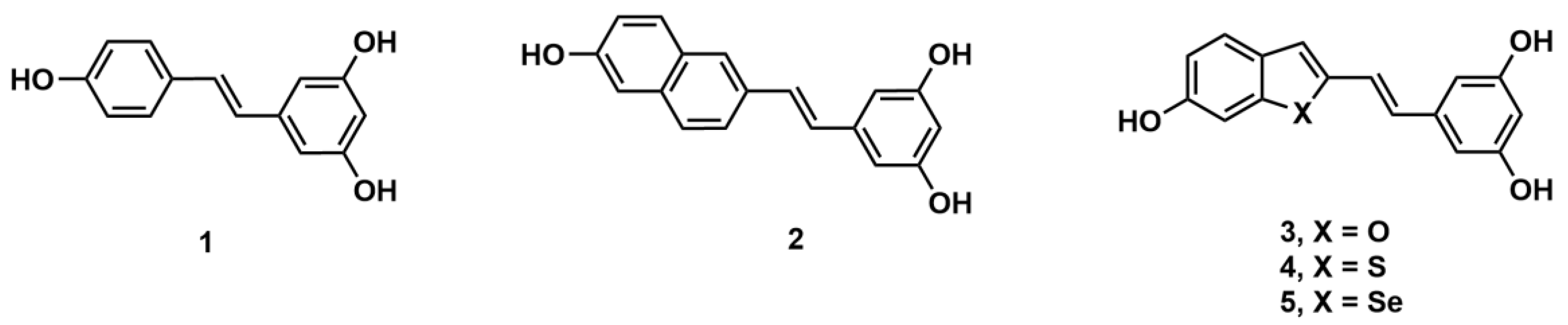
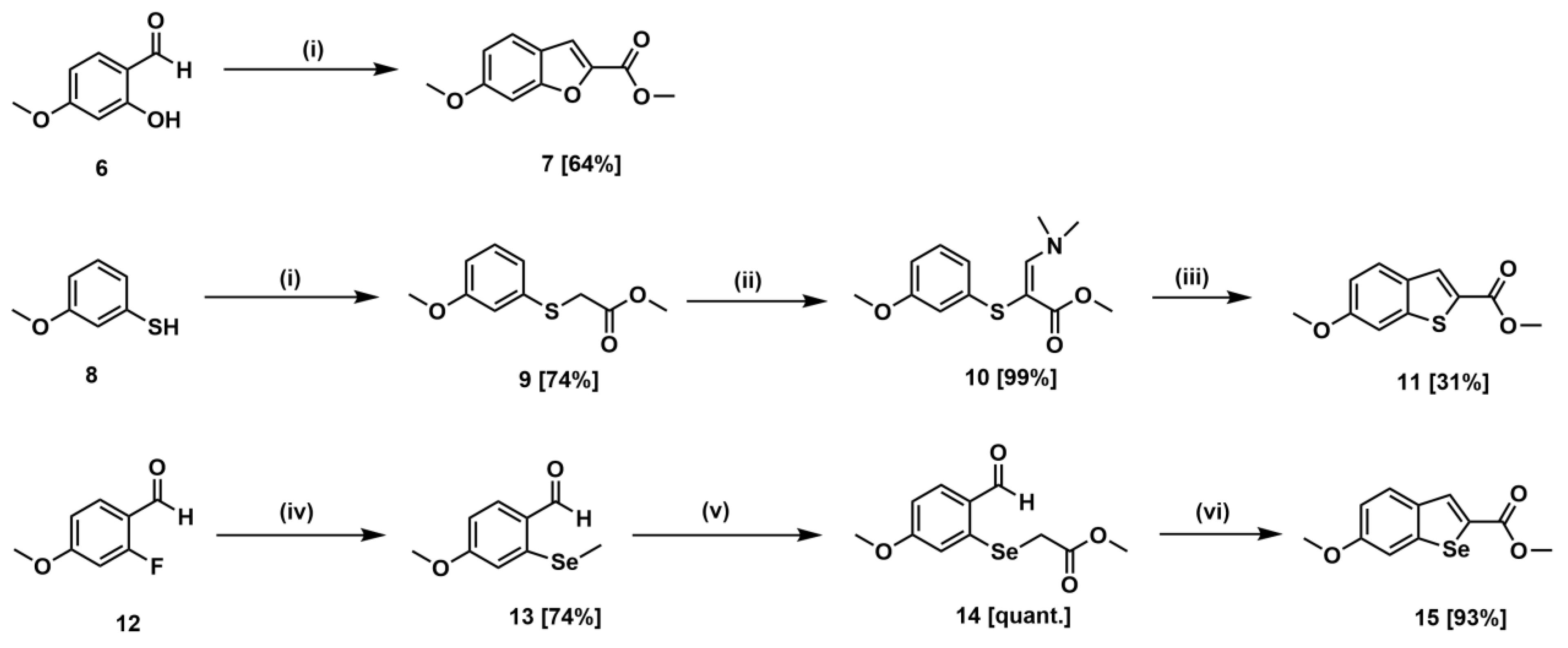
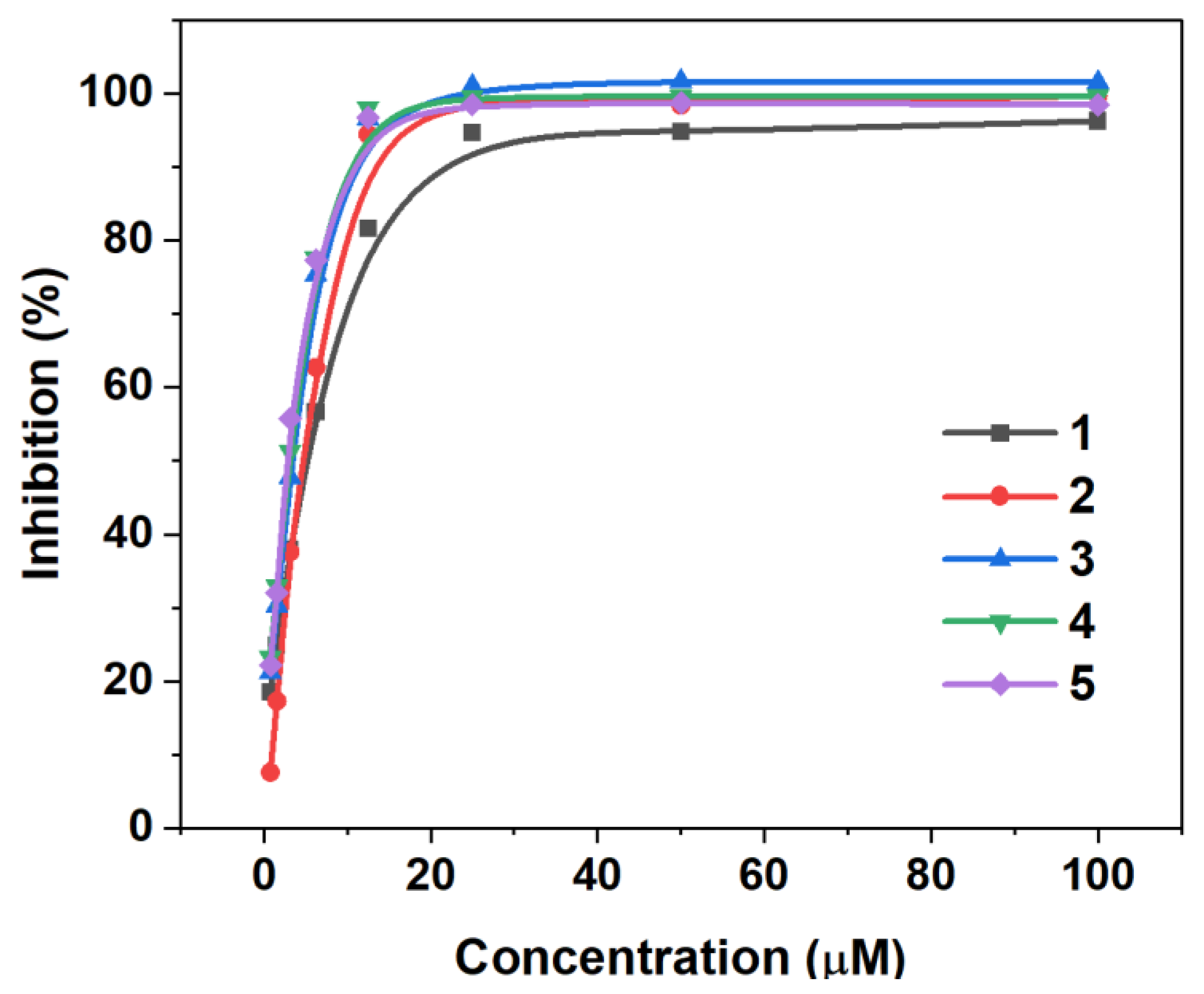
3.2. Syntheses
3.2.1. Methyl 6-Methoxybenzofuran-2-carboxylate (7)
K2CO3 (2.73 g, 19.72 mmol) and methyl bromoacetate (0.75 mL, 7.89 mmol) were added to a stirred solution of compound 6 (1.0 g, 6.57 mmol) in CH3CN (65 mL). The reaction mixture was refluxed for 4 h under argon. After the reaction was completed, the reaction mixture was brought to room temperature, filtered through a Celite® pad, and washed with CH3CN (15 mL). Then, the filtrate was concentrated under reduced pressure. The residue was purified by column chromatography (EtOAc/hexane = 1/8) to yield compound 7 (0.88 g, 4.27 mmol, 64.7%) as a white solid. 1H NMR (300 MHz, CDCl3) δ: 3.84 (s, 3H), 3.93 (s, 3H), 6.91 (dd, J = 8.7, 2.1 Hz, 1H), 7.02 (d, J = 2.1 Hz, 1H), 7.43 (s, 1H), 7.50 (d, J = 8.7 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ: 52.2, 55.5, 95.4, 113.8, 114.1, 119.9, 122.9, 144.3, 156.8, 159.7, 160.3; GC-MS m/z 206 (M+, base).
