Beyond Blood Pressure: Emerging Pathways and Precision Approaches in Hypertension-Induced Kidney Damage
Abstract
1. Introduction
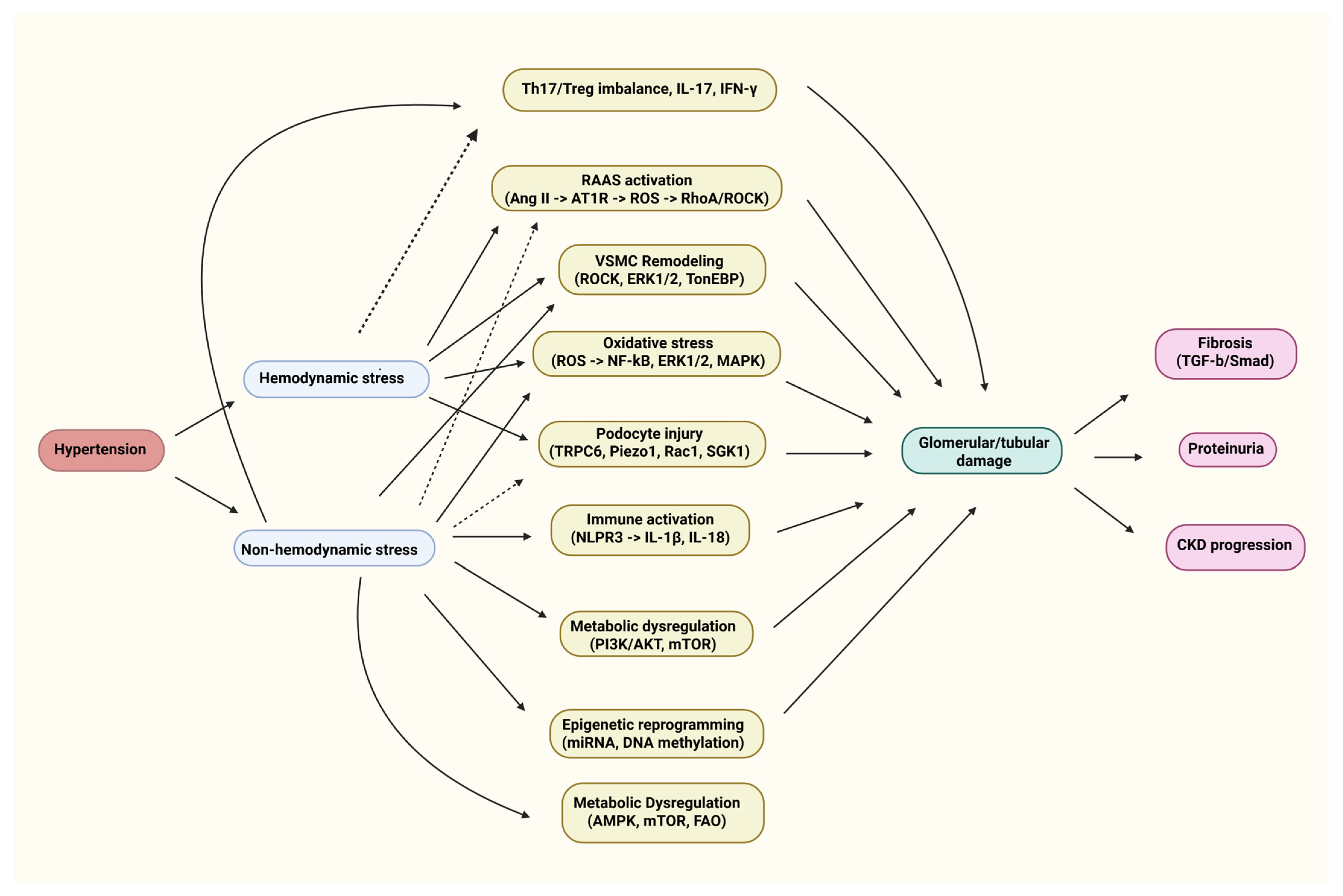
2. Emerging Molecular Pathways Beyond Hemodynamics
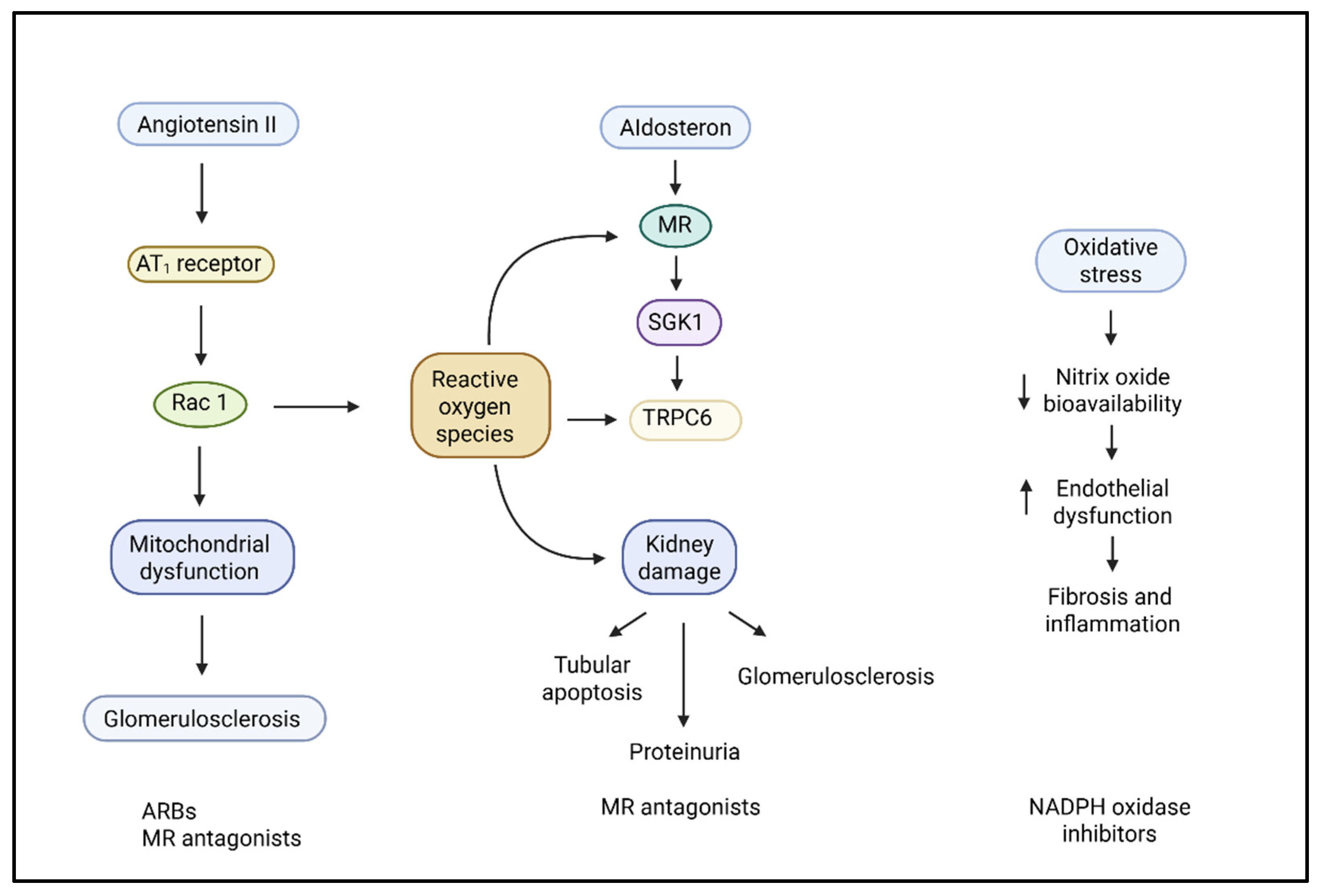
2.1. Renin–Angiotensin–Aldosterone System
2.2. Oxidative Stress and Reactive Oxygen Species
2.3. Immune and Inflammatory Mechanisms
2.4. Mechanical Stress and Podocyte Injury
2.5. Endothelial Dysfunction and Hypoxia
2.6. Metabolic and Energy Dysregulation
2.7. Epigenetic and MicroRNA Regulation
2.8. Vascular Smooth Muscle and Cytoskeletal Dynamics
3. Immune Mechanisms Underpinning Renal Injury
3.1. T-Cell Activation and Cytokine Responses
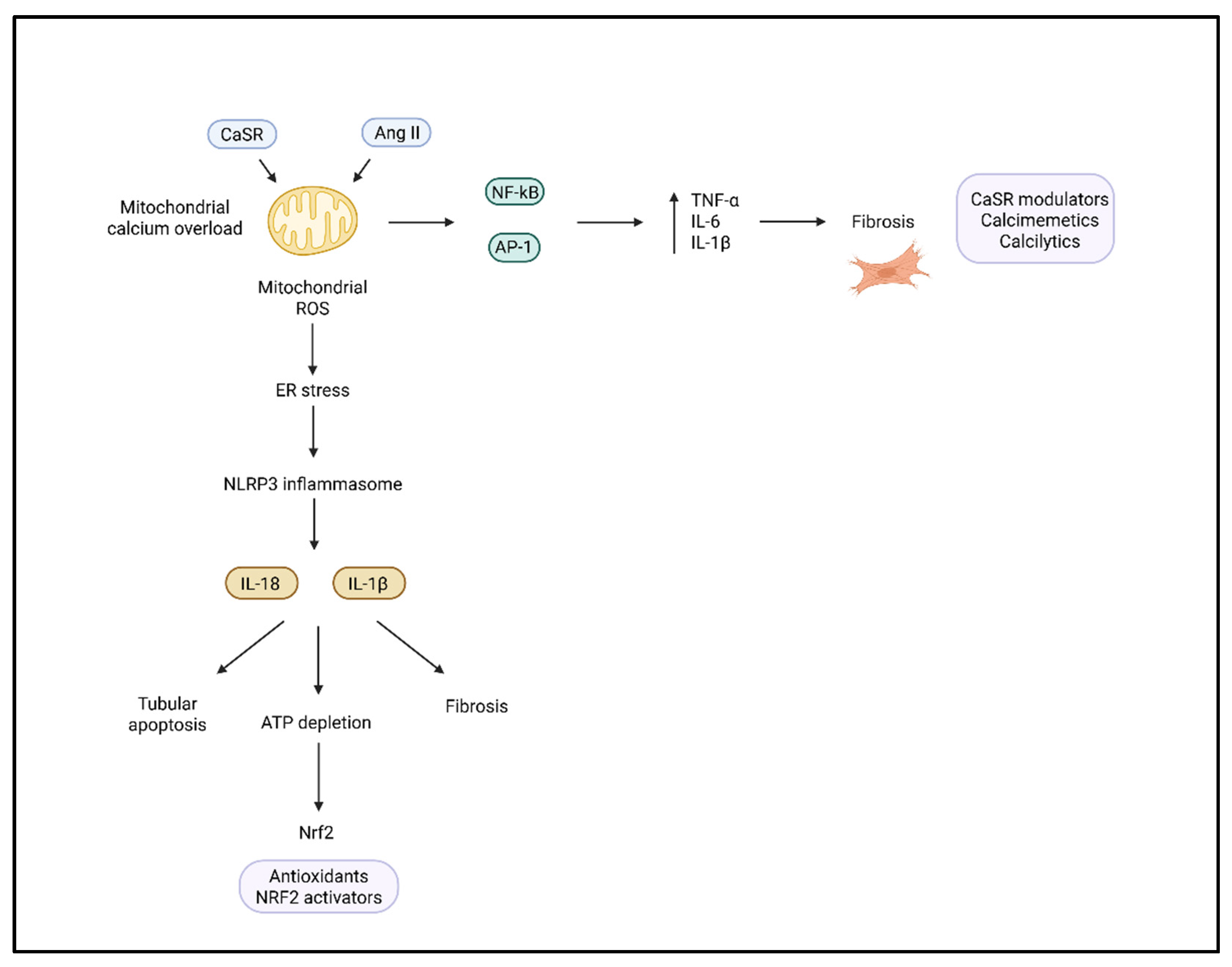
3.2. Role of the NLRP3 Inflammasome
3.3. Neuroimmune Interactions
4. Genetic Susceptibility and Epigenetic Regulation
4.1. Genetic Variants and Renal Hemodynamics
4.2. The Role of P66SHC and Cytoskeletal Regulators
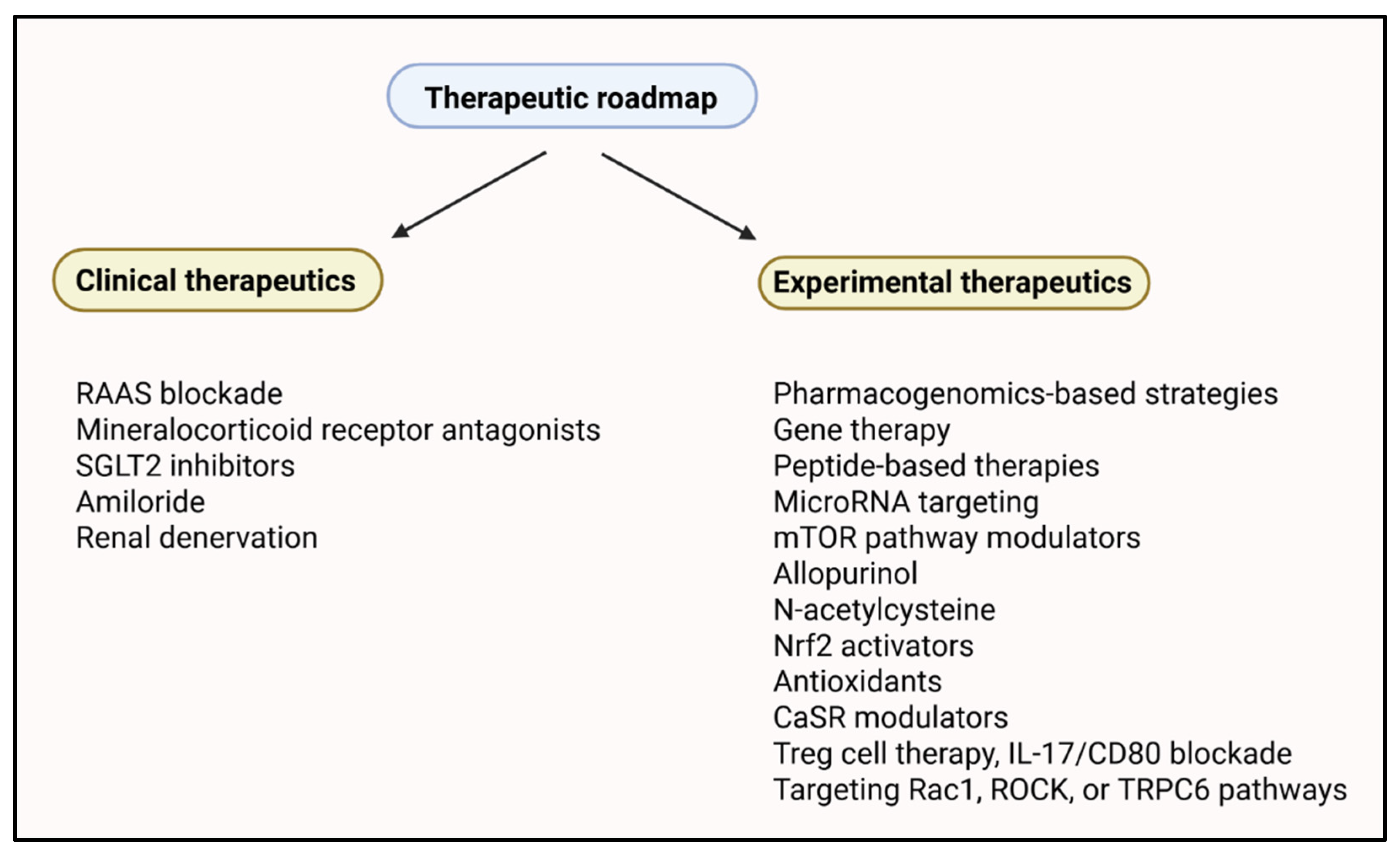
5. Targeted Therapeutics
5.1. Genetically Guided Pharmacotherapy
5.2. Device-Based Interventions
5.3. Targeting Inflammatory and Immune Pathways
5.4. Metabolic Modulators and mTOR Inhibition
5.5. Personalized Therapeutic Combinations
5.6. Pediatric Precision Approaches
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| ACE2 | Angiotensin-Converting Enzyme 2 |
| ADD3 | Adducin 3 |
| ADMA | Asymmetric Dimethylarginine |
| Ang II | Angiotensin II |
| AMPK | AMP-Activated Protein Kinase |
| AP1 | Activator Protein 1 |
| ARB | Angiotensin Receptor Blocker |
| AREs | Antioxidant-Responsive Elements |
| AT1R | Angiotensin II Type 1 Receptor |
| Cas9 | CRISPR-associated protein 9 |
| CASP1 | Caspase 1 |
| CKD | Chronic Kidney Disease |
| CPT1 | Carnitine Palmitoyltransferase-1 |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CTGF | Connective Tissue Growth Factor |
| DPP-4 | Dipeptidyl Peptidase-4 |
| ECM | Extracellular Matrix |
| EMT | Epithelial–Mesenchymal Transition |
| eGFR | Estimated Glomerular Filtration Rate |
| ENaC | Epithelial Sodium Channel |
| eNOS | Endothelial Nitric Oxide Synthase |
| ER | Endoplasmic Reticulum |
| ESKD | End-Stage Kidney Disease |
| FAO | Fatty Acid Oxidation |
| FGF-23 | Fibroblast Growth Factor 23 |
| FGFR1 | Fibroblast Growth Factor Receptor 1 |
| FXR1 | Fragile X Mental Retardation Syndrome-Related Protein 1 |
| GLP-1 | Glucagon-Like Peptide 1 |
| GWAS | Genome-Wide Association Study |
| HIF | Hypoxia-Inducible Factor |
| Hsp27 | Heat Shock Protein 27 |
| ICAM | Intercellular Adhesion Molecule 1 |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-17 | Interleukin-17 |
| JAK-STAT | Janus Kinase–Signal Transducer and Activator of Transcription |
| JNK | c-Jun N-Terminal Kinase |
| KIM-1 | Kidney Injury Molecule-1 |
| L-FABP | Liver-Type Fatty Acid-Binding Protein |
| MAPK | Mitogen-Activated Protein Kinase |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| miRNA | MicroRNA |
| MitoQ | Mitoquinone |
| MR | Mineralocorticoid Receptor |
| MRTF-A | Myocardin-Related Transcription Factor-A |
| mtDNA | Mitochondrial DNA |
| mTOR | Mammalian Target of Rapamycin |
| NAC | N-Acetylcysteine |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NFATc1 | Nuclear Factor of Activated T Cells, Cytoplasmic 1 |
| NGAL | Neutrophil Gelatinase-Associated Lipocalin |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NLRP3 | NOD-, LRR-, and Pyrin Domain-Containing Protein 3 |
| NO | Nitric Oxide |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| PAI-1 | Plasminogen Activator Inhibitor 1 |
| PHRASE | Pediatric Hypertension and the Renin–Angiotensin SystEm Study |
| PLC-IP3 | Phospholipase C-Inositol 1,4,5-Trisphosphate Pathway |
| PPARα | Peroxisome Proliferator-Activated Receptor Alpha |
| RAAS | Renin–Angiotensin–Aldosterone System |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| RDN | Renal Denervation |
| ROCK | Rho-Associated Coiled-Coil Containing Protein Kinase |
| ROS | Reactive Oxygen Species |
| RAS | Renin–Angiotensin System |
| SRF | Serum Response Factor |
| SGK1 | Serum- and Glucocorticoid-Regulated Kinase 1 |
| SGLT2 | Sodium–Glucose Cotransporter 2 |
| SHR | Spontaneously Hypertensive Rat |
| siRNA | Small Interfering RNA |
| SNS | Sympathetic Nervous System |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STING | Stimulator of Interferon Genes |
| TAZ | Transcriptional Co-Activator |
| TGF-β | Transforming Growth Factor Beta |
| TNF-α | Tumor Necrosis Factor-Alpha |
| Th17 | T Helper 17 Cells |
| TIMP-1 | Tissue Inhibitor of Metalloproteinases-1 |
| TonEBP/NFAT5 | Tonicity-Responsive Enhancer Binding Protein/Nuclear Factor of Activated T Cells 5 |
| Treg | Regulatory T Cell |
| TRP | Transient Receptor Potential |
| TRPC6 | Transient Receptor Potential Cation Channel, Subfamily C, Member 6 |
| UMOD | Uromodulin |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| VEGF | Vascular Endothelial Growth Factor |
| VSMC | Vascular Smooth Muscle Cell |
| YAP | Yes-Associated Protein |
References
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Camargo, L.L.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. Reactive oxygen species in hypertension. Nat. Rev. Cardiol. 2025, 22, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Atkins, R.; Coresh, J.; Cohen, E.P.; Collins, A.J.; Eckardt, K.-U.; Nahas, M.E.; Jaber, B.L.; Jadoul, M.; Levin, A.; et al. Chronic kidney disease as a global public health problem: Approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007, 72, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Pichler, R.; Afkarian, M.; Dieter, B.P.; Tuttle, K.R. Immunity and inflammation in diabetic kidney disease: Translating mechanisms to biomarkers and treatment targets. Am. J. Physiol. Physiol. 2017, 312, F716–F731. [Google Scholar] [CrossRef]
- Tsur, A.M.; Akavian, I.; Derazne, E.; Tzur, D.; Vivante, A.; Grossman, E.; Rotem, R.S.; Fishman, B.; Afek, A.; Coresh, J.; et al. Adolescent Blood Pressure and the Risk for Early Kidney Damage in Young Adulthood. Hypertension 2022, 79, 974–983. [Google Scholar] [CrossRef]
- Wenzel, U.O.; Ehmke, H.; Bode, M. Immune mechanisms in arterial hypertension. Recent. Adv. Cell Tissue Res. 2021, 385, 393–404. [Google Scholar] [CrossRef]
- Orejudo, M.; Rodrigues-Diez, R.R.; Rodrigues-Diez, R.; Garcia-Redondo, A.; Santos-Sánchez, L.; Rández-Garbayo, J.; Cannata-Ortiz, P.; Ramos, A.M.; Ortiz, A.; Selgas, R.; et al. Interleukin 17A Participates in Renal Inflammation Associated to Experimental and Human Hypertension. Front. Pharmacol. 2019, 10, 1015. [Google Scholar] [CrossRef]
- Ulrich, C.; Wildgrube, S.; Fiedler, R.; Seibert, E.; Kneser, L.; Fick, S.; Schäfer, C.; Markau, S.; Trojanowicz, B.; Girndt, M. NLRP3 Inflammasome Activation in Hemodialysis and Hypertensive Patients with Intact Kidney Function. Toxins 2020, 12, 675. [Google Scholar] [CrossRef] [PubMed]
- McBride, M.W.; Graham, D.; Delles, C.; Dominiczak, A.F. Functional genomics in hypertension. Curr. Opin. Nephrol. Hypertens. 2006, 15, 145–151. [Google Scholar] [CrossRef]
- Mira, F.S.; Oliveiros, B.; Carreira, I.M.; Alves, R.; Ribeiro, I.P. Genetic Variants Related to Increased CKD Progression-A Systematic Review. Biology 2025, 14, 68. [Google Scholar] [CrossRef]
- Villagomez Fuentes, L.E.; Algharably, E.A.; Toepfer, S.; König, M.; Demuth, I.; Bertram, L.; Kreutz, R.; Bolbrinker, J. Effect of a common UMOD variant on kidney function, blood pressure, cognitive and physical function in a community-based cohort of older adults. J. Hum. Hypertens. 2022, 36, 983–988. [Google Scholar] [CrossRef]
