A Review of Silica-Based Nanoplatforms for Anticancer Cargo Delivery
Abstract
1. Introduction
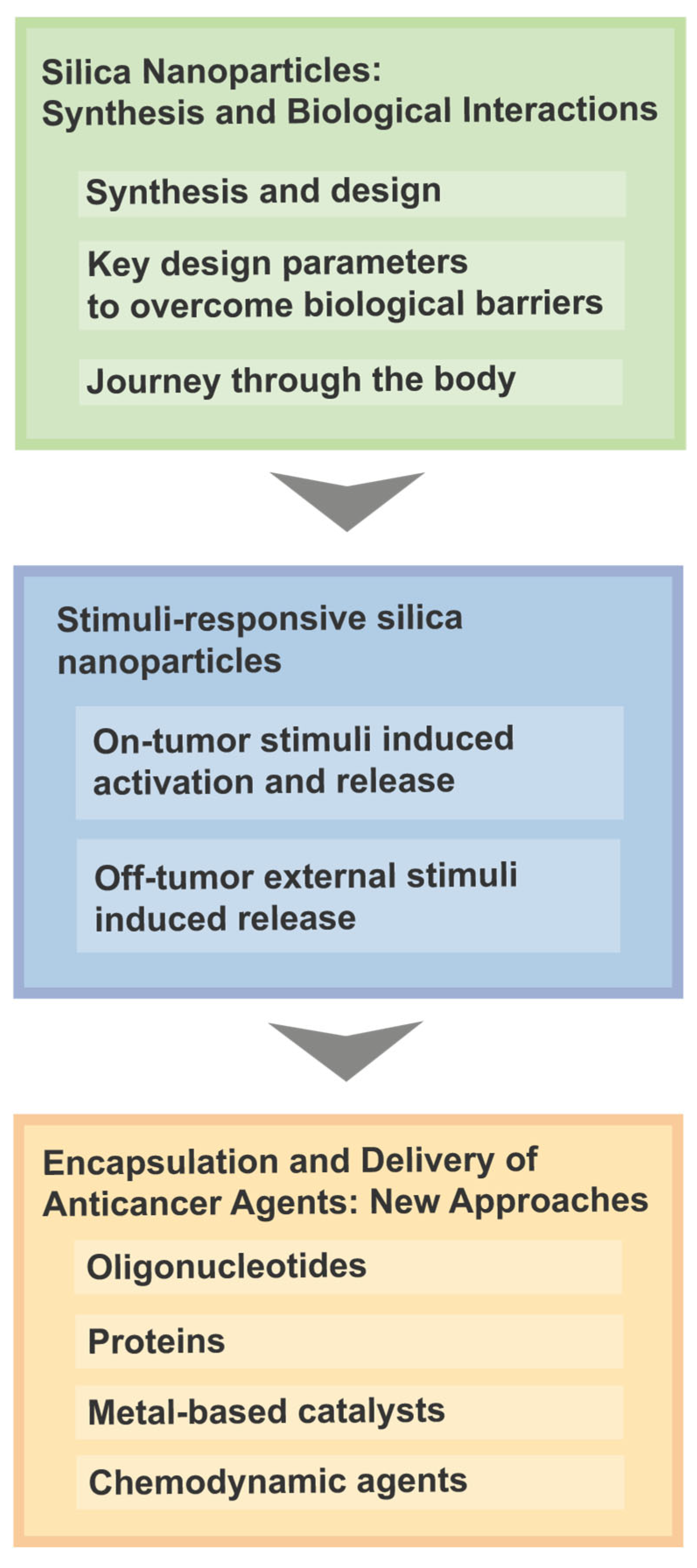
2. Silica Nanoparticles: Synthesis and Biological Interactions
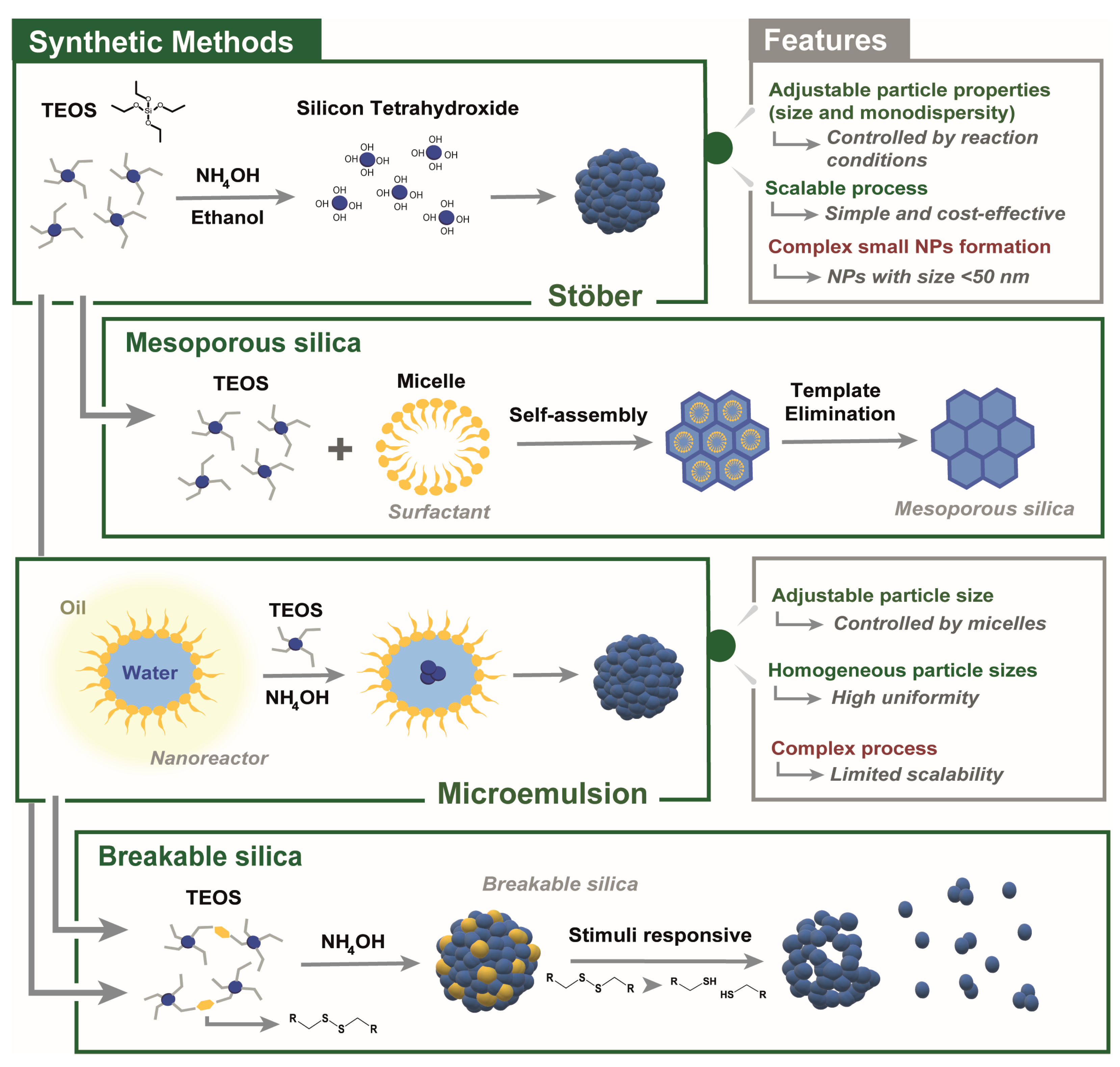
2.1. Synthesis and Design
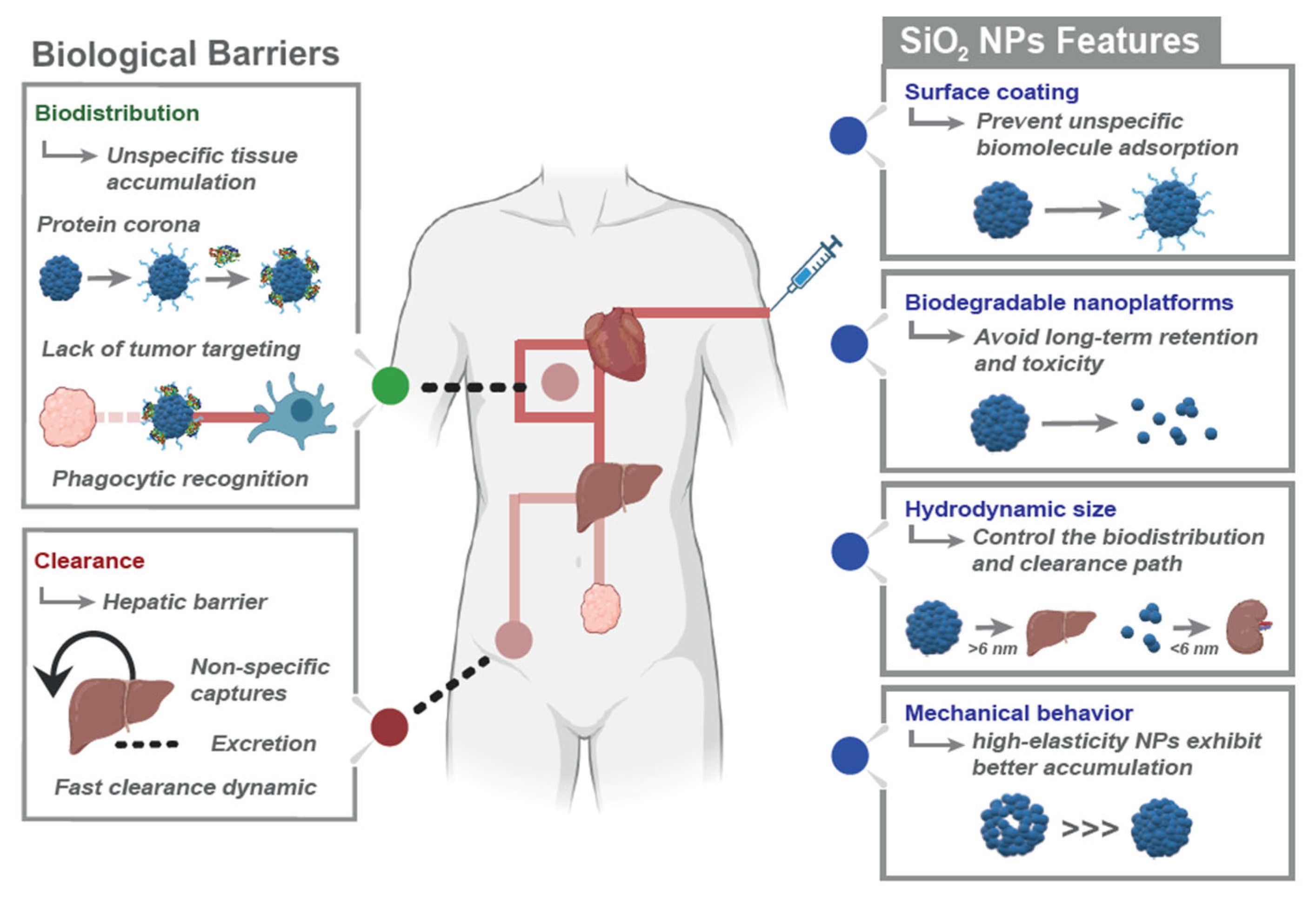
2.2. Key Design Parameters to Overcome Biological Barriers
2.2.1. Influence of Particle Size
2.2.2. Influence of Shape and Mechanical Properties
2.2.3. Influence of Surface Chemistry: Charge, Coating, Stealth, and Cloaking
2.3. Journey Through the Body
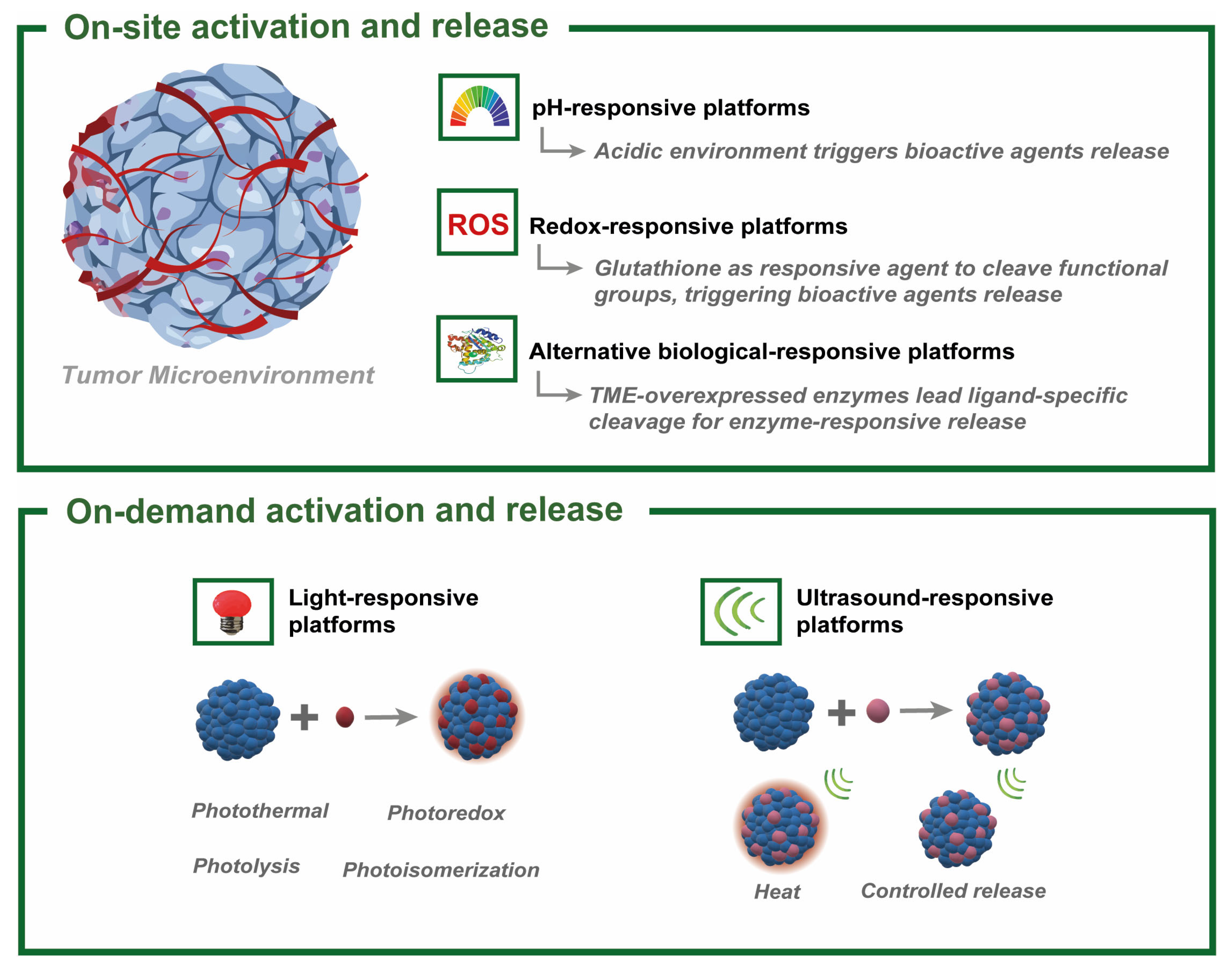
3. Stimuli-Responsive Silica Nanoparticles
3.1. On-Tumor Stimuli-Induced Activation and Release
3.1.1. pH
3.1.2. Reduction–Oxidation Processes (RedOx)
3.1.3. Enzymes and Other Biological Stimuli
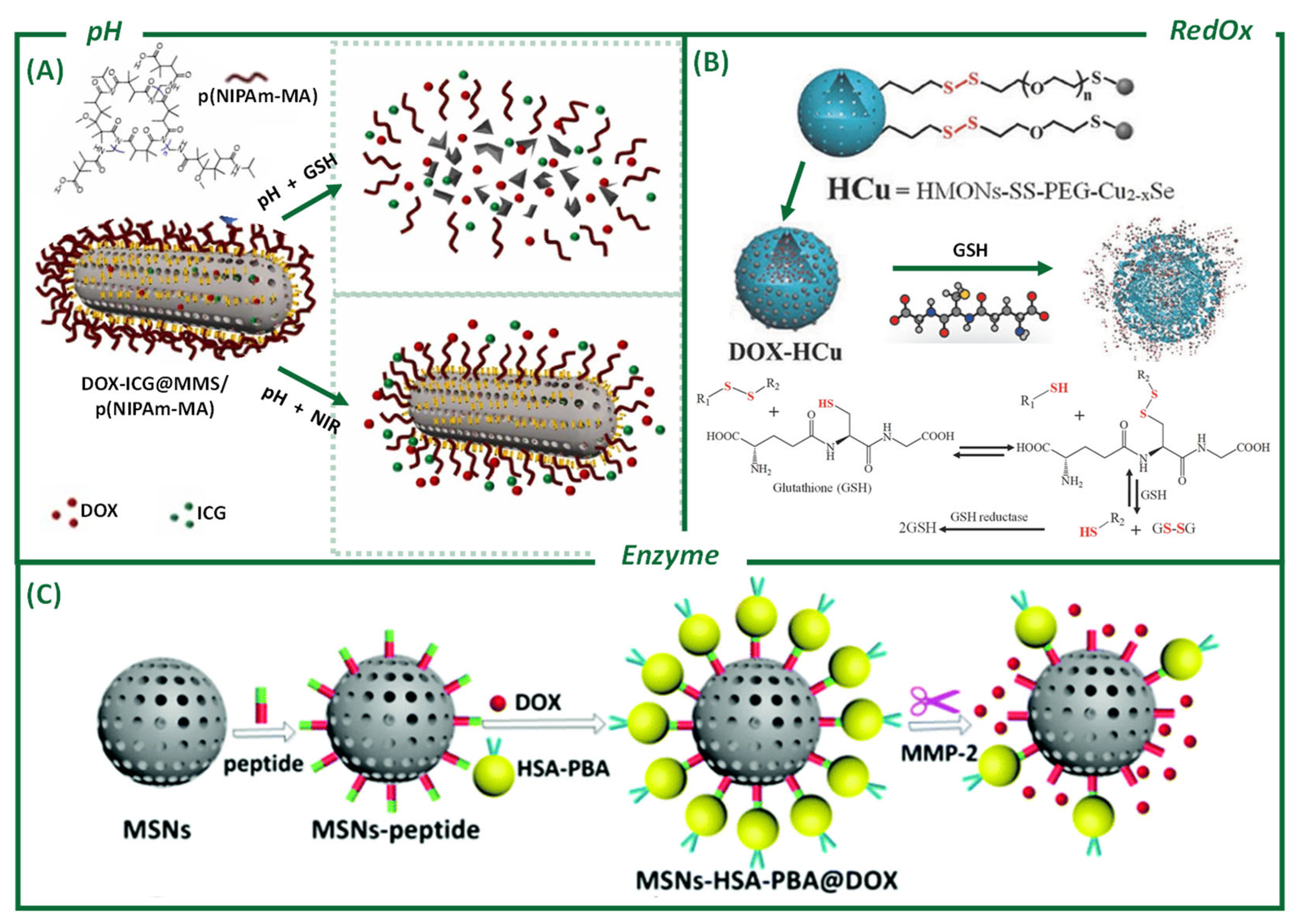
3.2. Off-Tumor External Stimuli-Induced Release
3.2.1. Light
3.2.2. Ultrasound

4. Encapsulation and Delivery of Anticancer Agents: New Approaches
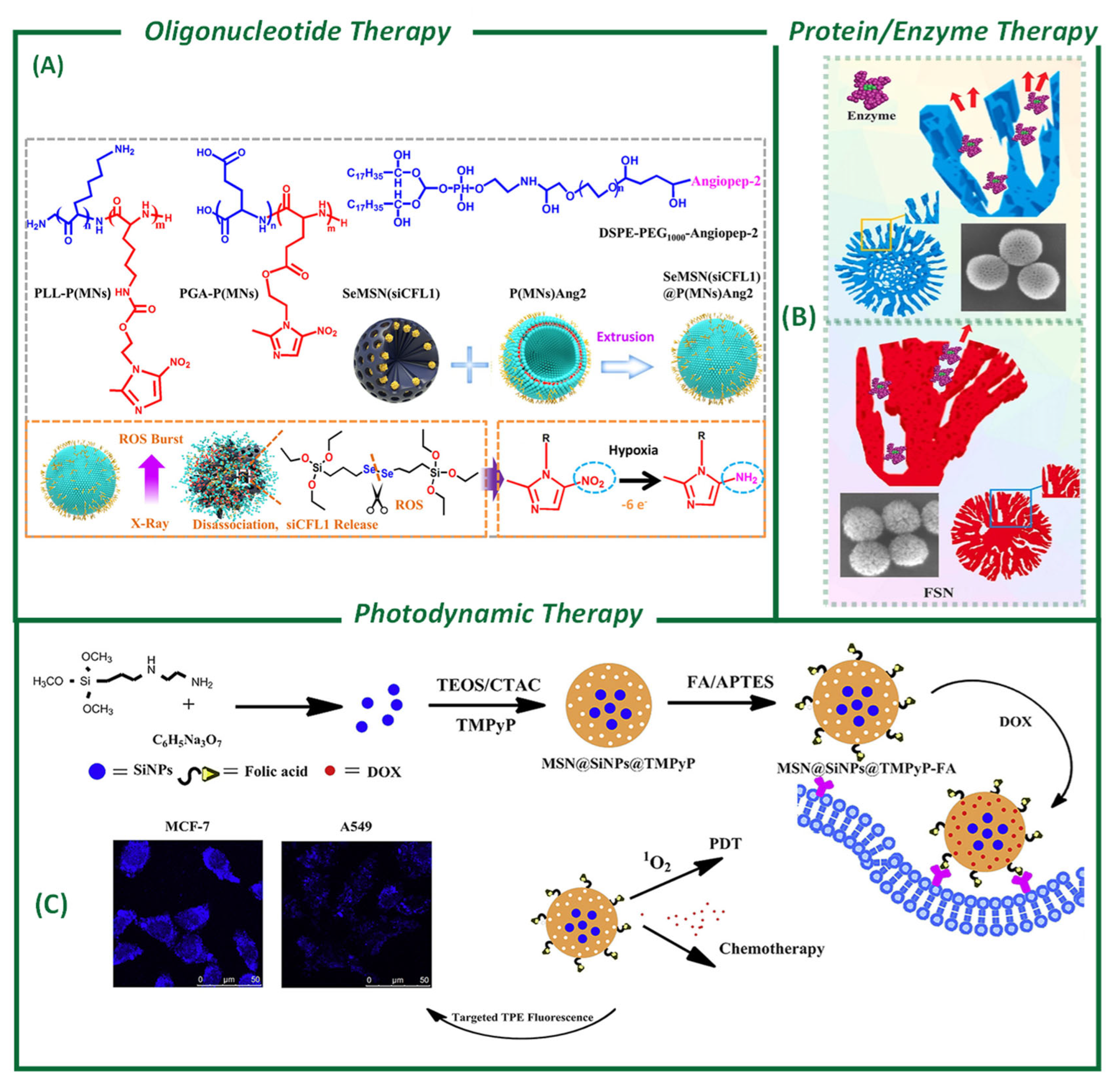
4.1. Oligonucleotides Loading and Delivery for Therapy
4.2. Protein-Based Delivery for Cancer Therapy
4.3. Photosensitizer-Based Photodynamic Therapy
4.4. Metal-Based Catalysts for Cancer Therapy
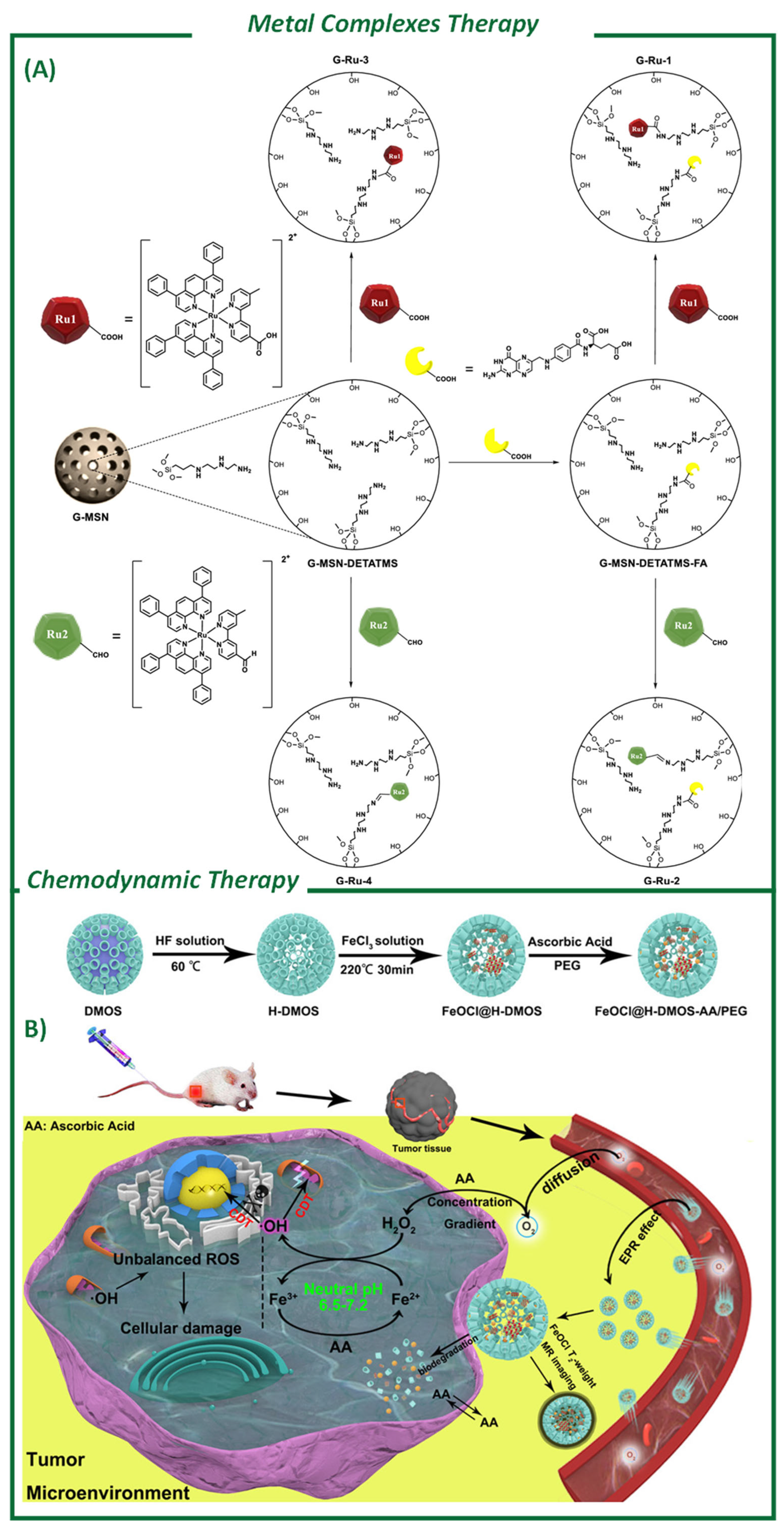
Chemodynamic Therapy
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Cui, X.; Chen, K.; Xing, H.; Yang, Q.; Krishna, R.; Bao, Z.; Wu, H.; Zhou, W.; Dong, X.; Han, Y.; et al. Pore Chemistry and Size Control in Hybrid Porous Materials for Acetylene Capture from Ethylene. Science 2016, 353, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Slater, A.G.; Cooper, A.I. Function-Led Design of New Porous Materials. Science 2015, 348, aaa8075. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Pan, H.; Zhang, J. Advances of Typical Mesoporous Materials and the Application in Drug Delivery. Mater. Res. Express 2023, 10, 042001. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kapoor, D.U.; Kukkar, R.R.; Gaur, M.; Elossaily, G.M.; Prajapati, B.G.; Chaiyasut, C. Mesoporous Silica Nanoparticles: Types, Synthesis, Role in the Treatment of Alzheimer’s Disease, and Other Applications. Pharmaceutics 2023, 15, 2666. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Maleki-Dizaji, N.; Barar, J.; Barzegar-Jalali, M.; Rameshrad, M.; Adibkia, K. Methylprednisolone Acetate-Loaded Hydroxyapatite Nanoparticles as a Potential Drug Delivery System for Treatment of Rheumatoid Arthritis: In Vitro and In Vivo Evaluations. Eur. J. Pharm. Sci. 2016, 91, 225–235. [Google Scholar] [CrossRef]
- Jafari, S.; Maleki-Dizaji, N.; Barar, J.; Barzegar-Jalali, M.; Rameshrad, M.; Adibkia, K. Physicochemical Characterization and in Vivo Evaluation of Triamcinolone Acetonide-Loaded Hydroxyapatite Nanocomposites for Treatment of Rheumatoid Arthritis. Colloids Surf. B Biointerfaces 2016, 140, 223–232. [Google Scholar] [CrossRef]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.-Y. Effect of Surface Functionalization of MCM-41-Type Mesoporous Silica Nanoparticles on the Endocytosis by Human Cancer Cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef]
- Shinde, P.S.; Suryawanshi, P.S.; Patil, K.K.; Belekar, V.M.; Sankpal, S.A.; Delekar, S.D.; Jadhav, S.A. A Brief Overview of Recent Progress in Porous Silica as Catalyst Supports. J. Compos. Sci. 2021, 5, 75. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, Y.; Zou, Y.; Lu, C.; Li, N.; Shi, Z.; Li, X.; Lai, X. Ultra-Sensitive PH Responsive Hydrogels with Injectable and Self-Healing Performance for Controlled Drug Delivery. Int. J. Pharm. X 2025, 9, 100334. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor Delivery of Macromolecular Drugs Based on the EPR Effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.H.; Park, K. Targeted Drug Delivery to Tumors: Myths, Reality and Possibility. J. Control. Release 2011, 153, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.M.; Ngo, W.; Lin, Z.P.; Sindhwani, S.; MacMillan, P.; Mladjenovic, S.M.; Chan, W.C.W. The Mechanisms of Nanoparticle Delivery to Solid Tumours. Nat. Rev. Bioeng. 2024, 2, 201–213. [Google Scholar] [CrossRef]
- Schöttler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailänder, V.; Wurm, F.R. Protein Adsorption Is Required for Stealth Effect of Poly(Ethylene Glycol)- and Poly(Phosphoester)-Coated Nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- La-Beck, N.M.; Gabizon, A.A. Nanoparticle Interactions with the Immune System: Clinical Implications for Liposome-Based Cancer Chemotherapy. Front. Immunol. 2017, 8, 416. [Google Scholar] [CrossRef]
- Chen, Z.-A.; Wu, C.-H.; Wu, S.-H.; Huang, C.-Y.; Mou, C.-Y.; Wei, K.-C.; Yen, Y.; Chien, I.-T.; Runa, S.; Chen, Y.-P.; et al. Receptor Ligand-Free Mesoporous Silica Nanoparticles: A Streamlined Strategy for Targeted Drug Delivery across the Blood–Brain Barrier. ACS Nano 2024, 18, 12716–12736. [Google Scholar] [CrossRef]
- Komatsu, A.; Higashi, Y.; Lin, C.-K.; Chen, Y.-P.; Wu, S.-H.; Suzuki, M.; Matsumoto, K.; Tamanoi, F. Accumulation of Small-Size, Highly Dispersive Mesoporous Silica Nanoparticles in a Tumor in Both Chorioallantoic Membrane and Mouse Models. Cells 2025, 14, 734. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, J.; Tang, L.; Ma, J.; Wang, Y.; Yang, H.; Wang, X.; Fan, W. Custom-Design of Multi-Stimuli-Responsive Degradable Silica Nanoparticles for Advanced Cancer-Specific Chemotherapy. Small 2024, 20, e2400353. [Google Scholar] [CrossRef]
- Díaz-Cervantes, E.; Navarro-García, N.E.; Rodríguez-González, V. Potential of Mesoporous Silica Nanoparticles for the Delivery of Anticancer Bioactive Compounds. Mater. Lett. 2024, 373, 137144. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef] [PubMed]
- Florek, J.; Caillard, R.; Kleitz, F. Evaluation of Mesoporous Silica Nanoparticles for Oral Drug Delivery—Current Status and Perspective of MSNs Drug Carriers. Nanoscale 2017, 9, 15252–15277. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Niu, Y.; Li, Y.; Gong, Y.; Shi, H.; Huo, Q.; Liu, Y.; Xu, Q. Stimuli-Responsive Delivery Vehicles Based on Mesoporous Silica Nanoparticles: Recent Advances and Challenges. J. Mater. Chem. B 2017, 5, 1339–1352. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2017, 23, 47. [Google Scholar] [CrossRef]
- Zhang, Y.; Hsu, B.Y.W.; Ren, C.; Li, X.; Wang, J. Silica-Based Nanocapsules: Synthesis, Structure Control and Biomedical Applications. Chem. Soc. Rev. 2015, 44, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of Silica Nanoparticles: An Update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef]
- Zou, H.; Ren, Y. Synthetic Strategies for Nonporous Organosilica Nanoparticles from Organosilanes. Nanoscale 2023, 15, 10484–10497. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Hu, X.-X.; Zhang, X.-B. Dye-Doped Fluorescent Silica Nanoparticles for Live Cell and In Vivo Bioimaging. Nanomaterials 2016, 6, 81. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid. Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Lee, M.H.; Beyer, F.L.; Furst, E.M. Synthesis of Monodisperse Fluorescent Core-Shell Silica Particles Using a Modified Stöber Method for Imaging Individual Particles in Dense Colloidal Suspensions. J. Colloid. Interface Sci. 2005, 288, 114–123. [Google Scholar] [CrossRef]
