Evaluating an Early Risk Model for Uncomplicated Hypertension in Pregnancy Based on Nighttime Blood Pressure, Uric Acid, and Angiogenesis-Related Factors
Abstract
1. Introduction
2. Results
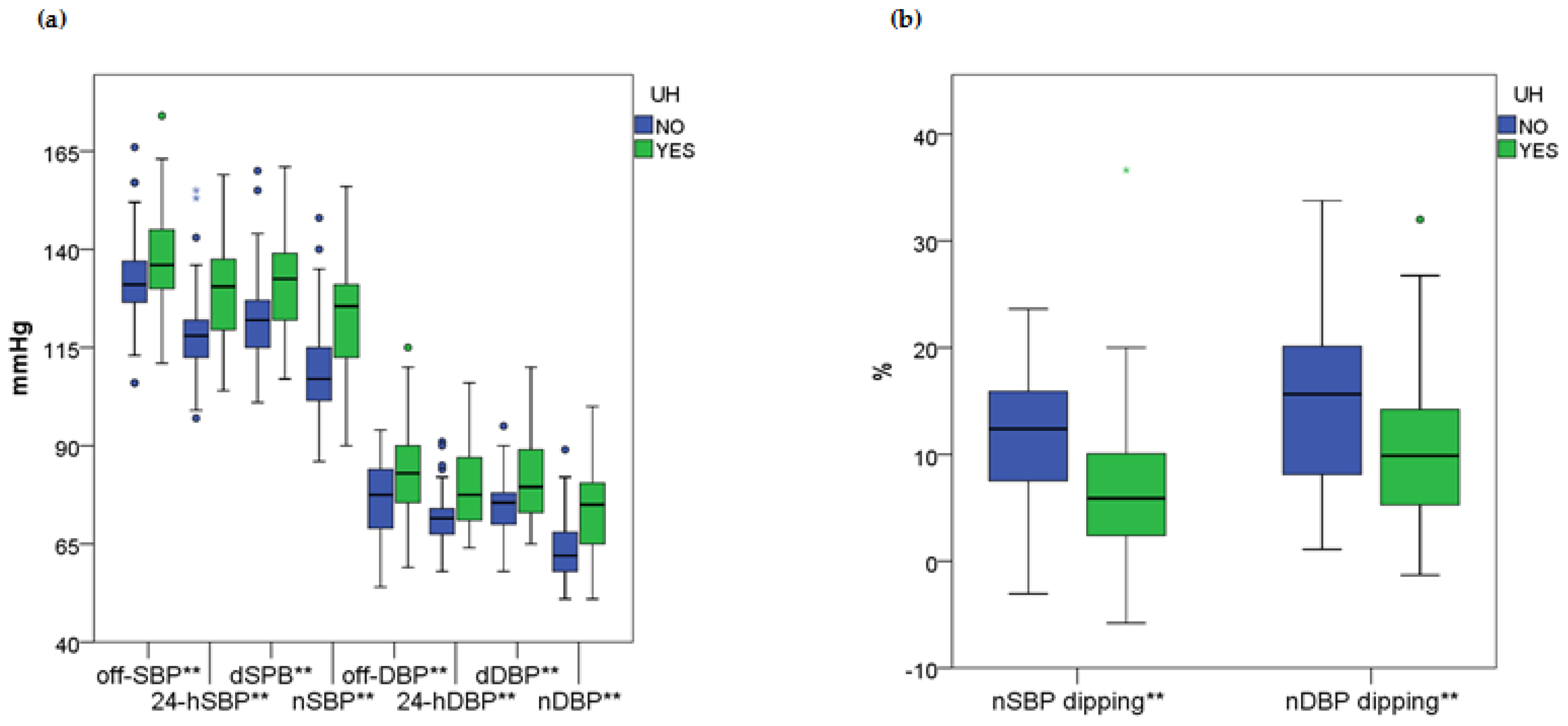
2.1. General and Comparative Findings: Relationship Between Blood Pressure Indices and Biochemical Markers with Uncomplicated Hypertension During Pregnancy
2.2. Combined Polynomial Transformation of Uric Acid and Angiogenesis-Related Factors Ratio (UA-AF Index) as a Marker for UH
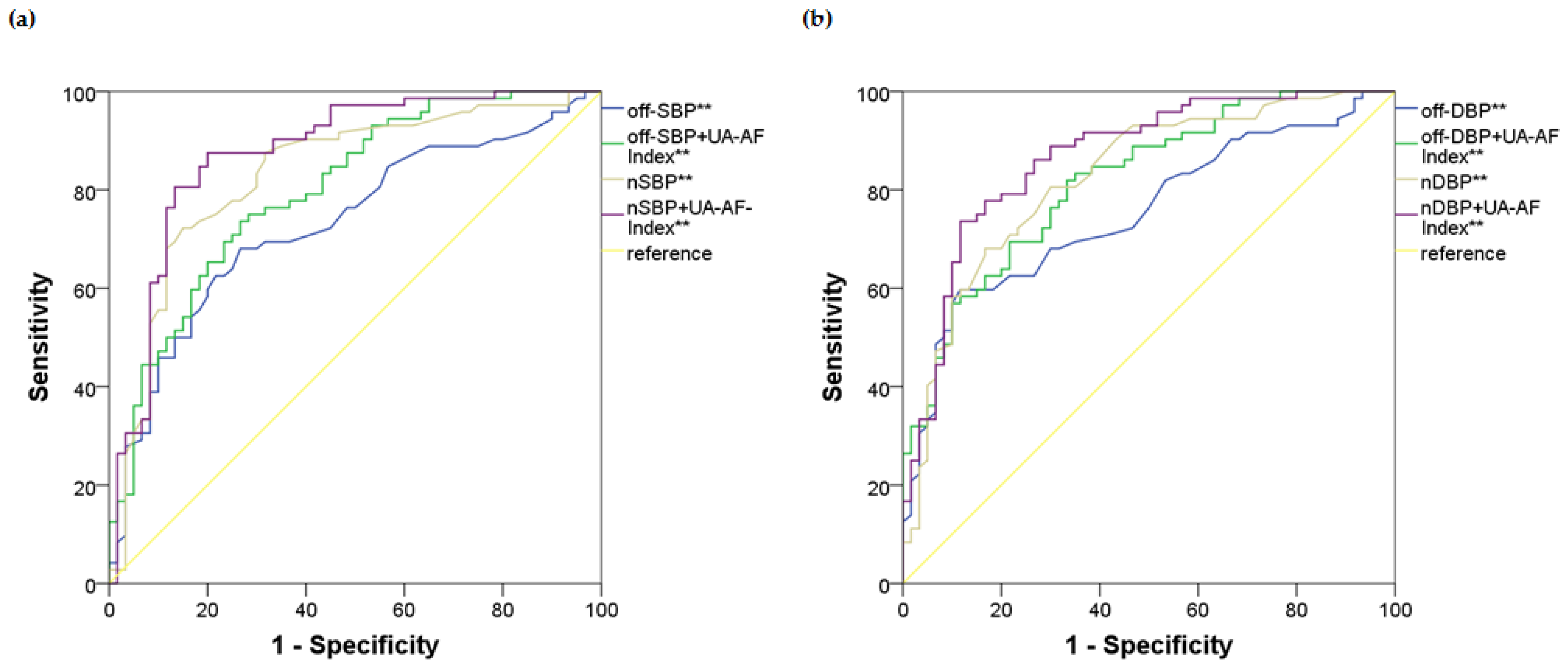
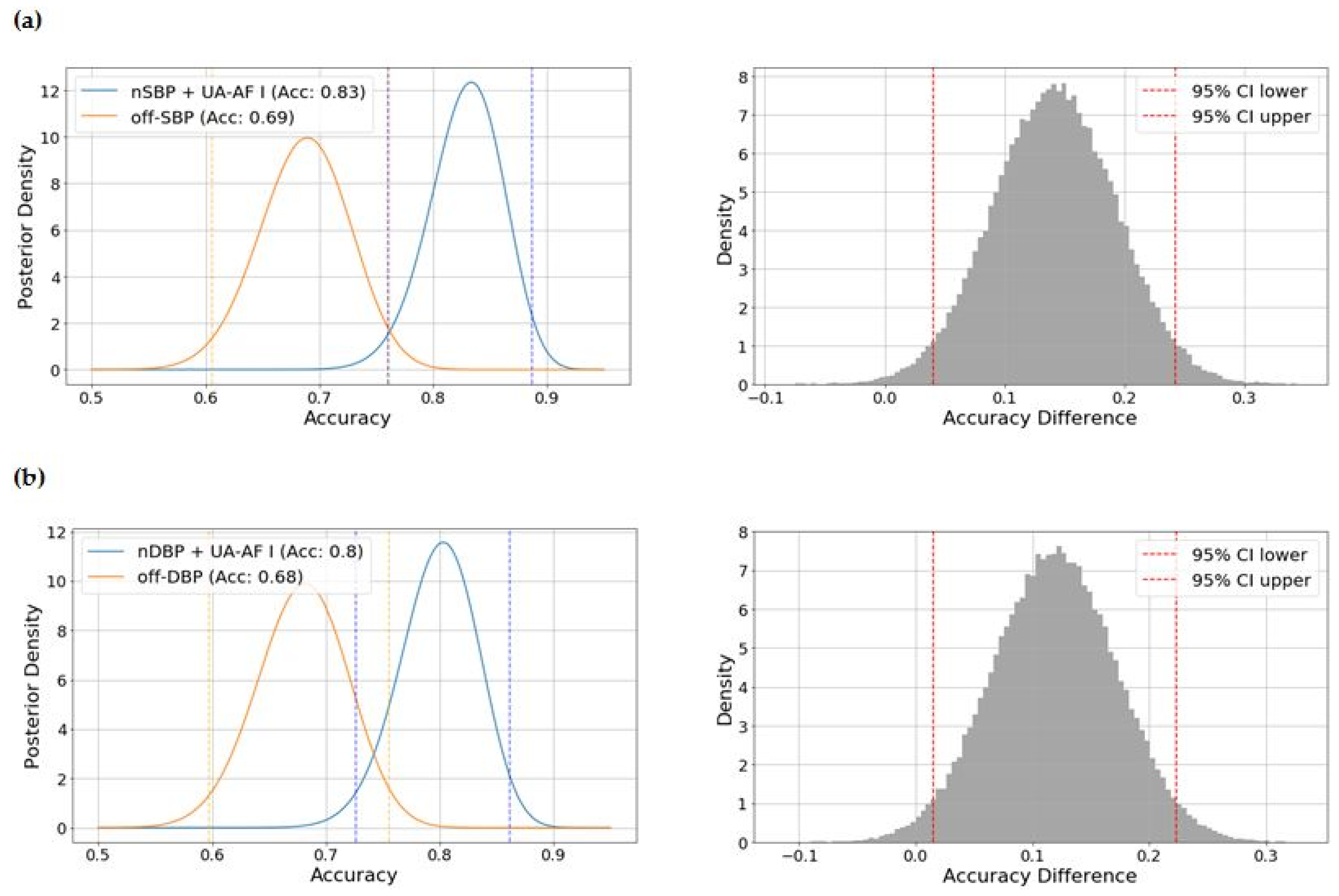
2.3. Identifying the Optimal Model Based on Blood Pressure Indices and the UA-AF Index for the Risk of UH
3. Discussion
Limitations and Strengths
4. Methods and Materials
4.1. Study Design, Setting, and Participants
4.2. Clinical and Laboratory Variables
4.3. Parameters of 24-h ABPM Collection
4.4. Uncomplicated Hypertension During Pregnancy
4.5. Ethical Statement
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, P.; Green, M.; Myers, J.E. Hypertensive disorders of pregnancy. BMJ 2023, 381, e071653. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Wenger, N.K. Hypertension During Pregnancy. Curr. Hypertens. Rep. 2020, 22, 64. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, M.; Rutherford, J.D. Cardiovascular physiology of pregnancy. Circulation 2014, 130, 1003–1008. [Google Scholar] [CrossRef]
- Qu, H.; Khalil, R.A. Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H661–H681. [Google Scholar] [CrossRef]
- Penny, J.A.; Halligan, A.W.; Shennan, A.H.; Lambert, P.C.; Jones, D.R.; de Swiet, M.; Taylor, D.J. Automated, ambulatory, or conventional blood pressure measurement in pregnancy: Which is the better predictor of severe hypertension? Am. J. Obstet. Gynecol. 1998, 178, 521–526. [Google Scholar] [CrossRef]
- Webster, J. Ambulatory blood pressure monitoring in pregnancy: A better guide to risk assessment? J. Hypertens. 2019, 37, 13–15. [Google Scholar] [CrossRef]
- Schmella, M.J.; Clifton, R.G.; Althouse, A.D.; Roberts, J.M. Uric Acid Determination in Gestational Hypertension: Is it as Effective a Delineator of Risk as Proteinuria in High-Risk Women? Reprod. Sci. 2015, 22, 1212–1219. [Google Scholar] [CrossRef]
