Comparing Protein Stability in Modern and Ancient Sabkha Environments: Implications for Molecular Remnants on Ancient Mars
Abstract
1. Introduction
2. Results and Discussion
2.1. Age and Mineralogical Characteristics
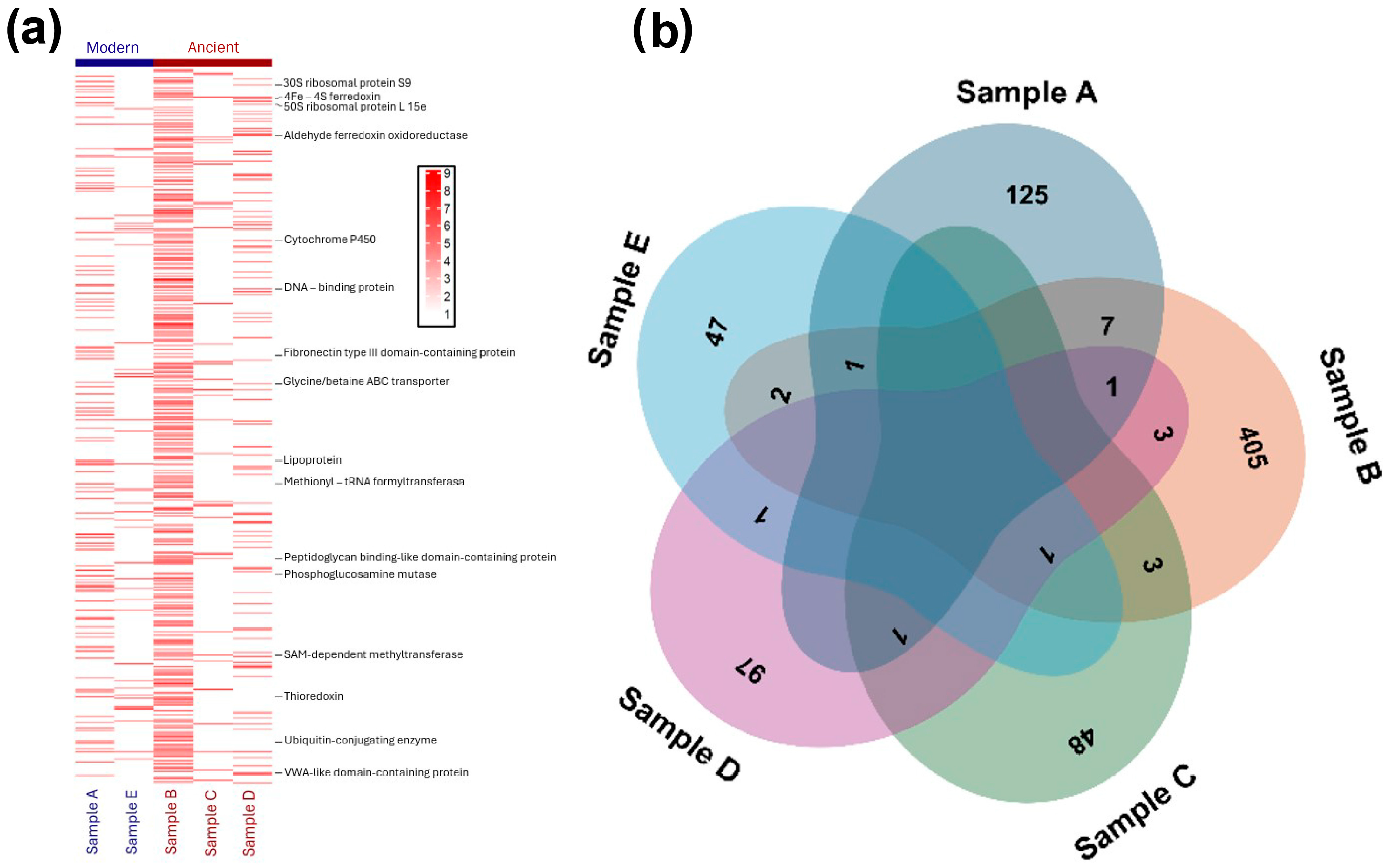
2.2. Protein Concentration and Distribution
2.3. Protein Identification and Classification
2.3.1. Classification of Preserved Proteins
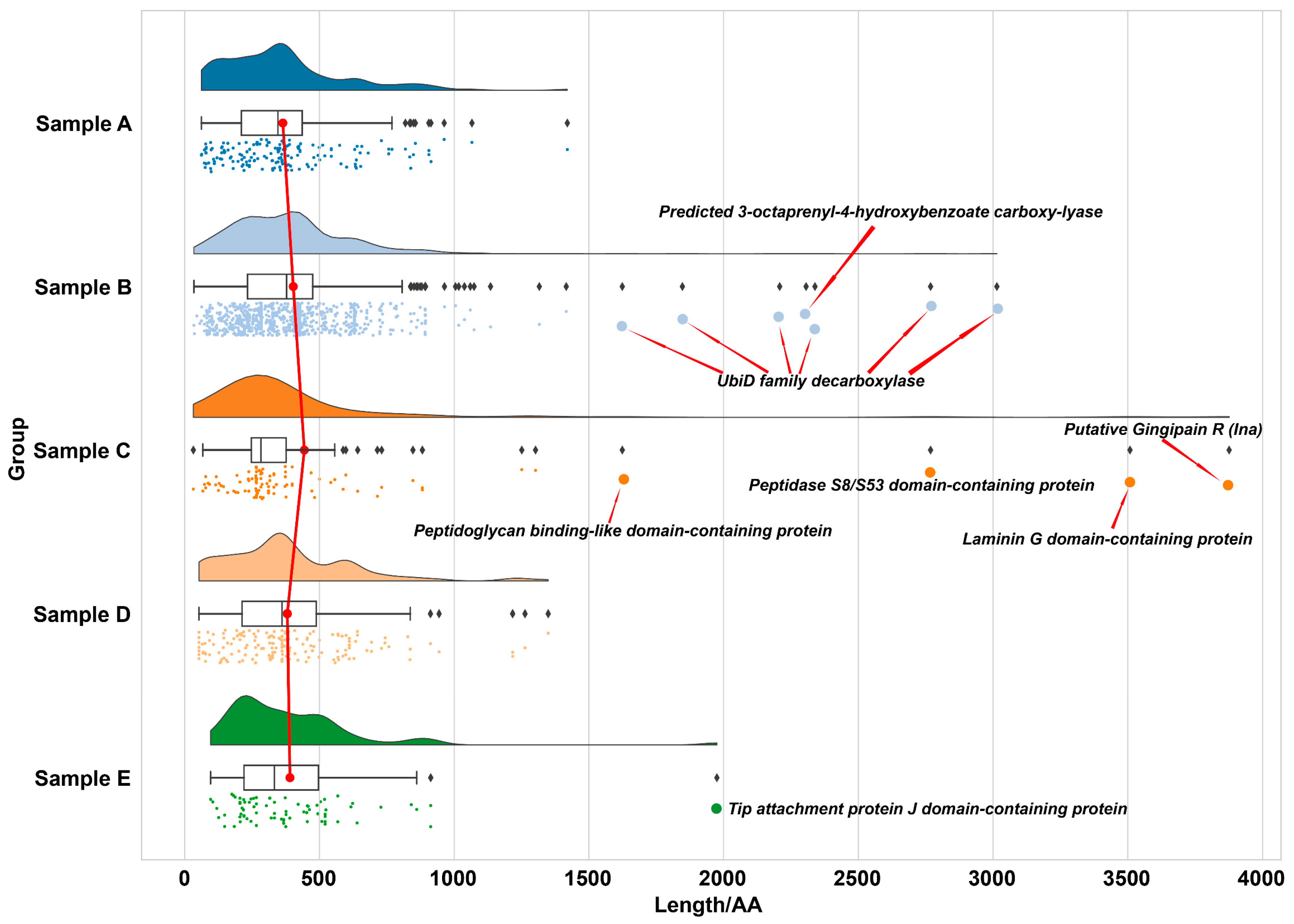
2.3.2. Length-Dependent Preservation Patterns in Ancient Proteomes
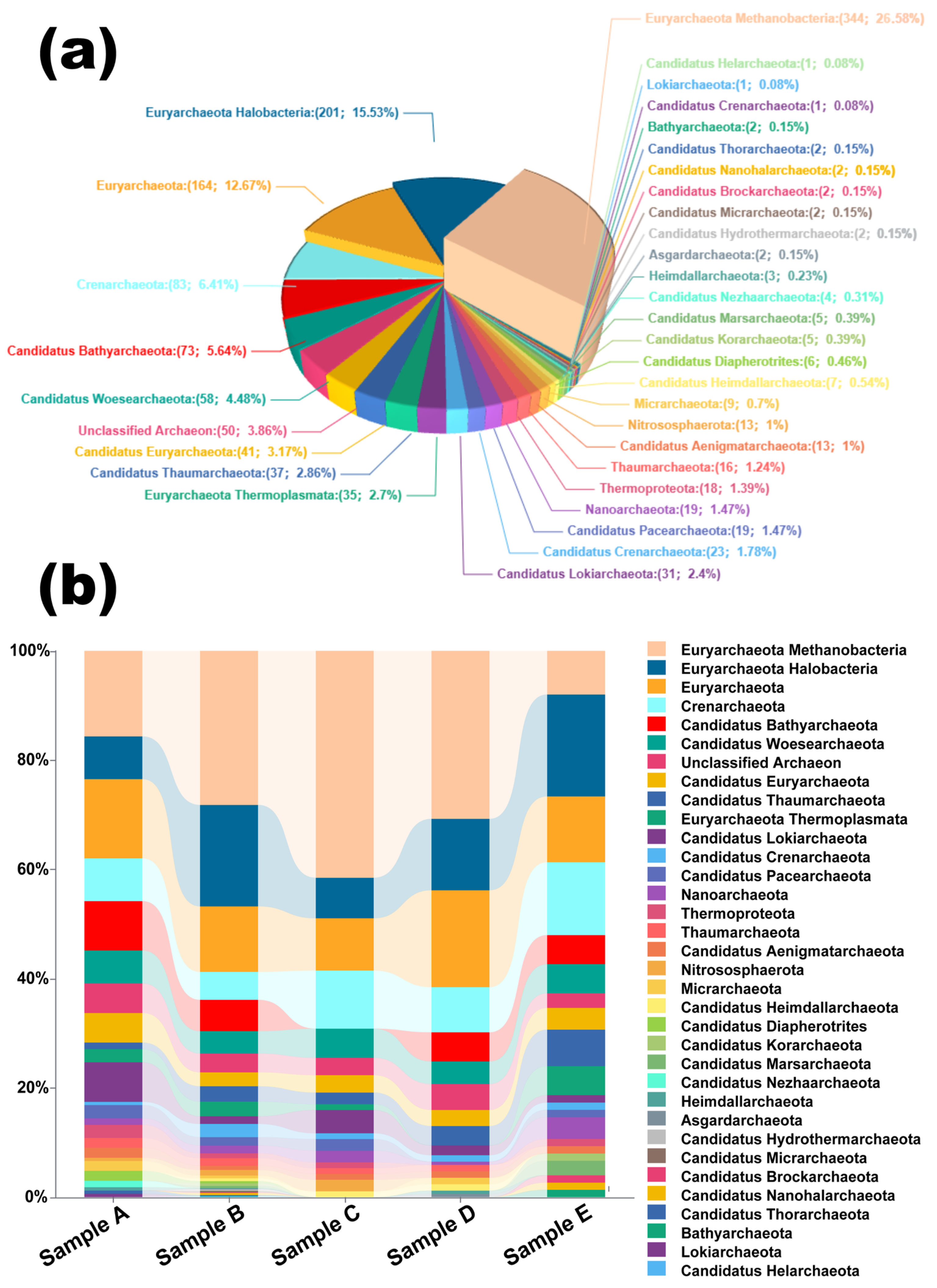
2.3.3. Taxonomic Characterization and Microbial Community Reconstruction Through Proteomics
2.4. Early Preservation Windows and the Astrobiological Significance of Poorly Characterized Proteins
3. Materials and Methods
3.1. Geologic Settings
3.2. Site Description and Sampling Procedures
3.3. Radiocarbon Dating (14C)
3.4. Mineral Identification by X-Ray Diffraction (XRD)
3.5. Protein Extraction and Concentration
3.6. Trypsin Digestion and Desalting
3.7. LC-MS Analysis
3.8. Direct DIA (Data-Independent Acquisition) Database Searching
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- McKay, C.P. The search for life on Mars. Orig. Life Evol. Biosph. 1997, 27, 263–289. [Google Scholar] [CrossRef]
- Westall, F.; Foucher, F.; Bost, N.; Bertrand, M.; Loizeau, D.; Vago, J.L.; Kminek, G.; Gaboyer, F.; Campbell, K.A.; Breheret, J.G.; et al. Biosignatures on Mars: What, Where, and How? Implications for the Search for Martian Life. Astrobiology 2015, 15, 998–1029. [Google Scholar] [CrossRef] [PubMed]
- Malin, M.C.; Edgett, K.S. Evidence for persistent flow and aqueous sedimentation on early Mars. Science 2003, 302, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Dohm, J.M.; Fink, W.; Williams, J.-P.; Mahaney, W.C.; Ferris, J.C. Chicxulub-like Gale impact into an ocean/land interface on Mars: An explanation for the formation of Mount Sharp. Icarus 2023, 390, 115306. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J. Hypothesis of an ancient northern ocean on Mars and insights from the Zhurong rover. Nat. Astron. 2024, 8, 1220–1229. [Google Scholar] [CrossRef]
- Liu, C.; Ling, Z.; Wu, Z.; Zhang, J.; Chen, J.; Fu, X.; Qiao, L.; Liu, P.; Li, B.; Zhang, L.; et al. Aqueous alteration of the Vastitas Borealis Formation at the Tianwen-1 landing site. Commun. Earth Environ. 2022, 3, 280. [Google Scholar] [CrossRef]
- Osterloo, M.M.; Hamilton, V.E.; Bandfield, J.L.; Glotch, T.D.; Baldridge, A.M.; Christensen, P.R.; Tornabene, L.L.; Anderson, F.S. Chloride-bearing materials in the southern highlands of Mars. Science 2008, 319, 1651–1654. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.C.; Phillips, R.J.; Zuber, M.T. Meridiani Planum and the global hydrology of Mars. Nature 2007, 446, 163–166. [Google Scholar] [CrossRef]
