Hypertension: A Continuing Public Healthcare Issue
Abstract
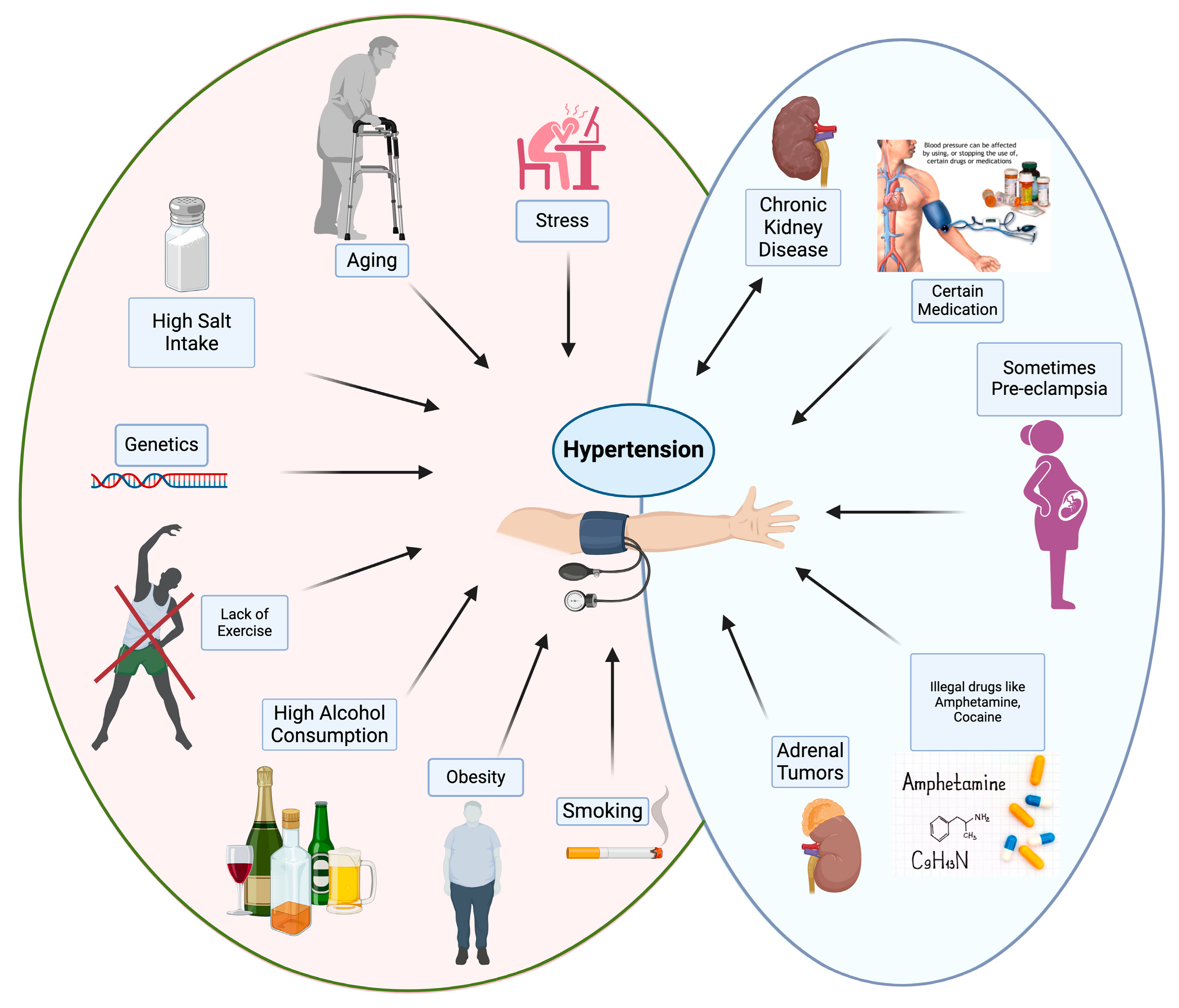
1. Introduction
2. Epidemiology of Hypertension
3. Disease Burden
4. Cardiovascular Disease Risk
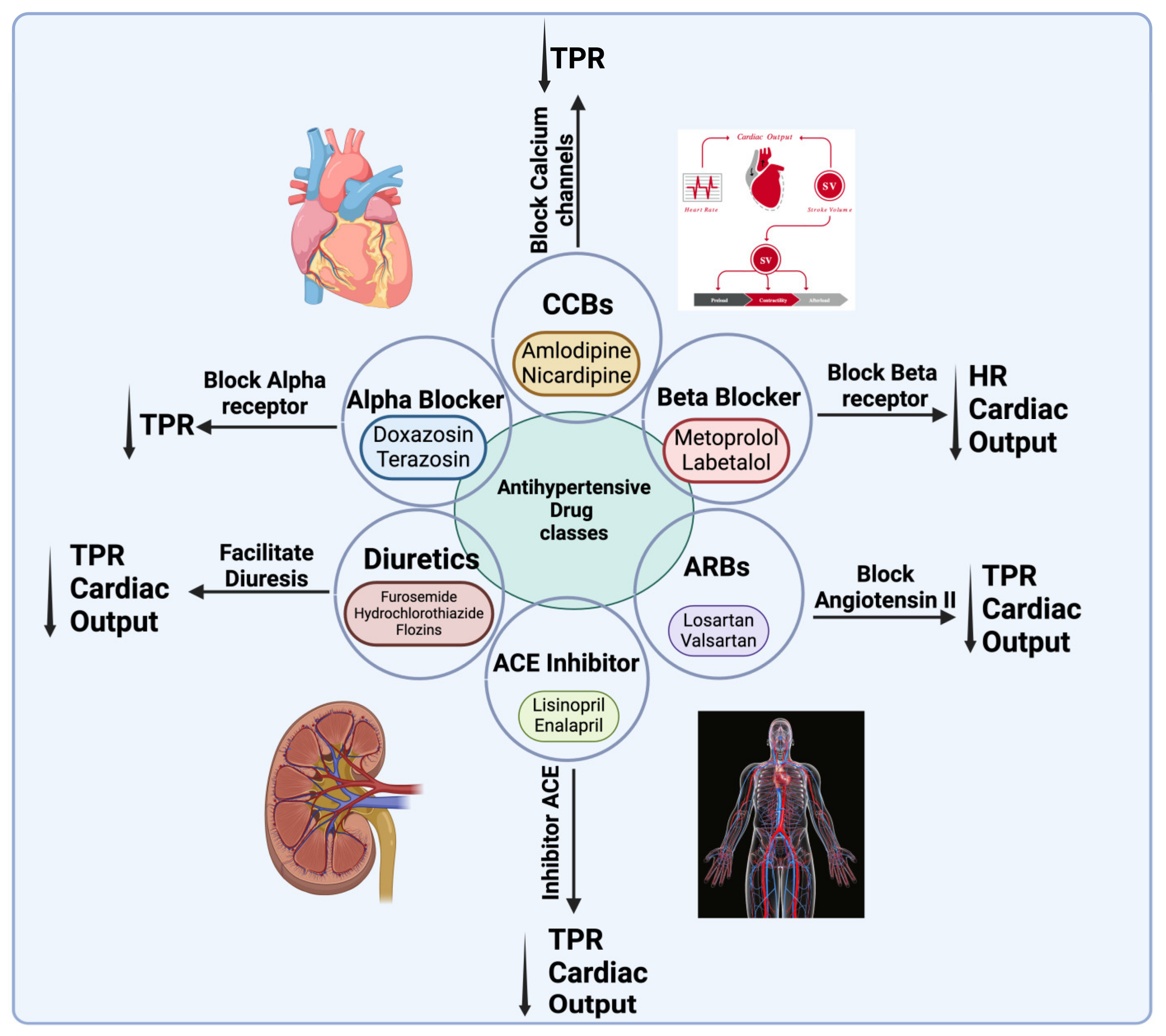
5. Pathophysiology and Treatment
6. Hypertension in Children and Adolescents
7. Hypertension in Middle Age and Old Age
8. Hypertension in Economic and Social Situations
9. Body Mass Index and Hypertension
10. Lifestyle Factors and Hypertension
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Francula-Zaninovic, S.; Nola, I.A. Management of measurable variable cardiovascular disease’risk factors. Curr. Cardiol. Rev. 2018, 14, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Kuneinen, S.M.; Kautiainen, H.; Ekblad, M.O.; Korhonen, P.E. Multifactorial prevention program for cardiovascular disease in primary care: Hypertension status and effect on mortality. J. Hum. Hypertens. 2024, 38, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef]
- Saiz, L.C.; Gorricho, J.; Garjón, J.; Celaya, M.C.; Erviti, J.; Leache, L. Blood pressure targets for the treatment of people with hypertension and cardiovascular disease. Cochrane Database Syst. Rev. 2022, 2022, CD010315. [Google Scholar] [CrossRef]
- Kario, K.; Okura, A.; Hoshide, S.; Mogi, M. The WHO Global report 2023 on hypertension warning the emerging hypertension burden in globe and its treatment strategy. Hypertens. Res. 2024, 47, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Ekesbo, R.; Midlöv, P.; Gerward, S.; Persson, K.; Nerbrand, C.; Johansson, L. Lack of adherence to hypertension treatment guidelines among GPs in southern Sweden—A case report-based survey. BMC Fam. Pr. 2012, 13, 34. [Google Scholar] [CrossRef]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A. Hypertension. Nat. Rev. Dis. Primers 2018, 4, 18014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carey, R.M.; Muntner, P.; Bosworth, H.B.; Whelton, P.K. Prevention and Control of Hypertension: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1278–1293. [Google Scholar] [CrossRef]
- Schutte, A.E.; Jafar, T.H.; Poulter, N.R.; Damasceno, A.; Khan, N.A.; Nilsson, P.M.; Alsaid, J.; Neupane, D.; Kario, K.; Beheiry, H.; et al. Addressing global disparities in blood pressure control: Perspectives of the International Society of Hypertension. Cardiovasc. Res. 2023, 119, 381–409. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Miller, M.A. Cardiovascular disease and hypertension in sub-Saharan Africa: Burden, risk and interventions. Int. Emerg. Med. 2016, 11, 299–305. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Zha, M.; Yang, Q.; Zhang, Y.; Hou, L.; Ye, X.; Chen, G.; Shao, J.; Xia, W.; Song, P. Trends in the prevalence of hypertension according to severity and phenotype in Chinese adults over two decades (1991–2015). J. Clin. Hypertens. 2021, 23, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L. Epidemiology of Heart Failure: A Contemporary Perspective. Circ. Res. 2021, 128, 1421–1434. [Google Scholar] [CrossRef]
- Kaplan, M.S.; Huguet, N.; Feeny, D.H.; McFarland, B.H. Self-reported hypertension prevalence and income among older adults in Canada and the United States. Soc. Sci. Med. 2010, 70, 844–849. [Google Scholar] [CrossRef]