- Gherghina, M.-E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric Acid and Oxidative Stress-Relationship with Cardiovascular, Metabolic, and Renal Impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
- Rana, S.; Powe, C.E.; Salahuddin, S.; Verlohren, S.; Perschel, F.H.; Levine, R.J.; Lim, K.-H.; Wenger, J.B.; Thadhani, R.; Karumanchi, S.A. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012, 125, 911–919. [Google Scholar] [CrossRef]
- O’Gorman, N.; Wright, D.; Poon, L.C.; Rolnik, D.L.; Syngelaki, A.; de Alvarado, M.; Carbone, I.F.; Dutemeyer, V.; Fiolna, M.; Frick, A.; et al. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation: Comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet. Gynecol. 2017, 49, 756–760. [Google Scholar] [CrossRef]
- Espeche, W.G.; Salazar, M.R.; Minetto, J.; Leiva Sisnieguez, C.E.; Cerri, G.; Balbín, E.; Stavile, R.N.; Ramos, P.C.; Soria, A.; Santillan, C.; et al. Hypertension arising after 20 weeks of gestation: Gestational hypertension or masked chronic hypertension? J. Hum. Hypertens. 2023, 37, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Woolcock, H.; Parra, N.; Zhang, Y.; Reddy, U.M.; Bello, N.A.; Miller, E.; Booker, W.A. Pregnancy Outcomes in Women Who Developed Elevated Blood Pressure and Stage I Hypertension after 20 Weeks, Gestation. Am. J. Perinatol. 2024, 41, 2135–2143. [Google Scholar] [CrossRef]
- Duffy, J.Y.; Getahun, D.; Chen, Q.; Fong, A. Pregnancy Outcomes Associated With a Single Elevated Blood Pressure Before 20 Weeks of Gestation. Obstet. Gynecol. 2021, 138, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Ayala, D.E. Prognostic value of office and ambulatory blood pressure measurements in pregnancy. Hypertension 2002, 40, 298–303. [Google Scholar] [CrossRef]
- Ayala, D.E.; Hermida, R.C. Ambulatory blood pressure monitoring for the early identification of hypertension in pregnancy. Chronobiol. Int. 2013, 30, 233–259. [Google Scholar] [CrossRef]
