Distinctive Features of Extracellular Vesicles Present in the Gastric Juice of Patients with Gastric Cancer and Healthy Subjects
Abstract
1. Introduction
2. Results
2.1. Characterization of GJ EVs by Cryo-EM
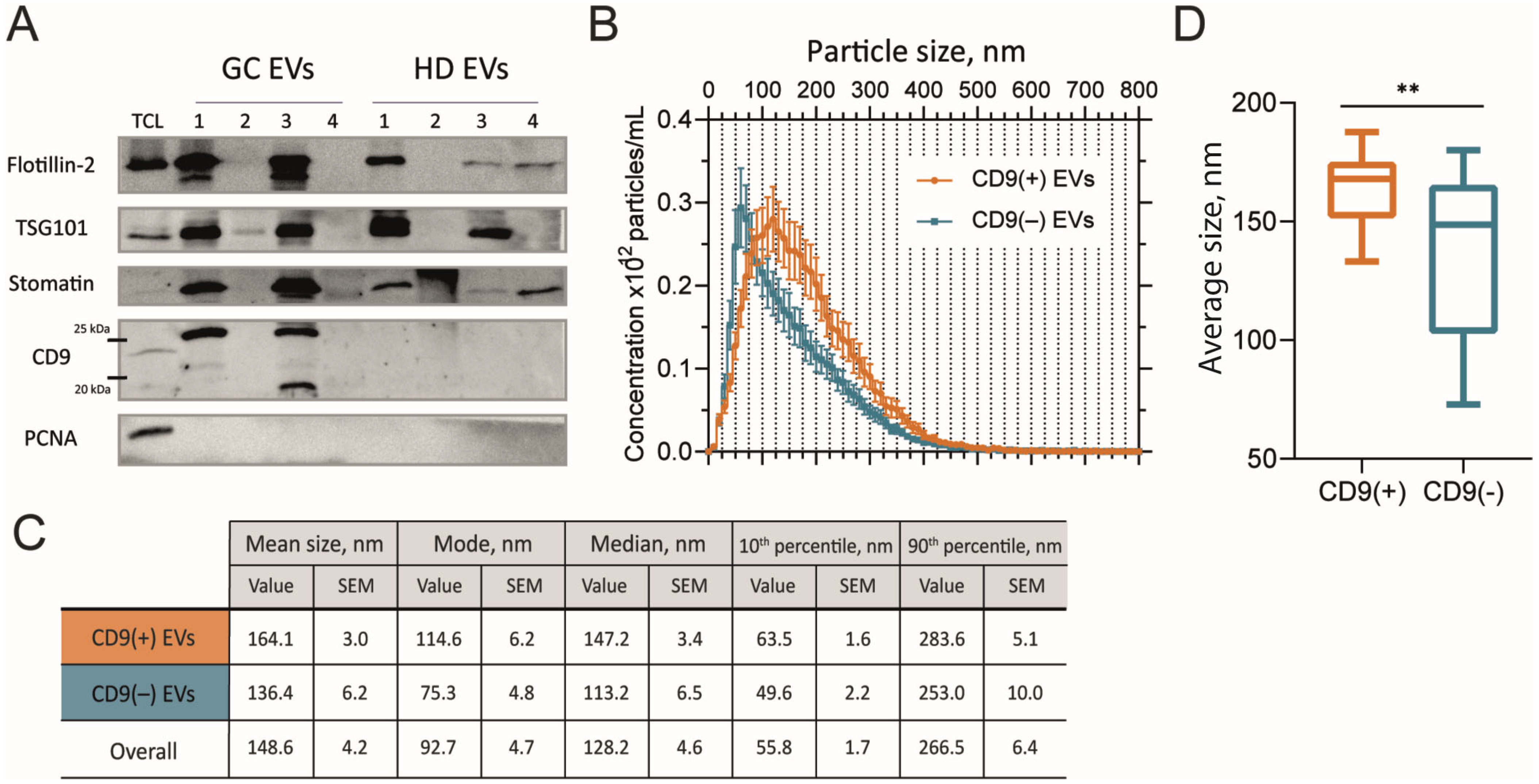
2.2. Size of GJ EVs Differs Between CD9(+) and CD9(–) Samples
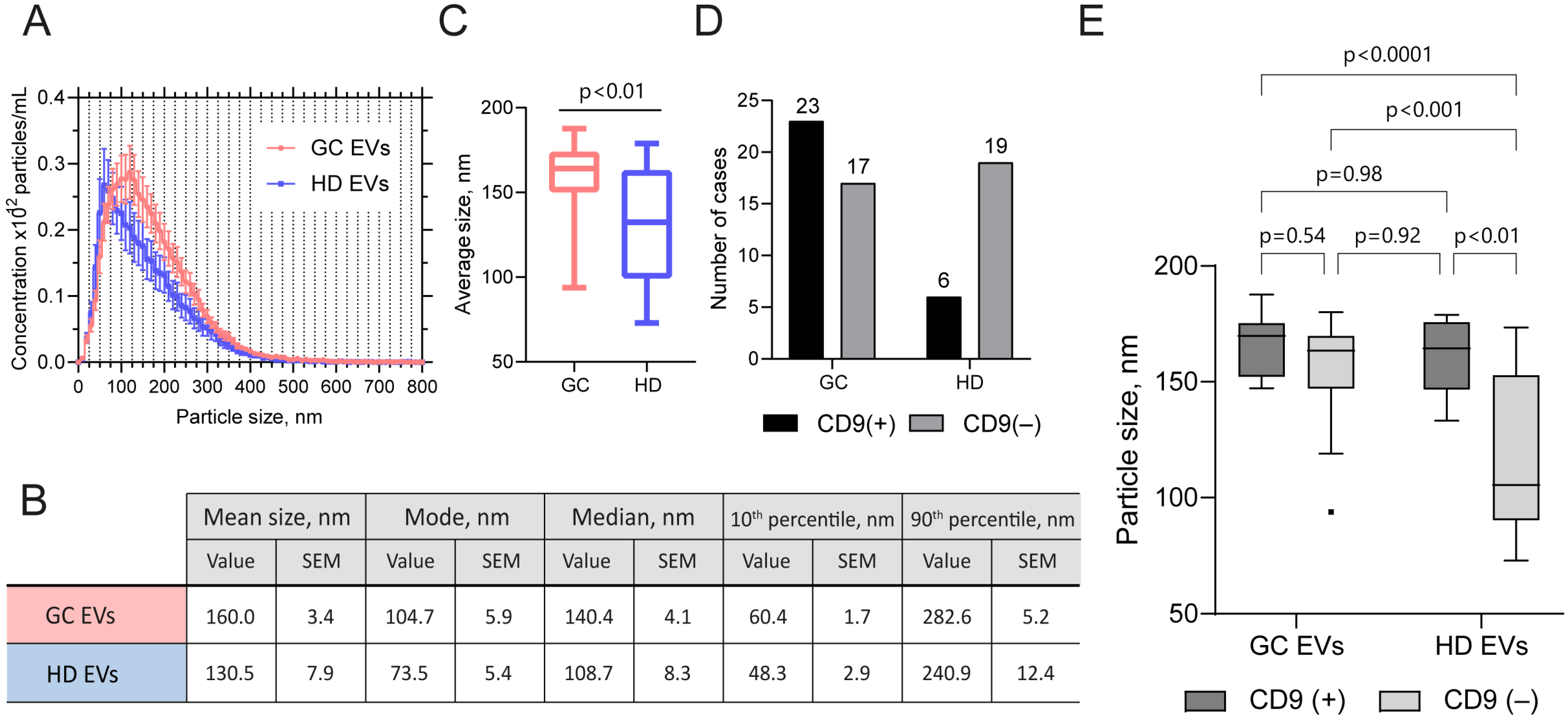
2.3. EV Samples from GJ of GC Patients and Healthy Subjects Differ in Size and in the Presence of CD9
3. Discussion
4. Materials and Methods
4.1. Clinical Specimens and Sample Processing
4.2. EV Isolation
4.3. EV Visualization
4.4. Nanoparticle Tracking Analysis
4.5. Immunoblotting and Antibodies
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC | Gastric cancer |
| EVs | Extracellular vesicles |
| sEVs | Small extracellular vesicles |
| GJ | Gastric juice |
| ISEV | International Society for Extracellular Vesicles |
| TEM | Transmission electron microscopy |
| NTA | Nanoparticle tracking analysis |
| Cryo-EM | Cryogenic electron microscopy |
| TEMs | Tetraspanin-enriched microdomains |
| MVs | Microvesicles |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. Gastric Cancer: Overview. Gastroenterol. Clin. N. Am. 2013, 42, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, A.; Zheng, S.; Chen, C.; Lyu, J. Recent Estimates and Predictions of 5-Year Survival in Patients with Gastric Cancer: A Model-Based Period Analysis. Cancer Control 2022, 29, 107327482210992. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, M.; Oda, I.; Matsuda, T.; Saito, Y. Epidemiological Trends and Future Perspectives of Gastric Cancer in Eastern Asia. Digestion 2021, 103, 22–28. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Li, Q.; Lai, H.; Zhang, H.; Hu, Z.; Li, Y.; Huang, S. EV-Origin: Enumerating the Tissue-Cellular Origin of Circulating Extracellular Vesicles Using exLR Profile. Comput. Struct. Biotechnol. J. 2020, 18, 2851–2859. [Google Scholar] [CrossRef]
- Hu, C.; Jiang, W.; Lv, M.; Fan, S.; Lu, Y.; Wu, Q.; Pi, J. Potentiality of Exosomal Proteins as Novel Cancer Biomarkers for Liquid Biopsy. Front. Immunol. 2022, 13, 792046. [Google Scholar] [CrossRef]