- Verhaegh, N.A.M.; van Blaaderen, A. Dispersions of Rhodamine-Labeled Silica Spheres: Synthesis, Characterization, and Fluorescence Confocal Scanning Laser Microscopy. Langmuir 1994, 10, 1427–1438. [Google Scholar] [CrossRef]
- Van Blaaderen, A.; Vrij, A. Synthesis and Characterization of Colloidal Dispersions of Fluorescent, Monodisperse Silica Spheres. Langmuir 1992, 8, 2921–2931. [Google Scholar] [CrossRef]
- Van Blaaderen, A.; Imhof, A.; Hage, W.; Vrij, A. Three-Dimensional Imaging of Submicrometer Colloidal Particles in Concentrated Suspensions Using Confocal Scanning Laser Microscopy. Langmuir 1992, 8, 1514–1517. [Google Scholar] [CrossRef]
- Guo, Y.; Gou, K.; Yang, B.; Wang, Y.; Pu, X.; Li, S.; Li, H. Enlarged Pore Size Chiral Mesoporous Silica Nanoparticles Loaded Poorly Water-Soluble Drug Perform Superior Delivery Effect. Molecules 2019, 24, 3552. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.H.; Blanford, C.F.; Stein, A. Synthesis of Ordered Microporous Silicates with Organosulfur Surface Groups and Their Applications as Solid Acid Catalysts. Chem. Mater. 1998, 10, 467–470. [Google Scholar] [CrossRef]
- Candela-Noguera, V.; Alfonso, M.; Amorós, P.; Aznar, E.; Marcos, M.D.; Martínez-Máñez, R. In-Depth Study of Factors Affecting the Formation of MCM-41-Type Mesoporous Silica Nanoparticles. Microporous Mesoporous Mater. 2024, 363, 112840. [Google Scholar] [CrossRef]
- Voss, R.; Brook, M.A.; Thompson, J.; Chen, Y.; Pelton, R.H.; Brennan, J.D. Non-Destructive Horseradish Peroxidase Immobilization in Porous Silica Nanoparticles. J. Mater. Chem. 2007, 17, 4854. [Google Scholar] [CrossRef]
- Santra, S.; Yang, H.; Dutta, D.; Stanley, J.T.; Holloway, P.H.; Tan, W.; Moudgil, B.M.; Mericle, R.A. TAT Conjugated, FITC Doped Silica Nanoparticles for Bioimaging Applications. Chem. Commun. 2004, 21, 2810–2811. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Schüth, F.; Lozano, D.; Colilla, M.; Manzano, M. Engineering Mesoporous Silica Nanoparticles for Drug Delivery: Where Are We after Two Decades? Chem. Soc. Rev. 2022, 51, 5365–5451. [Google Scholar] [CrossRef]
- Yoo, H.; Pak, J. Synthesis of Highly Fluorescent Silica Nanoparticles in a Reverse Microemulsion through Double-Layered Doping of Organic Fluorophores. J. Nanoparticle Res. 2013, 15, 1609. [Google Scholar] [CrossRef]
- Prasetyanto, E.A.; Bertucci, A.; Septiadi, D.; Corradini, R.; Castro-Hartmann, P.; De Cola, L. Breakable Hybrid Organosilica Nanocapsules for Protein Delivery. Angew. Chem. Int. Ed. 2016, 55, 3323–3327. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, H.; Ishikawa, T.; Kondo, S. Surface Characterization of Ultramicro Spherical Particles of Silica Prepared by w/o Microemulsion Method. Colloids Surf. 1989, 37, 71–80. [Google Scholar] [CrossRef]
- Osseo-Asare, K.; Arriagada, F.J. Preparation of SiO2 Nanoparticles in a Non-Ionic Reverse Micellar System. Colloids Surf. 1990, 50, 321–339. [Google Scholar] [CrossRef]
- Lindberg, R.; Sjöblom, J.; Sundholm, G. Preparation of Silica Particles Utilizing the Sol-Gel and the Emulsion-Gel Processes. Colloids Surf. A Physicochem. Eng. Asp. 1995, 99, 79–88. [Google Scholar] [CrossRef]
- Wang, L.; Tan, W. Multicolor FRET Silica Nanoparticles by Single Wavelength Excitation. Nano Lett. 2006, 6, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Tapec, R.; Theodoropoulou, N.; Dobson, J.; Hebard, A.; Tan, W. Synthesis and Characterization of Silica-Coated Iron Oxide Nanoparticles in Microemulsion: The Effect of Nonionic Surfactants. Langmuir 2001, 17, 2900–2906. [Google Scholar] [CrossRef]
- Santra, S.; Zhang, P.; Wang, K.; Tapec, R.; Tan, W. Conjugation of Biomolecules with Luminophore-Doped Silica Nanoparticles for Photostable Biomarkers. Anal. Chem. 2001, 73, 4988–4993. [Google Scholar] [CrossRef]
- Schmidt, J.; Guesdon, C.; Schomäcker, R. Engineering Aspects of Preparation of Nanocrystalline Particles in Microemulsions. J. Nanoparticle Res. 1999, 1, 267–276. [Google Scholar] [CrossRef]
- Travaglini, L.; Picchetti, P.; Totovao, R.; Prasetyanto, E.A.; De Cola, L. Highly Degradable Imine-Doped Mesoporous Silica Particles. Mater. Chem. Front. 2018, 3, 111–119. [Google Scholar] [CrossRef]
- Shao, D.; Li, M.; Wang, Z.; Zheng, X.; Lao, Y.-H.; Chang, Z.; Zhang, F.; Lu, M.; Yue, J.; Hu, H.; et al. Bioinspired Diselenide-Bridged Mesoporous Silica Nanoparticles for Dual-Responsive Protein Delivery. Adv. Mater. 2018, 30, 1801198. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, F.; Xu, N.; Yao, Q.; Wang, R.; Xie, X.; Zhang, F.; He, Y.; Shao, D.; Dong, W.-f.; et al. Red-Light-Triggered Self-Destructive Mesoporous Silica Nanoparticles for Cascade-Amplifying Chemo-Photodynamic Therapy Favoring Antitumor Immune Responses. Biomaterials 2022, 281, 121368. [Google Scholar] [CrossRef] [PubMed]
- Mai, N.X.D.; Nguyen, T.H.T.; Vong, L.B.; Dang, M.H.D.; Nguyen, T.T.T.; Nguyen, L.H.T.; Ta, H.K.T.; Nguyen, T.H.; Phan, T.B.; Doan, T.L.H. Tailoring Chemical Compositions of Biodegradable Mesoporous Organosilica Nanoparticles for Controlled Slow Release of Chemotherapeutic Drug. Mater. Sci. Eng. C 2021, 127, 112232. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Cattoën, X.; Durand, J.O.; Wong Chi Man, M.; Khashab, N.M. Organosilica Hybrid Nanomaterials with a High Organic Content: Syntheses and Applications of Silsesquioxanes. Nanoscale 2016, 8, 19945–19972. [Google Scholar] [CrossRef]
- Sanchez, C.; Shea, K.J.; Kitagawa, S.; Hu, L.-C. Organo—Silica Hybrid Functional Nanomaterials: How Do Organic Bridging Groups and Silsesquioxane Moieties Work Hand-in-Hand? Chem. Soc. Rev. 2011, 40, 688–695. [Google Scholar] [CrossRef]
- Song, Y.; Cheng, D.; Luo, J.; Zhang, M.; Yang, Y. Surfactant-Free Synthesis of Monodispersed Organosilica Particles with Pure Sulfide-Bridged Silsesquioxane Framework Chemistry via Extension of Stöber Method. J. Colloid. Interface Sci. 2021, 591, 129–138. [Google Scholar] [CrossRef]
- Yuan, P.; Mao, X.; Wu, X.; Liew, S.S.; Li, L.; Yao, S.Q. Mitochondria-Targeting, Intracellular Delivery of Native Proteins Using Biodegradable Silica Nanoparticles. Angew. Chem. 2019, 131, 7739–7743. [Google Scholar] [CrossRef]
- Fang, X.; Li, C.; Zheng, L.; Yang, F.; Chen, T. Dual-Targeted Selenium Nanoparticles for Synergistic Photothermal Therapy and Chemotherapy of Tumors. Chem. Asian J. 2018, 13, 996–1004. [Google Scholar] [CrossRef]
- Yuan, P.; Zhang, H.; Qian, L.; Mao, X.; Du, S.; Yu, C.; Peng, B.; Yao, S.Q. Intracellular Delivery of Functional Native Antibodies under Hypoxic Conditions by Using a Biodegradable Silica Nanoquencher. Angew. Chem. Int. Ed. 2017, 56, 12481–12485. [Google Scholar] [CrossRef]
