Iron Status in Sport Horses: Is It Important for Equine Athletes?
Abstract
1. Introduction
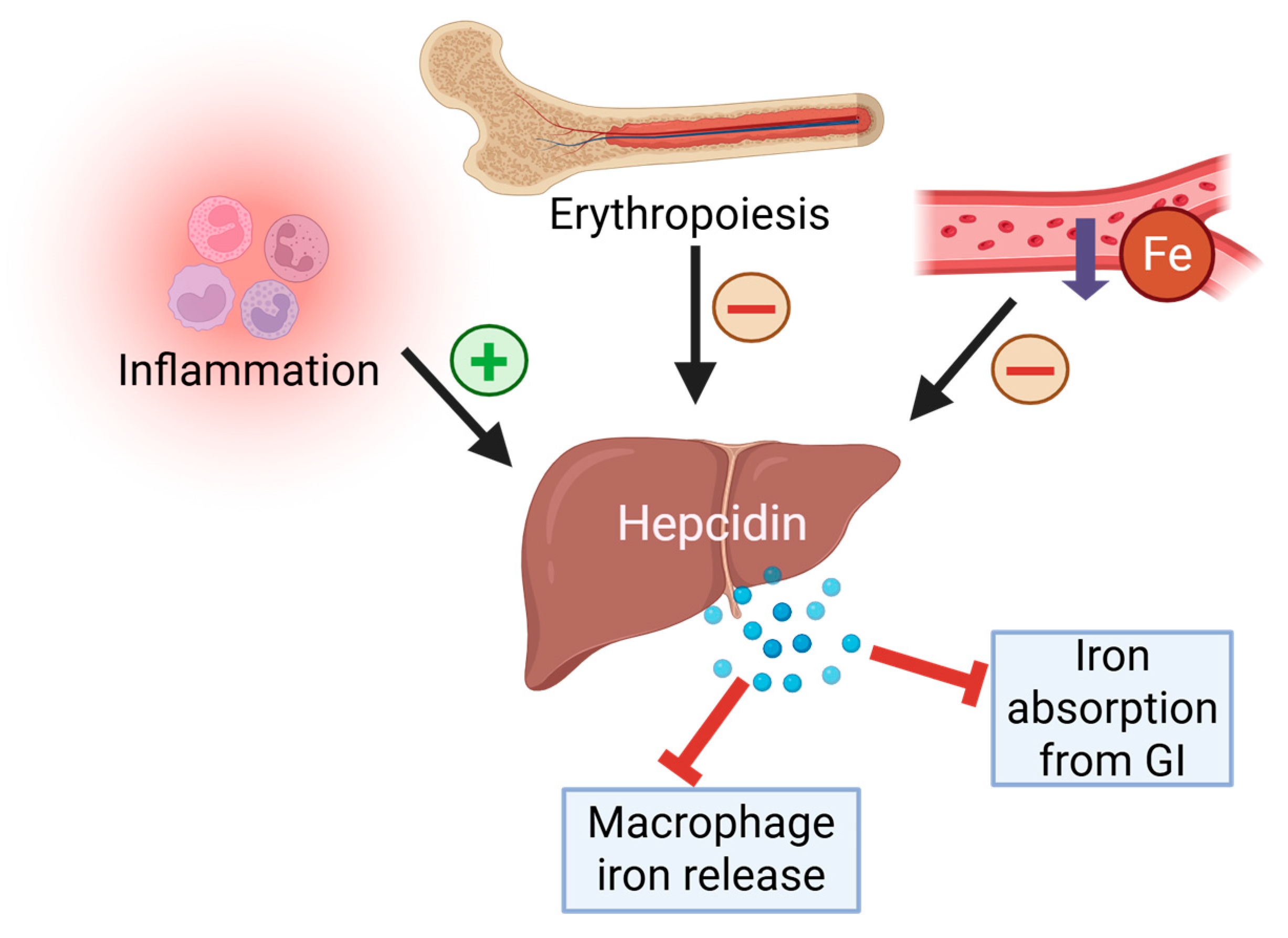
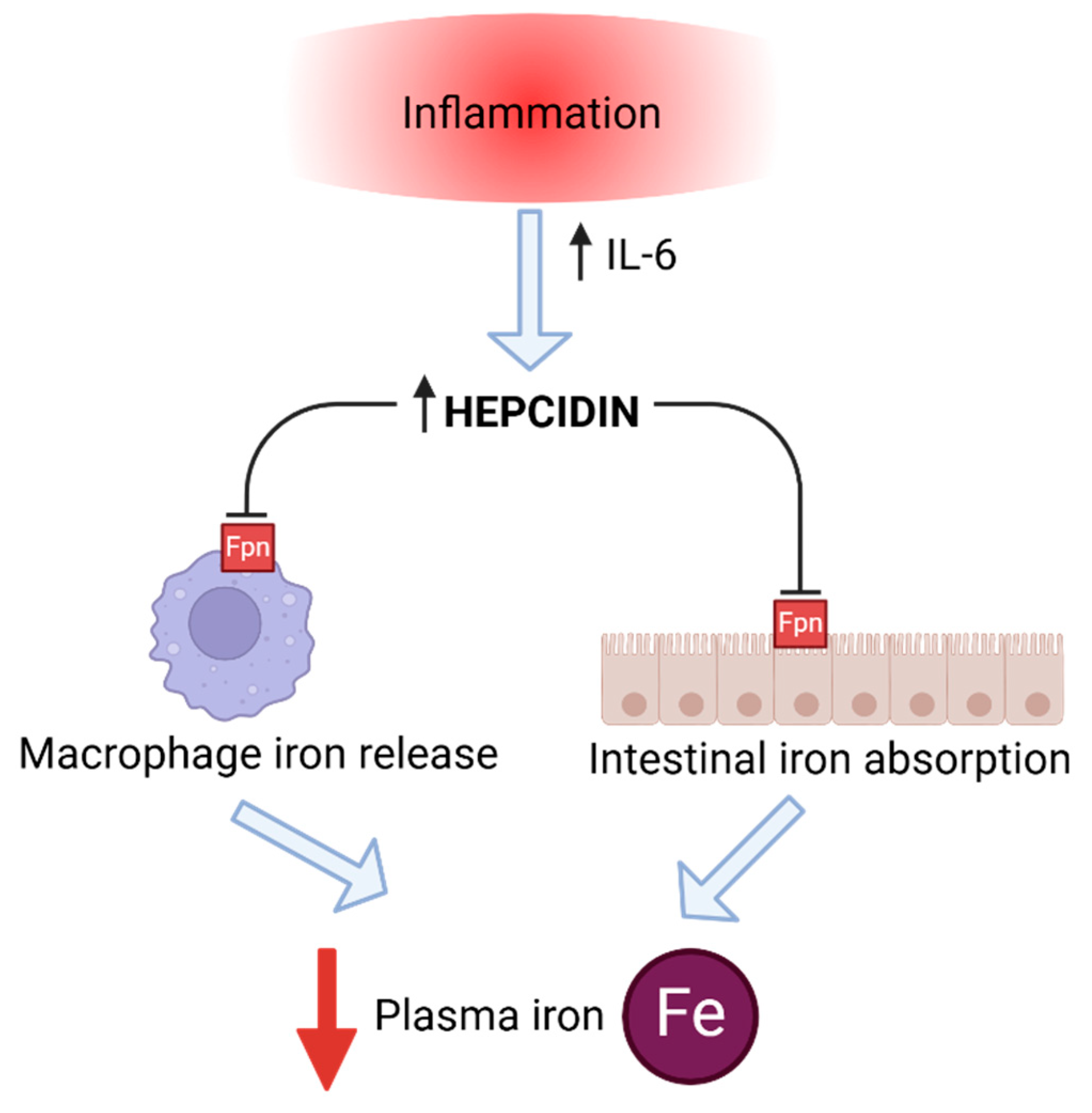
2. Iron Homeostasis
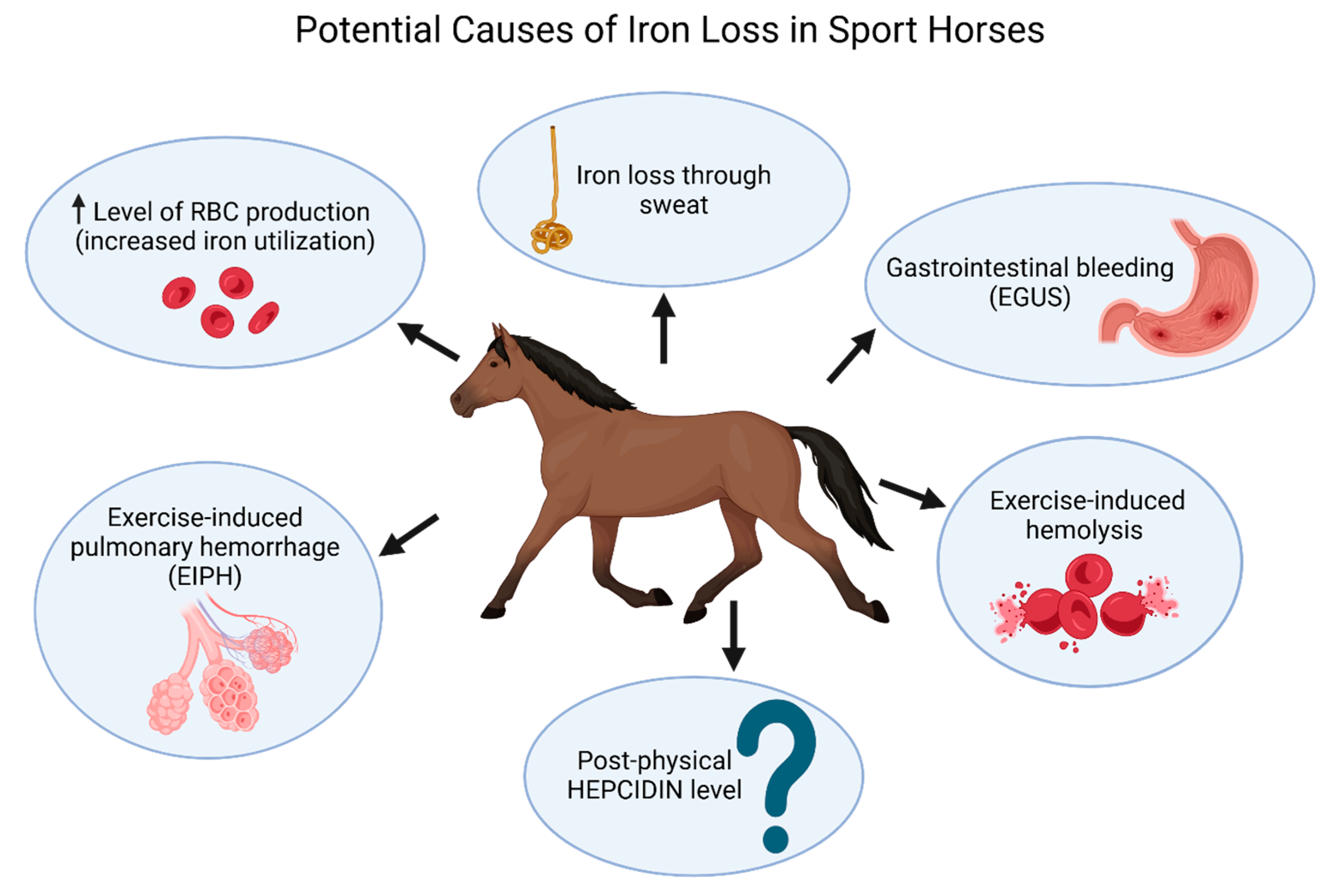
3. Iron and Sport Horses
4. Post-Physical Hepcidin Level, Iron Status, and Inflammation in Sport Horses
5. Hormones Influencing Hepcidin and Iron Status
6. Monitoring Iron Status
7. Performance-Enhancing Strategies Related to Iron Metabolism: Benefits and Risks
8. Clinical Relevance
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lasocki, S.; Gaillard, T.; Rineau, E. Iron is essential for living! Crit. Care 2014, 18, 678. [Google Scholar] [CrossRef] [PubMed]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Aspects Med. 2001, 22, 1–87. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Kück, M.; Radziwolek, L.; Kerling, A. Iron Deficiency in Adolescent and Young Adult German Athletes-A Retrospective Study. Nutrients 2022, 14, 4511. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.B.; Hetzel, S.J.; Brooks, M.A. Iron Deficiency and Anemia among Collegiate Athletes: A Retrospective Chart Review. Med. Sci. Sports Exerc. 2017, 49, 1711–1715. [Google Scholar] [CrossRef]
- Sim, M.; Garvican-Lewis, L.A.; Cox, G.R.; Govus, A.; McKay, A.K.A.; Stellingwerff, T.; Peeling, P. Iron considerations for the athlete: A narrative review. Eur. J. Appl. Physiol. 2019, 119, 1463–1478. [Google Scholar] [CrossRef]
- Eichner, E.R. Sports anemia, iron supplements, and blood doping. Med. Sci. Sports Exerc. 1992, 24 (Suppl. 9), S315–S318. [Google Scholar] [CrossRef]
- Clénin, G.; Cordes, M.; Huber, A.; Schumacher, Y.O.; Noack, P.; Scales, J.; Kriemler, S. Iron deficiency in sports—Definition, influence on performance and therapy. Swiss Med. Wkly. 2015, 145, w14196. [Google Scholar] [CrossRef]
- Weaver, C.M.; Rajaram, S. Exercise and iron status. J. Nutr. 1992, 122 (Suppl. 3), 782–787. [Google Scholar] [CrossRef]
- Inoue, Y.; Matsui, A.; Asai, Y.; Aoki, F.; Matsui, T.; Yano, H. Effect of exercise on iron metabolism in horses. Biol. Trace Elem. Res. 2005, 107, 33–42. [Google Scholar] [CrossRef]
- Larsson, J.; Pilborg, P.H.; Johansen, M.; Christophersen, M.T.; Holte, A.; Roepstorff, L.; Olsen, L.H.; Harrison, A.P. Physiological parameters of endurance horses pre- compared to post-race, correlated with performance: A two race study from scandinavia. ISRN Vet. Sci. 2013, 2013, 684353. [Google Scholar] [CrossRef]
- Witkowska-Piłaszewicz, O.; Malin, K.; Dąbrowska, I.; Grzędzicka, J.; Ostaszewski, P.; Carter, C. Immunology of Physical Exercise: Is Equus caballus an Appropriate Animal Model for Human Athletes? Int. J. Mol. Sci. 2024, 25, 5210. [Google Scholar] [CrossRef] [PubMed]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E. The horse as a model of naturally occurring osteoarthritis. Bone Jt. Res. 2012, 1, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Patterson-Kane, J.C.; Becker, D.L.; Rich, T. The pathogenesis of tendon microdamage in athletes: The horse as a natural model for basic cellular research. J. Comp. Pathol. 2012, 147, 227–247. [Google Scholar] [CrossRef]
- Hooda, J.; Shah, A.; Zhang, L. Heme, an essential nutrient from dietary proteins, critically impacts diverse physiological and pathological processes. Nutrients 2014, 6, 1080–1102. [Google Scholar] [CrossRef]
- Skrzypczak, W.; Stefaniak, T.; Zabielski, R. Fizjologia Noworodka; PWRiL: Warsaw, Poland, 2011; pp. 286–291. [Google Scholar]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing acts: Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef]
- Malyszko, J. Hepcidin assays: Ironing out some details. Clin. J. Am. Soc. Nephrol. 2009, 4, 1015–1016. [Google Scholar] [CrossRef]
- Ganz, T. Molecular control of iron transport. J. Am. Soc. Nephrol. 2007, 18, 394–400. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). The Nutrient Requirements of Horses, 6th ed.; Natl Acad Press: Washington, DC, USA, 2022. [Google Scholar]