- István, B. Hypertension in the elderly. Lege. Artis. Med. 2019, 29, 531–536. [Google Scholar] [CrossRef]
- Lionakis, N.; Mendrinos, D.; Sanidas E, F.G. Hypertension in the elderly. World J. Cardiol. 2012, 4, 135. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, H.S. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef]
- Oliveros, E.; Patel, H.; Kyung, S.; Fugar, S.; Goldberg, A.; Madan, N.; Williams, K.A. Hypertension in older adults: Assessment, management, and challenges. Clin. Cardiol. 2020, 43, 99–107. [Google Scholar] [CrossRef]
- Abrahamowicz, A.A.; Ebinger, J.; Whelton, S.P.; Commodore-Mensah, Y.; Yang, E. Racial and Ethnic Disparities in Hypertension: Barriers and Opportunities to Improve Blood Pressure Control. Curr. Cardiol. Rep. 2023, 25, 17–27. [Google Scholar] [CrossRef]
- Urhoghide, E.; Onyechi, N.P.; Okobi, O.E.; Odoma, V.A.; Okunromade, O.; Moevi, A.A.; Louise-Oluwasanmi, O.; Ojo, S.; Harry, N.M.; Awoyemi, E.; et al. A Cross-Sect. Study Trends Cardiovasc. Mortality Among African Americans With Hypertension. Cureus 2023, 15, e40437. [Google Scholar] [CrossRef]
- Gheorghe, A.; Griffiths, U.; Murphy, A.; Legido-Quigley, H.; Lamptey, P.; Perel, P. The economic burden of cardiovascular disease and hypertension in low- and middle-income countries: A systematic review. BMC Public Health 2018, 18, 975. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, Z.; Ayala, C. Hospitalization costs associated with hypertension as a secondary diagnosis among insured patients aged 18–64 years. Am. J. Hypertens. 2010, 23, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Rehman, E.; Mumtaz, A.; Jianglin, Z. Cardiovascular Disease Mortality and Potential Risk Factor in China: A Multi-Dimensional Assessment by a Grey Relational Approach. Int. J. Public Health 2022, 67, 1604599. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Durao, S.; Toews, I.; Bavuma, C.M.; Hohlfeld, A.; Nury, E.; Meerpohl, J.J.; Kredo, T. Screening strategies for hypertension. Cochrane Database Syst. Rev. 2020, 2020, CD013212. [Google Scholar] [CrossRef]
- Gu, D.; He, J.; Coxson, P.G.; Rasmussen, P.W.; Huang, C.; Thanataveerat, A.; Tzong, K.Y.; Xiong, J.; Wang, M.; Zhao, D.; et al. The Cost-Effectiveness of Low-Cost Essential Antihypertensive Medicines for Hypertension Control in China: A Modelling Study. PLoS Med. 2015, 12, e1001860. [Google Scholar] [CrossRef]
- Khoong, E.C.; Commodore-Mensah, Y.; Lyles, C.R.; Fontil, V. Use of Self-Measured Blood Pressure Monitoring to Improve Hypertension Equity. Curr. Hypertens. Rep. 2022, 24, 599–613. [Google Scholar] [CrossRef]
- Bundy, J.D.; He, J. Hypertension and Related Cardiovascular Disease Burden in China. Ann. Glob. Health 2016, 82, 227–233. [Google Scholar] [CrossRef]
- Vasatova, M.; Pudil, R.; Horacek, J.M.; Buchler, T. Current Applications of Cardiac Troponin T for the Diagnosis of Myocardial Damage. Adv. Clin. Chem. 2013, 61, 33–65. [Google Scholar] [CrossRef]
- Ramani, G.V.; Uber, P.A.; Mehra, M.R. Chronic heart failure: Contemporary diagnosis and management. Mayo Clin. Proc. 2010, 85, 180–195. [Google Scholar] [CrossRef]
