1. Introduction
Osteoarthrosis (OA) affected 527.81 million people worldwide in 2019; this number has increased more than twofold since 1990 and is expected to rise further because of the aging society [
1]. Due to its increasing prevalence, OA is the most important joint disease that leads to disability and therefore holds a high social burden [
2]. Women are affected more often than men [
1]. OA leads to the destruction of the articular cartilage, and during its further progress, the subchondral bone is also affected. Clinical treatment is still challenging and controversial. Allogenic or autologous osteochondral tissue transplantation are promising, but they express several disadvantages, e.g., a risk of contamination, donor site morbidity, limited availability, and high costs [
3]. Thus, there is an increasing demand for biocompatible scaffolds that contribute to the self-healing of cartilage by stimulating signaling cascades and cell ingrowth but avoid blood coagulation and the increase in inflammatory factors. Several materials have been used with different methods for scaffold generation, but most of them are not suitable for 3D printing, which, following technological advantages, is the most promising method for producing finely structured pores that meet the requirements of cell ingrowth. Therefore, this study aimed to establish a suitable polymer through the 2D analysis of casted discs. In the future, the best polymer shall be used for the fabrication of osteochondral scaffolds by 3D printing, followed by analysis in 3D cell cultures and an in vivo model.
Articular cartilage has limited self-restoring capacities because of its low cell density, lack of vascularization, and alymphatic nature [
3]. Cartilage defects are usually filled with replacement tissue made of fibrocartilage, which contains a high proportion of collagen type I fibers, while hyaline cartilage contains mainly masked collagen type II fibers. The aim of implanting cell-free scaffolds is the ingrowth of chondroblast or mesenchymal stem cells (MSCs) from the sides where healthy cartilage and bone are localized.
MSCs are capable of differentiating into cartilage-synthesizing chondrocytes but also into bone-forming osteoblasts, adipocytes, and several other cell types. A possibility to direct MSCs to the chondrogenic cell fate is the addition of specific supplements into the cell culture medium. Besides drug supplementation, in vitro chondrogenesis can be stimulated by pressure or hypoxia. A low oxygen content of 1–6% instead of 20% oxygen stimulates chondrogenesis [
4] via hypoxia-inducible factor 1 subunit alpha (HIF-1α) [
5]. Hypoxia can also be chemically induced, e.g., by dimethyloxalylglycine (DMOG) [
6]. The combined application of DMOG and parathyroid hormone-related protein (PTHrP) facilitated chondrogenesis and prevented hypertrophy [
7]. On the contrary, the application of DMOG to porcine synovial-derived MSCs co-cultured with chondrocytes only resulted in a general upregulation of collagen type II deposition [
8]. Recently, Dong et al., 2024 [
9], summarized in a literature review that DMOG applied to MSCs stimulates the osteogenic as well as the chondrogenic fate and additionally supports angiogenesis. Thus, the effect of DMOG is still controversial.
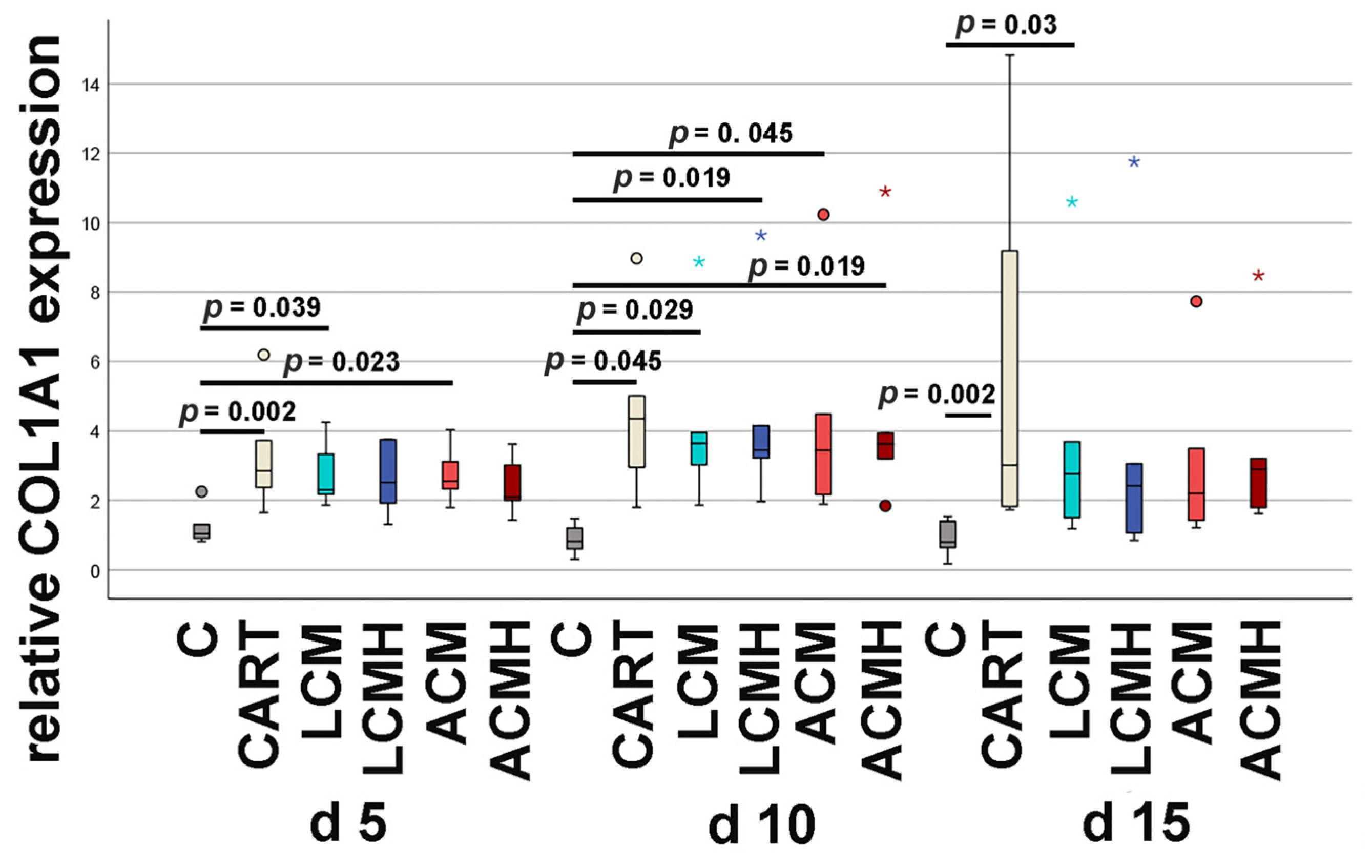
The differentiation of MSCs into chondrocytes can be characterized by the serial synthesis and release of matrix proteins. Here, changes in the matrix components transient receptor potential vanilloid cation channel 4 (TRPV4), sex-determining region Y (SRY)-box transcription factor 9 (SOX9), aggrecan (ACAN), hyaluronan and proteoglycan link protein 1 (HAPLN1), runt-related transcription factor 2 (RUNX2), collagen type I, and collagen type II were determined by relative mRNA expression measured by real-time reverse transcriptase polymerase chain reaction (real-time RT-PCR).
TRPV4 is a mechano- and osteosensitive ion channel that is highly expressed in chondrocytes, where its activation leads to an anabolic response with increased matrix production [
10]. TRPV4 also activates SOX9, which is the most important regulatory factor in the differentiation of MSCs to early chondrocytes. In cooperation with SOX5 and SOX6, it stimulates the synthesis of collagen type II through the activation of a chondrocyte-specific enhancer [
11]. As a structuring proteoglycan of hyaline cartilage, ACAN crosslinks collagen II fibrils, forms aggregates with glycosaminoglycans (GAGs), and binds hyaluronic acid [
12], thereby forming a negatively charged structure trapping high amounts of water and positively charged ions. According to this, the basic function of ACAN is its resistance to compressive force which is characteristic of articular cartilage [
13]. RUNX2 is required for chondrocyte maturation and involved in the decision whether MSCs are differentiated into an osteoblast or chondrocyte lineage. It is expressed by MSCs and regulates the expression of transcription factor SP7 which directs osteoblast progenitors to immature osteoblasts and suppresses the differentiation of MSCs into chondrocytes. RUNX2 is weakly expressed in proliferating chondrocytes and upregulated in hypertrophic and terminal chondrocytes. Its function is to keep the cells in the proliferation stage and to prevent them from changing into the phenotype of permanent cartilage [
14]. In the osteoblast lineage, RUNX2 additionally stimulates the osteogenic differentiation of MSCs into osteoblasts. During late osteogenesis, RUNX2 inhibits the differentiation of mature osteoblasts into osteocytes [
15]. As in chondrogenic differentiation, osteoblasts are protected from final differentiation and maintained in a state of proliferation and bone matrix production. Collagen type I is associated with cartilage of a fibrocartilaginous phenotype but also expressed in culture systems during chondrogenic differentiation [
16]. In hyaline cartilage, the occurrence of collagen type II predominates over collagen type I. In hyaline articular cartilage, 90–95% of the extracellular matrix is collagen type II, whereas in fibrocartilage, collagen type I is most prominent [
17]. In the present study, the detection of collagen type II is demonstrated at the mRNA level by real-time RT-PCR and at the protein level using ELISA.
