Preparing O/W/O Emulsion for Curcumin (Curcuma longa) Delivery and In Vitro Digestibility Assay
Abstract
1. Introduction
2. Results and Discussion
2.1. Particle Size of Emulsions
2.2. Viscoelatic Properties of Curcumin Emulsions
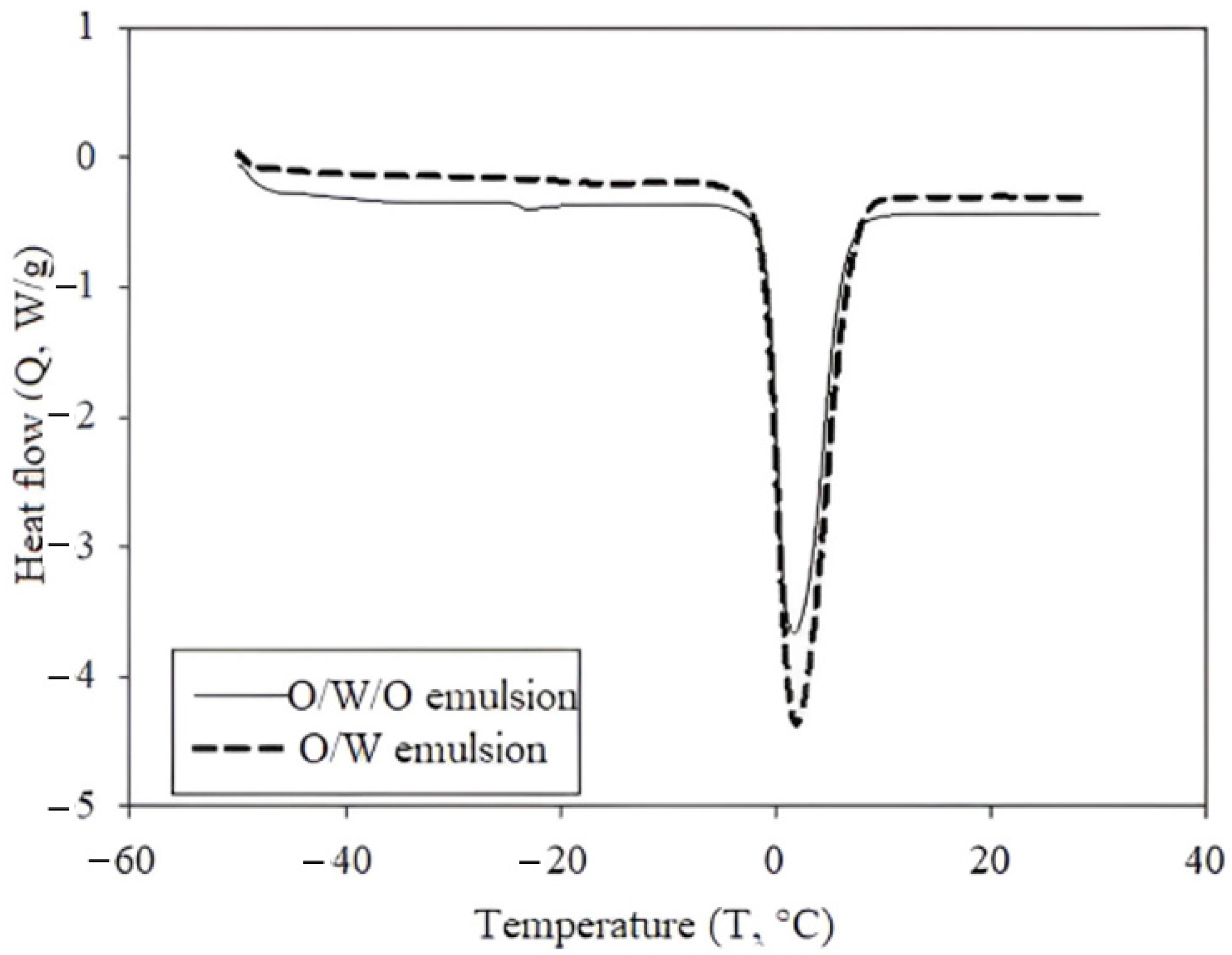
2.3. Differential Scanning Calorimetry
2.4. Results of Curcumin Release During Simulated Digestion in Emulsion Matrices
2.5. Effect of In Vitro Digestion on Curcumin Retention in the Matrix of Curcuma Root Samples
3. Materials and Methods
3.1. Material
3.2. Preparation of Model Emulsion Samples
3.3. Particle Size Measurement
3.4. Dynamic Oscillatory Rheology
3.5. Determination of Freezable Water Content in Emulsions by DSC
3.6. Release of Curcumin Complex Encapsulated in Emulsions During Simulated Digestion In Vitro
3.7. Determination of Curcumin Content Using HPLC Method
3.8. The Effect of Sample Matrix on Curcumin Release During Simulated In Vitro Digestion
3.8.1. Preparation of Samples from Native and Undigested Parts of Curcuma Root
3.8.2. Determination of In Vitro Digestibility of Curcuma Samples
3.8.3. Evaluation of Retention Factor (RF) for Curcumin
3.8.4. Determination of the Dry Matter and Ash Content
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DLS | Dynamic light scattering |
| DMD | Dry matter digestibility |
| DMR | Mass of the sample after digestion and drying |
| DSC | Differential scanning calorimetry |
| DW | Dry weight of the sample |
| FIG-U | Federation Internationale Pharmaceutique Units |
| GIT | Gastrointestinal tract |
| HIPE | High internal phase emulsion |
| HPLC | High-performance liquid chromatography |
| O/W | Oil-in-water emulsion |
| O/W/O | Oil-in-water-in-oil emulsion |
| RF | Retention factor |
| RPM | Rotations per minute |
References
- Hayakawa, H.; Minaniya, Y.; Ito, K.; Yamamoto, Y.; Fukuda, T. Difference of Curcumin Content in Curcuma longa L. (Zingiberaceae) Caused by Hybridization with Other Curcuma Species. Am. J. Plant Sci. 2011, 2, 111–119. [Google Scholar] [CrossRef]
- Agrawal, S.; Nair, R.; Thomas, M.; Anjana, G.; Patel, S.K.; Uikey, P.; Birla, S.; Singh, J.; Tripathi, N. Morphological Characterization of Turmeric (Curcuma spp.) Genotypes. J. Eco-Friendly Agric. 2024, 19, 67–72. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Syed, H.K.; Liew, K.B.; Loh, G.O.K.; Peh, K.K. Stability Indicating HPLC–UV Method for Detection of Curcumin in Curcuma Longa Extract and Emulsion Formulation. Food Chem. 2015, 170, 321–326. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.; Jung, Y.; Noh, J.; Syed, A.; Lee, M.; Lim, K.; Bae, O.; Chung, J. Cyclocurcumin, an Antivasoconstrictive Constituent of Curcuma longa (Turmeric). J. Nat. Prod. 2017, 80, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Afzal, A.; Khan, U.; Abdul, H.; Mohiuddin, E.; Asif, M. Curcuma longa and Curcumin: A Review Article. Rom. J. Biol-Plant Biol. 2010, 55, 65–70. Available online: https://www.ibiol.ro/plant/revue55n2.html (accessed on 6 June 2025).
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Kushwaha, P.; Prajapati, K.; Shuaib, M.; Gupta, S. Identification of Compounds from Curcuma longa with in Silico Binding Potential Against SARS-CoV-2 and Human Host Proteins Involve in Virus Entry and Pathogenesis. Indian J. Pharm. Sci. 2021, 83, 1181–1195. [Google Scholar] [CrossRef]
