Potential Therapeutic Appliances of Dietary Polyphenols: Resveratrol and Curcumin in Treatment of Gliomas
Abstract
1. Introduction
2. Gliomas: Overview
3. Resveratrol’s Role in Treatment of Gliomas
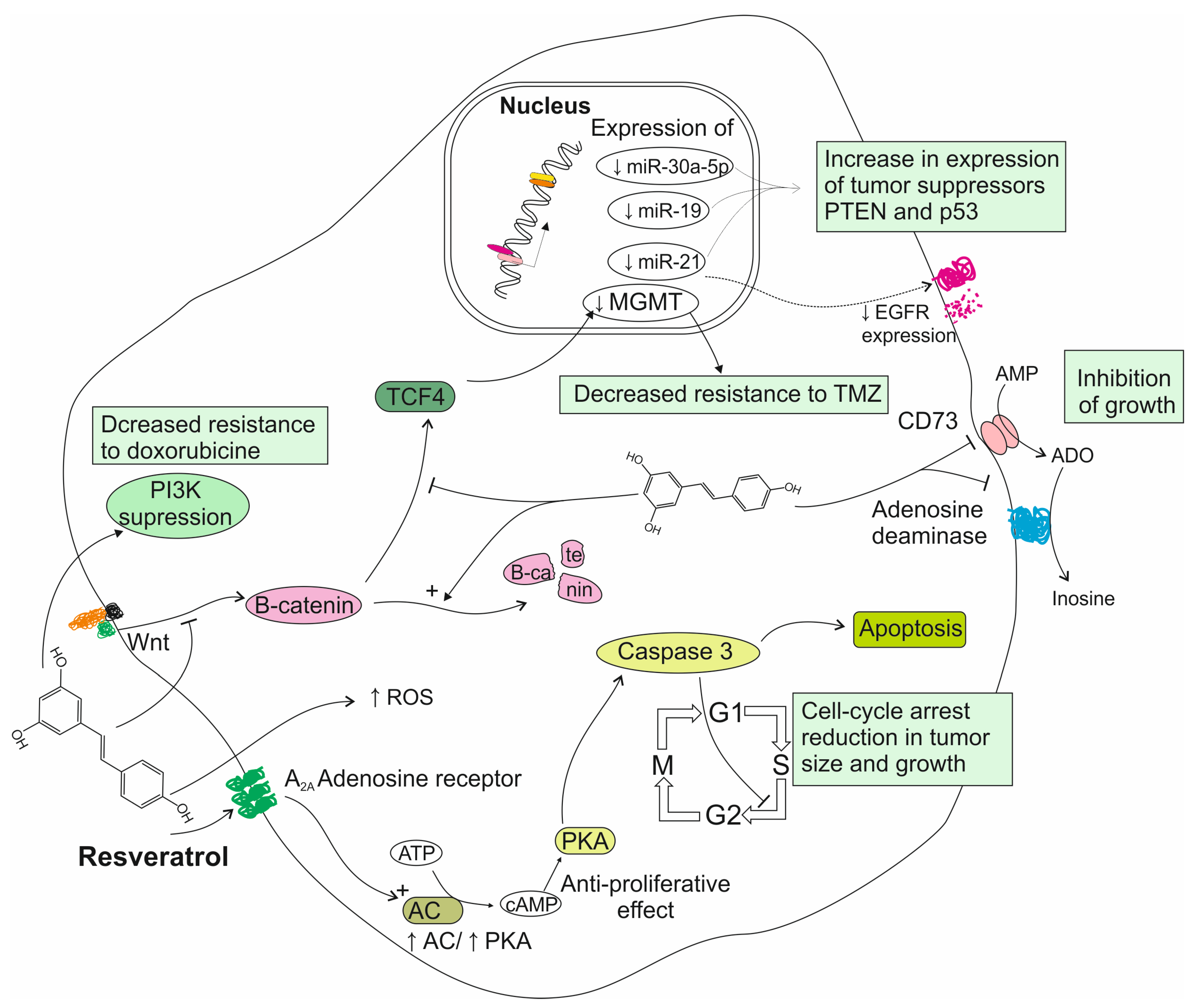
3.1. Reports from In Vitro Studies on Resveratrol Activity
3.2. Reports from Animal Models on Resveratrol Activity
4. Curcumin’s Role in Treatment of Gliomas
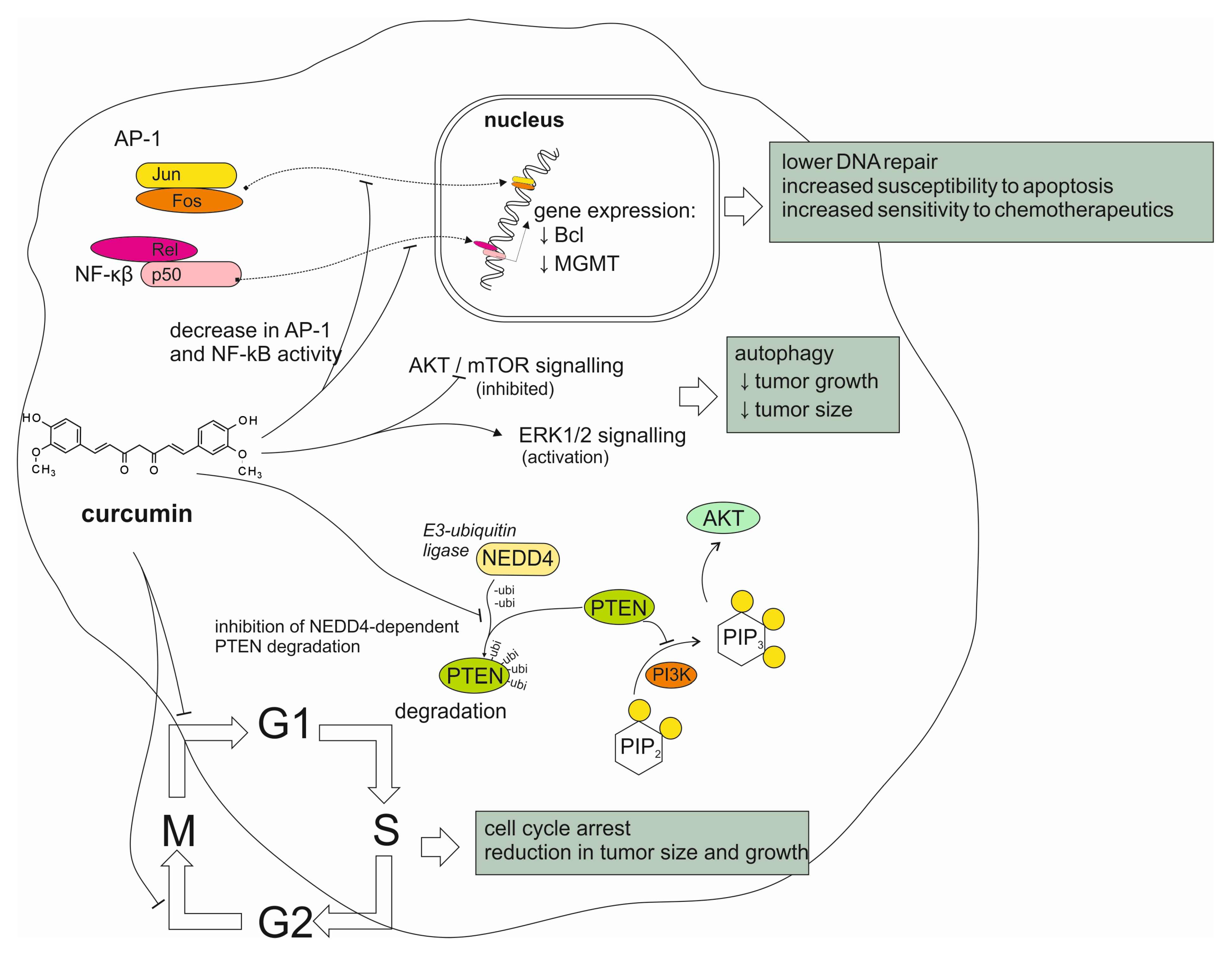
4.1. Reports from In Vitro Studies on Curcumin Activity
4.2. Reports from Animal Models on Curcumin Activity
4.3. Reports from Studies Conducted on Humans
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Abbas, M.; Farhan, S.; Faqir Muhammad, A.; Muhammad, A.; Tabussam, T.; Muhammad Shakeel, B.; Adnan, I.; Shahzad, H.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French Paradox Revisited. Front. Pharmacol. 2012, 3, 141. [Google Scholar] [CrossRef]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and Its Effects on the Vascular System. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of Resveratrol in Prevention and Therapy of Cancer: Preclinical and Clinical Studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar]