- Chao, C.-T.; Kuo, F.-C.; Lin, S.-H. Epigenetically regulated inflammation in vascular senescence and renal progression of chronic kidney disease. Semin. Cell Dev. Biol. 2024, 154, 305–315. [Google Scholar] [CrossRef]
- Ameer, O.Z. Hypertension in chronic kidney disease: What lies behind the scene. Front. Pharmacol. 2022, 13, 949260. [Google Scholar] [CrossRef]
- Ma, C.; Li, X.; Li, W.; Li, Y.; Shui, F.; Zhu, P. The efficacy and safety of SGLT2 inhibitors in patients with non-diabetic chronic kidney disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2023, 55, 3167–3174. [Google Scholar] [CrossRef]
- Saliba, A.; Du, Y.; Feng, T.; Garmire, L. Multi-Omics Integration in Nephrology: Advances, Challenges, and Future Directions. Semin. Nephrol. 2025, 44, 151584. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, N.R.; Harrison, D.G. Markers or Makers: Inflammatory Cytokines in Treatment-Resistant Hypertension. Hypertension 2019, 73, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Cisek, K.; Krochmal, M.; Klein, J.; Mischak, H. The application of multi-omics and systems biology to identify therapeutic targets in chronic kidney disease. Nephrol. Dial. Transplant. 2016, 31, 2003–2011. [Google Scholar] [CrossRef]
- Basile, D.P.; Abais-Battad, J.M.; Mattson, D.L. Contribution of Th17 cells to tissue injury in hypertension. Curr. Opin. Nephrol. Hypertens. 2021, 30, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Palygin, O.; Rufanova, V.A.; Chong, A.; Lazar, J.; Jacob, H.J.; Mattson, D.; Roman, R.J.; Williams, J.M.; Cowley, A.W.; et al. p66Shc regulates renal vascular tone in hypertension-induced nephropathy. J. Clin. Investig. 2016, 126, 2533–2546. [Google Scholar] [CrossRef][Green Version]
- Bitzer, M.; Ju, W.; Subramanian, L.; Troost, J.P.; Tychewicz, J.; Steck, B.; Wiggins, R.C.; Gipson, D.S.; Gadegbeku, C.A.; Brosius, F.C.; et al. The Michigan O’Brien Kidney Research Center: Transforming translational kidney research through systems biology. Am. J. Physiol. Physiol. 2022, 323, F401–F410. [Google Scholar] [CrossRef]
- Xu, Z.; Luo, W.; Chen, L.; Zhuang, Z.; Yang, D.; Qian, J.; Khan, Z.A.; Guan, X.; Wang, Y.; Li, X.; et al. Ang II (Angiotensin II)–Induced FGFR1 (Fibroblast Growth Factor Receptor 1) Activation in Tubular Epithelial Cells Promotes Hypertensive Kidney Fibrosis and Injury. Hypertension 2022, 79, 2028–2041. [Google Scholar] [CrossRef] [PubMed]
- Kawarazaki, W.; Nagase, M.; Yoshida, S.; Takeuchi, M.; Ishizawa, K.; Ayuzawa, N.; Ueda, K.; Fujita, T. Angiotensin II- and Salt-Induced Kidney Injury through Rac1-Mediated Mineralocorticoid Receptor Activation. J. Am. Soc. Nephrol. 2012, 23, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Hilgers, K.F.; Mann, J.F.E. Role of Angiotensin II in Glomerular Injury: Lessons from Experimental and Clinical Studies. Kidney Blood Press. Res. 1996, 19, 254–262. [Google Scholar] [CrossRef]
- Nijenhuis, T.; Sloan, A.J.; Hoenderop, J.G.J.; Flesche, J.; Van Goor, H.; Kistler, A.D.; Bakker, M.; Bindels, R.J.M.; De Boer, R.A.; Möller, C.C.; et al. Angiotensin II Contributes to Podocyte Injury by Increasing TRPC6 Expression via an NFAT-Mediated Positive Feedback Signaling Pathway. Am. J. Pathol. 2011, 179, 1719–1732. [Google Scholar] [CrossRef]
- Rupérez, M.; Ruiz-Ortega, M.; Esteban, V.; Lorenzo, Ó.; Mezzano, S.; Plaza, J.J.; Egido, J. Angiotensin II Increases Connective Tissue Growth Factor in the Kidney. Am. J. Pathol. 2003, 163, 1937–1947. [Google Scholar] [CrossRef][Green Version]
- Ruiz-Ortega, M.; Ruperez, M.; Lorenzo, O.; Esteban, V.; Blanco, J.; Mezzano, S.; Egido, J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int. 2002, 62, S12–S22. [Google Scholar] [CrossRef] [PubMed]
- Long, A.; Salvo, M. Finerenone: A Novel Mineralocorticoid Receptor Antagonist for Cardiorenal Protection in CKD and T2DM. Ann. Pharmacother. 2022, 56, 1041–1048. [Google Scholar] [CrossRef]
- Lefranc, C.; Friederich-Persson, M.; Foufelle, F.; Nguyen Dinh Cat, A.; Jaisser, F. Adipocyte-Mineralocorticoid Receptor Alters Mitochondrial Quality Control Leading to Mitochondrial Dysfunction and Senescence of Visceral Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 2881. [Google Scholar] [CrossRef]
- Nagase, M.; Ayuzawa, N.; Kawarazaki, W.; Ishizawa, K.; Ueda, K.; Yoshida, S.; Fujita, T. Oxidative Stress Causes Mineralocorticoid Receptor Activation in Rat Cardiomyocytes: Role of Small GTPase Rac1. Hypertension 2012, 59, 500–506. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Pan, C.-T.; Chang, Y.-Y.; Peng, S.-Y.; Lee, P.-C.; Liao, C.-W.; Shun, C.-T.; Li, P.-T.; Wu, V.-C.; Chou, C.-H.; et al. Aldosterone Excess Induced Mitochondria Decrease and Dysfunction via Mineralocorticoid Receptor and Oxidative Stress In Vitro and In Vivo. Biomedicines 2021, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Abe, M.; Kobayashi, H. The Effect of Aldosterone on Cardiorenal and Metabolic Systems. Int. J. Mol. Sci. 2023, 24, 5370. [Google Scholar] [CrossRef]
- Masuo, K.; Lambert, G.W.; Esler, M.D.; Rakugi, H.; Ogihara, T.; Schlaich, M.P. The role of sympathetic nervous activity in renal injury and end-stage renal disease. Hypertens. Res. 2010, 33, 521–528. [Google Scholar] [CrossRef]
- Miura, T.; Fukuda, M.; Sato, R.; Fukuta, H.; Watanabe, S.; Mizuno, M.; Sakata, S.; Kimura, G. 745 Estimating Autonomic Nerve Activity Using ABPM in Patients with CKD. J. Hypertens. 2012, 30, e215–e216. [Google Scholar] [CrossRef]
- Frame, A.A.; Nist, K.M.; Kim, K.; Puleo, F.; Moreira, J.D.; Swaldi, H.; McKenna, J.; Wainford, R.D. Integrated renal and sympathetic mechanisms underlying the development of sex- and age-dependent hypertension and the salt sensitivity of blood pressure. Geroscience 2024, 46, 6435–6458. [Google Scholar] [CrossRef] [PubMed]
- Dzau, V.J.; Hodgkinson, C.P. Precision Hypertension. Hypertension 2024, 81, 702–708. [Google Scholar] [CrossRef]
- Ueno, M.; Fujii, W.; Ono, W.; Murata, H.; Fujigaki, Y.; Shibata, S. Renin Inhibition and the Long-Term Renal Function in Patients with Hypertensive Emergency: A Retrospective Cohort Study. Am. J. Hypertens. 2024, 37, 407–414. [Google Scholar] [CrossRef]
- Casas, J.P.; Chua, W.; Loukogeorgakis, S.; Vallance, P.; Smeeth, L.; Hingorani, A.D.; MacAllister, R.J. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: Systematic review and meta-analysis. Lancet 2005, 366, 2026–2033. [Google Scholar] [CrossRef]
- Douglas, J.G.; Agodoa, L. ACE inhibition is effective and renoprotective in hypertensive nephrosclerosis: The African American Study of Kidney Disease and Hypertension (AASK) trial. Kidney Int. 2003, 63, S74–S76. [Google Scholar] [CrossRef]
- Tonneijck, L.; Muskiet, M.H.A.; Smits, M.M.; Van Bommel, E.J.; Heerspink, H.J.L.; Van Raalte, D.H.; Joles, J.A. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. JASN 2017, 28, 1023–1039. [Google Scholar] [CrossRef]
- Egan, B.M.; Pohl, F.; Anderson, X.; Williams, S.C.; Gregory Adodo, I.; Hunt, P.; Wang, Z.; Chiu, C.-H.; Scharf, A.; Mosley, M.; et al. The ACE inhibitor captopril inhibits ACN-1 to control dauer formation and aging. Development 2024, 151, dev202146. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Ebert, T.; Anker, S.D.; Ruilope, L.M.; Fioretto, P.; Fonseca, V.; Umpierrez, G.E.; Birkenfeld, A.L.; Lawatscheck, R.; Scott, C.; Rohwedder, K.; et al. Outcomes with Finerenone in Patients with Chronic Kidney Disease and Type 2 Diabetes by Baseline Insulin Resistance. Diabetes Care 2024, 47, 362–370. [Google Scholar] [CrossRef]
- Garty, H.; Palmer, L.G. Epithelial sodium channels: Function, structure, and regulation. Physiol. Rev. 1997, 77, 359–396. [Google Scholar] [CrossRef]