- Gao, J.; Li, A.; Hu, J.; Feng, L.; Liu, L.; Shen, Z. Recent Developments in Isolating Methods for Exosomes. Front. Bioeng. Biotechnol. 2022, 10, 1100892. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Gudbergsson, J.M.; Andresen, T.L.; Simonsen, J.B. What Is the Blood Concentration of Extracellular Vesicles? Implications for the Use of Extracellular Vesicles as Blood-Borne Biomarkers of Cancer. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 109–116. [Google Scholar] [CrossRef]
- Skryabin, G.O.; Komelkov, A.V.; Zhordania, K.I.; Bagrov, D.V.; Enikeev, A.D.; Galetsky, S.A.; Beliaeva, A.A.; Kopnin, P.B.; Moiseenko, A.V.; Senkovenko, A.M.; et al. Integrated miRNA Profiling of Extracellular Vesicles from Uterine Aspirates, Malignant Ascites and Primary-Cultured Ascites Cells for Ovarian Cancer Screening. Pharmaceutics 2024, 16, 902. [Google Scholar] [CrossRef]
- Yamamoto, H.; Watanabe, Y.; Oikawa, R.; Morita, R.; Yoshida, Y.; Maehata, T.; Yasuda, H.; Itoh, F. BARHL2 Methylation Using Gastric Wash DNA or Gastric Juice Exosomal DNA Is a Useful Marker For Early Detection of Gastric Cancer in an H. Pylori-Independent Manner. Clin. Transl. Gastroenterol. 2016, 7, e184. [Google Scholar] [CrossRef]
- Kagota, S.; Taniguchi, K.; Lee, S.-W.; Ito, Y.; Kuranaga, Y.; Hashiguchi, Y.; Inomata, Y.; Imai, Y.; Tanaka, R.; Tashiro, K.; et al. Analysis of Extracellular Vesicles in Gastric Juice from Gastric Cancer Patients. Int. J. Mol. Sci. 2019, 20, 953. [Google Scholar] [CrossRef] [PubMed]
- Skryabin, G.O.; Vinokurova, S.V.; Galetsky, S.A.; Elkin, D.S.; Senkovenko, A.M.; Denisova, D.A.; Komelkov, A.V.; Stilidi, I.S.; Peregorodiev, I.N.; Malikhova, O.A.; et al. Isolation and Characterization of Extracellular Vesicles from Gastric Juice. Cancers 2022, 14, 3314. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Skryabin, G.O.; Komelkov, A.V.; Galetsky, S.A.; Bagrov, D.V.; Evtushenko, E.G.; Nikishin, I.I.; Zhordaniia, K.I.; Savelyeva, E.E.; Akselrod, M.E.; Paianidi, I.G.; et al. Stomatin Is Highly Expressed in Exosomes of Different Origin and Is a Promising Candidate as an Exosomal Marker. J. Cell. Biochem. 