Molecular Mechanisms of Vitexin: An Update on Its Anti-Cancer Functions
Abstract
1. Introduction
2. Sources, Chemistry, and Structural Activity Relationship of Vitexin
2.1. Botanical Sources and Pharmacological Properties
2.2. Chemical Profile and Pharmacokinetic
2.3. Biosynthesis and Production Strategies
3. Anticancer Effect of Vitexin
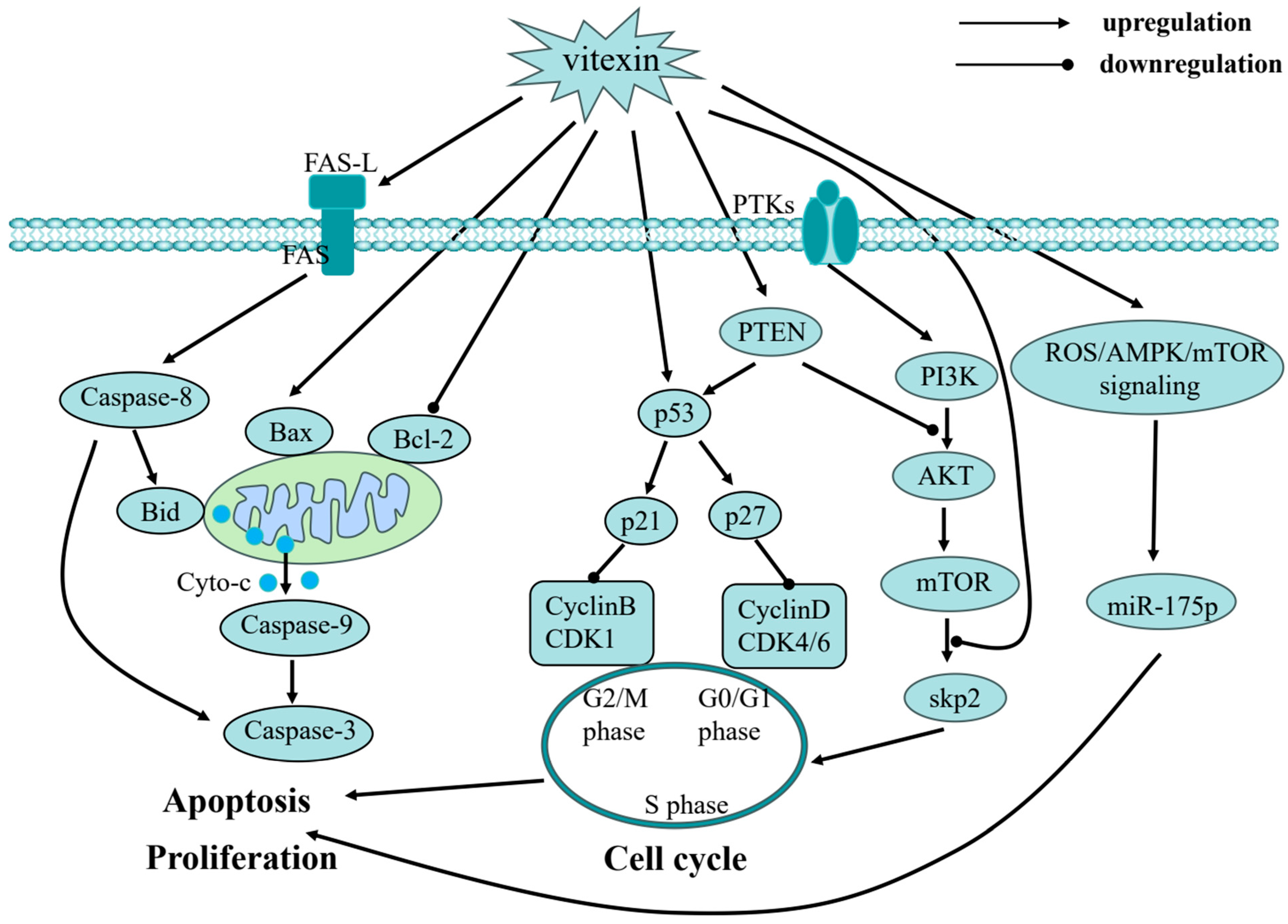
3.1. Cell Cycle Arrest
3.2. Apoptosis Induction
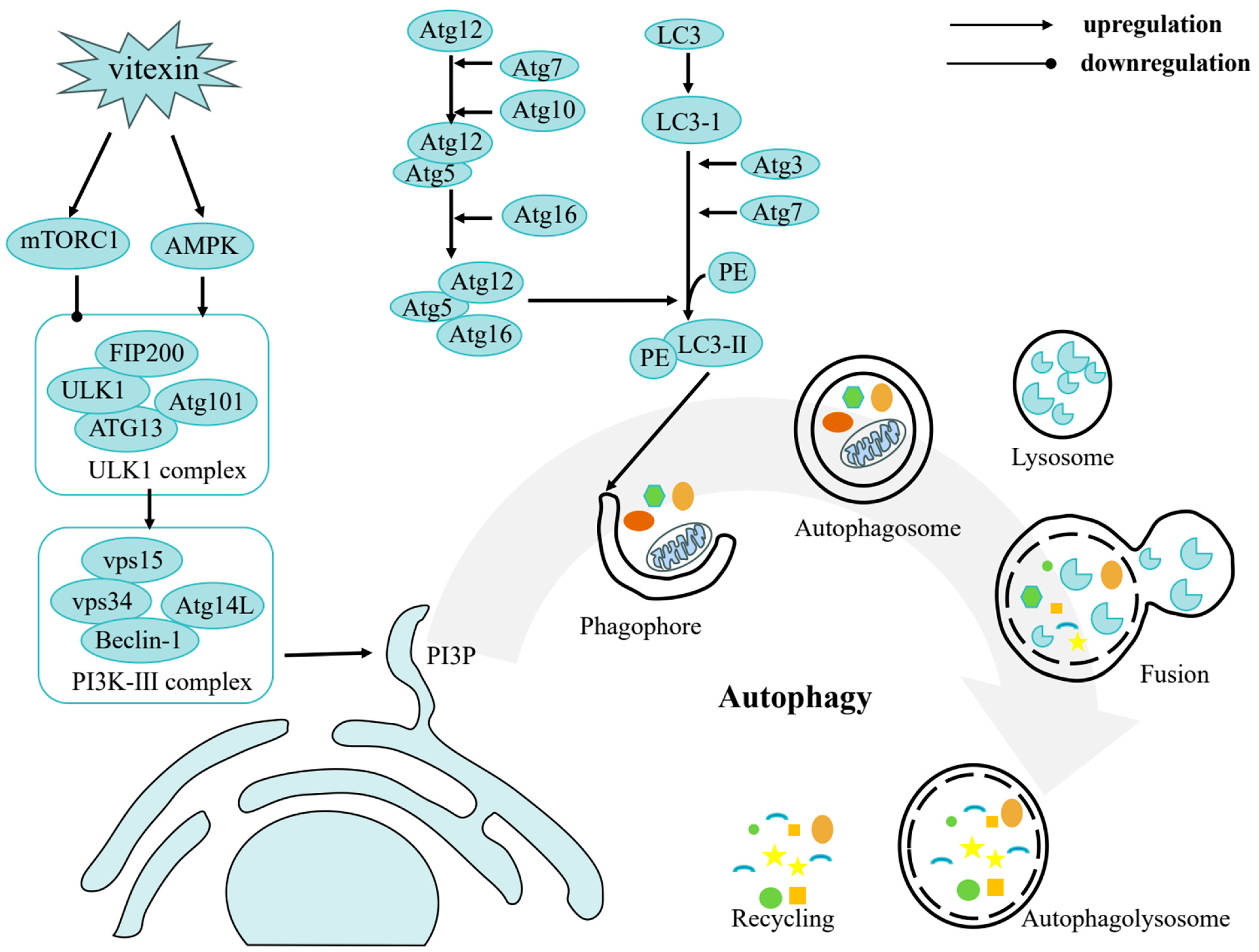
3.3. Autophagy Induction
3.4. Anti-Proliferation
3.5. Metastasis and Angiogenesis
3.6. Epigenetic Modification
3.7. Inhibition of Tumor Glycolysis (Warburg Effect)
4. Synergistic Effects and Safety Considerations of Vitexin
5. Delivery Strategies for Vitexin in Cancer Therapy
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, M.; Lu, J. Cancer statistics and trends in China: The potential of natural product application. Chin. J. Nat. Med. 2024, 22, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-A.; Moon, S.Y.; Kim, W.-Y.; Paek, S.-M.; Park, H.H.; Lee, C.S. Structure-Based Classification and Anti-Cancer Effects of Plant Metabolites. Int. J. Mol. Sci. 2018, 19, 2651. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Anticancer action of plant products: Changing stereotyped attitudes. Explor. Drug Sci. 2022, 3, 423–427. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Luo, J.; Cai, W.; Wu, T.; Xu, B. Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis L.) and mung bean (Vigna radiate L.), and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities. Food Chem. 2016, 201, 350–360. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonini, E.; Frati, A.; Scarpa, E.-S. C-Glycosyl Flavonoids from Beta vulgaris Cicla and Betalains from Beta vulgaris rubra: Antioxidant, Anticancer and Antiinflammatory Activities—A Review. Phytother. Res. PTR 2017, 31, 871–884. [Google Scholar] [CrossRef]
- Liang, M.; Xu, W.; Zhang, W.; Zhang, C.; Liu, R.; Shen, Y.; Li, H.; Wang, X.; Wang, X.; Pan, Q.; et al. Quantitative LC/MS/MS method and in vivo pharmacokinetic studies of vitexin rhamnoside, a bioactive constituent on cardiovascular system from hawthorn. Biomed. Chromatogr. 2007, 21, 422–429. [Google Scholar] [CrossRef]
- Yang, J.H.; Choi, M.-H.; Yang, S.H.; Cho, S.S.; Park, S.J.; Shin, H.-J.; Ki, S.H. Potent Anti-Inflammatory and Antiadipogenic Properties of Bamboo (Sasa coreana Nakai) Leaves Extract and Its Major Constituent Flavonoids. J. Agric. Food Chem. 2017, 65, 6665–6673. [Google Scholar] [CrossRef]
- Gadioli, I.L.; da Cunha, M.d.S.B.; de Carvalho, M.V.O.; Costa, A.M.; Pineli, L.d.L.d.O. A systematic review on phenolic compounds in Passiflora plants: Exploring biodiversity for food, nutrition, and popular medicine. Crit. Rev. Food Sci. Nutr. 2018, 58, 785–807. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, B.; Yu, W.Y.; Yao, X.; Ma, X.; Sheng, G.; Ma, Q. Vitexin attenuates acute doxorubicin cardiotoxicity in rats via the suppression of oxidative stress, inflammation and apoptosis and the activation of FOXO3a. Exp. Ther. Med. 2016, 12, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Huan, R.; Meng, D.; Qi, J.; Xia, L. Progress in the study of anti-tumor effects and mechanisms of vitexin. Pharmacol. Rep. 2025, 77, 124–134. [Google Scholar] [CrossRef]
- Malar, D.S.; Suryanarayanan, V.; Prasanth, M.I.; Singh, S.K.; Balamurugan, K.; Devi, K.P. Vitexin inhibits Aβ25-35 induced toxicity in Neuro-2a cells by augmenting Nrf-2/HO-1 dependent antioxidant pathway and regulating lipid homeostasis by the activation of LXR-α. Toxicol. Vitr. 2018, 50, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liang, T.; Zhang, Y.; Huang, K.; Yang, S.; Lv, H.; Chen, Y.; Zhang, C.; Guan, X. Vitexin alleviates high-fat diet induced brain oxidative stress and inflammation via anti-oxidant, anti-inflammatory and gut microbiota modulating properties. Free Radic. Biol. Med. 2021, 171, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Jia, Y.; Lu, X.; Zhang, T.; Zhao, K.; Fu, Z.; Pang, C.; Qian, Y. Vitexin suppresses RANKL-induced osteoclastogenesis and prevents lipopolysaccharide (LPS)-induced osteolysis. J. Cell. Physiol. 2019, 234, 17549–17560. [Google Scholar] [CrossRef]
