Evaluation of the Effects of the Sodium–Glucose Cotransporter 2 Inhibitors and Sacubitril/Valsartan Combined Therapy in Patients with HFrEF: An Echocardiographic Study
Abstract
1. Introduction
1.1. Heart Failure and Diabetes
1.2. Literature Review
1.3. Heart Failure Therapy
1.4. Global Longitudinal Strain
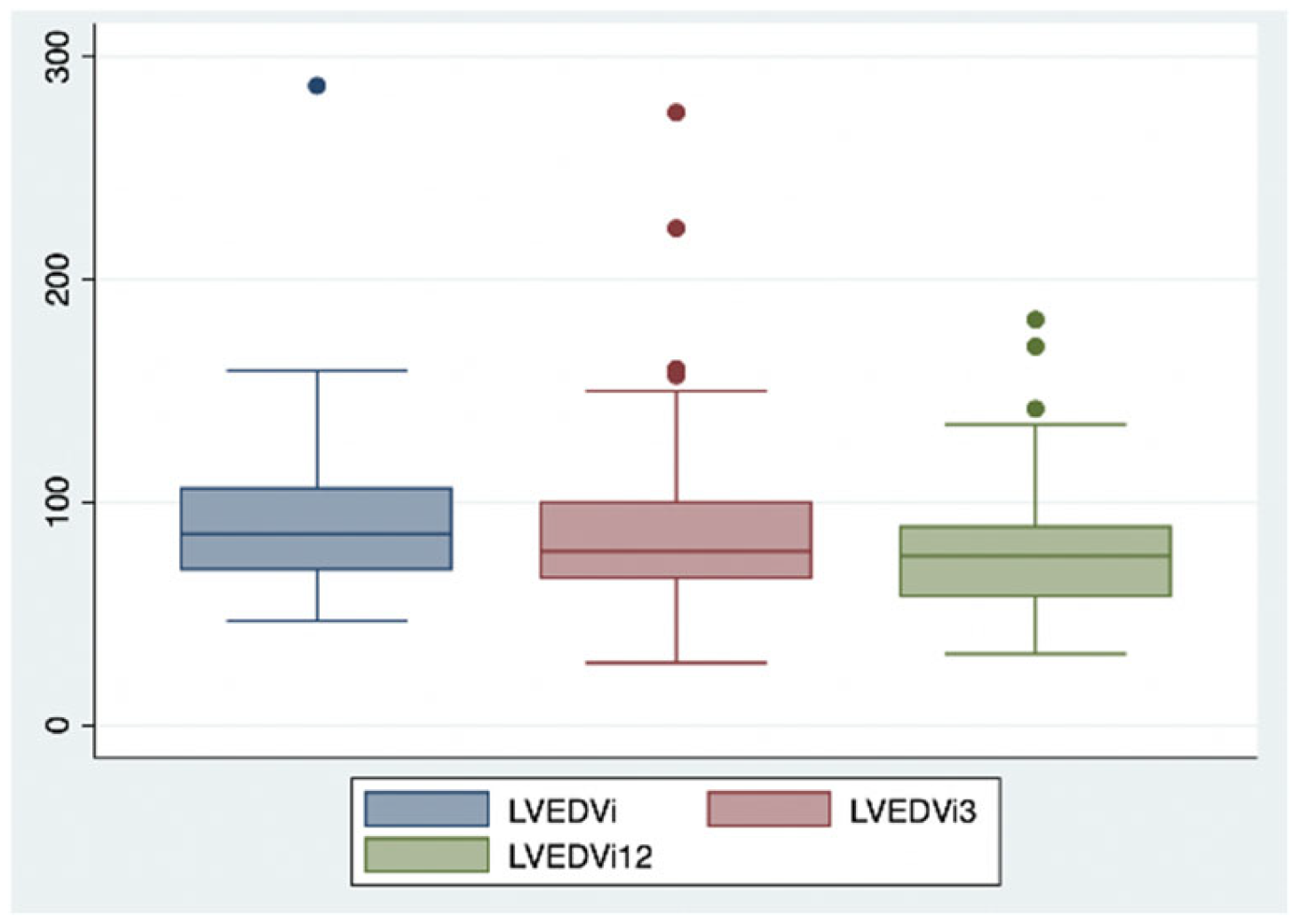
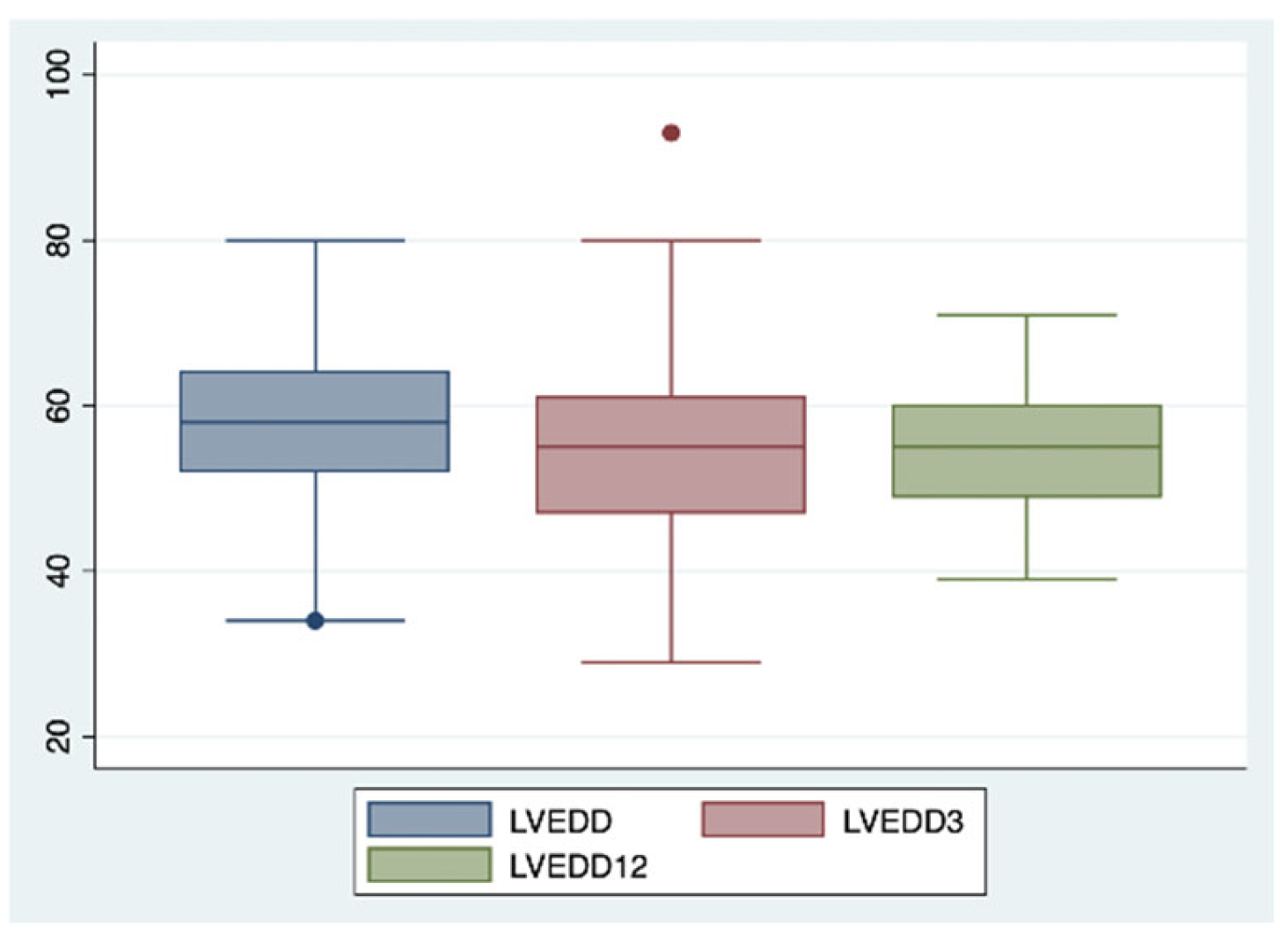
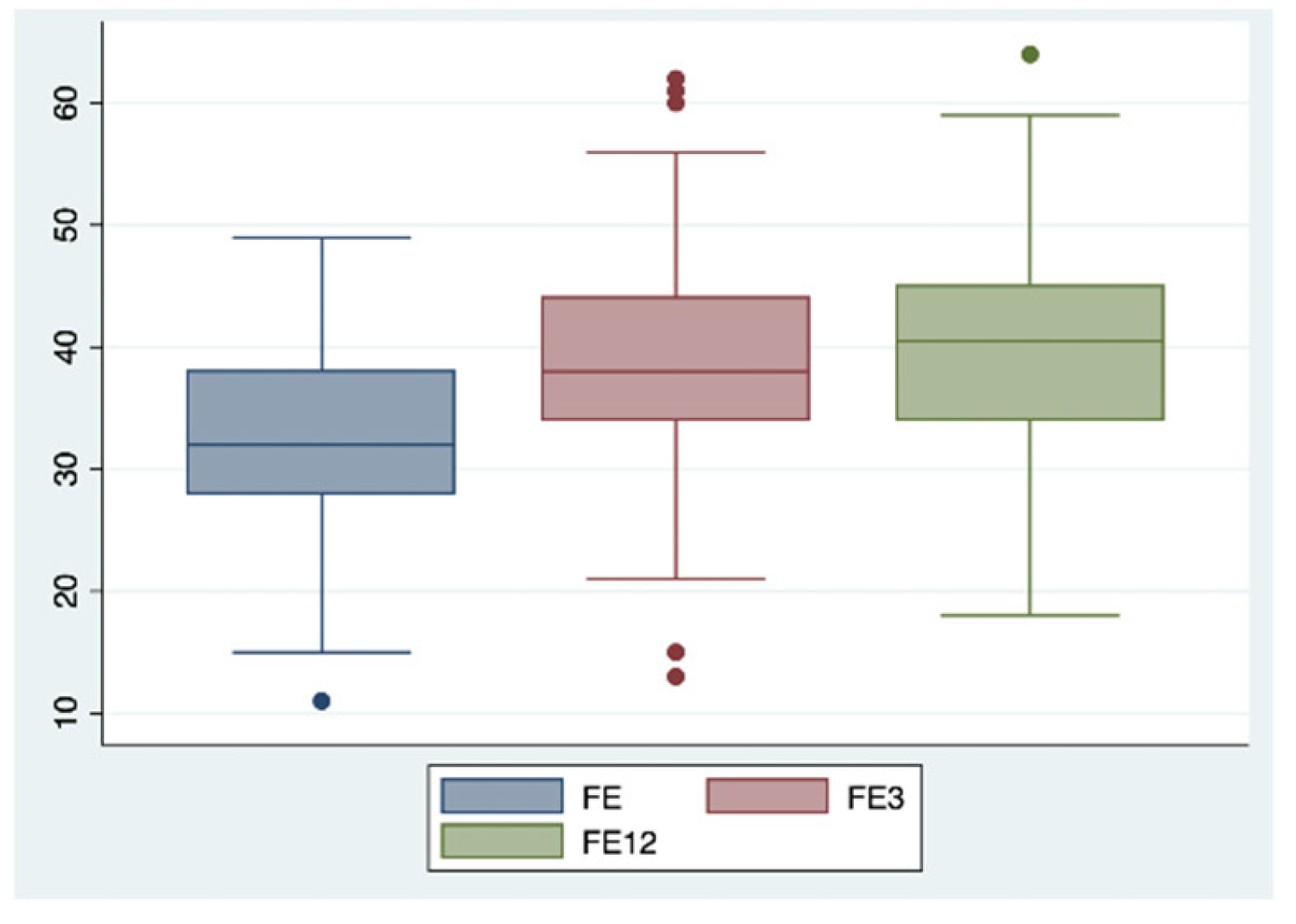
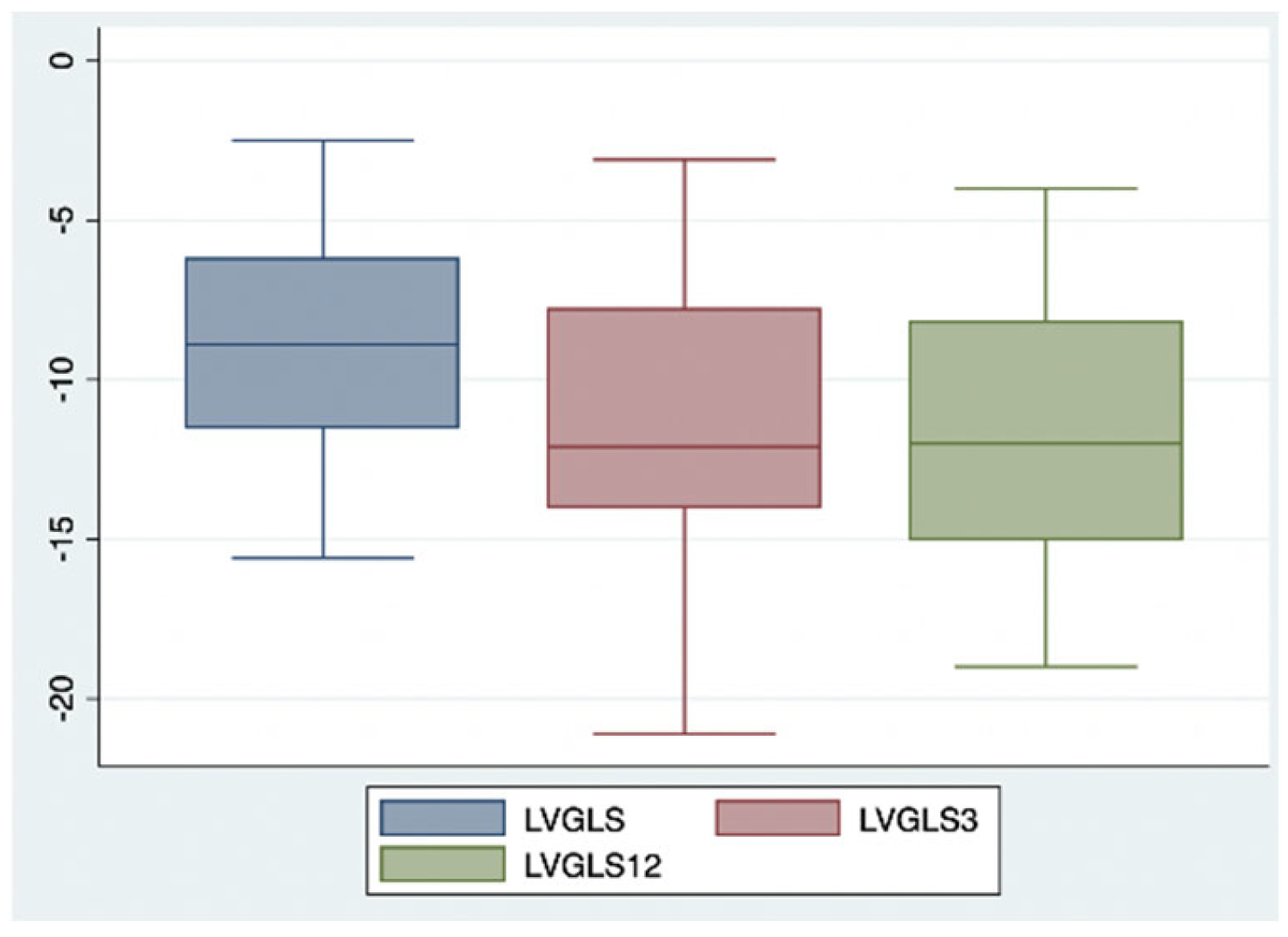
2. Results
3. Discussion
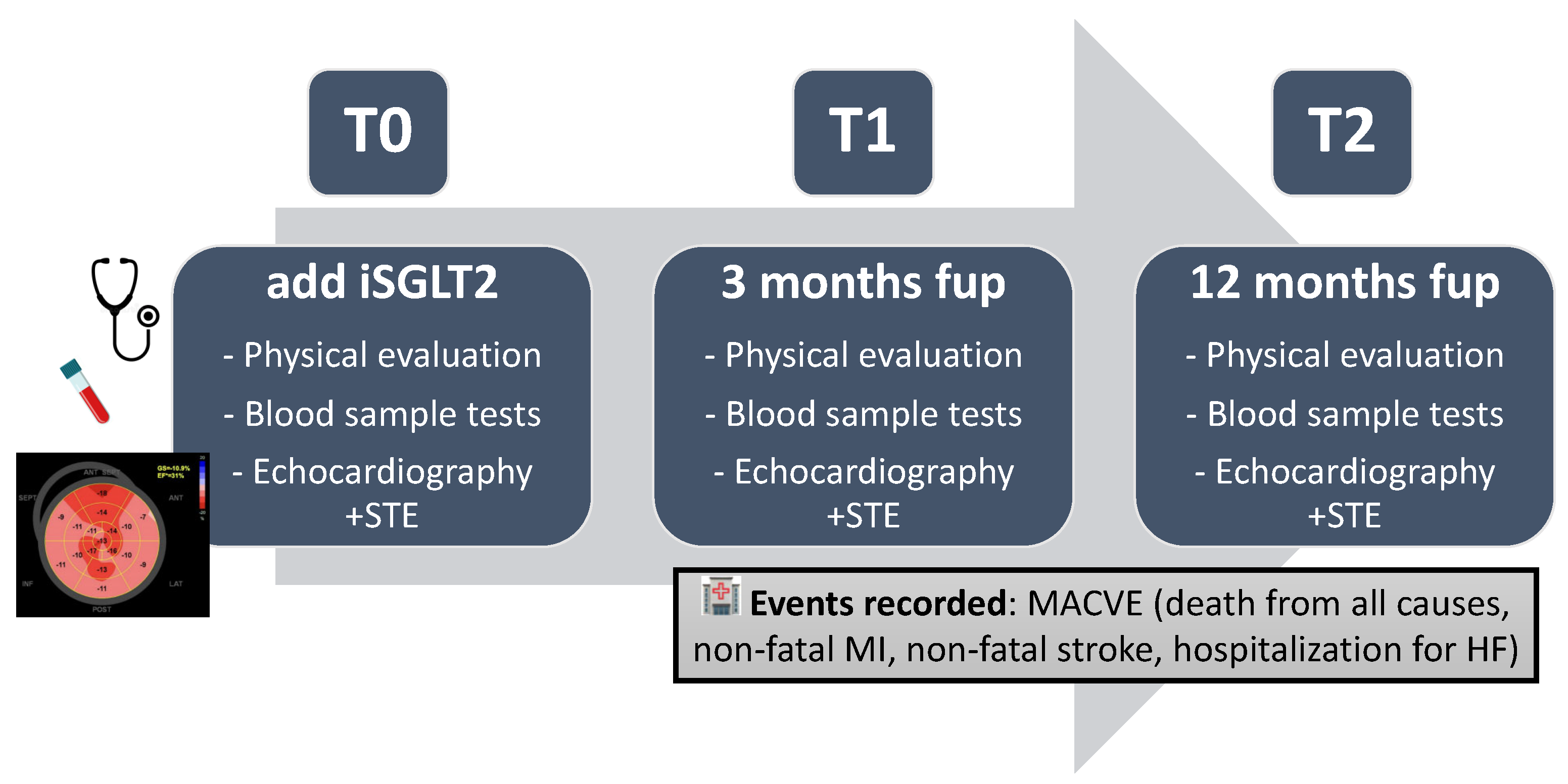
4. Materials and Methods
4.1. Study Design and Population
- Primary endpoint:
- Assessing the mid-term (3 months) echocardiographic impact of the combination of ARNI + SGLT2 inhibitors in terms of ventricular remodeling, studied as variations in myocardial strain indices (GLS%) in patients with HFrEF receiving optimized medical therapy.
- Secondary Endpoints:
- Evaluate the long-term (12 months) echocardiographic impact of the ARNI + SGLT2 inhibitor combination in terms of ventricular remodeling, studied as variations in myocardial strain indices (GLS%) in patients with HFrEF receiving optimized medical therapy.
- Evaluate the clinical impact of the ARNI + SGLT2 inhibitor combination in terms of variation in laboratory data indicative of heart failure (NT-pro-BNP) in the mid-to-long term (3–12 months) in patients with HFrEF receiving optimized medical therapy.
- Evaluate the echocardiographic mid-to-long-term impact (3–12 months) of the ARNI + SGLT2 inhibitor combination on left ventricular remodeling, studied as variations in volumetric indices and contractile function (LVEDV, LVEDD, EF%) in patients with HFrEF receiving optimized medical therapy.
- Evaluate the clinical impact of the ARNI + SGLT2 inhibitor combination in terms of major adverse cardiovascular events (MACVE: composite endpoint of death from all causes, non-fatal myocardial infarction, non-fatal stroke and hospitalization for HF) in the mid-to-long term (3–12 months) in patients with HFrEF receiving optimized medical therapy.
- Identify echocardiographic parameters predictive of MACVE in the mid-to-long term (3–12 months) in patients with HFrEF after the introduction of combination therapy with ARNI + SGLT2 inhibitor.
- Identify laboratory parameters predictive of major adverse cardiovascular events (MACVE) in the mid-to-long term (3–12 months) in patients with HFrEF after the introduction of combination therapy with ARNI + SGLT2 inhibitor.
- Identify echocardiographic parameters predictive of ventricular remodeling (LVEDV, LVEDD, EF%) and variation in myocardial strain indices (GLS%) in the mid-to-long term (3–12 months) in patients with HFrEF after the introduction of combination therapy with ARNI + SGLT2 inhibitor.
- Identify laboratory parameters predictive of ventricular remodeling (LVEDV, LVEDD, EF%) and variation in myocardial strain indices (GLS%) in the mid-to-long term (3–12 months) in patients with HFrEF after the introduction of combination therapy with ARNI + SGLT2 inhibitor (Figure 6).
