Modeling Possible G-Quadruplexes and i-Motifs at DNA–DNA Contact Sites: Strategy, Classification, and Examples
Abstract
1. Introduction
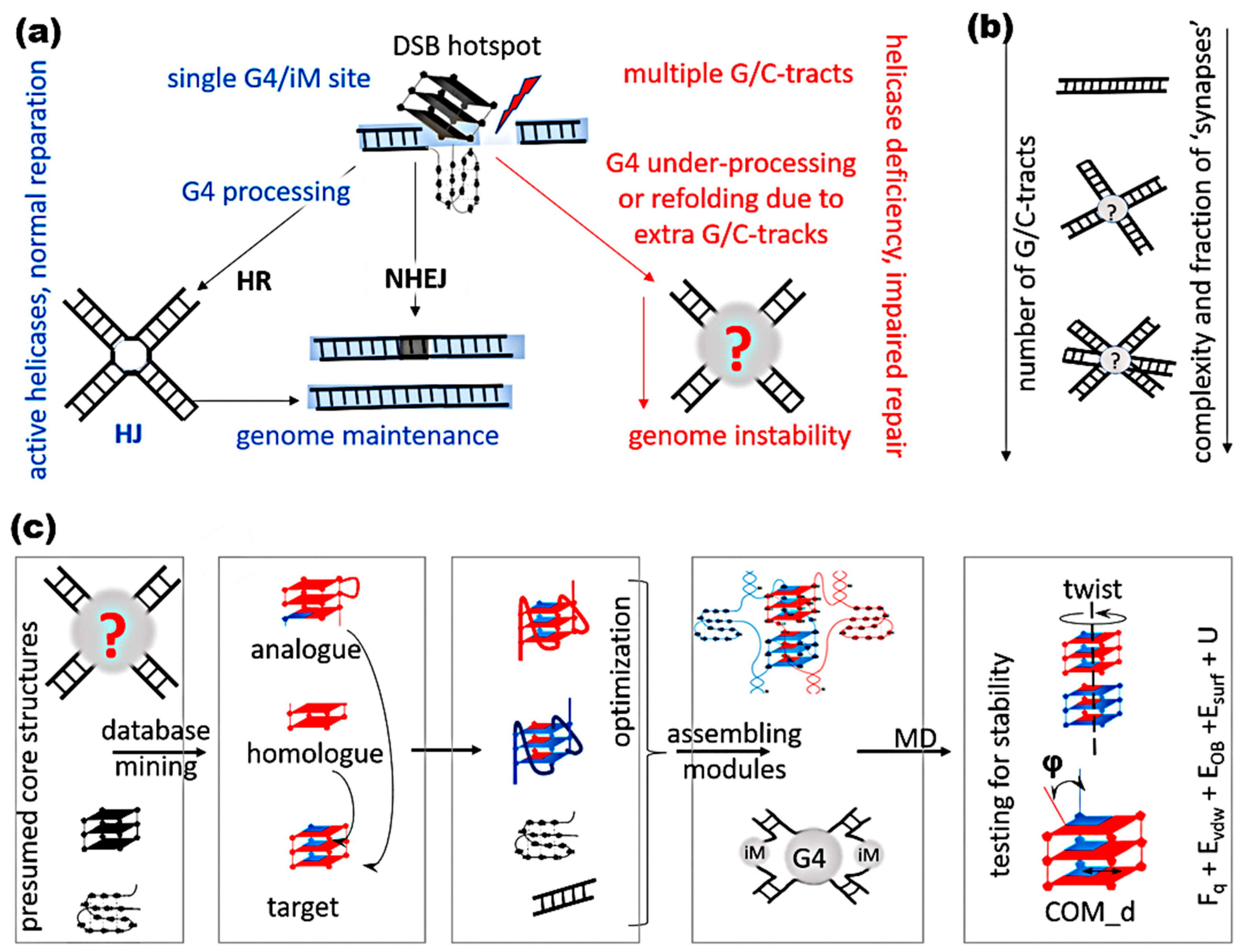
2. Results and Discussion
2.1. General Strategy for Creating and Testing Models of Possible Complexes with G/C-Tracts
- To score the planar deformation of tetrads in G4s—the distances from the centers of mass of the guanine bases to the center of mass (COM) of their containing tetrad and the angles between the normals to the guanine bases and the vector connecting the COMs of the boundary tetrads.
- To make an evaluation of the twisting deformation in G4s—the angles of rotation of one tetrad with respect to another.
- To estimate the structural deformation of cytosine pairs in iMs—the distances between the COMs of the cytosine bases and the angles between the normals and the bases of the cytosines forming the pair.
- Distances between the COMs of G4s, if they did not have stacking tetrads, and distances between the COMs of stacking boundary tetrads otherwise.
- Twist angles of G4s relative to each other, i.e., rotation angles between stacking tetrads (in the case of stacking between boundary tetrads)
- The angle between the axes of the G4s—straight lines passing through the COMs of the boundary tetrads.
- In the case of the formation of G4s located in the same plane, the angles between the axis of each of the G4s and the normal to the plane, in which the G4s are located, were estimated
- When G4s were packed into a stack or stacks parallel to each other, the angles between the axis of each of the G4s and the axis of the stack (a straight line, passing through the COMs of the boundary tetrads of the boundary G4s) were estimated
- Angles between the axes of the iMs (straight lines passing through the COMs of the boundary cytosine pairs) and the normal to the plane in which the G4s are located, or the axis of the stack.
- In the schemes of the studied models, all nucleotides in (G3T)nG3 and (C3A)nC3 involved in the formation of non-canonical forms are numbered.
- The legends for the graphs of parameters describing the arrangement of nucleotides in non-canonical forms indicate the name of the nucleotide by means of the first letter of its name and its number.
- In the description of the behavior of entire tetrads in G4s, in the case when the tetrads are from different G4s, the G4’s number is indicated in the legend for each tetrad. If the curve depicts the behavior of tetrads of the same G4 relative to each other, then the number of the G4 to which both tetrads belong is indicated at the beginning of the legend and then the numbers of the tetrads themselves.
- The numbers of G4s and iMs are marked with Roman numerals, and the numbers of tetrads in G4s are marked with Arabic.