3.2.2. Methyl 6-Hydroxybenzofuran-2-carboxylate (7.1)
A stirred solution of compound 7 (0.4 g, 1.94 mmol) in anhydrous CH2Cl2 (12 mL) was cooled to −20 °C under an argon atmosphere. BBr3 (1.0 M in CH2Cl2, 0.4 mL, 4.27 mmol) was then added dropwise. After 30 min of stirring at −20 °C, the reaction mixture was brought to room temperature and stirred for an additional 3 h. Then, the reaction mixture was cooled again to 0 °C, and MeOH (2 mL) was slowly added to quench the excess BBr3, followed by stirring for 10–15 min. The reaction mixture was extracted with CH2Cl2 (3 × 30 mL), and the combined organic layers were washed with distilled H2O (2 × 25 mL) and brine (25 mL), dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (EtOAc/hexane = 1/2) to afford compound 7.1 (0.23 g, 62.2%) as a white solid. 1H NMR (300 MHz, CD3OD) δ: 3.98 (s, 3H), 6.82 (dd, J = 8.7, 2.1 Hz, 1H), 6.90 (d, J = 2.1 Hz, 1H), 7.47 (s, 1H), 7.49 (d, J = 8.7 Hz, 1H); 13C NMR (75 MHz, CD3OD) δ: 52.7, 98.6, 115.3, 115.7, 120.7, 124. 5, 145.3, 158.8, 160.0, 161.6; GC-MS m/z 192 (M+, base).
3.2.3. Methyl 6-(Benzyloxy)benzofuran-2-carboxylate (7.2)
K2CO3 (0.80 g, 5.78 mmol) and benzyl bromide (0.55 mL, 4.62 mmol) were added to a solution of compound 7.1 (0.74 g, 3.85 mmol) in acetone (10 mL) under an argon atmosphere at room temperature. The solution was refluxed for 4 h with stirring, and the reaction mixture was brought to room temperature, filtered through a Celite® pad, and washed with acetone (15 mL). The filtrate was concentrated in vacuo. The residue was dissolved in EtOAc (50 mL) and washed with distilled H2O (2 × 15 mL) and brine (15 mL), dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (EtOAc/hexane = 1/4) to afford compound 7.2 (1.00 g, 3.54 mmol, 92.6%) as a white solid. 1H NMR (300 MHz, CDCl3) δ: 3.94 (s, 3H), 5.10 (s, 2H), 7.00 (dd, J = 8.7, 2.1 Hz, 1H), 7.10 (d, J = 2.1 Hz, 1H), 7.31–7.44 (m, 6H), 7.52 (d, J = 8.7 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ: 52.5, 71.0, 97.5, 114.2, 114.8, 120.7, 123.2, 127.6, 128.2, 128.8, 136.7, 145.1, 157.2, 159.8, 159.9; GC-MS m/z 282 (M+, base).
3.2.4. (6-(Benzyloxy)benzofuran-2-yl)methanol (7.3)
LiAlH4 (2.0 M in THF, 2.71 mL, 5.31 mmol, 1.5 eq.) was added dropwise to a stirred solution of compound 7.2 (1.0 g, 3.54 mmol) in THF (5 mL) at 0 °C under an argon atmosphere. After completion of addition, the reaction mixture was brought to room temperature and stirred for an additional 90 min. After the reaction was completed, the reaction mixture was cooled to 0 °C, and the excess LiAlH4 was quenched by aq. sat. K2CO3 solution (1 mL). Then, the resulting mixture was filtered through a Celite® pad and washed with EtOAc (20 mL). The filtrate was dried over Na2SO4 and concentrated in vacuo to afford compound 7.3 (0.90 g, quantitative) as a white solid. 1H NMR (300 MHz, CDCl3) δ: 1.65 (br s, 1H), 4.70 (s, 2H), 5.08 (s, 2H), 6.56 (s, 1H), 6.92 (dd, J = 8.1, 2.1 Hz, 1H), 7.04 (d, J = 2.1 Hz, 1H), 7.30–7.44 (m, 6H); 13C NMR (75 MHz, CDCl3) δ: 52.3, 71.0, 97.5, 114.2, 114.8, 120.7, 123.2, 127.6, 128.2, 128.8, 136.7, 145.1, 157.2, 159.8; GC-MS m/z 254 (M+, base).
3.2.5. 6-(Benzyloxy)benzofuran-2-carbaldehyde (7.4)
A mixture of compound 7.3 (0.29 g, 1.14 mmol) and IBX (2-iodoxybenzoic acid, 0.48 g, 1.71 mmol) in DMSO (5 mL) was stirred for 2 h at 90 °C under an argon atmosphere. After the addition of EtOAc (40 mL), the organic layer was washed with aq. sat. NaHCO3 solution (15 mL) and distilled H2O (3 × 15 mL) and brine (2 × 15 mL) and dried over anhydrous Na2SO4. Solvent evaporation under reduced pressure yielded compound 7.4 (0.24 g, 82.7%) as a yellow solid. 1H NMR (300 MHz, CDCl3) δ: 5.13 (s, 2H), 7.07 (dd, J = 8.7, 2.1 Hz, 1H), 7.09 (d, J = 2.1 Hz, 1H), 7.03–7.47 (m, 6H), 7.59 (d, J = 8.7 Hz, 1H), 9.72 (s, 1H); 13C NMR (75 MHz, CDCl3) δ: 70.9, 97.3, 115.4, 117.7, 120.4, 123.9, 127.4, 128.2, 128.6, 136.3, 152.8, 157.9, 160.8, 178.4; GC-MS m/z 252 (M+, base).