2021, 122, 100–115. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Koning, R.I.; Koster, A.J.; Sharp, T.H. Advances in Cryo-Electron Tomography for Biology and Medicine. Ann. Anat. 2018, 217, 82–96. [Google Scholar] [CrossRef]
- Tatischeff, I.; Larquet, E.; Falcón-Pérez, J.M.; Turpin, P.-Y.; Kruglik, S.G. Fast Characterisation of Cell-derived Extracellular Vesicles by Nanoparticles Tracking Analysis, Cryo-electron Microscopy, and Raman Tweezers Microspectroscopy. J. Extracell.Vesicles 2012, 1, 19179. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Ehab, S.; Cho, J. Relevance of Multilamellar and Multicompartmental Vesicles in Biological Fluids: Understanding the Significance of Proportional Variations and Disease Correlation. Biomark. Res. 2023, 11, 77. [Google Scholar] [CrossRef]
- Konoshenko, M.; Sagaradze, G.; Orlova, E.; Shtam, T.; Proskura, K.; Kamyshinsky, R.; Yunusova, N.; Alexandrova, A.; Efimenko, A.; Tamkovich, S. Total Blood Exosomes in Breast Cancer: Potential Role in Crucial Steps of Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 7341. [Google Scholar] [CrossRef]
- Emelyanov, A.; Shtam, T.; Kamyshinsky, R.; Garaeva, L.; Verlov, N.; Miliukhina, I.; Kudrevatykh, A.; Gavrilov, G.; Zabrodskaya, Y.; Pchelina, S.; et al. Cryo-Electron Microscopy of Extracellular Vesicles from Cerebrospinal Fluid. PLoS ONE 2020, 15, e0227949. [Google Scholar] [CrossRef]
- Höög, J.L.; Lötvall, J. Diversity of Extracellular Vesicles in Human Ejaculates Revealed by Cryo-Electron Microscopy. J. Extracell. Vesicles 2015, 4, 28680. [Google Scholar] [CrossRef] [PubMed]
- Peyret, A.; Ibarboure, E.; Pippa, N.; Lecommandoux, S. Liposomes in Polymersomes: Multicompartment System with Temperature-Triggered Release. Langmuir 2017, 33, 7079–7085. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.S.; Yun, M.E.; Park, S.N. Surfactant-Stable and pH-Sensitive Liposomes Coated with N-Succinyl-Chitosan and Chitooligosaccharide for Delivery of Quercetin. Carbohydr. Polym. 