- Luís, Â.; Marcelino, H.; Domingues, F.; Pereira, L.; Cascalheira, J.F. Therapeutic Potential of Resveratrol for Glioma: A Systematic Review and Meta-Analysis of Animal Model Studies. Int. J. Mol. Sci. 2023, 24, 16597. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Kismali, G.; Aggarwal, B.B. Curcumin, a component of turmeric: From farm to pharmacy. BioFactors 2013, 39, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Chandran, B.; Goel, A. A Randomized, Pilot Study to Assess the Efficacy and Safety of Curcumin in Patients with Active Rheumatoid Arthritis. Phyther. Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef]
- Lang, A.; Salomon, N.; Wu, J.C.Y.; Kopylov, U.; Lahat, A.; Har-Noy, O.; Ching, J.Y.L.; Cheong, P.K.; Avidan, B.; Gamus, D.; et al. Curcumin in Combination with Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1444–1449.e1. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef]
- Earhart, R.; PHASE I/II STUDY OF THE TOLERABILITY, SAFETY AND EFFICACY OF LIPOSOMAL CURCUMIN IN COMBINATION WITH RADIATION AND TEMOZOLOMIDE IN PATIENTS WITH NEWLY DIAGNOSED HIGH-GRADE GLIOMAS. Protocol Number: 1004 Compound: Liposomal Curcumin (LipoCurc) Study Phase: 1; 2022. Available online: https://clinicaltrials.gov/study/NCT05768919 (accessed on 1 December 2024).