- Saran, T.; Zawadka, M.; Chmiel, S.; Mazur, A. Sweat iron concentration during 4-week exercise training. Ann. Agric. Environ. Med. 2018, 25, 500–503. [Google Scholar] [CrossRef]
- Waller, M.F.; Haymes, E.M. The effects of heat and exercise on sweat iron loss. Med. Sci. Sports Exerc. 1996, 28, 197–203. [Google Scholar] [CrossRef]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Trinder, D. Athletic induced iron deficiency: New insights into the role of inflammation, cytokines and hormones. Eur. J. Appl. Physiol. 2008, 103, 381–391. [Google Scholar] [CrossRef]
- Pakula, P.D.; Halama, A.; Al-Dous, E.K.; Johnson, S.J.; Filho, S.A.; Suhre, K.; Vinardell, T. Characterization of exercise-induced hemolysis in endurance horses. Front. Vet. Sci. 2023, 10, 1115776. [Google Scholar] [CrossRef] [PubMed]
- Masini, A.; Tedeschi, D.; Baragli, P.; Sighieri, C.; Lubas, G. Exercise-induced intravascular haemolysis in standardbred horses. Comp. Clin. Pathol. 2003, 12, 45–48. [Google Scholar] [CrossRef]
- Cywinska, A.; Szarska, E.; Kowalska, A.; Ostaszewski, P.; Schollenberger, A. Gender differences in exercise-induced intravascular haemolysis during race training in thoroughbred horses. Res. Vet. Sci. 2011, 90, 133–137. [Google Scholar] [CrossRef]
- Schott, H.C., 2nd; Hodgson, D.R.; Bayly, W.M. Haematuria, pigmenturia and proteinuria in exercising horses. Equine Vet. J. 1995, 27, 67–72. [Google Scholar]
- Fisher, R.L.; McMahon, L.F., Jr.; Ryan, M.J.; Larson, D.; Brand, M. Gastrointestinal bleeding in competitive runners. Dig. Dis. Sci. 1986, 31, 1226–1228. [Google Scholar] [CrossRef] [PubMed]
- Baska, R.S.; Moses, F.M.; Graeber, G.; Kearney, G. Gastrointestinal bleeding during an ultramarathon. Dig. Dis. Sci. 1990, 35, 276–279. [Google Scholar] [CrossRef]
- Grooteman, K.; van Geenen, E.; Kievit, W.; Drenth, J. Chronic anemia due to gastrointestinal bleeding: When do gastroenterologists transfuse? United Eur. Gastroenterol. J. 2017, 5, 967–973. [Google Scholar] [CrossRef][Green Version]
- Nieto, J.E.; Snyder, J.R.; Beldomenico, P.; Aleman, M.; Kerr, J.W.; Spier, S.J. Prevalence of gastric ulcers in endurance horses—A preliminary report. Vet. J. 2004, 167, 33–37. [Google Scholar] [CrossRef]
- Vatistas, N.J.; Snyder, J.R.; Carlson, G.; Johnson, B.; Arthur, R.M.; Thurmond, M.; Zhou, H.; Lloyd, K.L. Cross-sectional study of gastric ulcers of the squamous mucosa in thoroughbred racehorses. Equine Vet. J. Suppl. 1999, 31, 34–39. [Google Scholar] [CrossRef]
- Lo Feudo, C.M.; Stucchi, L.; Conturba, B.; Stancari, G.; Zucca, E.; Ferrucci, F. Equine Gastric Ulcer Syndrome affects fitness parameters in poorly performing Standardbred racehorses. Front. Vet. Sci. 2022, 9, 1014619. [Google Scholar] [CrossRef]
- Lo Feudo, C.M.; Stucchi, L.; Conturba, B.; Stancari, G.; Zucca, E.; Ferrucci, F. Medical causes of poor performance and their associations with fitness in Standardbred racehorses. J. Vet. Intern. Med. 2023, 37, 1514–1527. [Google Scholar] [CrossRef] [PubMed]
- Crispe, E.J.; Lester, G.D. Exercise-induced Pulmonary Hemorrhage: Is It Important and Can It Be Prevented? Vet. Clin. North. Am. Equine Pract. 2019, 35, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Cotroneo, E.; Ashek, A.; Wang, L.; Wharton, J.; Dubois, O.; Bozorgi, S.; Busbridge, M.; Alavian, K.N.; Wilkins, M.R.; Zhao, L. Iron homeostasis and pulmonary hypertension: Iron deficiency leads to pulmonary vascular remodeling in the rat. Circ. Res. 2015, 116, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.N.; Gao, G.; Chang, Y.Z. Hepcidin and sports anemia. Cell Biosci. 2014, 4, 19. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. The role of hepcidin in iron metabolism. Acta Haematol. 2009, 122, 78–86. [Google Scholar] [CrossRef]