Recently, tissue engineering has made progress in the generation of scaffolds that permit chondrogenic differentiation. Therefore, scaffold material must be biocompatible and allow MSC adhesion, proliferation, and differentiation. In addition, it should serve as a guide for the body’s own cells to grow into the cartilage defect. The scaffold therefore requires adapted biomechanical and biochemical properties and an architecture that allows the ingrowing cells to maintain their information exchange through cell–cell and cell–matrix contacts. For the generation of such scaffolds, 3D printing methods are most suitable. Recently established polyamid-ε-caprolactone-methacrylate (ACM) and poly-(D,L)-lactide-ε-caprolactone-methacrylate (LCM) can be printed in a 3D structure with a specific pore size [
18,
19]. The in vitro and in vivo biocompatibility of LCM has already been shown in human MSCs and a murine intracalvarial defect model [
20,
21]. LCM consists of polyesters, which have easily adjustable physical and chemical properties [
18]. During the degradation of polylactate-containing materials, lactic acid is released, so the local pH value is reduced during acetolysis [
22]. Due to the slow degradation of approximately 10–30% of the dry mass after 80 days at 37 °C, as shown by Felfel et al. in 2016, the immunological reactions of LCM cannot be ruled out by short-term analyses [
23]. The degradation of ACM produces the non-toxic monomer caprolactone and the amino acids alanine and glycine [
19]. The degradation rate is adjusted by the concentration of caprolactone. Thus, it is possible to adapt material degradation to cartilage regeneration. In addition, the degeneration products should not lead to an immunological foreign body reaction and an increase in proinflammatory factors. Inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1, and IL-6 induce chondrocyte death and the breakdown of the cartilage matrix, enhance the synthesis of proteolytic enzymes, and inhibit the production of collagen type II and GAGs. IL-6 is produced by, e.g., M1 macrophages, monocytes, B cells, lymphoid cells, and endothelial cells, as well as by chondrocytes and MSCs. It has essential functions for the innate and adaptive immune system and increases the inflammatory response by itself but also through the stimulation of TNF-α and IL-1 and the inhibition of anti-inflammatory factors. It is considered as the key cytokine of cartilage destruction [
24]. In addition, it enhances osteoclast development and the degradation of the subchondral bone, which makes it extremely relevant for the establishment of osteochondral implants.
Articular cartilage consists of a non-mineralized portion towards the joint and a mineralized layer at the transition to the subchondral bone. The mineralized cartilage is calcified but not ossified as the subchondral bone. The small, calcified cartilage layer builds a biological barrier that is necessary for the proper formation of the non-calcified hyaline cartilage. The glycosaminoglycan heparin has the ability to prevent cartilage mineralization [
25]. In addition, heparin is well known for its anticoagulation effect, and it is also able to inhibit angiogenesis and inflammation [