- Yang, G.; Kim, S.; Oh, J.Y.; Kim, D.; Jin, S.; Choi, E.; Ryu, J.H. Surface Protein-Retractive and Redox-Degradable Mesoporous Organosilica Nanoparticles for Enhanced Cancer Therapy. J. Colloid. Interface Sci. 2023, 649, 1014–1022. [Google Scholar] [CrossRef]
- Ngo, W.; Ahmed, S.; Blackadar, C.; Bussin, B.; Ji, Q.; Mladjenovic, S.M.; Sepahi, Z.; Chan, W.C.W. Why Nanoparticles Prefer Liver Macrophage Cell Uptake In Vivo. Adv. Drug Deliv. Rev. 2022, 185, 114238. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Bertrand, N.; Zope, H.; Farokhzad, O.C. Emerging Understanding of the Protein Corona at the Nano-Bio Interfaces. Nano Today 2016, 11, 817–832. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation, Successful Approach to Drug Delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica Nanoparticles: Biomedical Applications and Toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef] [PubMed]
- Izak-Nau, E.; Kenesei, K.; Murali, K.; Voetz, M.; Eiden, S.; Puntes, V.F.; Duschl, A.; Madarász, E. Interaction of Differently Functionalized Fluorescent Silica Nanoparticles with Neural Stem- and Tissue-Type Cells. Nanotoxicology 2014, 8 (Suppl. S1), 138–148. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.Y.; Li, X.; Sun, M.Z.; Wang, Y.P.; Wu, M.; Zhang, C.Y.; Wang, Y.N.; Liu, B.; Zhang, Y.S.; Zhao, X.; et al. An Assessment of the Impact of SiO2 Nanoparticles of Different Sizes on the Rest/Wake Behavior and the Developmental Profile of Zebrafish Larvae. Small 2013, 9, 3161–3168. [Google Scholar] [CrossRef]
- Yang, X.; He, C.; Li, J.; Chen, H.; Ma, Q.; Sui, X.; Tian, S.; Ying, M.; Zhang, Q.; Luo, Y.; et al. Uptake of Silica Nanoparticles: Neurotoxicity and Alzheimer-like Pathology in Human SK-N-SH and Mouse Neuro2a Neuroblastoma Cells. Toxicol. Lett. 2014, 229, 240–249. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Wang, J.; Li, Y.; Li, Y.; Wei, J.; Zheng, T.; Jin, M.; Sun, Z. Silica Nanoparticles Induced Intrinsic Apoptosis in Neuroblastoma SH-SY5Y Cells via CytC/Apaf-1 Pathway. Environ. Toxicol. Pharmacol. 2017, 52, 161–169. [Google Scholar] [CrossRef]
- Chauhan, S.; Manivasagam, G.; Kumar, P.; Ambasta, R.K. Cellular Toxicity of Mesoporous Silica Nanoparticle in SHSY5Y and BM-MNCs Cell. Pharm. Nanotechnol. 2018, 6, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Jeong, J.; Yoon, D.; Kim, S.; Choi, J. Global Metabolomics Approach in in Vitro and in Vivo Models Reveals Hepatic Glutathione Depletion Induced by Amorphous Silica Nanoparticles. Chem. Biol. Interact. 2018, 293, 100–106. [Google Scholar] [CrossRef]
- Ahmad, J.; Ahamed, M.; Akhtar, M.J.; Alrokayan, S.A.; Siddiqui, M.A.; Musarrat, J.; Al-Khedhairy, A.A. Apoptosis Induction by Silica Nanoparticles Mediated through Reactive Oxygen Species in Human Liver Cell Line HepG2. Toxicol. Appl. Pharmacol. 2012, 259, 160–168. [Google Scholar] [CrossRef]
- Zuo, D.; Duan, Z.; Jia, Y.; Chu, T.; He, Q.; Yuan, J.; Dai, W.; Li, Z.; Xing, L.; Wu, Y. Amphipathic Silica Nanoparticles Induce Cytotoxicity through Oxidative Stress Mediated and P53 Dependent Apoptosis Pathway in Human Liver Cell Line HL-7702 and Rat Liver Cell Line BRL-3A. Colloids Surf. B Biointerfaces 2016, 145, 232–240. [Google Scholar] [CrossRef]
- Hui, Y.; Yi, X.; Wibowo, D.; Yang, G.; Middelberg, A.P.J.; Gao, H.; Zhao, C.-X. Nanoparticle Elasticity Regulates Phagocytosis and Cancer Cell Uptake. Sci. Adv. 2020, 6, eaaz4316. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef]
- Arnida, M.M.J.-A.; Ray, A.; Peterson, C.M.; Ghandehari, H. Geometry and Surface Characteristics of Gold Nanoparticles Influence Their Biodistribution and Uptake by Macrophages. Eur. J. Pharm. Biopharm. 2011, 77, 417–423. [Google Scholar] [CrossRef]
- Albayati, T.M.; Alardhi, S.M.; Khalbas, A.H.; Humdi, Z.J.; Ali, N.S.; Salih, I.K.; Saady, N.M.C.; Zendehboudi, S.; Abdulrahman, M.A. Comprehensive Review of Mesoporous Silica Nanoparticles: Drug Loading, Release, and Applications as Hemostatic Agents. ChemistrySelect 2024, 9, e202400450. [Google Scholar] [CrossRef]
- Clemments, A.M.; Muniesa, C.; Landry, C.C.; Botella, P. Effect of Surface Properties in Protein Corona Development on Mesoporous Silica Nanoparticles. RSC Adv. 2014, 4, 29134–29138. [Google Scholar] [CrossRef]
- Ferraz, N.; Hong, J.; Karlsson Ott, M. Procoagulant Behavior and Platelet Microparticle Generation on Nanoporous Alumina. J. Biomater. Appl. 2009, 24, 675–692. [Google Scholar] [CrossRef]
- Yu, T.; Greish, K.; McGill, L.D.; Ray, A.; Ghandehari, H. Influence of Geometry, Porosity, and Surface Characteristics of Silica Nanoparticles on Acute Toxicity: Their Vasculature Effect and Tolerance Threshold. ACS Nano 2012, 6, 2289–2301. [Google Scholar] [CrossRef]
- Chen, Y.; Ju, L.; Rushdi, M.; Ge, C.; Zhu, C. Receptor-Mediated Cell Mechanosensing. Mol. Biol. Cell 2017, 28, 3134–3155. [Google Scholar] [CrossRef]
- Tonbul, H.; Sahin, A.; Tavukcuoglu, E.; Ultav, G.; Akbas, S.; Aktas, Y.; Esendaglı, G.; Capan, Y. Folic Acid Decoration of Mesoporous Silica Nanoparticles to Increase Cellular Uptake and Cytotoxic Activity of Doxorubicin in Human Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2021, 63, 102535. [Google Scholar] [CrossRef]
- Souris, J.S.; Lee, C.H.; Cheng, S.H.; Chen, C.T.; Yang, C.S.; Ho, J.-a.A.; Mou, C.Y.; Lo, L.W. Surface Charge-Mediated Rapid Hepatobiliary Excretion of Mesoporous Silica Nanoparticles. Biomaterials 2010, 31, 5564–5574. [Google Scholar] [CrossRef]
- Scheffer, F.R.; Silveira, C.P.; Morais, J.; Bettini, J.; Cardoso, M.B. Tailoring Pseudo-Zwitterionic Bifunctionalized Silica Nanoparticles: From Colloidal Stability to Biological Interactions. Langmuir 2020, 36, 10756–10763. [Google Scholar] [CrossRef]
- Colilla, M.; Izquierdo-Barba, I.; Sánchez-Salcedo, S.; Fierro, J.L.G.; Hueso, J.L.; Vallet-Regí, M. Synthesis and Characterization of Zwitterionic SBA-15 Nanostructured Materials. Chem. Mater. 2010, 22, 6459–6466. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Chou, C.-M.; Chang, T.-Y.; Ting, H.; Dembélé, J.; Chu, Y.-T.; Liu, T.-P.; Changou, C.A.; Liu, C.-W.; Chen, C.-T. Bridging Size and Charge Effects of Mesoporous Silica Nanoparticles for Crossing the Blood–Brain Barrier. Front. Chem. 2022, 10, 931584. [Google Scholar] [CrossRef]
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, Shape, Charge and “Stealthy” Surface: Carrier Properties Affect the Drug Circulation Time in Vivo. Asian J. Pharm. Sci. 2021, 16, 444–458. [Google Scholar] [CrossRef]
- Xie, P.; Liu, P. PH-Responsive Surface Charge Reversal Carboxymethyl Chitosan-Based Drug Delivery System for PH and Reduction Dual-Responsive Triggered DOX Release. Carbohydr. Polym. 2020, 236, 116093. [Google Scholar] [CrossRef]