3.2.6. (E)-6-(Benzyloxy)-2-(3,5-bis(benzyloxy)styryl)benzofuran (7.5)
Diethyl 3,5-bis(benzyloxy)benzylphosphonate (0.80 g, 1.82 mmol) in DMF (4 mL) was added dropwise to a suspension of NaH (60% in mineral oil, 0.13 g, 5.47 mmol) in DMF (3 mL) at 0 °C and stirred for 20~30 min. Compound 7.4 (0.46 g, 1.82 mmol) in DMF (5 mL) was transferred dropwise to the above mixture under an argon atmosphere. The resulting mixture was brought to room temperature and stirred for 3 h. After the reaction was completed, distilled H2O (5 mL) was added to the reaction mixture slowly to quench excess NaH. The resulting mixture was extracted with EtOAc (3 × 30 mL). The combined organic layer was washed with distilled H2O (2 × 15 mL) and brine (15 mL), dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (EtOAc/hexane = 1/8) to afford compound 7.5 (0.75g, 76.5%) as a yellow solid. 1H NMR (300 MHz, CDCl3) δ: 5.04 (s, 4H), 5.09 (s, 2H), 6.54 (t, J = 2.2 Hz, 1H), 6.57 (s, 1H) 6.74 (d, J = 2.2 Hz, 2H) 6.88 (d, J = 15.9 Hz, 1H), 6.90 (dd, J = 8.4, 2.2 Hz, 1H), 7.05 (d, J = 2.2 Hz, 1H), 7.11 (d, J = 15.9 Hz, 1H), 7.35–7.45 (m, 16H); 13C NMR (75 MHz, CDCl3) δ: 70.2, 70.6, 96.9, 101.9, 105.4, 105.8, 112.5, 117.0, 120.9, 122.6, 127.3, 127.4, 127.9, 128.5, 128.7, 136.7, 136,8, 138.7, 154.1, 155.8, 157.4, 160.0; MS (EI+) m/z 538 (M+, base), 447, 356, 265.
3.2.7. (E)-5-(2-(6-Hydroxybenzofuran-2-yl)vinyl)benzene-1,3-diol (3)
AlCl3 (4.34 g, 32.53 mmol) was added to a mixture of compound 7.5 (0.73 g, 1.36 mmol) and N,N-dimethylaniline (2.96 mL, 24.40 mmol) in DCM (10 mL) under an argon atmosphere and stirred for 3 h at room temperature. After the reaction was completed, 1M HCl (10 mL) was added to the mixture dropwise at 0 °C and stirred for 10 min. The reaction mixture was extracted with EtOAc (3 × 30 mL). The combined organic layer was washed with aq. sat. NaHCO3 solution (15 mL) and distilled H2O (2 × 15 mL) and brine (15 mL) dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (CH2Cl2/MeOH = 97/3), yielding 3 (0.13 g, 36.1%) as a yellow solid. 1H NMR (300 MHz, (CD3)2CO) δ 8.58 (br s, 1H), 8.33 (br s, 2H), 7.35 (dd, J = 8.4, 0.8 Hz, 1H), 7.03 (d, J = 16.0 Hz, 1H), 6.98 (d, J = 16.0 Hz, 1H), 6.94 (d, J = 2.0 Hz, 1H) 6.77 (dd, J = 8.4, 2.0 Hz, 1H), 6.71 (d, J = 0.8 Hz, 1H), 6.57 (d, J = 2.0 Hz, 1H), 6.31 (t, J = 2.0 Hz, 1H); 13C NMR (75 MHz, (CD3)2CO) δ 98.5, 103.7, 106.2, 113.2, 117.5, 121.9, 122.7, 129.6, 139.8, 154.9, 157.0, 157.1, 159.6; MS (EI+) m/z 268 (M+, base); HRMS m/z (M+) calcd for C16H12O4: 268.0736, Found: 268.0734.
3.2.8. Methyl 2-((3-Methoxyphenyl)thio)acetate (9)
Methyl bromoacetate (0.18 mL, 1.95 mmol) was added to a stirred suspension of compound 8 (0.228 g, 1.63 mmol) and K2CO3 (0.67 g, 4.88 mmol) in acetone (6 mL) under an argon atmosphere at room temperature. After 5 h of refluxing, the reaction mixture was brought to room temperature, filtered through Celite®, and washed with acetone (10 mL). Then, the filtrate was concentrated in vacuo. The residue was dissolved in EtOAc (50 mL), washed with 1N HCl (10 mL), distilled H2O (2 × 15 mL) and brine (15 mL), dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (EtOAc/hexane = 1/8) to afford compound 9 (0.26 g, 74.3%) as a clear liquid. 1H NMR (300 MHz, CDCl3) δ: 3.65 (s, 2H), 3.71 (s, 3H), 3.78 (s, 3H), 6.74 (d, J = 7.2 Hz, 1H), 6.93 (s, 1H), 6.94 (d, J = 7.8 Hz, 1H), 7.19 (t, J = 7.9 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ: 36.5, 52.5, 55,3, 112.9, 115.1, 121.8, 130.0, 136.3, 159.9, 169.8; GC-MS m/z 212 (M+, base).