- Ronzaud, C.; Loffing-Cueni, D.; Hausel, P.; Debonneville, A.; Malsure, S.R.; Fowler-Jaeger, N.; Boase, N.A.; Perrier, R.; Maillard, M.; Yang, B.; et al. Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J. Clin. Investing. 2013, 123, JCI61110. [Google Scholar] [CrossRef]
- Wulff, P.; Vallon, V.; Huang, D.Y.; Völkl, H.; Yu, F.; Richter, K.; Jansen, M.; Schlünz, M.; Klingel, K.; Loffing, J.; et al. Impaired renal Na+ retention in the sgk1-knockout mouse. J. Clin. Investing. 2002, 110, 1263–1268. [Google Scholar] [CrossRef]
- Norlander, A.E.; Saleh, M.A.; Pandey, A.K.; Itani, H.A.; Wu, J.; Xiao, L.; Kang, J.; Dale, B.L.; Goleva, S.B.; Laroumanie, F.; et al. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight 2017, 2, e92801. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Saleh, M.A.; Kirabo, A.; Itani, H.A.; Montaniel, K.R.C.; Xiao, L.; Chen, W.; Mernaugh, R.L.; Cai, H.; Bernstein, K.E.; et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J. Clin. Investig. 2015, 126, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Pitzer, A.L.; Van Beusecum, J.P.; Kleyman, T.R.; Kirabo, A. ENaC in Salt-Sensitive Hypertension: Kidney and Beyond. Curr. Hypertens. Rep. 2020, 22, 69. [Google Scholar] [CrossRef]
- Barbaro, N.R.; Foss, J.D.; Kryshtal, D.O.; Tsyba, N.; Kumaresan, S.; Xiao, L.; Mernaugh, R.L.; Itani, H.A.; Loperena, R.; Chen, W.; et al. Dendritic Cell Amiloride-Sensitive Channels Mediate Sodium-Induced Inflammation and Hypertension. Cell Rep. 2017, 21, 1009–1020. [Google Scholar] [CrossRef]
- Andersen, H.; Hansen, M.H.; Buhl, K.B.; Stæhr, M.; Friis, U.G.; Enggaard, C.; Supramaniyam, S.; Lund, I.K.; Svenningsen, P.; Hansen, P.B.L.; et al. Plasminogen Deficiency and Amiloride Mitigate Angiotensin II–Induced Hypertension in Type 1 Diabetic Mice Suggesting Effects Through the Epithelial Sodium Channel. JAHA 2020, 9, e016387. [Google Scholar] [CrossRef]
- Touyz, R.M.; Chignalia, A.; Sedeek, M. Reactive Oxygen Species, Oxidative Stress, and Hypertension. In Studies on Cardiovascular Disorders; Sauer, H., Shah, A.M., Laurindo, F.R.M., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 281–315. ISBN 978-1-60761-599-6. [Google Scholar]
- Xu, N.; Jiang, S.; Persson, P.B.; Persson, E.A.G.; Lai, E.Y.; Patzak, A. Reactive oxygen species in renal vascular function. Acta Physiol. 2020, 229, e13477. [Google Scholar] [CrossRef]
- Just, A.; Arendshorst, W.J. The Role of Reactive Oxygen Species in Renal Blood Flow Autoregulation. FASEB J. 2008, 22, 761.19. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Garvin, J. Effects of Reactive Oxygen Species on Tubular Transport along the Nephron. Antioxidants 2017, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Yisireyili, M.; Higashiyama, Y.; Nishijima, F.; Niwa, T. Indoxyl sulfate upregulates renal expression of ICAM-1 via production of ROS and activation of NF-κB and p53 in proximal tubular cells. Life Sci. 2013, 92, 143–148. [Google Scholar] [CrossRef]
- Kim, J.; Seok, Y.M.; Jung, K.-J.; Park, K.M. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am. J. Physiol. Physiol. 2009, 297, F461–F470. [Google Scholar] [CrossRef]
- Araujo, M.; Wilcox, C.S. Oxidative Stress in Hypertension: Role of the Kidney. Antioxid. Redox Signal. 2014, 20, 74–101. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fallahzadeh, M.K.; McCullough, P.A. Aging Male Spontaneously Hypertensive Rat as an Animal Model for the Evaluation of the Interplay between Contrast-Induced Acute Kidney Injury and Cardiorenal Syndrome in Humans. Cardiorenal Med. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Griffin, K.A.; Bidani, A.K. Hypertensive renal damage: Insights from animal models and clinical relevance. Curr. Sci. Inc. 2004, 6, 145–153. [Google Scholar] [CrossRef]
- Wei, Q.; Xiao, X.; Huo, E.; Guo, C.; Zhou, X.; Hu, X.; Dong, C.; Shi, H.; Dong, Z. Hypermethylation and suppression of microRNA219a-2 activates the ALDH1L2/GSH/PAI-1 pathway for fibronectin degradation in renal fibrosis. Mol. Ther. 2025, 33, 249–262. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, J.; Yang, T.; Jiang, L.; Liu, X.; Wang, S.; Wang, X.; Huang, Y.; Wang, H.; Zhang, M.; et al. STING/ACSL4 axis-dependent ferroptosis and inflammation promote hypertension-associated chronic kidney disease. Mol. Ther. 2023, 31, 3084–3103. [Google Scholar] [CrossRef]
- Russo, E.; Bussalino, E.; Macciò, L.; Verzola, D.; Saio, M.; Esposito, P.; Leoncini, G.; Pontremoli, R.; Viazzi, F. Non-Haemodynamic Mechanisms Underlying Hypertension-Associated Damage in Target Kidney Components. Int. J. Mol. Sci. 2023, 24, 9422. [Google Scholar] [CrossRef]
- Chu, H.; Qin, Z.; Ma, J.; Xie, Y.; Shi, H.; Gu, J.; Shi, B. Calcium-Sensing Receptor (CaSR)-Mediated Intracellular Communication in Cardiovascular Diseases. Cells 2022, 11, 3075. [Google Scholar] [CrossRef]
- Sundararaman, S.S.; Van Der Vorst, E.P.C. Calcium-Sensing Receptor (CaSR), Its Impact on Inflammation and the Consequences on Cardiovascular Health. Int. J. Mol. Sci. 2021, 22, 2478. [Google Scholar] [CrossRef]
- Iamartino, L.; Brandi, M.L. The calcium-sensing receptor in inflammation: Recent updates. Front. Physiol. 2022, 13, 1059369. [Google Scholar] [CrossRef] [PubMed]
- Werner, L.E.; Wagner, U. Calcium-sensing receptor-mediated NLRP3 inflammasome activation in rheumatoid arthritis and autoinflammation. Front. Physiol. 2023, 13, 1078569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, R.; Zhong, H.; Tang, N.; Liu, Y.; Zhao, Y.; Zhang, T.; He, F. CaSR participates in the regulation of vascular tension in the mesentery of hypertensive rats via the PLC-IP3/AC-V/cAMP/RAS pathway. Mol. Med. Rep. 2019, 20, 4433–4448. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, D.; Kemp, P.J. The Calcium-Sensing Receptor Beyond Extracellular Calcium Homeostasis: Conception, Development, Adult Physiology, and Disease. Annu. Rev. Physiol. 2012, 74, 271–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hong, S.; Qi, S.; Liu, W.; Zhang, X.; Shi, Z.; Chen, W.; Zhao, M.; Yin, X. NLRP3 Inflammasome Is Involved in Calcium-Sensing Receptor-Induced Aortic Remodeling in SHRs. Mediat. Inflamm. 2019, 2019, 6847087. [Google Scholar] [CrossRef]
- De Zeeuw, D.; Akizawa, T.; Audhya, P.; Bakris, G.L.; Chin, M.; Christ-Schmidt, H.; Goldsberry, A.; Houser, M.; Krauth, M.; Lambers Heerspink, H.J.; et al. Bardoxolone Methyl in Type 2 Diabetes and Stage 4 Chronic Kidney Disease. N. Engl. J. Med. 2013, 369, 2492–2503. [Google Scholar] [CrossRef]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef]
- Feig, D.I.; Soletsky, B.; Johnson, R.J. Effect of Allopurinol on Blood Pressure of Adolescents with Newly Diagnosed Essential Hypertension: A Randomized Trial. JAMA 2008, 300, 924. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Bikineyeva, A.T.; Budzyn, K.; Nazarewicz, R.R.; McCann, L.; Lewis, W.; Harrison, D.G.; Dikalov, S.I. Therapeutic Targeting of Mitochondrial Superoxide in Hypertension. Circ. Res. 2010, 107, 106–116. [Google Scholar] [CrossRef]
- Petrova, G.; Mattson, D. Time Course of Immune Cell Infiltration and Cytokine Production in the Kidneys of Dahl Salt-Sensitive (SS) Rats. FASEB J. 2015, 29, 667.8. [Google Scholar] [CrossRef]
- Kirabo, A.; Fontana, V.; de Faria, A.P.C.; Loperena, R.; Galindo, C.L.; Wu, J.; Bikineyeva, A.T.; Dikalov, S.; Xiao, L.; Chen, W.; et al. DC isoketal-modified proteins activate T cells and promote hypertension. J. Clin. Investing. 2014, 124, 4642–4656. [Google Scholar] [CrossRef]
- Lu, X.; Crowley, S.D. Actions of immune cells in the hypertensive kidney. Curr. Opin. Nephrol. Hypertens. 2020, 29, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L. S-40-1: Inflammation and immunity in hypertension. J. Hypertens. 2023, 41, e90. [Google Scholar] [CrossRef]