- Flahaut, J.; Carter, J.; Poulet, F.; Bibring, J.P.; van Westrenen, W.; Davies, G.R.; Murchie, S.L. Embedded clays and sulfates in Meridiani Planum, Mars. Icarus 2015, 248, 269–288. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.C.; Lewis, K.W. Early Mars hydrology: 2. Hydrological evolution in the Noachian and Hesperian epochs. J. Geophys. Res. 2011, 116, E06002. [Google Scholar] [CrossRef]
- Jaumann, R.; Tirsch, D.; Adeli, S.; Bahia, R.; Michael, G.; Le Deit, L.; Grau Galofre, A.; Head, J.; Bohacek, E.; Gross, C.; et al. Geological Record of Water and Wind Processes on Mars as Observed by the Mars Express High Resolution Stereo Camera. Space Sci. Rev. 2024, 220, 45. [Google Scholar] [CrossRef]
- Kite, E.S.; Steele, L.J.; Mischna, M.A.; Richardson, M.I. Warm early Mars surface enabled by high-altitude water ice clouds. Proc. Natl. Acad. Sci. USA 2021, 118, e2101959118. [Google Scholar] [CrossRef] [PubMed]
- Al-Farraj, A. An evolutionary model for sabkha development on the north coast of the UAE. J. Arid Environ. 2005, 63, 740–755. [Google Scholar] [CrossRef]
- Evans, G.; Schmidt, V.; Bush, P.; Nelson, H. Stratigraphy and geologic history of the sabkha, Abu Dhabi, persian gulf. Sedimentology 1969, 12, 145–159. [Google Scholar] [CrossRef]
- Abbas, K.; Deroin, J.-P.; Bouaziz, S. Monitoring of playa evaporites as seen with optical remote sensing sensors: Case of Chott El Jerid, Tunisia, from 2003 to present. Arab. J. Geosci. 2018, 11, 92. [Google Scholar] [CrossRef]
- Nair, C.P.R.; Unnikrishnan, V. Stability of the Liquid Water Phase on Mars: A Thermodynamic Analysis Considering Martian Atmospheric Conditions and Perchlorate Brine Solutions. ACS Omega 2020, 5, 9391–9397. [Google Scholar] [CrossRef]
- Warinner, C.; Korzow Richter, K.; Collins, M.J. Paleoproteomics. Chem. Rev. 2022, 122, 13401–13446. [Google Scholar] [CrossRef]
- Hendy, J. Ancient protein analysis in archaeology. Sci. Adv. 2021, 7, eabb9314. [Google Scholar] [CrossRef]
- Hendy, J.; Welker, F.; Demarchi, B.; Speller, C.; Warinner, C.; Collins, M.J. A guide to ancient protein studies. Nat. Ecol. Evol. 2018, 2, 791–799. [Google Scholar] [CrossRef]
- Herrero-Alfonso, P.; Pejenaute, A.; Millet, O.; Ortega-Quintanilla, G. Electrostatics introduce a trade-off between mesophilic stability and adaptation in halophilic proteins. Protein Sci. 2024, 33, e5003. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Plemenitas, A.; Oren, A. Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiol. Rev. 2018, 42, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.; Dev, K.; Sourirajan, A. Distinct Osmoadaptation Strategies in the Strict Halophilic and Halotolerant Bacteria Isolated from Lunsu Salt Water Body of North West Himalayas. Curr Microbiol 2018, 75, 888–895. [Google Scholar] [CrossRef]
- deMenocal, P.; Ortiz, J.; Guilderson, T.; Sarnthein, M. Coherent high- and low-latitude climate variability during the holocene warm period. Science 2000, 288, 2198–2202. [Google Scholar] [CrossRef]