- Ramic-Catak, A.; Mesihovic-Dinarevic, S.; Prnjavorac, B.; Nabil Naser, I.M. Public Health Dimensions of Cardiovascular Diseases (CVD) Prevention and Control—Global Perspectives and Current Situation in the Federation of Bosnia and Herzegovina. Mater. Sociomed. 2023, 35, 88–93. [Google Scholar] [CrossRef] [PubMed]
- De la Sierra, A. New American and European Hypertension Guidelines, Reconciling the Differences. Cardiol. Ther. 2019, 8, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Wright, J.T.; Taler, S.J.; Whelton, P.K. Guideline-Driven Management of Hypertension: An Evidence-Based Update. Circ. Res. 2021, 128, 827–846. [Google Scholar] [CrossRef] [PubMed]
- Brunström, M.; Carlberg, B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels a systematic review and meta-analysis. JAMA Int. Med. 2018, 178, 28–36. [Google Scholar] [CrossRef]
- Hsu, T.W.; Liu, J.S.; Hung, S.C.; Kuo, K.L.; Chang, Y.K.; Chen, Y.C.; Hsu, C.C.; Tarng, D.C. Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Int. Med. 2014, 174, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Loutradis, C.; Price, A.; Ferro, C.J.; Sarafidis, P. Renin-angiotensin system blockade in patients with chronic kidney disease: Benefits, problems in everyday clinical use, and open questions for advanced renal dysfunction. J. Hum. Hypertens. 2021, 35, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Ghatage, T.; Goyal, S.G.; Dhar, A.; Bhat, A. Novel therapeutics for the treatment of hypertension and its associated complications: Peptide- and nonpeptide-based strategies. Hypertens. Res. 2021, 44, 740–755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Padda, R.S.; Shi, Y.; Lo, C.S.; Zhang, S.L.; Chan, J.S. Angiotensin-(1–7): A Novel Peptide to Treat Hypertension and Nephropathy in Diabetes? J. Diabetes Metab. 2015, 6, 1000615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jasleen, B.; Vishal, G.K.; Sameera, M.; Fahad, M.; Brendan, O.; Deion, S.; Pemminati, S. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors: Benefits Versus Risk. Cureus. 2023, 15, e33939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piechocki, M.; Przewłocki, T.; Pieniążek, P.; Trystuła, M.; Podolec, J.; Kabłak-Ziembicka, A. A Non-Coronary, Peripheral Arterial Atherosclerotic Disease (Carotid, Renal, Lower Limb) in Elderly Patients—A Review PART II-Pharmacological Approach for Management of Elderly Patients with Peripheral Atherosclerotic Lesions outside Coronary Territory. J. Clin. Med. 2024, 13, 1508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hall, J.E.; Omoto, A.C.M.; Wang, Z.; Mouton, A.; Li, X.; Hall, M.E. 5-Pathophysiology of Hypertension. In Hypertension: A Companion to Braunwald’s Heart Disease, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- DeMarco, V.G.; Aroor, A.R.; Sowers, J.R. The pathophysiology of hypertension in patients with obesity. Nat. Rev. Endocrinol. 2014, 10, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Tackling, G.; Borhade, M.B. Hypertensive Heart Disease. In StatPearls—NCBI Bookshelf; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Spruill, T.M. Chronic psychosocial stress and hypertension. Curr. Hypertens. Rep. 2010, 12, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Beevers, G.; Lip, G.Y.H.; O’Brien, E. ABC of hypertension: The pathophysiology of hypertension. BMJ 2001, 322, 912–916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cabandugama, P.K.; Gardner, M.J.; Sowers, J.R. The Renin Angiotensin Aldosterone System in Obesity and Hypertension: Roles in the Cardiorenal Metabolic Syndrome. Med. Clin. N. Am. 2017, 101, 129–137. [Google Scholar] [CrossRef]