- Dützmann, S.; Schiborr, C.; Kocher, A.; Pilatus, U.; Hattingen, E.; Weissenberger, J.; Geßler, F.; Quick-Weller, J.; Franz, K.; Seifert, V.; et al. Intratumoral Concentrations and Effects of Orally Administered Micellar Curcuminoids in Glioblastoma Patients. Nutr. Cancer 2016, 68, 943–948. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- McFaline-Figueroa, J.R.; Lee, E.Q. Brain Tumors. Am. J. Med. 2018, 131, 874–882. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical Report: Primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro. Oncol. 2015, 17, iv1–iv62. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fei, Z.; Zhen, H.N.; Zhang, J.N.; Zhang, X. Resveratrol inhibits cell growth and induces apoptosis of rat C6 glioma cells. J. Neurooncol. 2007, 81, 231–240. [Google Scholar] [CrossRef]
- Wang, G.; Dai, F.; Yu, K.; Jia, Z.; Zhang, A.; Huang, Q.; Kang, C.; Jiang, H.; Pu, P. Resveratrol inhibits glioma cell growth via targeting oncogenic microRNAs and multiple signaling pathways. Int. J. Oncol. 2015, 46, 1739–1747. [Google Scholar] [CrossRef]
- Sánchez-Melgar, A.; Albasanz, J.L.; Guixà-González, R.; Saleh, N.; Selent, J.; Martín, M. The antioxidant resveratrol acts as a non-selective adenosine receptor agonist. Free Radic. Biol. Med. 2019, 135, 261–273. [Google Scholar] [CrossRef]
- Sánchez-Melgar, A.; Muñoz-López, S.; Albasanz, J.L.; Martín, M. Antitumoral Action of Resveratrol Through Adenosinergic Signaling in C6 Glioma Cells. Front. Neurosci. 2021, 15, 702817. [Google Scholar] [CrossRef]
- Jhaveri, A.; Luther, E.; Torchilin, V. The effect of transferrin-targeted, resveratrol-loaded liposomes on neurosphere cultures of glioblastoma: Implications for targeting tumour-initiating cells. J. Drug Target. 2019, 27, 601–613. [Google Scholar] [CrossRef]
- Yang, H.C.; Wang, J.Y.; Bu, X.Y.; Yang, B.; Wang, B.Q.; Hu, S.; Yan, Z.Y.; Gao, Y.S.; Han, S.Y.; Qu, M.Q. Resveratrol restores sensitivity of glioma cells to temozolamide through inhibiting the activation of Wnt signaling pathway. J. Cell. Physiol. 2019, 234, 6783–6800. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; Wu, M.; Wu, J.; Liu, J. Synergistic effects of resveratrol and temozolomide against glioblastoma cells: Underlying mechanism and therapeutic implications. Cancer Manag. Res. 2020, 12, 8341–8354. [Google Scholar] [CrossRef]
- Wu, M.; Song, D.; Li, H.; Ahmad, N.; Xu, H.; Yang, X.; Wang, Q.; Cheng, X.; Deng, S.; Shu, X. Resveratrol Enhances Temozolomide Efficacy in Glioblastoma Cells through Downregulated MGMT and Negative Regulators-Related STAT3 Inactivation. Int. J. Mol. Sci. 2023, 24, 9453. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Mousavi, M.; Moliani, A.; Bahman, Y.; Bagheri, H. Resveratrol inhibits glioblastoma cells and chemoresistance progression through blockade P-glycoprotein and targeting AKT/PTEN signaling pathway. Chem. Biol. Interact. 2023, 376, 110409. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-P.; Chang, Y.-L.; Huang, P.-I.; Chiou, G.-Y.; Tseng, L.-M.; Chiou, S.-H.; Chen, M.-H.; Chen, M.-T.; Shih, Y.-H.; Chang, C.-H.; et al. Resveratrol suppresses tumorigenicity and enhances radiosensitivity in primary glioblastoma tumor initiating cells by inhibiting the STAT3 axis. J. Cell. Physiol. 2012, 227, 976–993. [Google Scholar] [CrossRef] [PubMed]
- Arabzadeh, A.; Mortezazadeh, T.; Aryafar, T.; Gharepapagh, E.; Majdaeen, M.; Farhood, B. Therapeutic potentials of resveratrol in combination with radiotherapy and chemotherapy during glioblastoma treatment: A mechanistic review. Cancer Cell Int. 2021, 21, 391. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.H.; Wang, L.L.; Li, H.; Song, X.; Shi, S.; Gu, J.Y.; Wu, M.L.; Chen, X.Y.; Kong, Q.Y.; Liu, J. Diffusion Efficiency and Bioavailability of Resveratrol Administered to Rat Brain by Different Routes: Therapeutic Implications. Neurotherapeutics 2015, 12, 491–501. [Google Scholar] [CrossRef]
