–2447. [Google Scholar] [CrossRef]
- Prasad, S.; Mingrino, R.; Kaukinen, K.; Hayes, K.L.; Powell, R.M.; MacDonald, T.T.; Collins, J.E. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab. Investig. 2005, 85, 1139–1162. [Google Scholar] [CrossRef]
- Denizot, J.; Sivignon, A.; Barreau, F.; Darcha, C.; Chan, C.H.; Stanners, C.P.; Hofman, P.; Darfeuille-Michaud, A.; Barnich, N. Adherent-invasive Escherichia coli induce claudin-2 expression and barrier defect in CEABAC10 mice and Crohn’s disease patients. Inflamm. Bowel. Dis. 2012, 18, 294–304. [Google Scholar] [CrossRef]
- Weber, C.R.; Nalle, S.C.; Tretiakova, M.; Rubin, D.T.; Turner, J.R. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab. Investig. 2008, 88, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.; Jeon, K.; Moon, S.; Lee, K.; Kim, W.-K.; Jeong, H.; Cha, K.H.; Lim, M.Y.; Kang, W.; Kweon, M.-N.; et al. Roseburia spp. Abundance Associates with Alcohol Consumption in Humans and Its Administration Ameliorates Alcoholic Fatty Liver in Mice. Cell Host Microbe 2020, 27, 25–40.e6. [Google Scholar] [CrossRef] [PubMed]
- Jirsova, Z.; Heczkova, M.; Dankova, H.; Malinska, H.; Videnska, P.; Vespalcova, H.; Micenkova, L.; Bartonova, L.; Sticova, E.; Lodererova, A.; et al. The Effect of Butyrate-Supplemented Parenteral Nutrition on Intestinal Defence Mechanisms and the Parenteral Nutrition-Induced Shift in the Gut Microbiota in the Rat Model. Biomed. Res. Int. 2019, 2019, 7084734. [Google Scholar] [CrossRef]
- Chen, L.; Ruan, G.; Cheng, Y.; Yi, A.; Chen, D.; Wei, Y. The role of Th17 cells in inflammatory bowel disease and the research progress. Front. Immunol. 2023, 13, 1055914. [Google Scholar] [CrossRef]
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; Macdonald, T.T.; Pallone, F.; Monteleone, G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 2011, 141, 237–248.e1. [Google Scholar] [CrossRef]
- Rannug, A. How the AHR became important in intestinal homeostasis—A diurnal FICZ/AHR/CYP1A1 feedback controls both immunity and immunopathology. Int. J. Mol. Sci. 2020, 21, 5681. [Google Scholar] [CrossRef]
- Marinelli, L.; Martin-Gallausiaux, C.; Bourhis, J.-M.; Béguet-Crespel, F.; Blottière, H.M.; Lapaque, N. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci. Rep. 2019, 9, 643. [Google Scholar] [CrossRef]
- Sabihi, M.; Böttcher, M.; Pelczar, P.; Huber, S. Microbiota-Dependent Effects of IL-22. Cells 2020, 9, 2205. [Google Scholar] [CrossRef]
- Leonard, W.J.; Wan, C.K. IL-21 Signaling in Immunity. F1000Research 2016, 5, F1000 Faculty Rev-224. [Google Scholar] [CrossRef]
- Vyas, S.P.; Goswami, R. A decade of Th9 cells: Role of Th9 cells in inflammatory bowel disease. Front. Immunol. 2018, 9, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Cho, K.-A.; Kang, J.L.; Kim, K.H.; Woo, S.-Y. Comparison of experimental mouse models of inflammatory bowel disease. Int. J. Mol. Med. 2014, 33, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Ameho, C.K.; A Adjei, A.; Harrison, E.K.; Takeshita, K.; Morioka, T.; Arakaki, Y.; Ito, E.; Suzuki, I.; Kulkarni, A.D.; Kawajiri, A.; et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut 1997, 41, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Melgar, S.; Karlsson, A.; Michaëlsson, E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: Correlation between symptoms and inflammation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 288, G1328–G1338. [Google Scholar] [CrossRef]
