Abstract
Grass pollen allergies significantly contribute to atopic diseases such as asthma and allergic rhinitis, resulting in considerable healthcare burdens. Objective: In this study, molecular sensitization patterns to grass pollen in Swiss patients were addressed. The research utilized a retrospective cohort approach using ImmunoCAP™ ISAC testing from October 2015 to July 2020. Clinical histories, demographics, and skin prick test results were collected for analysis. The minimum patient age was 18 years and the average patient age was 41.3 years, with a female predominance (68.5%). In total, 4814 measurements were analyzed. Allergic rhinitis was the most common clinical symptom, followed by asthma and urticaria. A total of 1963 patients (40.8%) revealed sensitization to grass pollen. The most common sensitizations were found to the major allergens Phl p 1 (86%) and Phl p 5 (65%), but also to Phl p 4 (62%). Monosensitization was mostly found to allergens Phl p 1 (266/13.5%) and Phl p 4 (157/7.9%), and less so to Phl p 5 (33/1.7%). Notably, the Phl p 4-monosensitized subgroup showed only an 18% positivity rate in skin prick tests and presented mostly with urticaria. This study gives insights into the spectrum of grass pollen allergies in a Central European setting and underscores the possibly underestimated role of Phl p 4 among grass pollen allergens, especially in a subgroup that suffers mainly from seasonal urticaria. Monovalent sensitization to Phl p 4 can also cause seasonal rhinitis and might therefore be missed if only Phl p 1/p 5 are tested. A better understanding of sensitization patterns will further improve diagnosis and treatment options.
1. Introduction
Grass pollen allergy has a significant impact on patients’ quality of life [1,2] and plays a major role in managing atopic diseases such as asthma, atopic dermatitis, or allergic rhinoconjunctivitis. Therefore, it is a considerable burden for the healthcare system [3,4,5,6].
In Europe, grass pollen is one of the most important airborne allergen sources [7], with an average sensitization rate of 38% [7,8]. Further increases in these numbers in various regions due to changes in environmental factors are to be expected [9]. Regarding grass pollen allergy, Timothy grass (Phleum pratense) is the most relevant allergen in Europe and can be used as a representative marker of other homologous grass allergens in the Poaceae family [10]. Therefore, it is often used for diagnostics and epidemiologic studies [11].
Differentiation is possible between grass-specific allergen components such as Phl p 1, Phl p 2, Phl p 5, and Phl p 6 and cross-reactive components like Phl p 4, Phl p 7, Phl p 11, and Phl p 12 [12]. According to its IgE-binding frequency and capacity, an allergen can be classified as a major (>50%) or minor (<50%) allergen [13]. Phleum pratense sensitization patterns have been analyzed in different European countries, and IgE-binding patterns are heterogeneous and vary depending on the geographical region [14,15,16]. Consequently, knowledge of sensitization profiles provides important data for allergen-specific immunotherapy (SIT) decisions and should be identified to improve the management of allergies [17,18].
The aim of this study was to analyze grass-pollen-sensitized people in a cross-sectional cohort of allergy patients from Zurich, Switzerland and to describe the molecular sensitization patterns of IgE to Phleum pratense, as well as to compare individual patterns regarding differences in demographic information, clinical symptoms, and skin prick test results.
2. Results
2.1. Demographics and Patient Characteristics
A total of 4814 ISACs were performed in 4814 patients and were screened for relevant sensitizations. Clinical information such as symptoms, skin prick test (SPT) results, and personal medical history was available and previously collected for 2481 patients. A total of 812 patients were excluded from the study due to being underage. A total of 1256 patients did not show any sensitization at all. A total of 2746 were positive to at least one allergen component, and 1963 were positive to at least one grass-pollen-specific molecule and represented our study population. Patients were 18 to 90 years old, with a mean age of 41 years. The sex distribution was 68% female and 32% male.