- Clark, P.A.; Bhattacharya, S.; Elmayan, A.; Darjatmoko, S.R.; Thuro, B.A.; Yan, M.B.; Van Ginkel, P.R.; Polans, A.S.; Kuo, J.S. Resveratrol targeting of AKT and p53 in glioblastoma and glioblastoma stem-like cells to suppress growth and infiltration. J. Neurosurg. 2017, 126, 1448–1460. [Google Scholar] [CrossRef]
- Tseng, S.H.; Lin, S.M.; Chen, J.C.; Su, Y.H.; Huang, H.Y.; Chen, C.K.; Lin, P.Y.; Chen, Y. Resveratrol Suppresses the Angiogenesis and Tumor Growth of Gliomas in Rats. Clin. Cancer Res. 2004, 10, 2190–2202. [Google Scholar] [CrossRef]
- Figueiró, F.; Bernardi, A.; Frozza, R.L.; Terroso, T.; Zanotto-Filho, A.; Jandrey, E.H.F.; Moreira, J.C.F.; Salbego, C.G.; Edelweiss, M.I.; Pohlmann, A.R.; et al. Resveratrol-loaded lipid-core nanocapsules treatment reduces in vitro and in vivo glioma growth. J. Biomed. Nanotechnol. 2013, 9, 516–526. [Google Scholar] [CrossRef]
- Guo, W.; Li, A.; Jia, Z.; Yuan, Y.; Dai, H.; Li, H. Transferrin modified PEG-PLA-resveratrol conjugates: In vitro and in vivo studies for glioma. Eur. J. Pharmacol. 2013, 718, 41–47. [Google Scholar] [CrossRef]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control. Release 2018, 277, 89–101. [Google Scholar] [CrossRef]
- Yi, G.; Liu, H.; Sun, F.; Du, R.; Kong, J.; Wang, H.; Cheng, H.; Wang, G.; Gao, F.; Liang, P. Intratumor Injection of Thermosensitive Polypeptide with Resveratrol Inhibits Glioblastoma Growth. Tissue Eng. Part C Methods 2023, 29, 103–109. [Google Scholar] [CrossRef]
- Peñalva, R.; Morales, J.; González-Navarro, C.J.; Larrañeta, E.; Quincoces, G.; Peñuelas, I.; Irache, J.M. Increased oral bioavailability of resveratrol by its encapsulation in casein nanoparticles. Int. J. Mol. Sci. 2018, 19, 2816. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.S.; Park, H.; Lee, S.K.; Sim, W.Y.; Jeong, J.S.; Baek, I.H.; Kim, M.S. Pure Trans-resveratrol nanoparticles prepared by a supercritical antisolvent process using alcohol and dichloromethane mixtures: Effect of particle size on dissolution and bioavailability in rats. Antioxidants 2020, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Lee, C.C.; Shih, Y.L.; Lin, T.Y.; Wang, S.H.; Lin, Y.F.; Shih, C.M. Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy. Free Radic. Biol. Med. 2012, 52, 377–391. [Google Scholar] [CrossRef]
- Xu, H.; Jia, F.; Singh, P.K.; Ruan, S.; Zhang, H.; Li, X. Synergistic anti-glioma effect of a coloaded nano-drug delivery system. Int. J. Nanomed. 2017, 12, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, K.M.; Mahesh, V.B.; Brann, D.W. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFκB transcription factors. J. Neurochem. 2007, 102, 522–538. [Google Scholar] [CrossRef]
- Zanotto-Filho, A.; Braganhol, E.; Edelweiss, M.I.; Behr, G.A.; Zanin, R.; Schröder, R.; Simões-Pires, A.; Battastini, A.M.O.; Moreira, J.C.F. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J. Nutr. Biochem. 2012, 23, 591–601. [Google Scholar] [CrossRef]
- Fratantonio, D.; Maria Sofia, M.; Bashllari, R.; Muscarà, C.; Ferlazzo, G.; Costa, G.; Saija, A.; Cimino, F.; Speciale, A. Curcumin potentiates the antitumor activity of Paclitaxel in rat glioma C6 cells. Phytomedicine 2019, 55, 23–30. [Google Scholar] [CrossRef]
- Zhuo, W.; Wang, W.; Zhou, W.; Duan, Z.; He, S.; Zhang, X.; Yi, L.; Zhang, R.; Guo, A.; Gou, X.; et al. A Targeted and Responsive Nanoprodrug Delivery System for Synergistic Glioma Chemotherapy. Small 2024, 20, e2400630. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, X.; Wu, H. Curcumin with Thymoquinone Controls C6 Glioma Cell Proliferation by Modulating Mitochondrial Functions and SIRT1 Expressions. Lat. Am. J. Pharm. 2023, 42, 907–916. [Google Scholar]
- Aoki, H.; Takada, Y.; Kondo, S.; Sawaya, R.; Aggarwal, B.B.; Kondo, Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: Role of akt and extracellular signal-regulated kinase signaling pathways. Mol. Pharmacol. 2007, 72, 29–39. [Google Scholar] [CrossRef]
