Novel Au(I)- and Ag(I)-NHC Complexes with N-Boc-Protected Proline as Potential Candidates for Neurodegenerative Disorders
Abstract
1. Introduction
2. Results and Discussion
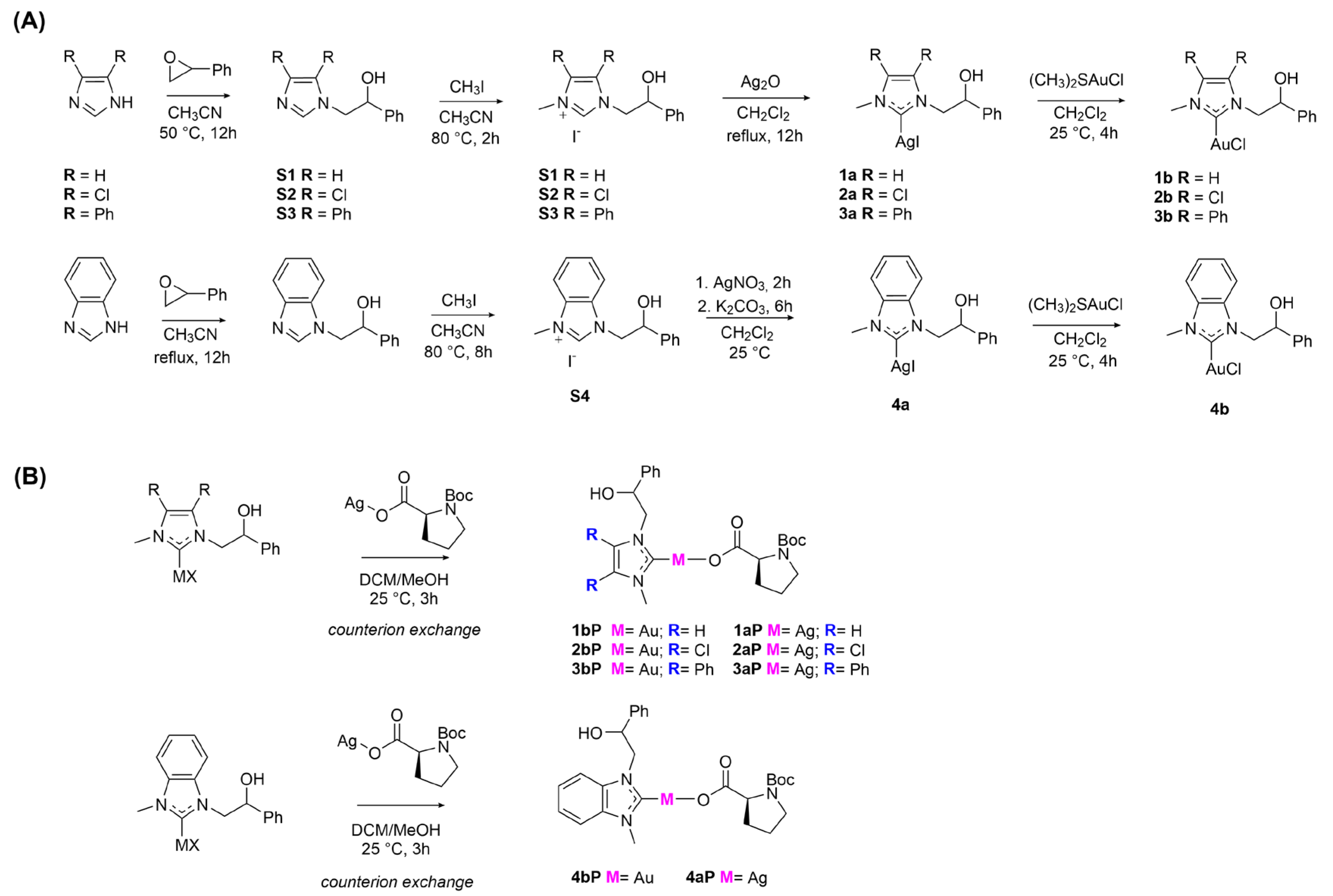
2.1. Chemistry
2.2. Cholinesterase-Inhibitory Activity
2.3. MAO-A/B-Inhibitory Activity
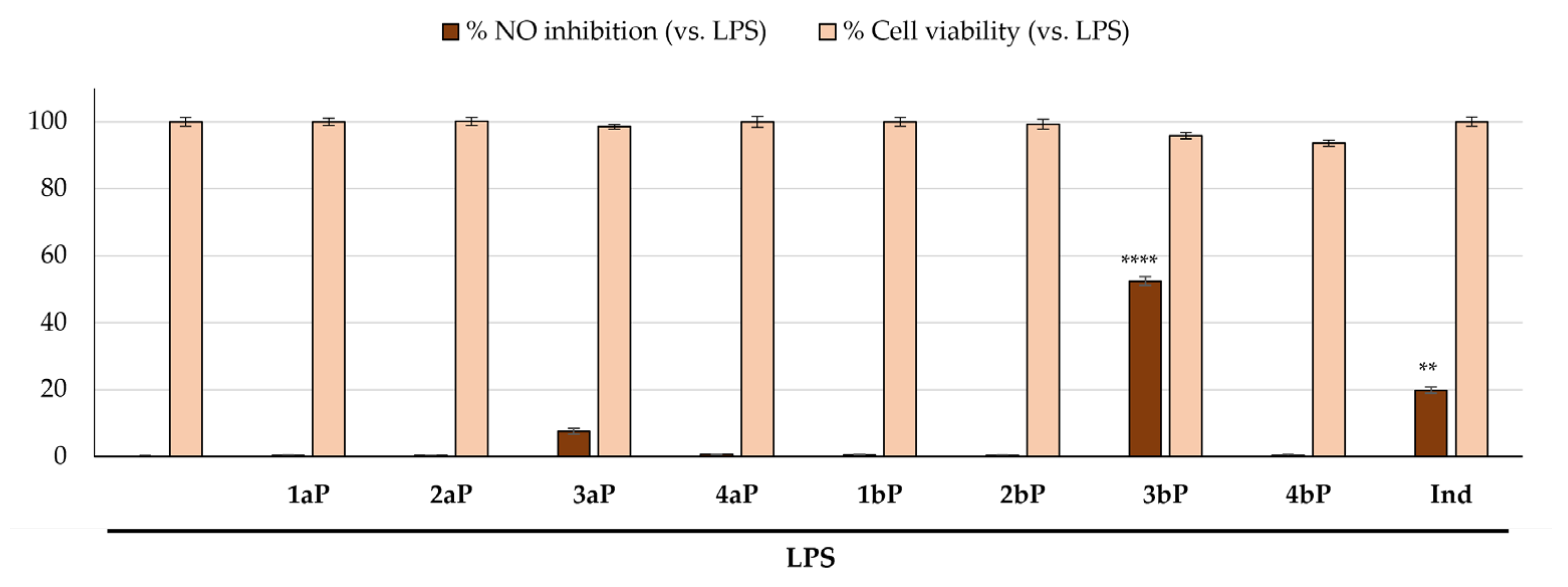
2.4. Anti-Inflammatory Activity
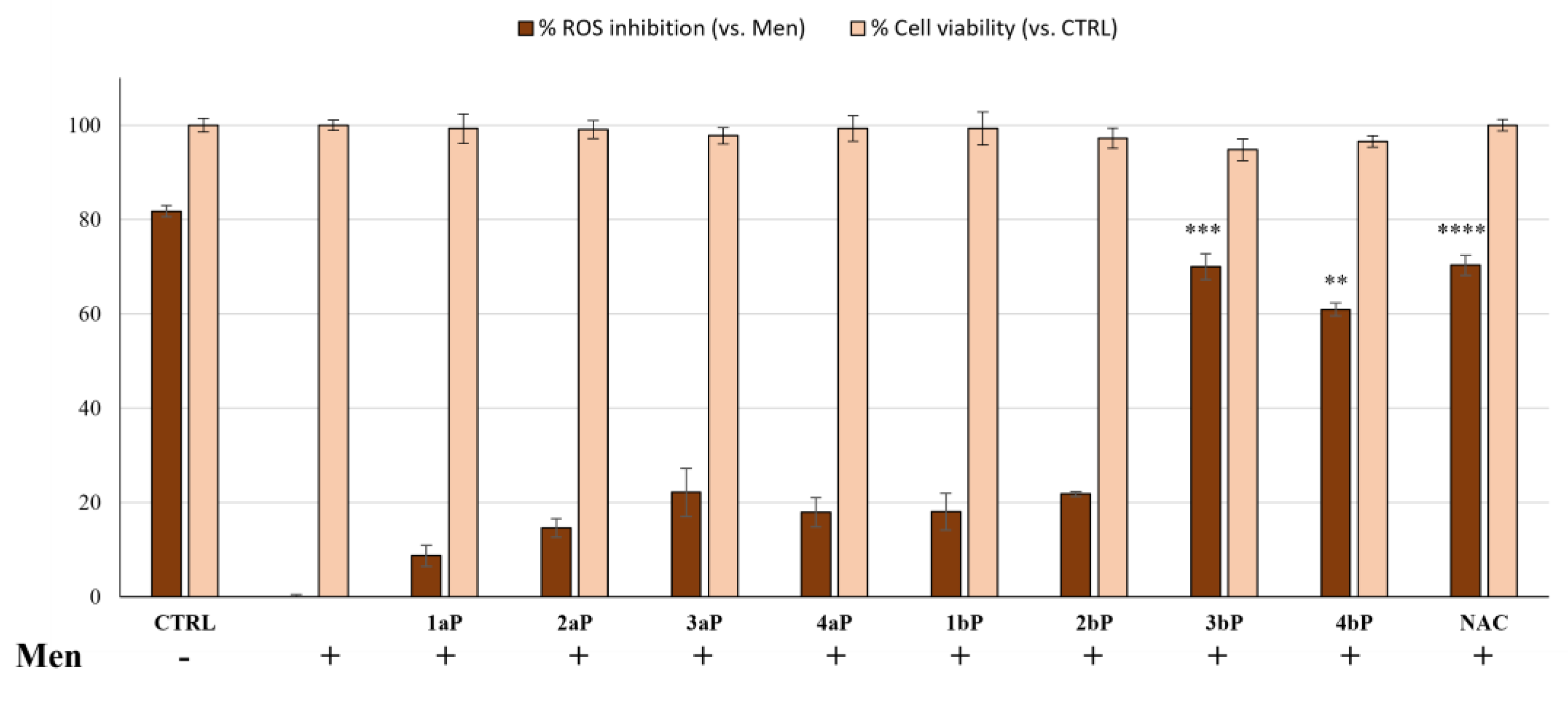
2.5. Antioxidant Activity
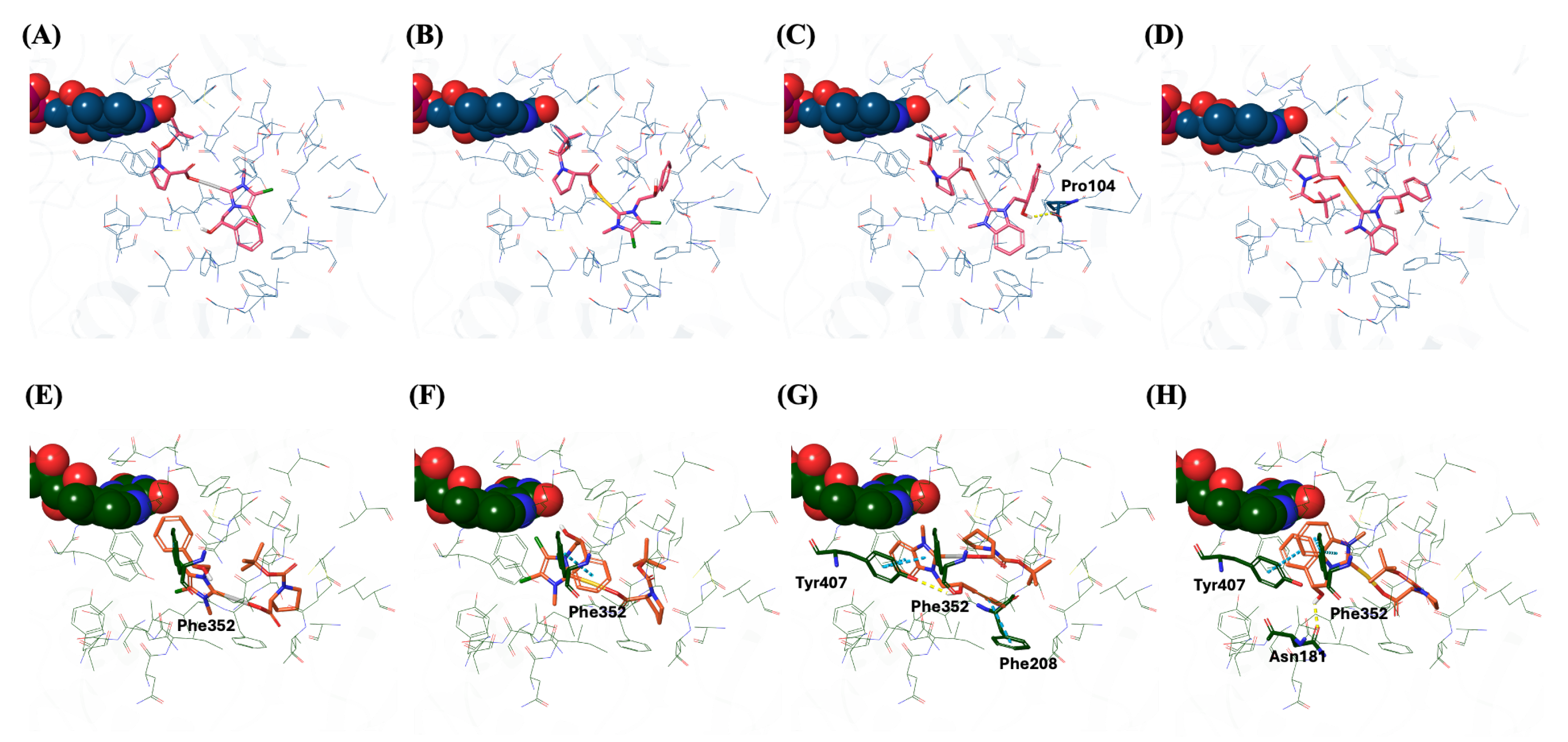
2.6. Docking Studies
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of Boc-L-Proline Ag(I) Salt
Synthesis of Co-Assembled Boc-Proline Silver(I) Complexes 1–4aP [23]
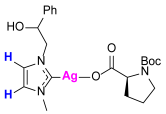
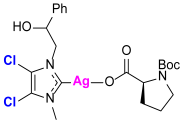
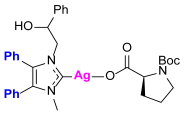
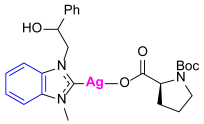
Synthesis of Co-Assembled Boc-Proline Gold(I) Complexes 1–4bP [23]
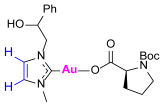
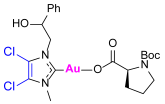

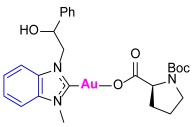
3.2. Evaluation of Cholinesterase-Inhibitory Activity
3.3. Evaluation of Human Monoamine Oxidase (hMAO)-Inhibitory Activity
3.4. Anti-Inflammatory Activity
3.5. Antioxidant Activity
3.6. Docking Studies
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ateleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Niculescu, A.-G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters—Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef]
- Talesa, V.N. Acetylcholinesterase in Alzheimer’s disease. Mech. Ageing Dev. 2001, 122, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huang, G. The biological activities of butyrylcholinesterase inhibitors. Biomed. Pharmacother. 2022, 146, 112556. [Google Scholar] [CrossRef]