- Hydrogen bonds between nucleotides are marked with black dotted lines.
- The duplexes on the drawings and diagrams are colored and named as follows: the first symbol is a capital letter of the color of this duplex on the drawing (for instance R is red color), and the second symbol is its number in Roman numerals.
2.2. Implementation of the Proposed Strategy for (G3T)n/(C3A)n Duplexes and Classification of the Respective Complexes
- Monomeric G4s and monomeric iMs
- Monomeric G4s and dimeric iMs
- N-molecular G4s (where N is the number of chains forming them) and monomeric iMs
- N-molecular quadruplexes and dimeric iMs.
- N-molecular G4s form stacks by stacking, and these G4s can be either right-handed or left-handed [15]; mixed stacks containing G4 units of both types are labeled “r-l” (right-left). In addition, a flip-flop case is possible. In this variant, two cases are possible when quadruplexes in stacks are connected by chain transitions from one quadruplex to another, and vice versa.
- G4s are not connected to each other by chain transitions from one to another and do not form stacking
- G4s are connected to each other by chain transitions from one to another and located in the same plane, and in this class, variants are possible when the COMs of G4s lie on the same line while G4s forms G4′ tapes and when the COMs of G4s form a triangle.
- Without the girth of one chain around the other and transition of the chain from one flanking duplex to another;
- With the girth of one chain around the other, but without transition of the chain from one flanking duplex to another;
- Transition of the chain from one flanking duplex to another, but without girthing one chain around another;
- Both with the girth of one chain around the other and with transition of the chain from one flanking duplex to another
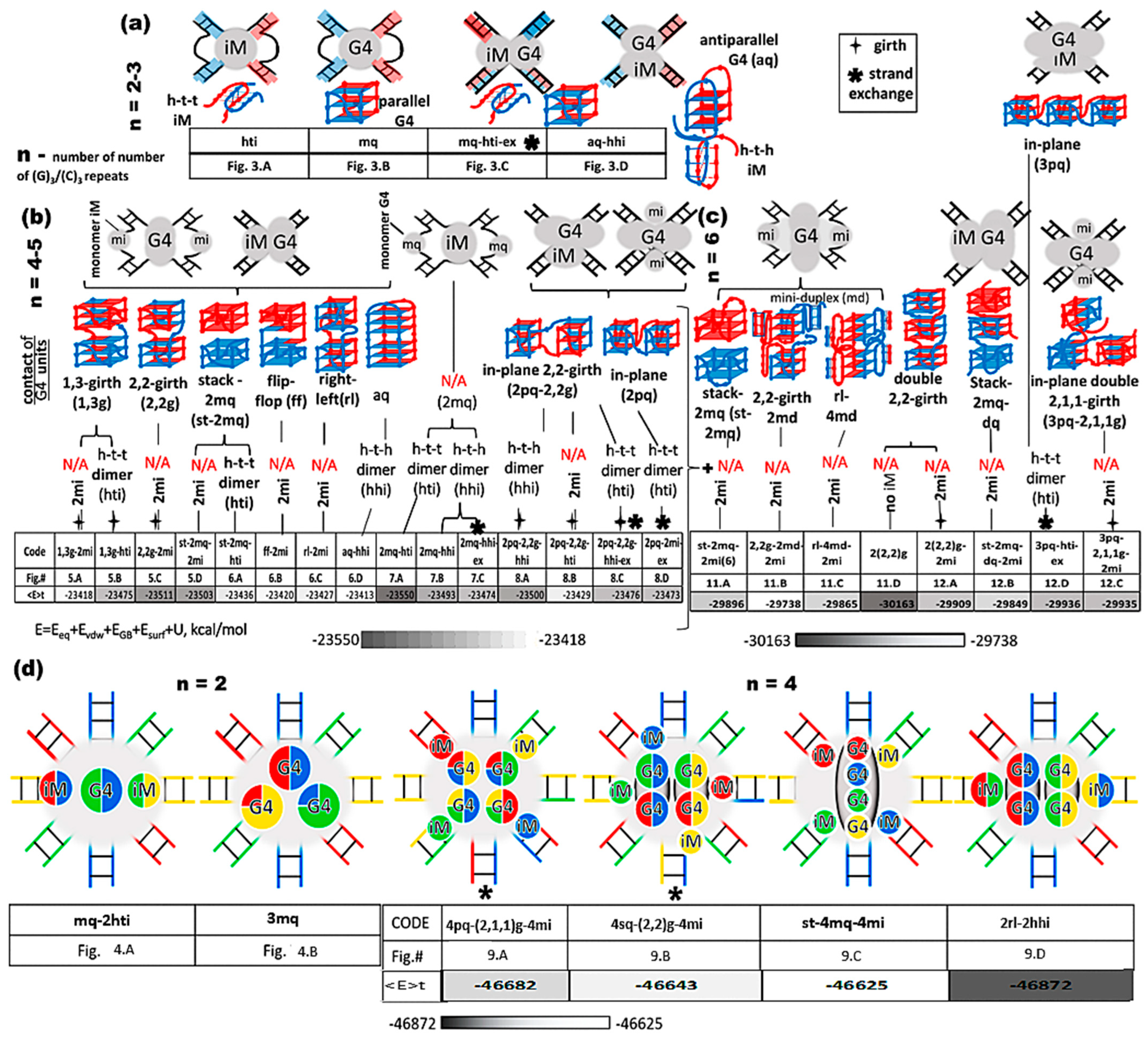
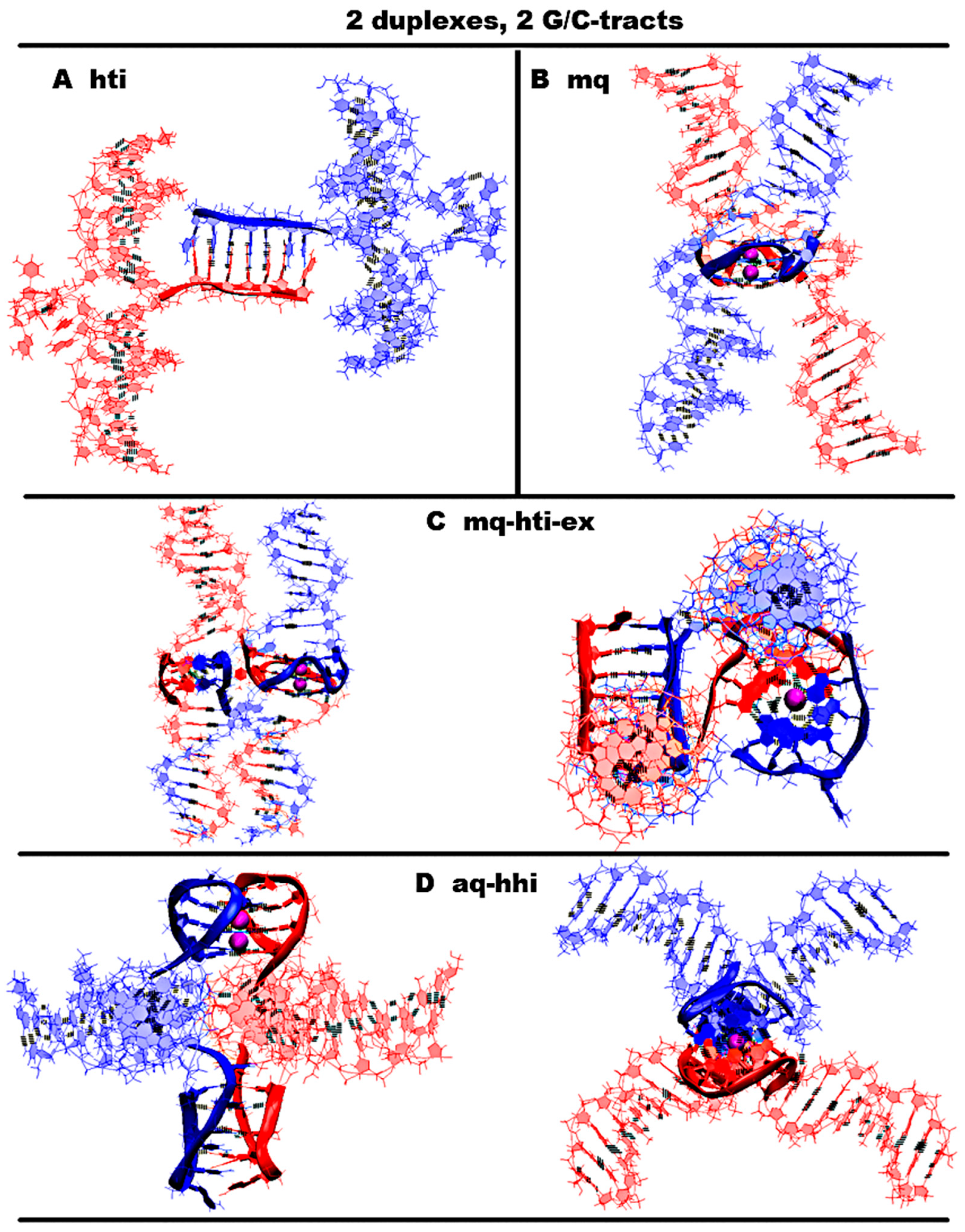
2.3. Contacts of Duplexes with 2 G/C-Tracts
2.4. Contacts of Duplexes with 3 G/C-Tracts
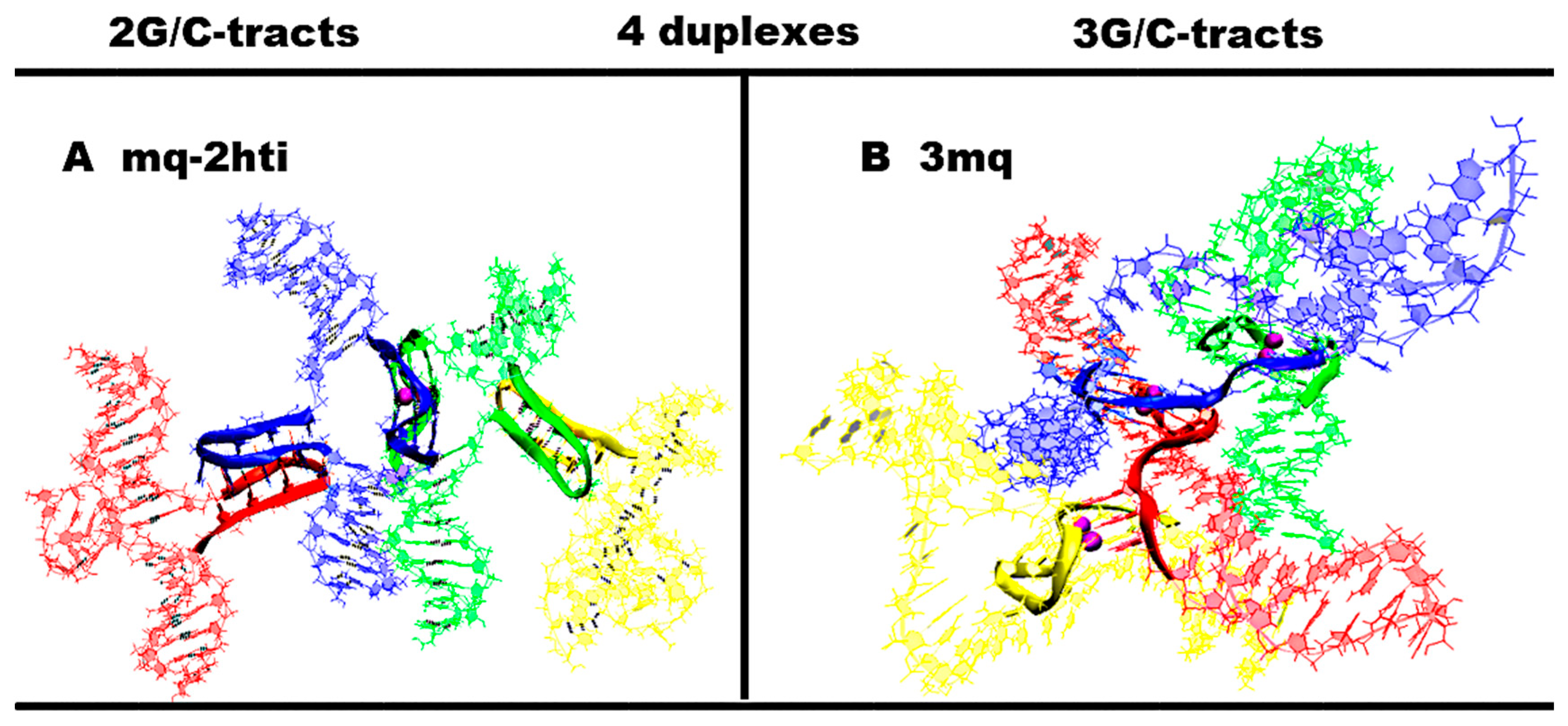
2.5. Contacts of Duplexes with 4 G/C-Tracts
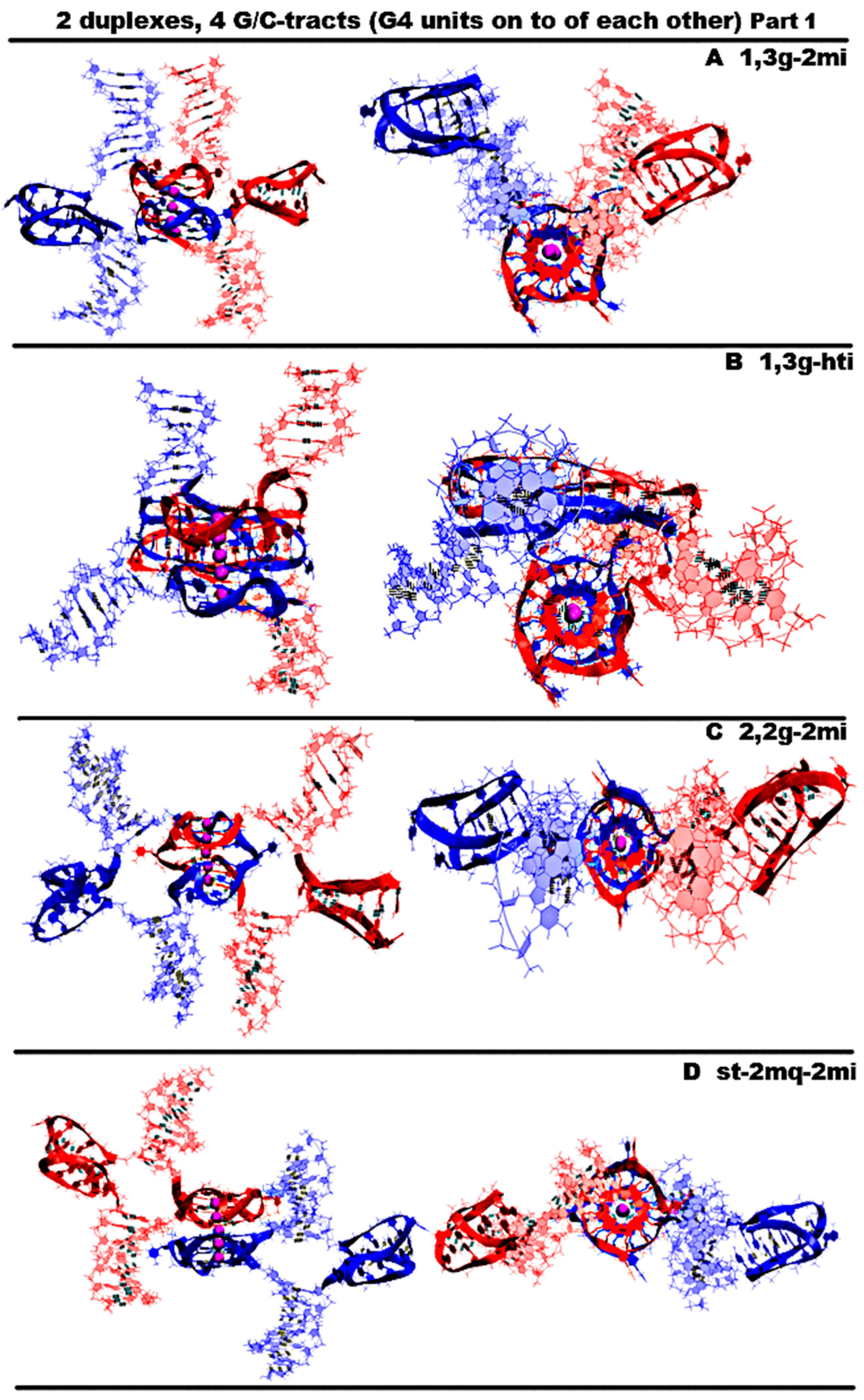
- “1,3 girth and two iM-monomers” (1,3g-2mi) is presented in Figure 5A and Figure S5.A.1. In this option, G-strands form a stack consisting of two G4s with side bulges of T. Each of these G4s is formed in the ratio of 3/4:1/4, i.e., 3/4 of each G-fragment takes part in the formation one G4, and the remaining 1/4 of the same G-fragment belongs to another G4. It should be noted that while folding, the G4s form the G-strand from one duplex girths the similar G-strand of another duplex. Each of the C-strands forms the monomeric iM consisting of six cytosine pairs. MD showed, see Figure S5.A.2-3, that the G4s and their location relative to each other are stable, but the penultimate pairs in the iMs were not stable.