- Wang, X.; Deng, J.; Yuan, J.; Tang, X.; Wang, Y.; Chen, H.; Liu, Y.; Zhou, L. Curcumin exerts its tumor suppressive function via inhibition of NEDD4 oncoprotein in glioma cancer cells. Int. J. Oncol. 2017, 51, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Seyithanoglu, M.H.; Abdallah, A.; Kitis, S.; Güler, E.M.; Koçyigit, A.; Dündar, T.T.; Papaker, M.G. Investigation of cytotoxic, genotoxic, and apoptotic effects of curcumin on glioma cells. Cell. Mol. Biol. 2019, 65, 101–108. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L. Curcumin induces human glioma cell apoptosis by promoting reactive oxygen species production. Indian J. Pharm. Sci. 2021, 83, 714–722. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, M.; Zeng, F.; Jin, H.; Xu, Q.; Huang, Y. Dual-Targeting Magnetic PLGA Nanoparticles for Codelivery of Paclitaxel and Curcumin for Brain Tumor Therapy. ACS Appl. Mater. Interfaces 2016, 8, 32159–32169. [Google Scholar] [CrossRef]
- Rahman, M.; Afzal, O.; Altamimi, A.S.A.; Alrobaian, M.; Barkat, M.A.; Ullah, S.N.M.N.; Almalki, W.H.; Singh, T.; Beg, S.; Choudhry, H. Paclitaxel and Curcumin as Dual-Drug-Loaded Lipid Nanocapsules in the Management of Brain Tumour. J. Clust. Sci. 2023, 34, 1927–1938. [Google Scholar] [CrossRef]
- Piwowarczyk, L.; Mlynarczyk, D.T.; Krajka-Kuźniak, V.; Majchrzak-Celińska, A.; Budzianowska, A.; Tomczak, S.; Budzianowski, J.; Woźniak-Braszak, A.; Pietrzyk, R.; Baranowski, M.; et al. Natural Compounds in Liposomal Nanoformulations of Potential Clinical Application in Glioblastoma. Cancers 2022, 14, 6222. [Google Scholar] [CrossRef]
- Sahab-Negah, S.; Ariakia, F.; Jalili-Nik, M.; Afshari, A.R.; Salehi, S.; Samini, F.; Rajabzadeh, G.; Gorji, A. Curcumin Loaded in Niosomal Nanoparticles Improved the Anti-tumor Effects of Free Curcumin on Glioblastoma Stem-like Cells: An In Vitro Study. Mol. Neurobiol. 2020, 57, 3391–3411. [Google Scholar] [CrossRef]
- Tondro, G.; Mohammadi, A.; Rajabzadeh, G.; Moradi, H.R.; Negah, S.S. Niosomal Curcumin Inhibited Gliomagenesis-Related Markers in U87 Cell Line. Preprint 2023, 1–19. [Google Scholar] [CrossRef]
- Nowacka, A.; Ziółkowska, E.; Smuczyński, W.; Bożiłow, D.; Śniegocki, M. Potential of Curcumin and Its Analogs in Glioblastoma Therapy. Antioxidants 2025, 14, 351. [Google Scholar] [CrossRef]
- Hasan, U.; Chauhan, M.; Basu, S.M.; Jayakumar, R.; Giri, J. Overcoming multidrug resistance by reversan and exterminating glioblastoma and glioblastoma stem cells by delivering drug-loaded nanostructure hybrid lipid capsules (nHLCs). Drug Deliv. Transl. Res. 2024, 14, 342–359. [Google Scholar] [CrossRef]
- Orbay, S.; Sanyal, R.; Sanyal, A. Porous Microgels for Delivery of Curcumin: Microfluidics-Based Fabrication and Cytotoxicity Evaluation. Micromachines 2023, 14, 1969. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S.S.R. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Zoi, V.; Galani, V.; Vartholomatos, E.; Zacharopoulou, N.; Tsoumeleka, E.; Gkizas, G.; Bozios, G.; Tsekeris, P.; Chousidis, I.; Leonardos, I.; et al. Curcumin and radiotherapy exert synergistic anti-glioma effect in vitro. Biomedicines 2021, 9, 1562. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.; Sun, T.; Yang, Y.; He, Y.; Yang, S.; Xue, X.; Yang, W. Curcumin Enhanced Ionizing Radiation-Induced Immunogenic Cell Death in Glioma Cells Through Endoplasmic Reticulum Stress Signaling Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 424411. [Google Scholar] [CrossRef]
- Ghanbari, B.; Riz, A.N.; Moghaddam, Z.H. The effect of curcumin in combination with radiation therapy and hyperthermia for a glioblastoma spheroid model. Int. J. Radiat. Res. 2024, 22, 145–153. [Google Scholar] [CrossRef]
