Tracking Drug Resistance in Plasmodium falciparum: Genetic Diversity of Key Resistance Markers in Brazilian Malaria Hotspots
Abstract
1. Introduction
2. Results
2.1. Prevalence of Drug Resistance Markers
2.2. Combined Haplotypes of pfk13, pfcrt, and pfmdr1
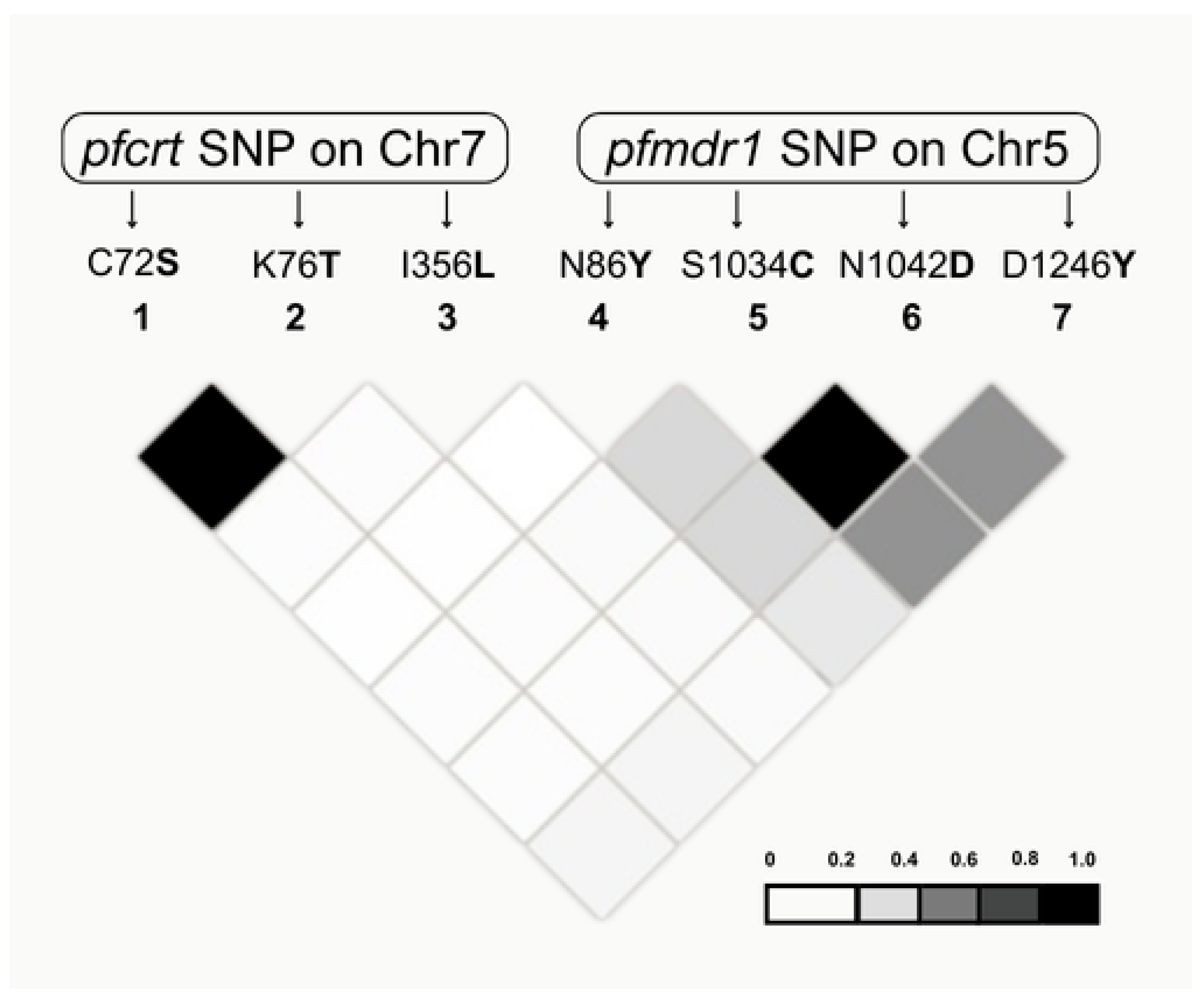
2.3. Genetic Diversity and Linkage Disequilibrium (LD) Analysis
3. Discussion
4. Materials and Methods
4.1. Blood Samples and Study Areas
4.2. Ethical Aspects and Consent to Participate
4.3. Malaria Diagnosis and Nucleic Acid Extraction
4.4. Gene Amplification and DNA Sequencing
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oladipo, H.J.; Tajudeen, Y.A.; Oladunjoye, I.O.; Yusuff, S.I.; Yusuf, R.O.; Oluwaseyi, E.M.; AbdulBasit, M.O.; Adebisi, Y.A.; El-Sherbini, M.S. Increasing challenges of malaria control in sub-Saharan Africa: Priorities for public health research and policymakers. Ann. Med. Surg. 2022, 81, 104366. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2024; World Health Organization: Geneva, Switzerland, 2024; pp. 1–293. ISBN 9789240064898. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 13 January 2025).