- Bergel, E.; Carroli, G.; Althabe, F. Ambulatory versus conventional methods for monitoring blood pressure during pregnancy. Cochrane Database Syst. Rev. 2002, 2002, CD001231. [Google Scholar] [CrossRef]
- Salazar, M.R.; Espeche, W.G.; Leiva Sisnieguez, C.E.; Minetto, J.; Balbín, E.; Soria, A.; Yoma, O.; Prudente, M.; Torres, S.; Grassi, F.; et al. Nocturnal hypertension and risk of developing early-onset preeclampsia in high-risk pregnancies. Hypertens. Res. 2021, 44, 1633–1640. [Google Scholar] [CrossRef]
- Salazar, M.R.; Espeche, W.G.; Leiva Sisnieguez, C.E.; Leiva Sisnieguez, B.C.; Balbín, E.; Stavile, R.N.; March, C.; Olano, R.D.; Soria, A.; Yoma, O.; et al. Nocturnal hypertension in high-risk mid-pregnancies predict the development of preeclampsia/eclampsia. J. Hypertens. 2019, 37, 182–186. [Google Scholar] [CrossRef]
- Espeche, W.G.; Salazar, M.R.; Minetto, J.; Cerri, G.; Carrera Ramos, P.; Soria, A.; Santillan, C.; Grassi, F.; Torres, S.; Carbajal, H.A. Relationship between serum uric acid, nocturnal hypertension and risk for preeclampsia in high-risk pregnancies. J. Hum. Hypertens. 2024, 38, 642–648. [Google Scholar] [CrossRef]
- Melchiorre, K.; Sharma, R.; Khalil, A.; Thilaganathan, B. Maternal Cardiovascular Function in Normal Pregnancy: Evidence of Maladaptation to Chronic Volume Overload. Hypertension 2016, 67, 754–762. [Google Scholar] [CrossRef]
- Lv, L.-J.; Wu, L.-L.; Wen, J.-Y.; Lei, Q.; Miao, J.; Duan, H.-L.; Ji, W.-J.; Duan, D.-M.; Luo, Y.-F.; Xiao, L.; et al. Excessive umbilical cord coiling confers risk of elevated nocturnal blood pressure and severe/early-onset preeclampsia. J. Hypertens. 2019, 37, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.R.; Khalil, R.A. Regulation of vascular angiotensin II type 1 and type 2 receptor and angiotensin-(1-7)/MasR signaling in normal and hypertensive pregnancy. Biochem. Pharmacol. 2024, 220, 115963. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Niehoff, M.; Hering, L.; Geusens, N.; Herse, F.; Tintu, A.N.; Plagemann, A.; LeNoble, F.; Pijnenborg, R.; Muller, D.N.; et al. Uterine vascular function in a transgenic preeclampsia rat model. Hypertension 2008, 51, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Cotechini, T.; Graham, C.H. Aberrant maternal inflammation as a cause of pregnancy complications: A potential therapeutic target? Placenta 2015, 36, 960–966. [Google Scholar] [CrossRef]