- Finberg, J.P.M.; Rabey, J.M. Inhibitors of MAO-A and MAO-B in Psychiatry and Neurology. Front. Pharmacol. 2016, 7, 340. [Google Scholar] [CrossRef]
- Shaban, N.Z.; Masoud, M.S.; Awad, D.; Mawlawi, M.A.; Sadek, O.M. Effect of Cd, Zn and Hg complexes of barbituric acid and thiouracil on rat brain monoamine oxidase-B (in vitro). Chem.-Biol. Interact. 2014, 208, 37–46. [Google Scholar] [CrossRef]
- Nitin, V.; Anand, T.; Singh, C.K.; Sodhi, K.K. Role of organometallic complexes in targeted therapies of different diseases: Infectious diseases, cancer and neurodegenerative Diseases. J. Organomet. Chem. 2024, 1022, 123389. [Google Scholar] [CrossRef]
- Sales, T.A.; Prandi, I.G.; de Castro, A.A.; Leal, D.H.S.; da Cunha, E.F.F.; Kuca, K.; Ramalho, T.C. Recent Developments in Metal-Based Drugs and Chelating Agents for Neurodegenerative Diseases Treatments. Int. J. Mol. Sci. 2019, 20, 1829. [Google Scholar] [CrossRef]
- Navale, G.R.; Ahmed, I.; Lim, M.H.; Ghosh, K. Transition Metal Complexes as Therapeutics: A New Frontier in Combatting Neurodegenerative Disorders through Protein Aggregation Modulation. Adv. Healthc. Mater. 2024, 13, 2401991. [Google Scholar] [CrossRef]
- Iacopetta, D.; Costabile, C.; La Chimia, M.; Mariconda, A.; Ceramella, J.; Scumaci, D.; Catalano, A.; Rosano, C.; Cuda, G.; Sinicropi, M.S.; et al. NHC-Ag(I) and NHC-Au(I) Complexes with N-Boc-Protected α-Amino Acidate Counterions Powerfully Affect the Growth of MDA-MB-231 Cells. ACS Med. Chem. Lett. 2023, 14, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.A.; Southerland, M.R.; Youngs, W.J. Recent Developments in the Medicinal Applications of Silver-NHC Complexes and Imidazolium Salts. Molecules 2017, 22, 1263. [Google Scholar] [CrossRef] [PubMed]