- Eeckhaut, V.; Van Immerseel, F.; Teirlynck, E.; Pasmans, F.; Fievez, V.; Snauwaert, C.; Haesebrouck, F.; Ducatelle, R.; Louis, P.; Vandamme, P. Butyricicoccus pullicaecorum gen. nov., sp. nov., an anaerobic, butyrate-producing bacterium isolated from the caecal content of a broiler chicken. Int. J. Syst. Evol. Microbiol. 2008, 58, 2799–2802. [Google Scholar] [CrossRef]
- Geirnaert, A.; Calatayud, M.; Grootaert, C.; Laukens, D.; Devriese, S.; Smagghe, G.; De Vos, M.; Boon, N.; Van de Wiele, T. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci. Rep. 2017, 7, 11450. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. (Eds.) The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Possemiers, S.; Verthé, K.; Uyttendaele, S.; Verstraete, W. PCR-DGGE-based quantification of stability of the microbial community in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol. Ecol. 2004, 49, 495–507. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19712298 (accessed on 10 July 2015). [CrossRef]
- Molly, K.; Vande Woestyne, M.; Verstraete, W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Appl. Microbiol. Biotechnol. 1993, 39, 254–258. Available online: http://link.springer.com/10.1007/BF00228615 (accessed on 9 June 2015). [CrossRef]
- Hoefman, S.; Pommerening-Röser, A.; Samyn, E.; De Vos, P.; Heylen, K. Efficient cryopreservation protocol enables accessibility of a broad range of ammonia-oxidizing bacteria for the scientific community. Res. Microbiol. 2013, 164, 288–292. [Google Scholar] [CrossRef]
- Vanhove, W.; Nys, K.; Arijs, I.; Cleynen, I.; Noben, M.; De Schepper, S.; Van Assche, G.; Ferrante, M.; Vermeire, S. Biopsy-derived intestinal epithelial cell cultures for pathway based stratification of patients with inflammatory bowel disease. J. Crohn’s Colitis 2018, 12, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Keenan, C.M.; Gale, D.; Shoupe, T.S. Exacerbation of experimental colitis by nonsteroidal anti-inflammatory drugs is not related to elevated leukotriene B4 synthesis. Gastroenterology 1992, 102, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Peña, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef]

| Medium | ConA | ConA + NaB | ConA + MH002 | ||
|---|---|---|---|---|---|
| IL-4 | Mean ± SEM (n) | 5.72 ± 1.86 (6) | 51.56 ± 10.08 (6) | 16.09 ± 1.48 (6) | 6.48 ± 4.29 (6) |
| p-value | 0.019 | - | 0.044 | 0.040 | |
| IL-9 | Mean ± SEM (n) | 1.73 ± 0.63 (6) | 296.50 ± 91.89 (6) | 11.13 ± 3.13 (6) | 10.60 ± 3.67 (6) |
| p-value | 0.056 | - | 0.059 | 0.062 | |
| IL-17A | Mean ± SEM (n) | 1.04 ± 0.67 (6) | 582.50 ± 88.13 (6) | 138.2 ± 26.69 (6) | 156.90 ± 37.66 (6) |
| p-value | 0.003 | - | 0.004 | 0.010 | |