- Kaur, R. Turmeric: A Golden Herb with Health-Promoting Components. Just Agric. 2024, 4, 1–5. Available online: https://justagriculture.in/files/newsletter/2024/january (accessed on 1 April 2025).
- Dima, C.; Assadpour, E.; Nechifor, A.; Dima, S.; Li, Y.; Jafari, S.M. Oral Bioavailability of Bioactive Compounds; Modulating Factors, in vitro Analysis Methods, and Enhancing Strategies. Crit. Rev. Food Sci. Nutr. 2024, 64, 8501–8539. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.J.; Donadkar, A.D.; Nair, B.; Kumar, A.R.; Sabitha, M.; Sethi, G.; Chauhan, A.S.; Nath, L.R. Smart Polymer-Based Delivery Systems for Curcumin in Colon Cancer Therapy: A Review. Phytother. Res. 2025, 39, 698–713. [Google Scholar] [CrossRef]
- Chauhan, M.; Saha, S.; Roy, A. Curcumin: A Review. J. Appl. Pharm. Res. 2014, 2, 18–28. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent Advances in Encapsulation of Curcumin in Nanoemulsions: A Review of Encapsulation Technologies, Bioaccessibility and Applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef]
- Ghasemi, H.; Darjani, S.; Mazloomi, H.; Mozaffari, S. Preparation of Stable Multiple Emulsions using Food-Grade Emulsifiers: Evaluating the Effects of Emulsifier Concentration, W/O Phase Ratio, and Emulsification Process. SN Appl. Sci. 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; XV, J.; Yuan, F. Curcumin-Loaded Nano-Emulsion Prepared by High Pressure Homogenization: Impact of Emulsifiers on Physicochemical Stability and in vitro Digestion. Food Sci. Technol. 2022, 42, e115121. [Google Scholar] [CrossRef]
- Opustilová, K.; Lapčíková, B.; Lapčík, L.; Gautam, S.; Valenta, T.; Li, P. Physico-Chemical Study of Curcumin and its Application in O/W/O Multiple Emulsion. Foods 2023, 12, 1394. [Google Scholar] [CrossRef]
- Sousa, F.L.; Santos, M.; Rocha, S.M.; Trindade, T. Encapsulation of Essential Oils in SiO2 Microcapsules and Release Behaviour of Volatile Compounds. J. Microencapsul. 2014, 31, 627–635. [Google Scholar] [CrossRef]
- Pal, R. Rheology of Double Emulsions. J. Colloid Interface Sci. 2007, 307, 509–515. [Google Scholar] [CrossRef]
- Tan, C.; McClements, D.J. Application of Advanced Emulsion Technology in the Food Industry: A Review and Critical Evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef]
- Schuch, A.; Deiters, P.; Henne, J.; Köhler, K.; Schuchmann, H.P. Production of W/O/W (Water-in-Oil-in-Water) Multiple Emulsions: Droplet Breakup and Release of Water. J. Colloid Interface Sci. 2013, 402, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Dalmazzone, C.; Noïk, C.; Clausse, D. Application of DSC for Emulsified System Characterization. Oil Gas Sci. Technol. 2008, 64, 543–555. [Google Scholar] [CrossRef]
- Khatoon, S.; Kalam, N. Mechanistic Insight of Curcumin: A Potential Pharmacological Candidate for Epilepsy. Front. Pharmacol. 2025, 15, 1531288. [Google Scholar] [CrossRef]
- Lu, X.; Huang, Q. Stability and in vitro Digestion Study of Curcumin-encapsulated in Different Milled Cellulose Particle Stabilized Pickering Emulsions. Food Funct. 2020, 11, 606–616. [Google Scholar] [CrossRef]