- Wen, Y.; Crowley, S.D. Renal effects of cytokines in hypertension. Curr. Opin. Nephrol. Hypertens. 2018, 27, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Arifaj, D.; Hering, L.; Yakoub, M.; Rump, L.C.; Stegbauer, J. Abstract 129: Role Of Human T Cells In Experimental Hypertension And Hypertensive Kidney Damage. Hypertension 2022, 79, 1.129. [Google Scholar] [CrossRef]
- Jevnikar, A.M.; Brennan, D.C.; Singer, G.G.; Heng, J.E.; Maslinski, W.; Wuthrich, R.P.; Glimcher, L.H.; Kelley, V.E.R. Stimulated kidney tubular epithelial cells express membrane associated and secreted TNFα. Kidney Int. 1991, 40, 203–211. [Google Scholar] [CrossRef]
- Vielhauer, V.; Mayadas, T.N. Functions of TNF and its Receptors in Renal Disease: Distinct Roles in Inflammatory Tissue Injury and Immune Regulation. Semin. Nephrol. 2007, 27, 286–308. [Google Scholar] [CrossRef]
- Xue, L.; Xie, K.; Han, X.; Yang, Z.; Qiu, J.; Zhao, Z.; Bao, T. Detrimental Functions of IL-17A in Renal Ischemia-Reperfusion Injury in Mice. J. Surg. Res. 2011, 171, 266–274. [Google Scholar] [CrossRef]
- Hao, X.; Liu, Y.; Hailaiti, D.; Gong, Y.; Zhang, X.; Yue, B.; Liu, J.; Wu, X.; Yang, K.; Wang, J.; et al. Mechanisms of inflammation modulation by different immune cells in hypertensive nephropathy. Front. Immunol. 2024, 15, 1333170. [Google Scholar] [CrossRef]
- Mattson, D.L. Immune mechanisms of salt-sensitive hypertension and renal end-organ damage. Nat. Rev. Nephrol. 2019, 15, 290–300. [Google Scholar] [CrossRef]
- Fanelli, C.; Arias, S.C.A.; Machado, F.G.; Okuma, J.K.; Malheiros, D.M.A.C.; Azevedo, H.; Moreira-Filho, C.A.; Camara, N.O.S.; Fujihara, C.K.; Zatz, R. Innate And Adaptive Immunity are Progressively Activated in Parallel with Renal Injury in the 5/6 Renal Ablation Model. Sci. Rep. 2017, 7, 3192. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Brown, S.H.M.; Bello, A.; El Nahas, M. Chronic Kidney Disease in Older People: Physiology, Pathology or Both? Nephron Clin. Pract. 2010, 116, c19–c24. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E.; Plante, G.E.; Mimran, A. Arterial Stiffness, Pulse Pressure, and the Kidney. Am. J. Hypertens. 2015, 28, 561–569. [Google Scholar] [CrossRef]
- Blacher, J.; London, G.M.; Safar, M.E.; Mourad, J.-J. Influence of age and end-stage renal disease on the stiffness of carotid wall material in hypertension. J. Hypertens. 1999, 17, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.E.; Schächinger, H.; Messerli, F.H. Accelerated decline in renal perfusion with aging in essential hypertension. Hypertension 1994, 23, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Caillon, A.; Paradis, P.; Schiffrin, E.L. Role of immune cells in hypertension. Br. J. Pharmacol. 2019, 176, 1818–1828. [Google Scholar] [CrossRef]
- Neumann, K.; Tiegs, G. Immune regulation in renal inflammation. Cell Tissue Res. 2021, 385, 305–322. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Peng, Z.; Hu, H.; Cui, X.; Zhu, Z.; Qi, Y.; Chen, W.; Liu, H.; Liang, W.; et al. PIEZO1-Mediated Calcium Signaling and Podocyte Injury in Diabetic Kidney Disease. JASN 2025, 36, 1310–1326. [Google Scholar] [CrossRef]
- Ogino, S.; Yoshikawa, K.; Nagase, T.; Mikami, K.; Nagase, M. Roles of the mechanosensitive ion channel Piezo1 in the renal podocyte injury of experimental hypertensive nephropathy. Hypertens. Res. 2024, 47, 747–759. [Google Scholar] [CrossRef]
- Kliewe, F.; Siegerist, F.; Hammer, E.; Al-Hasani, J.; Amling, T.R.J.; Hollemann, J.Z.E.; Schindler, M.; Drenic, V.; Simm, S.; Amann, K.; et al. Zyxin is important for the stability and function of podocytes, especially during mechanical stretch. Commun. Biol. 2024, 7, 446. [Google Scholar] [CrossRef]
- Koehler, S.; Brinkkoetter, P.; Rinschen, M.M. Podocyte proteome analysis reveals stress responses in glomerular sclerosis. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Endlich, K.; Kliewe, F.; Endlich, N. Stressed podocytes—Mechanical forces, sensors, signaling and response. Pflug. Arch. Eur. J. Physiol. 2017, 469, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Ilatovskaya, D.V.; Behr, A.; Staruschenko, A.; Hall, G.; Palygin, O. Mechanistic Insights Into Redox Damage of the Podocyte in Hypertension. Hypertension 2025, 82, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Wang, W.; Huo, Y.; Li, L.; Chen, R.; Lin, Z.; Tao, Y.; Peng, X.; Huang, W.; Guo, C. The mechanosensitive ion channel Piezo1 contributes to podocyte cytoskeleton remodeling and development of proteinuria in lupus nephritis. Kidney Int. 2024, 106, 625–639. [Google Scholar] [CrossRef]
- Naik, A.S.; Le, D.; Aqeel, J.; Wang, S.Q.; Chowdhury, M.; Walters, L.M.; Cibrik, D.M.; Samaniego, M.; Wiggins, R.C. Podocyte stress and detachment measured in urine are related to mean arterial pressure in healthy humans. Kidney Int. 2020, 98, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Kapitsinou, P.P.; Sano, H.; Michael, M.; Kobayashi, H.; Davidoff, O.; Bian, A.; Yao, B.; Zhang, M.-Z.; Harris, R.C.; Duffy, K.J.; et al. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J. Clin. Investing. 2014, 124, 2396–2409. [Google Scholar] [CrossRef]
- Tanaka, T. A mechanistic link between renal ischemia and fibrosis. Med. Mol. Morphol. 2017, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Luo, G.; Fang, Q.; Sun, Z. Stable Expression of Hypoxia-Inducible Factor-1α in Human Renal Proximal Tubular Epithelial Cells Promotes Epithelial to Mesenchymal Transition. Transplant. Proc. 2014, 46, 130–134. [Google Scholar] [CrossRef]
- Mihout, F.; Shweke, N.; Bigé, N.; Jouanneau, C.; Dussaule, J.; Ronco, P.; Chatziantoniou, C.; Boffa, J. Asymmetric dimethylarginine (ADMA) induces chronic kidney disease through a mechanism involving collagen and TGF-β1 synthesis. J. Pathol. 2011, 223, 37–45. [Google Scholar] [CrossRef]
- Hajime, N.; Kidokoro, K.; Satoh, M.; Itano, S.; Sasaki, T.; Kashihara, N. A14340 Endothelial dysfunction exacerbates renal tubular cell injury through inflammasome activation in hypertensive model mice. J. Hypertens. 2018, 36, e76. [Google Scholar] [CrossRef]
- Koo, J.-R. Oxidative Stress in Diabetes and Hypertension. Korean J. Electrolyte Metab. 2004, 2, 26. [Google Scholar] [CrossRef]
- Matsui, T.; Yamagishi, S.; Takeuchi, M.; Ueda, S.; Fukami, K.; Okuda, S. Irbesartan inhibits advanced glycation end product (AGE)-induced proximal tubular cell injury in vitro by suppressing receptor for AGEs (RAGE) expression. Pharmacol. Res. 2010, 61, 34–39. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, M.; Zhang, M.; Li, C.; Wang, X.; Long, Y.; Wang, Y.; Hu, J.; Chen, C.; Chen, X.; et al. Forkhead Box Protein K1 Promotes Chronic Kidney Disease by Driving Glycolysis in Tubular Epithelial Cells. Adv. Sci. 2024, 11, e2405325. [Google Scholar] [CrossRef]
- Clark, A.J.; Parikh, S.M. Mitochondrial Metabolism in Acute Kidney Injury. Semin. Nephrol. 2020, 40, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Zhu, S.; Gui, Y.; Zhou, D. Metabolic Chaos in Kidney Disease: Unraveling Energy Dysregulation. J. Clin. Med. 2024, 13, 6772. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Z.; Xu, J.; Ma, H.; Jin, K.; Xu, T.; Pan, X.; Feng, X.; Zhang, W. C3aR Antagonist Alleviates C3a Induced Tubular Profibrotic Phenotype Transition via Restoring PPARα/CPT-1α Mediated Mitochondrial Fatty Acid Oxidation in Renin-Dependent Hypertension. Front. Biosci. 2023, 28, 238. [Google Scholar] [CrossRef]