- Rosa, S.I.G.; Rios-Santos, F.; Balogun, S.O.; Martins, D.T.d.O. Vitexin reduces neutrophil migration to inflammatory focus by down-regulating pro-inflammatory mediators via inhibition of p38, ERK1/2 and JNK pathway. Phytomedicine 2016, 23, 9–17. [Google Scholar] [CrossRef]
- Kim, G.-H.; Lim, K.; Yang, H.S.; Lee, J.-K.; Kim, Y.; Park, S.-K.; Kim, S.-H.; Park, S.; Kim, T.-H.; Moon, J.-S.; et al. Improvement in neurogenesis and memory function by administration of Passiflora incarnata L. extract applied to sleep disorder in rodent models. J. Chem. Neuroanat. 2019, 98, 27–40. [Google Scholar] [CrossRef]
- Pahlavan, S.; Tousi, M.S.; Ayyari, M.; Alirezalu, A.; Ansari, H.; Saric, T.; Baharvand, H. Effects of hawthorn (Crataegus pentagyna) leaf extract on electrophysiologic properties of cardiomyocytes derived from human cardiac arrhythmia-specific induced pluripotent stem cells. FASEB J. 2018, 32, 1440–1451. [Google Scholar] [CrossRef]
- Wirawan, E.; Lippens, S.; Vanden Berghe, T.; Romagnoli, A.; Fimia, G.M.; Piacentini, M.; Vandenabeele, P. Beclin1: A role in membrane dynamics and beyond. Autophagy 2012, 8, 6–17. [Google Scholar] [CrossRef]
- Bedell, S.; Wells, J.; Liu, Q.; Breivogel, C. Vitexin as an active ingredient in passion flower with potential as an agent for nicotine cessation: Vitexin antagonism of the expression of nicotine locomotor sensitization in rats. Pharm. Biol. 2019, 57, 8–12. [Google Scholar] [CrossRef]
- Luo, J.; Chen, M.; Liu, Y.; Xie, H.; Yuan, J.; Zhou, Y.; Ding, J.; Deng, Z.; Li, J. Nature-derived lignan compound VB-1 exerts hair growth-promoting effects by augmenting Wnt/β-catenin signaling in human dermal papilla cells. PeerJ 2018, 6, e4737. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Mao, L.-N.; Liu, C.-P.; Sun, Y.-H.; Jiang, B.; Zhang, W.; Li, J.-X. Antinociceptive effects of vitexin in a mouse model of postoperative pain. Sci. Rep. 2016, 6, 19266. [Google Scholar] [CrossRef] [PubMed]
- Kenmogne, C.F.; Ponou, B.K.; Kemkuignou, B.M.; Kühlborn, J.; Tchuenguem, R.T.; Teponno, R.B.; Dzoyem, J.P.; Opatz, T.; Tapondjou, L.A. Mimonoside D: A new triterpenoid saponin from Mimosa diplotricha Sauvalle (Fabaceae). Nat. Prod. Res. 2023, 37, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Chedjou, I.N.; Ngouafong, F.T.; Tchuenguem, R.T.; Dzoyem, J.P.; Ponou, B.K.; Teponno, R.B.; Barboni, L.; Tapondjou, L.A. Siamoside A: A new C-glycosylated flavone from Senna siamea (Lam.) H. S. Irwin & Barneby (Caesalpiniaceae). Nat. Prod. Res. 2023, 37, 3461–3469. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Nguyen, V.T.; Van Cuong, P.; Nguyen Thanh, T.; Le Thi, T.A.; Mai Huong, D.T.; Truong, B.N.; Litaudon, M.; Ninh The, S. A new flavonoid from the leaves of Garcinia mckeaniana Craib and α-glucosidase and acetylcholinesterase inhibitory activities. Nat. Prod. Res. 2022, 36, 5074–5080. [Google Scholar] [CrossRef]
- Díaz, J.G. Chemical Composition of Hypericum Coadunatum Chr. from the Canary Islands. J. Mol. Struct. 2022, 1248, 131447. [Google Scholar] [CrossRef]
- Yang, H.H. Characterization of tyrosinase inhibitory constituents from the aerial parts of Humulus japonicus using LC-MS/MS coupled online assay. Med. Chem. 2018, 26, 509–515. [Google Scholar] [CrossRef]