- “1,3 girth and head-to-tail iM-dimer” (1,3g-hti) is shown in Figure 5B and Figure S5.C.1. In contrast to the previous case, the C-strands form the head-to-tail dimeric iM containing 10 cytosine pairs, divided into two equal halves by a fragment, containing adenines and cytosines, separated from each other the way that they become unable to form hydrogen bonds; see diagram Figure S5.B.1.C. An analysis of the values of the parameters presented in Figure S5.B.2-3 shows that during MD there were no changes in the G4 structures, the iMs, and their geometry of location relative to each other.
- “2,2 girth and two iM-monomers” (2,2g-2mi) is shown in Figure 5C and Figure S5.C.1. As in the previous two cases, the G-strands with mutual girth of each other form a stack of two dimeric parallel G4s, for which boundary tetrads form a stacking interaction. Each G4 is formed in the ratio of 2/4:2/4, i.e., two of the four G-tracts that form the G4 belong to one strand, and the other two belong to another one. In this case, the G-tracts formed by one strand are located on opposite sides of the stack. As in the first case considered above, each of the C-strands forms an iM monomer consisting of six cytosine pairs. From Figure S5.C.2., it can be seen that the G4s and their localizations relative to each other are stable, and the penultimate pairs in the iMs, as in case 1, were deformed during the MD.
- “Stacking and two iM-monomers” (st-2mq-2mi) is presented in Figure 5D and Figure S5.D.1. Earlier [17,18] it was shown that in the duplex containing GGGT repeats and melted in the area of their localization, the simultaneous existence of the G4 in the strand containing G-repeats and in the iM in the complementary strand is impossible due to their steric overlap. However, the author constructed a 3D model of the DNA duplex corresponding to this case. The stability of the G4 and the iM located opposite to each other was tested using the MD method. The analysis of the calculated trajectory has demonstrated the stability of non-canonical forms in this model. As a result, the possibility of contact was considered, in which the lower tetrad of the G4 formed by the G-strand of one duplex is located above the upper tetrad of the G4 formed by the similar strand of another duplex. In this variant, as in cases 1 and 3, each of the C-strands forms the monomeric iM consisting of six cytosine pairs. During the MD calculations, the G4s themselves did not undergo significant changes. As can be seen from the graphs in Figure S5.D.2.D-E, the distance between the G4s increased by 0.5 angstroms, and one G4 rotated relative to the other by an angle equal to 30 degrees during the modeling. According to Figure S5.D.2.J-M, as well as in cases 1 and 3, the penultimate pairs in the iMs were deformed.
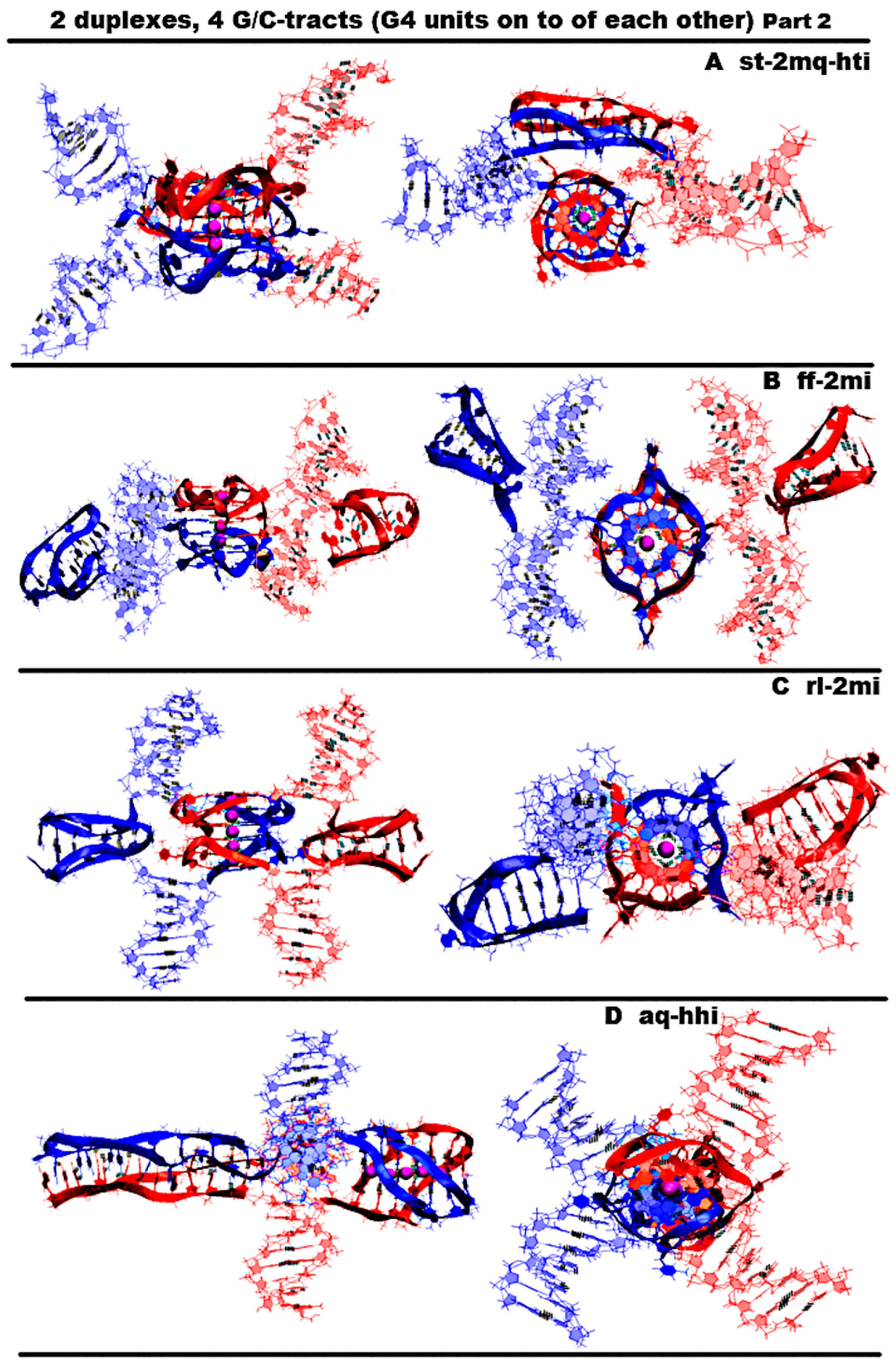
- “Stacking and head-to-tail iM-dimer” (st-2mq-hti) is shown in Figure 6A and Figure S6.A.1. In this case, the G-strands form the G4s located relative to each other as in the previous variant 4. In its turn, the C-strands form the head-to-tail dimeric iM, as in variant 2. During the dynamics, the G4s remained stable. In contrast to previous case 4, the distance between the G4s and the angle of rotation between neighboring tetrads did not change significantly during the calculation; see Figure S6.B.1.E-F. In the iM, only the boundary pairs were deformed; see Figure S6.B.2.J-M.
- “Flip-flop and two iM-monomers” (ff-2mi) is shown in Figure 6B and Figure S6.B.1. In this option, G-strands form the two-tetrad G4s located one above the other. But unlike the cases considered earlier, the axes, passing through the COMs of tetrads, are directed to the opposite sides. In this case, the boundary stacking tetrads consist of three guanines from one strand and one guanine from the other. The second tetrads are joined by the tetrads formed from two guanines and two thymines; see the scheme Figure S6.B.1.C. An analysis of the values of the quantities, characterizing the geometry of the arrangement of guanines and thymines in tetrads, showed that tetrads, composed exclusively of guanines, are stable; see Figure S6.B.1-2.F-H. And the tetrads, formed from two guanines and two thymines, slightly spread. The thymines, included in the tetrads, slightly move away from their original location relative to the tetrads’ COMs, while the thymine bases remain in the tetrads’ planes. Each of the C-strands forms the monomeric iM consisting of six cytosine pairs, as in previous cases 1, 3, and 4. The geometry of the location of the G4s relative to each other has not changed during the MD; see Figure S6.B.2.F-I. As follows from Figure S6.B.2.J-M, in the iMs, in contrast to cases 1, 3, and 4, during the calculation, not the penultimate, but the last cytosine pairs were subjected to deformations.