- Tableau Public. Malaria 2024. Available online: https://public.tableau.com/app/profile/mal.ria.brasil/viz/Dadosparacidado_201925_03_2020/Incio (accessed on 13 January 2025).
- Ministério da Saúde. Guia de tratamento da malária no Brasil; Secretaria de Vigilância em Saúde, Departamento de Imunização e Doenças Transmissíveis, 2nd ed.; Ministério da Saúde: Brasília, Brazil, 2021.
- Packard, R.M. The origins of antimalarial-drug resistance. N. Engl. J. Med. 2014, 371, 397–399. [Google Scholar] [CrossRef]
- Naidoo, I.; Roper, C. Mapping ‘partially resistant’, ‘fully resistant’, and ‘super resistant’ malaria. Trends Parasitol. 2013, 29, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Khammanee, T.; Sawangjaroen, N.; Buncherd, H.; Tun, A.W.; Thanapongpichat, S. Molecular Surveillance of Pfkelch13 and Pfmdr1 Mutations in Plasmodium falciparum Isolates from Southern Thailand. Korean J. Parasitol. 2019, 57, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Dhorda, M.; Fairhurst, R.M.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Anderson, J.M.; Mao, S.; Sam, B.; et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef]
- Pelleau, S.; Moss, E.L.; Dhingra, S.K.; Volney, B.; Casteras, J.; Gabryszewski, S.J.; Volkman, S.K.; Wirth, D.F.; Legrand, E.; Fidock, D.A.; et al. Adaptive evolution of malaria parasites in French Guiana: Reversal of chloroquine resistance by acquisition of a mutation in pfcrt. Proc. Natl. Acad. Sci. USA 2015, 112, 11672–11677. [Google Scholar] [CrossRef]
- Noedl, H.; Se, Y.; Schaecher, K.; Smith, B.L.; Socheat, D.; Fukuda, M.M.; Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 2008, 359, 2619–2620. [Google Scholar] [CrossRef]
- Mvumbi, D.M.; Kayembe, J.M.; Situakibanza, H.; Bobanga, T.L.; Nsibu, C.N.; Mvumbi, G.L.; Melin, P.; De Mol, P.; Hayette, M.-P. Falciparum malaria molecular drug resistance in the Democratic Republic of Congo: A systematic review. Malar. J. 2015, 14, 354. [Google Scholar] [CrossRef]
- Huang, F.; Yan, H.; Xue, J.B.; Cui, Y.-W.; Zhou, S.-S.; Xia, Z.-G.; Abeyasinghe, R.; Ringwald, P.; Zhou, X.-N. Molecular surveillance of pfcrt, pfmdr1, and pfk13-propeller mutations in Plasmodium falciparum isolates imported from Africa to China. Malar. J. 2021, 20, 73. [Google Scholar] [CrossRef]
- Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A.C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014, 505, 50–55. [Google Scholar] [CrossRef]
- World Health Organization. Malaria: Artemisinin Partial Resistance [Internet]; World Health Organization: Geneva, Switzerland, 2025. Available online: https://www.who.int/news-room/questions-and-answers/item/artemisinin-resistance (accessed on 18 June 2025).