- Costello, H.M.; Gumz, M.L. Circadian Rhythm, Clock Genes, and Hypertension: Recent Advances in Hypertension. Hypertension 2021, 78, 1185–1196. [Google Scholar] [CrossRef]
- Chang, L.; Xiong, W.; Zhao, X.; Fan, Y.; Guo, Y.; Garcia-Barrio, M.; Zhang, J.; Jiang, Z.; Lin, J.D.; Chen, Y.E. Bmal1 in perivascular adipose tissue regulates resting phase blood pressure through transcriptional regulation of angiotensinogen. Circulation 2018, 138, 67–79. [Google Scholar] [CrossRef]
- Rodrigo, G.C.; Herbert, K.E. Regulation of vascular function and blood pressure by circadian variation in redox signalling. Free Radic. Biol. Med. 2018, 119, 115–120. [Google Scholar] [CrossRef]
- Drăgoi, C.M.; Nicolae, A.C.; Ungurianu, A.; Margină, D.M.; Grădinaru, D.; Dumitrescu, I.-B. Circadian Rhythms, Chrononutrition, Physical Training, and Redox Homeostasis-Molecular Mechanisms in Human Health. Cells 2024, 13, 138. [Google Scholar] [CrossRef]
- Belovic, D.K.; Plešinac, S.; Dotlić, J.; Radojević, A.S.; Akšam, S.; Cvjetićanin, M.M.; Kocijančić, A. Biochemical Markers for Prediction of Hypertensive Disorders of Pregnancy. J. Med. Biochem. 2019, 38, 71–82. [Google Scholar] [CrossRef]
- Verlohren, S.; Perschel, F.H.; Thilaganathan, B.; Dröge, L.A.; Henrich, W.; Busjahn, A.; Khalil, A. Angiogenic Markers and Cardiovascular Indices in the Prediction of Hypertensive Disorders of Pregnancy. Hypertension 2017, 69, 1192–1197. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Z.; Wang, X.; Xiao, Q.; Lu, X.; Lu, S.; Shi, Y.; Lv, S. A risk model of prenatal screening markers in first trimester for predicting hypertensive disorders of pregnancy. EPMA J. 2020, 11, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Friedman, S.A.; Ecker, J.L.; Kao, L.; Kilpatrick, S.J. The clinical utility of serum uric acid measurements in hypertensive diseases of pregnancy. Am. J. Obstet. Gynecol. 1998, 178, 1067–1071. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K. Maternal Serum Uric Acid as a Predictor of Severity of Hypertensive Disorders of Pregnancy: A Prospective Cohort Study. Curr. Hypertens. Rev. 2019, 15, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010, 459, 923–939. [Google Scholar] [CrossRef]
