Composite Polylactide/Polycaprolactone Foams with Hierarchical Porous Structure for Pre-Vascularized Tissue Engineering
Abstract
1. Introduction
2. Results and Discussions
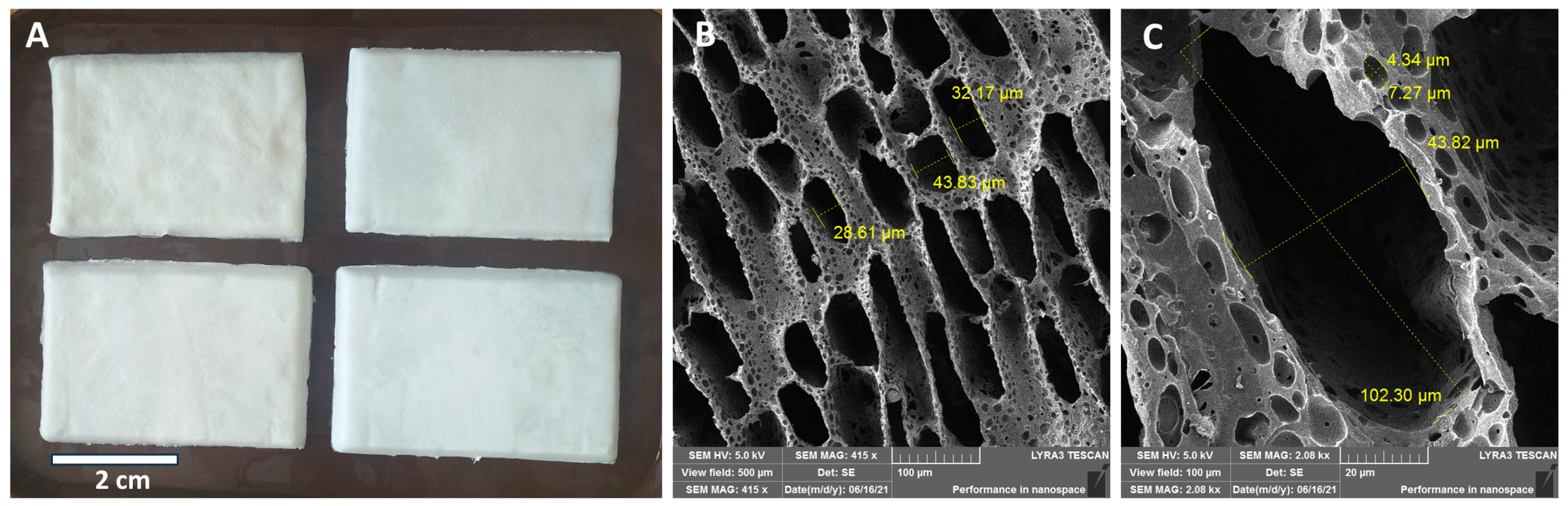
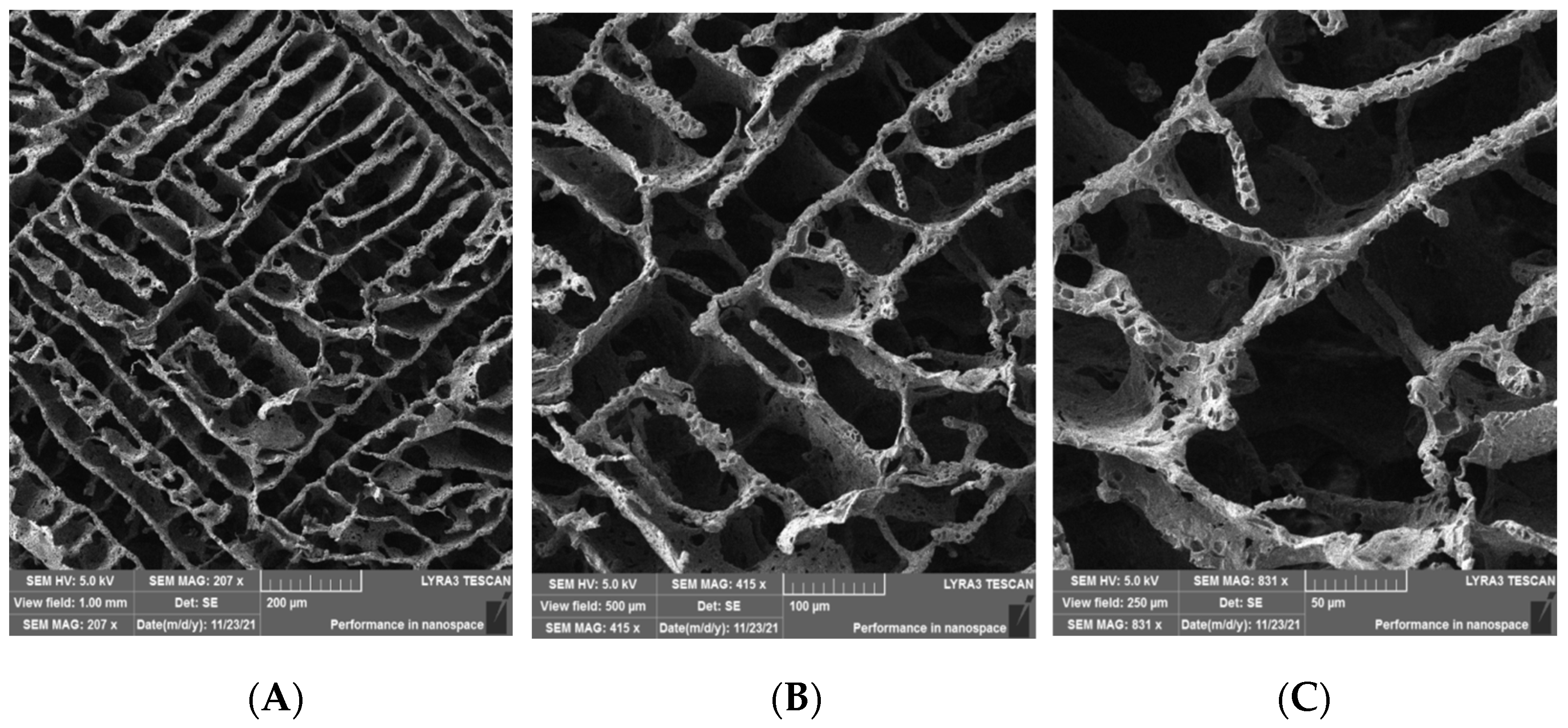
2.1. Scanning Electron Microscopy (SEM) Morphology of the Scaffolds
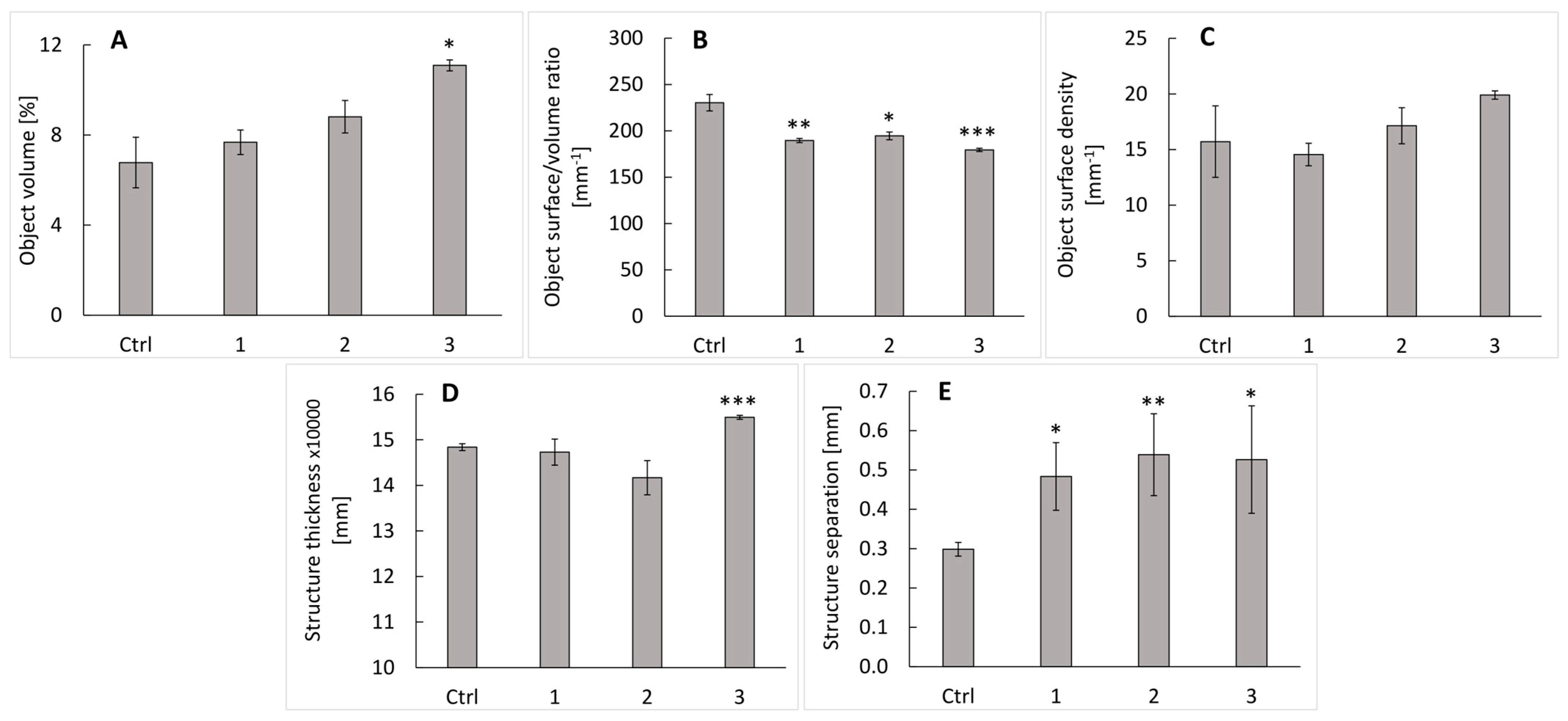
2.2. Micro-CT Analysis of the Scaffolds
2.3. Brunauer–Emmett–Teller (BET) Analysis of the Scaffolds
2.4. Water Uptake by the Scaffolds