- Sim, M.; Dawson, B.; Landers, G.; Swinkels, D.W.; Tjalsma, H.; Trinder, D.; Peeling, P. Effect of exercise modality and intensity on post-exercise interleukin-6 and hepcidin levels. Int. J. Sport. Nutr. Exerc. Metab. 2013, 23, 178–186. [Google Scholar] [CrossRef]
- Peeling, P.; McKay, A.K.A.; Pyne, D.B.; Guelfi, K.J.; McCormick, R.H.; Laarakkers, C.M.; Swinkels, D.W.; Garvican-Lewis, L.A.; Ross, M.L.R.; Sharma, A.P.; et al. Factors influencing the post-exercise hepcidin-25 response in elite athletes. Eur. J. Appl. Physiol. 2017, 117, 1233–1239. [Google Scholar] [CrossRef]
- Badenhorst, C.E.; Dawson, B.; Goodman, C.; Sim, M.; Cox, G.R.; Gore, C.J.; Tjalsma, H.; Swinkels, D.W.; Peeling, P. Influence of post-exercise hypoxic exposure on hepcidin response in athletes. Eur. J. Appl. Physiol. 2014, 114, 951–959. [Google Scholar] [CrossRef]
- Newlin, M.K.; Williams, S.; McNamara, T.; Tjalsma, H.; Swinkels, D.W.; Haymes, E.M. The effects of acute exercise bouts on hepcidin in women. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 79–88. [Google Scholar] [CrossRef]
- Peeling, P.; Sim, M.; Badenhorst, C.E.; Dawson, B.; Govus, A.D.; Abbiss, C.R.; Swinkels, D.W.; Trinder, D. Iron status and the acute post-exercise hepcidin response in athletes. PLoS ONE 2014, 9, e93002. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Li, Q.; Wu, Y.; Jia, X.; Feng, W.; Li, Z.; Shi, Y.; Hou, Q.; Ma, J.; et al. Lactate modulates iron metabolism by binding soluble adenylyl cyclase. Cell Metab. 2023, 35, 1597–1612.e6. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise-A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef]
- Beavers, K.M.; Brinkley, T.E.; Nicklas, B.J. Effect of exercise training on chronic inflammation. Clin. Chim. Acta 2010, 411, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. Hepcidin-The Culprit Explaining Disturbed Iron Homeostasis in Chronic Renal Disease?: IL-6 Mediates Hypoferremia of Inflammation by Inducing the Synthesis of the Iron Regulatory Hormone Hepcidin. J Clin Invest 113:1271-1276, 2004. J. Am. Soc. Nephrol. 2005, 16, 287–290. [Google Scholar] [CrossRef]
- Banzet, S.; Sanchez, H.; Chapot, R.; Bigard, X.; Vaulont, S.; Koulmann, N. Interleukin-6 contributes to hepcidin mRNA increase in response to exercise. Cytokine 2012, 58, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Wlefting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef]
- Witkowska-Piłaszewicz, O.; Bąska, P.; Czopowicz, M.; Żmigrodzka, M.; Szarska, E.; Szczepaniak, J.; Nowak, Z.; Winnicka, A.; Cywińska, A. Anti-Inflammatory State in Arabian Horses Introduced to the Endurance Training. Animals 2019, 9, 616. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Keller, P.; Plomgaard, P.; Febbraio, M.; Saltin, B. Searching for the exercise factor: Is IL-6 a candidate? J. Muscle Res. Cell Motil. 2003, 24, 113–119. [Google Scholar] [CrossRef]
- Oliveira-Filho, J.P.; Badial, P.R.; Cunha, P.H.; Peiró, J.R.; Araújo, J.P., Jr.; Divers, T.J.; Winand, N.J.; Borges, A.S. Lipopolysaccharide infusion up-regulates hepcidin mRNA expression in equine liver. Innate Immun. 2012, 18, 438–446. [Google Scholar] [CrossRef]
- Arfuso, F.; Giannetto, C.; Fazio, F.; Panzera, F.; Piccione, G. Training Program Intensity Induces an Acute Phase Response in Clinically Healthy Horses. J. Equine Vet. Sci. 2020, 88, 102986. [Google Scholar] [CrossRef]
- Kristensen, L.; Buhl, R.; Nostell, K.; Bak, L.; Petersen, E.; Lindholm, M.; Jacobsen, S. Acute exercise does not induce an acute phase response (APR) in Standardbred trotters. Can. J. Vet. Res. 2014, 78, 97–102. [Google Scholar] [PubMed]
- Colahan, P.T.; Kollias-Bakert, C.; Leutenegger, C.M.; Jones, J.H. Does training affect mRNA transciption for cytokine production in circulating leucocytes? Equine Vet. J. Suppl. 2002, 34, 154–158. [Google Scholar] [CrossRef]