- Mariconda, A.; Iacopetta, D.; Sirignano, M.; Ceramella, J.; Costabile, C.; Pellegrino, M.; Rosano, C.; Catalano, A.; Saturnino, C.; El-Kashef, H.; et al. N-Heterocyclic Carbene (NHC) Silver Complexes as Versatile Chemotherapeutic Agents Targeting Human Topoisomerases and Actin. ChemMedChem 2022, 17, e202200345. [Google Scholar] [CrossRef]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent advances in gold–NHC complexes with biological properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef]
- Bal, S.; Demirci, Ö.; Şen, B.; Taşkın Tok, T.; Taslimi, P.; Aktaş, A.; Gök, Y.; Aygün, M.; Gülçin, İ. Silver N-heterocyclic carbene complexes bearing fluorinated benzyl group: Synthesis, characterization, crystal structure, computational studies, and inhibitory properties against some metabolic enzymes. Appl. Organomet. Chem. 2021, 35, e6312. [Google Scholar] [CrossRef]
- Kazancı, A.; Gök, Y.; Kaya, R.; Aktaş, A.; Taslimi, P.; Gülçin, İ. Synthesis, characterization and bioactivities of dative donor ligand N-heterocyclic carbene (NHC) precursors and their Ag(I)NHC coordination compounds. Polyhedron 2021, 193, 114866. [Google Scholar] [CrossRef]
- Lasmari, S.; Ikhlef, S.; Boulcina, R.; Mokrani, E.H.; Bensouici, C.; Gürbüz, N.; Dündar, M.; Karcı, H.; Özdemir, İ.; Koç, A.; et al. New silver Nheterocyclic carbenes complexes: Synthesis, molecular docking study and biological activities evaluation as cholinesterase inhibitors and antimicrobials. J. Mol. Struct. 2021, 1238, 130399. [Google Scholar] [CrossRef]