- Sminia, P.; van den Berg, J.; van Kootwijk, A.; Hageman, E.; Slotman, B.J.; Verbakel, W.F.A.R. Experimental and clinical studies on radiation and curcumin in human glioma. J. Cancer Res. Clin. Oncol. 2021, 147, 403–409. [Google Scholar] [CrossRef]
- Purkayastha, S.; Berliner, A.; Fernando, S.S.; Ranasinghe, B.; Ray, I.; Tariq, H.; Banerjee, P. Curcumin blocks brain tumor formation. Brain Res. 2009, 1266, 130–138. [Google Scholar] [CrossRef]
- Weissenberger, J.; Priester, M.; Bernreuther, C.; Rakel, S.; Glatzel, M.; Seifert, V.; Kögel, D. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin. Cancer Res. 2010, 16, 5781–5795. [Google Scholar] [CrossRef]
- Orunoğlu, M.; Kaffashi, A.; Pehlivan, S.B.; Şahin, S.; Söylemezoğlu, F.; Karlı-Oğuz, K.; Mut, M. Effects of curcumin-loaded PLGA nanoparticles on the RG2 rat glioma model. Mater. Sci. Eng. C 2017, 78, 32–38. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, M.; Fu, C.; Yu, Y.; Fu, A. Targeted therapy of intracranial glioma model mice with curcumin nanoliposomes. Int. J. Nanomed. 2018, 13, 1601–1610. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Yu, H.; Chen, M. Glioma-targeted multifunctional nanoparticles to co-deliver camptothecin and curcumin for enhanced chemo-immunotherapy. Biomater. Sci. 2022, 10, 1292–1303. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Amaral, L.; Domingues, F.; Pereira, L.; Cascalheira, J.F. Action of Curcumin on Glioblastoma Growth: A Systematic Review with Meta-Analysis of Animal Model Studies. Biomedicines 2024, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Hocking, A.J.; Farrall, A.L.; Newhouse, S.; Sordillo, P.; Greco, K.; Karapetis, C.S.; Dougherty, B.; Klebe, S. Study protocol of a phase 1 clinical trial establishing the safety of intrapleural administration of liposomal curcumin: Curcumin as a palliative treatment for malignant pleural effusion (IPAL-MPE). BMJ Open 2021, 11, e047075. [Google Scholar] [CrossRef] [PubMed]
- Greil, R.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; Bolger, G.T.; Vcelar, B.; Sordillo, P.P. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (LipocurcTM) in patients with locally advanced or metastatic cancer. Cancer Chemother. Pharmacol. 2018, 82, 695–706. [Google Scholar] [CrossRef]
| Dosage | Animal Species | Tumor Type | Way of Administration | Treatment Period | Results | Ref. |
|---|---|---|---|---|---|---|
| 8 mg/kg | Wistar rats | Glioma (C6 cell line) | Oral administration (1 time/day) | Until death of animal | ↑ survival time ↑ apoptosis of tumor cells ↑ tumor cells expression of EGFR, MMP-9, NF-κβ, PCNA and COX-2 ↓ tumor cells expression of VEGF and GFAP | [25] |
| 100 mg/kg | Fischer-344 rats | Glioma (RT-2 cell line) | Intraperitoneal injection (1 time/day) | Until 28 days of treatment or animal death | ↑ survival time | [37] |
| 0.1 mg/mL (water solution concentration) 50 mg/kg (oral gavage) 5 mg (intra-tumoral injection) | Female BALB/c nude mice | Glioma (U87 cell line) | Water solution of resveratrol served ad libitum Administration through oral gavage (1 time/day) Intra-tumor injections (1 time/day) | Oral administration for 18 days (water or food) Intra-tumor injections for 14 days | In all groups: ↓ tumor volume ↑ apoptosis of tumor cells | [36] |
| 50 μL of 500 μM solution | Sprague-Dawley rats | Glioma (RG-2 cell line) | Lumbar puncture (3 times/day) | 3 days | ↑ expression of LC-3 and Beclin-1 in tumor cells and surrounding tissues ↑ apoptosis of tumor cells | [35] |
| 5 mg/kg (nanoencapsulated form) | Wistar rats | Glioma (C6 cell line) | Oral administration (1 time/day) | 10 days | ↓ tumor volume | [38] |
| 15 mg/kg (transferrin (Tf)-modified PEG-PLA nanoparticles conjugated with resveratrol) | Male Sprague-Dawley rats | Glioma (C6 cell line) | Intraperitoneal injection (1 time every 2 days) | Continuous until animal death | ↑ survival time ↓ tumor volume | [39] |