- Witkowska-Piłaszewicz, O.; Pingwara, R.; Winnicka, A. The Effect of Physical Training on Peripheral Blood Mononuclear Cell Ex Vivo Proliferation, Differentiation, Activity, and Reactive Oxygen Species Production in Racehorses. Antioxidants 2020, 9, 1155. [Google Scholar] [CrossRef] [PubMed]
- Cadegiani, F.A.; Kater, C.E. Hormonal aspects of overtraining syndrome: A systematic review. BMC Sports Sci. Med. Rehabil. 2017, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Nederhof, E.; Buyse, L.; Roelands, B.; de Schutter, G.; Piacentini, M.F. Diagnosing overtraining in athletes using the two-bout exercise protocol. Br. J. Sports Med. 2010, 44, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Piacentini, M.F.; Busschaert, B.; Buyse, L.; De Schutter, G.; Stray-Gundersen, J. Hormonal responses in athletes: The use of a two bout exercise protocol to detect subtle differences in (over)training status. Eur. J. Appl. Physiol. 2004, 91, 140–146. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Xu, Z.; Dai, J.; Song, P.; Shi, J.; Hu, Y.; Hu, Z.; Nie, G.; Chang, Y.Z.; et al. Hepcidin levels in hyperprolactinemic women monitored by nanopore thin film based assay: Correlation with pregnancy-associated hormone prolactin. Nanomedicine 2015, 11, 871–878. [Google Scholar] [CrossRef]
- Lehtihet, M.; Bonde, Y.; Beckman, L.; Berinder, K.; Hoybye, C.; Rudling, M.; Sloan, J.H.; Konrad, R.J.; Angelin, B. Circulating Hepcidin-25 Is Reduced by Endogenous Estrogen in Humans. PLoS ONE 2016, 11, e0148802. [Google Scholar] [CrossRef]
- Moreno-Carranza, B.; Bravo-Manríquez, M.; Baez, A.; Ledesma-Colunga, M.G.; Ruiz-Herrera, X.; Reyes-Ortega, P.; de Los Ríos, E.A.; Macotela, Y.; Martínez de la Escalera, G.; Clapp, C. Prolactin regulates liver growth during postnatal development in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R902–R908. [Google Scholar] [CrossRef]
- Thompson, D.L., Jr.; DePew, C.L.; Ortiz, A.; Sticker, L.S.; Rahmanian, M.S. Growth hormone and prolactin concentrations in plasma of horses: Sex differences and the effects of acute exercise and administration of growth hormone-releasing hormone. J. Anim. Sci. 1994, 72, 2911–2918. [Google Scholar] [CrossRef][Green Version]
- Kitaura, T.; Sato, F.; Hada, T.; Ishimaru, M.; Kodama, R.; Nambo, Y.; Watanabe, G.; Taya, K. Influence of exercise and emotional stresses on secretion of prolactin and growth hormone in Thoroughbred horses. J. Equine Sci. 2021, 32, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Assenza, A.; Arfuso, F.; Fazio, F.; Giannetto, C.; Rizzo, M.; Zumbo, A.; Piccione, G. Effect of gender and jumping exercise on leukocyte number, dopamine and prolactin levels in horses. Thai J. Vet. Med. 2018, 48, 95–101. [Google Scholar] [CrossRef]
- Bachman, E.; Feng, R.; Travison, T.; Li, M.; Olbina, G.; Ostland, V.; Ulloor, J.; Zhang, A.; Basaria, S.; Ganz, T.; et al. Testosterone suppresses hepcidin in men: A potential mechanism for testosterone-induced erythrocytosis. J. Clin. Endocrinol. Metab. 2010, 95, 4743–4747. [Google Scholar] [CrossRef]
- Guo, W.; Bachman, E.; Li, M.; Roy, C.N.; Blusztajn, J.; Wong, S.; Chan, S.Y.; Serra, C.; Jasuja, R.; Travison, T.G.; et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell 2013, 12, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Latour, C.; Kautz, L.; Besson-Fournier, C.; Island, M.L.; Canonne-Hergaux, F.; Loréal, O.; Ganz, T.; Coppin, H.; Roth, M.P. Testosterone perturbs systemic iron balance through activation of epidermal growth factor receptor signaling in the liver and repression of hepcidin. Hepatology 2014, 59, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Bachman, E.; Travison, T.G.; Basaria, S.; Davda, M.N.; Guo, W.; Li, M.; Connor Westfall, J.; Bae, H.; Gordeuk, V.; Bhasin, S. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: Evidence for a new erythropoietin/hemoglobin set point. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 725–735. [Google Scholar] [CrossRef]