- Zhao, Y.; Liu, Z.; Soe, M.T.; Wang, L.; Soe, T.N.; Wei, H.; Than, A.; Aung, P.L.; Li, Y.; Zhang, X.; et al. Genetic Variations Associated with Drug Resistance Markers in Asymptomatic Plasmodium falciparum Infections in Myanmar. Genes 2019, 10, 692. [Google Scholar] [CrossRef]
- de Abreu-Fernandes, R.; Almeida-de-Oliveira, N.K.; Gama, B.E.; Gomes, L.R.; De Lavigne Mello, A.R.; Queiroz, L.T.; Barros, J.A.; Alecrim, M.D.G.C.; Medeiros de Souza, R.; Pratt-Riccio, L.R.; et al. Plasmodium falciparum Chloroquine-pfcrt Resistant Haplotypes in Brazilian Endemic Areas Four Decades after CQ Withdrawn. Pathogens 2023, 12, 731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chenet, S.M.; Okoth, S.A.; Kelley, J.; Lucchi, N.; Huber, C.S.; Vreden, S.; Macedo de Oliveira, A.; Barnwell, J.W.; Udhayakumar, V.; Adhin, M.R. Molecular profile of malaria drug resistance markers of Plasmodium falciparum in Suriname. Antimicrob. Agents Chemother. 2017, 61, e00303-17. [Google Scholar] [CrossRef] [PubMed]
- Avcı, K.D.; Karakuş, M.; Kart Yaşar, K. Molecular survey of pfmdr-1, pfcrt, and pfk13 gene mutations among patients returning from Plasmodium falciparum endemic areas to Turkey. Malar. J. 2024, 23, 286. [Google Scholar] [CrossRef] [PubMed]
- Veiga, M.I.; Dhingra, S.K.; Henrich, P.P.; Straimer, J.; Gnädig, N.; Uhlemann, A.C.; Martin, R.E.; Lehane, A.M.; Fidock, D.A. Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies. Nat. Commun. 2016, 7, 11553. [Google Scholar] [CrossRef] [PubMed]
- Happi, C.; Gbotosho, G.; Folarin, O.; Sowunmi, A.; Hudson, T.; O’Neil, M.; Milhous, W.; Wirth, D.; Oduola, A. Selection of Plasmodium falciparum multidrug resistance gene 1 alleles in asexual stages and gametocytes by artemether-lumefantrine in Nigerian children with uncomplicated falciparum malaria. Antimicrob. Agents Chemother. 2009, 53, 888–895. [Google Scholar] [CrossRef]
- Ndong Ngomo, J.M.; Mawili-Mboumba, D.P.; M’Bondoukwé, N.P.; Ditombi, B.M.; Koumba Lengongo, J.V.; Batchy Ognagosso, F.B.; Bouyou-Akotet, M.K. Drug Resistance Molecular Markers of Plasmodium falciparum and Severity of Malaria in Febrile Children in the Sentinel Site for Malaria Surveillance of Melen in Gabon: Additional Data from the Plasmodium Diversity Network African Network. Trop. Med. Infect. Dis. 2023, 8, 184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moyeh, M.N.; Njimoh, D.L.; Evehe, M.S.; Ali, I.M.; Nji, A.M.; Nkafu, D.N.; Masumbe, P.N.; Barbara, A.T.; Ndikum, V.N.; Mbacham, W.F. Effects of Drug Policy Changes on Evolution of Molecular Markers of Plasmodium falciparum Resistance to Chloroquine, Amodiaquine, and Sulphadoxine-Pyrimethamine in the South West Region of Cameroon. Malar. Res. Treat. 2018, 2018, 7071383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Global Malaria Programme. Artemisinin Resistance and Artemisinin-Based Combination Therapy Efficacy, Status Report; World Health Organization: Geneva, Switzerland, 2018; Available online: https://iris.who.int/handle/10665/274362 (accessed on 27 June 2024).