- “Stacking of right and left handed parallel G4-dimers, and two iM-monomers” (rl-2mi) is demonstrated in Figure 6C and Figure S6.C.1. In this option, the G-strands form two parallel G4s located one above the other, wherein the lower one is right-handed, and the upper one is left-handed. Each of them is formed by half of one strand and half of the other. As in case 6, the axes, passing through the tetrads’ COMs, are directed to the opposite sides. The C-strands form monomeric iMs, consisting of six cytosine pairs. An analysis of the evolution of the parameter values presented in Figure S6.C.1-2 shows that the structures of both right-handed and left-handed G4, as well as their localization relative to each other, did not change during the calculations, and in the iM, as in previous cases 1, 3, and 4, the penultimate pairs underwent deformation.
- “Antiparallel G4-dimer and head-to-head iM-dimer” (aq-hhi) is shown in Figure 6D and Figure S6.D.1. This option has already been considered for n = 2. As n increases to 3, the number of all tetrads in the antiparallel G4s increases to 5. Thymines, which are part of G-motive and are not included in the lateral loops, form side bulges. The guanines in lateral loops compared to the previously considered variant with n = 2, during MD calculations, showed a stronger deviation from the original location; see Figures S3.D.2.E-F and S6.D.2.F-I. The C-strands form the head-to-head dimeric iM, which, in contrast to cases 2 and 5, contains 12 cytosine pairs, divided in half by the fragment containing adenines. In this variant of the iM, all cytosines form the C-C pairs. From the analysis of the data presented in Figure S6.D.2.J-M, it can be concluded that the iM structure demonstrated stability during the dynamics.The next group of possible options of the geometry of the arrangement of non-canonical forms relative to each other in the variant of contacts of two duplexes is formed by cases in which G4 are not connected to each other by chain transitions from one to another and do not form stacks.
- “Head-to-tail iM-dimer between two parallel G4-monomers” (2mq-hti) is demonstrated in Figure 7A and Figure S7.A.1. In this option, as in previous cases 2 and 5, the C-strands form the head-to-tail dimeric iM, but unlike the mentioned cases, it is located localized between the parallel G4s formed by the G-strands. In this case, the COMs of all non-canonical structures are located on a straight line passing through these COMs. Also, as in cases 2 and 5, the iM contains 10 cytosine pairs, divided in half by the area containing adenines. A comparison of the initial conformation and the one obtained at the last step of the trajectory, as well as the analysis of the behavior of the curve QIQII, see Figure S7.A.1.D, indicates that the G4s located in the starting conformation at a 90-degree angle tend to be located parallel to each other during the calculations. An analysis of the parameter values given in Figure S7.A.1-2.F-M showed that the G4s and the iM did not undergo deformations during the calculations.
- “Head-to-head iM-dimer between two parallel G4-monomers” (2mq-hhi) is demonstrated in Figure 7B and Figure S7.B.1. In contrast to the previous case, in this one, as in case 8, the G-strands forming the dimeric head-to-head iM are localized between the parallel G4s, formed by G-strands. The G4 arrangement in the starting conformation is similar to the previous case; see Figure 7A,B. However, in the analysis of the parameters describing the geometry arrangement of the G4s relative to each other, the angle between the axes passing through the tetrads’ COMs (curve QIQII in Figure S7.B.1.D), the distance between the COMs of internal tetrads (curve Iq2IIq2 in Figure S7.B.1.G-S.2.K,M), indicated that the G4s converged on each other during the calculations and the axes passing through the tetrads’ COMs turned to become parallel to each other in the final conformation; see Figure S7.B.1.A-B. T51 and T113 thymines belonging to propeller loops can form a stacking interaction. The COMs of all non-canonical structures, as in the previous case, are located in the same plane. An analysis of the data presented in Figure S7.B.1-2.E-N showed the stability of the structure of the G4s and the iM during MD calculations.
- “Head-to-head iM-dimer between two parallel G4-monomers in case of strands exchange” (2mq-hhi-ex) is demonstrated in Figure 7C and Figure S7.C.1. The localization of non-canonical structures, their geometry, and behavior in the process of MD calculations are identical to case 10 just described. However, unlike case 10, in this variant, the strands forming the iM are exchanged in the duplexes. As in the previous case, in the process of the MD calculations, the structures of the G4s and the iM demonstrated stability; see Figure S7.C.1-2.E-N.The third group of possible options of the geometry of the arrangement of non-canonical forms relative to each other in the variant of contacts of two duplexes is formed by cases in which G4s are connected to each other by chain transitions from one to another and located in the same plane.
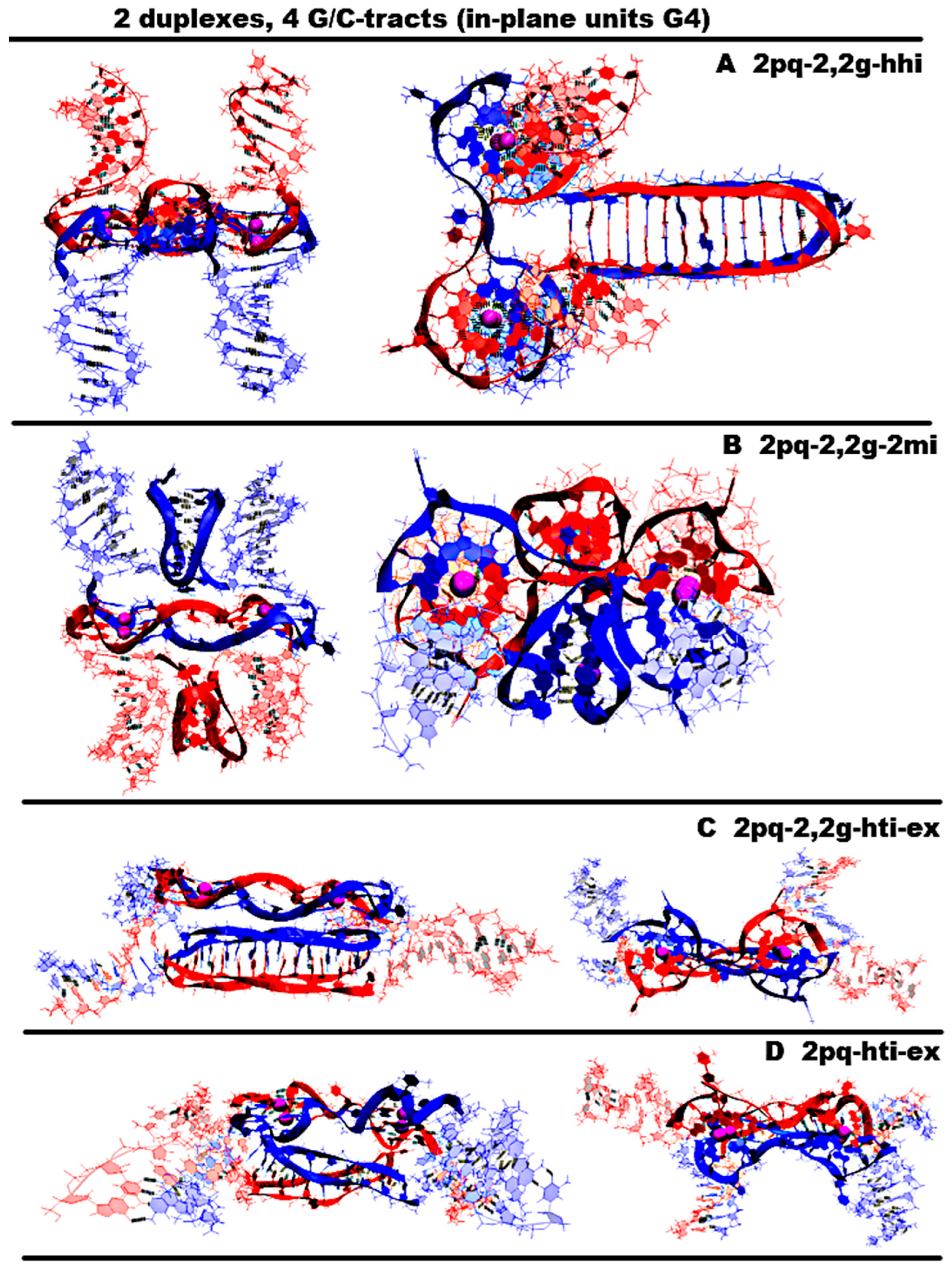
- “Two parallel G4-dimers in the same plane and head-to-head iM-dimer between two duplexes with mutual girth of the strands” (2pq-2,2g-hhi) is demonstrated in Figure 8A and Figure S8.A.1. In this option, the parallel G4s formed by G-strands are located in the same plane, and each strand forms G4′s half. During the transition of the strands from one G4 to another, the sugar-phosphate backbone turns in such a way that the side formed by the strand in one G4 is opposite to the side formed by the same strand in the neighboring G4. As a result, the strands mutually girth each other, and the angle between the axes passing through the tetrads’ COMs is 180 degrees, and it remains so throughout the entire MD trajectory (QIQII curve in Figure S8.A.1.D). The head-to-head iM, formed by the C-strands, is the same as in case 8 and 10 and is located parallel to the tetrads of the G4s located in the same plane. As follows from Figure S8.A.2.J-M, only two cytosine pairs were subjected to deformation during the MD.
- “Two parallel G4-dimers in the same plane and two iM-monomers clamped duplexes with mutual girth of the strands” (2pq-2,2g-2mi) is demonstrated in Figure 8B and Figure S8.B.1. In this option, the G4 topology and the G4 arrangement relative to each other are the same as in case 12. But unlike the previous case, the C-strands form monomeric iMs consisting of six cytosine pairs as in previous cases 1, 3, and 5–7. The iMs are localized between parts of the duplexes, the strands of which are involved in the formation of these iMs. The data analysis in Figure S8.B.1-2 indicates that the G4s did not undergo significant changes during the MD, and only one cytosine pair in the iMs turned to be unstable.
- “Two parallel G4-dimers in the same plane and head-to-tail iM-dimer between the duplexes with exchange and mutual girth of the strands” (2pq-2,2g-hti-ex) is demonstrated in Figure 8C and Figure S8.C.1. In this option, the localization and the topology of the G4s formed by G-strands are similar to the ones in previous cases 12 and 13. But in contrast to cases 12 and 13, the C-strand forms the head-to-tail dimeric iM, similar to those that were formed in cases 2, 5, and 9. In addition to mutual girth of the G-strands, in this variant, the exchange of the strands in the duplexes also exists. During the MD process, all non-canonical structures showed stability; see Figure S8.C.1-2.