- Antelo-Pais, P.; Prieto-Díaz, M.Á.; Micó-Pérez, R.M.; Pallarés-Carratalá, V.; Velilla-Zancada, S.; Polo-García, J.; Barquilla-García, A.; Ginel-Mendoza, L.; Segura-Fragoso, A.; Vitelli-Storelli, F.; et al. Urate Levels as a Predictor of the Prevalence and Level of Cardiovascular Risk Factors: An Identificación de La PoBlación Española de Riesgo Cardiovascular y Renal Study. Biomolecules 2024, 14, 1530. [Google Scholar] [CrossRef]
- Vazquez-Agra, N.; Marques-Afonso, A.-T.; Cruces-Sande, A.; Novo-Veleiro, I.; Lopez-Paz, J.-E.; Pose-Reino, A.; Hermida-Ameijeiras, A. Are Differences in Inflammatory Markers Between Patients with and Without Hypertension-Mediated Organ Damage Influenced by Circadian Blood Pressure Abnormalities? J. Clin. Med. 2022, 11, 1252. [Google Scholar] [CrossRef]
- Cross, J.C.; Hemberger, M.; Lu, Y.; Nozaki, T.; Whiteley, K.; Masutani, M.; Kozuma, S.; Taketani, Y. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol. Cell Endocrinol. 2002, 187, 207–212. [Google Scholar] [CrossRef]
- Nagamatsu, T.; Fujii, T.; Kusumi, M.; Zou, L.; Yamashita, T.; Osuga, Y.; Momoeda, M.; Kozuma, S.; Taketani, Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: An implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 2004, 145, 4838–4845. [Google Scholar] [CrossRef]
- Matin, M.; Mörgelin, M.; Stetefeld, J.; Schermer, B.; Brinkkoetter, P.T.; Benzing, T.; Koch, M.; Hagmann, H. Affinity-Enhanced Multimeric VEGF (Vascular Endothelial Growth Factor) and PlGF (Placental Growth Factor) Variants for Specific Adsorption of sFlt-1 to Restore Angiogenic Balance in Preeclampsia. Hypertension 2020, 76, 1176–1184. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874. [Google Scholar] [CrossRef] [PubMed]
- Alcohol Use and Your Health|alcohol|Alcohol|CDC 2025. Available online: https://www.cdc.gov/alcohol/about-alcohol-use/index.html (accessed on 24 June 2025).