- Gladkevich, A.; Bosker, F.; Korf, J.; Yenkoyan, K.; Vahradyan, H.; Aghajanov, M. Proline-rich polypeptides in Alzheimer’s disease and neurodegenerative disorders—Therapeutic potential or a mirage? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1347–1355. [Google Scholar] [CrossRef]
- Yenkoyan, K.; Safaryan, K.; Chavushyan, V.; Meliksetyan, I.; Navasardyan, G.; Sarkissian, J.; Galoyan, A.; Aghajanov, M. Neuroprotective action of proline-rich polypeptide-1 in β-amyloid induced neurodegeneration in rats. Brain Res. Bull. 2011, 86, 262–271. [Google Scholar] [CrossRef]
- Sirignano, M.; D’Amato, A.; Costabile, C.; Mariconda, A.; Crispini, A.; Scarpelli, F.; Longo, P. Hydroamination of alkynes catalyzed by NHC-Gold(I) complexes: The non-monotonic effect of substituted arylamines on the catalyst activity. Front. Chem. 2023, 11, 1260726. [Google Scholar] [CrossRef]
- Ivry, E.; Ben-Asuly, A.; Goldberg, I.; Lemcoff, N.G. Amino acids as chiral anionic ligands for ruthenium based asymmetric olefin metathesis. Chem. Commun. 2015, 51, 3870–3873. [Google Scholar] [CrossRef] [PubMed]
- Legault, C.Y.; Kendall, C.; Charette, A.B. Structure and reactivity of a new anionic N-heterocyclic carbene silver (i) complex. Chem. Commun. 2005, 30, 3826–3828. [Google Scholar] [CrossRef] [PubMed]
- Ciardulli, M.C.; Mariconda, A.; Sirignano, M.; Lamparelli, E.P.; Longo, R.; Scala, P.; D’Auria, R.; Santoro, A.; Guadagno, L.; Della Porta, G.; et al. Activity and Selectivity of Novel Chemical Metallic Complexes with Potential Anticancer Effects on Melanoma Cells. Molecules 2023, 28, 4851. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Sandeli, A.E.-K.; Khiri-Meribout, N.; Benzerka, S.; Gürbüz, N.; Dündar, M.; Karcı, H.; Bensouici, C.; Mokrani, E.H.; Özdemir, İ.; Koç, A.; et al. Silver (I)-N-heterocyclic carbene complexes: Synthesis and characterization, biological evaluation of Anti-Cholinesterase, anti-alpha-amylase, anti-lipase, and antibacterial activities, and molecular docking study. Inorg. Chim. Acta 2021, 525, 120486. [Google Scholar] [CrossRef]
- Behçet, A.; Aktaş, A.; Gök, Y.; Kaya, R.; Taslimi, P.; Gülçin, İ. Novel silver(I)N-heterocyclic carbene complexes bearing 2-(4-hydroxyphenyl)ethyl group: Synthesis, characterization, and enzyme inhibition properties. J. Heterocycl. Chem. 2020, 58, 603–611. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, D.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Andronie-Cioara, F.L.; Toma, M.M.; Bungau, S.; Bumbu, A.G. Role of Monoamine Oxidase Activity in Alzheimer’s Disease: An Insight into the Therapeutic Potential of Inhibitors. Molecules 2021, 26, 3724. [Google Scholar] [CrossRef]
- Mateev, E.; Georgieva, M.; Mateeva, A.; Zlatkov, A.; Ahmad, S.; Raza, K.; Azevedo, V.; Barh, D. Structure-Based Design of Novel MAO-B Inhibitors: A Review. Molecules 2023, 28, 4814. [Google Scholar] [CrossRef]
- Shaban, N.Z.; Ali, A.E.; Masoud, M.S. Effect of cadmium and zinc ethanolamine complexes on rat brain monoamine oxidase-B activity in vitro. J. Inorg. Biochem. 2003, 95, 141–148. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Nalamachu, S.; Wortmann, R. Role of Indomethacin in Acute Pain and Inflammation Management: A Review of the Literature. Postgrad. Med. 2015, 126, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Mariconda, A.; Sirignano, M.; Costabile, C.; Longo, P. New NHC- silver and gold complexes active in A3-coupling (aldehyde-alkyne-amine) reaction. Mol. Catal. 2020, 480, 110570. [Google Scholar] [CrossRef]
- Saturnino, C.; Barone, I.; Iacopetta, D.; Mariconda, A.; Sinicropi, M.S.; Rosano, C.; Campana, A.; Catalano, S.; Longo, P.; Andò, S.N. Heterocyclic Carbene Complexes of Silver and Gold as Novel Tools Against Breast Cancer Progression. Future Med. Chem. 2016, 8, 2213–2229. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Cagide, F.; Teixeira, J.; Amorim, R.; Sequeira, L.; Mesiti, F.; Silva, T.; Garrido, J.; Remião, F.; Vilar, S.; et al. Hydroxybenzoic Acid Derivatives as Dual-Target Ligands: Mitochondriotropic Antioxidants and Cholinesterase Inhibitors. Front. Chem. 2018, 6, 126. [Google Scholar] [CrossRef]
- Chavarria, D.; Cagide, F.; Pinto, M.; Gomes, L.R.; Low, J.N.; Borges, F. Development of piperic acid-based monoamine oxidase inhibitors: Synthesis, structural characterization and biological evaluation. J. Mol. Struct. 2019, 1182, 298–307. [Google Scholar] [CrossRef]
- Mariconda, A.; Iacopetta, D.; Sirignano, M.; Ceramella, J.; D’Amato, A.; Marra, M.; Pellegrino, M.; Sinicropi, M.S.; Aquaro, S.; Longo, P. Silver and Gold Complexes with NHC-Ligands Derived from Caffeine: Catalytic and Pharmacological Activity. Int. J. Mol. Sci. 2024, 25, 2599. [Google Scholar] [CrossRef]
- Schrödinger Release 2024-1: Maestro, Schrödinger LLC. 2024. Available online: www.schrodinger.com (accessed on 2 December 2024).