- Rohling, E.J.; Foster, G.L.; Grant, K.M.; Marino, G.; Roberts, A.P.; Tamisiea, M.E.; Williams, F. Sea-level and deep-sea-temperature variability over the past 5.3 million years. Nature 2014, 508, 477–482. [Google Scholar] [CrossRef]
- Warren, J.K. Evaporites: A Geological Compendium; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Kendall, C.G.S.C.; Warren, J. A review of the origin and setting of tepees and their associated fabrics. Sedimentology 1987, 34, 1007–1027. [Google Scholar] [CrossRef]
- Wood, A.P.; Kelly, D.P.; Harrison, A.P. The role of microbial activity in saline and hypersaline evaporitic environments. In Evaporites Through Space and Time; Schreiber, B.C., Lugli, S., Bąbel, M., Eds.; Geological Society of London: London, UK, 2007; pp. 147–165. [Google Scholar]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Riding, R. Microbial carbonates: The geological record of calcified bacterial–algal mats and biofilms. Sedimentology 2000, 47, 179–214. [Google Scholar] [CrossRef]
- Benzerara, K.; Skouri-Panet, F.; Li, J.; Ferard, C.; Gugger, M.; Laurent, T.; Couradeau, E.; Ragon, M.; Cosmidis, J.; Menguy, N.; et al. Intracellular Ca-carbonate biomineralization is widespread in cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 10933–10938. [Google Scholar] [CrossRef]
- Guan, Y.; Jiang, N.; Wu, Y.; Yang, Z.; Bello, A.; Yang, W. Disentangling the role of salinity-sodicity in shaping soil microbiome along a natural saline-sodic gradient. Sci. Total Environ. 2021, 765, 142738. [Google Scholar] [CrossRef]
- Georgiou, C.D.; Sun, H.J.; McKay, C.P.; Grintzalis, K.; Papapostolou, I.; Zisimopoulos, D.; Panagiotidis, K.; Zhang, G.; Koutsopoulou, E.; Christidis, G.E.; et al. Evidence for photochemical production of reactive oxygen species in desert soils. Nat. Commun. 2015, 6, 7100. [Google Scholar] [CrossRef]
- Stan-Lotter, H.; Fendrihan, S. Halophilic Archaea: Life with Desiccation, Radiation and Oligotrophy over Geological Times. Life 2015, 5, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Life at high salt concentrations, intracellular KCl concentrations, and acidic proteomes. Front. Microbiol. 2013, 4, 315. [Google Scholar] [CrossRef]
- Schroeter, E.R.; DeHart, C.J.; Cleland, T.P.; Zheng, W.; Thomas, P.M.; Kelleher, N.L.; Bern, M.; Schweitzer, M.H. Expansion for the Brachylophosaurus canadensis Collagen I Sequence and Additional Evidence of the Preservation of Cretaceous Protein. J. Proteome Res. 2017, 16, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, J.; Fabbri, M.; Yang, T.R.; Stein, K.; Sander, P.M.; Norell, M.A.; Briggs, D.E.G. Fossilization transforms vertebrate hard tissue proteins into N-heterocyclic polymers. Nat. Commun. 2018, 9, 4741. [Google Scholar] [CrossRef]