- “Two parallel G4-dimers in the same plane with head-to-tail iM-dimer between two duplexes and the strands exchange” (2pq-hti-ex) is demonstrated in Figure 8D and Figure S8.D.1. In this option, in contrast to the cases 12, 13, and 14 considered earlier, the halves of the G4s formed by G-strands are localized on one side of the plane containing the vectors connecting the tetrads’ COMs in the G4s. As a result, the strands forming G4s do not mutually girth each other. The value of the angle between the axes passing through the COMs of the tetrads of the G4s fluctuates during the trajectory near the value equal to 90 degrees; see Figure S8.D.1.D. C-strands form the dimeric head-to-tail iM, similar to those formed in cases 2, 5, 9, and 14. In this option, as in the previous one, strand exchange takes place. As in previous case 14, no deformations occurred in the non-canonical structures during the MD; see Figure S8.D.1-2.
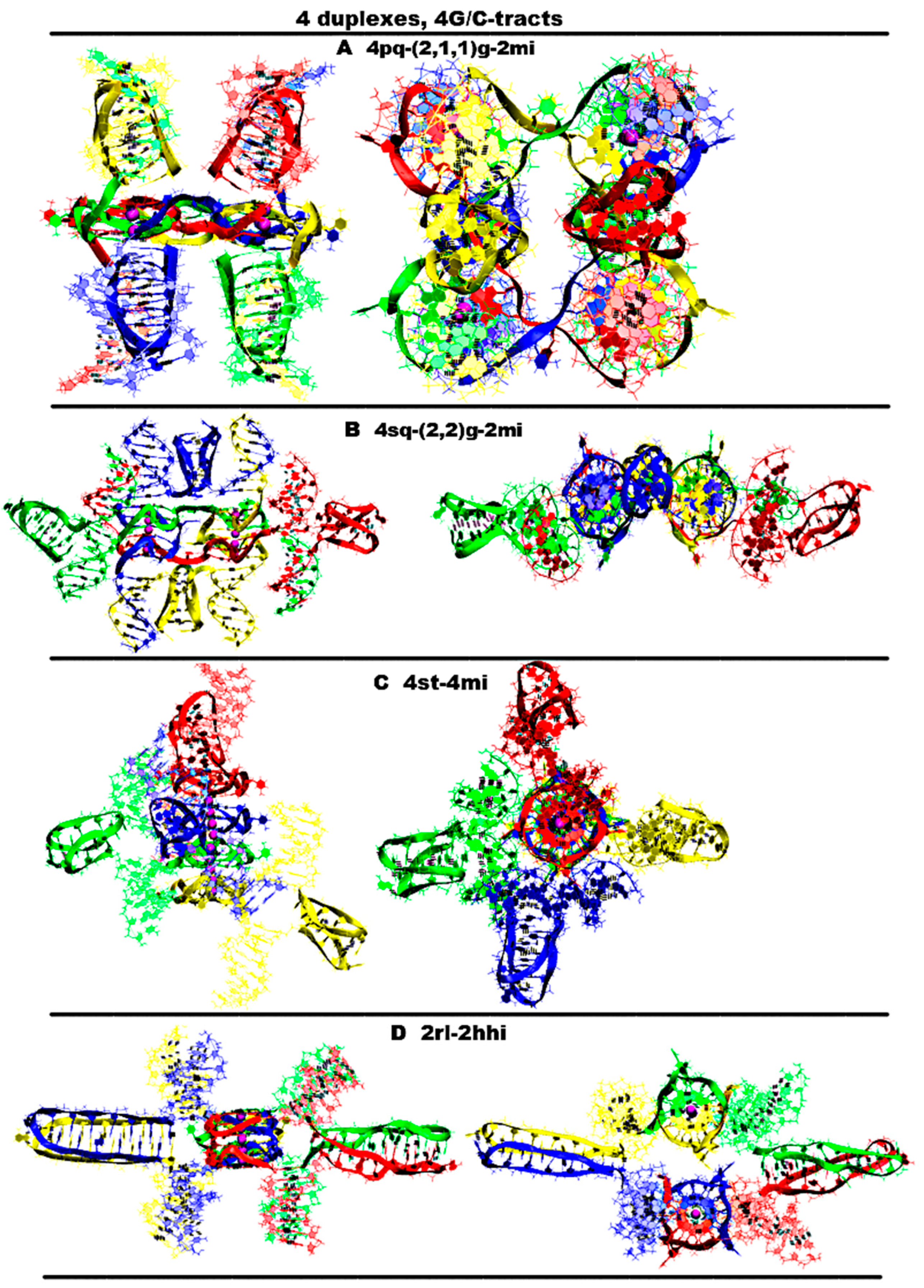
- “Four parallel G4-dimers in the same plane and four iM-monomers between the duplexes with exchange and mutual girth of the strands” (4pq-4(2,1,1)g-4mi) is demonstrated in Figure 9A and Figure S9.A.1. In this option, G-strands form the parallel G4s laying in the same plane. The top view of the G4 part shows that the COMs of the G4s form the square. Each G4 is formed by three strands in the ratio of 2/4:1/4:1/4. Each of the strands that form quadruplexes is arranged in them as follows: forms two of the four G-tracts in the first, then forms one in the next to the first, and then another in the next to the second one. If the G4s’ COMs are located at the vertices of the diagonals, then the G4 axes are unidirectional, and if the G4s’ COMs are located at the vertices of the sides of the square, then the axes are directed oppositely. The C-strands form the monomeric iMs consisting of six cytosine pairs, as in the previously considered cases for the contacts of two duplexes. The iMs are localized between the parts of the duplexes. In addition, at the start, the vectors, connecting the COMs of the first and last pair of cytosines of each iMs, are located perpendicular to the plane of the square, formed by the vertices in which the G4s’ COMs are located. During the dynamics, the angles between these vectors and the plane can change so that one of them can become parallel to the plane. The geometry of the iM localization is such that their heads are directed away from the plane of the square; at the same time, the heads of two iMs are oriented in one direction, and the other two are in the other. If the iMs’ COMs are connected by the line segments, then the square will be formed, which will be located in a plane perpendicular to the plane in which the G4s’ COMs are located. The G4s and the iMs are arranged in accordance with the following rule: each iM formed by the C-strand is located closest to the last quadruplex formed by the complementary G-strand. An analysis of the MD results showed, see Figure S9.A.3-4, that all G4s are stable, in three iMs one pair has undergone deformation, namely, in two of these three, cytosines in the penultimate pair have diverged, and in the third iM, the last cytosine pair turned to be unstable. The starting localization of the G4s relative to each other during the MD did not undergo significant changes. For two iMs, their positions relative to the G4s have changed. More precisely the slope of the vector, connecting the COMs of the first and last pair of cytosines, has changed so that for one iM, the angle between this vector and the normal to the plane, in which the G4s COMS are located, has changed from 0 to 90 degrees, and for the other iM, it has changed from 180 to 120; see Figure S9.A.2-4.
- “Four parallel G4-dimers in two stacks and four iM-monomers with exchange and mutual girth of the strands” (4sq-4(2,2)g-4mi) is demonstrated in Figure 9B and Figure S9.B.1. This case differs from the previous one in that G-strands form the dimeric parallel G4s packed in stacks parallel to each other. In this case, the geometry of the stacks is such that the axes passing through the G4s’ COMs included in the stacks are directed oppositely. Each G4 is formed by two strands in the ratio of 2/4:2/4. In this case, one of the strands involved in the formation of the G4 is involved in the formation of the neighboring G4 laying with the first G4 in the same plane, and the other strand is also involved in the formation of another neighboring quadruplex, which is located either under or above the first G4. Thus, two of the strands are involved in the formation of the G4s laying in the same plane, and the remaining two are involved in the formation of the G4s located one above the other. As in the previously considered case 1, C-strands form the monomeric iMs consisting of six cytosine pairs. In this case, two iMs are located above and below the planes in which the G4s’ COMs are located, and the other two are located on the sides of the stacks with the G4s. Those iMs that are located on the sides are formed by strands that are complementary to the strands involved in the formation of the G4s laying in the same plane. Both options are possible only through mutual girthing of the strands, forming G4s, and the transition of strands from one duplex to another. For this case the analysis of the MD results showed (see Figure S9.B.2.D-F) that the G4s and their geometry relative to each other have not changed. The iM location, in contrast to case 1, did not change significantly during the MD. However, as follows from Figure S9.B.4.O-R, two cytosine pairs in two iMs and one in the other two were deformed in the course of the calculations.
- “Stacking of four parallel G4-dimers with four iM-monomers” (st-4mq-4mi) is demonstrated in Figure 9C and Figure S9.C.1. In contrast to cases 1 and 2 described above, in this option, when forming the tetrameric complex through sequential stacking of the G4s formed by G-strands, neither mutual girthing of the strands nor the transition them from one duplex to another occurs. The formation of the G4 stacking occurs according to the following algorithm. Initially, the complexes described in option 4 for the case with two duplexes are formed. In these complexes, the iMs are opposite each other. Then, the pairs of duplexes in these complexes are localized relative to each other so that the line passing through the COMs of iMs included in one pair form a right angle with the same line for the other pair. In this case, the lower G4 of one pair of the duplexes is stacked with the upper G4 of the other. As follows from the analysis of the MD results, see Figure S9.C.2.D-F, the G4s and their localization relative to each other have not changed. The localization of the iMs relative to the stack of the G4s also did not qualitatively change during the MD. However, as follows from Figure S9.C.4.O-R, during the calculations, two iMs were stable, and in each of the other two remaining iMs, one cytosine pair was deformed.