- Huynh, C.; Ryu, J.; Lee, J.; Inoki, A.; Inoki, K. Nutrient-sensing mTORC1 and AMPK pathways in chronic kidney diseases. Nat. Rev. Nephrol. 2023, 19, 102–122. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Palygin, O.; Guijas, C.; Palermo, A.; Palacio-Escat, N.; Domingo-Almenara, X.; Montenegro-Burke, R.; Saez-Rodriguez, J.; Staruschenko, A.; Siuzdak, G. Metabolic rewiring of the hypertensive kidney. Sci. Signal. 2019, 12, eaax9760. [Google Scholar] [CrossRef]
- Tian, Z.; Liang, M. Renal metabolism and hypertension. Nat. Commun. 2021, 12, 963. [Google Scholar] [CrossRef]
- Chen, H.-H.; Zhang, Y.-X.; Lv, J.-L.; Liu, Y.-Y.; Guo, J.-Y.; Zhao, L.; Nan, Y.-X.; Wu, Q.-J.; Zhao, Y.-H. Role of sirtuins in metabolic disease-related renal injury. Biomed. Pharmacother. 2023, 161, 114417. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wei, Q. The metabolic pathway regulation in kidney injury and repair. Front. Physiol. 2024, 14, 1344271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z. Effect and Regulation of the NLRP3 Inflammasome During Renal Fibrosis. Front. Cell Dev. Biol. 2019, 7, 379. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, L.; Yang, Q.; Zhang, X.; Li, X. Epigenetics in kidney diseases. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; Volume 104, pp. 233–297. ISBN 978-0-12-824622-1. [Google Scholar]
- Kim, S.K.; Bae, G.S.; Bae, T.; Ku, S.-K.; Choi, B.; Kwak, M.-K. Renal microRNA-144-3p is associated with transforming growth factor-β1-induced oxidative stress and fibrosis by suppressing the NRF2 pathway in hypertensive diabetic kidney disease. Free Radic. Biol. Med. 2024, 225, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.J.; Pushpakumar, S.B.; Sen, U. Hydrogen sulfide alleviates hypertensive kidney dysfunction through an epigenetic mechanism. Am. J. Physiol. Circ. Physiol. 2017, 312, H874–H885. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Z.; Chen, Z.; Fu, C.; Huang, L.; Wang, J.; Li, J.; Barszczyk, A.; Yang, D. MicroRNA-29b suppresses TGF-β-induced epithelial-mesenchymal transition in renal interstitium of spontaneously hypertensive rats. Chin. Med. J. 2022, 135, 857–859. [Google Scholar] [CrossRef]
- Lu, C.; Chen, B.; Chen, C.; Li, H.; Wang, D.; Tan, Y.; Weng, H. CircNr1h4 regulates the pathological process of renal injury in salt-sensitive hypertensive mice by targeting miR-155-5p. J. Cell. Mol. Med. 2020, 24, 1700–1712. [Google Scholar] [CrossRef]
- Riffo-Campos, A.L.; Perez-Hernandez, J.; Ortega, A.; Martinez-Arroyo, O.; Flores-Chova, A.; Redon, J.; Cortes, R. Exosomal and Plasma Non-Coding RNA Signature Associated with Urinary Albumin Excretion in Hypertension. Int. J. Mol. Sci. 2022, 23, 823. [Google Scholar] [CrossRef]
- Chen, C.; Lu, C.; Qian, Y.; Li, H.; Tan, Y.; Cai, L.; Weng, H. Urinary miR-21 as a potential biomarker of hypertensive kidney injury and fibrosis. Sci. Rep. 2017, 7, 17737. [Google Scholar] [CrossRef] [PubMed]
- Berillo, O.; Huo, K.-G.; Fraulob-Aquino, J.C.; Richer, C.; Briet, M.; Boutouyrie, P.; Lipman, M.L.; Sinnett, D.; Paradis, P.; Schiffrin, E.L. Circulating let-7g-5p and miR-191-5p Are Independent Predictors of Chronic Kidney Disease in Hypertensive Patients. Am. J. Hypertens. 2020, 33, 505–513. [Google Scholar] [CrossRef]
- Petzuch, B.; Bénardeau, A.; Hofmeister, L.; Meyer, J.; Hartmann, E.; Pavkovic, M.; Mathar, I.; Sandner, P.; Ellinger-Ziegelbauer, H. Urinary miRNA Profiles in Chronic Kidney Injury—Benefits of Extracellular Vesicle Enrichment and miRNAs as Potential Biomarkers for Renal Fibrosis, Glomerular Injury, and Endothelial Dysfunction. Toxicol. Sci. 2022, 187, 35–50. [Google Scholar] [CrossRef]
- Yaacoub, S.; Boudaka, A.; AlKhatib, A.; Pintus, G.; Sahebkar, A.; Kobeissy, F.; Eid, A.H. The pharmaco-epigenetics of hypertension: A focus on microRNA. Mol. Cell Biochem. 2024, 479, 3255–3271. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Russo, E.; Bianca Bertolotto, M.; Zanetti, V.; Picciotto, D.; Cappadona, F.; Esposito, P.; Carbone, F.; Montecucco, F.; Viazzi, F. FC027: Uric Acid Stimulates Cytoskeleton Pathways in Vascular Smooth Muscle Cells Through F-ACTIN Polymerization and Atrogin, Asma and SM22 up Regulation. Nephrol. Dial. Transplant. 2022, 37, gfac100.003. [Google Scholar] [CrossRef]
- Tang, L.; Dai, F.; Liu, Y.; Yu, X.; Huang, C.; Wang, Y.; Yao, W. RhoA/ROCK signaling regulates smooth muscle phenotypic modulation and vascular remodeling via the JNK pathway and vimentin cytoskeleton. Pharmacol. Res. 2018, 133, 201–212. [Google Scholar] [CrossRef]
- Gu, Z.; Kordowska, J.; Williams, G.L.; Wang, C.-L.A.; Hai, C.-M. Erk1/2 MAPK and caldesmon differentially regulate podosome dynamics in A7r5 vascular smooth muscle cells. Exp. Cell Res. 2007, 313, 849–866. [Google Scholar] [CrossRef]
- Di-Luoffo, M.; Ben-Meriem, Z.; Lefebvre, P.; Delarue, M.; Guillermet-Guibert, J. PI3K functions as a hub in mechanotransduction. Trends Biochem. Sci. 2021, 46, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Sawma, T.; Shaito, A.; Najm, N.; Sidani, M.; Orekhov, A.; El-Yazbi, A.F.; Iratni, R.; Eid, A.H. Role of RhoA and Rho-associated kinase in phenotypic switching of vascular smooth muscle cells: Implications for vascular function. Atherosclerosis 2022, 358, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, C.; Qin, C.; Xie, L.; Wang, X.; Gao, Z.; Qiangbacuozhen; Wang, T.; Yu, L.; Liu, H. Role of the PI3K/AKT Pathway in Modulating Cytoskeleton Rearrangements and Phenotype Switching in Rat Pulmonary Arterial Vascular Smooth Muscle Cells. DNA Cell Biol. 2014, 33, 12–19. [Google Scholar] [CrossRef]
- Fan, F.; Geurts, A.M.; Pabbidi, M.R.; Ge, Y.; Zhang, C.; Wang, S.; Liu, Y.; Gao, W.; Guo, Y.; Li, L.; et al. A Mutation in γ-Adducin Impairs Autoregulation of Renal Blood Flow and Promotes the Development of Kidney Disease. JASN 2020, 31, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.J.C.; Nesmith, A.P.; Parker, K.K. The Role of Mechanotransduction on Vascular Smooth Muscle Myocytes Cytoskeleton and Contractile Function. Anat. Rec. 2014, 297, 1758–1769. [Google Scholar] [CrossRef]
- St. Paul, A.; Corbett, C.; Peluzzo, A.; Kelemen, S.; Okune, R.; Haines, D.S.; Preston, K.; Eguchi, S.; Autieri, M.V. FXR1 regulates vascular smooth muscle cell cytoskeleton, VSMC contractility, and blood pressure by multiple mechanisms. Cell Rep. 2023, 42, 112381. [Google Scholar] [CrossRef]
- Hödebeck, M.; Scherer, C.; Wagner, A.H.; Hecker, M.; Korff, T. TonEBP/NFAT5 regulates ACTBL2 expression in biomechanically activated vascular smooth muscle cells. Front. Physiol. 2014, 5, 467. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Arzaghi, H.; Ma, Z.; Roye, Y.; Musah, S. Epigenetics of Hypertensive Nephropathy. Biomedicines 2024, 12, 2622. [Google Scholar] [CrossRef]
- Cortvrindt, C.; Speeckaert, R.; Moerman, A.; Delanghe, J.R.; Speeckaert, M.M. The role of interleukin-17A in the pathogenesis of kidney diseases. Pathology 2017, 49, 247–258. [Google Scholar] [CrossRef]
- Comeau, K.; Caillon, A.; Paradis, P.; Schiffrin, E. Determination of Interleukin-17A and Interferon-γ Production in γδ, CD4+, and CD8+ T Cells Isolated from Murine Lymphoid Organs, Perivascular Adipose Tissue, Kidney, and Lung. BIO Protoc. 2023, 13, e4679. [Google Scholar] [CrossRef]
- Shao, J.; Nangaku, M.; Miyata, T.; Inagi, R.; Yamada, K.; Kurokawa, K.; Fujita, T. Imbalance of T-Cell Subsets in Angiotensin II–Infused Hypertensive Rats with Kidney Injury. Hypertension 2003, 42, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, Y.; Hu, X.; Li, H.; Li, X.; Xiao, C.; Meng, T.; Peng, L.; Gan, L.; Zhou, Q.; et al. Interleukin-22 exacerbates angiotensin II-induced hypertensive renal injury. Int. Immunopharmacol. 2022, 109, 108840. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.D.; Frey, C.W.; Gould, S.K.; Griffiths, R.; Ruiz, P.; Burchette, J.L.; Howell, D.N.; Makhanova, N.; Yan, M.; Kim, H.-S.; et al. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am. J. Physiol. Physiol. 2008, 295, F515–F524. [Google Scholar] [CrossRef] [PubMed]