- Gonçalves, L.A.; Cravo, P.; Ferreira, M.U. Emerging Plasmodium vivax resistance to chloroquine in South America: An overview. Mem. Inst. Oswaldo Cruz. 2014, 109, 534–539. [Google Scholar] [CrossRef]
- MalariaGEN Plasmodium falciparum Community Project. Genomic epidemiology of artemisinin-resistant malaria. eLife 2016, 5, e08714. [Google Scholar] [CrossRef]
- Gama, B.E.; de Oliveira, N.K.A.; de Souza, J.M.; Santos, F.; de Carvalho, L.J.; Melo, Y.F.; Rosenthal, P.J.; Daniel-Ribeiro, C.T.; Ferreira-Da-Cruz, M.d.F. Brazilian Plasmodium falciparum isolates: Investigation of candidate polymorphisms for artemisinin resistance before the introduction of artemisinin-based combination therapy. Malar. J. 2010, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.R.; Lavigne, A.; Peterka, C.L.; Brasil, P.; Ménard, D.; Daniel-Ribeiro, C.T.; Ferreira-da-Cruz, M.F. Absence of K13 polymorphism in Plasmodium falciparum from Brazilian areas where the parasite is endemic. Antimicrob. Agents Chemother. 2018, 62, e00354-18. [Google Scholar] [CrossRef]
- Mathieu, L.C.; Singh, P.; Monteiro, W.M.; Magris, M.; Cox, H.; Lazrek, Y.; Melo, G.C.; Marchesini, P.; Alexandre, J.S.F.; Alvarez, A.M.; et al. Kelch13 mutations in Plasmodium falciparum and risk of spreading in Amazon basin countries. J. Antimicrob. Chemother. 2021, 76, 2854–2862. [Google Scholar] [CrossRef]
- de Aguiar-Barros, J.; Granja, F.; de Abreu-Fernandes, R.; de Queiroz, L.T.; da Silva, E.S.; Citó, A.C.; Mocelin, N.K.A.; Daniel-Ribeiro, C.T.; Ferreira-da-Cruz, M.F. Molecular surveillance of artemisinin-resistant Plasmodium falciparum parasites in mining areas of the Roraima Indigenous Territory in Brazil. Int. J. Environ. Res. Public Health 2024, 21, 679. [Google Scholar] [CrossRef]
- Miotto, O.; Amato, R.; Ashley, E.A.; MacInnis, B.; Almagro-Garcia, J.; Amaratunga, C.; Lim, P.; Mead, D.; Oyola, S.O.; Dhorda, M.; et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet. 2015, 47, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Olivera, M.J.; Guerra, A.P.; Cortes, L.J.; Horth, R.Z.; Padilla, J.; Novoa, J.; Ade, M.P.; Ljolje, D.; Lucchi, N.W.; Marquiño, W.; et al. Artemether-Lumefantrine Efficacy for the Treatment of Uncomplicated Plasmodium falciparum Malaria in Choco, Colombia after 8 Years as First-Line Treatment. Am. J. Trop. Med. Hyg. 2020, 102, 1056–1063. [Google Scholar] [CrossRef]