- “Two parallel stack with right and left handed G4-dimers and two head-to-head iM-dimers” (2rl-2hhi), demonstrated is Figure 9D and Figure S9.D.1, is one more possible option for the contact of four duplexes with the formation of non-canonical forms, in which the strands do not mutually girth and are not transited from one duplex to another. As in case 2, the G-strands form dimeric G4s with a ratio of 2/4:2/4, which are packed in stacks arranged parallel to each other. As in case 7, for the two duplexes described above, each stack consists of two parallel dimeric G4s located one above the other, formed half by one strand and half by the other, wherein the lower G4 is right-handed and the upper one is left-handed. In this option, the axes passing through the tetrads’ COMs are oriented in the opposite directions. The C4-strands form two head-to-head dimeric iM. The geometry of the location of the G4s and the iMs relative to each other is as follows: their COMs are located in the same plane and form a rhombus, in which the COMs of stacks of G4s are located at the vertices placed at the ends of the small diagonal, and the iMs’ COMs are located at the vertices of the large diagonal. As in options 8, 10, 11, and 12 described earlier for the case of two duplexes, each of the dimeric iMs is formed from two blocks consisting of six cytosine pairs separated by adenine inserts. The location of the iMs relative to each other is such that the vectors connecting the COMs of the first and the twelfth pairs of cytosines are oriented in the opposite directions. From the analysis of the MD results presented in Figure S9.D.1-4, it can be concluded that the G4s, their localization relative to each other, and the geometry of the location of the iMs relative to the stack of the G4s did not change during the MD. As follows from Figure S9.D.4.O-R, only one cytosine pair in each of the iMs was deformed in the course of the calculations.

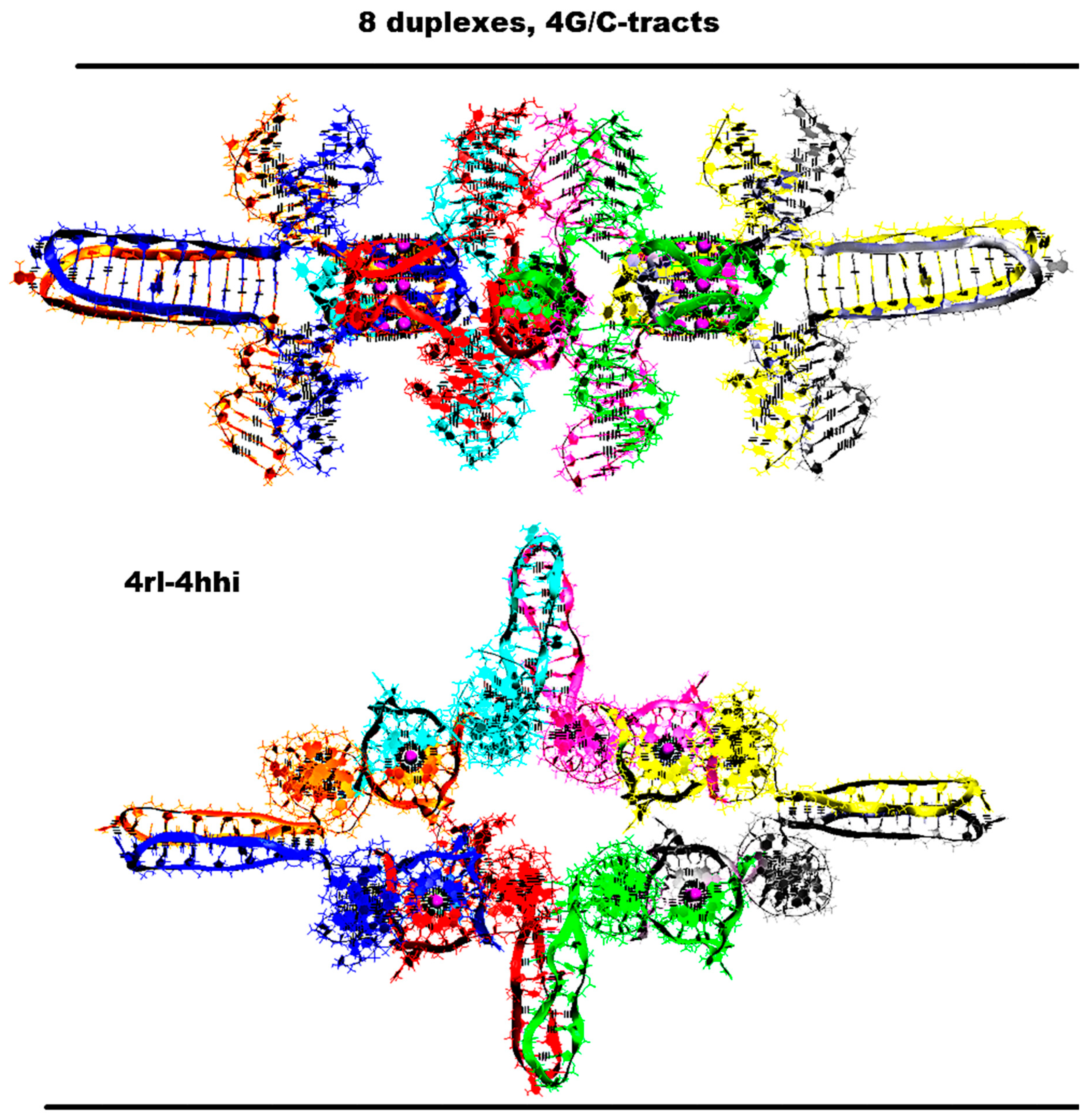
2.6. Contacts of Duplexes with 5 G/C-Tracts
2.7. Contacts of Duplexes with Six G/C-Tracts
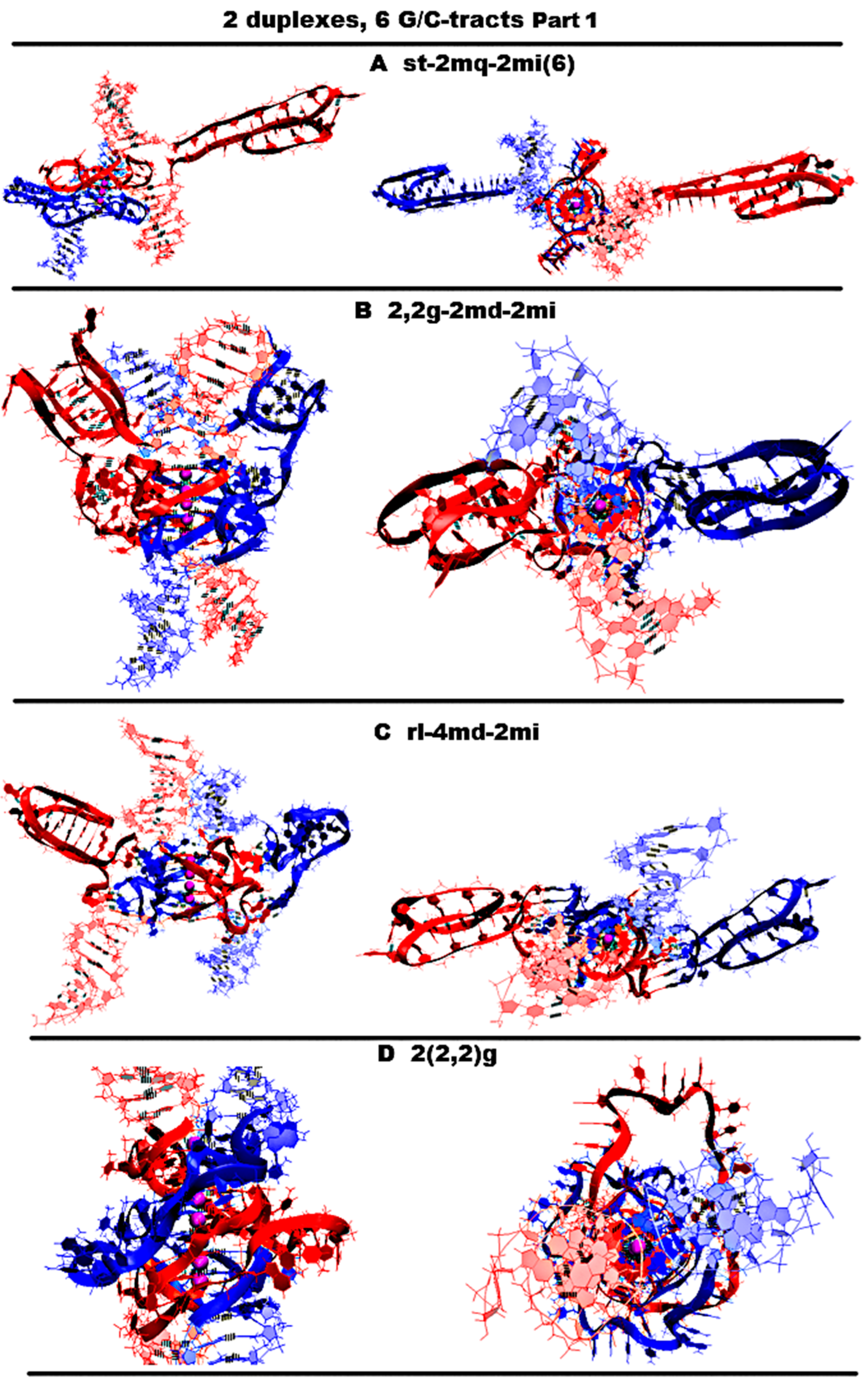
- Let us start by considering possible options with the case “stacking with two iM-monomers” (st-2mq-2mi(6)), which is presented in Figure 11A and Figure S11.A.1, and similar to the one discussed above for n = 4. As in the case considered for n = 4, the monomeric parallel G4s are arranged in the stack, while the lower tetrad of the upper G4 is stacked with the upper tetrad of the lower G4. Each of the G4s has two propeller five-nucleotide loops, between which there is the propeller single-nucleotide loop. At the same time, five-nucleotide loops, located one above another, diverge during the MD calculations, and these loops, if you look at the G4 stack from above, look like petals located at an angle of 90 degrees. The iMs located opposite each other are separated from the duplexes by CCCA fragments at each end. During the MD, as follows from Figure S11.A.2–3, the G4s, their stack, and the iMs were not deformed.
- “1,2. girth with two iM-monomers with two mini-duplexes” (2,2g-2md-2mi) is presented in Figure 11B and Figure S11.B.1. As in case 3 for n = 3, the G-strands form the stack consisting of the dimeric parallel G4s. The geometry of the strands forming the G4s is similar to the one considered in option 3 for n = 3, with the only difference that two of the propeller loops are five-nucleotide. But in contrast to the above option 1, five nucleotides are neighboring. As in the considered case 1, the iMs are monomeric head-to-head, but unlike variant 1, the iMs border on the duplexes at one end and are separated by a C3AC3 fragment from their other end. At the same time, the cytosines of the CCCA fragment, closest to the iM, form a mini-duplex with guanines in the five-nucleotide loop, the closest to the iM. Table S1 (Appendix to Figure S11.B.3.L) presents data of the percentage of MD trajectory snapshots during which the presence of hydrogen bonds, formed by G-C pairs in the mini-duplexes, was observed, and Figure S11.B.3.L shows the evolution of the number of hydrogen bonds in the mini-duplexes. An analysis of the data, presented in Table S1 and in Figure S11.B.3.L, shows that both mini-duplexes were preserved during the first 10 ns; then, one of them demonstrated instability as a result of thermal fluctuations. During the MD, as follows from Figure S11.B.2–3, the G4s’ structures were unchanged, the angle of rotation of one G4 relative to the other changed by 15 degrees (see Figure S11.B.2.D), and boundary pairs in the iMs had undergone deformations (see Figure S11.B.3.J-K).