- Neuberg-Zuchowicz, K.; Oedenberg, H. Changes in hematological parameters of show jumping horses during yearly training cycle. Med. Weter. 2011, 67, 765–769. [Google Scholar]
- Czech, A.; Kiesz, M.; Kiesz, A.; Próchniak, T.; Różański, P.; Klimiuk, K. Influence of Type of Use, Age and Gender on Haematological and Biochemical Blood Parameters of Małopolski Horses. Ann. Anim. Sci. 2019, 19, 85–96. [Google Scholar] [CrossRef]
- Dąbrowska, I.; Grzędzicka, J.; Malin, K.; Pawliński, B.; Mickiewicz, J.; Witkowska-Piłaszewicz, O. Intense Leisure Exploitation Influences on Horses Hormonal Reaction—Preliminary Study. Agriculture 2022, 12, 1777. [Google Scholar] [CrossRef]
- Grzędzicka, J.; Dąbrowska, I.; Malin, K.; Witkowska-Piłaszewicz, O. Exercise-related changes in the anabolic index (testosterone to cortisol ratio) and serum amyloid A concentration in endurance and racehorses at different fitness levels. Front. Vet. Sci. 2023, 10, 1148990. [Google Scholar] [CrossRef]
- Yang, Q.; Jian, J.; Katz, S.; Abramson, S.B.; Huang, X. 17β-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology 2012, 153, 3170–3178. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, S.; Wang, L.; Li, J.; Qu, G.; He, J.; Rong, H.; Ji, H.; Liu, S. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene 2012, 511, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Magallanes, V.M.; Barba-Moreno, L.; Romero-Parra, N.; Rael, B.; Benito, P.J.; Swinkels, D.W.; Laarakkers, C.M.; Díaz, Á.E.; Peinado, A.B.; IronFEMME Study Group. Menstrual cycle affects iron homeostasis and hepcidin following interval running exercise in endurance-trained women. Eur. J. Appl. Physiol. 2022, 122, 2683–2694. [Google Scholar] [CrossRef]
- Satué, K.; Fazio, E.; La Fauci, D.; Medica, P. Changes of Hepcidin, Ferritin and Iron Levels in Cycling Purebred Spanish Mares. Animals 2023, 13, 1229. [Google Scholar] [CrossRef]
- Li, X.; Rhee, D.K.; Malhotra, R.; Mayeur, C.; Hurst, L.A.; Ager, E.; Shelton, G.; Kramer, Y.; McCulloh, D.; Keefe, D.; et al. Progesterone receptor membrane component-1 regulates hepcidin biosynthesis. J. Clin. Investig. 2016, 126, 389–401. [Google Scholar] [CrossRef]
- Wideman, L.; Weltman, J.Y.; Hartman, M.L.; Veldhuis, J.D.; Weltman, A. Growth hormone release during acute and chronic aerobic and resistance exercise: Recent findings. Sports Med. 2002, 32, 987–1004. [Google Scholar] [CrossRef]
- Vigas, M.; Celko, J.; Koska, J. Role of body temperature in exercise-induced growth hormone and prolactin release in non-trained and physically fit subjects. Endocr. Regul. 2000, 34, 175–180. [Google Scholar]
- Roemmich, J.N.; Rogol, A.D. Exercise and growth hormone: Does one affect the other? J. Pediatr. 1997, 131 Pt 2, S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Troutt, J.S.; Rudling, M.; Persson, L.; Ståhle, L.; Angelin, B.; Butterfield, A.M.; Schade, A.E.; Cao, G.; Konrad, R.J. Circulating human hepcidin-25 concentrations display a diurnal rhythm, increase with prolonged fasting, and are reduced by growth hormone administration. Clin. Chem. 2012, 58, 1225–1232. [Google Scholar] [CrossRef]
- Krygier, A.; Szczepanek-Parulska, E.; Cieślewicz, M.; Wrotkowska, E.; Chanaj-Kaczmarek, J.; Ruchała, M. Iron Homeostasis and Hepcidin Concentration in Patients With Acromegaly. Front. Endocrinol. 2022, 12, 788247. [Google Scholar] [CrossRef]
- Goodnough, J.B.; Ramos, E.; Nemeth, E.; Ganz, T. Inhibition of hepcidin transcription by growth factors. Hepatology 2012, 56, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Solberg, A.; Reikvam, H. Iron Status and Physical Performance in Athletes. Life 2023, 13, 2007. [Google Scholar] [CrossRef] [PubMed]
- Fallon, K.E. Utility of hematological and iron-related screening in elite athletes. Clin. J. Sport Med. 2004, 14, 145–152. [Google Scholar] [CrossRef] [PubMed]