- Saitta, E.T.; Liang, R.; Lau, M.C.; Brown, C.M.; Longrich, N.R.; Kaye, T.G.; Novak, B.J.; Salzberg, S.L.; Norell, M.A.; Abbott, G.D.; et al. Cretaceous dinosaur bone contains recent organic material and provides an environment conducive to microbial communities. Elife 2019, 8, e46205. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gong, Y.; Yin, H.; Gong, D. Different Types of Peptide Detected by Mass Spectrometry among Fresh Silk and Archaeological Silk Remains for Distinguishing Modern Contamination. PLoS ONE 2015, 10, e0132827. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Suo, Z.; Avci, R.; Asara, J.M.; Allen, M.A.; Arce, F.T.; Horner, J.R. Analyses of Soft Tissue from Tyrannosaurus rex Suggest the Presence of Protein. Science 2007, 316, 277–280. [Google Scholar] [CrossRef]
- McNamara, M.E.; van Dongen, B.E.; Lockyer, N.P.; Bull, I.D.; Orr, P.J. Fossilization of melanosomes via sulfurization. Palaeontology 2016, 59, 337–350. [Google Scholar] [CrossRef]
- Alleon, J.; Bernard, S.; Le Guillou, C.; Marin-Carbonne, J.; Pont, S.; Beyssac, O.; McKeegan, K.D.; Robert, F. Molecular preservation of 1.88 Ga Gunflint organic microfossils as a function of temperature and mineralogy. Nat. Commun. 2016, 7, 11977. [Google Scholar] [CrossRef]
- Bill, R.M.; Revers, L.; Wilson, I.B.H. Protein Glycosylation; Springer: Boston, MA, USA, 1998. [Google Scholar]
- Demarchi, B.; Hall, S.; Roncal-Herrero, T.; Freeman, C.L.; Woolley, J.; Crisp, M.K.; Wilson, J.; Fotakis, A.; Fischer, R.; Kessler, B.M.; et al. Protein sequences bound to mineral surfaces persist into deep time. Elife 2016, 5, e17092. [Google Scholar] [CrossRef]
- Briggs, D.E.G.; McMahon, S. The role of experiments in investigating the taphonomy of exceptional preservation. Palaeontology 2016, 59, 1–11. [Google Scholar] [CrossRef]
- Bada, J.L.; Eglinton, G.; Curry, G.B. Amino acid cosmogeochemistry. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1997, 333, 349–358. [Google Scholar] [CrossRef]
- Aravind, L.; Koonin, E.V. DNA-binding proteins and evolution of transcription regulation in the archaea. Nucleic Acids Res. 1999, 27, 4658–4670. [Google Scholar] [CrossRef] [PubMed]
- Li, X. A Novel Transcriptional Regulator Involved in Pyrococcus Furiosus Cold Shock Response. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2008. [Google Scholar]
- Gauss, G.H.; Benas, P.; Wiedenheft, B.; Young, M.; Douglas, T.; Lawrence, C.M. Structure of the DPS-Like Protein from Sulfolobus solfataricus Reveals a Bacterioferritin-Like Dimetal Binding Site within a DPS-Like Dodecameric Assembly. Biochemistry 2006, 45, 10815–10827. [Google Scholar] [CrossRef]
- Robles-Fernández, A.; Areias, C.; Daffonchio, D.; Vahrenkamp, V.C.; Sánchez-Román, M. The Role of Microorganisms in the Nucleation of Carbonates, Environmental Implications and Applications. Minerals 2022, 12, 1562. [Google Scholar] [CrossRef]
- Warren, J.K. Sabkhas, Saline Mudflats and Pans. In Evaporites: A Geological Compendium; Warren, J.K., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 207–301. [Google Scholar]