2.2. Sensitization Patterns
The highest sensitization rates were found for Phl p 1 (86%; 1685/1963) and Phl p 5 (65%; 1274/1963), followed by Phl p 4 (62%; 1208/1963), Phl p 2 (48%; 945/1963), Phl p 6 (44%; 867/1963), and Phl p 11 (26%; 510/1963). The lowest sensitization frequencies were detected for Phl p 12 (13%; 238/1963) and Phl p 7 (5%; 97/1963). Therefore, only Phl p 1, Phl p 4, and Phl p 5 managed to classify as major allergens in our cohort, while Phl p 2 and Phl p 6 missed the sensitization frequency threshold of more than 50 percent by just a small margin.
2.3. Monosensitization
A total of 23% (460/1963) of patients were monosensitized to only one Phleum pratense molecule. The most frequently detected monomolecular sensitized components were Phl p 1 (12.5%, 246/1963) and Phl p 4 (7.9%, 157/1963), followed by Phl p 5 (1.6%, 33/1963), Phl p 11 (0.7%, 13/1963), Phl p 2 (0.3%, 6/1963, Phl p 12 (0.2%, 3/1963) and Phl p 6 (0.1%, 2/1963) There was no monomolecular sensitization observed for Phl p 7 (0%, 0/1963) (see Figure 1).
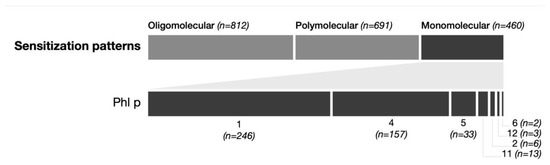
Figure 1.
Monosensitization patterns. The upper part shows the number of patients with sensitization to 2–4 allergens (oligomolecular), more than 4 allergens (polymolecular), and only 1 allergen (monomolecular). The lower part shows the distribution among the different Phl allergens in patients with monomolecular sensitization.
2.4. Oligomolecular and Polymolecular Sensitization
A total of 41% (812/1963) of patients showed an oligomolecular (2–4 molecules) sensitization pattern, and 35% (691/1963) displayed a polymolecular (5–8 molecules) IgE profile. A total of 14 patients (0.7%, 14/1963) showed IgE-binding activity to all eight grass pollen allergen components simultaneously. A very heterogenous and wide spectrum of 97 different sensitization patterns was found. In descending frequency of occurrence, the most common profiles were: Phl p 1 (12.5%), Phl p 1,2,4,5,6 (8.6%), Phl p 1,2,4,5,6,11 (8.5%), Phl p 4 (8%), Phl p 1,4 (4.3%), Phl p 1,5 (4.3%), Phl p 1,4,5,6, (4.2%), Phl p 1,2,4,5,6,12 (3.6%), Phl p 1,4,5 (3%), and Phl p 1,2 (2.9%) (see Figure 2).
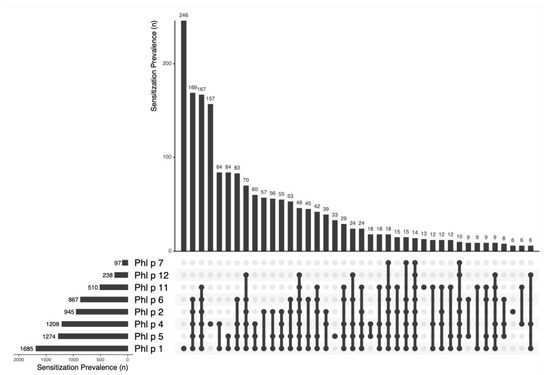
Figure 2.
The sensitization prevalence of Phl p molecules and the most frequent patterns. The upper part shows the frequency of sensitization in absolute numbers, while the lower part summarizes the different combinations of allergen sensitizations found, marked with dots for each allergen found.
Those ten profiles covered almost 60% of the study population and were subjected to further detailed examination, such as the prevalence of clinical symptoms and positivity of the skin prick test. The mean ages of all ten groups were between 30 and 44.