2018, 181, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, W.; Zhu, Y.; Luo, J.; Liu, R.; Liu, X. Synthesis of Temperature/pH Dual-Stimuli-Response Multicompartmental Microcapsules via Pickering Emulsion for Preprogrammable Payload Release. ACS Appl. Mater. Interfaces 2020, 12, 4821–4832. [Google Scholar] [CrossRef]
- Tatiana, S.; Stanislav, N.; Darya, K.; Luiza, G.; Konstantin, S.; Sergey, L.; Elena, V.; Galina, S.; Nikolai, V.; Arthur, K.; et al. Altered Level of Plasma Exosomes in Patients with Gaucher Disease. Eur. J. Med. Genet. 2020, 63, 104038. [Google Scholar] [CrossRef]
- Coleman, B.M.; Hanssen, E.; Lawson, V.A.; Hill, A.F. Prion-Infected Cells Regulate the Release of Exosomes with Distinct Ultrastructural Features. FASEB J. 2012, 26, 4160–4173. [Google Scholar] [CrossRef]
- Puhm, F.; Afonyushkin, T.; Resch, U.; Obermayer, G.; Rohde, M.; Penz, T.; Schuster, M.; Wagner, G.; Rendeiro, A.F.; Melki, I.; et al. Mitochondria Are a Subset of Extracellular Vesicles Released by Activated Monocytes and Induce Type I IFN and TNF Responses in Endothelial Cells. Circ. Res. 2019, 125, 43–52. [Google Scholar] [CrossRef]
- Broad, K.; Walker, S.A.; Davidovich, I.; Witwer, K.; Talmon, Y.; Wolfram, J. Unraveling Multilayered Extracellular Vesicles: Speculation on Cause. J. Extracell. Vesicles 2023, 12, e12309. [Google Scholar] [CrossRef]
- Zabeo, D.; Cvjetkovic, A.; Lässer, C.; Schorb, M.; Lötvall, J.; Höög, J.L. Exosomes Purified from a Single Cell Type Have Diverse Morphology. J. Extracell. Vesicles 2017, 6, 1329476. [Google Scholar] [CrossRef]
- Issman, L.; Brenner, B.; Talmon, Y.; Aharon, A. Cryogenic Transmission Electron Microscopy Nanostructural Study of Shed Microparticles. PLoS ONE 2013, 8, e83680. [Google Scholar] [CrossRef]
- Yuana, Y.; Koning, R.I.; Kuil, M.E.; Rensen, P.C.N.; Koster, A.J.; Bertina, R.M.; Osanto, S. Cryo-Electron Microscopy of Extracellular Vesicles in Fresh Plasma. J. Extracell. Vesicles 2013, 2, 21494. [Google Scholar] [CrossRef] [PubMed]