- Chapadense, F.; Machado, R.L.D.; Ventura, A.M.R.D.S.; Áreas, A.; Machado, R.B.; Viana, G.R.; Zalis, M.G.; de Castro, A.M.; Cravo, P. Plasmodium falciparum malarial parasites from Brazil lack artemisinin resistance-associated mutations in the kelch13 gene. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.M.; Zhang, H.W.; Yang, C.Y.; Liu, Y.; Zhao, Y.L.; Li, S.H.; Qian, D.; Xu, B.L. Molecular mutation profile of pfcrt in Plasmodium falciparum isolates imported from Africa in Henan Province. Malar. J. 2016, 15, 265. [Google Scholar] [CrossRef]
- Inoue, J.; Lopes, D.; do Rosário, V.; Machado, M.; Hristov, A.D.; Lima, G.F.; Costa-Nascimento, M.J.; Segurado, A.C.; Di Santi, S.M. Analysis of polymorphisms in Plasmodium falciparum genes related to drug resistance: A survey over four decades under different treatment policies in Brazil. Malar. J. 2014, 13, 372. [Google Scholar] [CrossRef]
- Costa, G.L.; Amaral, L.C.; Fontes, C.J.F.; Carvalho, L.H.; de Brito, C.F.A.; de Sousa, T.N. Assessment of copy number variation in genes related to drug resistance in Plasmodium vivax and Plasmodium falciparum isolates from the Brazilian Amazon and a systematic review of the literature. Malar. J. 2017, 16, 152. [Google Scholar] [CrossRef]
- Njiro, B.J.; Mutagonda, R.F.; Chamani, A.T.; Mwakyandile, T.; Sabas, D.; Bwire, G.M. Molecular surveillance of chloroquine-resistant Plasmodium falciparum in sub-Saharan African countries after withdrawal of chloroquine for treatment of uncomplicated malaria: A systematic review. J. Infect. Public Health 2022, 15, 550–557. [Google Scholar] [CrossRef]
- Foguim, F.T.; Bogreau, H.; Gendrot, M.; Mosnier, J.; Fonta, I.; Benoit, N.; Amalvict, R.; Madamet, M.; Wein, S.; Pradines, B. Prevalence of mutations in the Plasmodium falciparum chloroquine resistance transporter, PfCRT, and association with ex vivo susceptibility to common anti-malarial drugs against African Plasmodium falciparum isolates. Malar. J. 2020, 19, 201. [Google Scholar] [CrossRef]
- Ross, L.S.; Dhingra, S.K.; Mok, S.; Yeo, T.; Wicht, K.J.; Kümpornsin, K.; Takala-Harrison, S.; Witkowski, B.; Fairhurst, R.M.; Ariey, F.; et al. Emerging Southeast Asian PfCRT mutations confer Plasmodium falciparum resistance to the first-line antimalarial piperaquine. Nat. Commun. 2018, 9, 3314. [Google Scholar] [CrossRef]
- Otienoburu, S.D.; Maïga-Ascofaré, O.; Schramm, B.; Jullien, V.; Jones, J.J.; Zolia, Y.M.; Houze, P.; Ashley, E.A.; Kiechel, J.R.; Guerin, P.J.; et al. Selection of Plasmodium falciparum pfcrt and pfmdr1 polymorphisms after treatment with artesunate-amodiaquine fixed-dose combination or artemether-lumefantrine in Liberia. Malar. J. 2016, 15, 452. [Google Scholar] [CrossRef]