3.2.9. Methyl 3-(Dimethylamino)-2-((3-methoxyphenyl)thio)acrylate (10)
A solution of compound 9 (9.0 g, 42.40 mmol) in N,N-dimethylformamide dimethyl acetal (6.1 mL, 45.93 mmol) was stirred for 10 h at 80 °C under argon. After the completion of the reaction, the reaction mixture was brought to room temperature. The residue resulting from in vacuo solvent evaporation was purified by column chromatography (EtOAc/hexane = 1/4) to obtain compound 10 (11.31 g, 99.8%) as a brown liquid. 1H NMR (300 MHz, CDCl3) δ: 3.08 (s, 6H), 3.57 (s, 3H), 3.65 (s, 3H), 6.50 (d, J = 7.8 Hz, 1H), 6.60 (s, 1H), 6.63 (d, J = 8.1 Hz, 1H), 7.04 (t, J = 7.8 Hz, 1H) 7.98 (s, 1H); 13C NMR (75 MHz, CDCl3) δ: 42.6, 51.3, 54.8, 81.7, 109.6, 110.2, 116.8, 129.1, 129.3, 142.2, 155.3, 159.7, 169.6; GC-MS m/z 267 (M+, base).
3.2.10. Methyl 6-Methoxybenzo[b]thiophene-2-carboxylate (11)
A mixture of compound 10 (1.00 g, 3.74 mmol) and iodine (2.56 g, 10.10 mmol) in anhydrous CH2Cl2 (10 mL) was refluxed for 12 h under argon. After the completion of the reaction, the reaction mixture was diluted with CH2Cl2 (30 mL), washed sequentially with aq. sat. Na2S2O3 (2 × 15 mL), distilled H2O (2 × 15 mL) and brine (15 mL), dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified with column chromatography (EtOAc/hexane = 1/30) to afford compound 11 (0.26 g, 1.17 mmol, 31.3%) as a white solid. 1H NMR (300 MHz, CDCl3) δ 3.88 (s, 3H), 3.92 (s, 3H), 7.00 (dd, J = 8.8, 2.0 Hz, 1H), 7.26 (s, 1H), 7.72 (d, J = 8.8 Hz, 1H), 7.95 (s, 1H); 13C NMR (75 MHz, CDCl3) δ: 52.2, 55.7, 104.6, 115.7, 126.2, 130.3, 130.7, 132.8, 144.3, 159.5, 163.1; GC-MS m/z 222 (M+, base).
3.2.11. Methyl 6-Hydroxybenzo[b]thiophene-2-carboxylate (11.1)
BBr3 (1.0 M solution in CH2Cl2, 2.2 mL, 2.24 mmol) was added slowly to a stirred solution of compound 11 (0.25 g, 1.12 mmol) in anhydrous CH2Cl2 (5 mL) at 0 °C under an argon atmosphere. After stirring for 2 h at 0 °C and an additional 2 h at room temperature, the resulting mixture was cooled again to 0 °C. Then, MeOH (2 mL) was added slowly to quench the excess BBr3, followed by 20 min of stirring at room temperature. The reaction mixture was diluted with CH2Cl2 (35 mL), washed with distilled H2O (2 × 10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (EtOAc/hexane = 1/8) to obtain compound 11.1 (0.19 g, 82.6 %) as a white solid. 1H NMR (300 MHz, CD3OD) δ: 3.87 (s, 3H), 6.92 (d, J = 8.8 Hz, 1H), 7.20 (s, 1H), 7.71 (d, J = 8.8 Hz, 1H) 7.92 (s, 1H); 13C NMR (75 MHz, CD3OD) δ: 52.7, 107.8, 116.8, 127.5, 130.5, 131.7, 133.2, 145.6, 158.8, 164.8; GC-MS m/z 208 (M+, base).
3.2.12. Methyl 6-(Benzyloxy)benzo[b]thiophene-2-carboxylate (11.2)
Benzyl bromide (0.57 mL, 4.80 mmol) was added to a suspension of compound 11.1 (1.00 g, 4.80 mmol) and K2CO3 (1.33 g, 9.60 mmol) in acetone (20 mL) under an argon atmosphere at room temperature. After 10 h of refluxing, the reaction mixture was brought to room temperature, filtered through Celite®, and washed with acetone (10 mL). The filtrate was concentrated in vacuo. The residue was dissolved in EtOAc (45 mL), washed with 1N HCl (10 mL), distilled H2O (2 × 10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography (EtOAhc/hexane = 1/10) to obtain compound 11.2 (1.42 g, 4.76 mmol, 99.3%) as a white solid. 1H NMR (300 MHz, CDCl3) δ: 3.91 (s, 3H), 5.12, (s, 2H), 7.08 (dd, J = 8.8, 1.9 Hz, 1H), 7.34–7.45 (m, 6H), 7.73 (d, J = 8.8 Hz, 1H), 7.96 (s, 1H); 13C NMR (75 MHz, CDCl3) δ: 52.3, 70.6, 105.9, 116.2, 126.3, 127.4, 128.1, 128.6, 130.3, 130.9, 133.0, 136.5, 144.1, 158.6, 163.1; GC-MS m/z 298 (M+, base).
