Cold-Sensing TRP Channels and Temperature Preference Modulate Ovarian Development in the Model Organism Drosophila melanogaster
Abstract
1. Introduction
2. Results
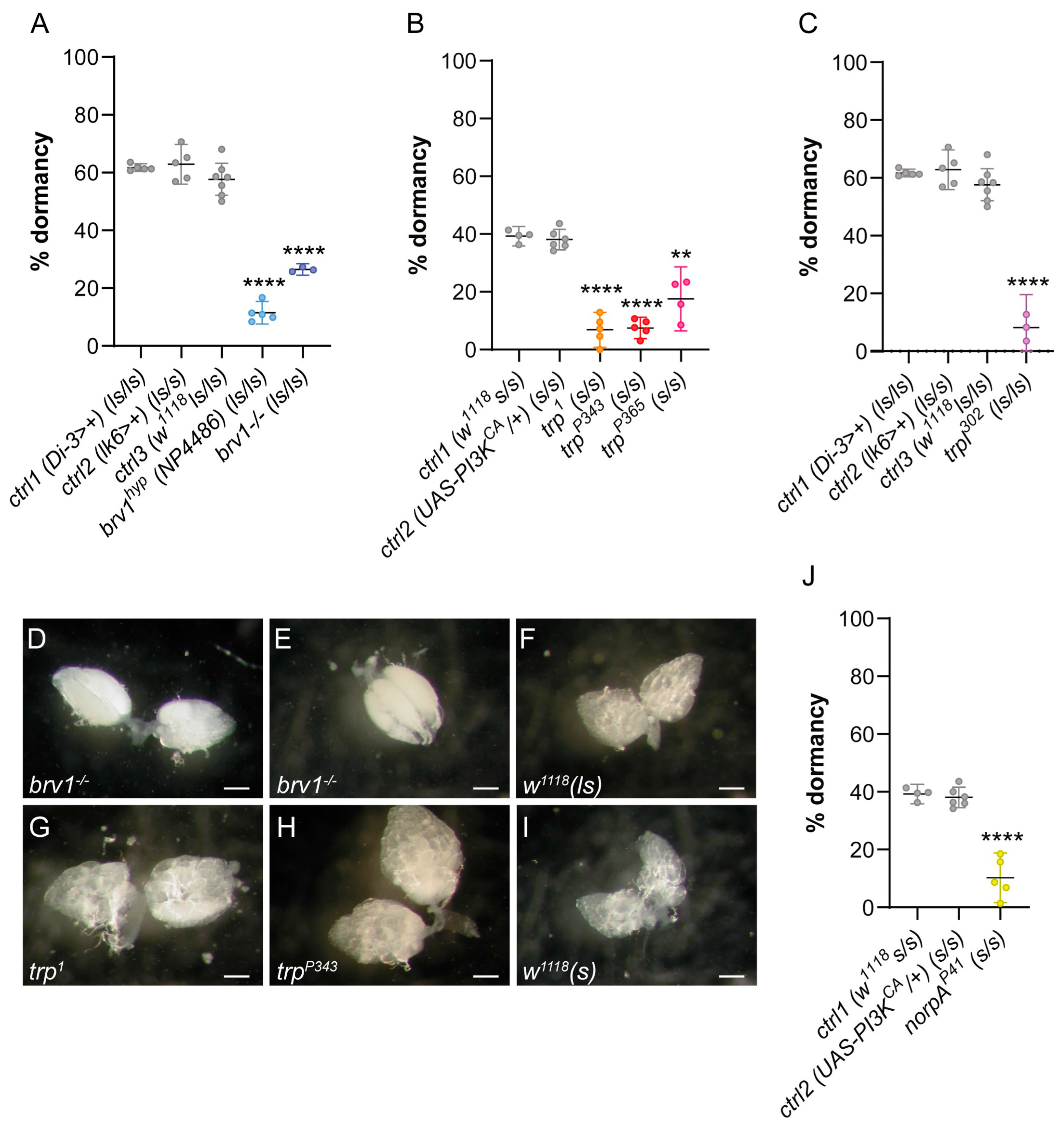
2.1. Drosophila Mutants for the Cold-Sensing TRP Channel Brivido 1 Exhibit Reduced Ovarian Dormancy at Low Temperatures
2.2. Loss-of-Function Mutations at the trp and trpl Genes Reduce Ovarian Dormancy at Low Temperatures
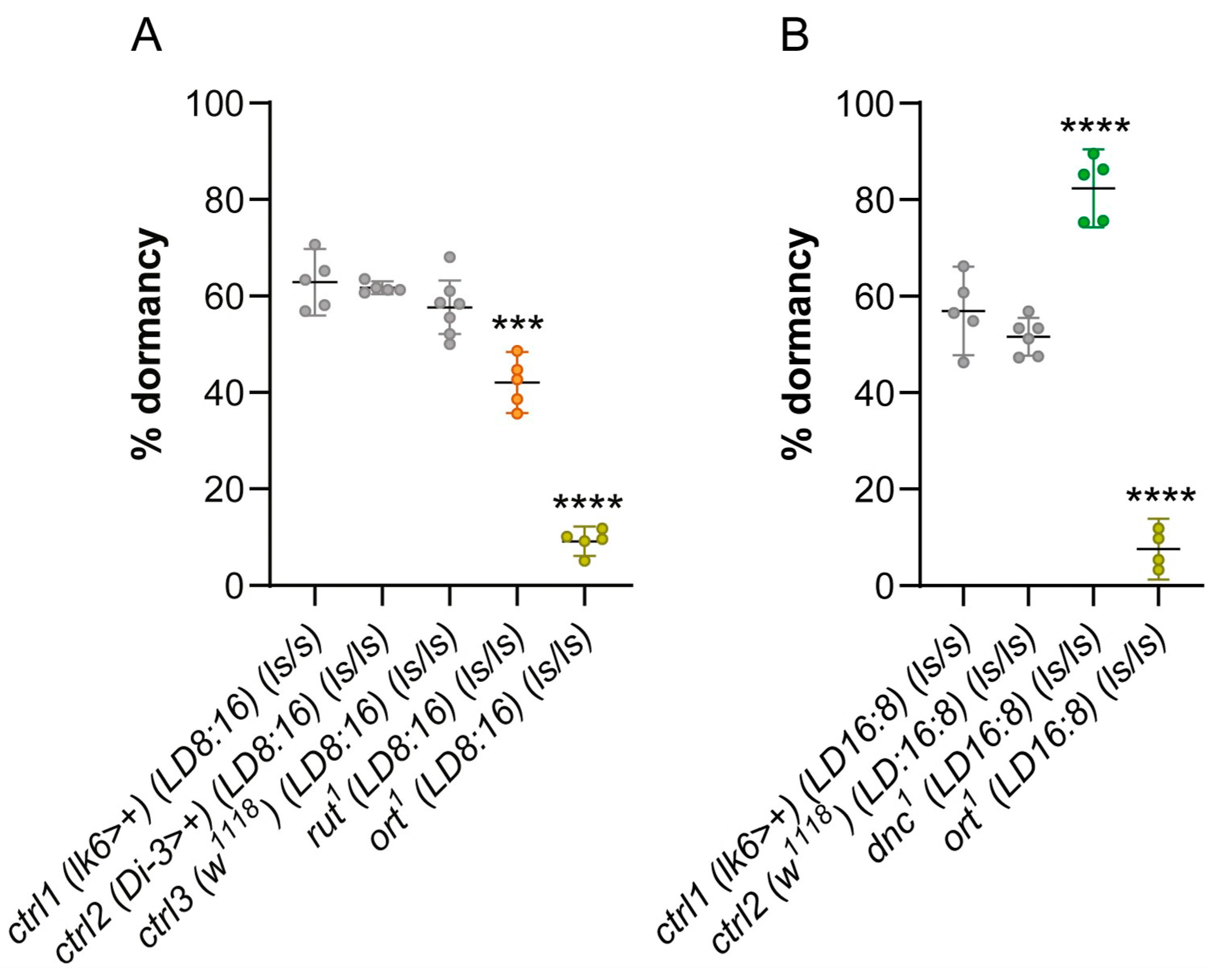
2.3. Mutations Modulating Temperature Preference Affect Ovarian Dormancy at Low Temperatures
3. Discussion
4. Materials and Methods
4.1. Fly Stocks and Maintenance
4.2. Genetic Controls and Genetic Background
4.3. Reproductive Dormancy Assays
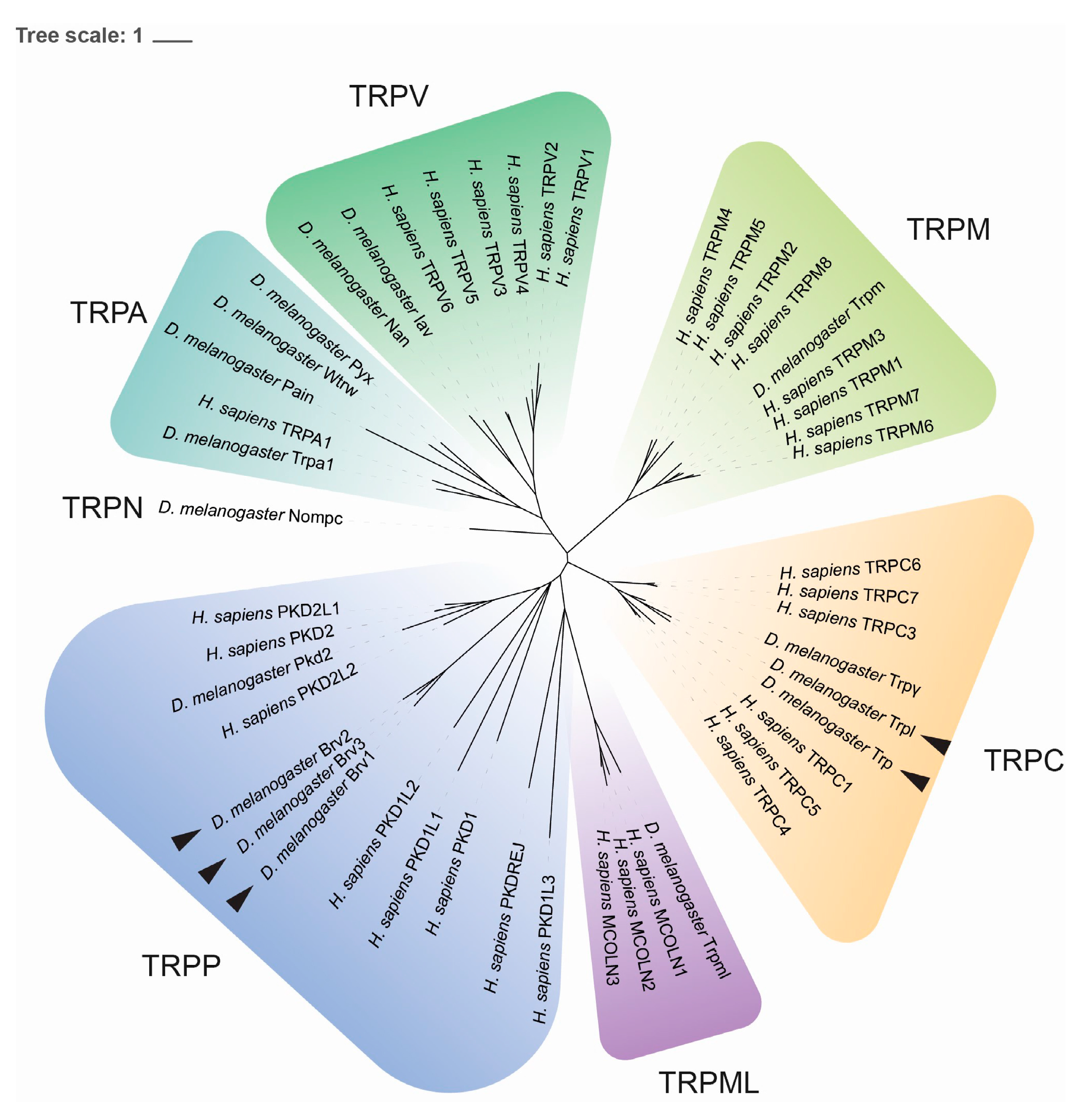
4.4. Phylogenetic Study and Sequence Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gallio, M.; Ofstad, T.A.; Macpherson, L.J.; Wang, J.W.; Zuker, C.S. The Coding of Temperature in the Drosophila Brain. Cell 2011, 144, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Panzano, V.C.; Chang, E.C.; Ni, L.; Dainis, A.M.; Jenkins, A.M.; Regna, K.; Muskavitch, M.A.T.; Garrity, P.A. Modulation of TRPA1 Thermal Sensitivity Enables Sensory Discrimination in Drosophila. Nature 2011, 481, 76–80. [Google Scholar] [CrossRef] [PubMed]
- McKemy, D.D. Temperature Sensing across Species. Pflug. Arch. 2007, 454, 777–791. [Google Scholar] [CrossRef]
- Gracheva, E.O.; Ingolia, N.T.; Kelly, Y.M.; Cordero-Morales, J.F.; Hollopeter, G.; Chesler, A.T.; Sánchez, E.E.; Perez, J.C.; Weissman, J.S.; Julius, D. Molecular Basis of Infrared Detection by Snakes. Nature 2010, 464, 1006–1011. [Google Scholar] [CrossRef]
- Abram, P.K.; Boivin, G.; Moiroux, J.; Brodeur, J. Behavioural Effects of Temperature on Ectothermic Animals: Unifying Thermal Physiology and Behavioural Plasticity. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1859–1876. [Google Scholar] [CrossRef]
- Bicego, K.C.; Barros, R.C.H.; Branco, L.G.S. Physiology of Temperature Regulation: Comparative Aspects. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 616–639. [Google Scholar] [CrossRef]
- Xiao, R.; Xu, X.Z.S. Temperature Sensation: From Molecular Thermosensors to Neural Circuits and Coding Principles. Annu. Rev. Physiol. 2021, 83, 205–230. [Google Scholar] [CrossRef]
- Himmel, N.J.; Cox, D.N. Transient Receptor Potential Channels: Current Perspectives on Evolution, Structure, Function and Nomenclature. Proc. Biol. Sci. 2020, 287, 20201309. [Google Scholar] [CrossRef]
- Montell, C. Drosophila Sensory Receptors—A Set of Molecular Swiss Army Knives. Genetics 2021, 217. [Google Scholar] [CrossRef]