2.5. Fourier-Transform Infrared Spectroscopy (FTIR) Characterization of the Scaffolds
2.6. X-Ray Diffraction (XRD) Analysis of the Scaffolds
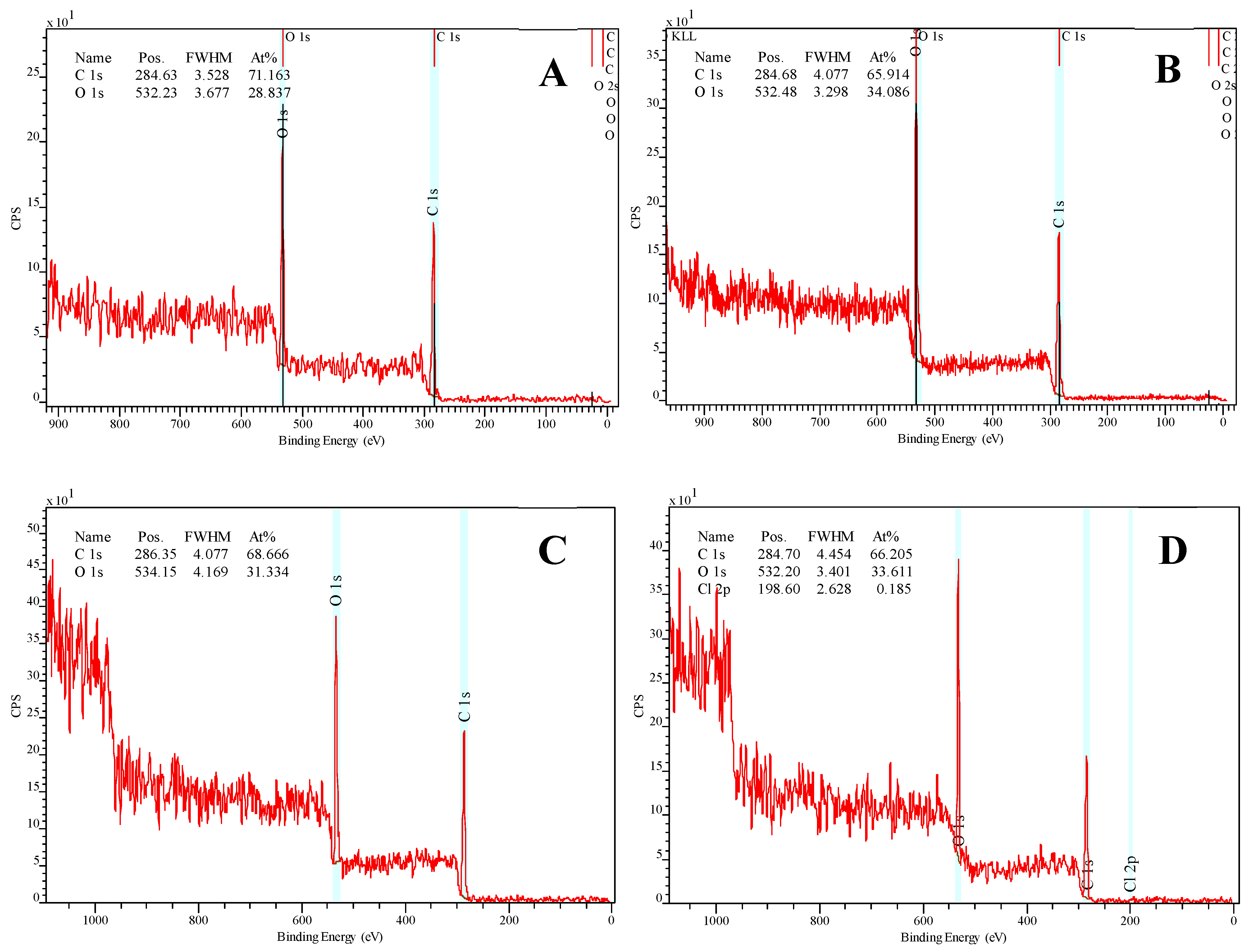
2.7. X-Ray Photoelectron Microscopy (XPS)
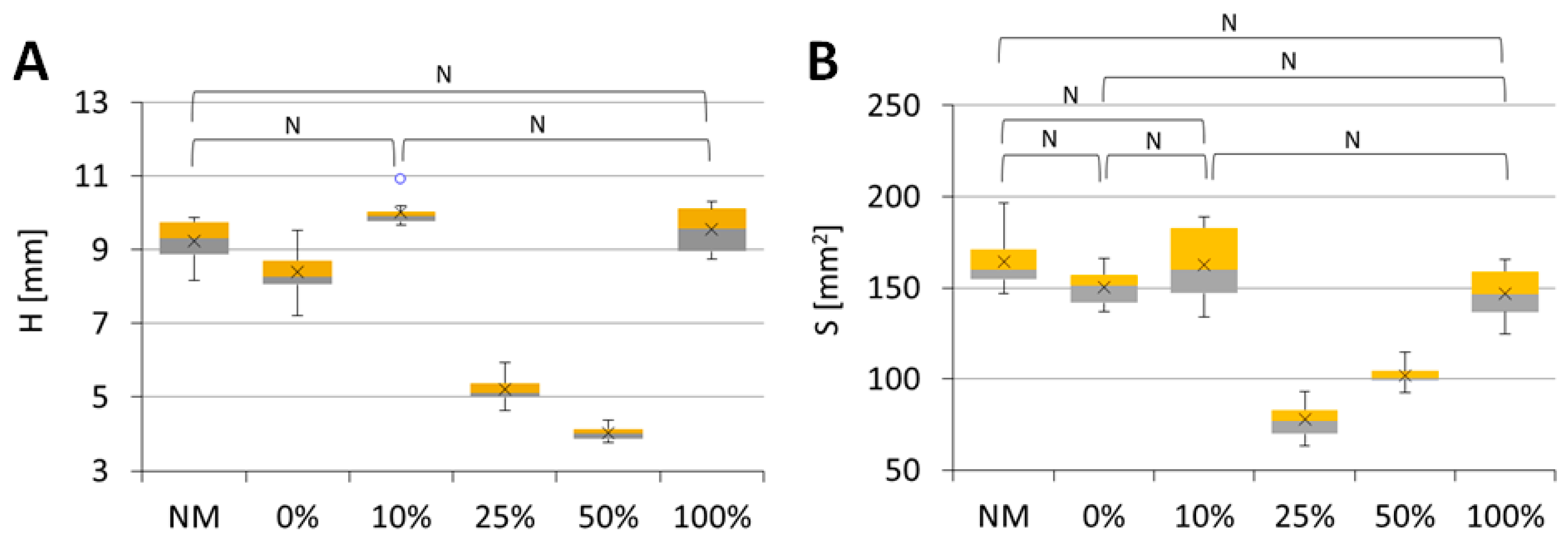
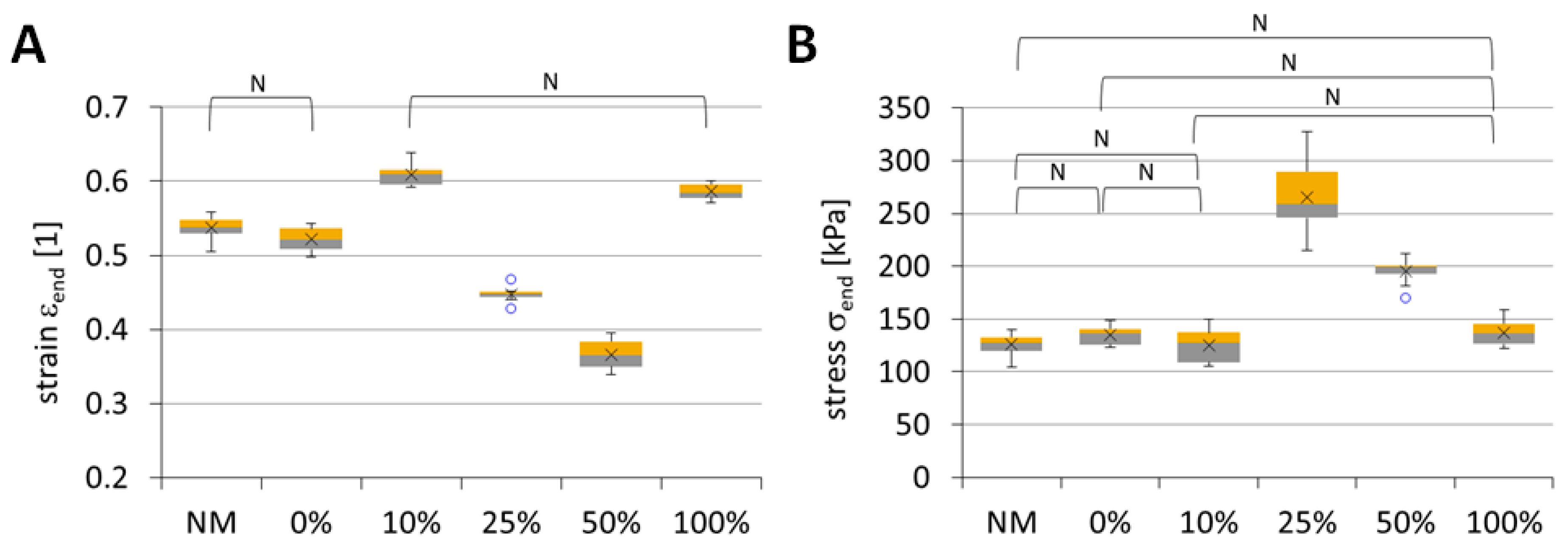
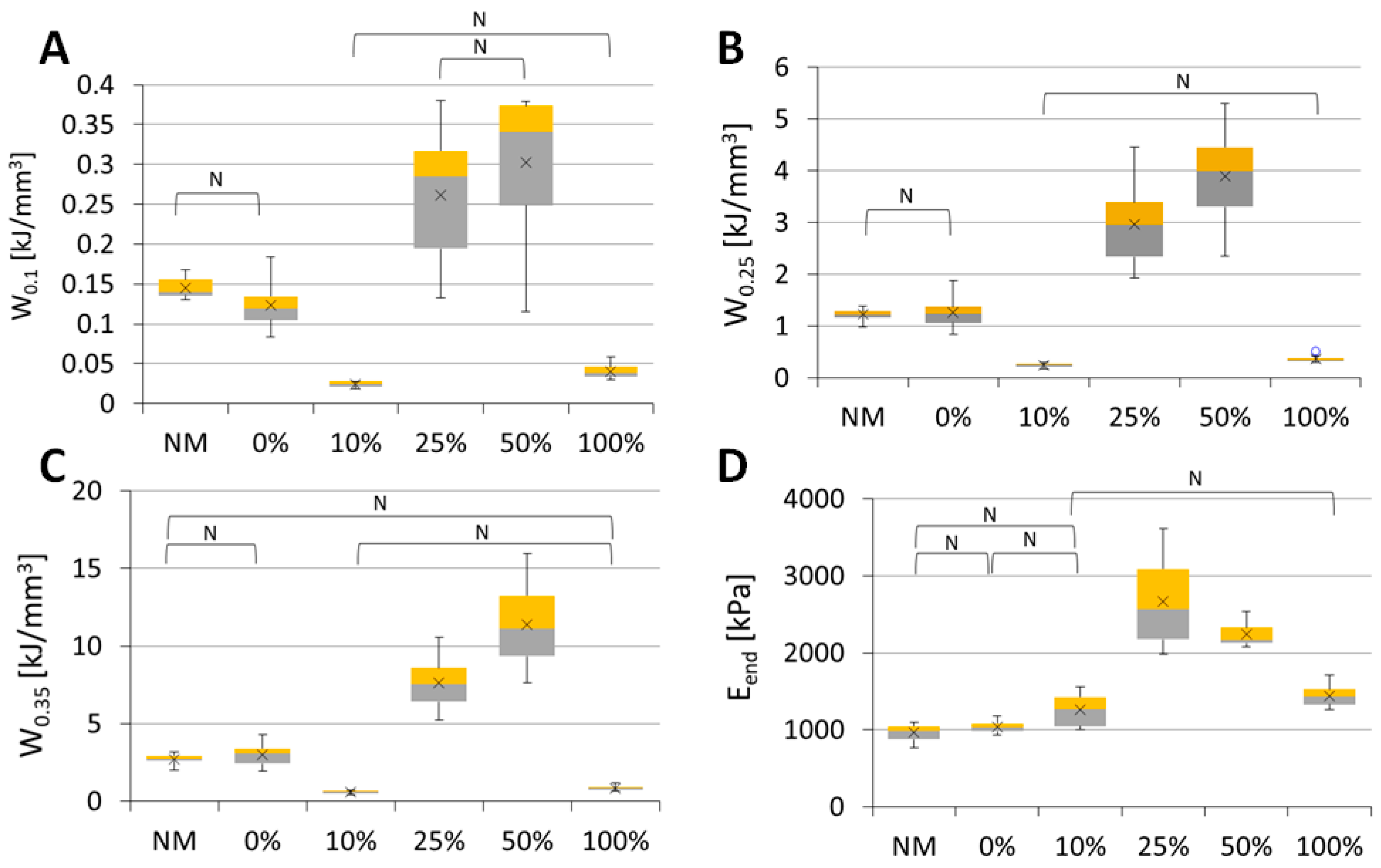
2.8. Mechanical Testing of the Scaffolds
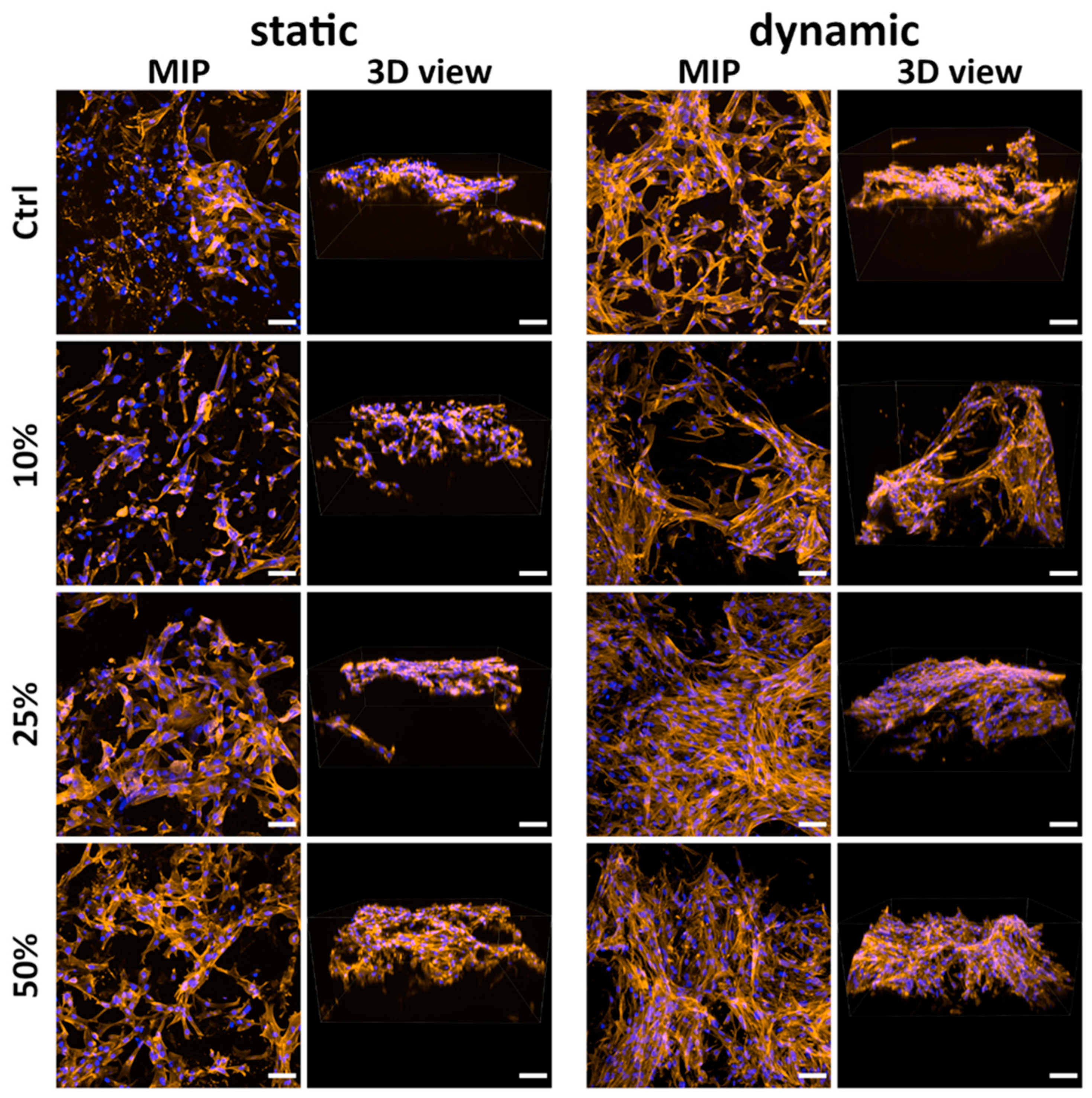
2.9. Cell Growth on the Scaffolds
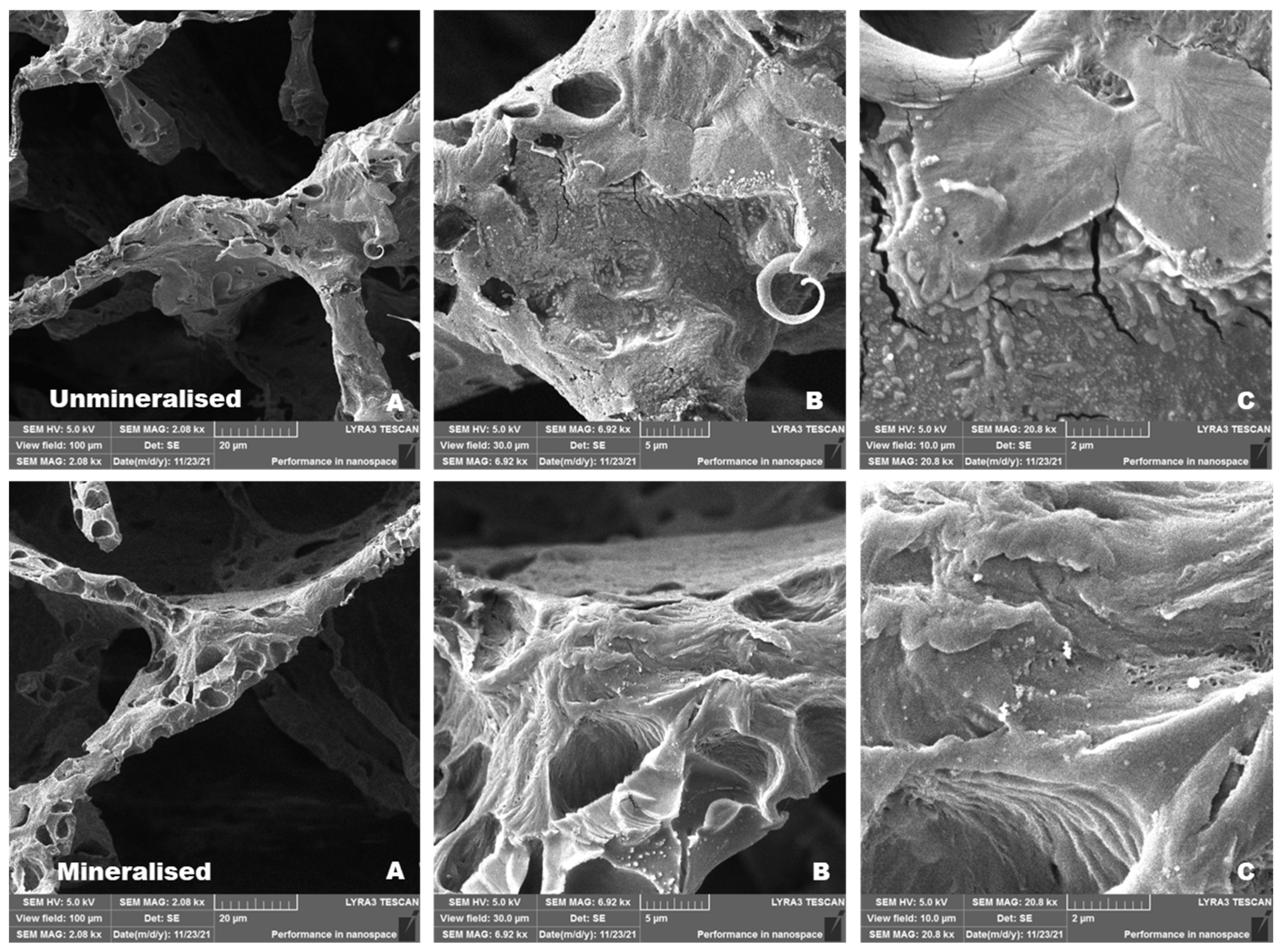