- Vilaysane, A.; Chun, J.; Seamone, M.E.; Wang, W.; Chin, R.; Hirota, S.; Li, Y.; Clark, S.A.; Tschopp, J.; Trpkov, K.; et al. The NLRP3 Inflammasome Promotes Renal Inflammation and Contributes to CKD. J. Am. Soc. Nephrol. 2010, 21, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Chung, H.; Komada, T.; Platnich, J.M.; Sandall, C.F.; Choudhury, S.R.; Chun, J.; Naumenko, V.; Surewaard, B.G.J.; Nelson, M.C.; et al. Renal immune surveillance and dipeptidase-1 contribute to contrast-induced acute kidney injury. J. Clin. Investig. 2018, 128, 2894–2913. [Google Scholar] [CrossRef]
- Komada, T.; Usui, F.; Kawashima, A.; Kimura, H.; Karasawa, T.; Inoue, Y.; Kobayashi, M.; Mizushina, Y.; Kasahara, T.; Taniguchi, S.; et al. Role of NLRP3 Inflammasomes for Rhabdomyolysis-induced Acute Kidney Injury. Sci. Rep. 2015, 5, 10901. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, Y.; Chen, C.; Wang, C. Anisodamine inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in rhabdomyolysis-induced acute kidney injury. Apoptosis 2017, 22, 1524–1531. [Google Scholar] [CrossRef]
- Huang, T.; Yin, H.; Ning, W.; Wang, X.; Chen, C.; Lin, W.; Li, J.; Zhou, Y.; Peng, Y.; Wang, M.; et al. Expression of inflammasomes NLRP1, NLRP3 and AIM2 in different pathologic classification of lupus nephritis. Clin. Exp. Rheumatol. 2020, 38, 680–690. [Google Scholar]
- Zhang, C.; Zhu, X.; Li, L.; Ma, T.; Shi, M.; Yang, Y.; Fan, Q. A small molecule inhibitor MCC950 ameliorates kidney injury in diabetic nephropathy by inhibiting NLRP3 inflammasome activation. DMSO 2019, 12, 1297–1309. [Google Scholar] [CrossRef]
- Krishnan, S.M.; Dowling, J.K.; Ling, Y.H.; Diep, H.; Chan, C.T.; Ferens, D.; Kett, M.M.; Pinar, A.; Samuel, C.S.; Vinh, A.; et al. Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br. J. Pharmacol. 2016, 173, 752–765. [Google Scholar] [CrossRef]
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 493–503. [Google Scholar] [CrossRef]
- Elizagaray, M.L.; Gomes, M.T.R.; Guimaraes, E.S.; Rumbo, M.; Hozbor, D.F.; Oliveira, S.C.; Moreno, G. Canonical and Non-canonical Inflammasome Activation by Outer Membrane Vesicles Derived From Bordetella pertussis. Front. Immunol. 2020, 11, 1879. [Google Scholar] [CrossRef]
- Krishnan, S.M.; Ling, Y.H.; Huuskes, B.M.; Ferens, D.M.; Saini, N.; Chan, C.T.; Diep, H.; Kett, M.M.; Samuel, C.S.; Kemp-Harper, B.K.; et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc. Res. 2019, 115, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, Q.; Shao, X.; Mou, S.; Gu, L.; Wang, L.; Zhang, Z.; Shen, J.; Zhou, Y.; Qi, C.; et al. NLRP3 inflammasome inhibition attenuates cisplatin-induced renal fibrosis by decreasing oxidative stress and inflammation. Exp. Cell Res. 2019, 383, 111488. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, L. The Selective NLRP3-Inflammasome Inhibitor CY-09 AmelioratesKidney Injury in Diabetic Nephropathy by Inhibiting NLRP3-inflammasome Activation. CMC 2023, 30, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Hutton, H.L.; Ooi, J.D.; Holdsworth, S.R.; Kitching, A.R. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology 2016, 21, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Hua, H.; Jia, Z.; Zhang, A.; Ding, G. Therapy Targeted to the NLRP3 Inflammasome in Chronic Kidney Disease. Kidney Dis. 2024, 10, 369–383. [Google Scholar] [CrossRef]
- Grassi, G.; Calhoun, D.A. Sympathetic–vascular interactions: Further evidence in kidney transplantation. J. Hypertens. 2002, 20, 379–381. [Google Scholar] [CrossRef]
- Linz, D.; Hohl, M.; Schutze, J.; Mahfoud, F.; Speer, T.; Linz, B.; Hubschle, T.; Juretschke, H.-P.; Dechend, R.; Geisel, J.; et al. Progression of Kidney Injury and Cardiac Remodeling in Obese Spontaneously Hypertensive Rats: The Role of Renal Sympathetic Innervation. Am. J. Hypertens. 2015, 28, 256–265. [Google Scholar] [CrossRef]
- Langston, J.B.; Guyton, A.C.; Gillespie, W.J. Acute effect of changes in renal arterial pressure and sympathetic blockade on kidney function. Am. J. Physiol. 1959, 197, 595–600. [Google Scholar] [CrossRef]
- Persson, P.B.; Ehmke, H.; Nafz, B.; Kirchheim, H.R. Sympathetic modulation of renal autoregulation by carotid occlusion in conscious dogs. Am. J. Physiol. 1990, 258, F364–F370. [Google Scholar] [CrossRef]
- Iliescu, R.; Irwin, E.D.; Georgakopoulos, D.; Lohmeier, T.E. Renal responses to chronic suppression of central sympathetic outflow. Hypertension 2012, 60, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.; Pabbidi, M.; Fan, F.; Ge, Y.; Liu, R.; Williams, J.M.; Sarkis, A.; Lazar, J.; Jacob, H.J.; Roman, R.J. Genetic basis of the impaired renal myogenic response in FHH rats. Am. J. Physiol. Physiol. 2013, 304, F565–F577. [Google Scholar] [CrossRef]
- Zhang, C.; He, X.; Murphy, S.R.; Zhang, H.; Wang, S.; Ge, Y.; Gao, W.; Williams, J.M.; Geurts, A.M.; Roman, R.J.; et al. Knockout of Dual-Specificity Protein Phosphatase 5 Protects Against Hypertension-Induced Renal Injury. J. Pharmacol. Exp. Ther. 2019, 370, 206–217. [Google Scholar] [CrossRef]
- Jia, Z.; Johnson, A.C.; Wang, X.; Guo, Z.; Dreisbach, A.W.; Lewin, J.R.; Kyle, P.B.; Garrett, M.R. Allelic Variants in Arhgef11 via the Rho-Rock Pathway Are Linked to Epithelial–Mesenchymal Transition and Contributes to Kidney Injury in the Dahl Salt-Sensitive Rat. PLoS ONE 2015, 10, e0132553. [Google Scholar] [CrossRef]
- Williams, J.M.; Johnson, A.C.; Stelloh, C.; Dreisbach, A.W.; Franceschini, N.; Regner, K.R.; Townsend, R.R.; Roman, R.J.; Garrett, M.R. Genetic Variants in Arhgef11 Are Associated with Kidney Injury in the Dahl Salt-Sensitive Rat. Hypertension 2012, 60, 1157–1168. [Google Scholar] [CrossRef]
- Parsa, A.; Kao, W.H.L.; Xie, D.; Astor, B.C.; Li, M.; Hsu, C.; Feldman, H.I.; Parekh, R.S.; Kusek, J.W.; Greene, T.H.; et al. APOL1 Risk Variants, Race, and Progression of Chronic Kidney Disease. N. Engl. J. Med. 2013, 369, 2183–2196. [Google Scholar] [CrossRef]
- Guo, J.; Guo, X.; Sun, Y.; Li, Z.; Jia, P. Application of omics in hypertension and resistant hypertension. Hypertens. Res. 2022, 45, 775–788. [Google Scholar] [CrossRef]
- El-Achkar, T.M.; Eadon, M.T.; Kretzler, M.; Himmelfarb, J.; Lake, B.; Zhang, K.; Lecker, S.; Morales, A.; Bogen, S.; Amodu, A.A.; et al. Precision Medicine in Nephrology: An Integrative Framework of Multidimensional Data in the Kidney Precision Medicine Project. Am. J. Kidney Dis. 2024, 83, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Palygin, O.; Miller, B.S.; Nishijima, Y.; Zhang, D.X.; Staruschenko, A.; Sorokin, A. Endothelin receptor A and p66Shc regulate spontaneous Ca2+ oscillations in smooth muscle cells controlling renal arterial spontaneous motion. FASEB J. 2019, 33, 2636–2645. [Google Scholar] [CrossRef] [PubMed]
- Arany, I.; Clark, J.S.; Reed, D.K.; Ember, I.; Juncos, L.A. Cisplatin enhances interaction between p66Shc and HSP27: Its role in reorganization of the actin cytoskeleton in renal proximal tubule cells. Anticancer. Res. 2012, 32, 4759–4763. [Google Scholar]
- Wu, I.-W.; Chang, L.-C.; Wu, Y.-L.; Yang, H.-Y.; Twu, Y.-C.; Tsai, P.-Y.; Paulus, S.; Resnick, R.; Chung, W.-H.; Yang, C.-W.; et al. Gut flora metagenomic analysis coupled with metabolic and deep immune profiling in chronic kidney disease. Nephrol. Dial. Transplant. 2024, 39, 1333–1343. [Google Scholar] [CrossRef]