- Vagner, T.; Spinelli, C.; Minciacchi, V.R.; Balaj, L.; Zandian, M.; Conley, A.; Zijlstra, A.; Freeman, M.R.; Demichelis, F.; De, S.; et al. Large Extracellular Vesicles Carry Most of the Tumour DNA Circulating in Prostate Cancer Patient Plasma. J. Extracell. Vesicles 2018, 7, 1505403. [Google Scholar] [CrossRef] [PubMed]
- Caponnetto, F.; Manini, I.; Skrap, M.; Palmai-Pallag, T.; Di Loreto, C.; Beltrami, A.P.; Cesselli, D.; Ferrari, E. Size-Dependent Cellular Uptake of Exosomes. Nanomedicine 2017, 13, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Akimov, S.A.; Polynkin, M.A.; Jiménez-Munguía, I.; Pavlov, K.V.; Batishchev, O.V. Phosphatidylcholine Membrane Fusion Is pH-Dependent. Int. J. Mol. Sci. 2018, 19, 1358. [Google Scholar] [CrossRef]
- Guo, J.; Li, S.; Bai, L.; Zhao, H.; Shang, W.; Zhong, Z.; Maimaiti, T.; Gao, X.; Ji, N.; Chao, Y.; et al. Structural Transition of GP64 Triggered by a pH-Sensitive Multi-Histidine Switch. Nat. Commun. 2024, 15, 7668. [Google Scholar] [CrossRef]
- Kreutzberger, A.J.B.; Sanyal, A.; Saminathan, A.; Bloyet, L.-M.; Stumpf, S.; Liu, Z.; Ojha, R.; Patjas, M.T.; Geneid, A.; Scanavachi, G.; et al. SARS-CoV-2 Requires Acidic pH to Infect Cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2209514119. [Google Scholar] [CrossRef]
- Morandi, M.I.; Busko, P.; Ozer-Partuk, E.; Khan, S.; Zarfati, G.; Elbaz-Alon, Y.; Abou Karam, P.; Napso Shogan, T.; Ginini, L.; Gil, Z.; et al. Extracellular Vesicle Fusion Visualized by Cryo-Electron Microscopy. PNAS Nexus 2022, 1, pgac156. [Google Scholar] [CrossRef]
- Capra, J.; Härkönen, K.; Kyykallio, H.; Vihinen, H.; Jokitalo, E.; Rilla, K. Microscopic Characterization Reveals the Diversity of EVs Secreted by GFP-HAS3 Expressing MCF7 Cells. Eur. J. Cell Biol. 2022, 101, 151235. [Google Scholar] [CrossRef]
- Dharan, R.; Sorkin, R. Tetraspanin Proteins in Membrane Remodeling Processes. J. Cell Sci. 2024, 137, jcs261532. [Google Scholar] [CrossRef]
- Jankovičová, J.; Sečová, P.; Michalková, K.; Antalíková, J. Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction. Int. J. Mol. Sci. 2020, 21, 7568. [Google Scholar] [CrossRef]