3.2.13. (6-(Benzyloxy)benzo[b]thiophen-2-yl)methanol (11.3)
Following the same method used for the preparation of compound 7.3, treatment of compound 11.2 (1.4 g, 4.69 mmol) in anhydrous THF (10 mL) with LiAlH4 (2.0 M in THF, 5.6 mL, 5.63 mmol, 1.2 eq.) afforded compound 11.3 (1.26 g, 99.2%) as a white solid. 1H NMR (300 MHz, CDCl3) δ: 2.13 (br s, 1H), 4.83 (s, 2H), 5.08 (s, 2H), 7.02 (dd, J = 8.8, 2.0 Hz, 1H), 7.01 (s, 1H), 7.31–7.44 (m, 6H), 7.57 (d, J = 8.8 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ: 60.9, 70.7, 106.8, 115.0, 121.1, 124.1, 127.4, 127.9, 128.5, 133.8, 137.0, 141.4, 142.3, 156.6; GC-MS m/z 270 (M+, 100%).
3.2.14. 6-(Benzyloxy)benzo[b]thiophene-2-carbaldehyde (11.4)
Following the same method used for the preparation of compound 7.4, treatment of compound 11.3 (1.26 g, 4.66 mmol) with IBX (1.96 g, 6.99 mmol) in DMSO (20 mL) afforded 11.4 (1.18 g, 94.4%) as a yellow solid. 1H NMR (300 MHz, CDCl3) δ: 5.15 (s, 2H), 7.11 (dd, J = 8.8, 2.4 Hz, 1H), 7.34–7.45 (m, 6H), 7.80 (d, J = 8.8 Hz, 1H), 7.92 (s, 1H), 9.99 (s, 1H); 13C NMR (75 MHz, CDCl3) δ: 70.9, 106.5, 117.0, 127.2, 127.6, 128.3, 128.8, 139.1, 134.2, 136.5, 141.6, 145.2, 159.7, 183.8; GC-MS m/z 268 ([M]+, 100%).
3.2.15. (E)-6-(Benzyloxy)-2-(3,5-bis(benzyloxy)styryl)benzo[b]thiophene (11.5)
Following the same method used for the preparation of compound 7.5, treatment of diethyl 3,5-bis(benzyloxy)benzylphosphonate (1.94 g, 4.40 mmol) in DMF (6 mL) with NaH (60% in mineral oil, 0.32 g, 13.19 mmol) in DMF (4 mL), followed by the addition of compound 11.4 (1.18 g, 4.40 mmol) in DMF (6 mL), afforded compound 11.5 (1.99 g, 81.6%) as a yellow solid. 1H NMR (300 MHz, CDCl3) δ: 5.03 (s, 4H), 5.70 (s, 2H), 6.53 (s, 1H), 6.71 (s, 2H), 6.78 (d, J = 16.1 Hz, 1H), 6.98 (d, J = 8.8 Hz, 1H), 7.11 (s, 1H), 7.20 (d, J = 16.1 Hz, 1H), 7.28–7.40 (m, 16H), 7.53 (d, J = 8.8 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ: 70.4, 70.7, 102.1, 102.2, 106.1, 106.3, 106.7, 115.0, 123.0, 124.0, 127.4, 127.9, 128.5, 129.3, 129.7, 134.5, 136.9, 137.0, 138.9, 139.3, 140.5, 140.6, 157.1, 160.2; MS (EI+) m/z 554 (M+, base), 463, 281, 91.
3.2.16. (E)-5-(2-(6-Hydroxybenzo[b]thiophen-2-yl)vinyl)benzene-1,3-diol (4)
Following the same method used for the preparation of compound 3, treatment of compound 11.5 (0.16 g, 0.29 mmol) and N,N-dimethylaniline (0.44 mL, 3.47 mmol) in anhydrous CH2Cl2 (10 mL) with AlCl3 (0.61 g, 4.61 mmol) afforded compound 4 (0.03 g, 37.5 %) as a yellow solid. 1H NMR (300 MHz, (CD3)2CO) δ: 6.31 (s, 1H), 6.56, (s, 2H), 6.75 (d, J = 16.1 Hz, 1H), 6.90 (d, J = 8.4 Hz, 1H), 7.25 (s, 1H), 7.27 (s, 1H) 7.33 (d, J = 16.1 Hz, 1H), 7.58 (d, J = 8.4 Hz, 1H) 8.41 (br s, 3H); 13C NMR (75 MHz, (CD3)2CO) δ: 103.6, 106.1, 108.3, 115.5, 123.4, 124.2, 125.1, 130.4, 134.6, 139.9, 140.5, 141.5, 156.6, 159.6; MS (EI+) m/z 284 (M+, base) HRMS m/z (M+) calcd for C16H12O3S: 284.0507, Found: 284.0510.