- Nam, T.-G.; Lee, S.M.; Park, J.-H.; Kim, D.-O.; Baek, N.; Eom, S.H. Flavonoid analysis of buckwheat sprouts. Food Chem. 2015, 170, 97–101. [Google Scholar] [CrossRef]
- Bai, Y.; Chang, J.; Xu, Y.; Cheng, D.; Liu, H.; Zhao, Y.; Yu, Z. Antioxidant and Myocardial Preservation Activities of Natural Phytochemicals from Mung Bean (Vigna radiata L.) Seeds. J. Agric. Food Chem. 2016, 64, 4648–4655. [Google Scholar] [CrossRef]
- De Carvalho, M.V.O.; De Oliveira, L.D.L.; Costa, A.M. Effect of training system and climate conditions on phytochemicals of Passiflora setacea, a wild Passiflora from Brazilian savannah. Food Chem. 2018, 266, 350–358. [Google Scholar] [CrossRef]
- Shin, H.; Park, Y.; Jeon, Y.H.; Yan, X.-T.; Lee, K.Y. Identification of Polygonum orientale constituents using high-performance liquid chromatography high-resolution tandem mass spectrometry. Biosci. Biotechnol. Biochem. 2018, 82, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zolkeflee, N.K.Z.; Maulidiani, M.; Khoo, L.W.; Shaari, K.; Abas, F. Chemical Constituents from the Butanol Fraction of Clinacanthus nutans Leaves. Chem. Nat. Compd. 2022, 58, 167–171. [Google Scholar] [CrossRef]
- Olennikov, D.N. Chemical Investigation of Anagallidium dichotomum and Anticholinesterase Activity of Its Constituents. Chem. Nat. Compd. 2014, 49, 1137–1139. [Google Scholar] [CrossRef]
- Agrawal, S.; Das, R.; Singh, A.K.; Kumar, P.; Shukla, P.K.; Bhattacharya, I.; Tripathi, A.K.; Mishra, S.K.; Tiwari, K.N. Network pharmacology-based anti-pancreatic cancer potential of kaempferol and catechin of Trema orientalis L. through computational approach. Med. Oncol. 2023, 40, 133. [Google Scholar] [CrossRef]
- Peng, Y.; Gan, R.; Li, H.; Yang, M.; McClements, D.J.; Gao, R.; Sun, Q. Absorption, metabolism, and bioactivity of vitexin: Recent advances in understanding the efficacy of an important nutraceutical. Crit. Rev. Food Sci. Nutr. 2021, 61, 1049–1064. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, C. Research progress on the mechanism of vitexin in preventing and treating diseases. Chin. J. Mod. Appl. Pharm. 2021, 38, 2156–2161. [Google Scholar] [CrossRef]
- He, M.; Min, J.-W.; Kong, W.-L.; He, X.-H.; Li, J.-X.; Peng, B.-W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef]
- Xue, H.-F.; Ying, Z.-M.; Zhang, W.-J.; Meng, Y.-H.; Ying, X.-X.; Kang, T.-G. Hepatic, gastric, and intestinal first-pass effects of vitexin in rats. Pharm. Biol. 2014, 52, 967–971. [Google Scholar] [CrossRef]
- Kerscher, F.; Franz, G. Bioynthesis of Vitexin and Isovitexin: C-Glycoxylation of Flavonoid Intermediates with Cell Free Preparation from Fagopyrum esculentum. Planta Med. 1986, 56, 517. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Liu, Y.; Zhao, L.; Pei, J. Screening and characterizing flavone synthases and its application in biosynthesizing vitexin from naringenin by a one-pot enzymatic cascade. Enzyme Microb. Technol. 2022, 160, 110101. [Google Scholar] [CrossRef]
- Liu, S.; Lyu, Y.; Yu, S.; Cheng, J.; Zhou, J. Efficient Production of Orientin and Vitexin from Luteolin and Apigenin Using Coupled Catalysis of Glycosyltransferase and Sucrose Synthase. J. Agric. Food Chem. 2021, 69, 6578–6587. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-Y.; Chien, Y.-S.; Chiu, T.-H.; Huang, W.-W.; Lu, C.-C.; Chiang, J.-H.; Yang, J.-S. Apoptosis triggered by vitexin in U937 human leukemia cells via a mitochondrial signaling pathway. Oncol. Rep. 2012, 28, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.K.; Kar, A.; Jayaraman, A.; Mahapatra, S.K.; Vadivel, V. Vitexin isolated from Prosopis cineraria leaves induce apoptosis in K-562 leukemia cells via inhibition of the BCR-ABL-Ras-Raf pathway. J. Pharm. Pharmacol. 2022, 74, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liao, P.; Pan, Y.; Chen, S.; Chou, S.; Chou, M. The Novel p53-Dependent Metastatic and Apoptotic Pathway Induced by Vitexin in Human Oral Cancer OC2 Cells. Phytother. Res. 2013, 27, 1154–1161. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Zeng, G.; Zhou, Y.; Yuan, H. Purified vitexin compound 1 inhibits growth and angiogenesis through activation of FOXO3a by inactivation of Akt in hepatocellular carcinoma. Int. J. Mol. Med. 2014, 33, 441–448. [Google Scholar] [CrossRef]