- DellaValle, D.M.; Haas, J.D. Iron supplementation improves energetic efficiency in iron-depleted female rowers. Med. Sci. Sports Exerc. 2014, 46, 1204–1215. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Sim, M.; Moretti, D.; Hall, R.; Stellingwerff, T.; Burden, R.J.; Peeling, P. Methodological Considerations for Investigating Iron Status and Regulation in Exercise and Sport Science Studies. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 359–370. [Google Scholar] [CrossRef]
- Satué, K.; Fazio, E.; La Fauci, D.; Medica, P. Hematological indexes and iron status in pregnant mares. Arch. Anim. Breed. 2023, 66, 197–205. [Google Scholar] [CrossRef]
- Animal Health Diagnostic Center. Available online: https://www.vet.cornell.edu/animal-health-diagnostic-center/laboratories/clinical-pathology/reference-intervals/chemistry (accessed on 7 January 2024).
- Burlikowska, K.; Bogusławska-Tryk, M.; Szymeczko, R.; Piotrowska, A. Haematological and biochemical blood parameters in horses used for sport and recreation. J. Cent. Eur. Agric. 2015, 16, 370–382. [Google Scholar] [CrossRef]
- Mills, P.C.; Smith, N.C.; Casas, I.; Harris, P.; Harris, R.C.; Marlin, D.J. Effects of exercise intensity and environmental stress on indices of oxidative stress and iron homeostasis during exercise in the horse. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 60–66. [Google Scholar] [CrossRef]
- Bollinger, L.; Bartel, A.; Weber, C.; Gehlen, H. Pre-Ride Biomarkers and Endurance Horse Welfare: Analyzing the Impact of the Elimination of Superoxide Dismutase, δ-Aminolevulinic-Dehydratase, Thiobarbituric Acid Reactive Substances, Iron, and Serum Amyloid A Levels in Elite 160 km Endurance Rides. Animals 2023, 13, 1670. [Google Scholar] [CrossRef]
- Hyyppä, S.; Höyhtyä, M.; Nevalainen, M.; Pösö, A.R. Effect of exercise on plasma ferritin concentrations: Implications for the measurement of iron status. Equine Vet. J. Suppl. 2002, 34, 186–190. [Google Scholar] [CrossRef]
- Assenza, A.; Congiu, F.; Giannetto, C.; Fazio, F.; Piccione, G. Serum iron, ferritin, transferrin and haptoglobin concentration variations during repeated show jumping competition in horse. Acta Vet. Brno 2017, 85, 343–347. [Google Scholar] [CrossRef][Green Version]
- Piccione, G.; Rizzo, M.; Arfuso, F.; Bruschetta, D.; Giudice, E.; Assenza, A. Iron Metabolism Modification During Repeated Show Jumping Event in Equine Athletes. Ann. Anim. Sci. 2017, 17, 197–204. [Google Scholar] [CrossRef][Green Version]
- Abramovitc, G.; Parra, A.C.; Fernandes, W.R. Changes in iron levels, total iron binding capacity, transferrin saturation in race horses, before and after of physical exercise. Braz. J. Vet. Med. 2014, 36, 289–293. [Google Scholar]
- Assenza, A.; Casella, S.; Giannetto, C.; Fazio, F.; Tosto, F.; Piccione, G. Iron profile in Thoroughbreds during a standard training program. Aust. Vet. J. 2016, 94, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Osawa, T.; Matsui, A.; Asai, Y.; Murakami, Y.; Matsui, T.; Yano, H. Changes of Serum Mineral Concentrations in Horses during Exercise. Anim. Biosci. 2002, 15, 531–536. [Google Scholar] [CrossRef]
- Ohira, Y.; Kariya, F.; Yasui, W.; Sugawara, S.; Koyanagi, K.; Kaihatsu, K.; Inoue, N.; Hirata, F.; Chen, C.; Ohno, H. Physical exercise and iron metabolism. In Sports Nutrition: Minerals and Electrolytes; Kies, C.V., Driskell, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 5–12. [Google Scholar]
- Allen, K.J.; van Erck-Westergren, E.; Franklin, S.H. Exercise Testing in the Equine Athlete. Equine Vet. Educ. 2016, 28, 89–98. [Google Scholar] [CrossRef]
- Hoes, M.F.; Grote Beverborg, N.; Kijlstra, J.D.; Kuipers, J.; Swinkels, D.W.; Giepmans, B.N.G.; Rodenburg, R.J.; van Veldhuisen, D.J.; de Boer, R.A.; van der Meer, P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur. J. Heart Fail. 2018, 20, 910–919. [Google Scholar] [CrossRef]