3.2.17. 4-Methoxy-2-(methylselanyl)benzaldehyde (13)
A mixture of dimethyl diselenide (1.99 g, 10.57 mmol) and dithiothreitol (0.98 g, 6.34 mmol) in anhydrous DMF (8 mL) was stirred for 1 h at 80 °C under an argon atmosphere. To this mixture, compound 12 (1.63 g, 10.57 mmol) and DBU (3.95 mL, 26.42 mmol) were added. The resulting mixture was heated with stirring for another 12 h. After the reaction was completed, the reaction mixture was brought to room temperature, and distilled H2O (10 mL) was added to it. After extraction with EtOAc (3 × 30 mL), the organic layer was washed with distilled H2O (3 × 15 mL) and brine (2 × 15 mL), dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography (EtOAc/hexane = 1/8) to obtain compound 13 (1.80 g, 74.4%) as a yellow solid. 1H NMR (300 MHz, CDCl3) δ: 2.26 (s, 3H), 3.90 (s, 3H), 6.81 (dd, J = 8.4, 2.2 Hz, 1H), 6.90 (s, 1H), 7.71 (d, J = 8.4 Hz, 1H), 9.95 (s, 1H); 13C NMR (75 MHz, CDCl3) δ: 5.8, 55.6, 110.1, 114.2, 128.5, 136.9, 140.8, 163.8, 190,3; MS (EI+) m/z 230 (M+), 215 (base), 187, 135.
3.2.18. Methyl 2-((2-Formyl-5-methoxyphenyl)selanyl)acetate (14)
A mixture of compound 13 (1.30 g, 5.67 mmol) and methyl bromoacetate (10.10 mL, 113.47 mmol) was refluxed for 3 h under an argon atmosphere. After the reaction was completed, the reaction mixture was brought to room temperature, and the evaporation of methyl bromoacetate yielded compound 14 (1.63 g, quantitative) as a white solid. 1H NMR (300 MHz, CDCl3) δ: 3.56 (s, 2H), 3.72 (s, 3H), 3.93 (s, 3H), 6.85 (dd, J = 8.7, 1.8 Hz, 1H), 7.29 (s, 1H), 7.72 (d, J = 8.7 Hz, 1H) 9.92 (s, 1H); 13C NMR (75 MHz, CDCl3) δ: 25.2, 52.5, 55.8, 111.9, 114.6, 128.2, 136.7, 139.2, 164.0, 171.1, 190.4; MS (EI+) m/z 288 (M+), 286, 214 (base), 187.
3.2.19. Methyl 6-Methoxybenzo[b]selenophene-2-carboxylate (15)
A suspension of compound 14 (0.48 g, 1.67 mmol) and K2CO3 (0.69 g, 5.01 mmol) in anhydrous CH3CN (15 mL) was refluxed for 4 h under an argon atmosphere. After the reaction was completed, the reaction mixture was brought to room temperature, filtered through a Celite® pad, and washed with CH3CN (10 mL). The filtrate was concentrated under reduced pressure. The residue was dissolved in EtOAc (45 mL), washed sequentially with 1N HCl (9 mL), H2O (2 × 10 mL) and brine (10 mL), dried over anhydrous Na2SO4 and concentrated under reduced pressure to yield compound 15 (0.42 g, 93.3%) as a white solid. 1H NMR (300 MHz, CDCl3) δ: 3.86 (s, 3H), 3.89 (s, 3H), 6.98 (dd, J = 8.7, 2.4 Hz, 1H), 7.34 (s, 1H), 7.73 (d, J = 8.7 Hz, 1H), 8.18 (s, 1H); 13C NMR (75 MHz, CDCl3) δ: 52.3, 55.6, 108.4, 115.0, 127.9, 132.7, 134.1, 135.0, 145.9, 159.3, 164.1; MS (EI+) m/z 270 (M+, base), 268, 239, 168.
3.2.20. Methyl 6-Hydroxybenzo[b]selenophene-2-carboxylate (15.1)
Following the method used to prepare compound 11.1, treatment of compound 15 (0.22 g, 0.82 mmol) in CH2Cl2 (10 mL) with BBr3 (1.0 M in CH2Cl2; 0.32 mL, 3.44 mmol) afforded compound 15.1 (0.20 g, 93.3%) as a white solid. 1H NMR (300 MHz, DMSO-d6) δ: 3.82 (s, 3H), 6.91 (dd, J = 8.4, 1.2 Hz, 1H), 7.44 (s, 1H), 7.82 (d, J = 8.4 Hz, 1H), 8.24 (s, 1H), 10.11 (br s, 1H); 13C NMR (75 MHz, DMSO-d6) δ: 62.0, 120.8, 125.2, 138.2, 140.3, 143.2, 144.2, 154.9, 167.1, 173.3; MS (EI+) m/z 256 (M+, base), 254, 225, 197.
3.2.21. Methyl 6-(Benzyloxy)benzo[b]selenophene-2-carboxylate (15.2)
Following the method used to prepare compound 7.2, treatment of compound 15.1 (0.19 g, 0.74 mmol) and K2CO3 (0.21 g, 1.49 mmol) in acetone (10 mL) with benzyl bromide (0.10 mL, 0.82 mmol) yielded compound 15.2 (0.26 g, quantitative) as a white solid. 1H NMR (300 MHz, CDCl3) δ: 3.89 (s, 3H), 5.11 (s, 2H), 7.06 (dd, J = 8.7, 2.3 Hz, 1H), 7.33–7.44 (m, 6H), 7.75 (d, J = 8.7 Hz, 1H), 8.18 (s, 1H); 13C NMR (75 MHz, CDCl3) δ:52.4, 70.6, 109.8, 115.7, 127.3, 128.1, 128.6, 133.0, 134.1, 135.3, 136.5, 145.9, 158.5, 164.2; MS (EI+) m/z 346 (M+, base), 344, 255, 168.