- Hemler, M.E. Tetraspanin Proteins Mediate Cellular Penetration, Invasion, and Fusion Events and Define a Novel Type of Membrane Microdomain. Annu. Rev. Cell Dev. Biol. 2003, 19, 397–422. [Google Scholar] [CrossRef] [PubMed]
- Jankovičová, J.; Neuerová, Z.; Sečová, P.; Bartóková, M.; Bubeníčková, F.; Komrsková, K.; Postlerová, P.; Antalíková, J. Tetraspanins in Mammalian Reproduction: Spermatozoa, Oocytes and Embryos. Med. Microbiol. Immunol. 2020, 209, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yue, S. EV Tetraspanins in Regenerative Medicine. In Extracellular Vesicle: Biology and Translational Application; Li, Z., Ed.; Springer Nature: Singapore, 2024; pp. 117–142. ISBN 978-981-97-5536-3. [Google Scholar]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Saito, T.; Wada, I. Unveiling a Novel Function of CD9 in Surface Compartmentalization of Oocytes. Development 2020, 147, dev189985. [Google Scholar] [CrossRef]
- Earnest, J.T.; Hantak, M.P.; Li, K.; Jr, P.B.M.; Perlman, S.; Gallagher, T. The Tetraspanin CD9 Facilitates MERS-Coronavirus Entry by Scaffolding Host Cell Receptors and Proteases. PLoS Pathog. 2017, 13, e1006546. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular Vesicles and Nanoparticles: Emerging Complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of Exosome versus Small Ectosome Secretion Revealed by Live Intracellular Tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef]
- Wang, T.; Gilkes, D.M.; Takano, N.; Xiang, L.; Luo, W.; Bishop, C.J.; Chaturvedi, P.; Green, J.J.; Semenza, G.L. Hypoxia-Inducible Factors and RAB22A Mediate Formation of Microvesicles That Stimulate Breast Cancer Invasion and Metastasis. Proc. Natl. Acad. Sci. USA 2014, 111, E3234–E3242. [Google Scholar] [CrossRef]
- Li, J.; Liao, T.; Chua, E.G.; Zhang, M.; Shen, Y.; Song, X.; Marshall, B.J.; Benghezal, M.; Tang, H.; Li, H. Helicobacter pylori Outer Membrane Vesicles: Biogenesis, Composition, and Biological Functions. Int. J. Biol. Sci. 2024, 20, 4029–4043. [Google Scholar] [CrossRef]