2.5. Positivity to Skin Prick Test
The prevalence of positive skin prick test responses to Dermatophagoides pteronyssinus, Dermatophagoides farina, Alternaria, Aspergillus, Cladosporium, Penicillium, grass pollen mix (Poa pratensis, Dactilis glomerata, Lolium perenne, Phleum pratense, Festuca pratensis, Helictotrichon pratense), Artemisia vulgaris, birch (Betula alba), alder (Alnus incana), hazel (Corylus avellana), ash, rye, and Ambrosia are shown in Table 1.

Table 1.
Positivity of the skin prick test for the ten most frequent sensitization patterns.
Positivity to the grass pollen mix in the SPT for the subgroup of Phl p 4-monosensitized patients was at 18%, which is much less compared to the other profiles, which had a positivity rate between 82 and 98%.
2.6. Prevalence of Clinical Symptoms
The prevalence of allergic rhinitis, asthma, eczema, urticaria, and anaphylaxis for each profile is demonstrated in Table 2 and shown in Figure 3. In all ten groups, allergic rhinitis was by far the most dominant symptom, followed by asthma. Regarding the prevalence of the aforementioned symptoms, there was a significant difference found between the ten patterns.

Table 2.
Prevalence of clinical symptoms for the ten most frequent sensitization patterns.
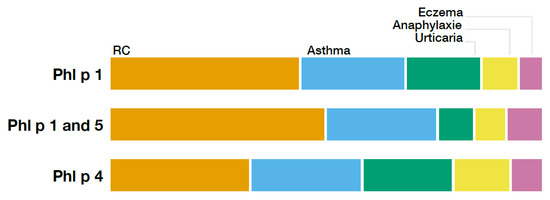
Figure 3.
Relative distribution of different clinical symptoms compared between the subgroups Phl p 1, Phl p 1 and 5, and Phl p 5.
3. Discussion
This is the first study of its size that describes the molecular sensitization patterns in grass-pollen-sensitized patients from Switzerland and summarizes characteristics of the most typical patterns [8,19]. Regarding their sensitization rates, all eight investigated Phleum pratense allergens have been examined in multiple prior studies. We can confirm that Phl p 1, Phl p 5, and Phl p 4 are the most common allergens, followed by Phl p 2 and Phl p 6, while sensitization to Phl p 11, Phl p 12, and Phl p 7 is by far less frequent [11,20,21]. Previous studies suggest sensitization rates around 90% for Phl p 1 and a range of 65 to 85% for Phl p 5, which is in line with our results of 86% and 65%, respectively [11]. The same applies for Phl p 2, where we reached 48%, with a previously suggested 40–60% range [11]. Our sensitization frequencies for the remaining allergens are noticeably lower compared to results from Italy [22] or Germany [21], with absolute differences of up to nearly 30%. Interestingly the work of Panzner et al. from the Czech Republic proved to be a much better match to our results, with maximum differences in frequencies of 5% [20]. It is known that geographical variations in prevalence and sensitization patterns exist due to varying reasons like allergen exposure, diverse climates, or levels of industrialization [23,24]. Another fact that could be a good explanation for the huge differences in the prevalence of sensitization is the number of patients who were included in the studies. While the studies from Italy and Germany only analyzed n = 77 and n = 101 patients, the study from the Czech Republic examined n = 669 grass-pollen-sensitized patients. It could be argued that the bigger sample size leads to more precise sensitization rates.