- “Stacking of right and left handed parallel G4-dimers with two iM-monomers and 4 mini-duplexes” (rl-4md-2mi) is presented in Figure 11C and Figure S11.C.1. In this option, the geometry of the location of the G4s relative to each other and their topology is the same as in the previously considered case 7 for n = 3 with two duplexes in contact. Namely, G-strands form with a ratio of 2/4:2/4 two parallel dimeric G4s located one above another, one of which is right-handed at the bottom and the other is left-handed at the top. At the same time, G-repeats 2 and 5 from (G3T)5G3 fragments with neighboring thymines form the five-nucleotide propeller loops. The C-strands form the monomeric iMs located opposite each other, consisting of six cytosine pairs. As in option 1, the iMs are separated from duplexes by CCCA fragments at each end. These CCCA fragments form the mini-duplexes with the five-nucleotide propeller loops. Table S2 (Appendix to Figure S11.C.3.L,M) presents data of the percentage of the MD trajectory snapshots, during which the presence of hydrogen bonds, formed by G-C pairs in the mini-duplexes, was observed. The evolution of the number of hydrogen bonds in the mini-duplexes is shown on Figure S11.C.3.L,M. An analysis of the data presented in Table S2 and Figure S11.C.3.L,M shows that all mini-duplexes are preserved throughout the entire computation time. During the calculations, as follows from Figure S11.C.3.J-K, only one cytosine pair from each iM was changed.
- “Stacking of three parallel G4-dimers” (2(2,2)g) is presented in Figure 11D and Figure S11.D.1. The G-strands form three parallel dimeric G4s located one above another with the ratio of 2/4:2/4. The vectors connecting the tetrads’ COMs are unidirectional for all G4s. The C-strands wind around the stack formed by the G4s. During the MD, no changes occurred in the G4 stack; see Figure S11.D.2-3.
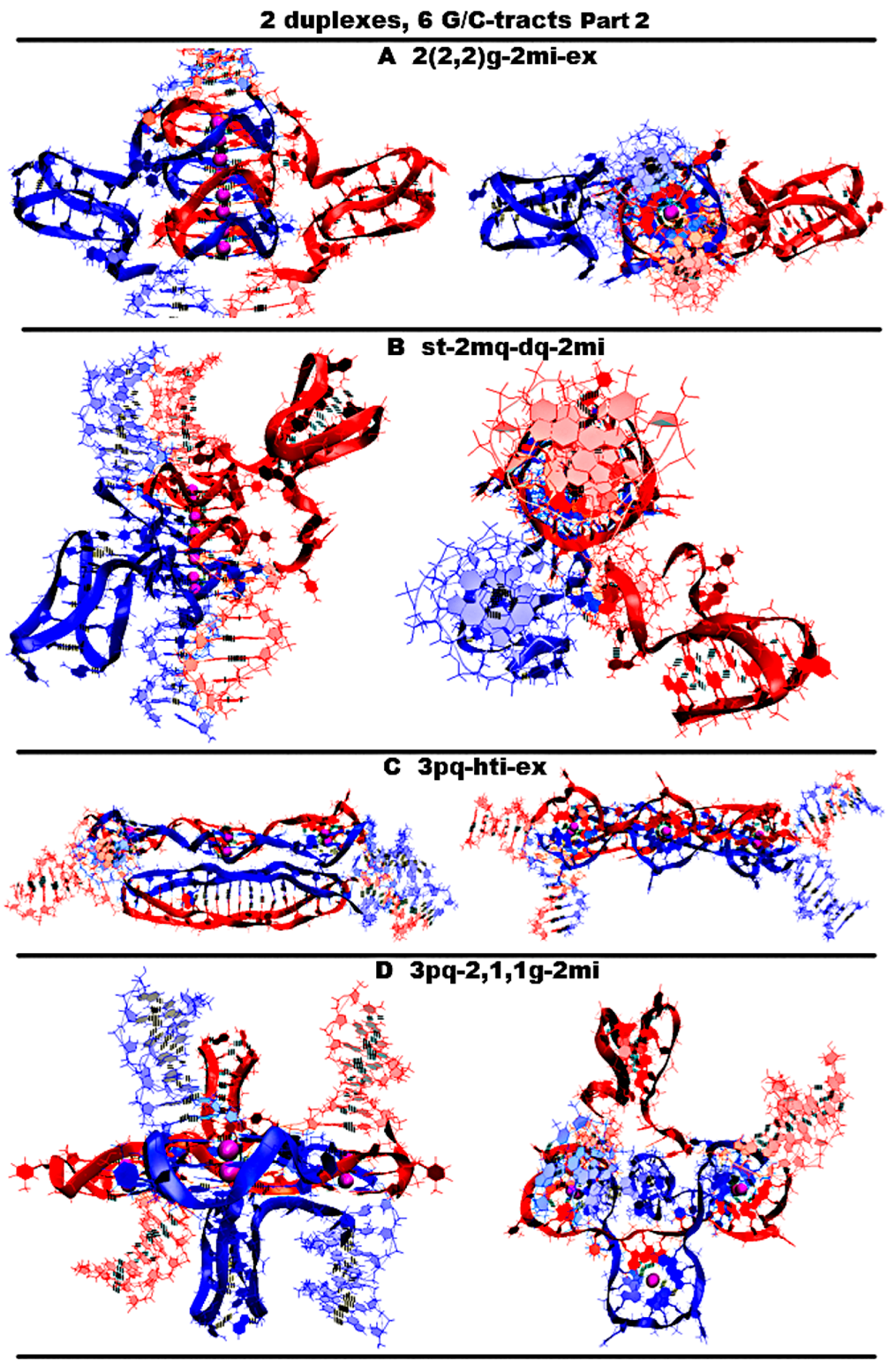
- “Stacking of three parallel G4-dimer and two iM-monomers with mutual girth of the strands” (2(2,2)g-2mi) is presented in Figure 12A and Figure S12.A.1. As in case 5, the G-strands form three parallel dimeric G4s located one above another with the strands’ ratio of 2/4:2/4. However, in contrast to case 5, C-strands form the monomeric iMs as in cases 1 and 6 considered above. This option is possible only in the case of the G-strands mutual girthing during G4 formation. As in case 6, as follows from Figure S12.A.2-3, during MD, only two cytosine pairs from the iMs turned out to be unstable.
- “Stacking of two parallel G4-monomers and G4-dimer, and two iM-monomers” (st-2mq-dq-2mi) is presented in Figure 12B and Figure S12.B.1. As in case 5, the G-strands form the stack of the parallel G4s located one above another. But unlike the previous variant, the boundary G4s are monomers, and the middle one is formed from two strands in the ratio 2/4:2/4. As in variant 1 considered above, the C-strand forms two monomeric iMs separated from duplexes by CCCA segments at each end. During the MD, as follows from Figure S12.B.2–3, the G4s’ stack did not change, and one cytosine pair was subjected to deformation in each of the iMs.
- “Three parallel G4-dimers in the same plane with head-to-tail iM-dimer with the strands exchange” (3pq-hti-ex) is presented in Figure 12C and Figure S12.C.1. As in the previously described case 15 for n = 3, the G-strands form the parallel dimeric G4, the COMS of which lay on the same straight line. Each G4 in this case is formed by two strands in the ratio of 2/4:2/4. The analysis of the angles between neighboring vectors connecting the tetrads’ COMs, see curves QIQII and QIIQIII on Figure S12.C.2.D, shows that during the MD calculations, the values of these angles fluctuated mainly around 45 degrees. The structures of the first and the third G4s were stable, and in the second G4, the guanine of one of the boundary tetrads moved slightly away from the tetrad’s COM; see Figure S12.C.2–3.F-H,J. The C-strands form the head-to-tail dimeric iM containing three fragments with five cytosine pairs each, separated by the parts containing adenines and cytosines, located so far away from each other that they cannot form hydrogen bonds; see diagram Figure S12.C.3.L-M. This variant is possible only on the condition of the exchange of the strands in the duplexes. As follows from Figure S12.C.3.L-M, during the MD, the iM structure remained unchanged.
- “Three parallel G4-dimers in the same plane and two iM-monomers with mutual girth of the strands” (3pq-2,1,1g-2mi) is presented in Figure 12D and Figure S12.D.1. In this option, the G-strands form the dimeric parallel G4s, the COMs of which lay in the same plane. The G4s’ COMs are located at the vertices of the triangle. Two of the three G4s are formed in the ratio of 3/4:1/4, i.e., three of four the G-tracts that form the G4 belong to one strand and the rest of another one. The third G4 is formed half by one strand and half by the other. The vectors connecting COMs of the tetrads of the G4s formed with the ratio 3/4:1/4 are oriented in one direction, and the third G4 is in the opposite direction. During the MD, the G4 structures demonstrated stability, and the localization of the G4s relative to each other did not change qualitatively, only the distances between the G4s changed; see Figure S12.D.2–3.D-KJ. The C-strands form the monomeric iMs as in cases 1, 6, and 7 considered above. The iMs are placed on the opposite sides of the plane in which the G4s’ COMs are located. At the same time, their end parts are directed towards each other. In each of the iMs, one cytosine pair was deformed during the MD calculations; see Figure S12.D.3.L-M.