- Theelen, M.J.P.; Beukers, M.; Grinwis, G.C.M.; Sloet van Oldruitenborgh-Oosterbaan, M.M. Chronic iron overload causing haemochromatosis and hepatopathy in 21 horses and one donkey. Equine Vet. J. 2019, 51, 304–309. [Google Scholar] [CrossRef]
- Pearson, E.G.; Andreasen, C.B. Effect of oral administration of excessive iron in adult ponies. J. Am. Vet. Med. Assoc. 2001, 218, 400–404. [Google Scholar] [CrossRef]
- McKeever, K.H.; Agans, J.M.; Geiser, S.; Lorimer, P.J.; Maylin, G.A. Low dose exogenous erythropoietin elicits an ergogenic effect in standardbred horses. Equine Vet. J. Suppl. 2006, 36, 233–238. [Google Scholar] [CrossRef]
- McKeever, K.H.; Agans, J.M.; Geiser, S.; Scali, R.; Guirnalda, P.D.; Kearns, C.F.; Lorimer, P.J. Effect of recombinant human erythropoietin administration on red cell volume, aerobic capacity and indices of performance in standardbred horses. In Proceedings of the 16th Equine Nutrition and Physiology Symposium, Raleigh, NC, USA, 2–5 June 1999; pp. 163–164. [Google Scholar]
- Wickler, S.J.; Greene, H.M. High altitude acclimatization and athletic performance in horses. Equine Comp. Exerc. Physiol. 2004, 1, 167–170. [Google Scholar] [CrossRef]
- Moerman, W. A Critical View on Hypoxia Training: Horse Versus Human. Master’s Thesis, Ghent Ghent University, Ghent, Belgium, 2018. [Google Scholar]
- Lewis, L.D.; Knight, A.; Lewis, B. Equine Clinical Nutrition: Feedings and Care; Williams & Wilkins: Baltimore, MD, USA, 1995. [Google Scholar]
- Auer, D.E.; Ng, J.C.; Thompson, H.L.; Inglis, S.; Seawright, A.A. Acute phase response in horses: Changes in plasma cation concentrations after localised tissue injury. Vet. Rec. 1989, 124, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Fleming, K.A.; Barton, M.H.; Latimer, K.S. Iron deficiency anemia in a neonatal foal. J. Vet. Intern. Med. 2006, 20, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.E.; Cipriano, J.E. Inflammation-induced changes in serum iron analytes and ceruloplasmin of Shetland ponies. Vet. Pathol. 1987, 24, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.S.; Divers, T.J.; Stokol, T.; Mohammed, O.H. Serum iron and plasma fibrinogen concentrations as indicators of systemic inflammatory diseases in horses. J. Vet. Intern. Med. 2007, 21, 489–494. [Google Scholar] [CrossRef]
- Piccione, G.; Casella, S.; Giannetto, C.; Messina, V.; Monteverde, V.; Caola, G.; Guttadauro, S. Haematological and haematochemical responses to training and competition in standardbred horses. Comp. Clin. Pathol. 2010, 19, 95–101. [Google Scholar] [CrossRef]
- Wood, S.C.; Fedde, M.R. Effects of racing and gender on viscoelastic properties of horse blood. Respir. Physiol. 1997, 107, 165–172. [Google Scholar] [CrossRef]
- Hassan, H.; Aly, M.; ELseady, Y.; Nayel, M.; Elsify, A.; Salama, A.; Hassan, M.; Elbarody, E.; Kamar, A. The Effect of Race in the Clinical, Hematological and Biochemical Biomarkers in Thoroughbred Horses. Alex. J. Vet. Sci. 2015, 46, 161–169. [Google Scholar] [CrossRef]
- Maśko, M.; Domino, M.; Jasiński, T.; Witkowska-Piłaszewicz, O. The Physical Activity-Dependent Hematological and Biochemical Changes in School Horses in Comparison to Blood Profiles in Endurance and Race Horses. Animals 2021, 11, 1128. [Google Scholar] [CrossRef]
- Hinchcliff, K.W.; Kaneps, A.J.; Geor, R.J. Equine Sports Medicine and Surgery: Basic and Clinical Sciences of the Equine Athlete, 2nd ed.; Elsevier: St. Louis, MO, USA, 2014. [Google Scholar]
- Schalm’s Veterinary Hematology, 6th ed.; Weiss, D.J., Wardrop, K.J., Eds.; Wiley-Blackwell: Ames, IA, USA, 2010. [Google Scholar]
- Padalino, B.; Rubino, G.; Lacinio, R.; Petazzi, F. A New Classification to Diagnose Type of Anemia in Standardbred Horses: A Retrospective Study. JEVS 2016, 44, 21–25. [Google Scholar] [CrossRef]
- Kellon, E.M. Equine Anaemia—It’s NOT About Iron Deficiency. Forageplus Talk. Available online: https://www.forageplustalk.co.uk/equine-anaemia-by-dr-kellon/ (accessed on 28 May 2025).