- Diver, M.M.; Lin King, J.V.; Julius, D.; Cheng, Y. Sensory TRP Channels in Three Dimensions. Annu. Rev. Biochem. 2022, 91, 629–649. [Google Scholar] [CrossRef]
- Dietrich, A. Transient Receptor Potential (TRP) Channels in Health and Disease. Cells 2019, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.-P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP Channel Drug Discovery: From Target Validation to Clinical Studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Kostál, V.; Zahradnícková, H.; Šimek, P. Hyperprolinemic Larvae of the Drosophilid Fly, Chymomyza costata, Survive Cryopreservation in Liquid Nitrogen. Proc. Natl. Acad. Sci. USA 2011, 108, 13041–13046. [Google Scholar] [CrossRef]
- Schiesari, L.; O’Connor, M.B. Diapause: Delaying the Developmental Clock in Response to a Changing Environment. Curr. Top. Dev. Biol. 2013, 105, 213–246. [Google Scholar] [CrossRef]
- Rosenzweig, M.; Kang, K.; Garrity, P.A. Distinct TRP Channels Are Required for Warm and Cool Avoidance in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2008, 105, 14668–14673. [Google Scholar] [CrossRef]
- Hong, S.-T.; Bang, S.; Hyun, S.; Kang, J.; Jeong, K.; Paik, D.; Chung, J.; Kim, J. cAMP Signalling in Mushroom Bodies Modulates Temperature Preference Behaviour in Drosophila. Nature 2008, 454, 771–775. [Google Scholar] [CrossRef]
- Kwon, Y.; Shen, W.L.; Shim, H.-S.; Montell, C. Fine Thermotactic Discrimination between the Optimal and Slightly Cooler Temperatures via a TRPV Channel in Chordotonal Neurons. J. Neurosci. 2010, 30, 10465–10471. [Google Scholar] [CrossRef]
- Ni, L.; Klein, M.; Svec, K.V.; Budelli, G.; Chang, E.C.; Ferrer, A.J.; Benton, R.; Samuel, A.D.; Garrity, P.A. The Ionotropic Receptors IR21a and IR25a Mediate Cool Sensing in Drosophila. eLife 2016, 5, e13254. [Google Scholar] [CrossRef]
- Knecht, Z.A.; Silbering, A.F.; Ni, L.; Klein, M.; Budelli, G.; Bell, R.; Abuin, L.; Ferrer, A.J.; Samuel, A.D.; Benton, R.; et al. Distinct Combinations of Variant Ionotropic Glutamate Receptors Mediate Thermosensation and Hygrosensation in Drosophila. eLife 2016, 5, e17879. [Google Scholar] [CrossRef]
- Turner, H.N.; Armengol, K.; Patel, A.A.; Himmel, N.J.; Sullivan, L.; Iyer, S.C.; Bhattacharya, S.; Iyer, E.P.R.; Landry, C.; Galko, M.J.; et al. The TRP Channels Pkd2, NompC, and Trpm Act in Cold-Sensing Neurons to Mediate Unique Aversive Behaviors to Noxious Cold in Drosophila. Curr. Biol. 2016, 26, 3116–3128. [Google Scholar] [CrossRef]
- Budelli, G.; Ni, L.; Berciu, C.; van Giesen, L.; Knecht, Z.A.; Chang, E.C.; Kaminski, B.; Silbering, A.F.; Samuel, A.; Klein, M.; et al. Ionotropic Receptors Specify the Morphogenesis of Phasic Sensors Controlling Rapid Thermal Preference in Drosophila. Neuron 2019, 101, 738–747.e3. [Google Scholar] [CrossRef] [PubMed]