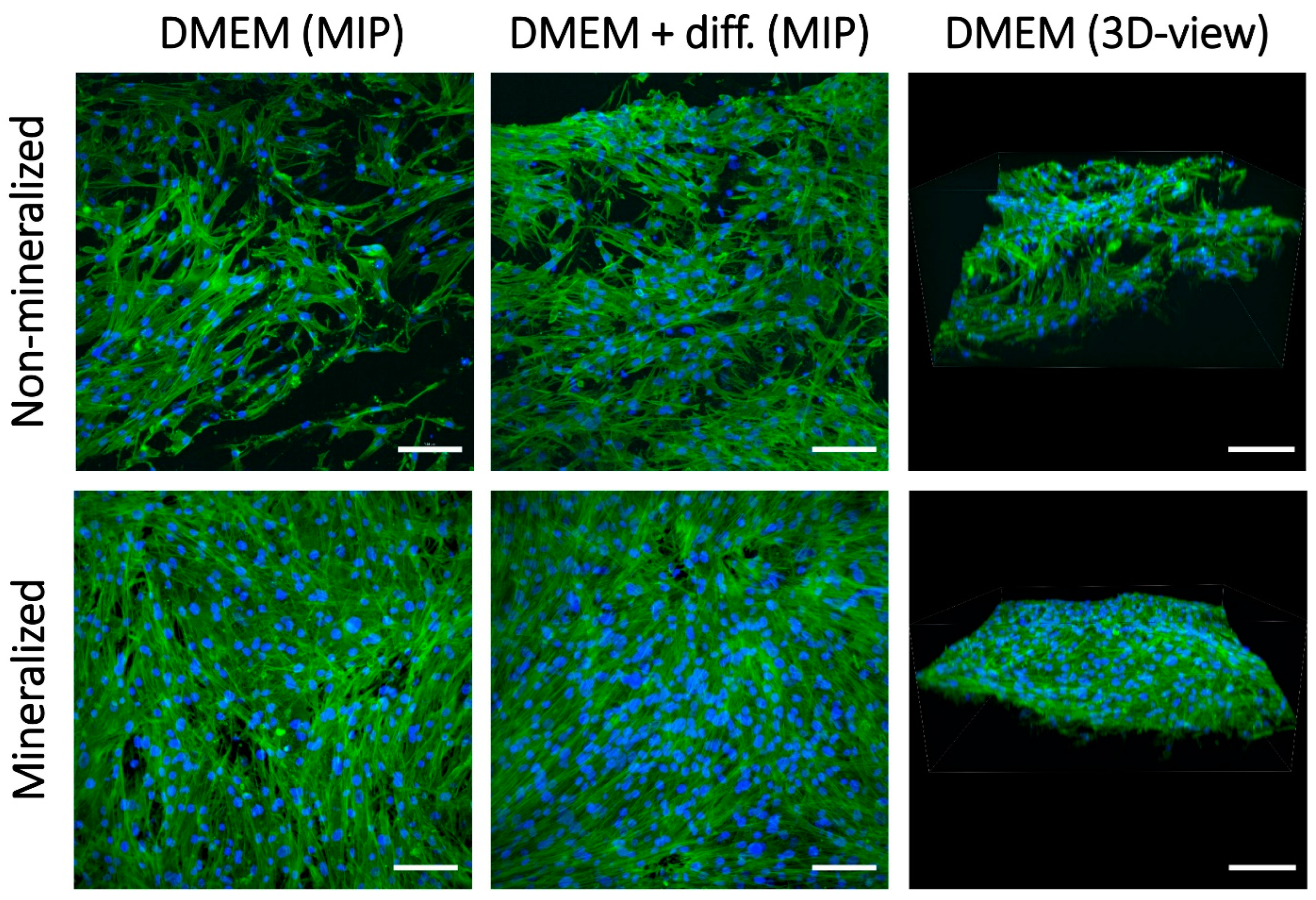
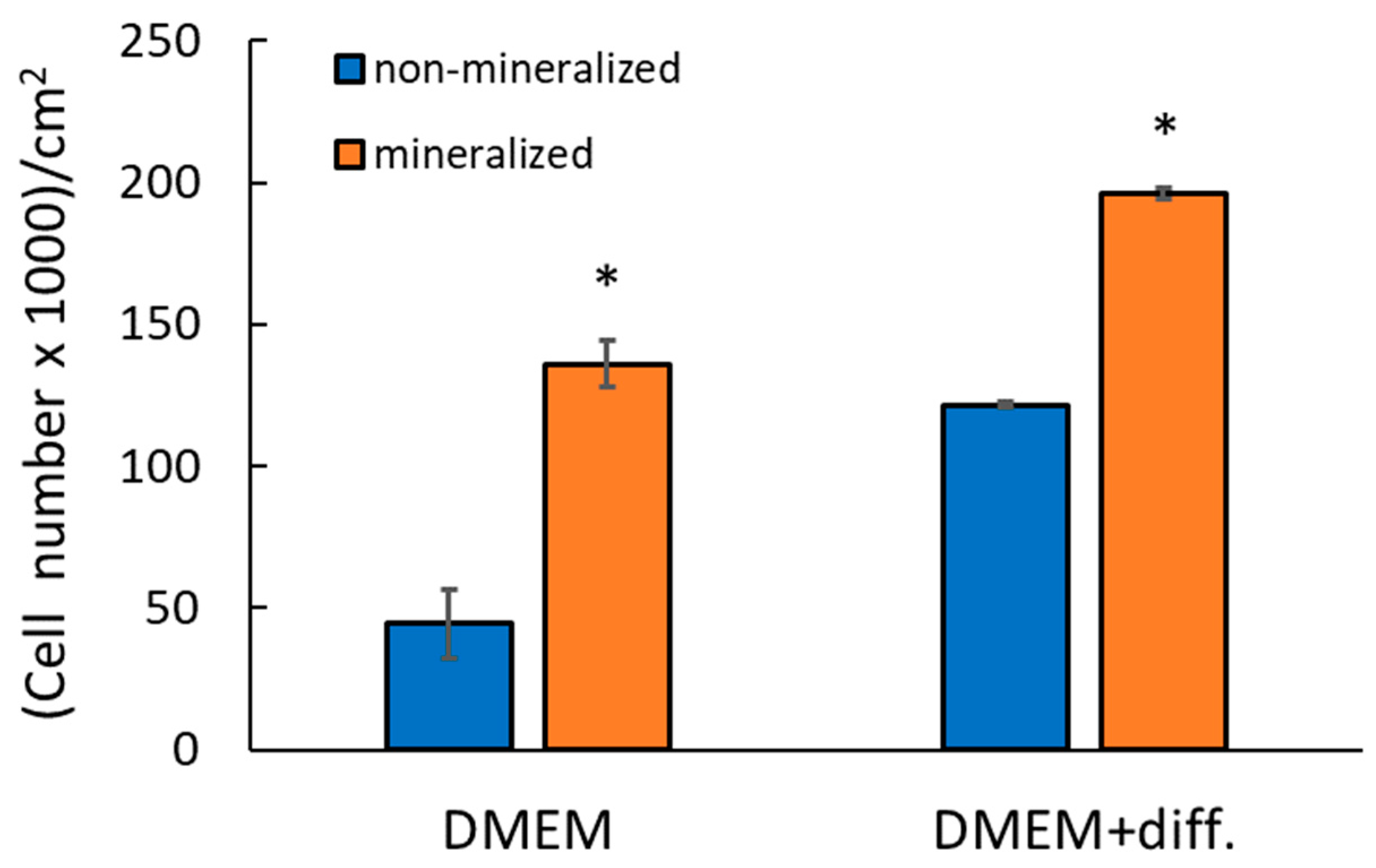
2.9.1. Comparison of Non-Mineralized and Mineralized Scaffolds Prepared Without Klucel
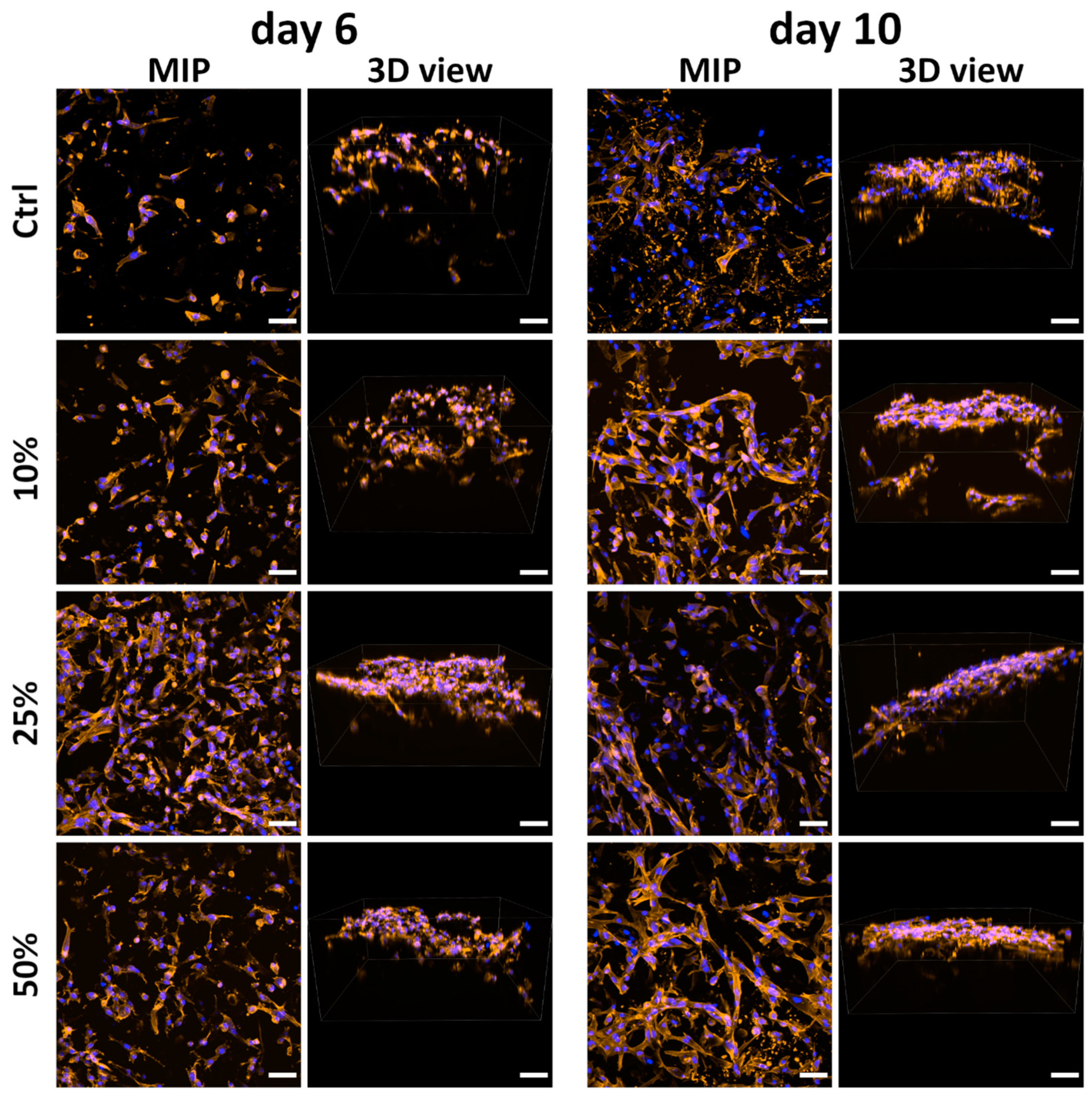
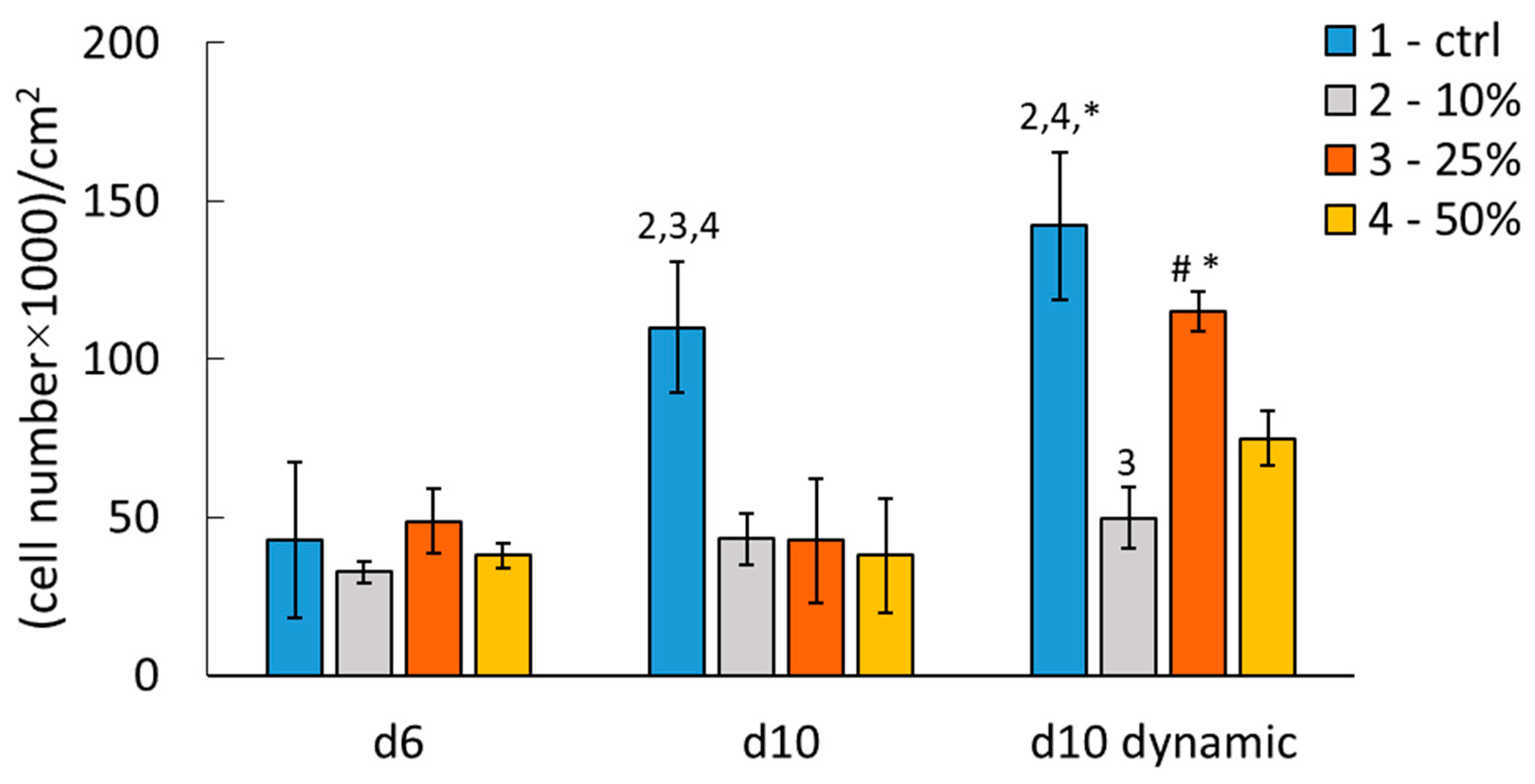
2.9.2. Comparison of Mineralized Scaffolds Prepared Without and with Klucel
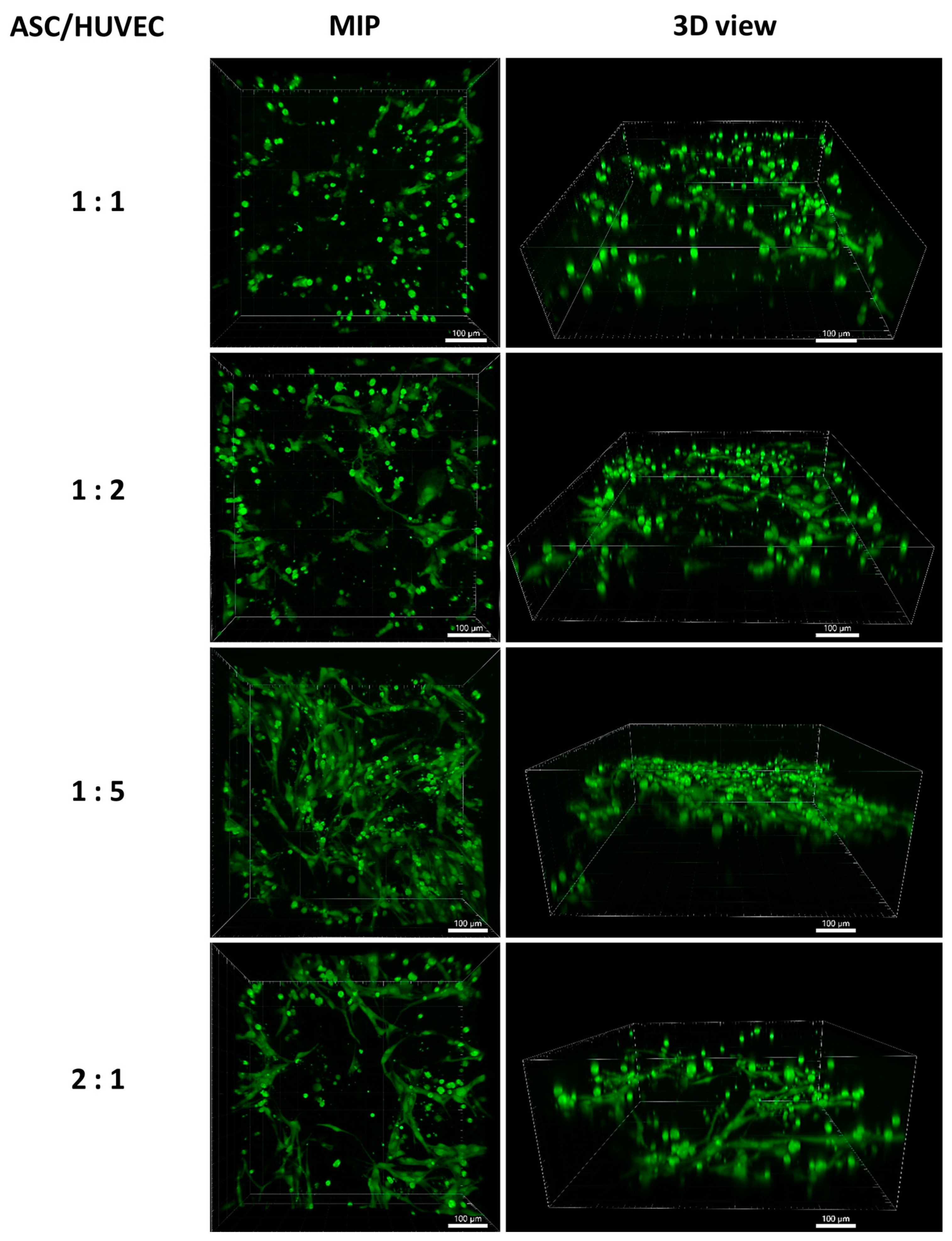
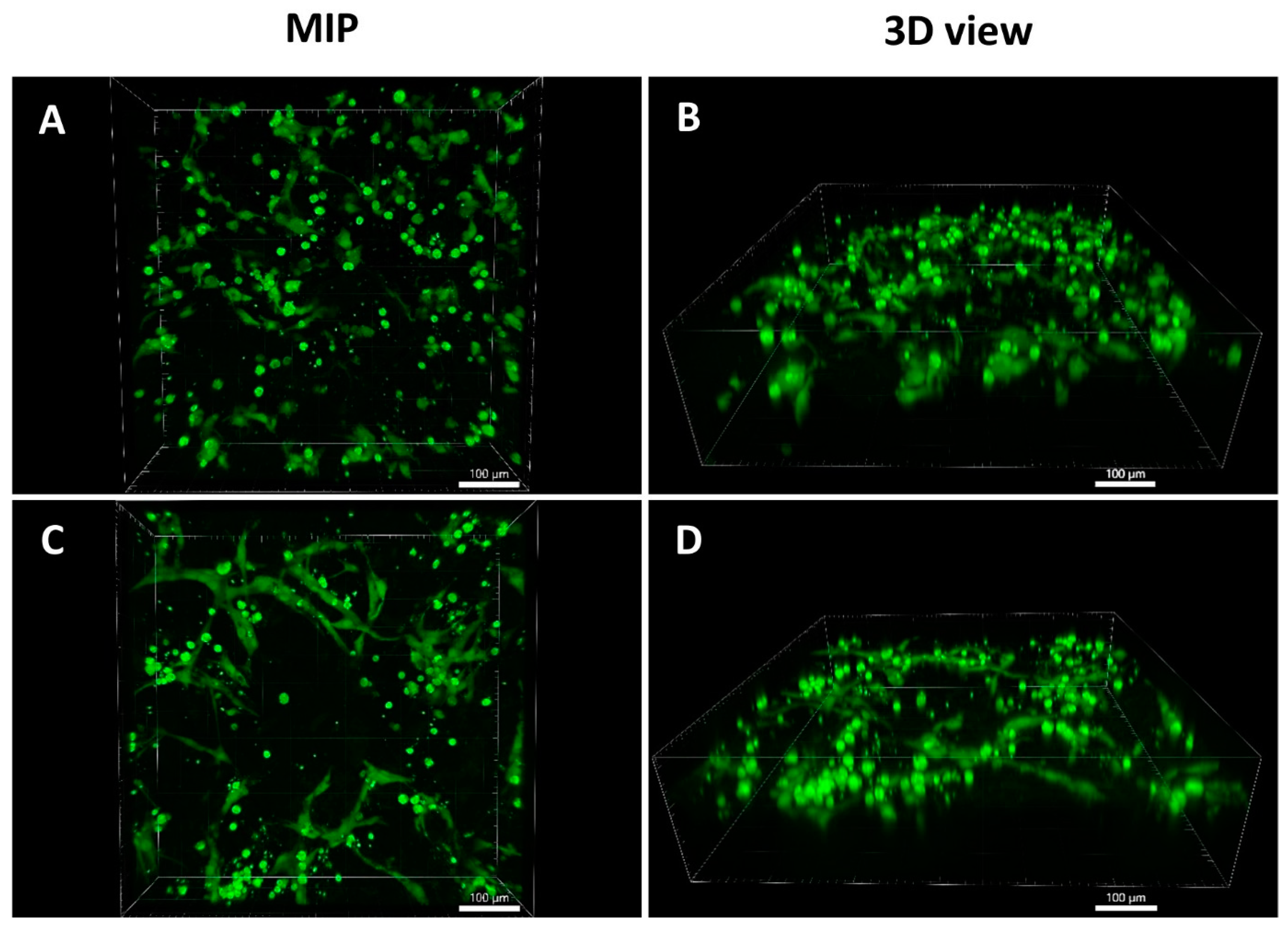
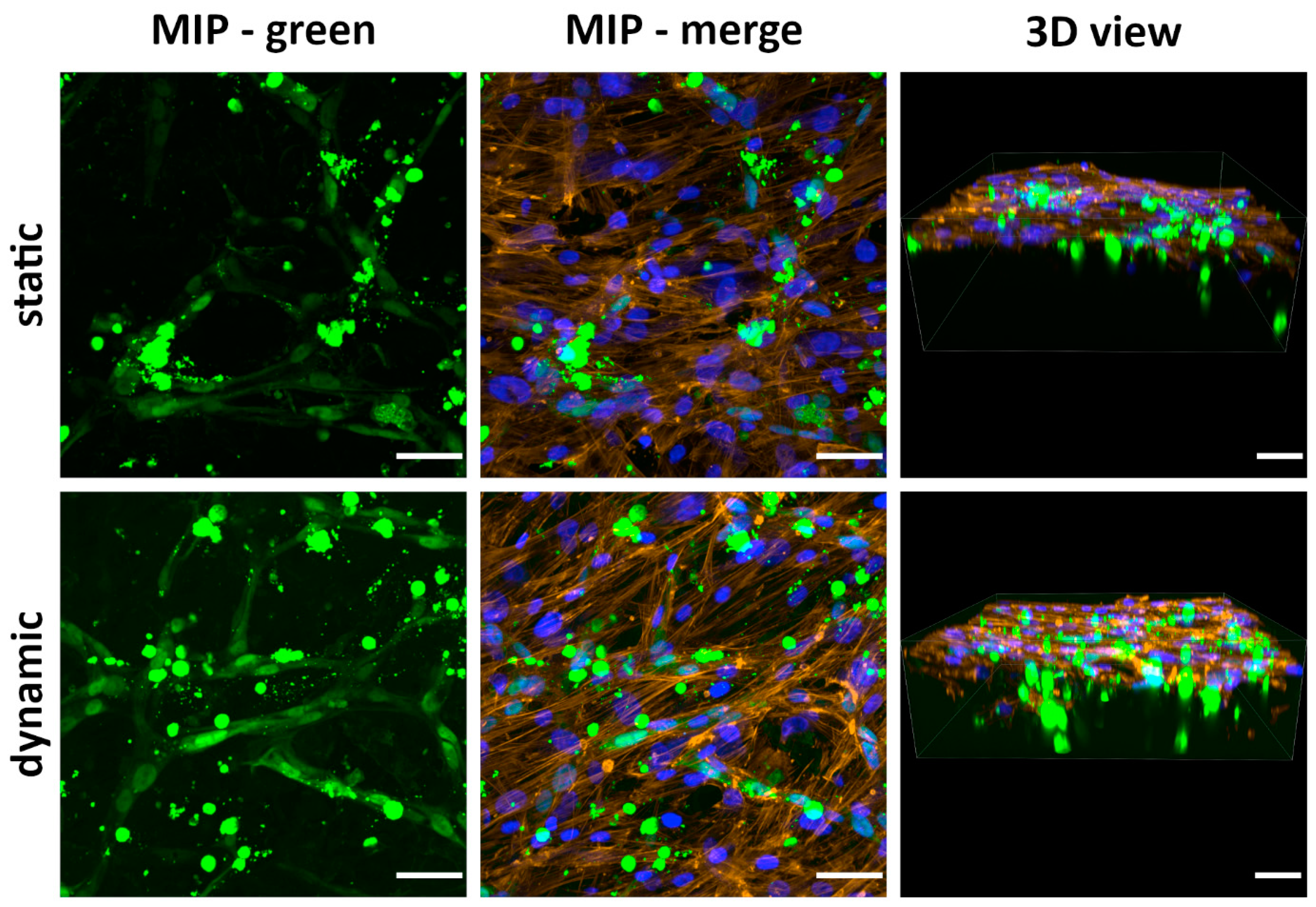
2.9.3. Cocultivation of ASCs with HUVECs on the Scaffolds
3. Materials and Methods
3.1. Preparation of the Scaffolds
3.1.1. Preparation of the Soft Foams with Hierarchical Porous Structure from PLA, PCL, and Their Blends
3.1.2. Mineralization of the Prepared Foams
3.1.3. Sterilization of the Prepared Foams
3.2. Material Characterization Techniques
3.2.1. Visualization of Scaffold Microstructure by Scanning Electron Microscopy (SEM)
3.2.2. Micro-CT
- Total Volume = analyzed volume of interest.
- Scaffold Volume = volume of scaffold material (without porosity).
- Percent Object Volume = scaffold volume/total volume.
- Object Surface to Object Volume ratio.
- Object Surface Density (object surface to volume of interest ratio).
- Pore Size = mean pore size.
- Pore Size Distribution.
- Structure Thickness.
- Porosity = total porosity of the scaffold (= open porosity + closed porosity):
- ○
- Open Porosity = volume of porosity communicating with the external space
- ○
- Closed Porosity = volume of porosity within the scaffold material without communication with the external space.