The vast heterogeneity of sensitization patterns was no surprise, since there have been similar observations made in the past [21,25,26,27]. In 2012 a study by Tripodi et al. described 39 different patterns in a cohort of 176 patients [27]. Five years later another research project managed to identify 87 patterns in a cohort of 1120 patients [25]. Our results showed 92 different IgE profiles in an even bigger study population of 1963 patients. These findings seem to underline the theory in the work of Cipriani et al. that all 256 mathematical possibilities of different sensitization patterns could be observed if the study population was large enough [25]. A comparison between the patterns detected in our study of Swiss patients and the patterns found in Germany [21] or Italy [25] indicates different frequencies of the existing profiles. Nevertheless, the following five patterns are found in the top ten of all three countries, underlining their importance in this part of the European population: “Phl p 1”, “Phl p 1,2,4,5,6”, “Phl p 1,2,4,5,6,11”, “Phl p 1,4”, and “Phl p 1,2,4,5,6,12”. Additionally, these observations seem to reflect the findings of a German study that analyzed the data of a birth cohort and described a mechanism called molecular spreading, suggesting Phl p 1 as the initiator molecule of sensitization to Phleum pratense followed by sensitization to Phl p 4 and Phl p 5, then to Phl p 2 and Phl p 6, and finally to Phl p 11, Phl p 12, and Phl p 7 [26].
When we compared the ten most common patterns among themselves regarding characteristics of the groups, such as age, sex ratio, or clinical symptoms, significant differences were observed, suggesting that there might be different phenotypes that could be characterized in further studies.
The frequencies of mono-, oligo- and polymolecular patterns are in concordance with results from Cipriani et al., displaying almost identical percentages [25]. Even though the most common pattern is a monomolecular one, it is known that monosensitization is still much rarer than sensitization to multiple Phleum pratense components [20]. While frequent monosensitization to Phl p 1 was no big surprise, given that in more than three-quarters of cases it is assumed to act as an initiator molecule, potentially leading to an oligo- or polymolecular sensitization [26,28,29], the large amount of monomolecular sensitization to Phl p 4 was rather astonishing. It was already observed to be one of the leading monomolecular sensitization components in other studies, but not to such a large extent [20,25]. The importance of Phl p 1 and Phl p 4 was stressed by a Swedish birth cohort study, which concluded both molecules to be crucial early indicators for predicting future grass pollen allergy [30].
Also, in a recent work from southern China, the high prevalence of Phl p 4 was outlined, being the main allergenic component in pollen-sensitized asthma patients [27]. In another study sensitization to Phl p 4 was discussed as an early indicator for pollen allergy [31]. In the MeDall study it was also noted that natural Phl p 4 is a hitherto unrecognized early indicator of grass pollen allergy, showing the second most common sensitization rate in children below 16 years old, just after Phl p 1 [30]. As our study group was 18 years old or older we cannot comment on the possibly predictive aspect of Phl p 4; however, our data demonstrate a relevant importance in adult patients also.
The fact that the subgroup of Phl p 4-monosensitized patients showed seasonal allergic symptoms during the gras pollen season, underlines the clinical importance of these findings. Sensitization to Phl p 4 is almost as frequent as Phl p 5 and monosensitization occurs even more often, therefore the number of affected individuals should not be overlooked. In our population monosensitization to Phl p 4 was the fourth most common pattern and therefore 8% of patients are affected.
In the SPT only 18% of the Phl p 4-monosensitized subgroup showed positivity to the grass pollen mix compared to 82–98% in the other subgroups. Therefore, the SPT seems not to be an appropriate diagnostic tool to diagnose an allergy in this subgroup of patients. Even a conventional ImmunoCAP test, which only tests sensitization to Phl p 1 and Phl p 5, would lead to a underdiagnosis in this specific subpopulation.
There are more clinical data and maybe even specific provocation tests needed to strengthen this theory. But at this point several factors indicate that Phl p 4 has been underestimated in its clinical relevance, potentially leading to underdiagnosis of grass pollen allergy, especially in the subgroup of Phl p 4-monosensitized patients. Urticaria might be an underestimated or underrecognized symptom of grass pollen allergy, as also shown by other authors [32,33].
Possibly sensitization patterns might also reflect distinct clinical phenotypes, as shown for other allergens such as pets or house dust mites [34,35,36,37].