26]. Thus, the coating of scaffolds with heparin seems to be promising. Here, we compared pure ACM and LCM discs with heparin-coated ones for their compatibility as future implant materials for the production of porous scaffolds for chondrogenic differentiation, which is one of the key processes during cartilage repair and the healing of osteochondral defects. To address this issue, we established a 2D in vitro system of chondrogenesis and evaluated the new polymer materials using real-time RT-PCR, light microscopy, collagen type II and IL-6 ELISA, and an MTT assay to determine material suitability and cellular viability.
3. Discussion
This study aimed at investigating the suitability of two different synthetic polymers for the generation of scaffolds for osteochondral regeneration. It was reported that porous 3D scaffolds were most suitable for cartilage regeneration because of enhanced matrix deposition [
31,
32,
33]. However, as a first pilot study, here, we examined material discs and not a porous 3D scaffold. Discs (1 mm in height) allow the clear documentation of the suitability of the material in 2D cell cultures without the overlapping effects of cell proliferation and migration caused by porous scaffolds.
Previously used and approved cartilage implant materials mainly consist of a matrix built with collagen and hyaluronate with short resorption times [
34]. In arthritis, catabolic processes predominate and lead to a fast breakdown of these natural polymers. Synthetic polymers offer greater stability and, thus, the necessary time for cartilage formation before the scaffold is degraded [
23,
35]. The polymers LCM and ACM contain a high proportion of semi-crystalline polycaprolactone, which means that implants made of this material have high biomechanical stiffness and are therefore ideal for cartilage scaffolds implanted into the joints where they should withstand weight loading [
19].
At day 5 of chondrogenic differentiation, the viability and metabolic activity of the cells on the materials were investigated by means of an MTT assay. Previous studies measured a higher viability of undifferentiated MSCs on a porous LCM scaffold compared to discs [
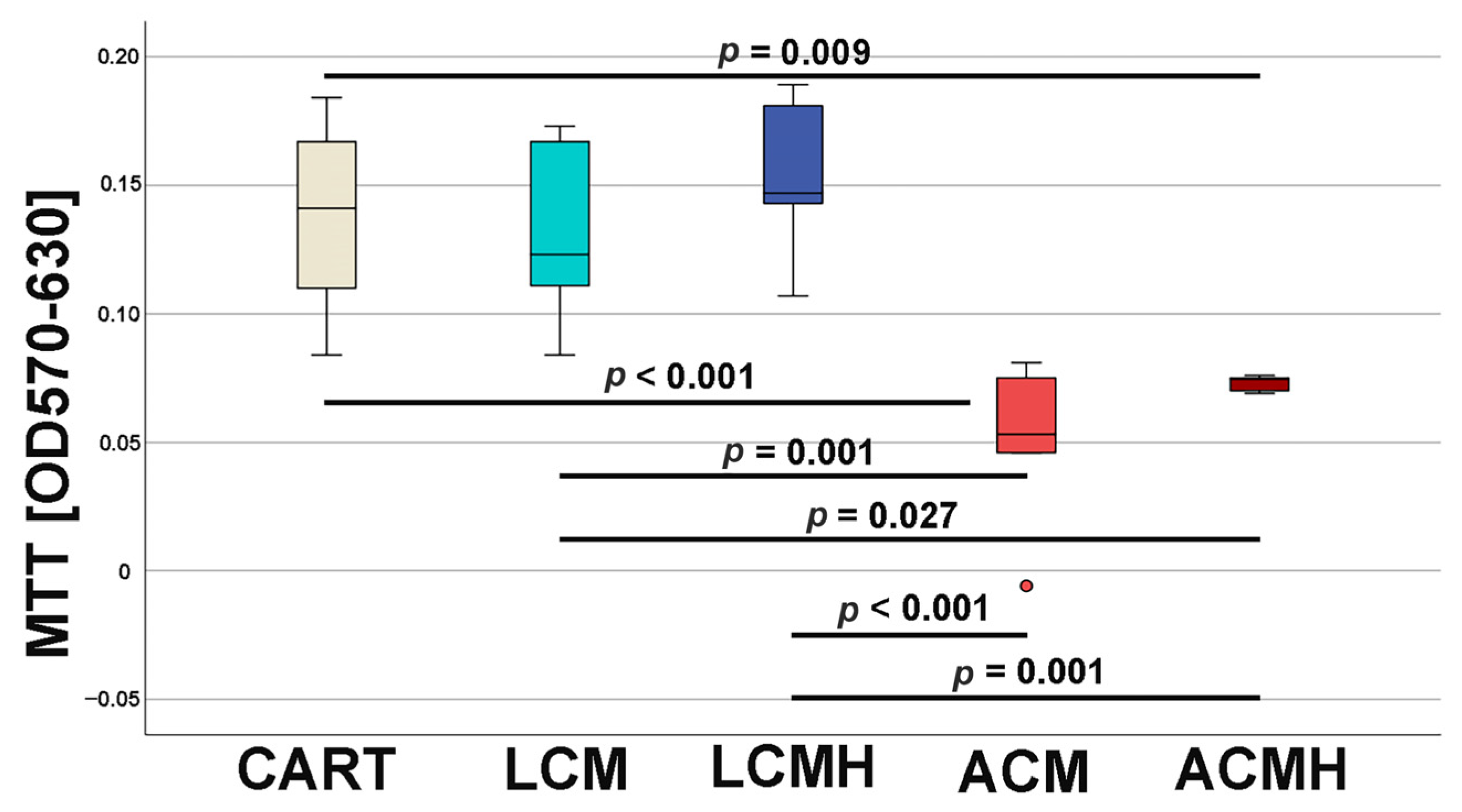
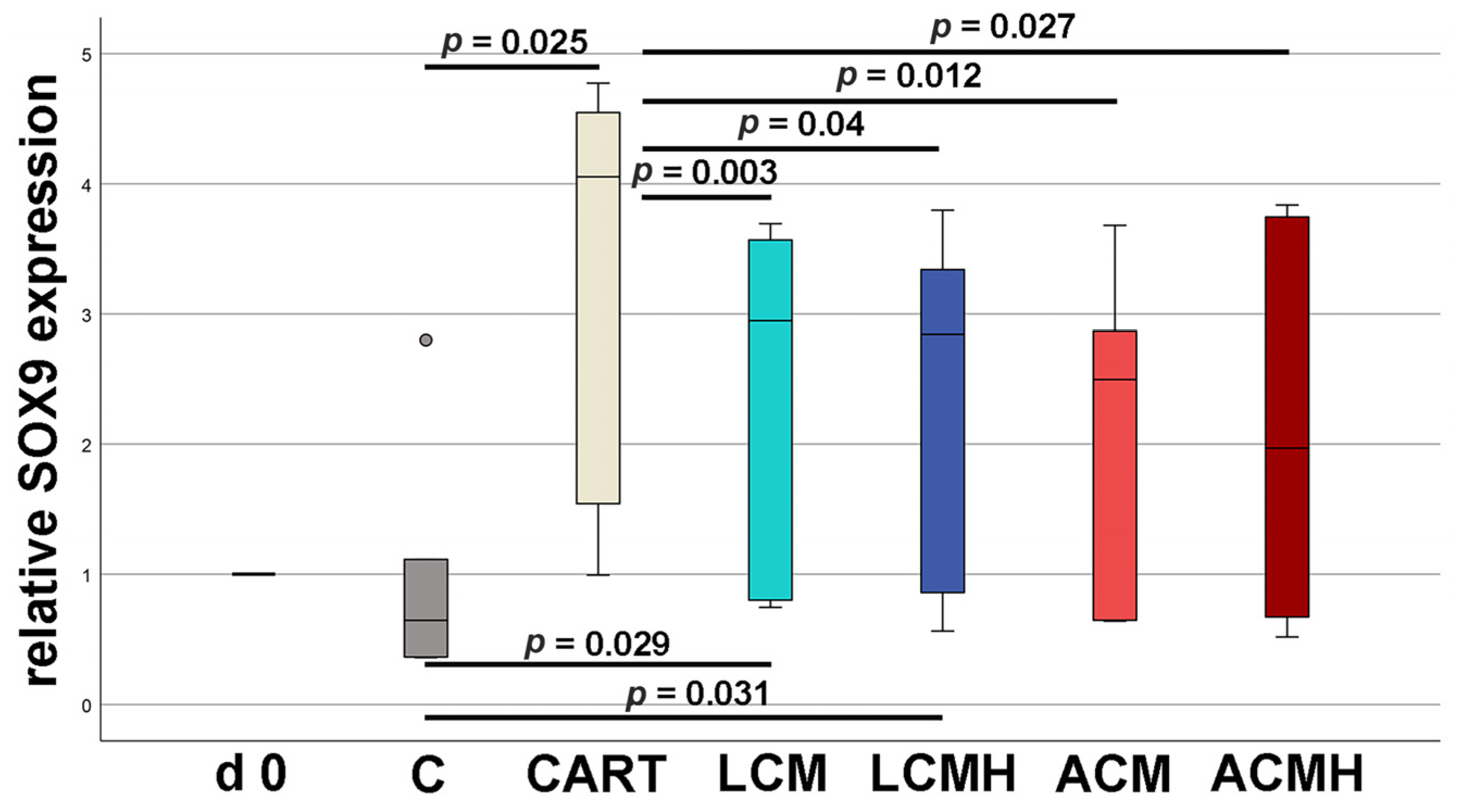
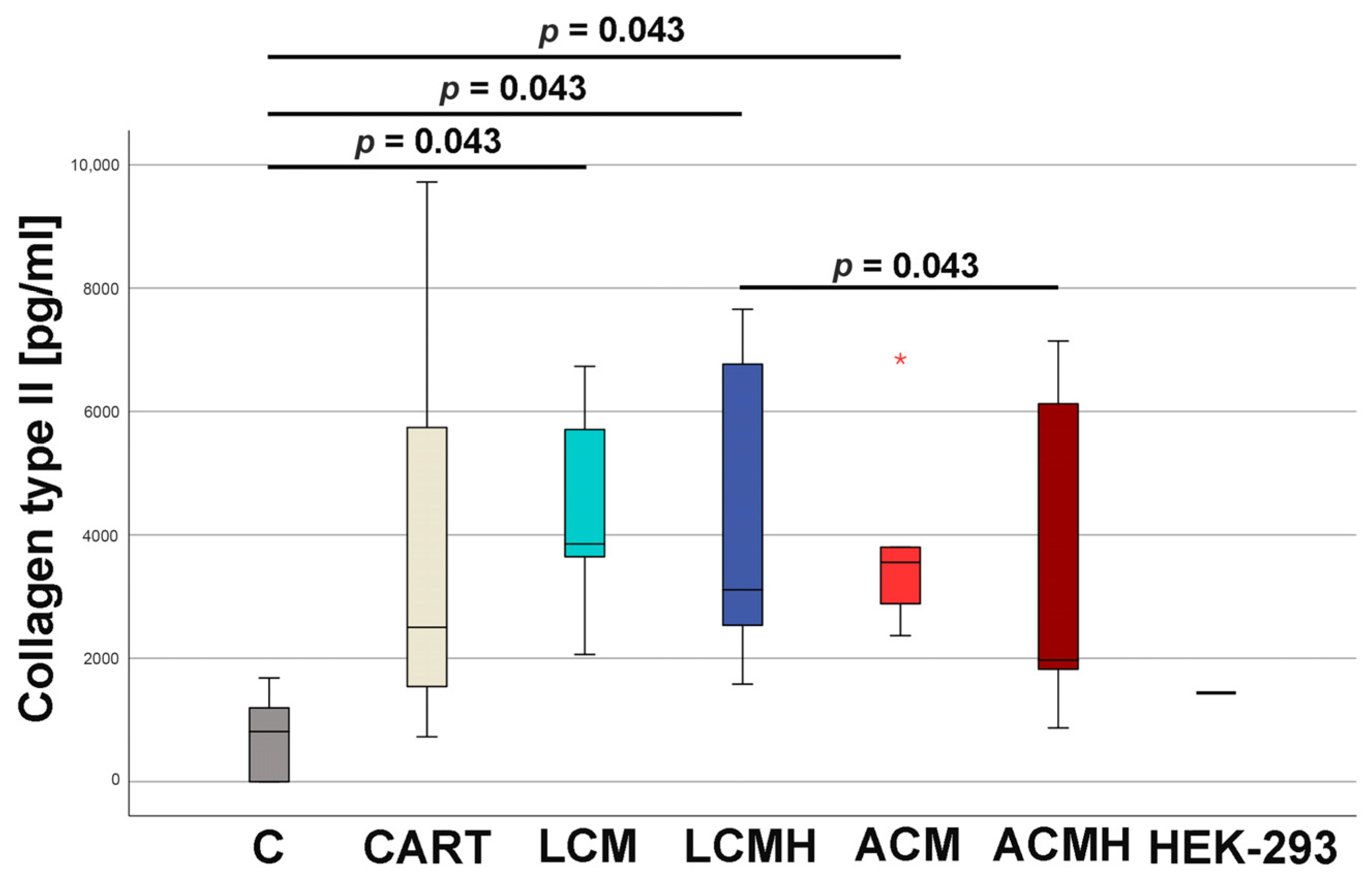
20]. Thus, when comparing the materials in this study, it was surprising that LCM discs performed better. LCM discs showed no difference to the chondrogenic control CART, while ACM and ACMH discs revealed a significant reduction in cell viability and metabolic activity. Since our study was the first direct comparison of LCM and ACM, the real-time RT-PCR of the early chondrogenic target SOX9 was used for verification. The relative SOX9 expression was upregulated on LCM and LCMH discs but not on ACM and ACMH. Additionally, a significant reduction in collagen type II was measured on ACMH compared to LCMH according to the ELISA. Thus, it was demonstrated with three independent methods that LCM and LCMH are more suitable for the generation of scaffolds for cartilage defect healing compared to ACM and ACMH.