- Xu, C.; Song, R.; Lu, P.; Chen, J.; Zhou, Y.; Shen, G.; Jiang, M.; Zhang, W. A PH-Responsive Charge-Reversal Drug Delivery System with Tumor-Specific Drug Release and ROS Generation for Cancer Therapy. Int. J. Nanomed. 2020, 15, 65–80. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Wei, H.; Shan, X.; Wang, X.; Ou, M.; Liu, Q.; Gao, N.; Chen, H.; Mei, L.; et al. Charge-Reversal Biodegradable MSNs for Tumor Synergetic Chemo/Photothermal and Visualized Therapy. J. Control. Release 2021, 338, 719–730. [Google Scholar] [CrossRef]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, e1706759. [Google Scholar] [CrossRef]
- Fang, R.H.; Gao, W.; Zhang, L. Targeting Drugs to Tumours Using Cell Membrane-Coated Nanoparticles. Nat. Rev. Clin. Oncol. 2023, 20, 33–48. [Google Scholar] [CrossRef]
- Dumontel, B.; Conejo-Rodríguez, V.; Vallet-Regí, M.; Manzano, M. Natural Biopolymers as Smart Coating Materials of Mesoporous Silica Nanoparticles for Drug Delivery. Pharmaceutics 2023, 15, 447. [Google Scholar] [CrossRef] [PubMed]
- Colilla, M.; Vallet-Regí, M. Organically Modified Mesoporous Silica Nanoparticles against Bacterial Resistance. Chem. Mater. 2023, 35, 8788–8805. [Google Scholar] [CrossRef]
- Dumontel, B.; Jiménez-Jiménez, C.; Vallet-Regí, M.; Manzano, M. Bioinspired Extracellular Vesicle-Coated Silica Nanoparticles as Selective Delivery Systems. Mater. Today Bio 2023, 23, 100850. [Google Scholar] [CrossRef]
- Su, J.; Sun, H.; Meng, Q.; Zhang, P.; Yin, Q.; Li, Y. Enhanced Blood Suspensibility and Laser-Activated Tumor-Specific Drug Release of Theranostic Mesoporous Silica Nanoparticles by Functionalizing with Erythrocyte Membranes: Erratum. Theranostics 2020, 10, 2401. [Google Scholar] [CrossRef]
- Wang, J.; Pan, H.; Li, J.; Nie, D.; Zhuo, Y.; Lv, Y.; Wang, N.; Chen, H.; Guo, S.; Gan, Y.; et al. Cell Membrane-Coated Mesoporous Silica Nanorods Overcome Sequential Drug Delivery Barriers against Colorectal Cancer. Chin. Chem. Lett. 2023, 34, 107828. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Lei, W.; Yang, C.; Wu, Y.; Ru, G.; He, X.; Tong, X.; Wang, S. Nanocarriers Surface Engineered with Cell Membranes for Cancer Targeted Chemotherapy. J. Nanobiotechnol. 2022, 20, 45. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Li, Y.; Chang, J.; Tian, F.; Zhao, F.; Ma, Y.; Sun, J. Microfluidic Sonication to Assemble Exosome Membrane-Coated Nanoparticles for Immune Evasion-Mediated Targeting. Nano Lett. 2019, 19, 7836–7844. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Chen, X.; Liu, W.; Chen, T. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications. Nano-Micro Lett. 2019, 11, 1–46. [Google Scholar] [CrossRef]
- Sancho-Albero, M.; Decio, A.; Akpinar, R.; De Luigi, A.; Giavazzi, R.; Terracciano, L.M.; De Cola, L. Melanoma Extracellular Vesicles Membrane Coated Nanoparticles as Targeted Delivery Carriers for Tumor and Lungs. Mater. Today Bio 2025, 30, 101433. [Google Scholar] [CrossRef]
- Qiao, L.; Hu, S.; Huang, K.; Su, T.; Li, Z.; Vandergriff, A.; Cores, J.; Dinh, P.U.; Allen, T.; Shen, D.; et al. Tumor Cell-Derived Exosomes Home to Their Cells of Origin and Can Be Used as Trojan Horses to Deliver Cancer Drugs. Theranostics 2020, 10, 3474–3487. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.D.; Kim, H.Y.; Choi, Y.H.; Park, J.O.; Choi, E. Tumor-Derived Extracellular Vesicles for the Active Targeting and Effective Treatment of Colorectal Tumors in Vivo. Drug Deliv. 2022, 29, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor Exosome-Based Nanoparticles Are Efficient Drug Carriers for Chemotherapy. Nat. Commun. 2019, 10, 3838. [Google Scholar] [CrossRef]
- Song, W.; Jia, P.; Zhang, T.; Dou, K.; Liu, L.; Ren, Y.; Liu, F.; Xue, J.; Hasanin, M.S.; Qi, H.; et al. Cell Membrane-Camouflaged Inorganic Nanoparticles for Cancer Therapy. J. Nanobiotechnol. 2022, 20, 289. [Google Scholar] [CrossRef]
- Huang, P.; Lian, D.; Ma, H.; Gao, N.; Zhao, L.; Luan, P.; Zeng, X. New Advances in Gated Materials of Mesoporous Silica for Drug Controlled Release. Chin. Chem. Lett. 2021, 32, 3696–3704. [Google Scholar] [CrossRef]
- Shao, D.; Zhang, F.; Chen, F.; Zheng, X.; Hu, H.; Yang, C.; Tu, Z.; Wang, Z.; Chang, Z.; Lu, J.; et al. Biomimetic Diselenide-Bridged Mesoporous Organosilica Nanoparticles as an X-Ray-Responsive Biodegradable Carrier for Chemo-Immunotherapy. Adv. Mater. 2020, 32, 2004385. [Google Scholar] [CrossRef]
- Nie, D.; Dai, Z.; Li, J.; Yang, Y.; Xi, Z.; Wang, J.; Zhang, W.; Qian, K.; Guo, S.; Zhu, C.; et al. Cancer-Cell-Membrane-Coated Nanoparticles with a Yolk-Shell Structure Augment Cancer Chemotherapy. Nano Lett. 2020, 20, 936–946. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, Z.; Zheng, Y.; Song, J.; Li, J.; Zeng, Y.; Liu, X. Ultrasound-Driven Biomimetic Nanosystem Suppresses Tumor Growth and Metastasis through Sonodynamic Therapy, CO Therapy, and Indoleamine 2,3-Dioxygenase Inhibition. ACS Nano 2020, 14, 8985–8999. [Google Scholar] [CrossRef]
- Hadipour Moghaddam, S.P.; Yazdimamaghani, M.; Ghandehari, H. Glutathione-Sensitive Hollow Mesoporous Silica Nanoparticles for Controlled Drug Delivery. J. Control. Release 2018, 282, 62–75. [Google Scholar] [CrossRef]
- Lérida-Viso, A.; Estepa-Fernández, A.; García-Fernández, A.; Martí-Centelles, V.; Martínez-Máñez, R. Biosafety of Mesoporous Silica Nanoparticles; towards Clinical Translation. Adv. Drug Deliv. Rev. 2023, 201, 115049. [Google Scholar] [CrossRef] [PubMed]
- Khalaji, A.; Yancheshmeh, F.B.; Farham, F.; Khorram, A.; Sheshbolouki, S.; Zokaei, M.; Vatankhah, F.; Soleymani-Goloujeh, M. Don’t Eat Me/Eat Me Signals as a Novel Strategy in Cancer Immunotherapy. Heliyon 2023, 9, e20507. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing Nanoparticles with Biological Molecules: Developing Chemistries That Facilitate Nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef]
- Licciardello, N.; Hunoldt, S.; Bergmann, R.; Singh, G.; Mamat, C.; Faramus, A.; Ddungu, J.L.Z.; Silvestrini, S.; Maggini, M.; De Cola, L.; et al. Biodistribution Studies of Ultrasmall Silicon Nanoparticles and Carbon Dots in Experimental Rats and Tumor Mice. Nanoscale 2018, 10, 9880–9891. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, J.; Milosevic, A.; Hauser, D.; Lehner, R.; Blank, F.; Petri-Fink, A.; Rothen-Rutishauser, B. Biodistribution, Clearance, and Long-Term Fate of Clinically Relevant Nanomaterials. Adv. Mater. 2018, 30, e1704307. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Chen, X.; Fang, W.; Yu, K.; Gu, W.; Wei, Y.; Zheng, H.; Piao, J.; Li, F. Strategies to Regulate the Degradation and Clearance of Mesoporous Silica Nanoparticles: A Review. Int. J. Nanomed. 2024, 19, 5859–5878. [Google Scholar] [CrossRef]