- Flegal, K.M. Body-mass index and all-cause mortality. Lancet 2017, 389, 2284–2285. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, G.S.; O’Brien, E.; Myers, M.; Palatini, P.; Parati, G.; Kollias, A.; Birmpas, D.; Kyriakoulis, K.; Bountzona, I.; Stambolliu, E.; et al. STRIDE BP international initiative for accurate blood pressure measurement: Systematic review of published validation studies of blood pressure measuring devices. J. Clin. Hypertens. 2019, 21, 1616–1622. [Google Scholar] [CrossRef]
- Vazquez-Agra, N.; Cruces-Sande, A.; Barbosa-Gouveia, S.; Lopez-Paz, J.-E.; Camafort, M.; Casariego-Vales, E.; Pose-Reino, A.; Hermida-Ameijeiras, A. Assessing the relationship between lipoprotein(a) levels and blood pressure among hypertensive patients beyond conventional measures. An observational study. Sci. Rep. 2024, 14, 14433. [Google Scholar] [CrossRef]
- Vazquez-Agra, N.; Cruces-Sande, A.; Mendez-Alvarez, E.; Soto-Otero, R.; Cinza-Sanjurjo, S.; Lopez-Paz, J.-E.; Pose-Reino, A.; Hermida-Ameijeiras, A. Correlation Between Blunted Nocturnal Decrease in Diastolic Blood Pressure and Oxidative Stress: An Observational Study. Antioxidants 2022, 11, 2430. [Google Scholar] [CrossRef]
- O’Brien, E.; Sheridan, J.; O’Malley, K. Dippers and non-dippers. Lancet 1988, 2, 397. [Google Scholar] [CrossRef]
- Parati, G.; Stergiou, G.; O’Brien, E.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leeuw, P.; Dolan, E.; et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 2014, 32, 1359–1366. [Google Scholar] [CrossRef]
- Fisher, M.J.; Marshall, A.P. Understanding descriptive statistics. Aust. Crit. Care 2009, 22, 93–97. [Google Scholar] [CrossRef]
- Szeghalmy, S.; Fazekas, A. A Comparative Study of the Use of Stratified Cross-Validation and Distribution-Balanced Stratified Cross-Validation in Imbalanced Learning. Sensors 2023, 23, 2333. [Google Scholar] [CrossRef]
- Domínguez-Almendros, S.; Benítez-Parejo, N.; Gonzalez-Ramirez, A.R. Logistic regression models. Allergol. Immunopathol. 2011, 39, 295–305. [Google Scholar] [CrossRef]
- Li, F.; Ding, P.; Mealli, F. Bayesian causal inference: A critical review. Philos. Trans. A Math. Phys. Eng. Sci. 2023, 381, 20220153. [Google Scholar] [CrossRef]
- Ma, W.J. Bayesian Decision Models: A Primer. Neuron 2019, 104, 164–175. [Google Scholar] [CrossRef]

| Variable | Total (n = 132) | UH a (No) (n = 60) | UH (Yes) (n = 72) | p-Value |
|---|---|---|---|---|
| Age (years) † | 36 (8.0) | 35.5 (9.8) | 37.0 (8.0) | 0.391 |
| Primipara (yes) ‡ | 49 (37.1) | 21 (35.0) | 28 (38.9) | 0.719 |
| N° of births (>1) ‡ | 15 (11.4) | 6 (10.0) | 9 (12.5) | 0.785 |
| Miscarriages (>1) ‡ | 5 (3.8) | 2 (3.3) | 3 (4.2) | 0.999 |
| Tobacco (yes) b ‡ | 16 (12.1) | 3 (5.0) | 13 (18.1) | 0.031 |
| CKD (yes) c ‡ | 6 (4.5) | 2 (3.3) | 4 (5.6) | 0.688 |
| DM (yes) ‡ | 9 (6.8) | 2 (3.3) | 7 (9.7) | 0.181 |
| Essential HT (yes) ‡ | 55 (41.7) | 19 (31.7) | 36 (50.0) | 0.036 |
| HT drugs (yes) ‡ | 22 (16.7) | 4 (6.7) | 18 (25.0) | 0.005 |