- Turner, H.N.; Patel, A.A.; Cox, D.N.; Galko, M.J. Injury-Induced Cold Sensitization in Drosophila Larvae Involves Behavioral Shifts That Require the TRP Channel Brv1. PLoS ONE 2018, 13, e0209577. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Hyun, S.; Hong, S.-T.; Kang, J.; Jeong, K.; Park, J.-J.; Choe, J.; Chung, J. Dopamine Signalling in Mushroom Bodies Regulates Temperature-Preference Behaviour in Drosophila. PLoS Genet. 2011, 7, e1001346. [Google Scholar] [CrossRef]
- Hong, S.-T.; Bang, S.; Paik, D.; Kang, J.; Hwang, S.; Jeon, K.; Chun, B.; Hyun, S.; Lee, Y.; Kim, J. Histamine and Its Receptors Modulate Temperature-Preference Behaviors in Drosophila. J. Neurosci. 2006, 26, 7245–7256. [Google Scholar] [CrossRef]
- Li, Q.; Gong, Z. Cold-Sensing Regulates Drosophila Growth through Insulin-Producing Cells. Nat. Commun. 2015, 6, 10083. [Google Scholar] [CrossRef]
- Andreatta, G.; Kyriacou, C.P.; Flatt, T.; Costa, R. Aminergic Signaling Controls Ovarian Dormancy in Drosophila. Sci. Rep. 2018, 8, 2030. [Google Scholar] [CrossRef]
- Sato, A.; Sokabe, T.; Kashio, M.; Yasukochi, Y.; Tominaga, M.; Shiomi, K. Embryonic Thermosensitive TRPA1 Determines Transgenerational Diapause Phenotype of the Silkworm, Bombyx mori. Proc. Natl. Acad. Sci. USA 2014, 111, E1249–E1255. [Google Scholar] [CrossRef]
- Schiesari, L.; Andreatta, G.; Kyriacou, C.P.; O’Connor, M.B.; Costa, R. The Insulin-like Proteins dILPs-2/5 Determine Diapause Inducibility in Drosophila. PLoS ONE 2016, 11, e0163680. [Google Scholar] [CrossRef]
- Zonato, V.; Collins, L.; Pegoraro, M.; Tauber, E.; Kyriacou, C.P. Is Diapause an Ancient Adaptation in Drosophila? J. Insect Physiol. 2017, 98, 267–274. [Google Scholar] [CrossRef]
- Kubrak, O.I.; Kucerová, L.; Theopold, U.; Nylin, S.; Nässel, D.R. Characterization of Reproductive Dormancy in Male Drosophila melanogaster. Front. Physiol. 2016, 7, 572. [Google Scholar] [CrossRef]
- Saunders, D.S.; Henricht, V.C.; Gilbertt, L.I. Induction of Diapause in Drosophila melanogaster: Photoperiodic Regulation and the Impact of Arrhythmic Clock Mutations on Time Measurement. Proc. Natl. Acad. Sci. USA 1989, 86, 3748–3752. [Google Scholar] [CrossRef] [PubMed]
- Gandara, A.C.P.; Drummond-Barbosa, D. Warm and Cold Temperatures Have Distinct Germline Stem Cell Lineage Effects during Drosophila Oogenesis. Development 2022, 149, dev200149. [Google Scholar] [CrossRef] [PubMed]