- Hu, Y.; Bai, S.; Wu, X.; Tan, S.; He, Y. Biodegradability of Mesoporous Silica Nanoparticles. Ceram. Int. 2021, 47, 31031–31041. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Teng, X.; Huang, X.; Liu, H.; Chen, D.; Ren, J.; He, J.; Tang, F. Single and Repeated Dose Toxicity of Mesoporous Hollow Silica Nanoparticles in Intravenously Exposed Mice. Biomaterials 2011, 32, 1657–1668. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Liu, X.; Wang, J.; Li, S.; Gao, P. Review and Future Perspectives of Stimuli-Responsive Bridged Polysilsesquioxanes in Controlled Release Applications. Polymers 2024, 16, 3163. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Zhao, Y.; Sun, L. Fabrication of Biodegradable Mn-Doped Mesoporous Silica Nanoparticles for PH/Redox Dual Response Drug Delivery. J. Inorg. Biochem. 2020, 202, 110887. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lu, J.; Hou, J.; Zhao, Y. Fabrication of Hybrid Rod-like Mesoporous Silica Nanocarriers for Responsive Drug Release and Combined Photo-Chemotherapy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129227. [Google Scholar] [CrossRef]
- He, Y.; Shao, L.; Hu, Y.; Zhao, F.; Tan, S.; He, D.; Pan, A. Redox and PH Dual-Responsive Biodegradable Mesoporous Silica Nanoparticle as a Potential Drug Carrier for Synergistic Cancer Therapy. Ceram. Int. 2021, 47, 4572–4578. [Google Scholar] [CrossRef]
- Liu, L.; Kong, C.; Huo, M.; Liu, C.; Peng, L.; Zhao, T.; Wei, Y.; Qian, F.; Yuan, J. Schiff Base Interaction Tuned Mesoporous Organosilica Nanoplatforms with PH-Responsive Degradability for Efficient Anti-Cancer Drug Delivery in Vivo. Chem. Commun. 2018, 54, 9190–9193. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.S.; Bae, J.H.; Kim, M.J.; Kim, S.H.; Ha, C.S. Design of a Novel Mesoporous Organosilica Hybrid Microcarrier: A PH Stimuli-Responsive Dual-Drug-Delivery Vehicle for Intracellular Delivery of Anticancer Agents. Part. Part. Syst. Charact. 2013, 30, 1044–1055. [Google Scholar] [CrossRef]
- Chen, F.; Hong, H.; Goel, S.; Graves, S.A.; Orbay, H.; Ehlerding, E.B.; Shi, S.; Theuer, C.P.; Nickles, R.J.; Cai, W. In Vivo Tumor Vasculature Targeting of CuS@MSN Based Theranostic Nanomedicine. ACS Nano 2015, 9, 3926–3934. [Google Scholar] [CrossRef]
- Wu, M.; Meng, Q.; Chen, Y.; Xu, P.; Zhang, S.; Li, Y.; Zhang, L.; Wang, M.; Yao, H.; Shi, J. Ultrasmall Confined Iron Oxide Nanoparticle MSNs as a PH-Responsive Theranostic Platform. Adv. Funct. Mater. 2014, 24, 4273–4283. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Z.; Chen, Z.; Liu, X.; Hou, C.; Zhang, X.; Zhang, H. Fabrication of Single-Hole Glutathione-Responsive Degradable Hollow Silica Nanoparticles for Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 12600–12608. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, L.; Chang, Y.; Cao, Y.; Chen, C.; Wang, D. PH and Redox Dual-Responsive MSN-S-S-CS as a Drug Delivery System in Cancer Therapy. Materials 2020, 13, 1279. [Google Scholar] [CrossRef]
- Du, X.; Kleitz, F.; Li, X.; Huang, H.; Zhang, X.; Qiao, S. Disulfide-Bridged Organosilica Frameworks: Designed, Synthesis, Redox-Triggered Biodegradation, and Nanobiomedical Applications. Adv. Funct. Mater. 2018, 28, 1707325. [Google Scholar] [CrossRef]
- Wei, W.; Du, J.; Li, J.; Yan, M.; Zhu, Q.; Jin, X.; Zhu, X.; Hu, Z.; Tang, Y.; Lu, Y.; et al. Construction of Robust Enzyme Nanocapsules for Effective Organophosphate Decontamination, Detoxification, and Protection. Adv. Mater. 2013, 25, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yuan, S.; Qian, P.; Wu, Y. Enzymes in Nanomedicine for Anti-Tumor Therapy. Chem. Res. Chin. Univ. 2023, 39, 72–82. [Google Scholar] [CrossRef]
- Teng, Z.; Li, W.; Tang, Y.; Elzatahry, A.; Lu, G.; Zhao, D. Mesoporous Organosilica Hollow Nanoparticles: Synthesis and Applications. Adv. Mater. 2019, 31, 1707612. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, B.; Luo, Z.; Ding, X.; Li, J.; Dai, L.; Zhou, J.; Zhao, X.; Ye, J.; Cai, K. Enzyme Responsive Mesoporous Silica Nanoparticles for Targeted Tumor Therapy in Vitro and in Vivo. Nanoscale 2015, 7, 3614–3626. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, C.; Casanova, I.; Acosta, G.; Coll, C.; Moreno, M.J.; Albericio, F.; Aznar, E.; Mangues, R.; Royo, M.; Sancenón, F.; et al. Gated Mesoporous Silica Nanoparticles Using a Double-Role Circular Peptide for the Controlled and Target-Preferential Release of Doxorubicin in CXCR4-Expresing Lymphoma Cells. Adv. Funct. Mater. 2015, 25, 687–695. [Google Scholar] [CrossRef]
- Barros, M.; Sáez, J.A.; Arroyo, P.; Vicente Ros-Lis, J.; Dolores Garrido, M.; Martínez-Máñez, R.; Carmen Terencio, M.; Carmen Montesinos, M.; Gaviña, P. Nitroreductase-Responsive Gated Mesoporous Silica Nanocarriers for Hypoxia-Targeted Drug Delivery. Int. J. Pharm. 2025, 672, 125326. [Google Scholar] [CrossRef]
- Oroval, M.; Díez, P.; Aznar, E.; Coll, C.; Marcos, M.D.; Sancenón, F.; Villalonga, R.; Martínez-Máñez, R. Self-Regulated Glucose-Sensitive Neoglycoenzyme-Capped Mesoporous Silica Nanoparticles for Insulin Delivery. Chemistry 2017, 23, 1353–1360. [Google Scholar] [CrossRef]
- Yang, Y.; Wan, J.; Niu, Y.; Gu, Z.; Zhang, J.; Yu, M.; Yu, C. Structure-Dependent and Glutathione-Responsive Biodegradable Dendritic Mesoporous Organosilica Nanoparticles for Safe Protein Delivery. Chem. Mater. 2016, 28, 9008–9016. [Google Scholar] [CrossRef]
- Rizzi, F.; Fanizza, E.; Giancaspro, M.; Depalo, N.; Curri, M.L.; González, B.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous Silica Nanostructures Embedding NIR Active Plasmonic Nanoparticles: Harnessing Antimicrobial Agents Delivery System for Photo-Assisted Eradicating Gram-Positive Bacteria. Microporous Mesoporous Mater. 2025, 383, 113414. [Google Scholar] [CrossRef]
- Liu, J.-N.; Bu, W.-B.; Shi, J.-L. Silica Coated Upconversion Nanoparticles: A Versatile Platform for the Development of Efficient Theranostics. Acc. Chem. Res. 2015, 48, 1797–1805. [Google Scholar] [CrossRef]
- Vila, M.; Hueso, J.L.; Manzano, M.; Izquierdo-Barba, I.; de Andrés, A.; Sánchez-Marcos, J.; Prieto, C.; Vallet-Regí, M. Carbon Nanotubes—Mesoporous Silica Composites as Controllable Biomaterials. J. Mater. Chem. 2009, 19, 7745. [Google Scholar] [CrossRef]
- Li, B.; Harlepp, S.; Gensbittel, V.; Wells, C.J.R.; Bringel, O.; Goetz, J.G.; Begin-Colin, S.; Tasso, M.; Begin, D.; Mertz, D. Near Infra-Red Light Responsive Carbon Nanotubes@mesoporous Silica for Photothermia and Drug Delivery to Cancer Cells. Mater. Today Chem. 2020, 17, 100308. [Google Scholar] [CrossRef]
- Tam, V.; Picchetti, P.; Liu, Y.; Skripka, A.; Carofiglio, M.; Tamboia, G.; Bresci, A.; Manetti, F.; Cerullo, G.; Polli, D.; et al. Upconverting Nanoparticles Coated with Light-Breakable Mesoporous Silica for NIR-Triggered Release of Hydrophobic Molecules. ACS Appl. Mater. Interfaces 2024, 16, 29029–29041. [Google Scholar] [CrossRef]
- Picchetti, P.; Dimarco, B.N.; Travaglini, L.; Zhang, Y.; Ortega-Liebana, M.C.; De Cola, L. Breaking with Light: Stimuli-Responsive Mesoporous Organosilica Particles. Chem. Mater. 2020, 32, 392–399. [Google Scholar] [CrossRef]