- Shi, Y.-Y.; Deng, L.-D.; Rao, W.-W.; XU, Q. Inhibiting effects and mechanism of vitexin against proliferation of SMMC-7721 cancer cells. Chin. Hosp. Pharm. J. 2016, 36, 366–371. [Google Scholar] [CrossRef]
- He, J.-D.; Wang, Z.; Li, S.-P.; Xu, Y.-J.; Yu, Y.; Ding, Y.-J.; Yu, W.-L.; Zhang, R.-X.; Zhang, H.-M.; Du, H.-Y. Vitexin suppresses autophagy to induce apoptosis in hepatocellular carcinoma via activation of the JNK signaling pathway. Oncotarget 2016, 7, 84520–84532. [Google Scholar] [CrossRef]
- Lee, J.H.; Mohan, C.D.; Shanmugam, M.K.; Rangappa, S.; Sethi, G.; Siveen, K.S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Basappa, S.; et al. Vitexin abrogates invasion and survival of hepatocellular carcinoma cells through targeting STAT3 signaling pathway. Biochimie 2020, 175, 58–68. [Google Scholar] [CrossRef]
- An, F.; Wang, S.; Tian, Q.; Zhu, D. Effects of orientin and vitexin from Trollius chinensis on the growth and apoptosis of esophageal cancer EC-109 cells. Oncol. Lett. 2015, 10, 2627–2633. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Paul, S.; Jakhar, R.; Khan, I.; Kang, J.I.; Kim, H.M.; Yun, J.W.; Lee, S.-J.; Cho, H.J.; Lee, H.G.; et al. Vitexin confers HSF-1 mediated autophagic cell death by activating JNK and ApoL1 in colorectal carcinoma cells. Oncotarget 2017, 8, 112426–112441. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Cho, H.J.; Paul, S.; Jakhar, R.; Khan, I.; Lee, S.-J.; Kim, B.-Y.; Krishnan, M.; Khaket, T.P.; Lee, H.G.; et al. Vitexin induces apoptosis by suppressing autophagy in multi-drug resistant colorectal cancer cells. Oncotarget 2018, 9, 3278–3291. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhong, J.; Liu, Y.; Huang, Y.; Luo, F.; Zhou, Y.; Pan, X.; Cao, S.; Zhang, L.; Zhang, Y.; et al. Purified vitexin compound 1, a new neolignan isolated compound, promotes PUMA-dependent apoptosis in colorectal cancer. Cancer Med. 2018, 7, 6158–6169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, D.; Chen, H.; Zhang, J.; Jin, X. Vitexin induces G2/M-phase arrest and apoptosis via Akt/mTOR signaling pathway in human glioblastoma cells. Mol. Med. Rep. 2018, 17, 4599–4604. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhou, Y.; Zhong, X.; Su, F.; Xu, L. Effects of Vitexin, a Natural Flavonoid Glycoside, on the Proliferation, Invasion, and Apoptosis of Human U251 Glioblastoma Cells. Oxid. Med. Cell. Longev. 2022, 2022, 3129155. [Google Scholar] [CrossRef]
- Xie, T.; Wang, J.-R.; Dai, C.-G.; Fu, X.-A.; Dong, J.; Huang, Q. Vitexin, an inhibitor of hypoxia-inducible factor-1α, enhances the radiotherapy sensitization of hyperbaric oxygen on glioma. Clin. Transl. Oncol. 2020, 22, 1086–1093. [Google Scholar] [CrossRef]
- Liu, N.; Wang, K.S.; Qi, M.; Zhou, Y.J.; Zeng, G.Y.; Tao, J.; Zhou, J.D.; Zhang, J.L.; Chen, X.; Peng, C. Vitexin compound 1, a novel extraction from a Chinese herb, suppresses melanoma cell growth through DNA damage by increasing ROS levels. J. Exp. Clin. Cancer Res. 2018, 37, 269. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, H.; Gu, X.; Yin, X. The natural flavonoid glycoside vitexin displays preclinical antitumor activity by suppressing NF-κB signaling in nasopharyngeal carcinoma. OncoTargets Ther. 2019, 12, 4461–4468. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Q.; Liu, H.; Luo, S. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol. Res. 2019, 52, 7. [Google Scholar] [CrossRef]