- Hu, Z.; Feng, T.; Zeng, X.; Janaswamy, S.; Wang, H.; Campanella, O. Structural Characterization and Digestibility of Curcumin Loaded Octenyl Succinic Nanoparticles. Nanomaterials 2019, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Sabet, S.; Rashidinejad, A.; Melton, L.D.; McGillivray, D.J. Recent Advances to Improve Curcumin Oral Bioavailability. Trends Food Sci. Technol. 2021, 110, 253–266. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Maurelli, A.M.; Giotta, L.; Daniello, V.; Di Gioia, S.; Conese, M.; Ingrosso, C.; Ciriaco, F.; Catucci, L. Polymer Encapsulated Liposomes for Oral Co-Delivery of Curcumin and Hydroxytyrosol. Int. J. Mol. Sci. 2023, 24, 790. [Google Scholar] [CrossRef]
- Chen, Q.; Di, X.; Zhai, Y.; Zhao, Q.; Song, X. Influence of Oil Phases on the Digestibility and Curcumin Delivery Properties of Pickering Emulsions. Food Chem. X 2025, 26, 102270. [Google Scholar] [CrossRef]
- Koláčková, T.; Sumczynski, D.; Minařík, A.; Yalçin, E.; Orsavová, J. The Effect of in vitro Digestion on Matcha Tea (Camellia sinensis) Active Components and Antioxidant Activity. Antioxidants 2022, 11, 889. [Google Scholar] [CrossRef]
- Sumczynski, D.; Kotásková, E.; Družbíková, H.; Mlček, J. Determination of Contents and Antioxidant Activity of Free and Bound Phenolics Compounds and in vitro Digestibility of Commercial Black and Red Rice (Oryza sativa L.) Varieties. Food Chem. 2016, 211, 339–346. [Google Scholar] [CrossRef]
- Sumczynski, D.; Fišera, M.; Salek, R.N.; Orsavová, J. The Effect of Flake Production and in vitro Digestion on Releasing Minerals and Trace Elements from Wheat Flakes: The Extended Study of Dietary Intakes for Individual Life Stage Groups. Nutrients 2023, 15, 2509. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Wang, W.; Dou, Z.; Chen, X.; Chen, X.; Chen, H.; Fu, X. Multiscale Combined Techniques for Evaluating Emulsion Stability: A Critical Review. Adv. Colloid Interface Sci. 2023, 311, 102813. [Google Scholar] [CrossRef]
- Tylewicz, U.; Aganovic, K.; Vannini, M.; Toepfl, S.; Bortolotti, V.; Dalla Rosa, M.; Oey, I.; Heinz, V. Effect of Pulsed Electric Field Treatment on Water Distribution of Freeze-Dried Apple Tissue Evaluated with DSC and TD-NMR Techniques. Innov. Food Sci. Emerg. Technol. 2016, 37, 352–358. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, B.; McClements, D.J. Encapsulation of Lipophilic Polyphenols in Plant-Based Nanoemulsions: Impact of Carrier Oil on Lipid Digestion and Curcumin, Resveratrol and Quercetin Bioaccessibility. Food Funct. 2021, 12, 3420–3432. [Google Scholar] [CrossRef] [PubMed]
- Wichitnithad, W.; Jongaroonngamsang, N.; Pummangura, S.; Rojsitthisak, P. A Simple Isocratic HPLC Method for the Simultaneous Determination of Curcuminoids in Commercial Turmeric Extracts. Phytochem. Anal. 2009, 20, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sánchez, G.; Pérez-Jiménez, J.; Saura-Calixto, F. Current Advances for in vitro Protein Digestibility. Front. Nutr. 2024, 11, 1404538. [Google Scholar] [CrossRef]
- Muttakin, S.; Moxon, T.E.; Gouseti, O. In vivo, in vitro, and in silico Studies of the GI Tract. In Interdisciplinary Approaches to Food Digestion; Gouseti, O., Bornhorst, G., Bakalis, S., Mackie, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 29–67. [Google Scholar] [CrossRef]
- EN ISO 712:2009; Cereals and Cereal Products—Determination of Moisture Content—Reference Method. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 2171:2007; Cereals, Pulses and By-Products—Determination of Ash Yield by Incineration. International Organization for Standardization: Geneva, Switzerland, 2007.