- Bochevarov, A.D.; Harder, E.; Hughes, T.F.; Greenwood, J.R.; Braden, D.A.; Philipp, D.M.; Rinaldo, D.; Halls, M.D.; Zhang, J.; Friesner, R.A. Jaguar: A high-performance quantum chemistry software program with strengths in life and materials sciences. Int. J. Quantum Chem. 2013, 113, 2110–2142. [Google Scholar] [CrossRef]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Kobe, B.; Levy-Assaraf, M.; Voronov-Goldman, M.; Rozman Grinberg, I.; Weiserman, G.; Shimon, L.J.W.; Jindou, S.; Borovok, I.; White, B.A.; Bayer, E.A.; et al. Crystal Structure of an Uncommon Cellulosome-Related Protein Module from Ruminococcus flavefaciens That Resembles Papain-Like Cysteine Peptidases. PLoS ONE 2013, 8, e56138. [Google Scholar] [CrossRef]
- Košak, U.; Brus, B.; Knez, D.; Žakelj, S.; Trontelj, J.; Pišlar, A.; Šink, R.; Jukič, M.; Živin, M.; Podkowa, A.; et al. The Magic of Crystal Structure-Based Inhibitor Optimization: Development of a Butyrylcholinesterase Inhibitor with Picomolar Affinity and In Vivo Activity. J. Med. Chem. 2017, 61, 119–139. [Google Scholar] [CrossRef]
- Son, S.-Y.; Ma, J.; Kondou, Y.; Yoshimura, M.; Yamashita, E.; Tsukihara, T. Structure of human monoamine oxidase A at 2.2-Å resolution: The control of opening the entry for substrates/inhibitors. Proc. Natl. Acad. Sci. USA 2008, 105, 5739–5744. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Manzella, N.; Cagide, F.; Mialet-Perez, J.; Uriarte, E.; Parini, A.; Borges, F.; Binda, C. Tight-Binding Inhibition of Human Monoamine Oxidase B by Chromone Analogs: A Kinetic, Crystallographic, and Biological Analysis. J. Med. Chem. 2018, 61, 4203–4212. [Google Scholar] [CrossRef] [PubMed]
- Garcin, E.D.; Arvai, A.S.; Rosenfeld, R.J.; Kroeger, M.D.; Crane, B.R.; Andersson, G.; Andrews, G.; Hamley, P.J.; Mallinder, P.R.; Nicholls, D.J.; et al. Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat. Chem. Biol. 2008, 4, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Rodríguez Arce, E.; Mosquillo, M.F.; Pérez-Díaz, L.; Echeverría, G.A.; Piro, O.E.; Merlino, A.; Coitiño, E.L.; Maríngolo Ribeiro, C.; Leite, C.Q.F.; Pavan, F.R.; et al. Aromatic amine N-oxide organometallic compounds: Searching for prospective agents against infectious diseases. Dalton Trans. 2015, 44, 14453–14464. [Google Scholar] [CrossRef]
- Forum, A. ADL, Parameters for Docking with Metal Ions in Receptor. Available online: https://www.researchgate.net/profile/Dr-Rajesh-Patil/post/Autodock-docking-with-gold-nanoparticles/attachment/59d6477f79197b80779a2567/AS%3A462677517574144%401487322324251/download/ADL_+Parameters+for+docking+with+metal+ions+in+receptor.pdf (accessed on 2 December 2024).