| 10 mg/kg (encapsulated in Tf-targeted liposomes) | Female athymic NCr-nu/nu nude mice | Glioblastoma (U87MG cell line) | Intravenous injection (1 time every 3 days) | 30 days | ↑ survival time ↓ tumor volume | [40] |
| 12.5 mg/kg | BALB/c nu/nu female mice | Glioblastoma (U87MG cell line) | Intraperitoneal injection (1 time a day) | 12 days | ↓ tumor volume ↑ cytotoxicity of TMZ ↑ TMZ-mediated downregulation of ERK and LC3-II activity ↑ TMZ-mediated cleavage of PARP | [44] |
| 10 mg/kg (conjugated with mPEG–PCL copolymers) | Nude mice | Glioblastoma (U87MG cell line) | Intraperitoneal injection (1 time/day) | 14 days | ↑ TMZ-mediated anti-tumor effect when compared with control groups | [45] |
| Dosage | Animal Species | Tumor Type | Way of Administration | Time Period | Results | Ref. |
|---|---|---|---|---|---|---|
| Western-type mouse chow enriched with 0.05% curcumin | C6B3F1 mice | Glioma (Tu-2449 or Tu-9648 cell line) | Oral administration ad libitum | The administration of curcumin was started 7 days before the implantation of malignant cells and continued for 65 or 80 days or until the animal started showing symptoms of tumor presence. | Tu-2449 group: ↑ survival time ↓ tumor size ↓ STAT3 activity Tu-9648 group: ↑ survival time ↓ tumor cell migration to contralateral side of brain ↓ STAT3 activity | [69] |
| 50 mg/kg | Wistar rats | Glioma (C6 cell line) | Intraperitoneal injection (1 time/day) | The administration of curcumin was continued for 10 days. | ↓ tumor volume ↓ tumor development | [47] |
| 25 μM (encapsulated in PLGA nanoparticles) | Wistar rats | Glioma (RG2 cell line) | Intra-tumoral injection (1 time/day) | The administration of curcumin was continued until the animals started expressing clear symptoms of significant tumor advancement. | ↓ tumor volume | [70] |
| 4 mg/kg (encapsulated in PLGA modified with T7 transferrin receptor ligands and magnetic nanoparticles) | Male BALB/C nude mice | Glioblastoma (U87 cell line) | Intravenous injection (1 time every 3 days) | The administration of curcumin was started 15 days after the implantation of malignant cells and continued for 20 days. | ↑ paclitaxel-mediated reduction in tumor volume ↑ paclitaxel-mediated reduction in tumor development ↓ weight loss rate | [55] |
| 20 mg/kg (encapsulated in nanoliposomes modified with RDP peptide) | Male BALB/c nude mice | Glioma (U251MG cell line) | Intravenous injection (1 time every 2 days) | The administration of curcumin was continued for 7 days. | ↓ tumor volume ↓ tumor cell proliferation rate ↑ survival time ↓ symptoms severity | [71] |
| 800 μg (encapsulated in exosomes modified with neuropilin-1-targeted ligand—RGE) | Female BALB/c nude mice | Glioma (U251MG cell line) | Intravenous injection (1 time every 2 days) | The administration of curcumin was continued for 28 days. | ↓ tumor volume | [73] |
| 30 μM (concentration of curcumin in glioma cells breeding medium) | Male C57BL/6J mice | Glioma (GL261 cell line) | Subcutaneous injection of glioma cells previously treated with curcumin (vaccination) 7 days after initial vaccination, animals inoculated with live glioma cells not previously treated with polyphenol | - | ↑ tumor rejection rate | [65] |
| Aim of the Study | Intervention: | Study Design | Results | Ref. |
|---|---|---|---|---|
| The determination of intra-tumoral and serum levels of curcumin and related curcuminoids that are possible to achieve after the administration of a liquid micellar formulation. Assessment of its clinical tolerance in GBM patients, and the impact of highly bioavailable curcumin on metabolism and energy status in GBM. | 1 g of a micellar curcumin formulation (57.4 mg curcumin, 11.2 mg demethoxycurcumin and 1.4 mg bisdemethoxycurcumin) mixed in 200 mL of pear-juice beverage administered three times a day after regular meals for the 4 days before surgery (12 g in total, corresponding to 840 