- Mehra-Chaudhary, R.; Mick, J.; Tanner, J.J.; Beamer, L.J. Quaternary structure, conformational variability and global motions of phosphoglucosamine mutase. FEBS J. 2011, 278, 3298–3307. [Google Scholar] [CrossRef] [PubMed]
- DasSarma, S.; Arora, P. Genetic analysis of the gas vesicle gene cluster in haloarchaea. FEMS Microbiol. Lett. 1997, 153, 1–10. [Google Scholar] [CrossRef]
- Oren, A. The bioenergetic basis for the decrease in metabolic diversity at increasing salt concentrations: Implications for the functioning of salt lake ecosystems. In Saline Lakes: Publications from the 7th International Conference on Salt Lakes, held in Death Valley National Park, California, U.S.A., September 1999; Melack, J.M., Jellison, R., Herbst, D.B., Eds.; Springer Netherlands: Dordrecht, The Netherland, 2001; pp. 61–72. [Google Scholar]
- Javor, B.; Brock, T.D. Hypersaline Environments: Microbiology and Biogeochemistry; Springer: Berlin/Heidelberg, Germany, 1989; pp. VIII, 328. [Google Scholar]
- Kumar, V.; Tiwari, S.K. Halocin Diversity Among Halophilic Archaea and Their Applications. In Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications: Volume 1. Microbial Diversity in Normal & Extreme Environments; Satyanarayana, T., Johri, B.N., Das, S.K., Eds.; Springer: Singapore, 2019; pp. 497–532. [Google Scholar]
- Lennon, J.T.; Jones, S.E. Microbial seed banks: The ecological and evolutionary implications of dormancy. Nat. Rev. Microbiol. 2011, 9, 119–130. [Google Scholar] [CrossRef]
- Johnson, S.S.; Chevrette, M.G.; Ehlmann, B.L.; Benison, K.C. Insights from the metagenome of an acid salt lake: The role of biology in an extreme depositional environment. PLoS ONE 2015, 10, e0122869. [Google Scholar] [CrossRef]
- Vreeland, R.H.; Rosenzweig, W.D.; Powers, D.W. Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature 2000, 407, 897–900. [Google Scholar] [CrossRef]
- Benzerara, K.; Menguy, N.; López-García, P.; Yoon, T.-H.; Kazmierczak, J.; Tyliszczak, T.; Guyot, F.; Brown, G.E. Nanoscale detection of organic signatures in carbonate microbialites. Proc. Natl. Acad. Sci. USA 2006, 103, 9440–9445. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.W.; Leys, D. Structural insights into UbiD reversible decarboxylation. Curr. Opin. Struct. Biol. 2022, 75, 102432. [Google Scholar] [CrossRef]
- Eichinger, A.; Beisel, H.G.; Jacob, U.; Huber, R.; Medrano, F.J.; Banbula, A.; Potempa, J.; Travis, J.; Bode, W. Crystal structure of gingipain R: An Arg-specific bacterial cysteine proteinase with a caspase-like fold. EMBO J. 1999, 18, 5453–5462. [Google Scholar] [CrossRef] [PubMed]
- Colin-Garcia, M.; Kanawati, B.; Harir, M.; Schmitt-Kopplin, P.; Amils, R.; Parro, V.; Garcia, M.; Fernandez-Remolar, D. Detection of peptidic sequences in the ancient acidic sediments of Rio Tinto, Spain. Orig. Life Evol. Biosph. 2011, 41, 523–527. [Google Scholar] [CrossRef]