- Jiang, X.; Mahfoud, F.; Li, W.; Dong, H.; Yu, J.; Yu, S.; Chen, X.; Wang, P.; Li, Z.; Lauder, L.; et al. Efficacy and Safety of Catheter-Based Radiofrequency Renal Denervation in Chinese Patients with Uncontrolled Hypertension: The Randomized, Sham-Controlled, Multi-Center Iberis-HTN Trial. Circulation 2024, 150, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Duffin, K.L.; Laska, D.A.; Voelker, J.R.; Breyer, M.D.; Mitchell, P.G. A prospective study of multiple protein biomarkers to predict progression in diabetic chronic kidney disease. Nephrol. Dial. Transplant. 2014, 29, 2293–2302. [Google Scholar] [CrossRef]
- Liu, T.; Li, R.; Wang, X.; Gao, X.; Zhang, X. Benefits of SGLT2 inhibitors combining with renin–angiotensin-system blockers on cardiovascular outcomes in chronic kidney disease patients: A systemic review and meta-analysis. Med. Clín. 2022, 159, 65–72. [Google Scholar] [CrossRef]
- Zhang, C.; Fang, X.; Zhang, H.; Gao, W.; Hsu, H.J.; Roman, R.J.; Fan, F. Genetic susceptibility of hypertension-induced kidney disease. Physiol. Rep. 2021, 9, e14688. [Google Scholar] [CrossRef]
- Liu, J. Gly389Arg polymorphism of β1-adrenergic receptor is associated with the cardiovascular response to metoprolol. Clin. Pharmacol. Ther. 2003, 74, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiao, T.; Chen, L.; Xie, S.; Deng, M.; Wu, D. The Association of ADRB1 and CYP2D6 Polymorphisms with Antihypertensive Effects and Analysis of Their Contribution to Hypertension Risk. Am. J. Med. Sci. 2018, 355, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Zineh, I.; Beitelshees, A.; Gaedigk, A.; Walker, J.; Pauly, D.; Eberst, K.; Leeder, J.; Phillips, M.; Gelfand, C.; Johnson, J. Pharmacokinetics and genotypes do not predict metoprolol adverse events or efficacy in hypertension. Clin. Pharmacol. Ther. 2004, 76, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, B.; Delanghe, J.; Buyzere, M.D. Angiotensin I-Converting Enzyme Insertion/Deletion Polymorphism: Clinical Implications. Acta Clin. Belg. 1997, 52, 338–349. [Google Scholar] [CrossRef]
- Eales, J.M.; Jiang, X.; Xu, X.; Saluja, S.; Akbarov, A.; Cano-Gamez, E.; McNulty, M.T.; Finan, C.; Guo, H.; Wystrychowski, W.; et al. Uncovering genetic mechanisms of hypertension through multi-omic analysis of the kidney. Nat. Genet. 2021, 53, 630–637. [Google Scholar] [CrossRef]
- Kelley, E.F.; Snyder, E.M.; Alkhatib, N.S.; Snyder, S.C.; Sprissler, R.; Olson, T.P.; Akre, M.K.; Abraham, I. Economic evaluation of a pharmacogenomic multi-gene panel test to optimize anti-hypertension therapy: Simulation study. J. Med. Econ. 2018, 21, 1246–1253. [Google Scholar] [CrossRef]
- Raizada, M.K.; Sarkissian, S.D. Potential of Gene Therapy Strategy for the Treatment of Hypertension. Hypertension 2006, 47, 6–9. [Google Scholar] [CrossRef]
- Champion, H.C.; Bivalacqua, T.J.; Greenberg, S.S.; Giles, T.D.; Hyman, A.L.; Kadowitz, P.J. Adenoviral gene transfer of endothelial nitric-oxide synthase (eNOS) partially restores normal pulmonary arterial pressure in eNOS-deficient mice. Proc. Natl. Acad. Sci. USA 2002, 99, 13248–13253. [Google Scholar] [CrossRef]
- Zhong, J.; Basu, R.; Guo, D.; Chow, F.L.; Byrns, S.; Schuster, M.; Loibner, H.; Wang, X.; Penninger, J.M.; Kassiri, Z.; et al. Angiotensin-Converting Enzyme 2 Suppresses Pathological Hypertrophy, Myocardial Fibrosis, and Cardiac Dysfunction. Circulation 2010, 122, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Bian, Y.; Li, M.; Gao, F.; Xiao, C. Effects of RNA interference targeting angiotensin 1 receptor and angiotensin-converting enzyme on blood pressure and myocardial remodeling in spontaneous hypertensive rats. Zhonghua Xin Xue Guan Bing Za Zhi 2010, 38, 60–66. [Google Scholar]
- Paulis, L.; Franke, H.; Simko, F. Gene therapy for hypertension. Expert Opin. Biol. Ther. 2017, 17, 1345–1361. [Google Scholar] [CrossRef] [PubMed]
- Katsurada, K.; Shinohara, K.; Aoki, J.; Nanto, S.; Kario, K. Renal denervation: Basic and clinical evidence. Hypertens. Res. 2022, 45, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Sievert, H.; Schofer, J.; Ormiston, J.A.; Hoppe, U.C.; Meredith, I.T.; Walters, D.; Azizi, M.; Diaz-Cartelle, J. TCT-764 Bipolar Radiofrequency Renal Denervation with the Vessix Catheter in Patients with Resistant Hypertension: 2-year Results from the REDUCE-HTN Trial. J. Am. Coll. Cardiol. 2015, 66, B311. [Google Scholar] [CrossRef][Green Version]
- Sharp, A.S.P.; Cao, K.N.; Esler, M.D.; Kandzari, D.E.; Lobo, M.D.; Schmieder, R.E.; Pietzsch, J.B. Cost-effectiveness of catheter-based radiofrequency renal denervation for the treatment of uncontrolled hypertension: An analysis for the UK based on recent clinical evidence. Eur. Heart J. Qual. Care Clin. Outcomes 2024, 10, 698–708. [Google Scholar] [CrossRef]
- Nishi, E.E.; Lopes, N.R.; Gomes, G.N.; Perry, J.C.; Sato, A.Y.S.; Naffah-Mazzacoratti, M.G.; Bergamaschi, C.T.; Campos, R.R. Renal denervation reduces sympathetic overactivation, brain oxidative stress, and renal injury in rats with renovascular hypertension independent of its effects on reducing blood pressure. Hypertens. Res. 2019, 42, 628–640. [Google Scholar] [CrossRef]
- Yao, Y.; Fomison-Nurse, I.C.; Harrison, J.C.; Walker, R.J.; Davis, G.; Sammut, I.A. Chronic bilateral renal denervation attenuates renal injury in a transgenic rat model of diabetic nephropathy. Am. J. Physiol. Physiol. 2014, 307, F251–F262. [Google Scholar] [CrossRef]
- Zou, X.; Lin, S.; Zhong, L.; Liu, J.; Meng, Y.; Zhu, Y.; Sun, J. Renal denervation alleviates renal ischemic reperfusion injury-induced acute and chronic kidney injury in rats partly by modulating miRNAs. Clin. Exp. Nephrol. 2022, 26, 13–21. [Google Scholar] [CrossRef]
- Schuetze, J.; Linz, D.; Hohl, M.; Mahfoud, F.; Ewen, S.; Urban, D.; Juretschke, H.P.; Neumann-Haeflin, C.; Linz, W.; Boehm, M. Renal denervation attenuates impairment of renal function and kidney injury in obese spontaneously hypertensive rats. Eur. Heart J. 2013, 34, P2367. [Google Scholar] [CrossRef]
- Camafort, M.; Ihm, S.H.; Ruilope, L.M. Renal denervation for the treatment of hypertension and kidney disease. Curr. Opin. Nephrol. Hypertens. 2023, 32, 544–550. [Google Scholar] [CrossRef]
- Götzinger, F.; Kunz, M.; Lauder, L.; Mahfoud, F.; Böhm, M. Radio Frequency-Based Renal Denervation: A Story of Simplicity? Future Cardiol. 2023, 19, 431–440. [Google Scholar] [CrossRef]
- Turner, J.-E.; Paust, H.-J.; Steinmetz, O.M.; Panzer, U. The Th17 immune response in renal inflammation. Kidney Int. 2010, 77, 1070–1075. [Google Scholar] [CrossRef]
- Nishihara, M.; Ogura, H.; Ueda, N.; Tsuruoka, M.; Kitabayashi, C.; Tsuji, F.; Aono, H.; Ishihara, K.; Huseby, E.; Betz, U.A.K.; et al. IL-6–gp130–STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state. Int. Immunol. 2007, 19, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-J.; Lee, S.H.; Lee, S.-H.; Lee, E.-J.; Kim, E.-K.; Choi, J.Y.; Cho, M.-L. IL-1 Receptor Blockade Alleviates Graft-versus-Host Disease through Downregulation of an Interleukin-1β-Dependent Glycolytic Pathway in Th17 Cells. Mediat. Inflamm. 2015, 2015, 631384. [Google Scholar] [CrossRef]
- Chaudhari, S.; Pham, G.S.; Brooks, C.D.; Dinh, V.Q.; Young-Stubbs, C.M.; Shimoura, C.G.; Mathis, K.W. Should Renal Inflammation Be Targeted While Treating Hypertension? Front. Physiol. 2022, 13, 886779. [Google Scholar] [CrossRef] [PubMed]
- Deussen, A.; Kopaliani, I. Targeting inflammation in hypertension. Curr. Opin. Nephrol. Hypertens. 2023, 32, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Basso, P.J.; Andrade-Oliveira, V.; Câmara, N.O.S. Targeting immune cell metabolism in kidney diseases. Nat. Rev. Nephrol. 2021, 17, 465–480. [Google Scholar] [CrossRef]