- Delong, C.; Sharma, S. Physiology, Peripheral Vascular Resistance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Schiffrin, E.L. Circulatory therapeutics: Use of antihypertensive agents and their effects on the vasculature. J. Cell. Mol. 2010, 14, 1018–1029. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Padmanabhan, T.N.C.; Dani, S.; Chopra, V.K.; Guha, S.; Vasnawala, H.; Ammar, R. Prevalence of sympathetic overactivity in hypertensive patients—A pan India, non-interventional, cross sectional study. Indian Heart J. 2014, 66, 686–690. [Google Scholar] [CrossRef]
- Imig, J.D.; Anderson, G.L. Small artery resistance increases during the development of renal hypertension. Hypertension 1991, 17, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Boedtkjer, E.; Aalkjaer, C. Disturbed acid-base transport: An emerging cause of hypertension. Front. Physiol. 2013, 4, 388. [Google Scholar] [CrossRef]
- Fountain, J.H.; Kaur, J.; Lappin, S.L. Physiology, Renin Angiotensin System. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kurtz, A. Renin release: Sites, mechanisms, and control. Annu. Rev. Physiol. 2011, 73, 377–399. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, A.; Majeed, J.; Thakur, A.K.; Aggarwal, G. Drugs acting on the renin-angiotensin-aldosterone system (RAAS) and deaths of COVID-19 patients: A systematic review and meta-analysis of observational studies. Egypt Heart J. 2022, 74, 64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, C.; Xue, J.; Ye, C.; Chen, A. Role of the central renin-angiotensin system in hypertension (Review). Int. J. Mol. Med. 2021, 47, 95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nair, R.; Vaqar, S. Renovascular Hypertension. [Updated 22 May 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551587/ (accessed on 21 December 2024).
- DiBona, G.F. The Sympathetic Nervous System and Hypertension Recent Developments. Hypertension 2004, 43, 147–150. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Yang, X.; Liu, K.; Zhang, X.; Zuo, X.; Ye, R.; Wang, Z.; Shi, R.; Meng, Q.; et al. Signaling pathways in vascular function and hypertension: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Ancion, A.; Tridetti, J.; Nguyen Trung, M.L.; Oury, C.; Lancellotti, P. A Review of the Role of Bradykinin and Nitric Oxide in the Cardioprotective Action of Angiotensin-Converting Enzyme Inhibitors: Focus on Perindopril. Cardiol. Ther. 2019, 8, 179–191. [Google Scholar] [CrossRef]
- Sinha, M.D.; Chowienczyk, P. Cardiovascular Influences on Blood Pressure. In Pediatric Hypertension, 5th ed.; Springer: London, UK, 2023. [Google Scholar] [CrossRef]
- Arendse, L.B.; Jan Danser, A.H.; Poglitsch, M.; Touyz, R.M.; Burnett, J.C.; Llorens-Cortes, C.; Ehlers, M.R.; Sturrock, E.D. Novel therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacol. Rev. 2019, 71, 539–570. [Google Scholar] [CrossRef]