3.2.3. Brunauer–Emmett–Teller (BET) Analysis
3.2.4. Determination of the Water Uptake of the Scaffolds
3.2.5. Fourier-Transform Infrared (FTIR) Analysis of Scaffold Surfaces
3.2.6. X-Ray Diffraction (XRD) Technique
3.2.7. X-Ray Photoelectron Spectroscopy (XPS) Analysis of the Scaffolds
3.2.8. Mechanical Testing of Scaffolds

| Sample/Parameter | A [mm] | B [mm] | H [mm] | S [mm2] |
|---|---|---|---|---|
| NM | 13.42 ± 0.93 | 12.24 ± 0.53 | 9.24 ± 0.59 | 164.4 ± 15.7 |
| 0% | 11.81 ± 0.54 | 12.75 ± 0.82 | 8.39 ± 0.72 | 150.5 ± 10.5 |
| 10% | 11.84 ± 1.18 | 13.74 ± 0.93 | 10.01 ± 0.4 | 162.8 ± 21.4 |
| 25% | 7.67 ± 0.48 | 10.14 ± 1.24 | 5.2 ± 0.39 | 77.8 ± 10.2 |
| 50% | 9.92 ± 0.47 | 10.31 ± 0.7 | 4.02 ± 0.2 | 102.1 ± 6.6 |
| 100% | 11.25 ± 1.21 | 13.15 ± 1.79 | 9.55 ± 0.63 | 146.6 ± 14.2 |
3.3. Cellular Component, Isolation, and Characterization
3.3.1. Adipose-Derived Stem Cells (ASCs)
3.3.2. Human Umbilical Vein Endothelial Cells (HUVECs)
3.4. Cell Cultivation
3.4.1. Cultivation of ASCs
3.4.2. Co-Cultivation of ASCs and HUVECs
3.5. Cell Visualization and Confocal Microscopy
3.6. Cell Number Counting and Statistics
4. Conclusions and Further Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PLA | Poly(lactic acid) |
| PCL | Polycaprolactone |
| NaCl | Sodium chloride |
| TM | Trademark |
| Micro-CT | Microcomputed tomography |
| 3D | Three-dimensional |
| ASCs | Adipose-derived stem cells |
| PGA | Polyglycolic acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| FDA | Food and Drug Administration |
| ECM | Extracellular matrix |
| PVA | Poly(vinyl alcohol) |
| PEO | Poly(ethylene oxide) |
| SBF | Simulated body fluid |
| HUVECs | Human umbilical vein endothelial cells |
| w/w | Weight in weight |
| SEM | Scanning electron microscopy |
| Ctrl, CTRL | Control |
| ANOVA | Analysis of variance |
| S.D. | Standard deviation |
| BET | Brunauer–Emmett–Teller |
| A, Å | Angstrom, Ångström |
| IUPAC | International Union of Pure and Applied Chemistry |
| vs. | Versus |
| FTIR | Fourier-transform infrared |
| IR | Infrared |
| Klu | Klucel |
| NM | Non-mineralized |
| HSD | Honest significant difference |
| Stress | |
| Strain | |
| W | Strain energy |
| E | Compressive modulus |
| Saos | Sarcoma osteogenic |
| MIP | Maximum Image Projection |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal bovine serum |
| DAPI | 4′,6-diamidino-2-phenylindole |
| F-actin | Filamenous actin |
| S.E.M. | Standard error of the mean |
| bmMSCs | Bone marrow mesenchymal stem cells |
| Ti-6Al-4V | Alloy of 90% titanium, 6% aluminium, and 4% vanadium |
| ECs | Endothelial cells |
| CMFDA | 5-Chloromethylfluorescein diacetate |
| RGD | Arginin-glycin-aspartic acid (Arg-Gly-Asp) |
| VEGF | Vascular endothelial growth factor |
| MVFs | Microvascular fragments |
| NRecon | Volumetric reconstruction software |
| CTVox | Micro-CT Volume Rendering Software |
| BJH | Barrett–Joyner–Halenda |
| PBS | Phosphate-buffered saline |
| BSA | Bovine serum albumin |
| FGF-2 | Fibroblast growth factor-2 |
| CD | Cluster of differentiation |
| FITC | fluorescein-5-isothiocyanate |
| EGM-2 | Endothelial cell growth medium 2 |
| EBM-2 | Endothelial cell basal medium 2 |
| EGF | Epidermal growth factor |
| IGF-1 | Insulin-like growth factor-1 |
References
- Bersini, S.; Gilardi, M.; Arrigoni, C.; Talo, G.; Zamai, M.; Zagra, L.; Caiolfa, V.; Moretti, M. Human in vitro 3D co-culture model to engineer vascularized bone-mimicking tissues combining computational tools and statistical experimental approach. Biomaterials 2016, 76, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, G.; Xu, H.; Hu, K.; Sun, J.F.; Liu, M.; Zhang, F.M.; Gu, N. Pre-vascularization in fibrin Gel/PLGA microsphere scaffolds designed for bone regeneration. NPG Asia Mater. 2018, 10, 827–839. [Google Scholar] [CrossRef]
- Forget, A.; Gianni-Barrera, R.; Uccelli, A.; Sarem, M.; Kohler, E.; Fogli, B.; Muraro, M.G.; Bichet, S.; Aumann, K.; Banfi, A.; et al. Mechanically Defined Microenvironment Promotes Stabilization of Microvasculature, Which Correlates with the Enrichment of a Novel Piezo-1+ Population of Circulating CD11b+/CD115+ Monocytes. Adv. Mater. 2019, 31, e1808050. [Google Scholar] [CrossRef]
- Schott, N.G.; Vu, H.; Stegemann, J.P. Multimodular vascularized bone construct comprised of vasculogenic and osteogenic microtissues. Biotechnol. Bioeng. 2022, 119, 3284–3296. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- Phua, Q.H.; Han, H.A.; Soh, B.S. Translational stem cell therapy: Vascularized skin grafts in skin repair and regeneration. J. Transl. Med. 2021, 19, 83. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16, discussion 16. [Google Scholar] [CrossRef]
- Pamula, E.; Filová, E.; Bacáková, L.; Lisá, V.; Adamczyk, D. Resorbable polymeric scaffolds for bone tissue engineering: The influence of their microstructure on the growth of human osteoblast-like MG 63 cells. J. Biomed. Mater. Res. A 2009, 89, 432–443. [Google Scholar] [CrossRef]
- Beran, M.; Musilkova, J.; Sedlar, A.; Slepicka, P.; Vesely, M.; Kolska, Z.; Vltavsky, O.; Molitor, M.; Bacakova, L. Evaluation of Polymeric Micro/Nanofibrous Hybrid Scaffolds Prepared via Centrifugal Nozzleless Spinning for Tissue Engineering Applications. Polymers 2025, 17, 386. [Google Scholar] [CrossRef] [PubMed]
- Maimouni, I.; Cejas, C.M.; Cossy, J.; Tabeling, P.; Russo, M. Microfluidics Mediated Production of Foams for Biomedical Applications. Micromachines 2020, 11, 83. [Google Scholar] [CrossRef]
- Socci, M.C.; Rodríguez, G.; Oliva, E.; Fushimi, S.; Takabatake, K.; Nagatsuka, H.; Felice, C.J.; Rodríguez, A.P. Polymeric Materials, Advances and Applications in Tissue Engineering: A Review. Bioengineering 2023, 10, 218. [Google Scholar] [CrossRef]
- Novotna, K.; Zajdlova, M.; Suchy, T.; Hadraba, D.; Lopot, F.; Zaloudkova, M.; Douglas, T.E.L.; Munzarova, M.; Juklickova, M.; Stranska, D.; et al. Polylactide nanofibers with hydroxyapatite as growth substrates for osteoblast-like cells. J. Biomed. Mater. Res. A 2014, 102, 3918–3930. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Pandit, V.; Elkin, J.; Denman, T.; Cooper, J.A.; Kotha, S.P. Large-scale and highly efficient synthesis of micro- and nano-fibers with controlled fiber morphology by centrifugal jet spinning for tissue regeneration. Nanoscale 2013, 5, 2337–2345. [Google Scholar] [CrossRef]
- Bacakova, L.; Novotna, K.; Hadraba, D.; Musilkova, J.; Slepicka, P.; Beran, M. Influence of Biomimetically Mineralized Collagen Scaffolds on Bone Cell Proliferation and Immune Activation. Polymers 2022, 14, 602. [Google Scholar] [CrossRef] [PubMed]