- Entzian, K.; Aigner, A. Drug Delivery by Ultrasound-Responsive Nanocarriers for Cancer Treatment. Pharmaceutics 2021, 13, 1135. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Ultrasound Responsive Mesoporous Silica Nanoparticles for Biomedical Applications. Chem. Commun. 2019, 55, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Sun, Y.; Zheng, Y.; Zeng, D.; Li, F.; Zhang, S.; Wang, X.; Zhang, K.; Ma, M.; et al. Multifunctional Mesoporous Composite Nanocapsules for Highly Efficient MRI-Guided High-Intensity Focused Ultrasound Cancer Surgery. Angew. Chem. Int. Ed. 2011, 50, 12505–12509. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Xia, H. Ultrasound Reversible Response Nanocarrier Based on Sodium Alginate Modified Mesoporous Silica Nanoparticles. Front. Chem. 2019, 7, 59. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kang, J.-H.; Jang, J.; Lee, E.-J.; Kim, J.H.; Byun, J.; Shin, U.S. Dual Stimuli-Responsive Mesoporous Silica Nanoparticles for Efficient Loading and Smart Delivery of Doxorubicin to Cancer with RGD-Integrin Targeting. Eur. J. Pharm. Sci. 2023, 188, 106525. [Google Scholar] [CrossRef]
- Tieo, G.; Bao Ying Lim, N.; Lim, K.W.; Dröge, P.; Phan, A.T.; Jeitany, M. Targeting FANCM by Antisense Oligonucleotides in ALT-Positive Cancers. Mol. Ther. Nucleic Acids 2025, 36, 102492. [Google Scholar] [CrossRef]
- Xu, H.; Liao, C.; Liang, S.; Ye, B.C. A Novel Peptide-Equipped Exosomes Platform for Delivery of Antisense Oligonucleotides. ACS Appl. Mater. Interfaces 2021, 13, 10760–10767. [Google Scholar] [CrossRef] [PubMed]
- Adamus, T.; Hung, C.Y.; Yu, C.; Kang, E.; Hammad, M.; Flores, L.; Nechaev, S.; Zhang, Q.; Gonzaga, J.M.; Muthaiyah, K.; et al. Glioma-Targeted Delivery of Exosome-Encapsulated Antisense Oligonucleotides Using Neural Stem Cells. Mol. Ther. Nucleic Acids 2022, 27, 611–620. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Z.; Xie, Y.; Liu, Y.; Yang, K.; Li, T.; Shen, H.; Zhao, M.; Jin, J.; Xiao, H.; et al. Radiation-Triggered Selenium-Engineered Mesoporous Silica Nanocapsules for RNAi Therapy in Radiotherapy-Resistant Glioblastoma. ACS Nano 2023, 17, 4062–4076. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Q.; Jia, L.; Du, X.; Hanagata, N. A Facilely Controlled Length, Cytotoxicity, Length-Dependent and Cell Type-Dependent Cellular Uptake of Silica Nanotubes and Their Applications in the Delivery of Immunostimulatory CpG Oligodeoxynucleotides. J. Mater. Chem. B 2015, 3, 7246–7254. [Google Scholar] [CrossRef]
- Zhu, M.; Ding, X.; Zhao, R.; Liu, X.; Shen, H.; Cai, C.; Ferrari, M.; Wang, H.Y.; Wang, R.F. Co-Delivery of Tumor Antigen and Dual Toll-like Receptor Ligands into Dendritic Cell by Silicon Microparticle Enables Efficient Immunotherapy against Melanoma. J. Control. Release 2018, 272, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Hatami, H.; Rahiman, N.; Mohammadi, M. Oligonucleotide Based Nanogels for Cancer Therapeutics. Int. J. Biol. Macromol. 2024, 267, 131401. [Google Scholar] [CrossRef] [PubMed]
- Ménard, M.; Meyer, F.; Parkhomenko, K.; Leuvrey, C.; Francius, G.; Bégin-Colin, S.; Mertz, D. Mesoporous Silica Templated-Albumin Nanoparticles with High Doxorubicin Payload for Drug Delivery Assessed with a 3-D Tumor Cell Model. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2019, 1863, 332–341. [Google Scholar] [CrossRef]
- Kothalawala, S.G.; Jiao, J.; Speight, R.; Song, H.; Yang, Y.; Zhang, J. Pore Architecture Influences the Enzyme Immobilization Performance of Mesoporous Silica Nanospheres. Microporous Mesoporous Mater. 2022, 338, 111963. [Google Scholar] [CrossRef]
- Ochsner, M. Photophysical and Photobiological Processes in the Photodynamic Therapy of Tumours. J. Photochem. Photobiol. B 1997, 39, 1–18. [Google Scholar] [CrossRef]
- Liu, R.; Gao, Y.; Liu, N.; Suo, Y. Nanoparticles Loading Porphyrin Sensitizers in Improvement of Photodynamic Therapy for Ovarian Cancer. Photodiagn. Photodyn. Ther. 2021, 33, 102156. [Google Scholar] [CrossRef]
- Borzęcka, W.; Pereira, P.M.R.; Fernandes, R.; Trindade, T.; Torres, T.; Tomé, J.P.C. Encapsulation of Glycosylated Porphyrins in Silica Nanoparticles to Enhance the Efficacy of Cancer Photodynamic Therapy. Mater. Adv. 2021, 2, 1613–1620. [Google Scholar] [CrossRef]
- Mehraban, N.; Freeman, H. Developments in PDT Sensitizers for Increased Selectivity and Singlet Oxygen Production. Materials 2015, 8, 4421–4456. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W. Nanoparticle-Based Targeted Drug Delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, K.; Zhang, L.; Madajewski, B.; Zanzonico, P.; Sequeira, S.; Gonen, M.; Wiesner, U.; Bradbury, M.S. Target-or-Clear Zirconium-89 Labeled Silica Nanoparticles for Enhanced Cancer-Directed Uptake in Melanoma: A Comparison of Radiolabeling Strategies. Chem. Mater. 2017, 29, 8269–8281. [Google Scholar] [CrossRef]
- Kohle, F.F.E.; Li, S.; Turker, M.Z.; Wiesner, U.B. Ultrasmall PEGylated and Targeted Core–Shell Silica Nanoparticles Carrying Methylene Blue Photosensitizer. ACS Biomater. Sci. Eng. 2020, 6, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Kuang, G.; Zhang, Q.; He, S.; Liu, Y. Curcumin-Loaded PEGylated Mesoporous Silica Nanoparticles for Effective Photodynamic Therapy. RSC Adv. 2020, 10, 24624–24630. [Google Scholar] [CrossRef]
- Spreckelmeyer, S.; Orvig, C.; Casini, A. Cellular Transport Mechanisms of Cytotoxic Metallodrugs: An Overview beyond Cisplatin. Molecules 2014, 19, 15584–15610. [Google Scholar] [CrossRef]
- Wani, W.A.; Prashar, S.; Shreaz, S.; Gómez-Ruiz, S. Nanostructured Materials Functionalized with Metal Complexes: In Search of Alternatives for Administering Anticancer Metallodrugs. Coord. Chem. Rev. 2016, 312, 67–98. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; García-Peñas, A.; Prashar, S.; Rodríguez-Diéguez, A.; Fischer-Fodor, E. Anticancer Applications of Nanostructured Silica-Based Materials Functionalized with Titanocene Derivatives: Induction of Cell Death Mechanism through TNFR1 Modulation. Materials 2018, 11, 224. [Google Scholar] [CrossRef]
- Rubio-Ruiz, B.; Pérez-López, A.M.; Uson, L.; Ortega-Liebana, M.C.; Valero, T.; Arruebo, M.; Hueso, J.L.; Sebastian, V.; Santamaria, J.; Unciti-Broceta, A. In Cellulo Bioorthogonal Catalysis by Encapsulated AuPd Nanoalloys: Overcoming Intracellular Deactivation. Nano Lett. 2023, 23, 804–811. [Google Scholar] [CrossRef]
- Karges, J.; Díaz-García, D.; Prashar, S.; Gómez-Ruiz, S.; Gasser, G. Ru(II) Polypyridine Complex-Functionalized Mesoporous Silica Nanoparticles as Photosensitizers for Cancer Targeted Photodynamic Therapy. ACS Appl. Bio Mater. 2021, 4, 4394–4405. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, W.; Tan, W. Bioconjugated Silica Nanoparticles: Development and Applications. Nano Res. 2008, 1, 99–115. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Multifunctional Mesoporous Silica Nanoplatform Based on Silicon Nanoparticles for Targeted Two-Photon-Excited Fluorescence Imaging-Guided Chemo/Photodynamic Synergetic Therapy in Vitro. Talanta 2020, 209, 120552. [Google Scholar] [CrossRef]