| BMI (kg/m2) † | 30.8 (11.3) | 31.4 (13.12) | 30.4 (10.6) | 0.500 |
| Office SBP (mmHg) † | 134.5 (12.8) | 131.0 (10.8) | 136.0 (15.0) | 0.014 |
| Office DBP (mmHg) † | 81.0 (13.8) | 77.5 (15.0) | 83.0 (14.8) | 0.002 |
| Office HR (bpm) † | 90.0 (18.0) | 89.5 (22.8) | 90.5 (16.8) | 0.557 |
| FPG (mg/dL) † | 78.0 (13.3) | 78.5 (11.8) | 78.0 (17.8) | 0.634 |
| Urea (mg/dL) † | 21.0 (7.0) | 21.0 (7.0) | 21.0 (7.0) | 0.864 |
| Uric acid (mg/dL) † | 4.1 (1.38) | 4.1 (1.90) | 4.2 (1.08) | 0.460 |
| sFlt-1 (U/L) † | 1908.0 (2683.3) | 1789.0 (2869.0) | 2045.5 (2302.8) | 0.810 |
| PlGF (U/L) † | 215.5 (250.8) | 226.5 (274.3) | 201.5 (232.5) | 0.805 |
| sFlt-1/PlGF † | 7.0 (22.0) | 8.0 (26.0) | 7.0 (19.0) | 0.544 |
| Accuracy | AUC | Log Loss | ||||
|---|---|---|---|---|---|---|
| Transformation Degree | Train Set | Test Set | Train Set | Test Set | Train Set | Test Set |
| 1 | 0.56 | 0.53 | 0.55 | 0.56 | 0.68 | 0.69 |
| 2 a | 0.70 | 0.67 | 0.74 | 0.69 | 0.57 | 0.62 |
| 3 | 0.72 | 0.67 | 0.77 | 0.68 | 0.55 | 0.62 |
| 4 | 0.73 | 0.66 | 0.77 | 0.66 | 0.54 | 0.80 |
| 5 | 0.73 | 0.65 | 0.78 | 0.67 | 0.53 | 1.05 |
| Variable | B | SE | Wald | p-Value | Exp(B) | 95%CI (Lower) | 95%CI (Upper) | |
|---|---|---|---|---|---|---|---|---|
| Office SBP (accuracy: 69%. Nagelkerke R2: 0.173) | ||||||||
| Former smoker (yes) | 1.678 | 0.685 | 5.997 | 0.014 | 5.354 | 1.398 | 20.507 | |
| HT drugs (yes) | 1.790 | 0.607 | 8.682 | 0.003 | 5.987 | 1.821 | 19.686 | |
| Office SBP (mmHg) | 0.043 | 0.017 | 6.348 | 0.012 | 1.044 | 1.010 | 1.079 | |
| Constant | −6.032 | 2.312 | 6.805 | 0.009 | ||||
| Office SBP + UA-AF index (accuracy: 74%. Nagelkerke R2: 0.371) | ||||||||
| Former smoker (yes) | 1.964 | 0.759 | 6.693 | 0.010 | 7.128 | 1.610 | 31.561 | |
| HT drugs (yes) | 1.989 | 0.676 | 8.667 | 0.003 | 7.311 | 1.944 | 27.488 | |
| Office SBP (mmHg) | 0.046 | 0.018 | 6.341 | 0.012 | 1.047 | 1.010 | 1.086 | |
| UA-AF index (SU) | 1.029 | 0.293 | 12.308 | <0.001 | 2.799 | 1.575 | 4.973 | |
| Constant | −6.735 | 2.515 | 7.172 | 0.007 | ||||
| Nighttime SBP (accuracy: 76%. Nagelkerke R2: 0.372) | ||||||||
| HT drugs (yes) | 1.555 | 0.661 | 5.539 | 0.019 | 4.736 | 1.297 | 17.292 | |
| nSBP (mmHg) | 0.087 | 0.018 | 23.925 | <0.001 | 1.091 | 1.054 | 1.130 | |
| Constant | −10.116 | 2.061 | 24.091 | <0.001 | ||||
| Nighttime SBP + UA-AF index (accuracy: 83%. Nagelkerke R2: 0.505) | ||||||||
| HT drugs (yes) | 1.264 | 0.662 | 3.650 | 0.056 | 3.540 | 0.968 | 12.952 | |
| nSBP (mmHg) | 0.098 | 0.020 | 23.041 | <0.001 | 1.103 | 1.060 | 1.149 | |
| UA-AF index (SU) | 1.039 | 0.327 | 10.113 | 0.001 | 2.826 | 1.490 | 5.361 | |
| Constant | −11.573 | 2.382 | 23.604 | <0.001 | ||||
| Office DBP (accuracy: 68%. Nagelkerke R2: 0.264) | ||||||||
| Former smoker (yes) | 1.811 | 0.705 | 6.604 | 0.010 | 6.115 | 1.537 | 24.335 | |
| HT drugs (yes) | 1.764 | 0.608 | 8.423 | 0.004 | 5.838 | 1.773 | 19.22 | |
| Office DBP (mmHg) | 0.063 | 0.019 | 11.08 | 0.001 | 1.065 | 1.026 | 1.106 | |
| Constant | −5.329 | 1.55 | 11.815 | 0.001 | ||||