| IC50 (µM) | SI a | ||
|---|---|---|---|
| Compounds | eeAChE | eqBChE | |
| Donepezil | (2.30 ± 0.53) × 10−2 | 2.13 ± 0.12 | 0.01 |
| 1aP | >25 | >25 | - |
| 2aP | >25 | 18.24 ± 2.4 | >1.37 |
| 3aP | >25 | 17.38 ± 0.98 | >1.44 |
| 4aP | 13.95 ± 0.72 | 5.19 ± 0.95 | 2.68 |
| 1bP | 18.37 ± 1.43 | 6.19 ± 0.91 | 2.97 |
| 2bP | 4.68 ± 0.47 | 3.14 ± 0.33 | 1.49 |
| 3bP | 5.89 ± 0.56 | >25 | >0.24 |
| 4bP | 3.72 ± 0.44 | 0.45 ± 0.06 | 8.27 |
| IC50 (µM) | SI a | ||
|---|---|---|---|
| Compounds | hMAO-A | hMAO-B | |
| Clorgyline | (2.00 ± 0.17) × 10−3 | 2.44 ± 0.17 | 8.19 × 10−4 |
| (R)-(–)-Deprenyl | 17.49 ± 2.67 | (4.92 ± 0.87) × 10−2 | 356.94 |
| 1aP | >25 | >25 | - |
| 2aP | 15.04 ± 1.02 | 9.82 ± 0.84 | 1.53 |
| 3aP | 24.97 ± 2.95 | >25 | 0.99 |
| 4aP | 3.32 ± 0.15 | 10.40 ± 1.95 | 0.32 |
| 1bP | 1.32 ± 0.02 | 3.78 ± 0.16 | 0.35 |
| 2bP | 0.72 ± 0.02 | 1.94 ± 0.09 | 0.37 |
| 3bP | 1.84 ± 0.18 | 4.75 ± 0.75 | 0.39 |
| 4bP | (7.13 ± 0.97) × 10−2 | 2.10 ± 0.20 | 0.03 |
| IC50 (µM) | ||
|---|---|---|
| Compounds | NO Inhibition | Cell Viability |
| Indomethacin | >50 | >50 |
| 1aP | >50 | >50 |
| 2aP | >50 | >50 |
| 3aP | 30.88 ± 1.74 | >50 |
| 4aP | >50 | >50 |
| 1bP | >50 | >50 |
| 2bP | 32.31 ± 1.82 | >50 |
| 3bP | 4.88 ± 0.58 | 20.27 ± 1.26 |
| 4bP | 13.05 ± 0.92 | 12.12 ± 1.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceramella, J.; D’Amato, A.; Procopio, F.; Mariconda, A.; Chavarria, D.; Iacopetta, D.; Ortuso, F.; Longo, P.; Borges, F.; Sinicropi, M.S. Novel Au(I)- and Ag(I)-NHC Complexes with N-Boc-Protected Proline as Potential Candidates for Neurodegenerative Disorders. Int. J. Mol. Sci. 2025, 26, 6116. https://doi.org/10.3390/ijms26136116
Ceramella J, D’Amato A, Procopio F, Mariconda A, Chavarria D, Iacopetta D, Ortuso F, Longo P, Borges F, Sinicropi MS. Novel Au(I)- and Ag(I)-NHC Complexes with N-Boc-Protected Proline as Potential Candidates for Neurodegenerative Disorders. International Journal of Molecular Sciences. 2025; 26(13):6116. https://doi.org/10.3390/ijms26136116
Chicago/Turabian StyleCeramella, Jessica, Assunta D’Amato, Francesca Procopio, Annaluisa Mariconda, Daniel Chavarria, Domenico Iacopetta, Francesco Ortuso, Pasquale Longo, Fernanda Borges, and Maria Stefania Sinicropi. 2025. "Novel Au(I)- and Ag(I)-NHC Complexes with N-Boc-Protected Proline as Potential Candidates for Neurodegenerative Disorders" International Journal of Molecular Sciences 26, no. 13: 6116. https://doi.org/10.3390/ijms26136116
APA StyleCeramella, J., D’Amato, A., Procopio, F., Mariconda, A., Chavarria, D., Iacopetta, D., Ortuso, F., Longo, P., Borges, F., & Sinicropi, M. S. (2025). Novel Au(I)- and Ag(I)-NHC Complexes with N-Boc-Protected Proline as Potential Candidates for Neurodegenerative Disorders. International Journal of Molecular Sciences, 26(13), 6116. https://doi.org/10.3390/ijms26136116