- Lirakis, M.; Dolezal, M.; Schlötterer, C. Redefining Reproductive Dormancy in Drosophila as a General Stress Response to Cold Temperatures. J. Insect Physiol. 2018, 107, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Emerson, K.J.; Uyemura, A.M.; McDaniel, K.L.; Schmidt, P.S.; Bradshaw, W.E.; Holzapfel, C.M. Environmental Control of Ovarian Dormancy in Natural Populations of Drosophila melanogaster. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2009, 195, 825–829. [Google Scholar] [CrossRef]
- Andreatta, G.; Montagnese, S.; Costa, R. Natural Alleles of the Clock Gene timeless Differentially Affect Life-History Traits in Drosophila. Front. Physiol. 2023, 13, 1092951. [Google Scholar] [CrossRef]
- Nagy, D.; Andreatta, G.; Bastianello, S.; Martín Anduaga, A.; Mazzotta, G.; Kyriacou, C.P.; Costa, R. A Semi-Natural Approach for Studying Seasonal Diapause in Drosophila melanogaster Reveals Robust Photoperiodicity. J. Biol. Rhythm. 2018, 33, 117–125. [Google Scholar] [CrossRef]
- Nagy, D.; Cusumano, P.; Andreatta, G.; Anduaga, A.M.; Hermann-Luibl, C.; Reinhard, N.; Gesto, J.; Wegener, C.; Mazzotta, G.; Rosato, E.; et al. Peptidergic Signaling from Clock Neurons Regulates Reproductive Dormancy in Drosophila melanogaster. PLoS Genet. 2019, 15, e1008158. [Google Scholar] [CrossRef]
- Kang, J.; Kim, J.; Choi, K.-W. Novel Cytochrome P450, Cyp6a17, Is Required for Temperature Preference Behavior in Drosophila. PLoS ONE 2011, 6, e29800. [Google Scholar] [CrossRef]
- Xu, W.-H.; Lu, Y.-X.; Denlinger, D.L. Cross-Talk between the Fat Body and Brain Regulates Insect Developmental Arrest. Proc. Natl. Acad. Sci. USA 2012, 109, 14687–14692. [Google Scholar] [CrossRef]
- Klein, M.; Afonso, B.; Vonner, A.J.; Hernandez-Nunez, L.; Berck, M.; Tabone, C.J.; Kane, E.A.; Pieribone, V.A.; Nitabach, M.N.; Cardona, A.; et al. Sensory Determinants of Behavioral Dynamics in Drosophila Thermotaxis. Proc. Natl. Acad. Sci. USA 2015, 112, E220–E229. [Google Scholar] [CrossRef]
- Shao, T.-L.; Ting, R.-T.; Lee, M.-C. Identification of Lsd1-Interacting Non-Coding RNAs as Regulators of Fly Oogenesis. Cell Rep. 2022, 40, 111294. [Google Scholar] [CrossRef] [PubMed]
- Menuz, K.; Larter, N.K.; Park, J.; Carlson, J.R. An RNA-Seq Screen of the Drosophila Antenna Identifies a Transporter Necessary for Ammonia Detection. PLoS Genet. 2014, 10, e1004810. [Google Scholar] [CrossRef] [PubMed]
- Shiao, M.-S.; Fan, W.-L.; Fang, S.; Lu, M.-Y.J.; Kondo, R.; Li, W.-H. Transcriptional Profiling of Adult Drosophila Antennae by High-Throughput Sequencing. Zool. Stud. 2013, 52, 42. [Google Scholar] [CrossRef]
- Niemeyer, B.A.; Suzuki, E.; Scott, K.; Jalink, K.; Zuker, C.S. The Drosophila Light-Activated Conductance Is Composed of the Two Channels TRP and TRPL. Cell 1996, 85, 651–659. [Google Scholar] [CrossRef]