- Hazzouri, K.M.; Sudalaimuthuasari, N.; Saeed, E.E.; Kundu, B.; Al-Maskari, R.S.; Nelson, D.; AlShehhi, A.A.; Aldhuhoori, M.A.; Almutawa, D.S.; Alshehhi, F.R.; et al. Salt flat microbial diversity and dynamics across salinity gradient. Sci Rep 2022, 12, 11293. [Google Scholar] [CrossRef]
- Zhong, H.; Lehtovirta-Morley, L.; Liu, J.; Zheng, Y.; Lin, H.; Song, D.; Todd, J.D.; Tian, J.; Zhang, X.H. Novel insights into the Thaumarchaeota in the deepest oceans: Their metabolism and potential adaptation mechanisms. Microbiome 2020, 8, 78. [Google Scholar] [CrossRef]
- Korzhenkov, A.A.; Toshchakov, S.V.; Bargiela, R.; Gibbard, H.; Ferrer, M.; Teplyuk, A.V.; Jones, D.L.; Kublanov, I.V.; Golyshin, P.N.; Golyshina, O.V. Archaea dominate the microbial community in an ecosystem with low-to-moderate temperature and extreme acidity. Microbiome 2019, 7, 11. [Google Scholar] [CrossRef]
- Butts, S.H.; Briggs, D.E.G. Silicification Through Time. In Taphonomy: Process and Bias Through Time; Allison, P.A., Bottjer, D.J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 411–434. [Google Scholar]
- Ehlmann, B.L.; Mustard, J.F.; Murchie, S.L.; Poulet, F.; Bishop, J.L.; Brown, A.J.; Calvin, W.M.; Clark, R.N.; Marais, D.J.D.; Milliken, R.E.; et al. Orbital Identification of Carbonate-Bearing Rocks on Mars. Science 2008, 322, 1828. [Google Scholar] [CrossRef] [PubMed]
- Tutolo, B.M.; Hausrath, E.M.; Kite, E.S.; Rampe, E.B.; Bristow, T.F.; Downs, R.T.; Treiman, A.; Peretyazhko, T.S.; Thorpe, M.T.; Grotzinger, J.P.; et al. Carbonates identified by the Curiosity rover indicate a carbon cycle operated on ancient Mars. Science 2025, 388, 292–297. [Google Scholar] [CrossRef]
- Fernández-Remolar, D.C.; Chong-Díaz, G.; Ruíz-Bermejo, M.; Harir, M.; Schmitt-Kopplin, P.; Tziotis, D.; Gómez-Ortíz, D.; García-Villadangos, M.; Martín-Redondo, M.P.; Gómez, F.; et al. Molecular preservation in halite- and perchlorate-rich hypersaline subsurface deposits in the Salar Grande basin (Atacama Desert, Chile): Implications for the search for molecular biomarkers on Mars. J. Geophys. Res. Biogeosciences 2013, 118, 922–939. [Google Scholar] [CrossRef]
- Alleon, J.; Bernard, S.; Le Guillou, C.; Daval, D.; Skouri-Panet, F.; Pont, S.; Delbes, L.; Robert, F. Early entombment within silica minimizes the molecular degradation of microorganisms during advanced diagenesis. Chem. Geol. 2016, 437, 98–108. [Google Scholar] [CrossRef]
- Rinke, C.; Schwientek, P.; Sczyrba, A.; Ivanova, N.N.; Anderson, I.J.; Cheng, J.-F.; Darling, A.; Malfatti, S.; Swan, B.K.; Gies, E.A.; et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature 2013, 499, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Spang, A.; Caceres, E.F.; Ettema, T.J.G. Genomic exploration of the diversity, ecology, and evolution of the archaeal domain of life. Science 2017, 357, eaaf3883. [Google Scholar] [CrossRef]