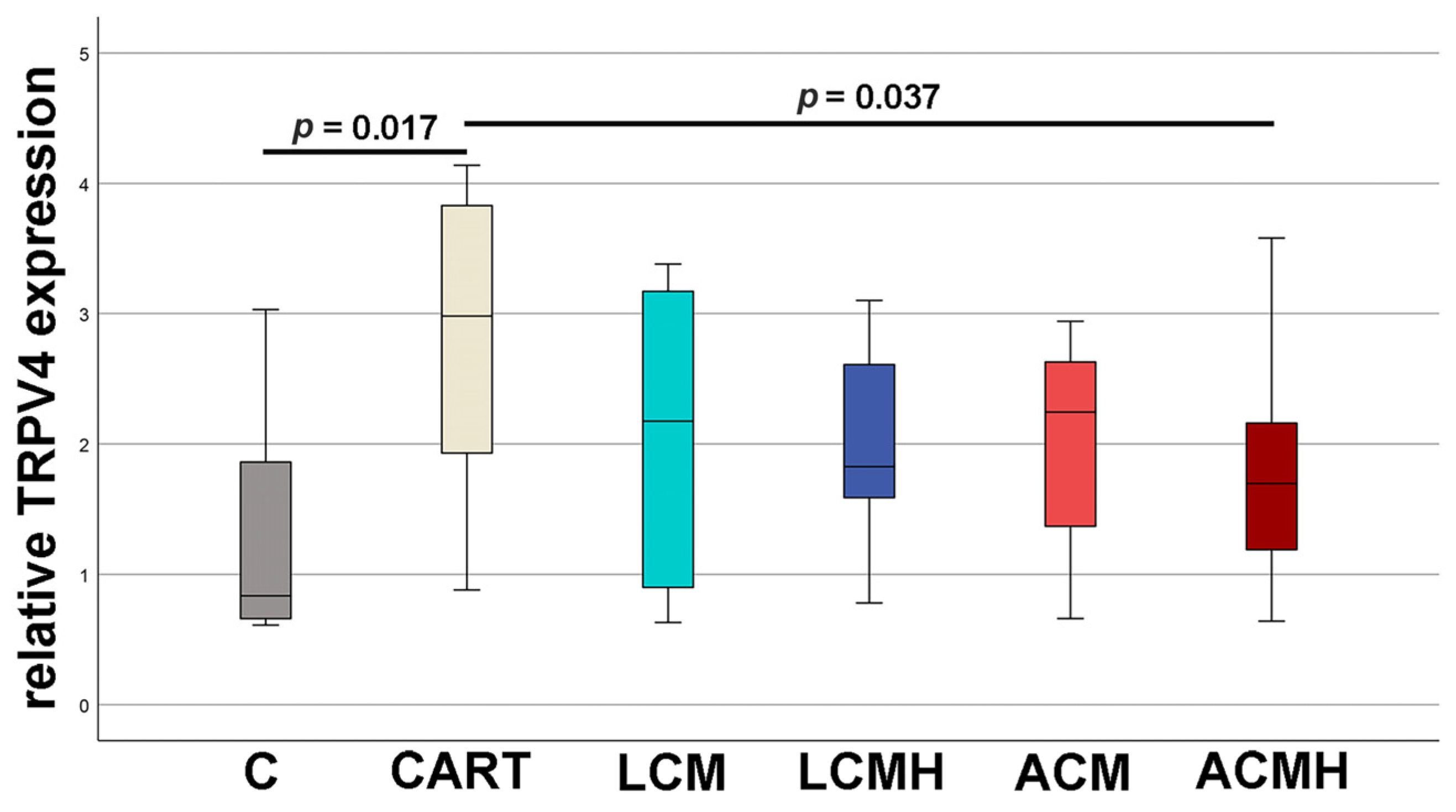
Cell adhesion to synthetic polymers is often reduced compared to that to natural materials because of the low number of adhesion sequences [
34]. Thus, coatings are often used to promote cell adhesion. A heparin coating is expected to improve cell adhesion and reduce hemostasis upon contact with blood. Hauptmann et al. (2022), analyzed cell adhesion and the proliferation of undifferentiated MSCs on ACM compared to ACMH by means of immunofluorescence labeling, followed by confocal laser scanning microscopy [
19]. They reported reduced proliferation and adhesion on ACMH compared to ACH [
19]. These results are in line with our observations for the early chondrogenic differentiation of MSCs on ACMH. The relative mRNA expression of TRPV4 was reduced on ACMH discs but not on ACM. Since the only difference between ACM and ACMH was the heparin coating, it seems that it is responsible for the bad outcome of ACMH. The negative effect of the heparin coating could be caused by the ability of heparin to bind growth factors such as TGF-ß1 and BMP-2 [
36]. We supplemented the chondrogenic differentiation medium with TGF-ß1 and BMP-2. Heparin might have absorbed these growth factors, and therefore they were no longer available to stimulate chondrogenic differentiation. This would explain the negative effect we detected for the heparin coating of ACMH. The collagen type II protein synthesis was reduced on ACMH discs compared to LCMH. This difference might be caused by the polymers but not the heparin coating since both polymer discs received the same coating. None of our results revealed significant differences between LCM and LCMH. Thus, the heparin coating did not have any negative or positive influence in combination with LCM in chondrogenic differentiation medium for up to 20 days. This could be due to the slow degradation of LCM described by Felfel et al., 2016 [
23], who showed only 10–30% of dry mass loss after 80 days of incubation in PBS at 37 °C. Thus, long-term incubation in chondrogenic medium would be of interest for further studies. At this time, heparin coating can be neglected for LCM, as it has shown no positive or negative effects. The use of heparin coating for ACM must be firmly rejected, as ACM without heparin coating was clearly shown to be more suitable as a material for cartilage implants.
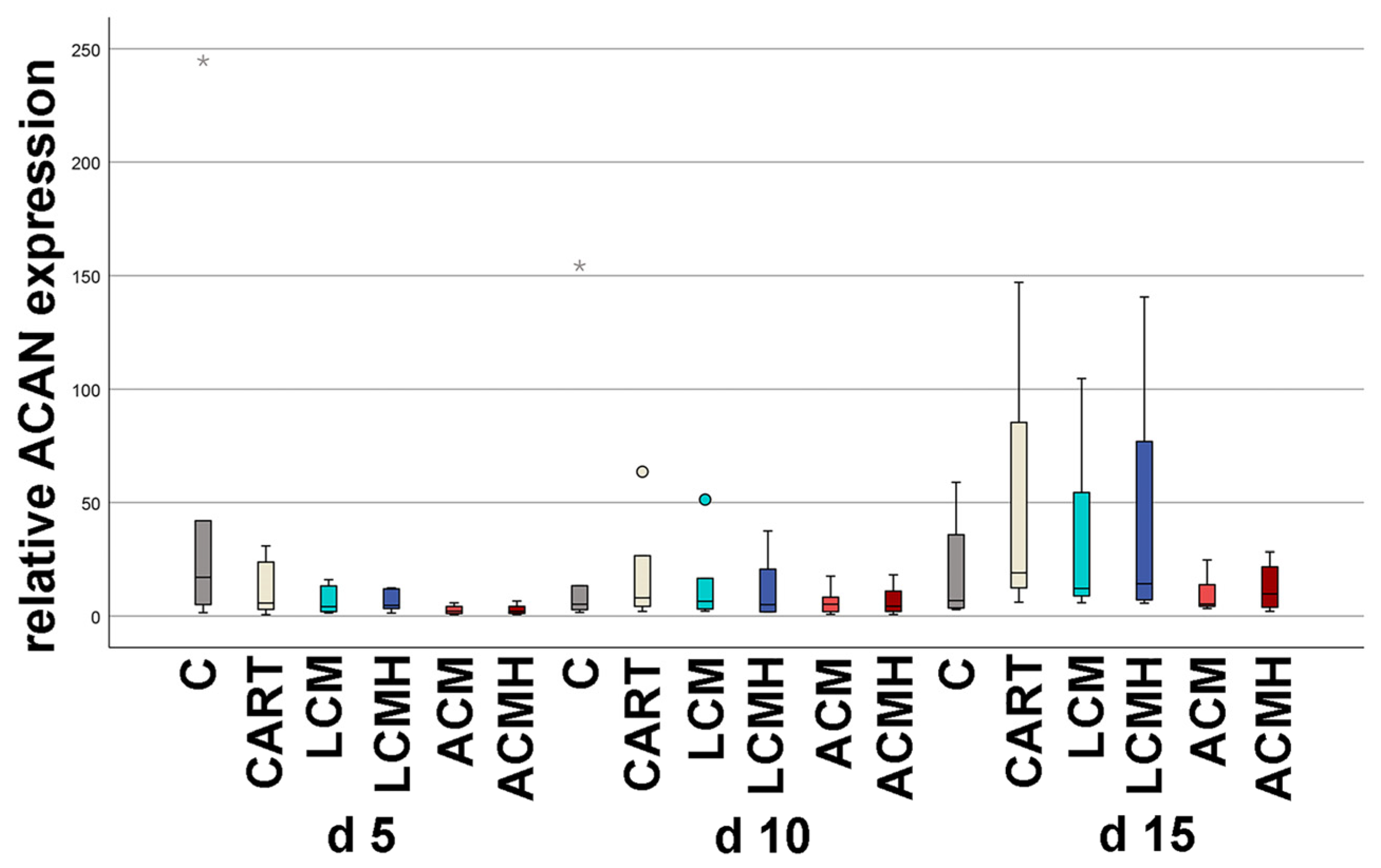
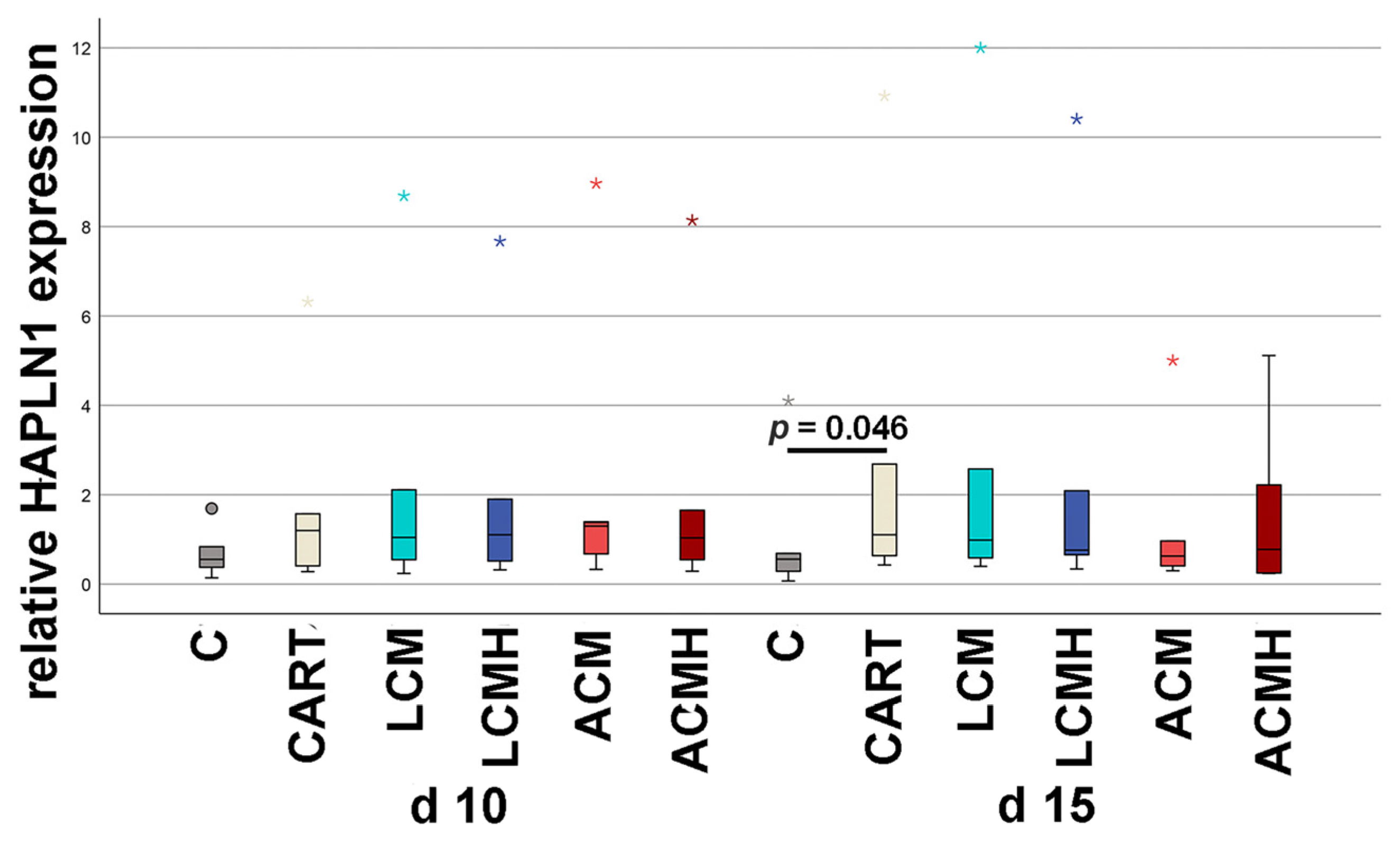
The chondrogenic differentiation of MSCs is still a challenge. Here, it was the basis for the comparison of the four implant discs. Therefore, not only were the materials compared but so was chondrogenic differentiation, assessing the quality of cartilage development. Worldwide, working groups have established protocols for the differentiation of MSCs into chondrocytes. The aim of the differentiation is the synthesis of an extracellular matrix (ECM) with the highest possible content of collagen type II and proteoglycans typical of cartilage such as aggrecan and HAPLN. In general, there are three main differentiation strategies: (i) the addition of growth factors, (ii) the induction of hypoxia, and (iii) the application of mechanical pressure. Here, we used a combination of growth factors and hypoxia that was induced by DMOG. Sathy et al. (2019) reported that MSC-, DMOG-, TGF-ß3-, and BMP-2-loaded hydrogels implanted in mice promoted the formation of a stable cartilaginous tissue with an accumulation of GAGs [
31]. DMOG was released for 72 h [
31], which is why we applied it for the same time span.