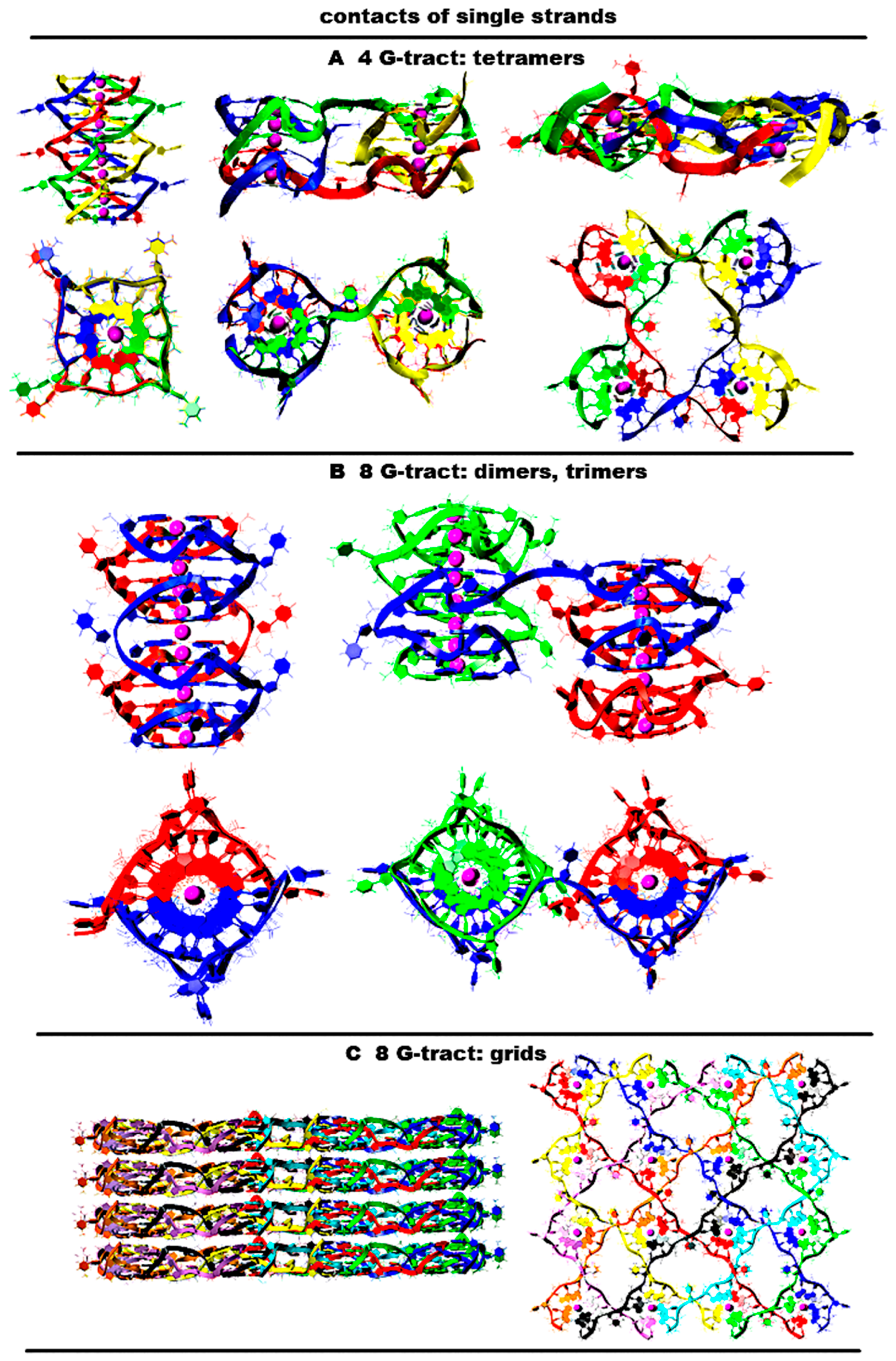
2.8. Possible Packing of Single-Stranded DNA with the Sequence (G3T)nG3 into G4 Forms in the Case of the Contact of Several Strands
3. Materials and Methods
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spiegel, J.; Adhikari, S.; Ba lasubramanian, S. The structure and function of DNA G-quadruplexes. Trends Chem. 2020, 2, 123–136. [Google Scholar] [CrossRef]
- Abou Assi, H.; Garavís, M.; González, C.; Damha, M.J. i-Motif DNA: Structural features and significance to cell biology. Nucleic Acids Res. 2018, 46, 8038–8056. [Google Scholar] [CrossRef] [PubMed]
- Zeraati, M.; Langley, D.B.; Schofield, P.; Moye, A.L.; Rouet, R.; Hughes, W.E.; Bryan, T.M.; Dinger, M.E.; Christ, D. I-motif DNA structures are formed in the nuclei of human cells. Nat. Chem. 2018, 10, 631–637. [Google Scholar] [CrossRef]
- Biffi, G.; Tannahill, D.; McCafferty, J.; Balasubramanian, S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat. Chem. 2013, 5, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Linke, R.; Limmer, M.; Juranek, S.A.; Heine, A.; Paeschke, K. The relevance of G-quadruplexes for DNA repair. Int. J. Mol. Sci. 2021, 22, 12599. [Google Scholar] [CrossRef] [PubMed]
- Dézé, O.; Laffleur, B.; Cogné, M. Roles of G4-DNA and G4-RNA in Class Switch Recombination and Additional Regulations in B-Lymphocytes. Molecules 2023, 28, 1159. [Google Scholar] [CrossRef]
- Wang, E.; Thombre, R.; Shah, Y.; Latanich, R.; Wang, J. G-Quadruplexes as pathogenic drivers in neurodegenerative disorders. Nucleic Acids Res. 2021, 49, 4816–4830. [Google Scholar] [CrossRef]
- Webba da Silva, M. Geometric Formalism for DNA Quadruplex Folding. Chem. Eur. J. 2007, 13, 9738–9745. [Google Scholar] [CrossRef]
- Frasson, I.; Pirota, V.; Richter, S.N.; Doria, F. Multimeric G-quadruplexes: A review on their biological roles and targeting. Int. J. Biol. Macromol. 2022, 204, 89–102. [Google Scholar] [CrossRef]
- Severov, V.; Tsvetkov, V.; Barinov, N.; Babenko, V.; Klinov, D.; Pozmogova, G. Spontaneous DNA synapsis by forming noncanonical intermolecular structures. Polymers 2022, 14, 2118. [Google Scholar] [CrossRef]
- Williams, J.D.; Houserova, D.; Johnson, B.R.; Dyniewski, B.; Berroyer, A.; French, H.; A Barchie, A.; Bilbrey, D.D.; Demeis, J.D.; Ghee, K.R.; et al. Characterization of long G4-rich enhancer-associated genomic regions engaging in a novel loop:loop ‘G4 Kissing’ interaction. Nucleic Acids Res. 2020, 48, 5907–5925. [Google Scholar] [CrossRef]
- Nambiar, M.; Srivastava, M.; Gopalakrishnan, V.; Sankaran, S.K.; Raghavan, S.C. G-Quadruplex Structures Formed at the HOX11 Breakpoint Region Contribute to Its Fragility during t(10;14) Translocation in T-Cell Leukemia. ASM J. Mol. Cell. Biol. 2013, 33, 4266–4281. [Google Scholar] [CrossRef]
- Hou, Y.; Li, F.; Zhang, R.; Li, S.; Liu, H.; Qin, Z.S.; Sun, X. Integrative characterization of G-Quadruplexes in the three-dimensional chromatin structure. Epigenetics 2019, 14, 894–911. [Google Scholar] [CrossRef]
- Protopopova, A.D.; Tsvetkov, V.B.; Varizhuk, A.M.; Barinov, N.A.; Podgorsky, V.V.; Klinov, D.V.; Pozmogova, G.E. The structural diversity of C-rich DNA aggregates: Unusual self-assembly of beetle-like nanostructures. Phys. Chem. Chem. Phys. 2018, 20, 3543–3553. [Google Scholar] [CrossRef]
- Chung, W.J.; Heddi, B.; Schmitt, E.; Lim, K.W.; Mechulam, Y.; Phan, A.T. Structure of a left-handed DNA G-quadruplex. Proc. Natl. Acad. Sci. USA 2015, 112, 2729–2733. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Gilbert, W. A sodium-potassium switch in the formation of four-stranded G4-DNA. Nature 1990, 344, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Yu, Z.; Konik, R.; Cui, Y.; Koirala, D.; Mao, H. G-Quadruplex and i-Motif Are Mutually Exclusive in ILPR Double-Stranded DNA. Biophys. J. 2012, 102, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Kong, D.; Ghimire, C.; Xu, C.; Mao, H. i-Motif is a General Phenomenon Governed by Steric Hindrance in Duplex DNA. Biochemistry 2016, 55, 2291–2299. [Google Scholar] [CrossRef]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Ben-Shalom, I.Y.; Berryman, J.T.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cisneros, G.A.; Cruzeiro, V.W.D.; et al. Amber 2022; University of California: San Francisco, CA, USA, 2022. [Google Scholar]
- Izadi, S.; Onufriev, A.V. Accuracy limit of rigid 3-point water models. J. Chem. Phys. 2016, 145, 074501. [Google Scholar] [CrossRef]
- Zgarbova, M.; Luque, F.J.; Sponer, J.; Cheatham, T.E., III; Otyepka, M.; Jurecka, P. Toward improved description of DNA backbone: Revisiting epsilon and zeta torsion force field parameters. J. Chem. Theory Comput. 2013, 9, 2339–2354. [Google Scholar] [CrossRef]
- Zgarbova, M.; Sponer, J.; Otyepka, M.; Cheatham, T.E., III; Galindo-Murillo, R.; Jurecka, P. Refinement of the sugar-phosphate backbone torsion beta for AMBER force fields improves the description of Z- and B-DNA. J. Chem. Theor. Comp. 2015, 12, 5723–5736. [Google Scholar] [CrossRef] [PubMed]
- Onufriev, A.; Case, D.A.; Bashford, D. Effective Born radii in the generalized Born approximation: The importance of being perfect. J. Comput. Chem. 2002, 23, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsvetkov, V.B. Modeling Possible G-Quadruplexes and i-Motifs at DNA–DNA Contact Sites: Strategy, Classification, and Examples. Int. J. Mol. Sci. 2025, 26, 5979. https://doi.org/10.3390/ijms26135979
Tsvetkov VB. Modeling Possible G-Quadruplexes and i-Motifs at DNA–DNA Contact Sites: Strategy, Classification, and Examples. International Journal of Molecular Sciences. 2025; 26(13):5979. https://doi.org/10.3390/ijms26135979
Chicago/Turabian StyleTsvetkov, Vladimir B. 2025. "Modeling Possible G-Quadruplexes and i-Motifs at DNA–DNA Contact Sites: Strategy, Classification, and Examples" International Journal of Molecular Sciences 26, no. 13: 5979. https://doi.org/10.3390/ijms26135979
APA StyleTsvetkov, V. B. (2025). Modeling Possible G-Quadruplexes and i-Motifs at DNA–DNA Contact Sites: Strategy, Classification, and Examples. International Journal of Molecular Sciences, 26(13), 5979. https://doi.org/10.3390/ijms26135979