- Zhao, B.; Jiao, L.; Jin, S.; Yang, W.; Bi, L.; Xu, L. Effect of vitexin on apoptosis and metastasis of A549 lung cancer cells and its mechanism. Lishizhen Med. Mater. Medica Res. 2020, 31, 65–68. [Google Scholar]
- Zhao, B.; Yin, Y.-N.; Wang, C.-Y.; Bi, L.; Xu, L.; Jiao, L. Mechanism of vitexin on regulation of M1/M2 macrophages polarization against metastasis of lung adenocarcinoma. Chin. J. Immunol. 2020, 36, 2456–2461. [Google Scholar]
- Li, Y.; Sun, Q.; Li, H.; Yang, B.; Wang, M. Vitexin suppresses renal cell carcinoma by regulating mTOR pathways. Transl. Androl. Urol. 2020, 9, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wang, K.; Zhou, Y.; Liu, N.; Guo, W.; Qi, J.; Hu, Z.; Su, S.; Tang, P.; Zhou, X. Purified Vitexin Compound 1 Serves as a Promising Antineoplastic Agent in Ovarian Cancer. Front. Oncol. 2021, 11, 734708. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Jiang, Y.; Sun, L. Vitexin suppresses the proliferation, angiogenesis and stemness of endometrial cancer through the PI3K/AKT pathway. Pharm. Biol. 2023, 61, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.M.J.; Yu, M.M.L.; Ma, M.M.Y.; Li, M.M.J. Vitexin Inhibits the Proliferation and Promotes the Apoptosis of Gastric Cancer Cells via Phosphatidylinositol-3-Kinase (PI3K)/Protein Kinase B (Akt)/The Mammalian Target of Rapamycin (mTOR) Signaling Pathway. J. Biomater. Tissue Eng. 2020, 10, 1843–1850. [Google Scholar] [CrossRef]
- Zhou, P.; Zheng, Z.-H.; Wan, T.; Wu, J.; Liao, C.-W.; Sun, X.-J. Vitexin Inhibits Gastric Cancer Growth and Metastasis through HMGB1-mediated Inactivation of the PI3K/AKT/mTOR/HIF-1α Signaling Pathway. J. Gastric Cancer 2021, 21, 439–456. [Google Scholar] [CrossRef]
- Zhang, L.; La, X.; Tian, J.; Li, H.; Li, A.; Liu, Y.; Wu, C.; Li, Z. The phytochemical vitexin and syringic acid derived from foxtail fillet bran inhibit breast cancer cells proliferation via GRP78/SREBP-1/SCD1 signaling axis. J. Funct. Foods 2021, 85, 104620. [Google Scholar] [CrossRef]
- Najafipour, R.; Momeni, A.M.; Mirmazloomi, Y.; Moghbelinejad, S. Vitexin Induces Apoptosis in MCF-7 Breast Cancer Cells through the Regulation of Specific miRNAs Expression. Int. J. Mol. Cell. Med. 2023, 11, 197–206. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Wang, Y.; Zhang, H.; Meng, Y. Effects of vitexin on apoptosis and cell cycle arrest in human cervical cancer HeLa cells. Chin. Tradit. Pat. Med. 2022, 44, 961–964. [Google Scholar]
- Wang, Q.; Zhang, J.; Ye, J.; Guo, J. Vitexin exerts anti-tumor and anti-angiogensis effects on cervical cancer through VEGFA/VEGFR2 pathway. Eur. J. Gynaecol. Oncol. 2022, 43, 86–91. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, S.; Mishra, N.; Soni, A.; Kumari, M.; Singh, S. Targeting cell cycle regulators: A new paradigm in cancer therapeutics. Biocell 2024, 48, 1639–1666. [Google Scholar] [CrossRef]
- Portugal, J.; Bataller, M.; Mansilla, S. Cell death pathways in response to antitumor therapy. Tumori 2009, 95, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Emi, M.; Tanabe, K.; Murakami, S. Role of the unfolded protein response in cell death. Apoptosis Int. J. Program. Cell Death 2006, 11, 5–13. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Jewell, J.L.; Russell, R.C.; Guan, K.-L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef]
- Wang, R.; Wang, G. Protein Modification and Autophagy Activation. Adv. Exp. Med. Biol. 2019, 1206, 237–259. [Google Scholar] [CrossRef]
- Ghazy, E.; Taghi, H.S. The Autophagy-Inducing Mechanisms of Vitexin, Cinobufacini, and Physalis alkekengi Hydroalcoholic Extract against Breast Cancer in vitro and in vivo. J. Gastrointest. Cancer 2022, 53, 592–596. [Google Scholar] [CrossRef]