The chondrogenic effect of TGF-β, which leads through the induction of SMAD signaling pathways to the formation of a chondrogenic cell type and inhibited hypertrophy, is well known. The application of TGF-β1 also activates the MAPK pathway by which cellular proliferation, survival, and chondrogenic differentiation are enhanced [
37,
38]. In pellet cultures, the exposure of TGF-β1 for only 24 h already induced chondrogenic differentiation, as measured by the increased gene expression of SOX9, aggrecan, and collagen type II. In addition, typical osteogenic genes were upregulated, e.g., RUNX2 and collagen type I. To avoid the well-known in vivo enhancement of enchondral ossification by TGF-β1 [
39], the usage was limited in time and concentration (10 ng/mL at day 0–5, 7.5 ng/mL at day 5–7, and 5 ng at day 7–17). A dual activity on osteogenic and chondrogenic fate is also known for BMP2 [
40]. The combination of TGF-β1 and BMP2 revealed an elevated proteoglycan synthesis [
41] and a cartilage-like correlation of collagen type II to type I [
42]. Since the long-term application of BMP2 was most promising in an in vivo model [
43], BMP2 was added during the whole cell culture period at a concentration of 100 ng/mL. The combination of BMP2 with dexamethasone increased SOX9 expression, while the late chondrogenic targets collagen type II and aggrecan were not enhanced. When dexamethasone was combined with TGF-β1, the chondrogenic differentiation of MSCs was stimulated, whereas the differentiation of synovial fibroblasts into chondrocytes was inhibited [
44]. Thus, the cell type also appears to have an influence on chondrogenic differentiation. Often, when articular cartilage is repaired, no hyaline cartilage is created but rather fibrocartilage, which has less compressive elasticity than hyaline cartilage and is, therefore, less suitable for reducing friction and buffering the effect of load-bearing in the joints. However, any replacement tissue is better than destructed hyaline cartilage. Fibrocartilage is characterized by a high amount of collagen type I, whereas 90–95% of the collagen in hyaline articular cartilage belongs to collagen type II [
17]. Our results demonstrated that the chondrocytes developed on the polymer discs were able to synthesize collagen type II. However, real-time RT-PCR also showed an upregulation of collagen type I on the implants and the positive control for chondrogenic differentiation (CART). We therefore hypothesize that the selected in vitro system for chondrogenic differentiation, although capable of producing type II collagen, synthesized only a small amount of collagen type II within the available time and instead increased the production of collagen type I. It is therefore possible that the resulting monolayer resembles fibrocartilage rather than hyaline articular cartilage. However, this effect is not due to the polymer discs, as shown by the positive control of chondrogenic differentiation, but rather to the selected in vitro system. We suppose that longer incubation times would have increased the proportion of type II collagen in the ECM but would also have entailed the risk that differences between the material groups would no longer have been noticeable due to advanced cartilage development. However, the length of most in vitro studies of cartilage synthesis using models is between 1 and 4 weeks, as reported in the review of Goldberg et al., 2017 [
45]. Therefore, the present study with an in vitro incubation time of 15 days for real-time RT-PCR and 20 days for collagen type II ELISA seems to fit well with the comparative literature. Cai et al. reported in a 3D system the presence of collagen type I in the extracellular matrix (ECM) of the early time points of chondrogenic differentiation when some of the MSCs first started their chondrogenic fate [
46]. On the other hand, it has been reported that collagen type I is associated with hypertrophic chondrocytes [
47]. During enchondral ossification, chondrocyte hypertrophy together with ECM calcification is a crucial step for the opening of the cartilage matrix by chondroclasts and the concomitant synthesis of the bone matrix directly onto the cartilage matrix by osteoblasts. RUNX2 is a key transcription factor for osteogenic differentiation and required for chondrocyte maturation. It is involved in the decision whether MSCs are differentiated into osteoblasts or a chondrocyte lineage. In proliferating chondrocytes, it is only weakly expressed but upregulated in hypertrophic and terminal chondrocytes where it suppresses the termination of chondrocyte differentiation and re-introduces the cells to a proliferative stage [
14]. Here, no significant changes in the relative RUNX2 expression were observed on the implant discs at days 5 and 15. Only at day 10 was RUNX2 mRNA increased on LCM and LCMH discs, which might be caused by residuals of the higher TGF-β1 concentration up to day 7 of incubation. Since RUNX2 mRNA expression decreased at day 15, we suppose that RUNX2 does not represent ossification but chondrogenic differentiation in our setting. Chondrogenic fate is also supported by the fact that collagen type II was detected at mRNA and protein level, even if only in small amounts. At the mRNA level, all test groups expressed significantly less collagen type II mRNA compared to the positive control HEK-293, whereas at the protein level, the median collagen type II concentration increased compared to the HEK-293 collagen type II synthesis. Since the real-time RT-PCR was performed on day 15 and the collagen type II ELISA was performed on day 20 of chondrogenic incubation, we suppose that during this last 5 days of cell culture incubation, the cartilage development increased sharply. The effect of the incubation time on collagen type II synthesis has been described in other publications. However, using 3D cell culture models, hydrogels, and collagen matrices, a significantly earlier peak in collagen type II synthesis was observed than in our study. Jahanbakhsh et al., 2020, described an increase in the collagen type II expression of MSCs encapsulated in different hydrogels from day 7 to day 14 with a slight decline to day 21 [
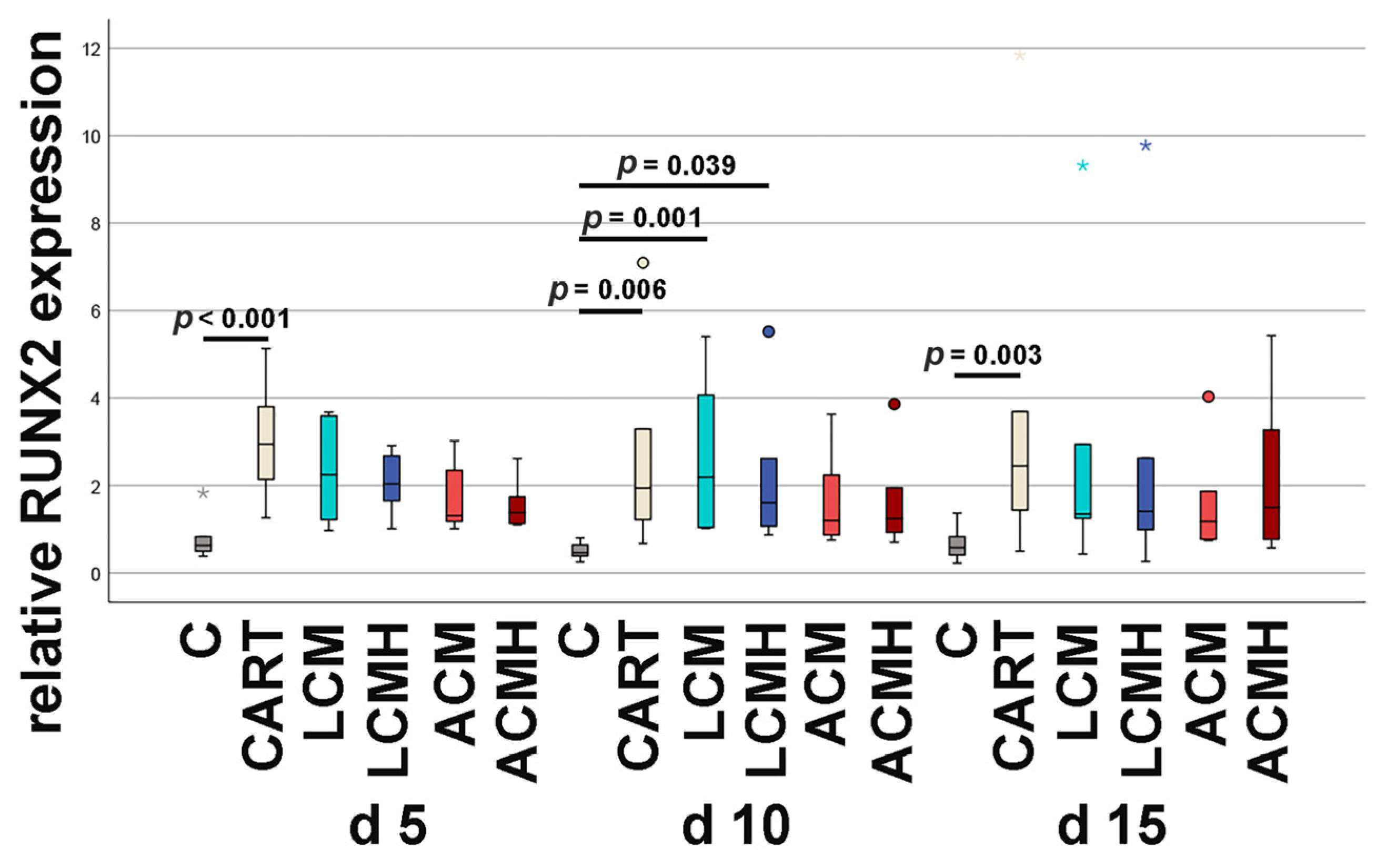
48]. We hypothesize that the delayed synthesis of collagen type II in our model is due to the lack of an additional stimulus such as hydrogels. The polymers failed to provide a similar stimulus for chondrogenic differentiation to that provided by hydrogels in various studies. However, hydrogels lack the biomechanical stability required for the repair of load-bearing joint regions. Future studies could use porous polymer scaffolds whose pores are filled with a hydrogel. This could provide an additional stimulus for collagen type II synthesis.