- Mbaye, A.; Dieye, B.; Ndiaye, Y.D.; Bei, A.K.; Muna, A.; Deme, A.B.; Yade, M.S.; Diongue, K.; Gaye, A.; Ndiaye, I.M.; et al. Selection of N86F184D1246 haplotype of pfmdr1 gene by artemether-lumefantrine drug pressure on Plasmodium falciparum populations in Senegal. Malar. J. 2016, 15, 433. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, N.W.; Abdallah, R.; Louzada, J.; Udhayakumar, V.; Oliveira-Ferreira, J. Molecular surveillance for polymorphisms associated with artemisinin-based combination therapy resistance in Plasmodium falciparum isolates collected in the state of Roraima, Brazil. Malar. J. 2020, 102, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Mukhtar, M.M.; Abubakar, U.F.; Damudi, H.A.; Muhammad, A.; Ibrahim, S.S. Polymorphism Analysis of pfmdr1 and pfcrt from Plasmodium falciparum Isolates in Northwestern Nigeria Revealed the Major Markers Associated with Antimalarial Resistance. Diseases 2021, 9, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sutar, S.K.; Gupta, B.; Ranjit, M.; Kar, S.K.; Das, A. Sequence analysis of coding DNA fragments of pfcrt and pfmdr-1 genes in Plasmodium falciparum isolates from Odisha, India. Mem. Inst. Oswaldo Cruz. 2011, 106, 78–84, Erratum in: Mem. Inst. Oswaldo Cruz. 2011, 106. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.A.; Forero-Peña, D.A.; Schneider, K.A.; Chavero, M.; Gamardo, A.; Figuera, L.; Kadakia, E.R.; Grillet, M.E.; Oliveira-Ferreira, J.; Escalante, A.A. Malaria in Venezuela: Changes in the complexity of infection reflects the increment in transmission intensity. Malar. J. 2020, 19, 176. [Google Scholar] [CrossRef]
- Delandre, O.; Daffe, S.M.; Gendrot, M.; Diallo, M.N.; Madamet, M.; Kounta, M.B.; Diop, M.N.; Bercion, R.; Sow, A.; Ngom, P.M.; et al. Absence of association between polymorphisms in the pfcoronin and pfk13 genes and the presence of Plasmodium falciparum parasites after treatment with artemisinin derivatives in Senegal. Int. J. Antimicrob. Agents 2020, 56, 106190. [Google Scholar] [CrossRef]
- Paloque, L.; Coppée, R.; Stokes, B.H.; Gnädig, N.F.; Niaré, K.; Augereau, J.M.; Fidock, D.A.; Clain, J.; Benoit-Vical, F. Mutation in the Plasmodium falciparum BTB/POZ domain of K13 protein confers artemisinin resistance. Antimicrob. Agents Chemother. 2022, 66, e01320-21. [Google Scholar] [CrossRef] [PubMed]