| IL-21 | Mean ± SEM (n) | 2.50 ± 1.54 (6) | 79.36 ± 12.60 (6) | 13.41 ± 2.20 (6) | 6.55 ± 3.92 (6) |
| p-value | 0.005 | - | 0.008 | 0.008 | |
| IL-22 | Mean ± SEM (n) | 1.21 ± 0.83 (6) | 117.80 ± 26.05 (6) | 289.4 ± 52.94 (6) | 378.40 ± 103.60 (6) |
| p-value | 0.016 | - | 0.009 | 0.051 | |
| LDH | Mean ± SEM (n) | 12.31 ± 0.87 (6) | 11.88 ± 1.01 (6) | 13.15 ± 1.19 (6) | 12.36 ± 1.39 (6) |
| p-value | 0.674 | - | 0.069 | 0.533 |
| (A) Acute DSS mice | ||||||||
| Water + VEH | n | DSS + VEH | n | DSS + 5-ASA | n | DSS + MH002 | n | |
| G-CSF | 0.00 ± 0.00 | 5 | 50.03 ± 10.84 ** | 7 | n/a | n/a | 29.35 ± 10.90 | 8 |
| Cxcl1 | 44.51 ± 11.71 | 6 | 160.7 ± 36.66 * | 7 | n/a | n/a | 130.0 ± 31.31 | 8 |
| IL-6 | 0.00 ± 0.00 | 5 | 27.89 ± 4.92 *** | 7 | n/a | n/a | 19.23 ± 2.67 | 7 |
| (B) Acute TNBS rats | ||||||||
| EtOH + VEH | n | TNBS + VEH | n | TNBS + 5-ASA | n | TNBS + MH002 | n | |
| Ifnγ | 14.83 ± 1.67 | 5 | 43.18 ± 7.47 * | 10 | 15.44 ± 1.43 ** | 10 | 31.01 ± 6.87 | 10 |
| Tnfα | 9.89 ± 2.66 | 5 | 93.65 ± 35.39 | 10 | 17.06 ± 9.89 * | 10 | 16.69 ± 6.71 * | 10 |
| IL-2 | 22.45 ± 5.83 | 5 | 160.2 ± 51.45 * | 10 | 16.58 ± 5.36 ** | 9 | 37.24 ± 12.22 * | 10 |
| (C) Recovery DSS mice | ||||||||
| Water + VEH | n | DSS + VEH | n | DSS + 5-ASA | n | DSS + MH002 | n | |
| G-CSF | 0.00 ± 0.00 | 5 | 94.10 ± 11.34 * | 5 | 51.97 ± 20.09 | 5 | 56.86 ± 23.95 | 5 |
| Cxcl1 | 11.05 ± 3.51 | 5 | 149.5 ± 41.03 ** | 5 | 37.28 ± 8.74 * | 5 | 50.50 ± 22.61 * | 5 |
| IL-6 | 0.00 ± 0.00 | 5 | 184.6 ± 59.97 * | 5 | 92.96 ± 55.11 | 5 | 22.57 ± 20.42 * | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, I.; Bolca, S.; Van den Bossche, L.; Vanhove, W.; Van Ryckeghem, S.; Gottardi, D.; Laukens, D.; Possemiers, S. MH002, a Novel Butyrate-Producing Consortium of Six Commensal Bacterial Strains Has Immune-Modulatory and Mucosal-Healing Properties. Int. J. Mol. Sci. 2025, 26, 6167. https://doi.org/10.3390/ijms26136167
Pinheiro I, Bolca S, Van den Bossche L, Vanhove W, Van Ryckeghem S, Gottardi D, Laukens D, Possemiers S. MH002, a Novel Butyrate-Producing Consortium of Six Commensal Bacterial Strains Has Immune-Modulatory and Mucosal-Healing Properties. International Journal of Molecular Sciences. 2025; 26(13):6167. https://doi.org/10.3390/ijms26136167
Chicago/Turabian StylePinheiro, Iris, Selin Bolca, Lien Van den Bossche, Wiebe Vanhove, Sara Van Ryckeghem, Davide Gottardi, Debby Laukens, and Sam Possemiers. 2025. "MH002, a Novel Butyrate-Producing Consortium of Six Commensal Bacterial Strains Has Immune-Modulatory and Mucosal-Healing Properties" International Journal of Molecular Sciences 26, no. 13: 6167. https://doi.org/10.3390/ijms26136167
APA StylePinheiro, I., Bolca, S., Van den Bossche, L., Vanhove, W., Van Ryckeghem, S., Gottardi, D., Laukens, D., & Possemiers, S. (2025). MH002, a Novel Butyrate-Producing Consortium of Six Commensal Bacterial Strains Has Immune-Modulatory and Mucosal-Healing Properties. International Journal of Molecular Sciences, 26(13), 6167. https://doi.org/10.3390/ijms26136167