- Hug, L.A.; Baker, B.J.; Anantharaman, K.; Brown, C.T.; Probst, A.J.; Castelle, C.J.; Butterfield, C.N.; Hernsdorf, A.W.; Amano, Y.; Ise, K.; et al. A new view of the tree of life. Nat. Microbiol. 2016, 1, 16048. [Google Scholar] [CrossRef] [PubMed]
- Maron, P.-A.; Ranjard, L.; Mougel, C.; Lemanceau, P. Metaproteomics: A New Approach for Studying Functional Microbial Ecology. Microb. Ecol. 2007, 53, 486–493. [Google Scholar] [CrossRef]
- Boadu, F.; Lee, A.; Cheng, J. Deep learning methods for protein function prediction. Proteomics 2025, 25, 2300471. [Google Scholar] [CrossRef]
- Lokier, S.W.; Fiorini, F. Temporal evolution of a carbonate coastal system, Abu Dhabi, United Arab Emirates. Mar. Geol. 2016, 381, 102–113. [Google Scholar] [CrossRef]
- Strohmenger, C.J.; Al-Mansoori, A.; Al-Jeelani, O.; Al-Shamry, A.; Al-Hosani, I.; Al-Mehsin, K.; Shebl, H. The sabkha sequence at Mussafah Channel (Abu Dhabi, United Arab Emirates): Facies stacking patterns, microbial-mediated dolomite and evaporite overprint. GeoArabia 2010, 15, 49–90. [Google Scholar] [CrossRef]
- Court, W.M.; Paul, A.; Lokier, S.W. The preservation potential of environmentally diagnostic sedimentary structures from a coastal sabkha. Mar. Geol. 2017, 386, 1–18. [Google Scholar] [CrossRef]
- Alsharhan, A.S.; Kendall, C.G.S.C. Holocene coastal carbonates and evaporites of the southern Arabian Gulf and their ancient analogues. Earth-Sci. Rev. 2003, 61, 191–243. [Google Scholar] [CrossRef]
- Lokier, S.W.; Knaf, A.; Kimiagar, S. A quantitative analysis of Recent arid coastal sedimentary facies from the Arabian Gulf Coastline of Abu Dhabi, United Arab Emirates. Mar. Geol. 2013, 346, 141–152. [Google Scholar] [CrossRef]
- Wood, W.W.; Bailey, R.M.; Hampton, B.A.; Kraemer, T.F.; Lu, Z.; Clark, D.W.; James, R.H.R.; Al Ramadan, K. Rapid late Pleistocene/Holocene uplift and coastal evolution of the southern Arabian (Persian) Gulf. Quat. Res. 2012, 77, 215–220. [Google Scholar] [CrossRef]
- Lokier, S.W.; Bateman, M.D.; Larkin, N.R.; Rye, P.; Stewart, J.R. Late Quaternary sea-level changes of the Persian Gulf. Quat. Res. 2015, 84, 69–81. [Google Scholar] [CrossRef]
- Stevens, T.; Jestico, M.J.; Evans, G.; Kirkham, A. Eustatic control of late Quaternary sea-level change in the Arabian/Persian Gulf. Quat. Res. 2014, 82, 175–184. [Google Scholar] [CrossRef]
- Engel, M.; Strohmenger, C.J.; Peis, K.T.; Pint, A.; Brill, D.; Brückner, H. High-resolution facies analysis of a coastal sabkha in the eastern Gulf of Salwa (Qatar): A spatio-temporal reconstruction. Sedimentology 2022, 69, 1119–1150. [Google Scholar] [CrossRef]
- Paulo, C.; Dittrich, M. 2D Raman spectroscopy study of dolomite and cyanobacterial extracellular polymeric substances from Khor Al-Adaid sabkha (Qatar). J. Raman Spectrosc. 2013, 44, 1563–1569. [Google Scholar] [CrossRef]