- Reuss, H.; Mojet, M.H.; Chyb, S.; Hardie, R.C. In Vivo Analysis of the Drosophila Light-Sensitive Channels, TRP and TRPL. Neuron 1997, 19, 1249–1259. [Google Scholar] [CrossRef]
- Shen, W.L.; Kwon, Y.; Adegbola, A.A.; Luo, J.; Chess, A.; Montell, C. Function of Rhodopsin in Temperature Discrimination in Drosophila. Science 2011, 331, 1333–1336. [Google Scholar] [CrossRef]
- Agam, K.; von Campenhausen, M.; Levy, S.; Ben-Ami, H.C.; Cook, B.; Kirschfeld, K.; Minke, B. Metabolic Stress Reversibly Activates the Drosophila Light-Sensitive Channels TRP and TRPL in Vivo. J. Neurosci. 2000, 20, 5748–5755. [Google Scholar] [CrossRef]
- Li, Y.; Cacciottolo, T.M.; Yin, N.; He, Y.; Liu, H.; Liu, H.; Yang, Y.; Henning, E.; Keogh, J.M.; Lawler, K.; et al. Loss of Transient Receptor Potential Channel 5 Causes Obesity and Postpartum Depression. Cell 2024, 187, 4176–4192.e17. [Google Scholar] [CrossRef]
- Stowers, L.; Holy, T.E.; Meister, M.; Dulac, C.; Koentges, G. Loss of Sex Discrimination and Male-Male Aggression in Mice Deficient for TRP2. Science 2002, 295, 1493–1500. [Google Scholar] [CrossRef]
- Leypold, B.G.; Yu, C.R.; Leinders-Zufall, T.; Kim, M.M.; Zufall, F.; Axel, R. Altered Sexual and Social Behaviors in Trp2 Mutant Mice. Proc. Natl. Acad. Sci. USA 2002, 99, 6376–6381. [Google Scholar] [CrossRef]
- Haga, S.; Hattori, T.; Sato, T.; Sato, K.; Matsuda, S.; Kobayakawa, R.; Sakano, H.; Yoshihara, Y.; Kikusui, T.; Touhara, K. The Male Mouse Pheromone ESP1 Enhances Female Sexual Receptive Behaviour through a Specific Vomeronasal Receptor. Nature 2010, 466, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, D.M.; Moeller, L.M.; Osakada, T.; Horio, N.; Li, Q.; Roy, D.S.; Cichy, A.; Spehr, M.; Touhara, K.; Liberles, S.D. A Juvenile Mouse Pheromone Inhibits Sexual Behaviour through the Vomeronasal System. Nature 2013, 502, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ruden, D.M.; Lu, X. PKD2 Cation Channel Is Required for Directional Sperm Movement and Male Fertility. Curr. Biol. 2003, 13, 2175–2178. [Google Scholar] [CrossRef] [PubMed]
- Sutton, K.A.; Jungnickel, M.K.; Florman, H.M. A Polycystin-1 Controls Postcopulatory Reproductive Selection in Mice. Proc. Natl. Acad. Sci. USA 2008, 105, 8661–8666. [Google Scholar] [CrossRef]
- Qian, F.; Boletta, A.; Bhunia, A.K.; Xu, H.; Liu, L.; Ahrabi, A.K.; Watnick, T.J.; Zhou, F.; Germino, G.G. Cleavage of Polycystin-1 Requires the Receptor for Egg Jelly Domain and Is Disrupted by Human Autosomal-Dominant Polycystic Kidney Disease 1-Associated Mutations. Proc. Natl. Acad. Sci. USA 2002, 99, 16981–16986. [Google Scholar] [CrossRef]