- Kramschuster, A.; Turng, L.S. An Injection Molding Process for Manufacturing Highly Porous and Interconnected Biodegradable Polymer Matrices for Use as Tissue Engineering Scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 366–376. [Google Scholar] [CrossRef]
- Bruzauskaite, I.; Bironaite, D.; Bagdonas, E.; Bernotiene, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Chen, X.N.; Fan, H.Y.; Deng, X.W.; Wu, L.N.; Yi, T.; Gu, L.X.; Zhou, C.C.; Fan, Y.J.; Zhang, X.D. Scaffold Structural Microenvironmental Cues to Guide Tissue Regeneration in Bone Tissue Applications. Nanomaterials 2018, 8, 960. [Google Scholar] [CrossRef]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef]
- Bianco, S.; Mancardi, D.; Merlino, A.; Bussolati, B.; Munaron, L. Hypoxia and hydrogen sulfide differentially affect normal and tumor-derived vascular endothelium. Redox. Biol. 2017, 12, 499–504. [Google Scholar] [CrossRef]
- Im, G.I.; Ko, J.Y.; Lee, J.H. Chondrogenesis of Adipose Stem Cells in a Porous Polymer Scaffold: Influence of the Pore Size. Cell Transplant. 2012, 21, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lian, M.F.; Wu, Q.; Qiao, Z.G.; Sun, B.B.; Dai, K.R. Effect of Pore Size on Cell Behavior Using Melt Electrowritten Scaffolds. Front. Bioeng. Biotechnol. 2021, 9, 629270. [Google Scholar] [CrossRef]
- Cheng, M.Q.; Wahafu, T.E.H.J.; Jiang, G.F.; Liu, W.; Qiao, Y.Q.; Peng, X.C.; Cheng, T.; Zhang, X.L.; He, G.; Liu, X.Y. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci. Rep. 2016, 6, 24134. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Wu, G.J.; Xie, P.C.; Yang, H.G.; Dang, K.F.; Xu, Y.X.; Sain, M.; Turng, L.S.; Yang, W.M. A review of thermoplastic polymer foams for functional applications. J. Mater. Sci. 2021, 56, 11579–11604. [Google Scholar] [CrossRef]
- Torres, E.; Dominguez-Candela, I.; Castello-Palacios, S.; Vallés-Lluch, A.; Fombuena, V. Development and Characterization of Polyester and Acrylate-Based Composites with Hydroxyapatite and Halloysite Nanotubes for Medical Applications. Polymers 2020, 12, 1703. [Google Scholar] [CrossRef]
- Broz, M.E.; VanderHart, D.L.; Washburn, N.R. Structure and mechanical properties of poly(D,L-lactic acid)/poly(ε-caprolactone) blends. Biomaterials 2003, 24, 4181–4190. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, T.T.; Li, L.Y.; Zhang, H.Y.; Wang, H.P.; Ke, F.Y. Fully biodegradable PLA composite with improved mechanical properties via 3D printing. Mater. Lett. 2023, 331, 133543. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Fullbrook, D.H.G.; Vilain, L.; Derrar, Y.; Nandi, U.; Grau, C.; Morales, A.; Hooper, G.; Hiezl, Z.; Douroumis, D. Personalised Tasted Masked Chewable 3D Printed Fruit-Chews for Paediatric Patients. Pharmaceutics 2021, 13, 1301. [Google Scholar] [CrossRef]
- Lima, S.G.B.; Pinho, L.A.G.; Pereira, M.N.; Gratieri, T.; Sa-Barreto, L.L.; Gelfuso, G.M.; Cunha, M. Preformulation studies of finasteride to design matrix systems for topical delivery. J. Pharm. Biomed. Anal. 2018, 161, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kang, S.M.; Park, S.A.; Kim, W.D.; Kwak, J.; Lee, H. Enhanced Adhesion of Preosteoblasts inside 3D PCL Scaffolds by Polydopamine Coating and Mineralization. Macromol. Biosci. 2013, 13, 1389–1395. [Google Scholar] [CrossRef]
- Thomas, M.; Arora, A.; Katti, D.S. Surface hydrophilicity of PLGA fibers governs in vitro mineralization and osteogenic differentiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 320–332. [Google Scholar] [CrossRef]
- Ebrahimi, Z.; Irani, S.; Ardeshirylajimi, A.; Seyedjafari, E. Enhanced osteogenic differentiation of stem cells by 3D printed PCL scaffolds coated with collagen and hydroxyapatite. Sci. Rep. 2022, 12, 12359. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Musilkova, J.; Kotelnikov, I.; Novotna, K.; Pop-Georgievski, O.; Rypacek, F.; Bacakova, L.; Proks, V. Cell adhesion and growth enabled by biomimetic oligopeptide modification of a polydopamine-poly(ethylene oxide) protein repulsive surface. J. Mater. Sci. Mater. Med. 2015, 26, 253. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Mei, S.L.; Chu, P.K.; Zhang, Y.M.; Wu, Z.F. The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions. Biomaterials 2010, 31, 5072–5082. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]
- Ghayor, C.; Weber, F.E. Osteoconductive Microarchitecture of Bone Substitutes for Bone Regeneration Revisited. Front. Physiol. 2018, 9, 960. [Google Scholar] [CrossRef]
- Yamahara, S.; Raudales, J.L.M.; Akiyama, Y.; Ito, M.; Chimedtseren, I.; Arai, Y.; Wakita, T.; Hiratsuka, T.; Miyazawa, K.; Goto, S.; et al. Appropriate pore size for bone formation potential of porous collagen type I-based recombinant peptide. Regen Ther. 2022, 21, 294–306. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Mays, T.J. A new classification of pore sizes. Stud. Surf. Sci. Catal. 2006, 160, 57–62. [Google Scholar] [CrossRef]
- Chanes-Cuevas, O.A.; Perez-Soria, A.; Cruz-Maya, I.; Guarino, V.; Alvarez-Perez, M.A. Macro-, micro- and mesoporous materials for tissue engineering applications. AIMS Mater. Sci. 2018, 5, 1124–1140. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M. Biporous nanocarbon foams and the effect of the structure on the capillary performance. Prog. Nat. Sci. Mater. Int. 2020, 30, 360–365. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Xiong, S.L.; Liu, M.; Yang, H.; Wei, P.; Yi, F.; Ouyang, M.; Xi, H.R.; Long, Z.S.; Liu, Y.Y.; et al. Study on the influence of scaffold morphology and structure on osteogenic performance. Front. Bioeng. Biotechnol. 2023, 11, 1127162. [Google Scholar] [CrossRef]
- Basta, A.H.; Lotfy, V.F.; Micky, J.A.; Salem, A.M. Hydroxypropylcellulose-based liquid crystal materials. Carbohydr. Polym. Technol. Appl. 2021, 2, 100103. [Google Scholar] [CrossRef]
- Tran, T.H.H.; Luu, C.H.; Nguyen, K.T.T.; Hoang, M.A.L.; Pham, Q.K.; Phan, C.M.; Thai, N.K.L.; Nguyen, H.T.; Thambi, T.; Phan, V.H.G. Mineralized Biopolymers-Based Scaffold Encapsulating with Dual Drugs for Alveolar Ridge Preservation. Macromol. Biosci. 2024, 25, e2400351. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, H.Y.; Poh, P.S.P.; Machens, H.G.; Schilling, A.F. Hydrogels for Engineering of Perfusable Vascular Networks. Int. J. Mol. Sci. 2015, 16, 15997–16016. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, S.; Arthurs, C.; Molina, M.I.E.; Pruitt, L.; Roy, A. An overview of the tribological and mechanical properties of PEEK and CFR-PEEK for use in total joint replacements. J. Mech. Behav. Biomed. Mater. 2023, 145, 105974. [Google Scholar] [CrossRef]