- Yuan, D.; Li, Y.; Lu, Z. Inhibitory effect and mechanism of vitexin on the growth of transplanted nasopharyngeal carcinoma CNE-2 cells in nude mice based on AMPK/ULK1 mediated autophagy. Drugs Clinic. 2024, 39, 281–289. [Google Scholar]
- Mori, H.; Sugie, S.; Yoshimi, N.; Hara, A.; Tanaka, T. Control of cell proliferation in cancer prevention. Mutat. Res. 1999, 428, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.K.; Mahapatra, S.K.; Vadivel, V. Oxidative stress mediated cytotoxicity in leukemia cells induced by active phyto-constituents isolated from traditional herbal drugs of West Bengal. J. Ethnopharmacol. 2020, 251, 112527. [Google Scholar] [CrossRef]
- De Craene, B.; Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewicz-Zając, M.; Mroczko, B.; Guzińska-Ustymowicz, K.; Pryczynicz, A.; Gryko, M.; Kemona, A.; Kędra, B.; Szmitkowski, M. Matrix metalloproteinase 2 (MMP-2) and their tissue inhibitor 2 (TIMP-2) in gastric cancer patients. Adv. Med. Sci. 2013, 58, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Dwivedi, S.K.D.; Bhattacharya, R.; Mukherjee, P.; Rao, G. VEGF signaling: Role in angiogenesis and beyond. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189079. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Wu, R.-H.; Wang, W.-S. Regorafenib diminishes the expression and secretion of angiogenesis and metastasis associated proteins and inhibits cell invasion via NF-κB inactivation in SK-Hep1 cells. Oncol. Lett. 2017, 14, 461–467. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, T.; Wang, S.; Mo, J.; Tian, T.; Zhou, X. Epigenetic modification of nucleic acids: From basic studies to medical applications. Chem. Soc. Rev. 2017, 46, 2844–2872. [Google Scholar] [CrossRef]
- Hogg, S.J.; Beavis, P.A.; Dawson, M.A.; Johnstone, R.W. Targeting the epigenetic regulation of antitumour immunity. Nat. Rev. Drug Discov. 2020, 19, 776–800. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, B.; Yang, Z. Research progress on the potential treatment of malignant renal rhabdoid tumor by vitexin. J. Contemp. Urol. Reprod. Onco. 2021, 13, 60–64. [Google Scholar]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; et al. The Effect of Dietary Polyphenols on Vascular Health and Hypertension: Current Evidence and Mechanisms of Action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Nian, H.; Delage, B.; Ho, E.; Dashwood, R.H. Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: Studies with sulforaphane and garlic organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 213–221. [Google Scholar] [CrossRef]
- Xu, T.; Cheng, Y.; Wen, L.; Feng, Q.; Zheng, J. The mechanism of vitexin regulating the sensitivity of human colorectal cancer cells to oxaliplatin. J. Chengde Med. Univ. 2023, 40, 1–5. [Google Scholar] [CrossRef]
- Chen, D.; Chen, Y.; Huang, F.; Zhang, X.; Zhou, Y.; Xu, L. The underlying regulatory mechanisms of colorectal carcinoma by combining Vitexin and Aspirin: Based on systems biology, molecular docking, molecular dynamics simulation, and in vitro study. Front. Endocrinol. 2023, 14, 1147132. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Shaedi, N.; Naharudin, I.; Choo, C.Y.; Wong, T.W. Design of oral intestinal-specific alginate-vitexin nanoparticulate system to modulate blood glucose level of diabetic rats. Carbohydr. Polym. 2021, 254, 117312. [Google Scholar] [CrossRef]
- Rashidi, N.; Davidson, M.; Apostolopoulos, V.; Nurgali, K. Nanoparticles in cancer diagnosis and treatment: Progress, challenges, and opportunities. J. Drug Deliv. Sci. Technol. 2024, 95, 105599. [Google Scholar] [CrossRef]