- Köttgen, M.; Buchholz, B.; Garcia-Gonzalez, M.A.; Kotsis, F.; Fu, X.; Doerken, M.; Boehlke, C.; Steffl, D.; Tauber, R.; Wegierski, T.; et al. TRPP2 and TRPV4 Form a Polymodal Sensory Channel Complex. J. Cell Biol. 2008, 182, 437–447. [Google Scholar] [CrossRef]
- Huque, T.; Cowart, B.J.; Dankulich-Nagrudny, L.; Pribitkin, E.A.; Bayley, D.L.; Spielman, A.I.; Feldman, R.S.; Mackler, S.A.; Brand, J.G. Sour Ageusia in Two Individuals Implicates Ion Channels of the ASIC and PKD Families in Human Sour Taste Perception at the Anterior Tongue. PLoS ONE 2009, 4, e7347. [Google Scholar] [CrossRef]
- Yu, Y.; Ulbrich, M.H.; Li, M.; Dobbins, S.; Zhang, W.K.; Tong, L.; Isacoff, E.Y.; Yang, J. Molecular Mechanism of the Assembly of an Acid-Sensing Receptor Ion Channel Complex. Nat. Commun. 2012, 3, 1252. [Google Scholar] [CrossRef]
- Bloomquist, B.T.; Shortridge, R.D.; Schneuwly, S.; Perdew, M.; Montell, C.; Steller, H.; Rubin, G.; Pak, W.L. Isolation of a Putative Phospholipase C Gene of Drosophila, norpA, and Its Role in Phototransduction. Cell 1988, 54, 723–733. [Google Scholar] [CrossRef]
- Jin, L.-Y.; Yu, J.-E.; Xu, H.-Y.; Chen, B.; Yang, Q.; Liu, Y.; Guo, M.-X.; Zhou, C.-L.; Cheng, Y.; Pang, H.-Y.; et al. Overexpression of Pde4d in Rat Granulosa Cells Inhibits Maturation and Atresia of Antral Follicles to Induce Polycystic Ovary. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166869. [Google Scholar] [CrossRef]
- Jin, S.-L.C.; Richard, F.J.; Kuo, W.-P.; D’Ercole, A.J.; Conti, M. Impaired Growth and Fertility of cAMP-Specific Phosphodiesterase PDE4D-Deficient Mice. Proc. Natl. Acad. Sci. USA 1999, 96, 11998–12003. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Richard, F.; Chun, S.-Y.; Park, J.-H.; Law, E.; Horner, K.; Jin, S.-L.C.; Conti, M. Phosphodiesterase Regulation Is Critical for the Differentiation and Pattern of Gene Expression in Granulosa Cells of the Ovarian Follicle. Mol. Endocrinol. 2003, 17, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Zhou, Q.; Cao, Z.; Jiang, Y.; Xu, M.; Liu, J.; Zhou, J.; Yan, G.; Sun, H. Downregulated INHBB in Endometrial Tissue of Recurrent Implantation Failure Patients Impeded Decidualization through the ADCY1/cAMP Signalling Pathway. J. Assist. Reprod. Genet. 2023, 40, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Aragon, I.V.; Rich, J.; McDonough, W.; Oditt, M.; Irelan, D.; Fiedler, E.; Abou Saleh, L.; Richter, W. Assessment of PDE4 Inhibitor-Induced Hypothermia as a Correlate of Nausea in Mice. Biology 2021, 10, 1355. [Google Scholar] [CrossRef]