- Hameed, S.; Bhattarai, P.; Gong, Z.; Liang, X.; Yue, X.; Dai, Z. Ultrasmall Porphyrin-Silica Core–Shell Dots for Enhanced Fluorescence Imaging-Guided Cancer Photodynamic Therapy. Nanoscale Adv. 2023, 5, 277–289. [Google Scholar] [CrossRef]
- Makhadmeh, G.N.; Abdul Aziz, A. Photodynamic Application of Protoporphyrin IX as a Photosensitizer Encapsulated by Silica Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Peiro, J.I.; Bonet-Aleta, J.; Hueso, J.L. Copper-Based Nanoplatforms and Their Role in Cancer Therapy. Coord. Chem. Rev. 2025, 534, 216542. [Google Scholar] [CrossRef]
- Bonet-Aleta, J.; Calzada-Funes, J.; Hueso, J.L. Recent Developments of Iron-Based Nanosystems as Enzyme-Mimicking Surrogates of Interest in Tumor Microenvironment Treatment. In Nanomaterials for Biocatalysis; Elsevier: Amsterdam, The Netherlands, 2022; pp. 237–265. [Google Scholar] [CrossRef]
- Bonet-Aleta, J.; Sancho-Albero, M.; Calzada-Funes, J.; Irusta, S.; Martin-Duque, P.; Hueso, J.L.; Santamaria, J. Glutathione-Triggered Catalytic Response of Copper-Iron Mixed Oxide Nanoparticles. Leveraging Tumor Microenvironment Conditions for Chemodynamic Therapy. J. Colloid. Interface Sci. 2022, 617, 704–717. [Google Scholar] [CrossRef]
- Sanchez-Uriel, L.; Bonet-Aleta, J.; Ibarra, A.; Hueso, J.L. Heterogeneous-Driven Glutathione Oxidation: Defining the Catalytic Role of Chalcopyrite Nanoparticles. J. Phys. Chem. C 2023, 127, 14146–14154. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, X.; Lin, W.; Chen, L.; Wu, Z.S. Persistent Targeting DNA Nanocarrier Made of 3D Structural Unit Assembled from Only One Basic Multi-Palindromic Oligonucleotide for Precise Gene Cancer Therapy. Adv. Healthc. Mater. 2024, 13, 2303865. [Google Scholar] [CrossRef]
- Yan, J.; Bhadane, R.; Ran, M.; Ma, X.; Li, Y.; Zheng, D.; Salo-Ahen, O.M.H.; Zhang, H. Development of Aptamer-DNAzyme Based Metal-Nucleic Acid Frameworks for Gastric Cancer Therapy. Nat. Commun. 2024, 15, 3684. [Google Scholar] [CrossRef]
- Ngamcherdtrakul, W.; Reda, M.; Nelson, M.A.; Wang, R.; Zaidan, H.Y.; Bejan, D.S.; Ha Hoang, N.; Lane, R.S.; Luoh, S.-W.; Leachman, S.A.; et al. In Situ Tumor Vaccination with Nanoparticle Co-Delivering CpG and STAT3 SiRNA to Effectively Induce Whole-Body Antitumor Immune Response. Adv. Mater. 2021, 33, 2100628. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wen, S.; Zhang, Y.; Sun, Z. Organosilane and Polyethylene Glycol Functionalized Magnetic Mesoporous Silica Nanoparticles as Carriers for CpG Immunotherapy In Vitro and In Vivo. PLoS ONE 2015, 10, e0140265. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yao, H.; Yi, K.; Lao, Y.H.; Shao, D.; Tao, Y. Emerging Nanoparticle Platforms for CpG Oligonucleotide Delivery. Biomater. Sci. 2024, 12, 2203–2228. [Google Scholar] [CrossRef]
- Liu, X.; Wu, F.; Ji, Y.; Yin, L. Recent Advances in Anti-Cancer Protein/Peptide Delivery. Bioconjug. Chem. 2019, 30, 305–324. [Google Scholar] [CrossRef]
- Omar, H.; Croissant, J.G.; Alamoudi, K.; Alsaiari, S.; Alradwan, I.; Majrashi, M.A.; Anjum, D.H.; Martins, P.; Laamarti, R.; Eppinger, J.; et al. Biodegradable Magnetic Silica@Iron Oxide Nanovectors with Ultra-Large Mesopores for High Protein Loading, Magnetothermal Release, and Delivery. J. Control. Release 2017, 259, 187–194. [Google Scholar] [CrossRef]
- Du, W.; Du, S.; Dong, X.; Bai, H.; Jiang, J.; Hao, S.; Yang, F.; Xiao, Q.; Zhang, B.; Ge, J.; et al. Biodegradable Silica Nanocapsules Enable Efficient Nuclear-Targeted Delivery of Native Proteins for Cancer Therapy. Biomaterials 2023, 294, 122000. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chang, C.; Liu, Z.; Zhao, Q.; Xu, Q.; Li, C.; Chen, Y.; Zhang, Y.; Lu, B. Hyaluronic Acid-Functionalized Hollow Mesoporous Silica Nanoparticles as PH-Sensitive Nanocarriers for Cancer Chemo-Photodynamic Therapy. Langmuir 2021, 37, 2619–2628. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Katsumiti, A.; Cajaraville, M.P.; Agarrabeitia, A.R.; Ortiz, M.J.; Martínez-Martínez, V. Functionalization of Photosensitized Silica Nanoparticles for Advanced Photodynamic Therapy of Cancer. Int. J. Mol. Sci. 2021, 22, 6618. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, N.; Chen, L.; Xie, Z. Reduction Responsive BODIPY Decorated Mesoporous Silica Nanoscale Platforms for Photodynamic Therapy. Microporous Mesoporous Mater. 2021, 311, 110689. [Google Scholar] [CrossRef]
- Chen, K.; Chang, C.; Liu, Z.; Zhou, Y.; Xu, Q.; Li, C.; Huang, Z.; Xu, H.; Xu, P.; Lu, B. Hyaluronic Acid Targeted and PH-Responsive Nanocarriers Based on Hollow Mesoporous Silica Nanoparticles for Chemo-Photodynamic Combination Therapy. Colloids Surf. B Biointerfaces 2020, 194, 111166. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Arbeloa, T.; Martínez-Martínez, V. Photosensitizer-Mesoporous Silica Nanoparticles Combination for Enhanced Photodynamic Therapy. Photochem. Photobiol. 2023, 99, 882–900. [Google Scholar] [CrossRef] [PubMed]
- Makhadmeh, G.N.; Abuelsamen, A.; Al-Akhras, M.A.H.; Aziz, A.A. Silica Nanoparticles Encapsulated Cichorium Pumilum as a Promising Photosensitizer for Osteosarcoma Photodynamic Therapy: In-Vitro Study. Photodiagn. Photodyn. Ther. 2022, 38, 102801. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yang, Y.; Lu, J.; Lin, Y.; Feng, S.; Luo, X.; Di, D.; Wang, S.; Zhao, Q. Recent Trends of Mesoporous Silica-Based Nanoplatforms for Nanodynamic Therapies. Coord. Chem. Rev. 2022, 469, 214687. [Google Scholar] [CrossRef]
- Garcia-Peiro, J.I.; Bonet-Aleta, J.; Tamayo-Fraile, M.L.; Hueso, J.L.; Santamaria, J. Platinum-Based Nanodendrites as Glucose Oxidase-Mimicking Surrogates. Nanoscale 2023, 15, 14399–14408. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Peiro, J.I.; Bonet-Aleta, J.; Santamaria, J.; Hueso, J.L. Platinum Nanoplatforms: Classic Catalysts Claiming a Prominent Role in Cancer Therapy. Chem. Soc. Rev. 2022, 51, 7662–7681. [Google Scholar] [CrossRef]
- Ortega-Liebana, M.C.; Bonet-Aleta, J.; Hueso, J.L.; Santamaria, J. Gold-Based Nanoparticles on Amino-Functionalized Mesoporous Silica Supports as Nanozymes for Glucose Oxidation. Catalysts 2020, 10, 333. [Google Scholar] [CrossRef]
- Bonet-Aleta, J.; Garcia-Peiro, J.I.; Hueso, J.L. Engineered Nanostructured Photocatalysts for Cancer Therapy. Catalysts 2022, 12, 167. [Google Scholar] [CrossRef]
- Rosenberg, B.; VanCamp, L. The Successful Regression of Large Solid Sarcoma 180 Tumors by Platinum Compounds. Cancer Res. 1970, 30, 1799–1802. [Google Scholar]
- Ndagi, U.; Mhlongo, N.; Soliman, M. Metal Complexes in Cancer Therapy – an Update from Drug Design Perspective. Drug Des. Devel Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef]
- Ellahioui, Y.; Prashar, S.; Gómez-Ruiz, S. A Short Overview on the Biomedical Applications of Silica, Alumina and Calcium Phosphate-Based Nanostructured Materials. Curr. Med. Chem. 2016, 23, 4450–4467. [Google Scholar] [CrossRef]