- Granato, D.; de Araújo Calado, V.M.; Jarvis, B. Observations on the use of Statistical Methods in Food Science and Technology. Food Res. Int. 2014, 55, 137–149. [Google Scholar] [CrossRef]




| Sample | Dry Matter (%) | Ash Content (%) | DMD 1 (%) | Curcumin in Native Part (mg/g) | Curcumin in Undigested Part (mg/g) |
|---|---|---|---|---|---|
| K1 | 88.4 ± 0.4 a | 8.13 ± 0.21 a | 56.2 ± 0.6 a | 33.4 ± 0.1 a | 36.9 ± 0.6 a |
| K2 | 92.2 ± 0.2 b | 6.38 ± 0.01 b | 52.9 ± 1.1 b | 38.4 ± 0.1 b | 30.2 ± 0.1 b |
| K3 | 90.0 ± 0.2 c | 5.05 ± 0.05 c | 59.6 ± 2.0 c | 24.1 ± 0.1 c | 33.6 ± 0.6 c |
| K4 | 89.4 ± 0.2 d | 7.84 ± 0.03 d | 78.8 ± 2.1 d | 32.8 ± 0.1 d | 36.2 ± 0.1 a |
| KF | 16.9 ± 1.2 e | 1.34 ± 0.11 e | 95.9 ± 3.2 e | 6.95 ± 0.01 e | 23.3 ± 0.2 d |
| Ingredients 1 | O/W | Ingredients 1 | O1/W/O2 |
|---|---|---|---|
| Curcumin (g) | 1.0 | Inner phase (mL) | 40 |
| Black cumin oil (mL) | 10.0 | SPAN 80 (mL) | 0.5 |
| Distilled water (mL) | 90.0 | Coconut oil (mL) | 60.0 |
| TWEEN 20 (g) | 0.5 | ||
| κ-carrageenan (g) | 1.0 | ||
| Processing Parameters | O/W | Processing Parameters | O1/W/O2 |
| Homogenization (RPM) 2 | 25,000 | Homogenization (RPM) 2 | 10,000 |
| Time of homogenization (min) | 5.0 | Time of homogenization (min) | 5.0 |
| Temperature (°C) | 25.0 | Temperature (°C) | 25.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opustilová, K.; Lapčíková, B.; Sumczynski, D.; Adámek, R. Preparing O/W/O Emulsion for Curcumin (Curcuma longa) Delivery and In Vitro Digestibility Assay. Int. J. Mol. Sci. 2025, 26, 5639. https://doi.org/10.3390/ijms26125639
Opustilová K, Lapčíková B, Sumczynski D, Adámek R. Preparing O/W/O Emulsion for Curcumin (Curcuma longa) Delivery and In Vitro Digestibility Assay. International Journal of Molecular Sciences. 2025; 26(12):5639. https://doi.org/10.3390/ijms26125639
Chicago/Turabian StyleOpustilová, Kristýna, Barbora Lapčíková, Daniela Sumczynski, and Richard Adámek. 2025. "Preparing O/W/O Emulsion for Curcumin (Curcuma longa) Delivery and In Vitro Digestibility Assay" International Journal of Molecular Sciences 26, no. 12: 5639. https://doi.org/10.3390/ijms26125639
APA StyleOpustilová, K., Lapčíková, B., Sumczynski, D., & Adámek, R. (2025). Preparing O/W/O Emulsion for Curcumin (Curcuma longa) Delivery and In Vitro Digestibility Assay. International Journal of Molecular Sciences, 26(12), 5639. https://doi.org/10.3390/ijms26125639