- Xie, D.; Geng, L.; Xiong, K.; Zhao, T.; Wang, S.; Xue, J.; Wang, C.; Wang, G.; Feng, Z.; Zhou, H.; et al. Cold-Inducible RNA-Binding Protein Prevents an Excessive Heart Rate Response to Stress by Targeting Phosphodiesterase. Circ. Res. 2020, 126, 1706–1720. [Google Scholar] [CrossRef]
- Paschapur, A.U.; Manoj, M.S.; Pavan, J.S.; Subramanian, S. Exploiting TRP Channel Diversity in Insects: A Pathway to next-Generation Pest Management. Arch. Toxicol. 2025, 1–21. [Google Scholar] [CrossRef]
- Swan, A.; Hijal, S.; Hilfiker, A.; Suter, B. Identification of New X-Chromosomal Genes Required for Drosophila Oogenesis and Novel Roles for fs(1)Yb, brainiac and dunce. Genome Res. 2001, 11, 67–77. [Google Scholar] [CrossRef]
- Iovchev, M.; Boutanaev, A.; Ivanov, I.; Wolstenholme, A.; Nurminsky, D.; Semenov, E. Phylogenetic Shadowing of a Histamine-Gated Chloride Channel Involved in Insect Vision. Insect Biochem. Mol. Biol. 2006, 36, 10–17. [Google Scholar] [CrossRef]
- Gisselmann, G.; Pusch, H.; Hovemann, B.T.; Hatt, H. Two cDNAs Coding for Histamine-Gated Ion Channels in D. melanogaster. Nat. Neurosci. 2002, 5, 11–12. [Google Scholar] [CrossRef]
- Pollock, J.A.; Assaf, A.; Peretz, A.; Nichols, C.D.; Mojet, M.H.; Hardie, R.C.; Minke, B. TRP, a Protein Essential for Inositide-Mediated Ca2+ Influx Is Localized Adjacent to the Calcium Stores in Drosophila Photoreceptors. J. Neurosci. 1995, 15, 3747–3760. [Google Scholar] [CrossRef]
- Wang, T.; Jiao, Y.; Montell, C. Dissecting Independent Channel and Scaffolding Roles of the Drosophila Transient Receptor Potential Channel. J. Cell Biol. 2005, 171, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Ben-Ami, H.C.; Hong, Y.S.; Park, S.; Strong, L.L.R.; Bowman, J.; Geng, C.; Baek, K.; Minke, B.; Pak, W.L. Novel Mechanism of Massive Photoreceptor Degeneration Caused by Mutations in the trp Gene of Drosophila. J. Neurosci. 2000, 20, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Szular, J.; Sehadova, H.; Gentile, C.; Szabo, G.; Chou, W.-H.; Britt, S.G.; Stanewsky, R. Rhodopsin 5– and Rhodopsin 6–Mediated Clock Synchronization in Drosophila melanogaster Is Independent of Retinal Phospholipase C-β Signaling. J. Biol. Rhythm. 2012, 27, 25–36. [Google Scholar] [CrossRef]
- Tauber, E.; Zordan, M.; Sandrelli, F.; Pegoraro, M.; Osterwalder, N.; Breda, C.; Daga, A.; Selmin, A.; Monger, K.; Benna, C.; et al. Natural Selection Favors a Newly Derived timeless Allele in Drosophila melanogaster. Science 2007, 316, 1895–1898. [Google Scholar] [CrossRef]
- Schmidt, P.S.; Zhu, C.T.; Das, J.; Batavia, M.; Yang, L.; Eanes, W.F. An Amino Acid Polymorphism in the couch potato Gene Forms the Basis for Climatic Adaptation in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2008, 105, 16207–16211. [Google Scholar] [CrossRef]