- Klimek, K.; Benko, A.; Vandrovcova, M.; Travnickova, M.; Douglas, T.E.L.; Tarczynska, M.; Broz, A.; Gaweda, K.; Ginalska, G.; Bacakova, L. Biomimetic biphasic curdlan-based scaffold for osteochondral tissue engineering applications—Characterization and preliminary evaluation of mesenchymal stem cell response. Biomater. Adv. 2022, 135, 212724. [Google Scholar] [CrossRef]
- Yang, X.; Cai, X.; Wang, J.; Tang, H.; Yuan, Q.; Gong, P.; Lin, Y. Mechanical stretch inhibits adipogenesis and stimulates osteogenesis of adipose stem cells. Cell Prolif. 2012, 45, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.E.; Denecke, B.; Kim, B.S.; Dreser, A.; Bernhagen, J.; Pallua, N. The effect of mechanical stress on the proliferation, adipogenic differentiation and gene expression of human adipose-derived stem cells. J. Tissue Eng. Regen. Med. 2018, 12, 276–284. [Google Scholar] [CrossRef]
- Tirkkonen, L.; Halonen, H.; Hyttinen, J.; Kuokkanen, H.; Sievänen, H.; Koivisto, A.M.; Mannerström, B.; Sándor, G.K.B.; Suuronen, R.; Miettinen, S.; et al. The effects of vibration loading on adipose stem cell number, viability and differentiation towards bone-forming cells. J. R. Soc. Interface 2011, 8, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, S.Y.; Yoo, Y.M.; Kim, C.H. Maturation of Adipocytes is Suppressed by Fluid Shear Stress. Cell Biochem. Biophys. 2017, 75, 87–94. [Google Scholar] [CrossRef]
- Baumgartner, W.; Schneider, I.; Hess, S.C.; Stark, W.J.; Märsmann, S.; Brunelli, M.; Calcagni, M.; Cinelli, P.; Buschmann, J. Cyclic uniaxial compression of human stem cells seeded on a bone biomimetic nanocomposite decreases anti-osteogenic commitment evoked by shear stress. J. Mech. Behav. Biomed. Mater. 2018, 83, 84–93. [Google Scholar] [CrossRef]
- Molitor, M.; Trávnícková, M.; Mesták, O.; Christodoulou, P.; Sedlár, A.; Bacáková, L.; Lucchina, S. The Influence of High and Low Negative Pressure Liposuction and Various Harvesting Techniques on the Viability and Function of Harvested Cells-a Systematic Review of Animal and Human Studies. Aesthetic Plast. Surg. 2021, 45, 2379–2394. [Google Scholar] [CrossRef] [PubMed]
- Tervaert, J.W.C.; Shoenfeld, Y.; Cruciani, C.; Scarpa, C.; Bassetto, F. Breast implant illness: Is it causally related to breast implants? Autoimmun. Rev. 2024, 23, 103448. [Google Scholar] [CrossRef]
- Broz, A.; Jirka, I.; Matejka, R.; Stepanovská, J.; Doubková, M.; Sajdl, P.; Drahokoupil, J.; Volochanskyi, O.; Futóová, T.; Bacáková, L. Surface modifications of a silicalite film designed for coating orthopaedic implants. Mater. Des. 2022, 224, 111373. [Google Scholar] [CrossRef]
- Bajek, A.; Gurtowska, N.; Gackowska, L.; Kubiszewska, I.; Bodnar, M.; Marszalek, A.; Januszewski, R.; Michalkiewicz, J.; Drewa, T. Does the liposuction method influence the phenotypic characteristic of human adipose-derived stem cells? Biosci. Rep. 2015, 35, e00212. [Google Scholar] [CrossRef]
- Volz, A.C.; Hack, L.; Atzinger, F.B.; Kluger, P.J. Completely Defined Co-Culture of Adipogenic Differentiated ASCs and Microvascular Endothelial Cells. ALTEX 2018, 35, 464–476. [Google Scholar] [CrossRef]
- Schott, N.G.; Stegemann, J.P. Coculture of Endothelial and Stromal Cells to Promote Concurrent Osteogenesis and Vasculogenesis. Tissue Eng. Part A 2021, 27, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Maisani, M.; Pezzoli, D.; Chassande, O.; Mantovani, D. Cellularizing hydrogel-based scaffolds to repair bone tissue: How to create a physiologically relevant micro-environment? J. Tissue Eng. 2017, 8, 2041731417712073. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.; Frueh, F.S.; McLuckie, M.; Sanchez-Macedo, N.; Wolint, P.; Lindenblatt, N.; Plock, J.A.; Calcagni, M.; Buschmann, J. Adipose tissue and the vascularization of biomaterials: Stem cells, microvascular fragments and nanofat-a review. Cytotherapy 2020, 22, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Estes, B.T.; Diekman, B.O.; Gimble, J.M.; Guilak, F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat. Protoc. 2010, 5, 1294–1311. [Google Scholar] [CrossRef]


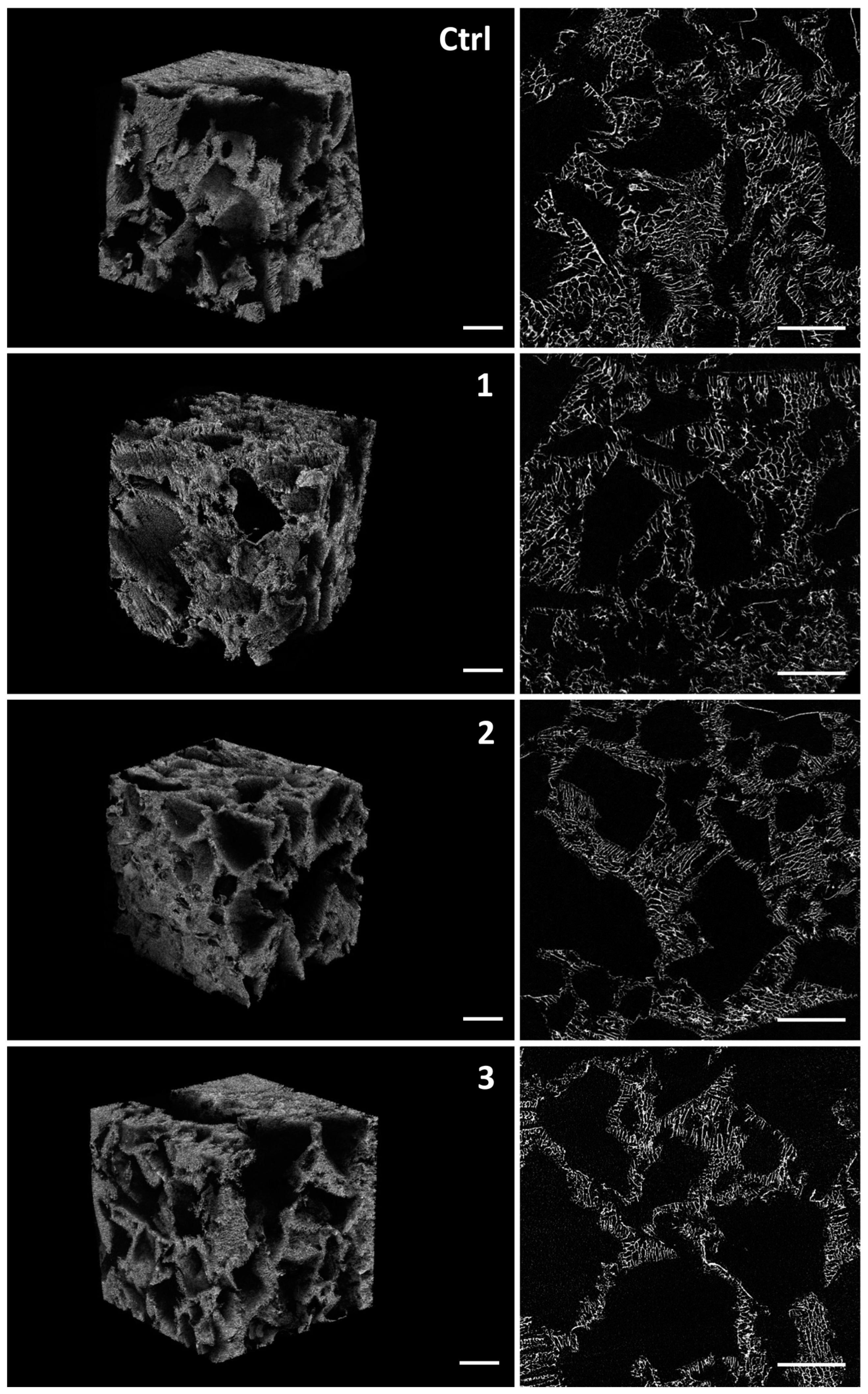

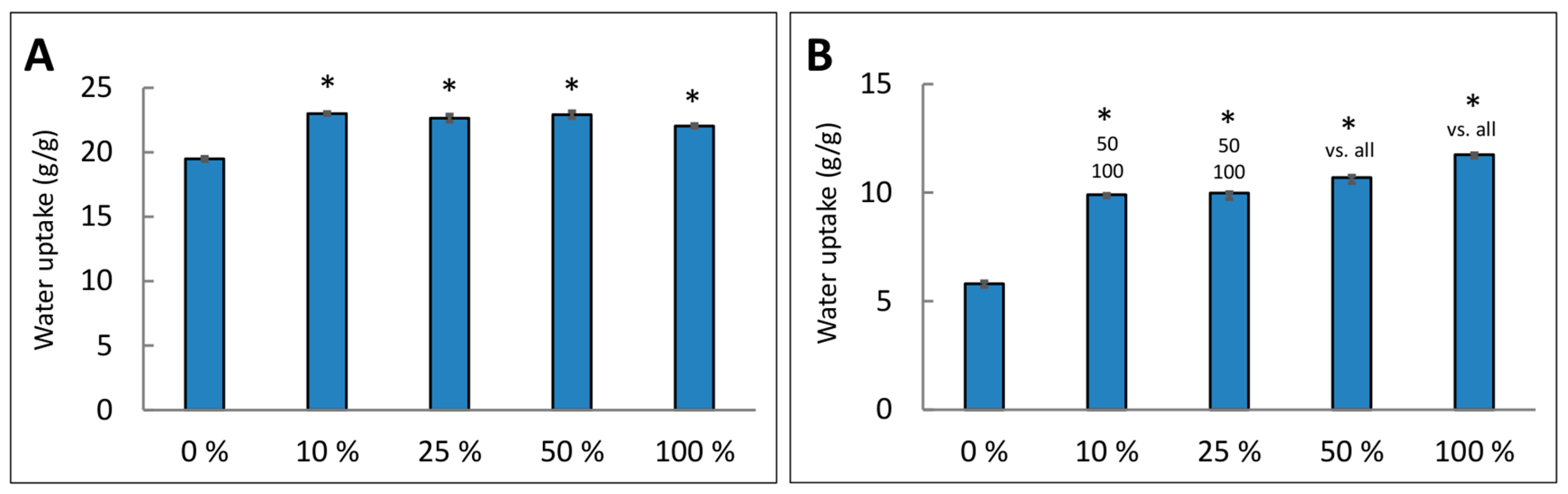
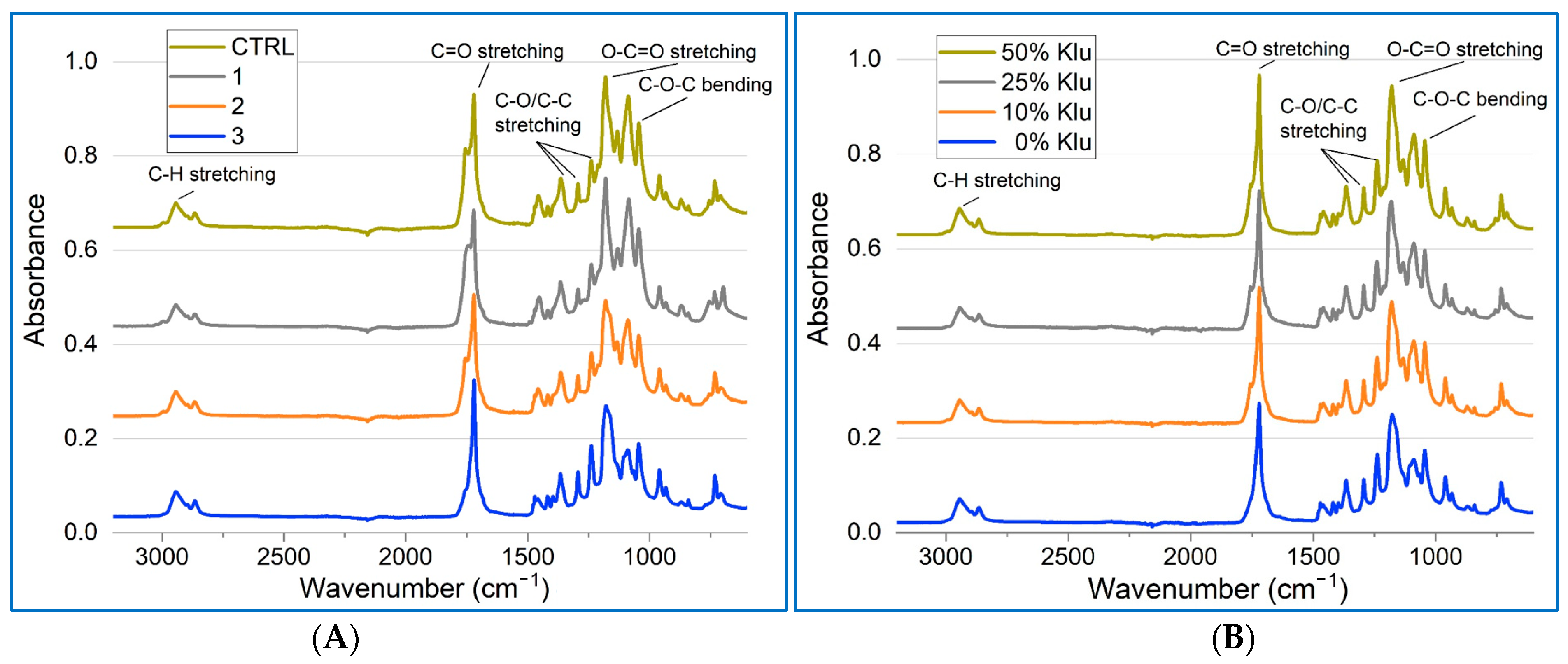
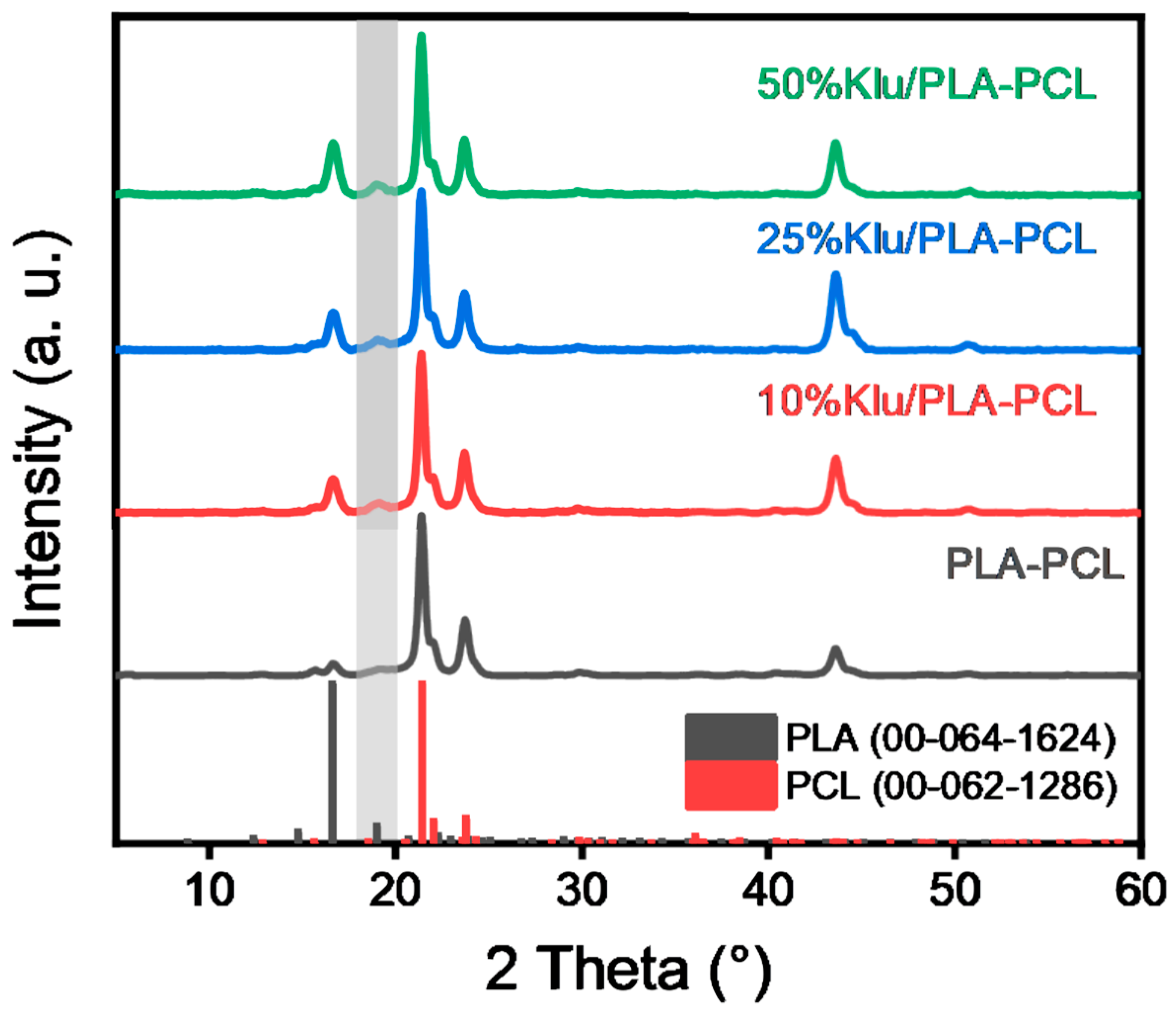

| Parameter/Sample | Ctrl | Klucel 10% | Klucel 25% | Klucel 50% |
|---|---|---|---|---|
| Total porosity (%) | 92.32 ± 2.34 | 92.32 ± 0.54 | 91.18 ± 0.72 | 88.91 ± 0.24 |
| Open porosity (%) | 92.32 ± 2.34 | 92.32 ± 0.54 | 91.18 ± 0.72 | 88.91 ± 0.24 |
| Closed porosity (%) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Sample/Parameter | Surface Area, m2/g | Pore Volume, cm3/g |
|---|---|---|
| CTRL | 4.2 ± 0.5 | 0.004 ± 0.001 |
| 10% | 7.9 ± 1.1 | 0.007 ± 0.001 |
| 25% | 8.7 ± 4.2 | 0.010 ± 0.005 |
| 50% | 10.3 ± 0.8 | 0.011 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musílková, J.; Beran, M.; Sedlář, A.; Slepička, P.; Bartoš, M.; Kolská, Z.; Havlíčková, Š.; Luňáčková, J.; Svobodová, L.; Froněk, M.; et al. Composite Polylactide/Polycaprolactone Foams with Hierarchical Porous Structure for Pre-Vascularized Tissue Engineering. Int. J. Mol. Sci. 2025, 26, 2974. https://doi.org/10.3390/ijms26072974
Musílková J, Beran M, Sedlář A, Slepička P, Bartoš M, Kolská Z, Havlíčková Š, Luňáčková J, Svobodová L, Froněk M, et al. Composite Polylactide/Polycaprolactone Foams with Hierarchical Porous Structure for Pre-Vascularized Tissue Engineering. International Journal of Molecular Sciences. 2025; 26(7):2974. https://doi.org/10.3390/ijms26072974
Chicago/Turabian StyleMusílková, Jana, Miloš Beran, Antonín Sedlář, Petr Slepička, Martin Bartoš, Zdeňka Kolská, Šárka Havlíčková, Jitka Luňáčková, Lucie Svobodová, Martin Froněk, and et al. 2025. "Composite Polylactide/Polycaprolactone Foams with Hierarchical Porous Structure for Pre-Vascularized Tissue Engineering" International Journal of Molecular Sciences 26, no. 7: 2974. https://doi.org/10.3390/ijms26072974
APA StyleMusílková, J., Beran, M., Sedlář, A., Slepička, P., Bartoš, M., Kolská, Z., Havlíčková, Š., Luňáčková, J., Svobodová, L., Froněk, M., Molitor, M., Chlup, H., & Bačáková, L. (2025). Composite Polylactide/Polycaprolactone Foams with Hierarchical Porous Structure for Pre-Vascularized Tissue Engineering. International Journal of Molecular Sciences, 26(7), 2974. https://doi.org/10.3390/ijms26072974