Our cell culture model for cartilage regeneration is based on the differentiation of human MSCs into chondrocytes synthesizing cartilage matrix. Stenderup et al. (2003) reported that the ability to proliferate and differentiate is reduced in the MSCs of older donors [
49]. Our donors ranged from 18 to 90 years of age, which explains the high variation that was found for some of the targets and assays. Despite the high variance, this wide age range of donors was used to represent all age groups affected by cartilage and osteochondral defects. The usage of cell culture passages P6 to P9 might also have negatively influenced the differentiation. It has been shown that higher passages possess increased senescence, which slows the matrix synthesis but increases the expression of proinflammatory factors such as IL-1β, IL-6, and TNF-α [
50] which induces matrix degradation [
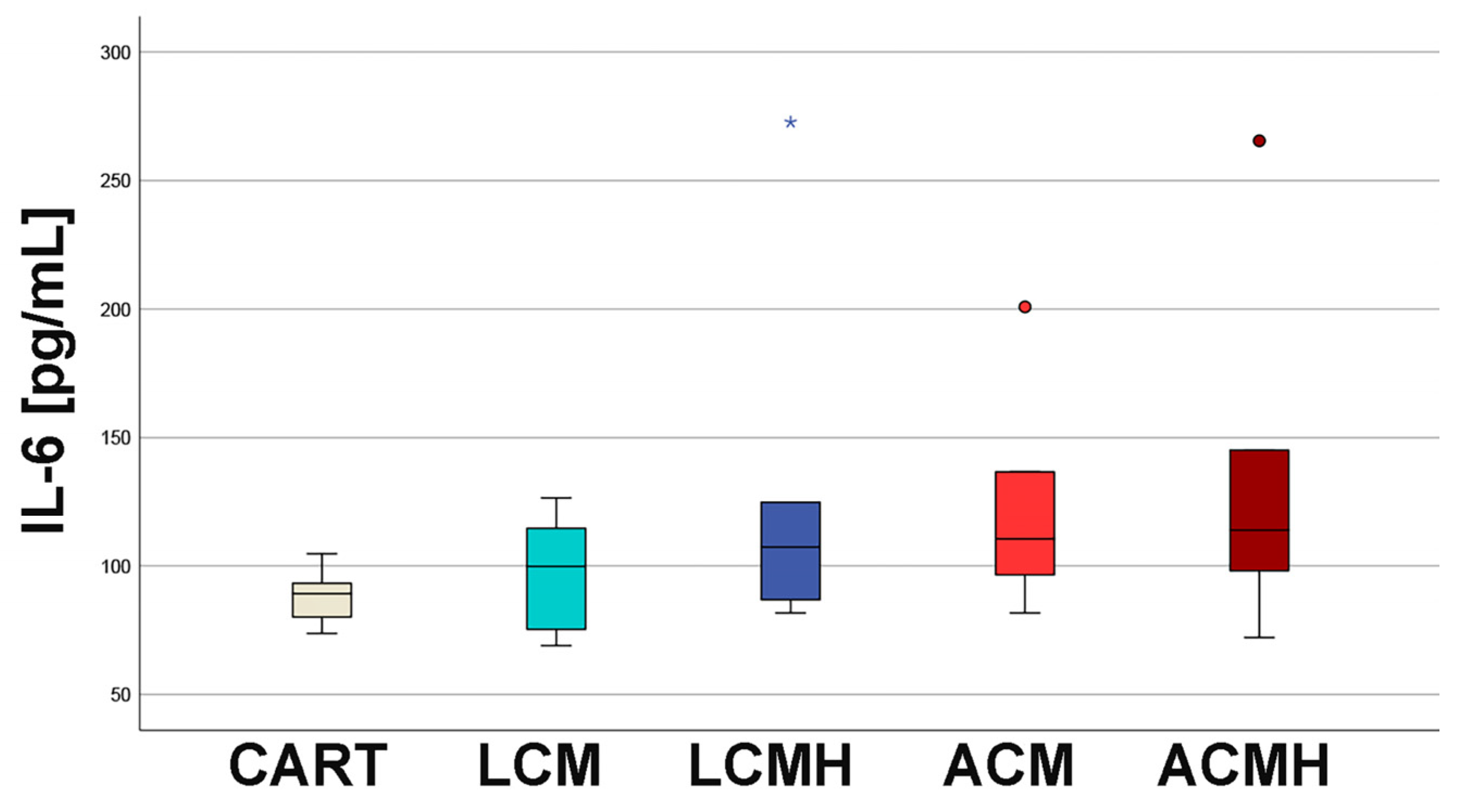
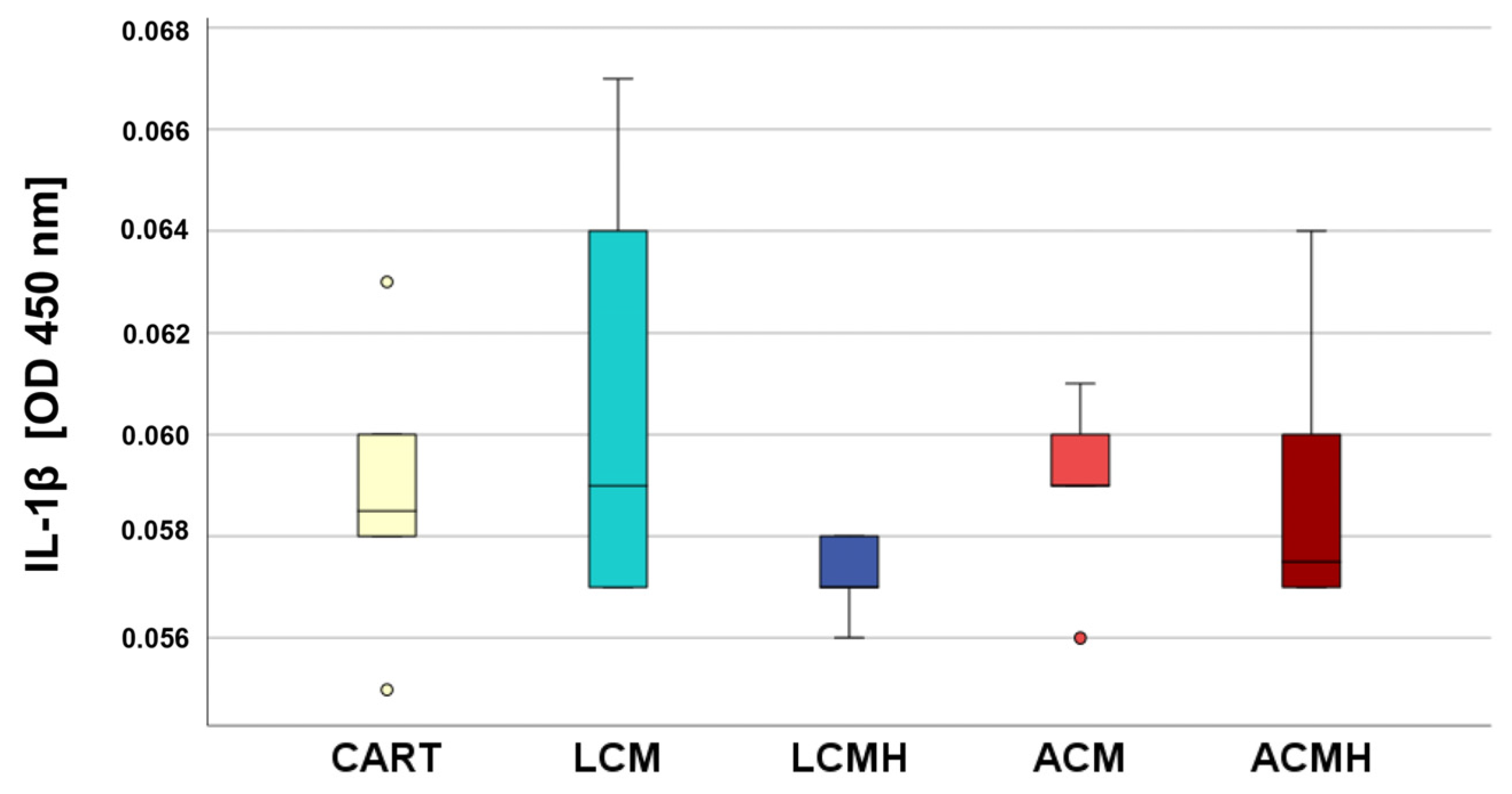
24]. The examination of the cell culture media from day 5 showed no significant differences in terms of IL-1β, IL-6, and TNF-α. This indicates that the materials did not trigger an inflammatory response and were not cytotoxic. In addition, the results from the MTT assay revealed a high metabolic activity on LCM discs that might be correlated with chondroblast proliferation and the high viability of the cells on the polymer discs. Thus, we recommend LCM as a suitable synthetic polymer for bioprinted porous 3D scaffolds.