- Estevão, B.M.; Vilela, R.R.C.; Geremias, I.P.; Zanoni, K.P.S.; de Camargo, A.S.S.; Zucolotto, V. Mesoporous Silica Nanoparticles Incorporated with Ir(III) Complexes: From Photophysics to Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2022, 40, 103052. [Google Scholar] [CrossRef] [PubMed]
- Pham, X.H.; Tran, V.K.; Hahm, E.; Kim, Y.H.; Kim, J.; Kim, W.; Jun, B.H. Synthesis of Gold-Platinum Core-Shell Nanoparticles Assembled on a Silica Template and Their Peroxidase Nanozyme Properties. Int. J. Mol. Sci. 2022, 23, 6424. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Noh, M.S.; Kim, Y.H.; Namgung, J.; Yoo, K.; Shin, M.S.; Yang, C.H.; Kim, Y.J.; Yu, S.J.; Chang, H.; et al. Recent Studies on Metal-Embedded Silica Nanoparticles for Biological Applications. Nanomaterials 2024, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Seong, B.; Kim, J.; Kim, W.; Lee, S.; Pham, X.H.; Jun, B.H. Synthesis of Finely Controllable Sizes of Au Nanoparticles on a Silica Template and Their Nanozyme Properties. Int. J. Mol. Sci. 2021, 22, 10382. [Google Scholar] [CrossRef]
- Pham, X.H.; Seong, B.; Bock, S.; Hahm, E.; Huynh, K.H.; Kim, Y.H.; Kim, W.; Kim, J.; Kim, D.E.; Jun, B.H. Nonenzymatic Hydrogen Peroxide Detection Using Surface-Enhanced Raman Scattering of Gold–Silver Core–Shell-Assembled Silica Nanostructures. Nanomaterials 2021, 11, 2748. [Google Scholar] [CrossRef]
- Kang, H.; Jeong, S.; Yang, J.K.; Jo, A.; Lee, H.; Heo, E.H.; Jeong, D.H.; Jun, B.H.; Chang, H.; Lee, Y.S. Template-Assisted Plasmonic Nanogap Shells for Highly Enhanced Detection of Cancer Biomarkers. Int. J. Mol. Sci. 2021, 22, 1752. [Google Scholar] [CrossRef]
- Li, T.; He, F.; Liu, B.; Jia, T.; Shao, B.; Zhao, R.; Zhu, H.; Yang, D.; Gai, S.; Yang, P. In Situ Synthesis of FeOCl in Hollow Dendritic Mesoporous Organosilicon for Ascorbic Acid-Enhanced and MR Imaging-Guided Chemodynamic Therapy in Neutral PH Conditions. ACS Appl. Mater. Interfaces 2020, 12, 56886–56897. [Google Scholar] [CrossRef]
- Bonet-Aleta, J.; Calzada-Funes, J.; Hueso, J.L. Manganese Oxide Nano-Platforms in Cancer Therapy: Recent Advances on the Development of Synergistic Strategies Targeting the Tumor Microenvironment. Appl. Mater. Today 2022, 29, 101628. [Google Scholar] [CrossRef]
- Bonet-Aleta, J.; Garcia-Peiro, J.I.; Irusta, S.; Hueso, J.L. Gold-Platinum Nanoparticles with Core-Shell Configuration as Efficient Oxidase-like Nanosensors for Glutathione Detection. Nanomaterials 2022, 12, 755. [Google Scholar] [CrossRef]
- Bonet-Aleta, J.; Hueso, J.L.; Sanchez-Uriel, L.; Encinas-Gimenez, M.; Irusta, S.; Martin-Duque, P.; Martinez, G.; Santamaria, J. Synergistic Assembly of Gold and Copper-Iron Oxide Nanocatalysts to Promote the Simultaneous Depletion of Glucose and Glutathione. Mater. Today Chem. 2023, 29, 101404. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, Y.; Bao, Q.; Shen, C.; Ni, D.; Hu, P.; Shi, J. Endogenous Copper for Nanocatalytic Oxidative Damage and Self-Protection Pathway Breakage of Cancer. ACS Nano 2021, 15, 16286–16297. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, Y.; Ye, J.; Li, C.; Wang, Q.; Liu, M.; Cui, Y.; Wang, C.; Jin, G.; Fu, Y.; et al. Targeted Delivery of Active Sites by Oxygen Vacancy-Engineered Bimetal Silicate Nanozymes for Intratumoral Aggregation-Potentiated Catalytic Therapy. ACS Nano 2024, 18, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- Begum, G.; Swathi, P.; Bandarapu, A.K.; Nayak, J.; Rana, R.K. Spatial Confinement of Enzyme and Nanozyme in Silica-Based Hollow Microreactors. ACS Appl. Mater. Interfaces 2020, 12, 45476–45484. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Xie, W.; Luo, X.; Zou, G.; Mo, Q.; Zhong, W. Manganese-Doped Stellate Mesoporous Silica Nanoparticles: A Bifunctional Nanoplatform for Enhanced Chemodynamic Therapy and Tumor Imaging. Microporous Mesoporous Mater. 2024, 370, 113012. [Google Scholar] [CrossRef]
- Porrang, S.; Davaran, S.; Rahemi, N.; Allahyari, S.; Mostafavi, E. How Advancing Are Mesoporous Silica Nanoparticles? A Comprehensive Review of the Literature. Int. J. Nanomed. 2022, 17, 1803–1827. [Google Scholar] [CrossRef]
- Gabizon, A.A.; Gabizon-Peretz, S.; Modaresahmadi, S.; La-Beck, N.M. Thirty Years from FDA Approval of Pegylated Liposomal Doxorubicin (Doxil/Caelyx): An Updated Analysis and Future Perspective. BMJ Oncol. 2025, 4, 573. [Google Scholar] [CrossRef]
| Therapy | Cargo | Texture | NP Size (nm) | Pore Size (nm) | Pore Volume (cm3/g) | Surface Area (m2/g) | References |
|---|---|---|---|---|---|---|---|
| Oligo-nucleotides | RNAi | Mesoporous | 70 | 6 nm | - | - | [150] |
| CpG | Mesoporous | 400–1850 | 2–10 | - | - | [151,152,153,154] | |
| Protein-based | HSA-DOX | Mesoporous | 100 | <1 | 1.62 | 708 | [155] |
| Protein | Capsule | 50 | - | - | - | [156] | |
| Phytase/Lipase | Dendritic mesoporous | 140 | 8 | ≤1 | 342–399 | [157] | |
| Photosensitizer | Silica as Carrier | Core–shell | <10 | - | - | - | [158] |
| TMPyP | Mesoporous | 90 | 2.1 | 0.28 | 223 | [159] | |
| BODYPYs | Mesoporous | 50, 80 | <6 | - | <300 | [160,161] | |
| ICG | Mesoporous | 100 | 2–5 | - | 83–981 | [119] | |
| Carrier | Proto-porphyrin | Non-porous | <10, 60–270 | - | - | - | [162,163,164,165] |
| Cichorium pumilum | Non-porous | 25 | - | - | - | [166] | |
| Metal-based | Ru | Mesoporous | 200 | <3 | 0.7 | 1045 | [167] |
| Ir | Mesoporous | 150 | - | - | - | [168] | |
| Au@Pt Au@Ag Au@Au | Non-porous | 150 Si, 12 Ag, <3 Au | - | - | - | [169,170,171,172] | |
| CDT/PDT | DOX-ICG-DA-HA | Hollow mesoporous | 120–270 | <3 | <2 | 28 | [173,174] |
| Curcumin | Mesoporous | <200 | <3.5 | - | 813 | [175] | |
| FeOCl | Hollow dendritic mesoporous | 125 | 10–22 | - | 29 | [176] | |
| TPEN | Mesoporous | 40–50 | - | <1 | 75–450 | [177] | |
| OFeCaSA-V@GA | Mesoporous | 130 | 10–20 | <1 | - | [178] | |
| Mn | Mesoporous | 80–100 | - | - | - | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosseri, A.; Sanchez-Uriel, L.; Garcia-Peiro, J.I.; Hornos, F.; Hueso, J.L. A Review of Silica-Based Nanoplatforms for Anticancer Cargo Delivery. Int. J. Mol. Sci. 2025, 26, 5850. https://doi.org/10.3390/ijms26125850
Mosseri A, Sanchez-Uriel L, Garcia-Peiro JI, Hornos F, Hueso JL. A Review of Silica-Based Nanoplatforms for Anticancer Cargo Delivery. International Journal of Molecular Sciences. 2025; 26(12):5850. https://doi.org/10.3390/ijms26125850
Chicago/Turabian StyleMosseri, Andrea, Leticia Sanchez-Uriel, Jose I. Garcia-Peiro, Felipe Hornos, and Jose L. Hueso. 2025. "A Review of Silica-Based Nanoplatforms for Anticancer Cargo Delivery" International Journal of Molecular Sciences 26, no. 12: 5850. https://doi.org/10.3390/ijms26125850
APA StyleMosseri, A., Sanchez-Uriel, L., Garcia-Peiro, J. I., Hornos, F., & Hueso, J. L. (2025). A Review of Silica-Based Nanoplatforms for Anticancer Cargo Delivery. International Journal of Molecular Sciences, 26(12), 5850. https://doi.org/10.3390/ijms26125850