- Daniels, M.A.; Fischer-Posovszky, P.; Boschmann, M.; Jumpertz-von Schwartzenberg, R.; Müller, T.D.; Sandforth, L.; Frank-Podlech, S.; Hülskämper, S.; Peter, A.; Wabitsch, M.; et al. Atrial natriuretic peptide and leptin interactions in healthy men. Front. Endocrinol. 2023, 14, 1195677. [Google Scholar] [CrossRef]
- Sayin, B.Y.; Oto, A. Left Ventricular Hypertrophy: Etiology-Based Therapeutic Options. Cardiol. Ther. 2022, 11, 203–230. [Google Scholar] [CrossRef]
- Chadda, K.R.; Fazmin, I.T.; Ahmad, S.; Valli, H.; Edling, C.E.; Huang, C.L.H.; Jeevaratnam, K. Arrhythmogenic mechanisms of obstructive sleep apnea in heart failure patients. Sleep 2018, 41, zsy136. [Google Scholar] [CrossRef]
- Ameer, O.Z. Hypertension in chronic kidney disease: What lies behind the scene. Front. Pharmacol. 2022, 13, 949260. [Google Scholar] [CrossRef]
- Kim, G.-H. Primary Role of the Kidney in Pathogenesis of Hypertension. Life 2024, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Iturbe, B.; Pons, H.; Johnson, R.J. Role of the immune system in hypertension. Physiol. Rev. 2017, 97, 1127–1164. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, X.; Cui, X.; Yuan, H.; Wang, H. The roles of immune system and autoimmunity in pulmonary arterial hypertension: A review. Pulm. Pharmacol. Ther. 2022, 72, 102094. [Google Scholar] [CrossRef] [PubMed]
- Navaneethabalakrishnan, S.; Smith, H.L.; Arenaz, C.M.; Goodlett, B.L.; McDermott, J.G.; Mitchell, B.M. Update on Immune Mechanisms in Hypertension. Am. J. Hypertens. 2022, 35, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.V.; Chapleau, M.W.; Harwani, S.C.; Abboud, F.M. The immune system and hypertension. Immunol. Res. 2014, 59, 243–253. [Google Scholar] [CrossRef]
- Huang, Y.T.; Lin, H.Y.; Wang, C.H.; Su, B.H.; Lin, C.C. Association of preterm birth and small for gestational age with metabolic outcomes in children and adolescents: A population-based cohort study from Taiwan. Pediatr. Neonatol. 2018, 59, 147–153. [Google Scholar] [CrossRef]
- Eitmann, S.; Németh, D.; Hegyi, P.; Szakács, Z.; Garami, A.; Balaskó, M.; Solymár, M.; Erőss, B.; Kovács, E.; Pétervári, E. Maternal overnutrition impairs offspring’s insulin sensitivity: A systematic review and meta-analysis. Matern. Child Nutr. 2020, 16, e13031. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Imig, J.D. Antihypertensive Drugs. Ref. Modul. Biomed. Sci. 2018. [Google Scholar] [CrossRef]
- Kim, Y.L. Impact of high-dose diuretic combination therapy on volume status in peritoneal dialysis patients. Kidney Res. Clin. Pract. 2019, 38, 3–5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masi, S.; Dalpiaz, H.; Piludu, S.; Piani, F.; Fiorini, G.; Borghi, C. New strategies for the treatment of hyperkalemia. Eur. J. Int. Med. 2024. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Markan, U.; Pasupuleti, S.; Pollard, C.M.; Perez, A.; Aukszi, B.; Lymperopoulos, A. The place of ARBs in heart failure therapy: Is aldosterone suppression the key? Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719868134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahfoud, F.; Wang, J.; Ray, S. The current position of β-blockers in hypertension: Guidelines and clinical practice. Curr. Med. Res. Opin. 2024, 40 (Suppl. S1), 25–32. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071, Erratum in J. Hypertens. 2024, 42, 194. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.; Hernandez, A.K.; Kuznia, A.L. High blood pressure in children and adolescents. Am. Fam. Physician 2018, 98, 486–494. [Google Scholar] [PubMed]