- Lorico, A.; Lorico-Rappa, M.; Karbanová, J.; Corbeil, D.; Pizzorno, G. CD9, a Tetraspanin Target for Cancer Therapy? Exp. Biol. Med. 2021, 246, 1121–1138. [Google Scholar] [CrossRef]
- Hwang, J.R.; Jo, K.; Lee, Y.; Sung, B.-J.; Park, Y.W.; Lee, J.-H. Upregulation of CD9 in Ovarian Cancer Is Related to the Induction of TNF-α Gene Expression and Constitutive NF-κB Activation. Carcinogenesis 2012, 33, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kischel, P.; Bellahcene, A.; Deux, B.; Lamour, V.; Dobson, R.; DE Pauw, E.; Clezardin, P.; Castronovo, V. Overexpression of CD9 in Human Breast Cancer Cells Promotes the Development of Bone Metastases. Anticancer Res. 2012, 32, 5211–5220. [Google Scholar] [PubMed]
- Baek, J.; Jang, N.; Choi, J.E.; Kim, J.-R.; Bae, Y.K. CD9 Expression in Tumor Cells Is Associated with Poor Prognosis in Patients with Invasive Lobular Carcinoma. J. Breast Cancer 2019, 22, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Choi, J.E.; Kang, S.H.; Son, Y.; Bae, Y.K. Prognostic Significance of CD9 Expression Differs between Tumour Cells and Stromal Immune Cells, and Depends on the Molecular Subtype of the Invasive Breast Carcinoma. Histopathology 2017, 70, 1155–1165. [Google Scholar] [CrossRef]
- Murayama, Y.; Oritani, K.; Tsutsui, S. Novel CD9-Targeted Therapies in Gastric Cancer. World J. Gastroenterol. 2015, 21, 3206–3213. [Google Scholar] [CrossRef]
- Miki, Y.; Yashiro, M.; Okuno, T.; Kitayama, K.; Masuda, G.; Hirakawa, K.; Ohira, M. CD9-Positive Exosomes from Cancer-Associated Fibroblasts Stimulate the Migration Ability of Scirrhous-Type Gastric Cancer Cells. Br. J. Cancer 2018, 118, 867–877. [Google Scholar] [CrossRef]
- Skryabin, G.O.; Komelkov, A.V.; Zhordania, K.I.; Bagrov, D.V.; Vinokurova, S.V.; Galetsky, S.A.; Elkina, N.V.; Denisova, D.A.; Enikeev, A.D.; Tchevkina, E.M. Extracellular Vesicles from Uterine Aspirates Represent a Promising Source for Screening Markers of Gynecologic Cancers. Cells 2022, 11, 1064. [Google Scholar] [CrossRef]


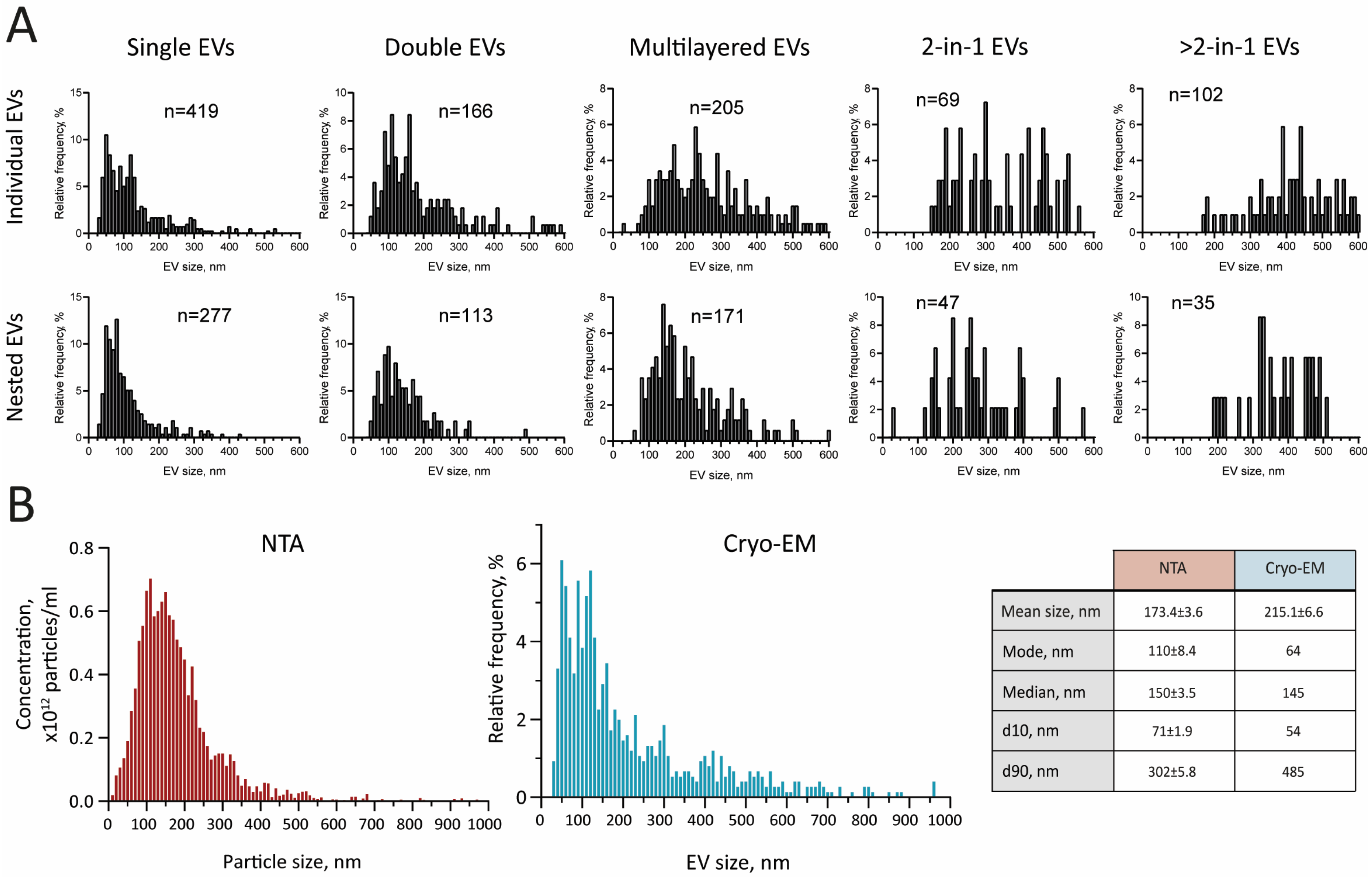
| Individual EVs | Nested EVs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Single | Double | Multilayered | 2-in-1 | >2-in-1 | Single | Double | Multilayered | 2-in-1 | >2-in-1 | |