- Rodklongtan, A.; Dumrongchai, T.; Chitprasert, P. Ultrasound-assisted pH-shifted mung bean protein isolate nanoparticles in calcium carbonate microparticles for oral delivery of vitexin. J. Food Sci. 2025, 90, e70032. [Google Scholar] [CrossRef]
- Yoksan, R.; Towongphaichayonte, P. Vitexin-loaded poly (ethylene glycol) methyl ether-grafted chitosan/alginate nanoparticles: Preparation, physicochemical properties and in vitro release behaviors. J. Sci. Food Agric. 2024, 104, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Chomchoey, S.; Klongdee, S.; Peanparkdee, M.; Klinkesorn, U. Fabrication and characterization of nanoemulsions for encapsulation and delivery of vitexin: Antioxidant activity, storage stability and in vitro digestibility. J. Sci. Food Agric. 2023, 103, 2532–2543. [Google Scholar] [CrossRef] [PubMed]


| No. | Plant Species (Latin Name) | Part Used | Reference |
|---|---|---|---|
| 1 | Mimosa diplotricha Sauvalle | aerial parts | [23] |
| 2 | Senna siamea | leaves and bark | [24] |
| 3 | Garcinia mckeaniana | leaves | [25] |
| 4 | Hypericum coadunatum Sm. ex Link Buch. | aerial parts | [26] |
| 5 | Humulus japonicus | aerial parts | [27] |
| 6 | Polygonum orientale | whole plants | [28] |
| 7 | Passiflora spp. | fruits | [29] |
| 8 | Vigna radiata | seeds | [30] |
| 9 | Fagopyrum esculentum | seeds | [31] |
| 10 | Anagallidium dichotomum (L.) Griseb | flowers, leaves, stems | [32] |
| 11 | Trema orientalis L. | ripe fruits | [33] |
| 12 | Clinacanthus nutans | leaves | [34] |
| Cancer | Models (In Vitro/In Vivo) | Biological Activities | Molecular Mechanisms | IC50 (μM) | Incubation Time | References |
|---|---|---|---|---|---|---|
| Leukemia | U937 cells (in vitro) | cytotoxicity and apoptosis induction | caspase-3, -7 and caspase-9 activities↑ Bcl-2↓ | 22.5 | 24 | [42] |
| K-562 cells (in vitro) | apoptosis induction | MMP↓ ROS↑ RAS↓ RAF↓ p38↑ BCL-2↓ procaspase-9↓ pro-caspase-3↓ BAX↑ | 65.7 | 48 | [43] | |
| Oral cancer | OC2 cells (in vitro) | induce apoptosis, inhibit proliferation and metastasis | caspase-3↑ p53↑ p21↑ Bax↑ PCNA↓ PAI-1↑ MMP-2↓ | 40 | 24 | [44] |
| Hepatocellular carcinoma | Hep3B, Huh-7, HepG2 and L-02 cells (in vitro) | induce G1/G0 phase arrest, inhibit growth and angiogenesis | P-PI3K↓ p-Akt↓ FOXO3a↑ p-FOXO3a↓ p21↑ p27↑ cyclin D1↓ VEGF↓ | 50 | 48 | [45] |
| SMMC-7721 cells (in vitro) | inhibit proliferation and induce apoptosis | Bcl-2↓ Casepase-3↑ Bax↑ P53↑ PARP↑ | 32.7 | 48 | [46] | |
| SK-Hep1 and Hepa1-6 cells (in vitro) | apoptosis induction and autophagy suppression, exert an inhibitory effect on HCC tumor growth | Caspase-3↑ Cleave Caspase-3↑ Bcl-2↓ LC3 II↓ p-JNK↑ p-Erk1/2↓ Ki67↓ MMP-2↓ | 35 | 48 | [47] | |
| HepG2, Hep3B, HCCLM3, and PLC/PRF5 cells (in vitro) | mitigate the survival and invasion of HCC cells | p-STAT3↓ cyclin D1↓VEGF↓ Bcl-2↓ Bcl-xL↓ Mcl-1↓ survivin↓ cleavage of procaspase-8 and procaspase-3↑ | 52.1 | 24 | [48] | |
| Esophageal cancer | EC-109 cells (in vitro) | inhibit cell growth and induce apoptosis | p53↑ bcl-2↓ | 35 | 24 | [49] |
| Colorectal carcinoma | HCT-116 cells (in vitro), xenograft model (in vivo) | autophagy induction, inhibit the cell growth | HSF-1↓ JNK↑ PI3K↓p-Akt↓ p-mTOR↓ p62↓ Bcl-2↓ Beclin-1↑Atg5↑ LC3-II↑ p-JNK↑ LC3-II↑ ApoL1↑ | 25 | 48 | [50] |
| HCT-116DR cells (in vitro), xenograft model (in vivo) | induce apoptosis through suppression of autophagy | ROS↑ BID↑ Bax↑ cytochrome c↑ ATG5↓ Beclin-1↓ LC3-II↓ | 55 | 72 | [51] | |
| HCT-116WT, HCT-116, p53-KO,HCT-116 PUMA-KO, HCT-116, BAX-KO and LoVo cells (in vitro), xenograft mouse model (in vivo) | suppress proliferation and induce apoptosis | p53↑ PUMA↑ Bax↑ | 20 | 48 | [52] | |
| Glioblastoma | LN-18 cells (in vitro) | induce G2/M cell cycle arrest and cell apoptosis | Akt/mTOR↓ cleaved-PARP↑ p-Akt↓ p-mTOR↓ | 30 | 48 | [53] |