mg curcuminoids). | Ten patients (four women, six men) scheduled for surgery, with histologically proven GBM, a mean age of 67 ± 8 years and a mean body mass index of 26.8 ± 1.8 kg/m2. Before and after the curcumin intervention, a 31P MRSI was performed to detect curcumin through changes in metabolites. The MRSIs of the brain were performed on a 3-T whole-body system and planned on T2-weighted images in three orientations. Tissue and blood samples were acquired and flash-frozen during surgery. | The mean intra-tumoral concentrations of curcuminoids among patients who completed the study were as follows: curcumin—56 pg/mg tissue; demethoxycurcumin—8.6 pg/mg; bisdemethoxycurcumin—0.866 pg/mg. The concentrations of curcumin within the tumor tissues correlated with the cumulative received dose. The mean serum concentration of total curcumin was 253 ng/mL. Curcumin admission significantly increased levels of intra-tumoral inorganic phosphate. Intra-tumoral pH increased from 7.06 ± 0.05 to 7.10 ± 0.05, but this result was not statistically significant. | [18] |
| To assess the safety, tolerability and efficacy of liposomal curcumin in combination with radiotherapy and TMZ in patients with high-grade glioma. | The patients treated with standard chemoradiation (radiation therapy + temozolomide treatment) will receive liposomal curcumin at doses of 300–400 mg/m2 weekly. | A phase I/II multi-center, open-label, dose-escalation study in patients with high-grade malignant gliomas. Dose finding will be performed using a time-to-event Bayesian optimal interval (TITE-BOIN) rule-based schema. Approximately 50 patients will be screened to achieve up to 30 patients assigned to the study’s intervention. The duration of treatment for each patient will be up to 34 weeks. Treatment starts with the beginning of infusion and ends, if tolerated, at the end of cycle 6 of adjuvant TMZ. The dose-limiting toxicity evaluation period is 10 weeks. Afterward, the patients will continue adjuvant TMZ and liposomal curcumin for an additional 24 weeks. Progression assessments accomplished with MRI evaluations are planned for the 10th, 18th and 26th weeks of treatment. Patients will be monitored for survival and progression for the first 2 years after the last dose of curcumin every 2 months. After the first 2 years, patients will be monitored every 6 months. | The study is currently in the recruitment phase. So far, no dose-limiting toxicity has been observed; the maximal tolerated dose of curcumin was assessed to 300 mg/m2. The research is projected to be completed in 2026–2027. | [17,75,76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolińska, E.; Grabarczyk, M.; Justyńska, W.; Bielenin, A.; Glabinski, A.; Szpakowski, P. Potential Therapeutic Appliances of Dietary Polyphenols: Resveratrol and Curcumin in Treatment of Gliomas. Int. J. Mol. Sci. 2025, 26, 6154. https://doi.org/10.3390/ijms26136154
Smolińska E, Grabarczyk M, Justyńska W, Bielenin A, Glabinski A, Szpakowski P. Potential Therapeutic Appliances of Dietary Polyphenols: Resveratrol and Curcumin in Treatment of Gliomas. International Journal of Molecular Sciences. 2025; 26(13):6154. https://doi.org/10.3390/ijms26136154
Chicago/Turabian StyleSmolińska, Ewa, Mikołaj Grabarczyk, Weronika Justyńska, Aleksandra Bielenin, Andrzej Glabinski, and Piotr Szpakowski. 2025. "Potential Therapeutic Appliances of Dietary Polyphenols: Resveratrol and Curcumin in Treatment of Gliomas" International Journal of Molecular Sciences 26, no. 13: 6154. https://doi.org/10.3390/ijms26136154
APA StyleSmolińska, E., Grabarczyk, M., Justyńska, W., Bielenin, A., Glabinski, A., & Szpakowski, P. (2025). Potential Therapeutic Appliances of Dietary Polyphenols: Resveratrol and Curcumin in Treatment of Gliomas. International Journal of Molecular Sciences, 26(13), 6154. https://doi.org/10.3390/ijms26136154