- Gama, B.E.; Silva-Pires, F.d.E.S.; Lopes, M.N.K.R.; Cardoso, M.A.B.; Britto, C.; Torres, K.L.; de Mendonça Lima, L.; de Souza, J.M.; Daniel-Ribeiro, C.T.; de Fátima Ferreira-da-Cruz, M. Real-Time PCR versus Conventional PCR for Malaria Parasite Detection in Low-Grade Parasitemia. Exp. Parasitol. 2007, 116, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Almeida-De-Oliveira, N.K.; Moreira, O.C.; De Lavigne, A.R.; Mendonça-Lima, L.; Werneck, G.L.; Daniel-Ribeiro, C.T.; Ferreira-Da-Cruz, M.D.F. Analytical Validation of Real-Time Quantitative PCR Assays for Optimum Diagnosis of Vivax Malaria. Mem. Inst. Oswaldo Cruz. 2019, 114, e180350. [Google Scholar] [CrossRef]
- Zalis, M.G.; Ferreira-da-Cruz, M.F.; Balthazar-Guedes, H.C.; Banic, D.M.; Alecrim, W.; Souza, J.M.; Druilhe, P.; Daniel-Ribeiro, C.T. Malaria Diagnosis: Standardization of a Polymerase Chain Reaction for the Detection of Plasmodium falciparum Parasites in Individuals with Low-Grade Parasitemia. Parasitol. Res. 1996, 82, 612–616. [Google Scholar] [CrossRef]
- Snounou, G.; Viriyakosol, S.; Zhu, X.P.; Jarra, W.; Pinheiro, L.; do Rosario, V.E.; Thaithong, S.; Brown, K.N. High Sensitivity of Detection of Human Malaria Parasites by the Use of Nested Polymerase Chain Reaction. Mol. Biochem. Parasitol. 1993, 61, 315–320. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
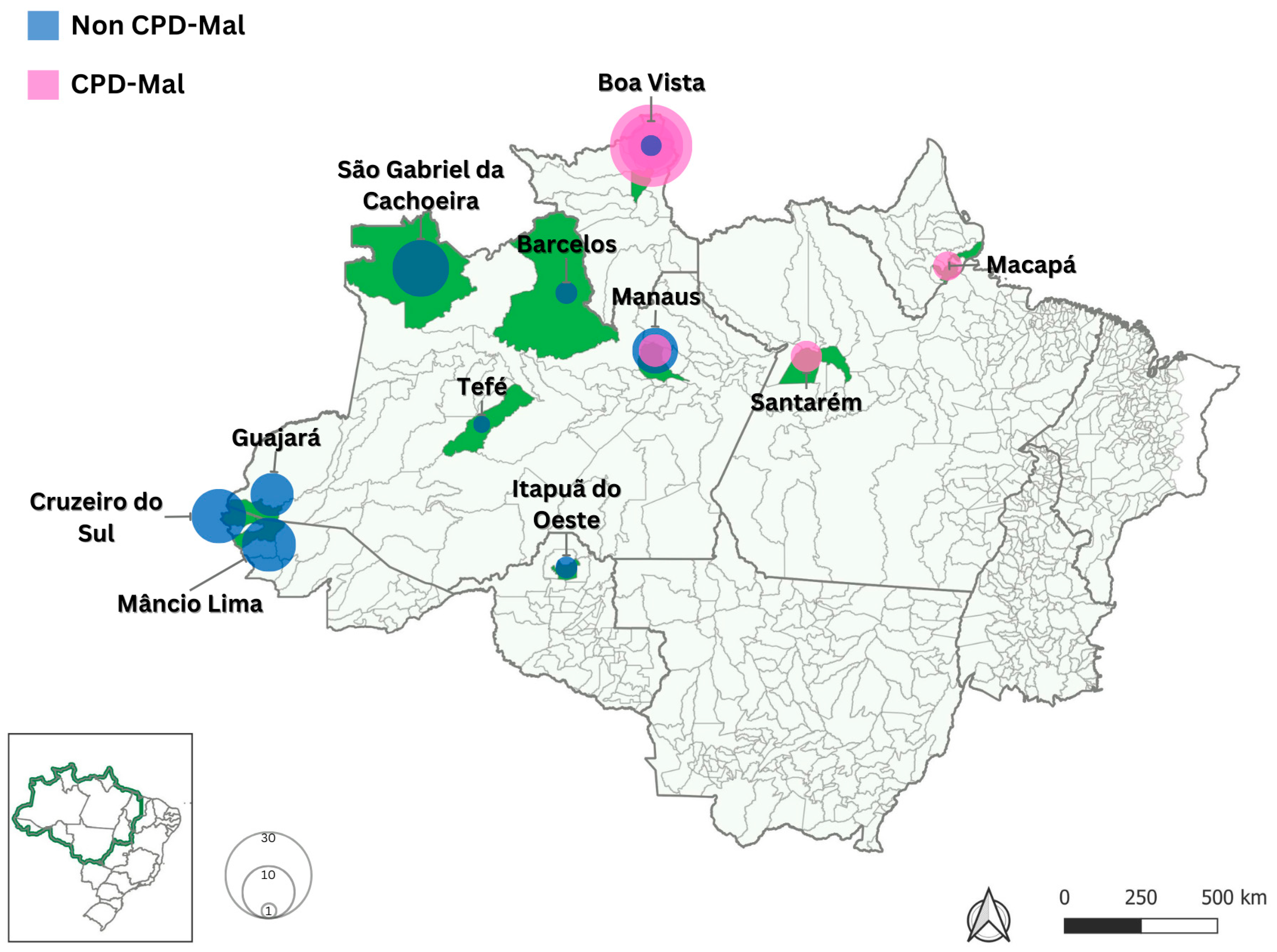
| Genotype 1 | Samples N(%) | Brazilian States | |||||
|---|---|---|---|---|---|---|---|
| Acre | Amazonas | Amapá | Roraima | Rondônia | Pará | ||
| (55 s) | (46 s) | (1 s) | (34 s ) | (2 s) | (3 s) | ||
| SVMNTMCGI 2 | 83 (59) | 44 | 27 | 1 | 7 | 2 | 2 |
| SVMNTMCGL 3 | 56 (40) | 11 | 17 | 0 | 27 | 0 | 1 |
| CVMNKMCGI 4 | 2 (1) | 0 | 2 | 0 | 0 | 0 | 0 |
| Genotype 1 | Number of Isolates (%) | Brazilian States | |||||
|---|---|---|---|---|---|---|---|
| Acre | Amazonas | Amapá | Roraima | Rondônia | Pará | ||
| (n = 55) | (n = 46) | (n = 1) | (n = 34) | (n = 2) | (n = 3) | ||
| NYCDD | 16 (11) | 12 | 4 | 0 | 0 | 0 | 0 |
| NYCDY 2 | 98 (70) | 35 | 27 | 1 | 31 | 1 | 3 |
| NYSND 3 | 19 (13) | 8 | 9 | 0 | 2 | 0 | 0 |
| NYSNY 4 | 3 (2) | 0 | 2 | 0 | 0 | 1 | 0 |
| YYSND 5 | 5 (4) | 0 | 4 | 0 | 1 | 0 | 0 |
| Haplotypes 1 | Combination Type | Samples N (%) | ||
|---|---|---|---|---|
| pfk13 | pfcrt | pfmdr1 | ||
| Wild-type 2 + | SVMNTMCGL+ | NYCDD | Quintuple | 5 (4) |
| NYCDY | Sextuple | 44 (31) | ||
| NYSND | Triple | 5 (4) | ||
| YYSND | Quadruple | 2 (1) | ||
| Wild-type 2 + | SVMNTMCGI+ | NYCDD | Quadruple | 10 (7) |
| NYCDY | Quintuple | 54 (38) | ||
| NYSND | Double | 13 (9) | ||
| NYSNY | Triple | 3 (2) | ||
| YYSND | Triple | 3 (2) | ||
| Wild-type 2 + | CVMNKMCGI 2 + | NYCDD | Double | 1 (1) |
| NYSND2 | Wild | 1 (1) | ||
| Parameters | pfcrt | pfmdr1 | pfk13 | |||