3.2.22. (6-(Benzyloxy)benzo[b]selenophen-2-yl)methanol (15.3)
Following the method used to prepare compound 7.3, treatment of compound 15.2 (1.1 g, 3.19 mmol) in THF (20 mL) with LiAlH4 (2.0 M in THF, 1.91 mL, 3.82 mmol) yielded compound 15.3 (1.01 g, 99.0%) as a white solid. 1H NMR (300 MHz, CDCl3) δ: 2.15 (br s, 1H), 4.85 (d, J = 5.4 Hz, 2H), 5.08 (s, 2H), 7.00 (d, J = 8.4 Hz, 1H), 7.24 (s, 1H), 7.34–7.44 (m, 6H), 7.56 (d, J = 8.4 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ: 62.9, 70.7, 110.5, 114.6, 124.0, 125.6, 127.4, 127.9, 128.5, 136.0, 137.0, 142.6, 146.4, 156.5; MS (EI+) m/z 318 (M+, base), 316, 227, 91.
3.2.23. 6-(Benzyloxy)benzo[b]selenophene-2-carbaldehyde (15.4)
Following the method used to prepare compound 7.4, treatment of compound 15.3 (0.21 g, 0.66 mmol) with IBX (0.28 g, 0.99 mmol) in DMSO (3 mL) yielded compound 15.4 (0.21 g, quantitative) as a yellow solid. 1H NMR (300 MHz, CDCl3) δ: 5.14 (s, 2H), 7.09 (dd, J = 8.7, 2.1 Hz, 1H), 7.34–7.45 (m, 6H), 7.82 (d, J = 8.7 Hz, 1H), 8.15 (s, 1H), 9.89 (s, 1H); 13C NMR (75 MHz, CDCl3) δ: 70.7, 110.2, 116.1, 127.4, 128.2, 128.6, 128.8, 135.1, 136.3, 138.1, 144.5, 146.1, 159.3, 184.9; LRMS (EI+) m/z 316 (M+, base), 225, 169, 167.
3.2.24. (E)-6-(Benzyloxy)-2-(3,5-bis(benzyloxy)styryl)benzo[b]selenophene (15.5)
Following the method used to prepare compound 7.5, treatment of diethyl 3,5-bis(benzyloxy)benzylphosphonate (0.28 g, 0.63 mmol) in DMF (6 mL) with NaH (60% in mineral oil, 0.046 g, 1.90 mmol) in DMF (4 mL), followed by the addition of compound 15.4 (0.2 g, 0.63 mmol) in DMF (6 mL), afforded compound 15.5 (0.32g, 84.2%) as a yellow solid. 1H NMR (300 MHz, CDCl3) δ: 5.04 (s, 4H), 5.09 (s, 2H), 6.53 (s, 1H), 6.62 (d, J = 15.9 Hz, 1H) 6.71 (s, 2H) 6.97 (d, J = 9.3 Hz, 1H), 7.22 (d, J = 15.9 Hz, 1H), 7.29–7.40 (m, 17H), 7.54 (d, J = 8.7 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ: 70.4, 70.7, 102.3, 106.1, 110.5, 114.7, 125.3, 125.6, 126.9, 127.3, 127.4, 127.9, 128.5, 130.9, 136.8, 136.9, 137.0, 139.0, 141.2, 143.3, 157.1, 160.3; MS (EI+) m/z 602 (M+, base), 600, 603, 329.
3.2.25. (E)-5-(2-(6-Hydroxybenzo[b]selenophen-2-yl)vinyl)benzene-1,3-diol (5)
Following the method used to prepare compound 3, treatment of compound 15.5 (0.27 g, 0.45 mmol) and N,N-dimethylaniline (1.02 mL, 8.08 mmol) in anhydrous CH2Cl2 (10 mL) with AlCl3 (1.44 g, 10.77 mmol) afforded compound 5 (0.06 g, 40.0%) as a yellow solid. 1H NMR (300 MHz, (CD3)2CO) δ: 6.30 (t, J = 1.5 Hz, 1H), 6.55, (d, J = 1.5 Hz, 2H), 6.61 (d, J = 15.6 Hz, 1H), 6.87 (dd, J = 8.4, 1.8 Hz, 1H), 7.32 (d, J = 15.6 Hz, 1H), 7.35 (d, J = 1.8 Hz, 1H), 7.40 (s, 1H), 7.56 (d, J = 8.4 Hz, 1H), 8.34 (br s, 3H); 13C NMR (75 MHz, (CD3)2CO) δ: 103.6, 106.1, 112.1, 115.3, 125.7, 126.6, 128.0, 131.6, 136.8, 140.0, 142.0, 143.2, 156.6, 159.6; MS (EI+) m/z 332 (M+, base), 330, 252; HRMS m/z (M+) calcd for C16H12O3Se: 331.9952, Found: 331.9954.