- Schryver, H.F. Mineral and vitamin intoxication in horses. Vet. Clin. N. Am. Equine Pract. 1990, 6, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.J.; Allen, J.R. Hematologic responses to exercise and training. Vet. Clin. N. Am. Equine Pract. 1985, 1, 461–476. [Google Scholar] [CrossRef] [PubMed]
| Process | Key Components | Iron Form | Function |
|---|---|---|---|
| Iron Absorption [14] | Duodenum, Enterocytes | Fe2+ (heme) or Fe3+ (non-heme) | Absorption of iron from food |
| Reduction of Non-Heme Iron [15,16] | Duodenal cytochrome b (Dcytb) | Fe3+ → Fe2+ | Reduction of non-heme iron to assimilable form |
| Iron Transport into Enterocytes [14,15,16] | Divalent metal transporter 1 (DMT1) | Fe2+ | Transport of Fe2+ into enterocyte cytoplasm |
| Iron Utilization or Export [14,15,16] | Ferroportin (Fpn), Hephaestin | Fe2+ → Fe3+ | Internal use or export to blood |
| Iron Transport in Blood [14,15,16] | Transferrin (Tf) | Fe3+ | Binding iron for transport to cells |
| Cellular Iron Uptake [15,16] | Transferrin Receptors (TfR1, TfR2), Cubilin (kidney) | Fe3+ → Cellular Iron | Cellular iron uptake via receptor-mediated endocytosis |
| Regulation of Iron Homeostasis [15,16] | Hepcidin (liver), Ferroportin degradation | Inhibits Fe2+ release by degrading ferroportin | Control of iron concentrations via hepcidin–ferroportin interaction |
| Research Area Related to Iron Status | Obtained Results | Publication |
|---|---|---|
| Resting serum iron concentrations in sport and recreational horses | Sport horses have higher serum Fe concentrations than recreational horses | Burlikowska et al. (2015) [90] |
| Effect of different exercise protocols (aerobic of varying intensity and duration) on iron homeostasis | Different types of physical exercise affect iron homeostasis in various ways | Mills et al. (1996) [91] |
| Iron concentrations as a potential indicator of elimination risk before an endurance ride | Iron concentrations are not reliable indicators for race elimination risk | Bollinger et al. (2023) [92] |
| Effect of aerobic exercise of varying intensity on plasma ferritin concentrations | Physical exertion elevates plasma ferritin concentrations, with a greater increase observed after prolonged and/or more intense exercise | Hyyppä et al., (2002) [93] |
| Effect of show jumping on iron homeostasis | Increased serum concentrations of ferritin, iron, and transferrin levels during repeated competition | Assenza et al. (2017) [94] |
| Effect of show jumping on iron homeostasis | Increased concentrations of iron, ferritin, transferrin, TIBC, and UIBC | Piccione et al. (2017) [95] |
| Effect of high intensity, short physical exercise (gallop) on iron homeostasis | Significant decrease in serum iron concentration, along with increased TIBC and transferrin saturation after exercise | Abramovitc et al. (2014) [96] |
| Effect of long-term physical exercise (80-day training program) on iron homeostasis | As the program progressed, transferrin, TIBC, and UIBC concentrations decreased, while ferritin and iron concentrations increased | Assenza et al. (2016) [97] |
| Effect of high-speed treadmill exercise (5-day protocol) on serum iron concentrations | Serum iron concentration significantly increased during exercise, followed by a gradual decrease | Inoue et al. (2002) [98] |
| Iron Monitoring Parameter | Significance | Optimal Sampling Time | Recommended Analytical Method |
|---|---|---|---|
| Serum Iron (Fe) | Measures circulating iron concentrations | Baseline, post-exercise (immediate, 30 min, 60 min) | Spectrophotometry |
| Ferritin | Indicator of iron stores and acute-phase response | Baseline, post-exercise (24 h, long-term) | ELISA |
| Transferrin | Iron transport protein; key for iron homeostasis | Baseline, post-exercise (immediate, 30 min) | ELISA |
| Total Iron-Binding Capacity (TIBC) | Measures total iron-binding potential of transferrin | Baseline, post-exercise (immediate, 30 min) | Spectrophotometry |
| Unsaturated Iron-Binding Capacity (UIBC) | Measures iron-binding capacity available in transferrin | Baseline, post-exercise (immediate, 30 min) | Spectrophotometry |
| Hemoglobin (Hb) | Essential for oxygen transport | Baseline, post-exercise (immediate, 30 min, long-term) | Hematology analyzer |
| Red Blood Cell Count (RBC) | Reflects erythropoiesis and overall red cell mass | Baseline and post-exercise (immediate, 30 min, long-term) | Hematology analyzer |
| Hematocrit (Hct) | Indicates blood volume occupied by red cells | Baseline and post-exercise (immediate, 30 min, long-term) | Hematology analyzer |
| Hepcidin | Regulates iron absorption and distribution | Baseline, post-exercise (24 h, long-term) | ELISA |
| Publication | Hct | RBC | Hb | Breed | Distance |
|---|---|---|---|---|---|
| Piccione et al. (2010) [113] | 36% | 34% | 37.5% | Standardbred Horses | 1600 m |
| Piccione et al. (2010) [113] | 23.5% | 20% | 12.3% | Standardbred Horses | 2000 m |
| Wood and Frede (1997) [114] | 57.5% | 48.1% | 46.3% | Quarter Horses and Thoroughbred Horses | from 320 m to 1700 m |
| Hassan et al. (2015) [115] | no data | 45.5% | 33.3% | Thoroughbred Horses | 1600 m |
| Clinical context | Suggested considerations (not Clinical Guidelines) |
| ↓ Performance/poor recovery | Consider evaluation of Hct, Hb, RBC, serum iron, ferritin, inflammatory markers (SAA, IL-6), and hepcidin (if available). Post-exercise samples (e.g., immediately after exercise and 24 h later) may reveal transient alterations. |
| Relevant blood tests | CBC (Hct, Hb, RBC), serum iron and ferritin, transferrin saturation (TIBC/UIBC), inflammatory markers (e.g., SAA, IL-6), hepcidin (if available). |
| Possible indicators of subclinical iron imbalance | Low-normal hematologic values in a fit horse with poor adaptation; history of exertional hemolysis, EGUS, or chronic inflammatory burden; suboptimal recovery after training. Interpretation should be cautious due to physiological variation. |
| Situations where iron status evaluation may be warranted | Prolonged recovery, post-surgical anemia, chronic blood loss, or suspected iron depletion. Supplementation should be considered only with laboratory confirmation and in the absence of inflammatory confounders. |
| Potential risks of iron over-supplementation (reported in the literature) | Iron overload (especially with repeated parenteral use), oxidative damage, hepatotoxicity, and in rare cases, acute toxicity. Injectable iron may bypass regulatory barriers; caution is advised. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiełbik, P.; Witkowska-Piłaszewicz, O. Iron Status in Sport Horses: Is It Important for Equine Athletes? Int. J. Mol. Sci. 2025, 26, 5653. https://doi.org/10.3390/ijms26125653
Kiełbik P, Witkowska-Piłaszewicz O. Iron Status in Sport Horses: Is It Important for Equine Athletes? International Journal of Molecular Sciences. 2025; 26(12):5653. https://doi.org/10.3390/ijms26125653
Chicago/Turabian StyleKiełbik, Paula, and Olga Witkowska-Piłaszewicz. 2025. "Iron Status in Sport Horses: Is It Important for Equine Athletes?" International Journal of Molecular Sciences 26, no. 12: 5653. https://doi.org/10.3390/ijms26125653
APA StyleKiełbik, P., & Witkowska-Piłaszewicz, O. (2025). Iron Status in Sport Horses: Is It Important for Equine Athletes? International Journal of Molecular Sciences, 26(12), 5653. https://doi.org/10.3390/ijms26125653