| Mean size, nm | 127.8 ± 94.5 | 191.3 ± 121.5 | 276.3 ± 147.7 | 361.3 ± 151.3 | 513.5 ± 190.2 | 107.1 ± 69.1 | 143.4 ± 70.39 | 215.8 ± 113.1 | 282.3 ± 104.4 | 405.3 ± 132.5 |
| Mode, nm | 87 | 164 | 171 | 419 | 430 | 61 | 89 | 112 | N/A | 324 |
| Median, nm | 105 | 158 | 241 | 338 | 485 | 84 | 128 | 185 | 255 | 394 |
| d10, nm | 46 | 84 | 123 | 185 | 297 | 47 | 71 | 105 | 144 | 216 |
| d90, nm | 257 | 356 | 478 | 530 | 786 | 202 | 233 | 359 | 494 | 627 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skryabin, G.; Enikeev, A.; Beliaeva, A.; Galetsky, S.; Bagrov, D.; Moiseenko, A.; Vnukova, A.; Imaraliev, O.; Karasev, I.; Tchevkina, E. Distinctive Features of Extracellular Vesicles Present in the Gastric Juice of Patients with Gastric Cancer and Healthy Subjects. Int. J. Mol. Sci. 2025, 26, 5857. https://doi.org/10.3390/ijms26125857
Skryabin G, Enikeev A, Beliaeva A, Galetsky S, Bagrov D, Moiseenko A, Vnukova A, Imaraliev O, Karasev I, Tchevkina E. Distinctive Features of Extracellular Vesicles Present in the Gastric Juice of Patients with Gastric Cancer and Healthy Subjects. International Journal of Molecular Sciences. 2025; 26(12):5857. https://doi.org/10.3390/ijms26125857
Chicago/Turabian StyleSkryabin, Gleb, Adel Enikeev, Anastasiia Beliaeva, Sergey Galetsky, Dmitry Bagrov, Andrey Moiseenko, Anna Vnukova, Oiatiddin Imaraliev, Ivan Karasev, and Elena Tchevkina. 2025. "Distinctive Features of Extracellular Vesicles Present in the Gastric Juice of Patients with Gastric Cancer and Healthy Subjects" International Journal of Molecular Sciences 26, no. 12: 5857. https://doi.org/10.3390/ijms26125857
APA StyleSkryabin, G., Enikeev, A., Beliaeva, A., Galetsky, S., Bagrov, D., Moiseenko, A., Vnukova, A., Imaraliev, O., Karasev, I., & Tchevkina, E. (2025). Distinctive Features of Extracellular Vesicles Present in the Gastric Juice of Patients with Gastric Cancer and Healthy Subjects. International Journal of Molecular Sciences, 26(12), 5857. https://doi.org/10.3390/ijms26125857