4. Materials and Methods
4.1. Polymer Samples
Polymer discs of 1 mm in height and 7 mm in diameter were kindly provided by cooperation partners at the Department of Biomaterials, Institute for Bioprocessing and Analytical Measurement Techniques e.V. (iba).
Discs were generated from biodegradable LCM with a (D,L)-lactide/ε-caprolactone ratio of 8:4 and ACM, with an L-alanine/ε-caprolactone ratio of 2:8. The synthesis of the polymers has been described previously in detail [
19,
23].
Both polymers were mixed with 2 wt.% of a commercially available UV photoinitiator, IRGACURE® 369 (Sigma Aldrich, Taufkirchen, Germany), filled into a silicone mask with the above-mentioned dimensions and cured under UV light for 9 min using a Vacuum UV Exposure Unit 2 from proMa systro which works at a wavelength of 365 nm and a power of 120 W. Subsequently, the cured plates were washed several times with acetone to remove uncured polymer and unreacted photoinitiator and then transferred in steps of 80:20, 60:40, 50:50, 40:60, and 20:80 from pure acetone to 70% ethanol for storage.
Heparin Modification of Polymer Samples
Coating solutions of the respective polyelectrolytes (poly-l-lysin/heparin) were prepared in HEPES/NaCl buffer (25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 137 mM NaCl, pH 7.4) at a concentration of 1 mg/mL and sterile-filtered (0.2 µm) before use. Automatic film build-up was performed by employing a dipping robot (DR3, Riegler&Kirstein, Postdam, Germany). The robot was equipped with specially designed sample-holding devices that carry up to 40 discs (diameter: 15 mm; thickness: 0.7 mm). The samples were immersed into the respective solutions at a speed of 5 mm/s (dip and withdraw) and left to incubate for 5 and 1 min in the polyelectrolyte and buffer solution, respectively. Ten dipping positions, corresponding to two polyelectrolyte solutions and four wash solutions (HEPES/NaCl buffer) per polyelectrolyte, were programmed. The film construction was carried out automatically, yielding a polyelectrolyte multilayer (PEM) architecture [PLL-HEP]20.
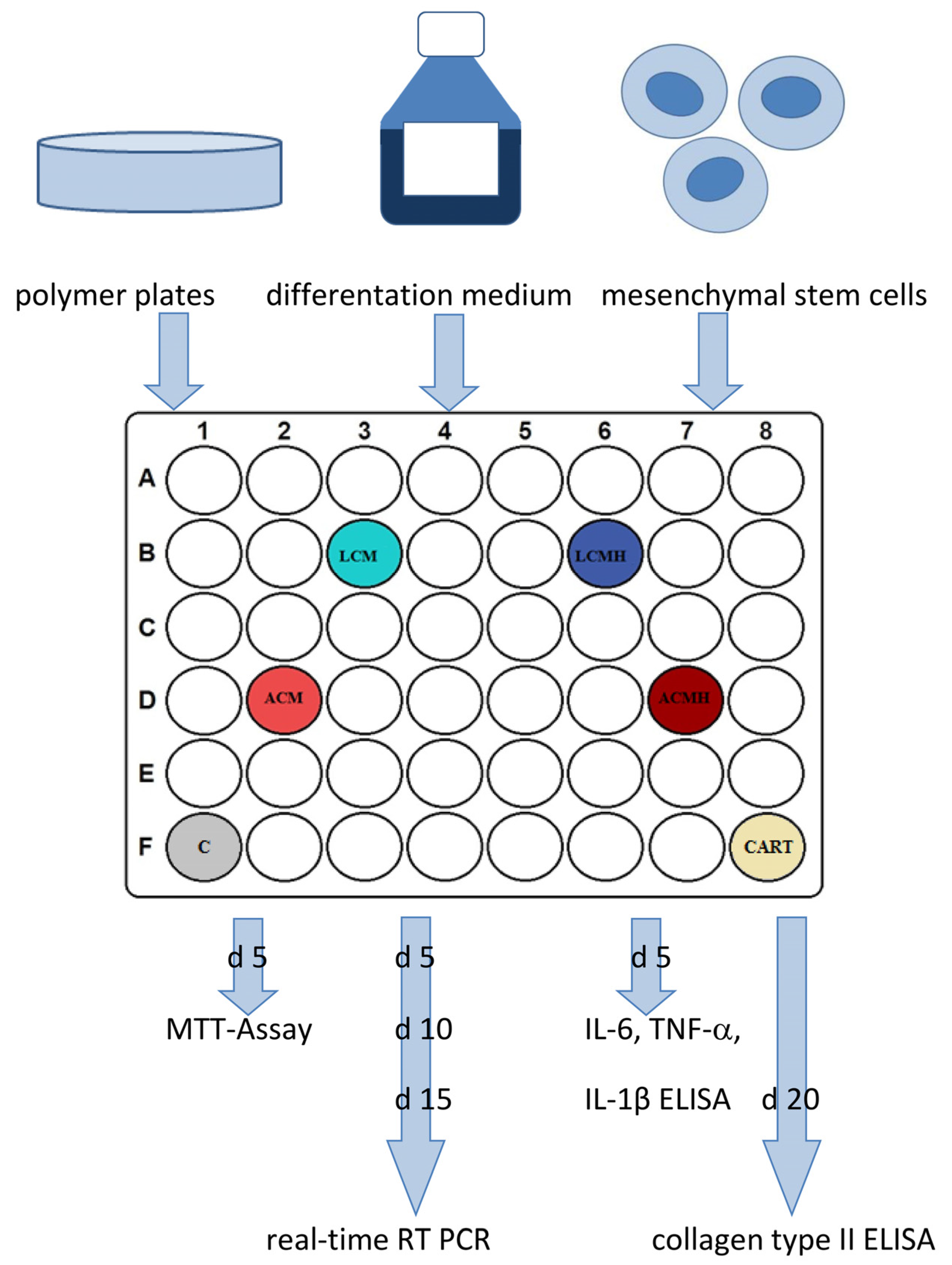
Before usage, the plates were washed again in 70% fresh ethanol for 24 h and in PBS for 24 h with 3 changes each. Afterwards, the plates were transferred to 48-well plates and covered with medium for 24 h before the cells were seeded to start the experiment. The study design is shown in
Figure 13. Technical duplicates were performed for every condition and readout parameter.
4.2. Cell Culture
Human MSCs were harvested from the spongy bone of femur heads that were collected during the routine insertion of endoprostheses at the University Hospital of Giessen (Department of Trauma, Hand, and Reconstructive Surgery) after receiving written informed consent from the patients and approval from the local ethic commission (reference number: 74/09), as described previously [
20]. In brief, spongy bone was treated with Hibernate A (Gibco, Thermo Fisher Scientific, Dreieich, Germany) containing 10 µg/mL collagenase (Fujifilm Wako, Neuss, Germany) and 0.1 M/mL calcium chloride at 37 °C for 1 h. Digestion was stopped by Hibernate A with 10% fetal bovine serum (FBS, PAN Biotech, Aidenbach, Germany). After erythrocytes were destroyed in lysis buffer for 2 min, the suspension was filtered and washed. Then, fluorescence-activated cell sorting was performed using the Aria II FACS sorter (BD Biosciences, San Jose, CA, USA). The following antibodies were used: (i) PB-conjugated mouse-anti-human CD73 (BioLegend, San Diego, CA, USA), (ii) APC-conjugated mouse-anti-human CD105 (BioLegend), (iii) mouse-anti-human CD31-APC/Cy7 (BioLegend), and (iv) mouse-anti-human CD45-APC/Cy7 (BioLegend). Thus, the sorted MSCs positively bound to CD73 and CD105, whereas they did not bind to CD31 and CD45.
Samples of 6 male donors without pre-existing diseases and bone-influencing medication were involved in the present study. Their mean age was 51.5 years with a range from 18 to 90 years, including one donor each aged 18, 29, 44, 56, 72, and 90 years. This high variance was chosen to reflect the patients’ situation as closely as possible. A separate set of experiments was performed for the cells of each donor and presented as n.