- Cogni, R.; Kuczynski, C.; Koury, S.; Lavington, E.; Behrman, E.L.; O’Brien, K.R.; Schmidt, P.S.; Eanes, W.F. The Intensity of Selection Acting on the couch potato Gene--Spatial-Temporal Variation in a Diapause Cline. Evolution 2014, 68, 538–548. [Google Scholar] [CrossRef]
- Sandrelli, F.; Tauber, E.; Pegoraro, M.; Mazzotta, G.; Cisotto, P.; Landskron, J.; Stanewsky, R.; Piccin, A.; Rosato, E.; Zordan, M.; et al. A Molecular Basis for Natural Selection at the timeless Locus in Drosophila melanogaster. Science 2007, 316, 1898–1900. [Google Scholar] [CrossRef]
- Zonato, V.; Fedele, G.; Kyriacou, C.P. An Intronic Polymorphism in couch potato Is Not Distributed Clinally in European Drosophila melanogaster Populations nor Does It Affect Diapause Inducibility. PLoS ONE 2016, 11, e0162370. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A Multiple Sequence Alignment Method with Reduced Time and Space Complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A Fast Online Phylogenetic Tool for Maximum Likelihood Analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Andreatta, G.; Broyart, C.; Borghgraef, C.; Vadiwala, K.; Kozin, V.; Polo, A.; Bileck, A.; Beets, I.; Schoofs, L.; Gerner, C.; et al. Corazonin Signaling Integrates Energy Homeostasis and Lunar Phase to Regulate Aspects of Growth and Sexual Maturation in Platynereis. Proc. Natl. Acad. Sci. USA 2020, 117, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreatta, G.; Montagnese, S.; Costa, R. Cold-Sensing TRP Channels and Temperature Preference Modulate Ovarian Development in the Model Organism Drosophila melanogaster. Int. J. Mol. Sci. 2025, 26, 5638. https://doi.org/10.3390/ijms26125638
Andreatta G, Montagnese S, Costa R. Cold-Sensing TRP Channels and Temperature Preference Modulate Ovarian Development in the Model Organism Drosophila melanogaster. International Journal of Molecular Sciences. 2025; 26(12):5638. https://doi.org/10.3390/ijms26125638
Chicago/Turabian StyleAndreatta, Gabriele, Sara Montagnese, and Rodolfo Costa. 2025. "Cold-Sensing TRP Channels and Temperature Preference Modulate Ovarian Development in the Model Organism Drosophila melanogaster" International Journal of Molecular Sciences 26, no. 12: 5638. https://doi.org/10.3390/ijms26125638
APA StyleAndreatta, G., Montagnese, S., & Costa, R. (2025). Cold-Sensing TRP Channels and Temperature Preference Modulate Ovarian Development in the Model Organism Drosophila melanogaster. International Journal of Molecular Sciences, 26(12), 5638. https://doi.org/10.3390/ijms26125638