4.2. Echocardiographic Protocol
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Leibundgut, G.; Brunner-La Rocca, H.P. End-stage chronic heart failure. Swiss Med. Wkly. 2007, 137, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Moroni, F.; Montone, R.A.; Azzalini, L.; Sanna, T.; Abbate, A. Ischemic Cardiomyopathy and Heart Failure After Acute Myocardial Infarction. Curr. Cardiol. Rep. 2022, 24, 1505–1515. [Google Scholar] [CrossRef]
- La Vecchia, G.; Fumarulo, I.; Caffè, A.; Chiatto, M.; Montone, R.A.; Aspromonte, N. Microvascular Dysfunction across the Spectrum of Heart Failure Pathology: Pathophysiology, Clinical Features and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 7628. [Google Scholar] [CrossRef]
- Paolillo, S.; Marsico, F.; Prastaro, M.; Renga, F.; Esposito, L.; De Martino, F.; Di Napoli, P.; Esposito, I.; Ambrosio, A.; Ianniruberto, M.; et al. Diabetic Cardiomyopathy: Definition, Diagnosis, and Therapeutic Implications. Heart Fail. Clin. 2019, 15, 341–347. [Google Scholar] [CrossRef]
- Aspromonte, N.; Zaninotto, M.; Aimo, A.; Fumarulo, I.; Plebani, M.; Clerico, A. Measurement of Cardiac-Specific Biomarkers in the Emergency Department: New Insight in Risk Evaluation. Int. J. Mol. Sci. 2023, 24, 15998. [Google Scholar] [CrossRef]
- Khan, S.S.; Shah, S.J. Pre-Heart Failure Risk Assessment: Don’t Get Lost in an Echo Chamber! J. Card. Fail. 2023, 29, 1490–1493. [Google Scholar] [CrossRef]
- Solomon, S.D.; Claggett, B.; Desai, A.S.; Packer, M.; Zile, M.; Swedberg, K.; Rouleau, J.L.; Shi, V.C.; Starling, R.C.; Kozan, Ö.; et al. Influence of Ejection Fraction on Outcomes and Efficacy of Sacubitril/Valsartan (LCZ696) in Heart Failure with Reduced Ejection Fraction: The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) Trial. Circ. Heart Fail. 2016, 9, e002744. [Google Scholar] [CrossRef]
- Bin Atan, N.M.A.S.; Bin Hadi, M.F.; Teoh, V.W.Y.; Danaee, M.; Loch, A. ARNI Versus Perindopril for Remodeling in HFrEF. A Cohort Study. J. Cardiovasc. Pharmacol. Ther. 2023, 28, 10742484231195019. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Pescariu, S.A.; Elagez, A.; Nallapati, B.; Bratosin, F.; Bucur, A.; Negru, A.; Gaita, L.; Citu, I.M.; Popa, Z.L.; Barata, P.I. Examining the Impact of Ertugliflozin on Cardiovascular Outcomes in Patients with Diabetes and Metabolic Syndrome: A Systematic Review of Clinical Trials. Pharmaceuticals 2024, 17, 929. [Google Scholar] [CrossRef]
- Theofilis, P.; Antonopoulos, A.S.; Katsimichas, T.; Oikonomou, E.; Siasos, G.; Aggeli, C.; Tsioufis, K.; Tousoulis, D. The impact of SGLT2 inhibition on imaging markers of cardiac function: A systematic review and meta-analysis. Pharmacol. Res. 2022, 180, 106243. [Google Scholar] [CrossRef]
- Mustapic, I.; Bakovic, D.; Susilovic-Grabovac, Z.; Borovac, J.A. Left Ventricular Systolic Function After 3 Months of SGLT2 Inhibitor Therapy in Heart Failure Patients with Reduced Ejection Fraction. J. Cardiovasc. Transl. Res. 2023, 16, 987–998. [Google Scholar] [CrossRef]
- Palmiero, G.; Cesaro, A.; Galiero, R.; Loffredo, G.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Salvatore, T.; Ruggiero, R.; Di Palo, M.R.; et al. Impact of gliflozins on cardiac remodeling in patients with type 2 diabetes mellitus & reduced ejection fraction heart failure: A pilot prospective study. GLISCAR study. Diabetes Res. Clin. Pract. 2023, 200, 110686. [Google Scholar] [CrossRef]
- Pastore, M.C.; Stefanini, A.; Mandoli, G.E.; Piu, P.; Diviggiano, E.E.; Iuliano, M.A.; Carli, L.; Marchese, A.; Martini, L.; Pecere, A.; et al. Dapagliflozin Effects on Cardiac Deformation in Heart Failure and Secondary Clinical Outcome. JACC Cardiovasc. Imaging 2024, 17, 1399–1408. [Google Scholar] [CrossRef]