- Okusa, M.D.; Rosin, D.L.; Tracey, K.J. Targeting neural reflex circuits in immunity to treat kidney disease. Nat. Rev. Nephrol. 2017, 13, 669–680. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Liang, X.; Li, Y.; Zhang, Y.; Zhang, C.; Wei, H.; Luo, R.; Ge, S.; Xu, G. Complement C3 Produced by Macrophages Promotes Renal Fibrosis via IL-17A Secretion. Front. Immunol. 2018, 9, 2385. [Google Scholar] [CrossRef]
- Kumar, V.; Kurth, T.; Cowley, A.W. Autophagy contributes to the development of salt-induced hypertension and kidney injury in Dahl S rats. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Jung, H.J.; An, H.-J.; Gwon, M.-G.; Gu, H.; Bae, S.; Lee, S.-J.; Kim, Y.-A.; Leem, J.; Park, K.-K. Anti-Fibrotic Effect of Synthetic Noncoding Oligodeoxynucleotide for Inhibiting mTOR and STAT3 via the Regulation of Autophagy in an Animal Model of Renal Injury. Molecules 2022, 27, 766. [Google Scholar] [CrossRef]
- Kim, Y.-A.; Gu, H.; Gwon, M.-G.; An, H.-J.; Bae, S.; Leem, J.; Jung, H.J.; Park, K.-K.; Lee, S.-J. Synthetic Non-Coding RNA for Suppressing mTOR Translation to Prevent Renal Fibrosis Related to Autophagy in UUO Mouse Model. Int. J. Mol. Sci. 2022, 23, 11365. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, L.; Chen, R.; Lu, H.; Sui, M.; Zhu, Y.; Zeng, L. SIRT3 Protects Against Acute Kidney Injury via AMPK/mTOR-Regulated Autophagy. Front. Physiol. 2018, 9, 1526. [Google Scholar] [CrossRef]
- Sheng, H.; Zhang, D.; Zhang, J.; Zhang, Y.; Lu, Z.; Mao, W.; Liu, X.; Zhang, L. Kaempferol attenuated diabetic nephropathy by reducing apoptosis and promoting autophagy through AMPK/mTOR pathways. Front. Med. 2022, 9, 986825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Howell, G.M.; Guo, L.; Collage, R.D.; Loughran, P.A.; Zuckerbraun, B.S.; Rosengart, M.R. CaMKIV-Dependent Preservation of mTOR Expression Is Required for Autophagy during Lipopolysaccharide-Induced Inflammation and Acute Kidney Injury. J. Immunol. 2014, 193, 2405–2415. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Rosenkranz, K.A.T.; Kusunoki, Y.; Li, C.; Klaus, M.; Gross, O.; Angelotti, M.-L.; Antonelli, G.; Cirillo, L.; Romagnani, P.; et al. Finerenone Added to RAS/SGLT2 Blockade for CKD in Alport Syndrome. Results of a Randomized Controlled Trial with Col4a3 −/− Mice. JASN 2023, 34, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Morino, J.; Hirai, K.; Kaneko, S.; Minato, S.; Yanai, K.; Mutsuyoshi, Y.; Ishii, H.; Matsuyama, M.; Kitano, T.; Shindo, M.; et al. Two cases of advanced stage rapidly progressive diabetic nephropathy effectively treated with combination therapy including RAS blocker, GLP-1 receptor agonist and SGLT-2 inhibitor. CEN Case Rep. 2019, 8, 128–133. [Google Scholar] [CrossRef]
- Møller, A.L.; Thöni, S.; Keller, F.; Sharifli, S.; Rasmussen, D.G.K.; Genovese, F.; Karsdal, M.A.; Mayer, G. Combination Therapy of RAS Inhibition and SGLT2 Inhibitors Decreases Levels of Endotrophin in Persons with Type 2 Diabetes. Biomedicines 2023, 11, 3084. [Google Scholar] [CrossRef]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2022, 43, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, B.; Ma, Z.; Huang, M.; Gao, Z.; Ni, J. Peptide 17 alleviates early hypertensive renal injury by regulating the Hippo/ YAP signalling pathway. Nephrology 2022, 27, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Ding, Y.-Y.; Zhang, T.; Cai, Q.; Hu, Y.; Gu, Q.; Gu, Z. Soybean-derived antihypertensive hydrolysates attenuate Ang II-induced renal damage by modulating MAPK and NF-κB signaling pathways. Food Funct. 2024, 15, 2485–2496. [Google Scholar] [CrossRef]
- Lin, L.; Ren, J.; Wang, C.; Mei, M.; Zheng, L.; Yang, J. A set of urinary peptides can predict early renal damage in primary hypertension. J. Hypertens. 2023, 41, 1653–1660. [Google Scholar] [CrossRef]
- Bernieh, D.; Lane, D.; Rufus, P.; Osman, H.; Salim, F.; Alghamdi, R.; Jenkins, S.; Gill, H.; Ng, L.; Jones, D.; et al. Development of a Targeted Proteomics Assay for Quantification of Angiotensin Peptides in the Renin Angiotensin Aldosterone System. J. Hypertens. 2022, 40, e284. [Google Scholar] [CrossRef]
- Degraeuwe, E.; Gasthuys, E.; Snauwaert, E.; Dossche, L.; Prytula, A.; Dehoorne, J.; Vermeulen, A.; Walle, J.V.; Raes, A. Real-world evidence of lisinopril in pediatric hypertension and nephroprotective management: A 10-year cohort study. Pediatr. Nephrol. 2025, 40, 797–809. [Google Scholar] [CrossRef]
- Van Den Belt, S.M.; Heerspink, H.J.L.; Kirchner, M.; Gracchi, V.; Thurn-Valsassina, D.; Bayazit, A.K.; Niemirska, A.; Canpolat, N.; Kaplan Bulut, I.; Azukaitis, K.; et al. Discontinuation of RAAS Inhibition in Children with Advanced CKD. CJASN 2020, 15, 625–632. [Google Scholar] [CrossRef]
- Serdaroglu, E.; Mir, S.; Berdeli, A. Hypertension and ace gene insertion/deletion polymorphism in pediatric renal transplant patients. Pediatr. Transplant. 2005, 9, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Keidar, S.; Strizevsky, A.; Raz, A.; Gamliel-Lazarovich, A. ACE2 activity is increased in monocyte-derived macrophages from prehypertensive subjects. Nephrol. Dial. Transplant. 2006, 22, 597–601. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Pathway | Mechanism | Key Mediators | Ref |
|---|---|---|---|
| Renin–angiotensin system | Vasoconstriction, fibrosis, inflammation | Ang II, AT1R, aldosterone | [17] |
| Oxidative stress | ROS-mediated endothelial and tubular damage | NOX enzymes, Nrf2, SOD | [18] |
| Immune activation | T-cell infiltration and cytokine-driven damage | IL-17, TNF-α, NF-κB | [19] |
| Mechanical stress | Cytoskeletal disruption in podocytes | TRPC6, Piezo1, actin regulators | [20] |
| Hypoxia | Capillary rarefaction and HIF pathway activation | HIF-1α, VEGF, ADMA | [18] |
| Metabolic dysregulation | ATP depletion, substrate shift, energy stress | mTOR, AMPK | [18] |
| Epigenetics | Altered gene expression via miRNA and methylation | miR-144-3p, miR-129, DNA methyltransferases | [21] |
| Strategy | Target | Example Therapy or Tool | Ref |
|---|---|---|---|
| Genetically guided therapy | Gene variants influencing drug response | LSD1 polymorphism → MR antagonist use | [173] |
| Device-based therapy | Sympathetic nerve activity | Renal denervation | [174] |
| Biomarker-guided treatment | Inflammation, fibrosis, oxidative stress | IL-6, TGF-β, NGAL, KIM-1 | [175] |
| Combination therapy | Multi-pathway blockade | RAS + SGLT2 + finerenone | [176] |
| RNA-targeted therapy | Disease-driving miRNA/mRNA | siRNA, antisense oligos | [20] |
| Systems biology | Patient stratification by omics | Multi-omics platforms, Nephroseq, scRNA-Seq | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delrue, C.; Speeckaert, M.M. Beyond Blood Pressure: Emerging Pathways and Precision Approaches in Hypertension-Induced Kidney Damage. Int. J. Mol. Sci. 2025, 26, 7606. https://doi.org/10.3390/ijms26157606
Delrue C, Speeckaert MM. Beyond Blood Pressure: Emerging Pathways and Precision Approaches in Hypertension-Induced Kidney Damage. International Journal of Molecular Sciences. 2025; 26(15):7606. https://doi.org/10.3390/ijms26157606
Chicago/Turabian StyleDelrue, Charlotte, and Marijn M. Speeckaert. 2025. "Beyond Blood Pressure: Emerging Pathways and Precision Approaches in Hypertension-Induced Kidney Damage" International Journal of Molecular Sciences 26, no. 15: 7606. https://doi.org/10.3390/ijms26157606
APA StyleDelrue, C., & Speeckaert, M. M. (2025). Beyond Blood Pressure: Emerging Pathways and Precision Approaches in Hypertension-Induced Kidney Damage. International Journal of Molecular Sciences, 26(15), 7606. https://doi.org/10.3390/ijms26157606