- Olsen, J.V.; Ong, S.E.; Mann, M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol. Cell Proteom. 2004, 3, 608–614. [Google Scholar] [CrossRef]
- Ma, J.; Chen, T.; Wu, S.; Yang, C.; Bai, M.; Shu, K.; Li, K.; Zhang, G.; Jin, Z.; He, F.; et al. iProX: An integrated proteome resource. Nucleic Acids Res. 2019, 47, D1211–D1217. [Google Scholar] [CrossRef]
- Chen, T.; Ma, J.; Liu, Y.; Chen, Z.; Xiao, N.; Lu, Y.; Fu, Y.; Yang, C.; Li, M.; Wu, S.; et al. iProX in 2021: Connecting proteomics data sharing with big data. Nucleic Acids Res. 2022, 50, D1522–D1527. [Google Scholar] [CrossRef]
- Sinitcyn, P.; Hamzeiy, H.; Salinas Soto, F.; Itzhak, D.; McCarthy, F.; Wichmann, C.; Steger, M.; Ohmayer, U.; Distler, U.; Kaspar-Schoenefeld, S.; et al. MaxDIA enables library-based and library-free data-independent acquisition proteomics. Nat. Biotechnol. 2021, 39, 1563–1573. [Google Scholar] [CrossRef]
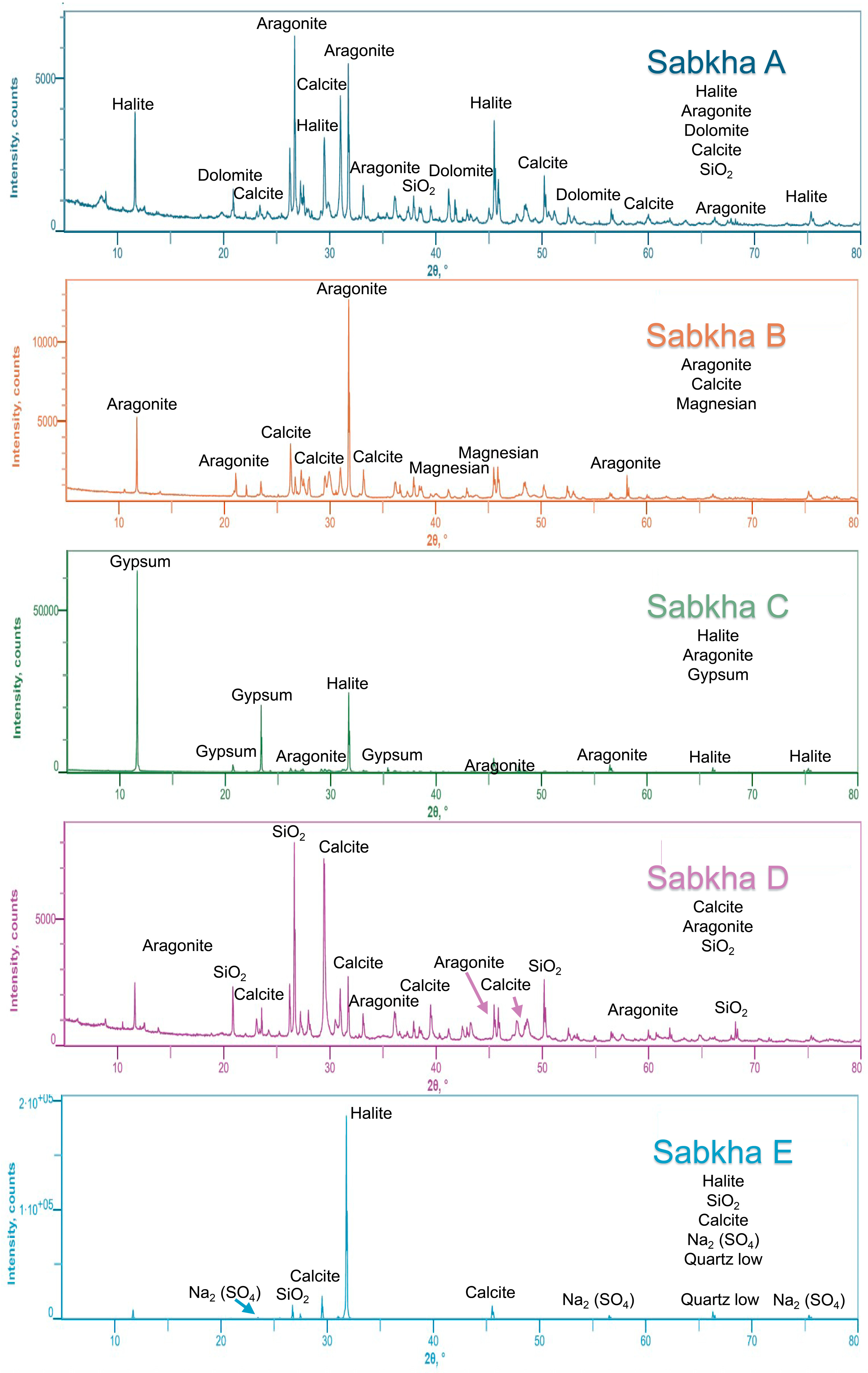


| Sample | TPC | Proteins | Peptides | Prot/Pep Rate | Length Range | Average Age Yr (14C) | Mineral Composition | Estimated Salinity |
|---|---|---|---|---|---|---|---|---|
| Sample A | 540 | 125 | 244 | 0.51 | 79–1420 | Modern Sediment | Aragonite/Calcite | 40–70 |
| Sample B | 438 | 405 | 539 | 0.75 | 92–964 | 2918–2502 Cal BP | Aragonite/Calcite/Mg Calcite | 60–90 |
| Sample C | 452 | 48 | 164 | 0.29 | 78–3876 | Gypsum/Halite | 100–150 | |
| Sample D | 494 | 97 | 213 | 0.46 | 86–1349 | 11,231–10,770 Cal BP | Calcite/SiO2 | 50–100 |
| Sample E | 332 | 47 | 140 | 0.34 | 96–1975 | Modern Sediment | Halite | 300–350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Huang, T.; Zhu, A.; Anglés, A.; Abdelghany, O.; Ahmed, A.; Fernández-Remolar, D.C. Comparing Protein Stability in Modern and Ancient Sabkha Environments: Implications for Molecular Remnants on Ancient Mars. Int. J. Mol. Sci. 2025, 26, 5978. https://doi.org/10.3390/ijms26135978
Hu Q, Huang T, Zhu A, Anglés A, Abdelghany O, Ahmed A, Fernández-Remolar DC. Comparing Protein Stability in Modern and Ancient Sabkha Environments: Implications for Molecular Remnants on Ancient Mars. International Journal of Molecular Sciences. 2025; 26(13):5978. https://doi.org/10.3390/ijms26135978
Chicago/Turabian StyleHu, Qitao, Ting Huang, Aili Zhu, Angélica Anglés, Osman Abdelghany, Alaa Ahmed, and David C. Fernández-Remolar. 2025. "Comparing Protein Stability in Modern and Ancient Sabkha Environments: Implications for Molecular Remnants on Ancient Mars" International Journal of Molecular Sciences 26, no. 13: 5978. https://doi.org/10.3390/ijms26135978
APA StyleHu, Q., Huang, T., Zhu, A., Anglés, A., Abdelghany, O., Ahmed, A., & Fernández-Remolar, D. C. (2025). Comparing Protein Stability in Modern and Ancient Sabkha Environments: Implications for Molecular Remnants on Ancient Mars. International Journal of Molecular Sciences, 26(13), 5978. https://doi.org/10.3390/ijms26135978