- Gartlehner, G.; Vander Schaaf, E.B.; Orr, C.; Kennedy, S.M.; Clark, R.; Viswanathan, M. Screening for Hypertension in Children and Adolescents: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA—J. Am. Med. Assoc. 2020, 324, 1884–1895. [Google Scholar] [CrossRef]
- Meher, M.; Pradhan, S.; Pradhan, S.R. Risk Factors Associated with Hypertension in Young Adults: A Systematic Review. Cureus 2023, 15, e37467. [Google Scholar] [CrossRef]
- Yang Lili Magnussen, C.G.; Yang Liu Bovet, P.; Xi, B. Elevated Blood Pressure in Childhood or Adolescence and Cardiovascular Outcomes in Adulthood: A Systematic Review. Hypertension 2020, 75, 948–955. [Google Scholar] [CrossRef]
- Perry, M. Hypertension: An overview. J. Community Nurs. 2023, 4, 1–7. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Neeland, I.J.; Tuttle, K.R.; Chow, S.L.; Mathew, R.O.; Khan, S.S.; Coresh, J.; Baker-Smith, C.M.; Carnethon, M.R.; Després, J.P.; et al. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 1636–1664. [Google Scholar] [CrossRef]
- Noone, C.; Dwyer, C.P.; Murphy, J.; Newell, J.; Molloy, G.J. Comparative effectiveness of physical activity interventions and anti-hypertensive pharmacological interventions in reducing blood pressure in people with hypertension: Protocol for a systematic review and network meta-analysis. Syst. Rev. 2018, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ren, L.; Wang, Y.; Ji, Z.; Zhu, R.; Sun, Y.; Li, J.; Zhang, L. Effect of body mass index trajectory on hypertension among children and adolescents aged 5–18 years: A retrospective cohort study. Ann. Med. 2023, 55, 2267572. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shang, S. Relationship between Sleep and Hypertension: Findings from the NHANES (2007–2014). Int. J. Environ. Res. Public Health 2021, 18, 7867. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Heizhati, M.; Wang, L.; Li, M.; Lin, M.; Gan, L.; Cai, X.; Yang, W.; Yao, L.; Wang, Z.; et al. Poor sleep quality is associated with new-onset hypertension in a diverse young and middle-aged population. Sleep Med. 2021, 88, 189–196. [Google Scholar] [CrossRef]
- Andrews, G.J. Aging and Health. In International Encyclopedia of Human Geography, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Jakovljevic, M.; Westerman, R.; Sharma, T.; Lamnisos, D. Aging and Global Health. In Handbook of Global Health: An Introduction to Current and Future Trends; Routledge: London, UK, 2021. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P. Arterial Stiffness and Hypertension in the Elderly. Front. Cardiovasc. Med. 2020, 7, e544302. [Google Scholar] [CrossRef]
- Mitchell, G.F. Arterial Stiffness in Aging: Does It Have a Place in Clinical Practice? Recent Advances in Hypertension. Hypertension 2021, 77, 768–780. [Google Scholar] [CrossRef]
- Sierra, C. Hypertension and the Risk of Dementia. Front. Cardiovasc. Med. 2020, 7, 5. [Google Scholar] [CrossRef]