| U251 cells (in vitro) | inhibit proliferation and invasion, induce apoptosis | JAK/STAT3↓ | 40 | 48 | [54] | |
| Glioma | SU3 cells (in vitro), BALB/c nude mice (in vivo) | cooperate with HBO to sensitize the glioma radiotherapy | HIF-1α↓ VGEF↓ GLUT-1↓ GLUT-3↓ | 29 | 48 | [55] |
| Melanoma | A375, Sk-Mel-5 and Sk-Mel-28 vemurafenib-resistant A375 cells (in vitro) | DNA damage, G2/M cell cycle arrest and apoptosis | ROS↑ P21↑ PUMA↑GADD45A↑ MCM6↓CDK1↓ CDK6↓ CYCE↓ CYCA↓ | 26 | 48 | [56] |
| Nasopharyngeal carcinoma | NPC cells CNE1, CNE2, HK1 and HNE1 cells (in vitro) | induce G0/G1 cell cycle arrest and apoptosis, inhibit NF-κB signaling | Cyclin D1↓ p21and p53↑ cleaved PARP↑ Bcl-2 and Mcl1↓ IKK↓ NF-κB↓ | 24 | 48 | [57] |
| Lung cancer | A549 and 16HBE cells (in vitro) | induce apoptosis and inactivate PI3K/Akt/mTOR signaling | Bcl-2↓ Bax↑ cleaved caspase-3↑ MMP↓ cytochrome c↑ p-PI3K, p-Akt and p-mTOR↓ | 28 | 48 | [58] |
| A549 cells (in vitro) | induce apoptosis, inhibit migration and invasion | caspase3, caspase9, Bcl-2 and bax↑ MMP2 and MMP9↓ | 27 | 48 | [59] | |
| RAW264.7 and A549 cells (in vitro) | decrease migration | iNOS, IL-1β, Arg-1,MR and p-STAT3↓ | N/A | N/A | [60] | |
| Renal cell carcinoma | OS-RC-2 and ACHN, HK-2 cells (in vitro) | induce apoptosis and hyperautophagy, up-regulate AMPK/mTOR and JNK pathways, down-regulate PI3K/Akt/mTOR pathways | caspase-3, caspase-9, cleaved caspase-3, and cleaved caspase-9↑Beclin1 and LC3↑ p62↓ p-AMPK↑ p-JNK↑ P-PI3K and p-AKT↓ | 25 | 48 | [61] |
| Ovarian cancer | HO8910 and SKOV3 cells (in vitro), xenograft tumor model (in vivo) | induce apoptosis and G2/M arrest | caspase-3↓ cleaved caspase-3↑ p21↑ | 26 | 48 | [62] |
| Endometrial cancer | HESCs, HEC-1B and Ishikawa cells (in vitro) | suppress the proliferation, angiogenesis, stemness and the PI3K/AKT pathway | Ki-67 and PCNA↓ VEGFA and FGF2↓ OCT4 and Nanog↓ P-PI3K and p-AKT↓ | 24 | 48 | [63] |
| Gastric cancer | AGS, CRL-1739, GES-1, SGC-7901cells (in vitro) | induce autophagy and apoptosis | p-PI3K, p-AKT and p-mTOR↓ | 25 | 48 | [64] |
| AGS, CRL-1739, GES-1, SGC-7901cells (in vitro) | suppress the migration, invasion, and EMT, inhibit the activation of PI3K/AKT/mTOR/HIF-1α pathway | cadherin↑ N-cadherin, MMP9 and MMP2 ↓HMGB1, p-PI3K,p-AKT, p-mTOR andHIF-1α↓ Ki67↓ | 25 | 48 | [65] | |
| Breast cancer | MDA-MB-231 and MCF-7 cells (in vitro) | inhibit proliferation | ki-67↓ SCD1↓ SFA↑ LDs↑ | 30 | 48 | [66] |
| MCF-7 cells (in vitro) | induce apoptosis | regulation of specific miRNAs expression | 32 | 48 | [67] | |
| Cervical cancer | HeLa cells (in vitro) | induce apoptosis and phase arrest | Bcl-2↓ Bax, caspase-3↑ p-P53↑ cyclin B1 and cyclin E↓ | 22 | 48 | [68] |
| Hela and Siha cells (in vitro) | reduce cell proliferation, migration, invasion and angiogenesis | VEGFA/VEGFR2↓ | 23 | 48 | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Deng, Y.; Li, J.; Feng, X.; Zou, H. Molecular Mechanisms of Vitexin: An Update on Its Anti-Cancer Functions. Int. J. Mol. Sci. 2025, 26, 5853. https://doi.org/10.3390/ijms26125853
Lu L, Deng Y, Li J, Feng X, Zou H. Molecular Mechanisms of Vitexin: An Update on Its Anti-Cancer Functions. International Journal of Molecular Sciences. 2025; 26(12):5853. https://doi.org/10.3390/ijms26125853
Chicago/Turabian StyleLu, Liyun, Yinhua Deng, Junnan Li, Xing Feng, and Hui Zou. 2025. "Molecular Mechanisms of Vitexin: An Update on Its Anti-Cancer Functions" International Journal of Molecular Sciences 26, no. 12: 5853. https://doi.org/10.3390/ijms26125853
APA StyleLu, L., Deng, Y., Li, J., Feng, X., & Zou, H. (2025). Molecular Mechanisms of Vitexin: An Update on Its Anti-Cancer Functions. International Journal of Molecular Sciences, 26(12), 5853. https://doi.org/10.3390/ijms26125853