- Masarone, D.; Kittleson, M.M.; D’Onofrio, A.; Falco, L.; Fumarulo, I.; Massetti, M.; Crea, F.; Aspromonte, N.; Pacileo, G. Basic science of cardiac contractility modulation therapy: Molecular and electrophysiological mechanisms. Heart Rhythm 2024, 21, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Fumarulo, I.; Stefanini, A.; Masarone, D.; Burzotta, F.; Cameli, M.; Aspromonte, N. Cardiac replacement therapy: Critical issues and future perspectives of heart transplantation and artificial heart. Curr. Probl. Cardiol. 2025, 50, 102971. [Google Scholar] [CrossRef] [PubMed]
- Al Saikhan, L.; Park, C.; Hardy, R.; Hughes, A. Prognostic implications of left ventricular strain by speckle-tracking echocardiography in the general population: A meta-analysis. Vasc. Health Risk Manag. 2019, 15, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.E.; Barac, A.; Thavendiranathan, P.; Scherrer-Crosbie, M. Strain Imaging in Cardio-Oncology. JACC Cardio Oncol. 2020, 2, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Geenty, P.; Sivapathan, S.; Stefani, L.D.; Zada, M.; Boyd, A.; Richards, D.; Kwok, F.; Thomas, L. A novel echocardiographic risk score for light-chain amyloidosis. Eur. Heart J. Open 2023, 3, oead040. [Google Scholar] [CrossRef]
- Chen, S.; Chen, C.; Zheng, L.; Cheng, W.; Bu, X.; Liu, Z. Assessment of new-onset heart failure prediction in a diabetic population using left ventricular global strain: A prospective cohort study based on UK Biobank. Front. Endocrinol. 2024, 15, 1365169. [Google Scholar] [CrossRef]
- Pescariu, S.A.; Şoşdean, R.; Tudoran, C.; Ionac, A.; Pop, G.N.; Timar, R.Z.; Pescariu, S.; Tudoran, M. Echocardiographic Parameters as Predictors for the Efficiency of Resynchronization Therapy in Patients with Dilated Cardiomyopathy and HFrEF. Diagnostics 2021, 12, 35. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Butler, J.; Fombu, E.; Maisel, A.; McCague, K.; Piña, I.L.; Prescott, M.F.; Riebman, J.B.; Solomon, S. Rationale and methods of the Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure (PROVE-HF). Am. Heart J. 2018, 199, 130–136. [Google Scholar] [CrossRef]
- Desai, A.S.; Solomon, S.D.; Shah, A.M.; Claggett, B.L.; Fang, J.C.; Izzo, J.; McCague, K.; Abbas, C.A.; Rocha, R.; Mitchell, G.F.; et al. Effect of Sacubitril-Valsartan vs Enalapril on Aortic Stiffness in Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2019, 322, 1077–1084. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Vargas-Delgado, A.P.; Requena-Ibanez, J.A.; Garcia-Ropero, A.; Mancini, D.; Pinney, S.; Macaluso, F.; Sartori, S.; Roque, M.; Sabatel-Perez, F.; et al. Randomized Trial of Empagliflozin in Nondiabetic Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 243–255. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Çetin, M.; Özer, S.; Çinier, G.; Yılmaz, A.S.; Erdoğan, T.; Şatıroğlu, Ö. Left atrial volume index and pulmonary arterial pressure predicted MACE among patients with STEMI during 8-year follow-up: Experience from a tertiary center. Herz 2021, 46, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Tsai, C.M.; Chiang, K.C.; Huang, C.C.; Lin, M.S.; Hung, C.L.; Ho, Y.L.; Nkomo, V.T.; Takeuchi, M.; Yang, L.T. Prognostic value of left ventricular and left atrial strain imaging in moderate to severe aortic stenosis: Insights from an Asian population. Int. J. Cardiol. 2024, 407, 132103. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Mondillo, S.; Righini, F.M.; Lisi, M.; Dokollari, A.; Lindqvist, P.; Maccherini, M.; Henein, M. Left Ventricular Deformation and Myocardial Fibrosis in Patients With Advanced Heart Failure Requiring Transplantation. J. Card. Fail. 2016, 22, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Čelutkienė, J.; Plymen, C.M.; Flachskampf, F.A.; de Boer, R.A.; Grapsa, J.; Manka, R.; Anderson, L.; Garbi, M.; Barberis, V.; Filardi, P.P.; et al. Innovative imaging methods in heart failure: A shifting paradigm in cardiac assessment. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1615–1633. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef]