- Ungvari, Z.; Toth, P.; Tarantini, S.; Prodan, C.I.; Sorond, F.; Merkely, B.; Csiszar, A. Hypertension-induced cognitive impairment: From pathophysiology to public health. Nat. Rev. Nephrol. 2021, 17, 639–654. [Google Scholar] [CrossRef]
- Pourmoghddas, A.; Gharipour, M.; Garakyaraghi, M.; Nouri, F.; Taheri, M.; Sadeghi, M. Association of socioeconomic status and hypertension based on habitual smoking among Iranian population: IHHP study. Acta Biomed. 2018, 89, 498–504. [Google Scholar] [CrossRef]
- Qin, Z.; Li, C.; Qi, S.; Zhou, H.; Wu, J.; Wang, W.; Ye, Q.; Yang, H.; Wang, C.; Hong, X. Association of socioeconomic status with hypertension prevalence and control in Nanjing: A cross-sectional study. BMC Public Health 2022, 22, 423. [Google Scholar] [CrossRef]
- Neufcourt, L.; Zins, M.; Berkman, L.F.; Grimaud, O. Socioeconomic disparities and risk of hypertension among older Americans: The Health and Retirement Study. J. Hypertens. 2021, 39, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Tasić, T.; Tadić, M.; Lozić, M. Hypertension in Women. Front. Cardiovasc. Med. 2022, 9, 905504. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Zhang, X.; Kan, B.; Vuong, A.M.; Xue, S.; Zhang, Y.; Li, B.; Zhao, Q.; Guo, D.; Shen, X.; et al. Hypertension, BMI, and cardiovascular and cerebrovascular diseases. Open Med. 2021, 16, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Picca, A.; Tosato, M.; Martone, A.M.; Ortolani, E.; Sisto, A.; D’angelo, E.; Serafini, E.; Desideri, G.; et al. Body mass index is strongly associated with hypertension: Results from the longevity check-up 7+ study. Nutrients 2018, 10, 1976. [Google Scholar] [CrossRef] [PubMed]
- El Meouchy, P.; Wahoud, M.; Allam, S.; Chedid, R.; Karam, W.; Karam, S. Hypertension Related to Obesity: Pathogenesis, Characteristics and Factors for Control. Int. J. Mol. Sci. 2022, 23, 12305. [Google Scholar] [CrossRef]
- Takagi, H.; Umemoto, T. The association between body mass index and abdominal aortic aneurysm growth: A systematic review. Vasa—Eur. J. Vasc. Med. 2016, 45, 119–124. [Google Scholar] [CrossRef]
- Tan, L.; Long, L.Z.; Ma, X.C.; Yang, W.W.; Liao, F.F.; Peng, Y.X.; Lu, J.M.; Shen, A.L.; An, D.Q.; Qu, H.; et al. Association of body mass index trajectory and hypertension risk: A systematic review of cohort studies and network meta-analysis of 89,094 participants. Front. Cardiovasc. Med. 2023, 9, 941341. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Carey, R.M.; Calhoun, D.A.; Bakris, G.L.; Brook, R.D.; Daugherty, S.L.; Dennison-Himmelfarb, C.R.; Egan, B.M.; Flack, J.M.; Gidding, S.S.; Judd, E.; et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension 2018, 72, e53–e90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Palo, K.E.; Barone, N.J. Hypertension and Heart Failure: Prevention, Targets, and Treatment. Heart Fail. Clin. 2020, 16, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Ma, Y.; Lei, X.; Ding, Y.; Yang, S.; He, D. Hypertension linked to Alzheimer’s disease via stroke: Mendelian randomization. Sci. Rep. 2023, 13, 21606. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Harada, N.; Okura, A. Digital Therapeutics in Hypertension: Evidence and Perspectives. Hypertension 2022, 79, 2148–2158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katz, M.E.; Mszar, R.; Grimshaw, A.A.; Gunderson, C.G.; Onuma, O.K.; Lu, Y.; Spatz, E.S. Digital Health Interventions for Hypertension Management in US Populations Experiencing Health Disparities: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2024, 7, e2356070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MacLeod, K.E.; Ye, Z.; Donald, B.; Wang, G. A Literature Review of Productivity Loss Associated with Hypertension in the United States. Popul. Health Manag. 2022, 25, 297–308. [Google Scholar] [CrossRef]
- Wang, G.; Grosse, S.D.; Schooley, M.W. Conducting Research on the Economics of Hypertension to Improve Cardiovascular Health. Am. J. Prev. Med. 2017, 53, S115–S117. [Google Scholar] [CrossRef]
- Franco, C.; Sciatti, E.; Favero, G.; Bonomini, F.; Vizzardi, E.; Rezzani, R. Essential Hypertension and Oxidative Stress: Novel Future Perspectives. Int. J. Mol. Sci. 2022, 23, 14489. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Community-Based Solutions to Promote Health Equity in the United States. The State of Health Disparities in the United States—Communities in Action—NCBI Bookshelf. In Communities in Action: Pathways to Health Equity; Baciu, A., Negussie, Y., Geller, A., Weinstein, J.N., Eds.; National Academies Press (US): Washington, DC, USA, 2017. [Google Scholar]
- Nagar, S.D.; Pemu, P.; Qian, J.; Boerwinkle, E.; Cicek, M.; Clark, C.R.; Cohn, E.; Gebo, K.; Loperena, R.; Mayo, K.; et al. Investigation of hypertension and type 2 diabetes as risk factors for dementia in the All of Us cohort. Sci. Rep. 2022, 12, 19797. [Google Scholar] [CrossRef]
- Elnaem, M.H.; Mosaad, M.; Abdelaziz, D.H.; Mansour, N.O.; Usman, A.; Elrggal, M.E.; Cheema, E. Disparities in Prevalence and Barriers to Hypertension Control: A Systematic Review. Int. J. Environ. Res. Public Health. 2022, 19, 14571. [Google Scholar] [CrossRef]
- Doumas, M.; Imprialos, K.P.; Kallistratos, M.S.; Manolis, A.J. Recent advances in understanding and managing resistant/refractory hypertension. F1000Research 2022, 19, 14571. [Google Scholar] [CrossRef]
- Carey, R.M. AT2 Receptors: Potential Therapeutic Targets for Hypertension. Am. J. Hypertens. 2017, 30, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Ram, C.V.S. A New Class of Drugs Approved in the United States for Hypertension: Endothelin Antagonists. Am. J. Med. 2024, 137, 795–798. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goorani, S.; Zangene, S.; Imig, J.D. Hypertension: A Continuing Public Healthcare Issue. Int. J. Mol. Sci. 2025, 26, 123. https://doi.org/10.3390/ijms26010123
Goorani S, Zangene S, Imig JD. Hypertension: A Continuing Public Healthcare Issue. International Journal of Molecular Sciences. 2025; 26(1):123. https://doi.org/10.3390/ijms26010123
Chicago/Turabian StyleGoorani, Samaneh, Somaye Zangene, and John D. Imig. 2025. "Hypertension: A Continuing Public Healthcare Issue" International Journal of Molecular Sciences 26, no. 1: 123. https://doi.org/10.3390/ijms26010123
APA StyleGoorani, S., Zangene, S., & Imig, J. D. (2025). Hypertension: A Continuing Public Healthcare Issue. International Journal of Molecular Sciences, 26(1), 123. https://doi.org/10.3390/ijms26010123