| Office DBP + UA-AF index (accuracy: 73%. Nagelkerke R2: 0.411) | ||||||||
| Former smoker (yes) | 2.081 | 0.782 | 7.076 | 0.008 | 8.010 | 1.729 | 37.110 | |
| HT drugs (yes) | 1.941 | 0.681 | 8.127 | 0.004 | 6.967 | 1.834 | 26.468 | |
| Office DBP (mmHg) | 0.067 | 0.020 | 10.710 | 0.001 | 1.069 | 1.027 | 1.113 | |
| UA-AF index (SU) | 0.998 | 0.286 | 12.145 | <0.001 | 2.713 | 1.548 | 4.757 | |
| Constant | −5.807 | 1.664 | 12.181 | <0.001 | ||||
| Nighttime DBP (accuracy: 74%. Nagelkerke R2: 0.380) | ||||||||
| Former smoker (yes) | 1.535 | 0.731 | 4.357 | 0.037 | 4.641 | 1.098 | 19.617 | |
| HT drugs (yes) | 1.653 | 0.649 | 6.492 | 0.011 | 5.223 | 1.464 | 18.625 | |
| nDBP (mmHg) | 0.117 | 0.026 | 20.861 | <0.001 | 1.124 | 1.069 | 1.181 | |
| Constant | −8.145 | 1.743 | 21.839 | <0.001 | ||||
| Nighttime DBP + UA-AF index (accuracy: 80%. Nagelkerke R2: 0.505) | ||||||||
| Former smoker (yes) | 1.828 | 0.876 | 4.352 | 0.037 | 6.221 | 1.117 | 34.653 | |
| HT drugs (yes) | 1.663 | 0.690 | 5.801 | 0.016 | 5.274 | 1.363 | 20.406 | |
| nDBP (mmHg) | 0.120 | 0.028 | 18.802 | <0.001 | 1.128 | 1.068 | 1.191 | |
| UA-AF index (SU) | 1.004 | 0.327 | 9.408 | 0.002 | 2.729 | 1.437 | 5.182 | |
| Constant | −8.569 | 1.889 | 20.570 | <0.001 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Castro, I.; Vazquez-Agra, N.; Alban-Salgado, A.; Sanchez-Andrade, M.; Lopez-Casal, S.; Cruces-Sande, A.; Seoane-Casqueiro, O.; Pose-Reino, A.; Hermida-Ameijeiras, A. Evaluating an Early Risk Model for Uncomplicated Hypertension in Pregnancy Based on Nighttime Blood Pressure, Uric Acid, and Angiogenesis-Related Factors. Int. J. Mol. Sci. 2025, 26, 6115. https://doi.org/10.3390/ijms26136115
Fernandez-Castro I, Vazquez-Agra N, Alban-Salgado A, Sanchez-Andrade M, Lopez-Casal S, Cruces-Sande A, Seoane-Casqueiro O, Pose-Reino A, Hermida-Ameijeiras A. Evaluating an Early Risk Model for Uncomplicated Hypertension in Pregnancy Based on Nighttime Blood Pressure, Uric Acid, and Angiogenesis-Related Factors. International Journal of Molecular Sciences. 2025; 26(13):6115. https://doi.org/10.3390/ijms26136115
Chicago/Turabian StyleFernandez-Castro, Isabel, Nestor Vazquez-Agra, Ana Alban-Salgado, Mariña Sanchez-Andrade, Susana Lopez-Casal, Anton Cruces-Sande, Oscar Seoane-Casqueiro, Antonio Pose-Reino, and Alvaro Hermida-Ameijeiras. 2025. "Evaluating an Early Risk Model for Uncomplicated Hypertension in Pregnancy Based on Nighttime Blood Pressure, Uric Acid, and Angiogenesis-Related Factors" International Journal of Molecular Sciences 26, no. 13: 6115. https://doi.org/10.3390/ijms26136115
APA StyleFernandez-Castro, I., Vazquez-Agra, N., Alban-Salgado, A., Sanchez-Andrade, M., Lopez-Casal, S., Cruces-Sande, A., Seoane-Casqueiro, O., Pose-Reino, A., & Hermida-Ameijeiras, A. (2025). Evaluating an Early Risk Model for Uncomplicated Hypertension in Pregnancy Based on Nighttime Blood Pressure, Uric Acid, and Angiogenesis-Related Factors. International Journal of Molecular Sciences, 26(13), 6115. https://doi.org/10.3390/ijms26136115