|---|---|---|---|---|---|---|
| Exon 2 | Exon 10 | Initial Region | Terminal Region | Helix Domain | ||
| SNPs (n) | 2 | 2 | 1 | 3 | 0 | |
| Haplotypes (n) | 3 | 2 | 2 | 4 | 1 | |
| Nucleotide diversity | π | 0.00182 | 0.00165 | 0.00012 | 0.00112 | 0 |
| θ | 0.00249 | 0.00063 | 0.00033 | 0.00058 | 0 | |
| Haplotype diversity | 0.146 | 0.471 | 0.068 | 0.483 | 0 | |
| Variance Hd | 0.00152 | 0.00045 | 0.00083 | 0.00184 | 0 | |
| SD Hd | 0.039 | 0.021 | 0.029 | 0.043 | 0 | |
| Neutrality Tests | ||||||
| Tajima’s D | −0.39181 | 1.72185 | −0.66673 | 1.58461 | 0 | |
| Fu and Li D * | 0.65945 | 0.4719 | 0.4719 | 0.79831 | 0 | |
| Fu and Li F * | 0.38727 | 0.9992 | 0.1451 | 1.2374 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Abreu-Fernandes, R.; de Queiroz, L.T.; Almeida-de-Oliveira, N.K.; de Lavigne Mello, A.R.; de Aguiar Barros, J.; Pratt-Riccio, L.R.; Melo, G.C.d.; Brasil, P.; Daniel-Ribeiro, C.T.; Menard, D.; et al. Tracking Drug Resistance in Plasmodium falciparum: Genetic Diversity of Key Resistance Markers in Brazilian Malaria Hotspots. Int. J. Mol. Sci. 2025, 26, 5977. https://doi.org/10.3390/ijms26135977
de Abreu-Fernandes R, de Queiroz LT, Almeida-de-Oliveira NK, de Lavigne Mello AR, de Aguiar Barros J, Pratt-Riccio LR, Melo GCd, Brasil P, Daniel-Ribeiro CT, Menard D, et al. Tracking Drug Resistance in Plasmodium falciparum: Genetic Diversity of Key Resistance Markers in Brazilian Malaria Hotspots. International Journal of Molecular Sciences. 2025; 26(13):5977. https://doi.org/10.3390/ijms26135977
Chicago/Turabian Stylede Abreu-Fernandes, Rebecca, Lucas Tavares de Queiroz, Natália Ketrin Almeida-de-Oliveira, Aline Rosa de Lavigne Mello, Jacqueline de Aguiar Barros, Lilian Rose Pratt-Riccio, Gisely Cardoso de Melo, Patrícia Brasil, Cláudio Tadeu Daniel-Ribeiro, Didier Menard, and et al. 2025. "Tracking Drug Resistance in Plasmodium falciparum: Genetic Diversity of Key Resistance Markers in Brazilian Malaria Hotspots" International Journal of Molecular Sciences 26, no. 13: 5977. https://doi.org/10.3390/ijms26135977
APA Stylede Abreu-Fernandes, R., de Queiroz, L. T., Almeida-de-Oliveira, N. K., de Lavigne Mello, A. R., de Aguiar Barros, J., Pratt-Riccio, L. R., Melo, G. C. d., Brasil, P., Daniel-Ribeiro, C. T., Menard, D., & Ferreira-da-Cruz, M. d. F. (2025). Tracking Drug Resistance in Plasmodium falciparum: Genetic Diversity of Key Resistance Markers in Brazilian Malaria Hotspots. International Journal of Molecular Sciences, 26(13), 5977. https://doi.org/10.3390/ijms26135977