MSCs were bred in a MesenPro RS medium kit (Gibco) with 0.2% gentamicin/amphotericin B, 1% glutamine (Capricorn scientific, Ebsdorfergrund, Germany), and 20% FBS at 37 °C with 6% CO2. Chondrogenic differentiation was performed in 48-well plates where 20.000 cells were seeded on the material plates or without material (positive control, chondro). Day 1–5 chondrogenic medium consisted of Dulbecco’s Modified Eagle Medium (DMEM) low glucose (Gibco, Thermo Fisher Scientific Waltham, MA, USA, #11054020) with 10% FBS, 10 ng/mL TGF-β1 (Sigma-Aldrich, Darmstadt, Germany), 100 µM DMOG (Sigma-Aldrich), 100 ng/mL BMP2 (Thermo Fisher Scientific), 1% Insulin–Transferrin–Selenium (ITS+, Merck, Darmstadt, Germany), 50 mg/mL sodium L-ascorbate (Sigma-Aldrich), 40 µg/mL L-proline (Sigma-Aldrich), 1% glutamine, 100 nmol/L dexamethasone (Merck), and 0.2% gentamicin/Amphotericin B. After day 5, DMOG was no longer used as a chondrogenic supplement, and FBS was also reduced to 1%. The concentration of TGF-β1 was reduced to 7.5 ng/mL from day 5 to 7 and to 5 ng/mL from day 7 to 17 of chondrogenic differentiation.
In addition, a negative control (C) was used that received a control medium consisting of DMEM with 10% FBS, 1% glutamine, and 0.2% gentamicin/Amphotericin B up to day 5. Afterwards, the FBS concentration was reduced to 1%.
4.3. MTT Assay
The polymer discs situated in a 48-well plate were covered with medium and 20,000 cells per well. At day 5 of cell culture, 50 µL MTT dye (3–4,5-dimethylthizol-2-yl)-2,5-diphenyltetrazoliumbromide, Merck) was added, and the well plate was incubated at 37 °C for 4 h. Afterwards, the supernatant was removed, 500 µL lysis buffer (0.04 N HCl in 2-Propanol) was added, and the well plate was shaken on ice for 10 min. Meanwhile, the color changed from yellow to purple. Then, 200 µL of the supernatant was transferred into a 96-well plate in doublets, and the absorbance was determined with a plate reader (Synergy HT, BioTek Instruments, Winooski, VT, USA) equipped with the software Gen 5 (BioTek Instruments). As background controls, (a) pure lysis buffer (blank value) and (b) material plates without cells in lysis buffer were used. As additional controls, (i) cells in chondrogenic differentiation medium (CART), (ii) cells in control medium without chondrogenic supplements (C), and (iii) cells after 2 h of cell culture were employed. Calculation and statistical analysis were performed after the subtraction of the background controls.
4.4. ELISAs for Proinflammatory Factors
MSCs were cultured on the polymer plates for 5 days, the supernatant was collected, and the occurrence of IL-1β, IL-6, and TNF-α was measured using an IL-1β-, IL-6-, and TNF-α-specific ELISA (Human IL-1β ELISA kit, Invitrogen, ThermoFisher Scientific; Quantikine ELISA Human IL-6, R&D Systems, Minneapolis, MN, USA; and Human TNF-α ELISA kit, Invitrogen; ThermoFisher Scientific) according to the instruction manuals. In brief, the thawed samples were diluted 1:2 (IL-1β) or 1:40 (IL-6) or used pure (TNF-a), and 50 µL (IL-1β), 2.5 µL (IL-6), or 200 µL (TNF-α) was added to the kit’s dilution buffer in a 96-well plate. A standard and the controls were also added to the well plate. Duplicates were performed for every condition. After 2 h of incubation in the dark on a horizontal shaker, the primary antibodies (kit components) were added, and the plate was again incubated and shaken for 2 h. Afterwards, the substrate (kit component) was added, and the color of the samples changed. The reaction was stopped after 15–20 min with 50 µL stop solution (kit component) that triggered a color change to yellow. Finally, the optical density was measured at 540 nm for IL-6 and at 450 nm (IL-1β and TNF-α) with a plate reader. The background control, obtained by replacing samples with PBS, was subtracted, and the samples’ optical density (OD) was determined. The IL-6 concentrations were calculated by a standard series. The ELISAs were verified by the immunoassay control group as well as by MSCs stimulated by lipopolysaccharides (LPSs, Thermo Fisher Scientific).
4.5. Real-Time Reverse Transcriptase Polymerase Chain Reaction (Real-Time RT-PCR)
Analyses of mRNA expression were performed at days 0, 5, 10, and 15 of cell culture incubation. A cell monolayer was washed with PBS, lysed with 750 µL Trizol (Life Technologies, Thermo Fisher Scientific), and stored at −80 °C. Then, the total mRNA was isolated with the RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany) and reverse-transcribed into cDNA using the QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer’s protocol. In brief, samples were mixed with 150 µL chloroform (Carl Roth, Karlsruhe, Germany) and centrifuged for 15 min with 14.000 rpm. The RNA phase was mixed with 70% ethanol and transferred to a column. Bound RNA was washed and eluted with 30 µL water, and the RNA concentration was determined by nanodrop (ND-1000, Nanodrop Technologies, Wilmington, DE, USA). RNA (125 ng) was treated with gDNA wipeout buffer (kit component) to destroy genomic DNA. Afterwards, RNA was reverse-transcribed into cDNA by reverse transcriptase diluted in a master mix containing primers and nucleic acids (kit components). After cDNA synthesis, real-time RT-PCR was performed with custom-designed intron-spanning primers (
Table 1) using the Quantifast SYBR Green PCR Kit (Qiagen) or the LightCycler Fast Start DNA Master Plus SYBR Green I (Roche Diagnostics GmbH, Mannheim Germany) for the target
RUNX2. Therefore, cDNA was diluted 1:4 in water and transferred into the master mix containing the buffered enzymes and nucleic acids. Real-time RT-PCR was carried out with 5 min degradation at 95 °C, followed by 40 cycles of 10 s at 95 °C and 30 s at 60 °C. Finally, a melting curve was used to control the sample’s purity. As additional controls, (a) cDNA and (b) reverse transcriptase were omitted during PCR and cDNA syntheses, respectively. The relative expression was calculated by the 2
−ΔΔCT method using cycle threshold (Ct) values that were normalized by the reference gene β-2 microglobulin (B2M).
4.6. Collagen Type II ELISA
The collagen type II concentration was measured at day 20 of cell culture incubation with a collagen type II ELISA (abbexa, Cambridge, UK). After washing, 220 µL of a papain solution (1 mg/mL diluted in 1 mM ethylenediaminetetraacetic acid (Versene, Gibco)), as well as 500 µL of ice-cold PBS, was added to the cells. Afterwards, the cells were centrifuged for 5 min at 300× g and 4 °C, washed with PBS, and homogenized first in a vibration mill (VWR, Darmstadt, Germany) for 1 min and then for 5 min in an ultrasonic bath (Bandelin electronic, Berlin, Germany). Samples were diluted in PBS and transferred to the precoated 96-well plate of the ELISA kit. Further procedures were carried out according to the manufacturer’s operating instructions. In brief, after 1 h of incubation at 37 °C, 100 µL detection reagent A was added, and the plate was incubated again for 1 h at 37 °C. Then, 100 µL detection reagent B was admitted, and the plate was incubated once again for 1 h at 37 °C. After washing, 90 µL of the substrate (kit component) was administered, and the plate was incubated for 30 min at 37 °C. The color changed from colorless to blue. The reaction was stopped by adding 50 µL stop solution and measured at 450 nm using a plate reader. For calculation, the value of the background control (PBS) was subtracted, and the concentration was quantified by the standard curve prepared within the plate with the supplied standard. HEK293 cells were used as a positive control.
4.7. Statistical Analyses
SPSS software (version 25, SPSS Institute Inc., Chicago, IL, USA) was used for statistical analyses. First, the values of every data set were tested for normal distribution using the Kolmogorov–Smirnov test. The data from the MTT assay showed a normal distribution. Thus, one-way analysis of variance (ANOVA) was applied to evaluate whether the differences between the group means were statistically significant. Following the ANOVA test, the post hoc test was performed to correct for multiple testing. Since the IL-6, IL-1β, and TNF-α ELISA values were not normally distributed, the non-parametric Kruskal–Wallis test was conducted to compare whether the differences in the 5 independent groups were significant. The values of the relative expression were used for the statistical analysis of real-time RT-PCR. Since the values for TRPV4 and SOX were normally distributed, a paired-samples
t-test was applied. The values of ACAN, HAPLN1, RUNX2, and COL1A1 were not normally distributed. Therefore, the non-parametric Friedman test for more than two dependent samples was used, since comparing multiple time points leads to the samples being considered related. The Friedman test was followed by Bonferroni correction to avoid statistical errors due to multiple testing. Because of the differences in the median values of the negative control (C) and positive control (CART) for HAPLN1 expression at day 15, we performed the non-parametric Wilcoxon signed-rank test for dependent samples for these two groups. The non-parametric Kruskal–Wallis test followed by the Mann–Whitney U-test was used for the statistical analysis of COL2A1 values because they were not normally distributed and only included one harvesting time point. The values of the collagen type II ELISA were also not normally distributed and were therefore analyzed with a Friedman test followed by Bonferroni correction and a Wilcoxon test. The significance level was set at a value of
p < 0.05, and the number of experiments and donors was n = 6. Graphs were generated as box plots with SPSS and are described in detail in the legend of
Figure 1.