- Tsujiuchi, M.; Yamauchi, T.; Ebato, M.; Maezawa, H.; Nogi, A.; Ikeda, N.; Mizukami, T.; Nagumo, S.; Iso, Y.; Nakadate, T.; et al. Prognostic Value of Left Atrial Size and Functional Indices Measured by 3-Dimensional Speckle-Tracking Analysis. Circ. J. Off. J. Jpn. Circ. Soc. 2019, 83, 801–808. [Google Scholar] [CrossRef]

| Age (y/o), mean ± sd | 64.3 ± 12.5 |
| eGFR (mL/min), mean ± SD | 69.7 ± 24.6 |
| Sex | |
| Male | 83.8% |
| Female | 16.2% |
| Prevalence of medical therapy | |
| B-blockers | 99% |
| MRA | 77.3% |
| iSGLT2 | 100% |
| Sac/Val 24/26 mg bid | 28.2% |
| Sac/Val 49/51 mg bid | 21.8% |
| Sac/Val 97/103 mg bid | 50% |
| Other diuretics | 61.8% |
| Anticoagulant | 50.9% |
| Cardiovascular risk factors | |
| Diabetes mellitus | 14.5% |
| Dyslipidemia | 64.5% |
| Hypertension | 68.2% |
| Active smoking | 9.1% |
| Previous smoking | 58.2% |
| Familiarity for CVD | 29.1% |
| Comorbidities | |
| CKD | 45.4% |
| History of AF | 44.5% |
| Previous TIA/stroke | 9.1% |
| Periferal arterial disease | 20.9% |
| Liver disease | 1.8% |
| Chronic anemia | 2.7% |
| Distiroidism | 17.2% |
| COPD | 26.4% |
| Cardiac diagnosis | |
| primitive/idiopathic DCM | 43% |
| ischemic cardiomyopathy | 35% |
| myocarditis | 7% |
| valvular heart disease | 6% |
| non-compacted myocardium | 5% |
| chemotoxicity | 2% |
| others | 2% |
| Parameter | Baseline (T0) | 3 Months (T1) | 12 Months (T2) |
|---|---|---|---|
| LVEDVi (mL/m2) | 95.3 | 87.8 | 78.9 |
| LVEDD (mm) | 58.8 | 54.7 | 54.7 |
| LAVi (mL/m2) | 49.2 | 46.5 | 43.6 |
| LVEF (%) | 31 | 39 | 40 |
| LVGLS (%) | −8.9 | −11.2 | −11.9 |
| Diastolic Function Grade | 1.7 | 1.3 | 1.3 |
| TAPSE (mm) | 18.4 | 18.8 | 19.6 |
| PAPs (mmHg) | 36.7 | 34.3 | 31.6 |
| MR Grade | 1.1 | 0.9 | 0.9 |
| TR Grade | 0.7 | 0.6 | 0.6 |
| NT-proBNP (pg/mL) | 2411 | 1276 | 1029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fumarulo, I.; Pasquini, A.; La Vecchia, G.; Pellizzeri, B.; Sten, A.; Garramone, B.; Vaccarella, M.; Ravenna, S.E.; Lombardo, A.; Burzotta, F.; et al. Evaluation of the Effects of the Sodium–Glucose Cotransporter 2 Inhibitors and Sacubitril/Valsartan Combined Therapy in Patients with HFrEF: An Echocardiographic Study. Int. J. Mol. Sci. 2025, 26, 5651. https://doi.org/10.3390/ijms26125651
Fumarulo I, Pasquini A, La Vecchia G, Pellizzeri B, Sten A, Garramone B, Vaccarella M, Ravenna SE, Lombardo A, Burzotta F, et al. Evaluation of the Effects of the Sodium–Glucose Cotransporter 2 Inhibitors and Sacubitril/Valsartan Combined Therapy in Patients with HFrEF: An Echocardiographic Study. International Journal of Molecular Sciences. 2025; 26(12):5651. https://doi.org/10.3390/ijms26125651
Chicago/Turabian StyleFumarulo, Isabella, Annalisa Pasquini, Giulia La Vecchia, Bianca Pellizzeri, Andriy Sten, Barbara Garramone, Marcello Vaccarella, Salvatore Emanuele Ravenna, Antonella Lombardo, Francesco Burzotta, and et al. 2025. "Evaluation of the Effects of the Sodium–Glucose Cotransporter 2 Inhibitors and Sacubitril/Valsartan Combined Therapy in Patients with HFrEF: An Echocardiographic Study" International Journal of Molecular Sciences 26, no. 12: 5651. https://doi.org/10.3390/ijms26125651
APA StyleFumarulo, I., Pasquini, A., La Vecchia, G., Pellizzeri, B., Sten, A., Garramone, B., Vaccarella, M., Ravenna, S. E., Lombardo, A., Burzotta, F., Pitocco, D., & Aspromonte, N. (2025). Evaluation of the Effects of the Sodium–Glucose Cotransporter 2 Inhibitors and Sacubitril/Valsartan Combined Therapy in Patients with HFrEF: An Echocardiographic Study. International Journal of Molecular Sciences, 26(12), 5651. https://doi.org/10.3390/ijms26125651