A strength of this study is the use of component-resolved diagnostics, which allowed precise measurements of specific IgE levels. This method is well established and reliable for epidemiological evaluations of sensitization patterns. Additionally, the size of its population represents a further strength which should not be underestimated. As already seen above, insight into sensitization pattern possibilities is enlarged by the rising number of patients assessed in a study. However, there are some limitations as well. Unfortunately, clinical data were very limited and could not be gathered for all patients, leading to the absence of important data. On top of that, the results of this study are only suitable for patients living in Switzerland and can only partially be applied to other European regions. We cannot exclude a selection bias when it comes to our study population. Due to the catchment area of the University Hospital Zurich, more than three-quarters of the patients were living in Zurich or the surrounding region, leading to an underrepresentation of people living in other regions with different environmental factors. It is absolutely possible that the role of Phl p 4 may vary in other Swiss, European, or also global regions; further studies focusing on the prevalence of sensitization to Phl p 4 in other regions are needed to shed light on this aspect.
This study focused mainly on an epidemiological approach, describing sensitization rates and patterns as well as summarizing characteristics of groups. Even though clinical information was present for some of the patients, specific provocation tests would be needed to further analyze the clinical relevance of these findings. Nonetheless this study provides information of the utmost importance and helps to improve the diagnostics and treatment of grass pollen allergy and associated allergic diseases.
4. Methods
4.1. Patient Characteristics and Data Acquisition
After ethical approval was obtained (BASEC Nr.: 2021-00070), we performed a retrospective cross-sectional cohort study. The electronic laboratory record database at the Department of Dermatology, University Hospital Zurich, Switzerland, was searched for patients in which an ImmunoCAP™ ISAC Test was performed between October 2015 and July 2020. All patients aged 18 or older who showed sensitization to at least one Phleum pratense allergen were included and comprised the study population. Thereafter, the electronic medical record database was screened, and the following clinical information was extracted, if available: gender, domicile, history of asthma, allergic rhinitis, atopic eczema, and chronic urticaria, past medical history, family allergy history, and current treatment. Furthermore, skin prick test results for seasonal and perennial inhalant allergens, including grass pollen, were collected.
4.2. Prick Testing
All skin prick tests were performed by an allergologist according to international standard criteria [38]. A negative control with sodium chloride and a positive control with histamine was applied. Test interpretation took place 15–20 min after the application of specific allergen extracts. The test result was defined as positive if the diameter of the wheal was ≥3 mm.
4.3. Molecular Analysis
The analysis of specific IgE concentrations was performed by ImmunoCAP ISAC allergen microarray immunoassay (Thermo Fisher, Uppsala, Sweden) based on fluorescence measurements, and the results are stated in ISU-E (ISAC Standardized Units). The sensitization threshold for an allergen component was defined as ≥0.3 ISU-E. All measurements below this value were considered not sensitized and set to zero.
4.4. Statistical Analysis
Statistical analysis and data visualization were conducted using R software (version 4.2.2), with p-values ≤ 0.05 indicating significance. The p-values for comparisons between the sensitization patterns for clinical symptoms and SPT were calculated using Pearson’s chi-squared test or Fisher’s exact test.
5. Conclusions
This study puts the role of Phleum p 4 in a new perspective, suggesting it to be a clinically relevant allergen with a potentially underestimated role in grass pollen allergy, especially in patients with seasonal urticarial as their leading symptom. Interestingly monosensitization to Phl p 4 was quite common and was only detected by a conventional skin prick test in 18% of patients, and might therefore be underdiagnosed. Furthermore, the results underline the importance of Phleum pratense sensitization in Switzerland and showcases the immense variability of sensitization patterns in a Phleum-pratense-sensitized population. The observed sensitization frequencies may be important when allergen-specific-immunotherapy for grass pollen allergy, and therefore the composition of therapeutic vaccines, is discussed.
Author Contributions
P.F. has acquired all data and written the original draft of the manuscript. P.C. has done all statistical analyses. P.S.-G. has been involved in the conceptualization of the study, C.G. has supervised and lead the study; P.S.-G. and C.G. have both been involved also in writing and editing the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an unrestricted grant attributed to P.S.-G., UZH Number F-85804-10-01.
Institutional Review Board Statement
The study has been approved by the University Hospital of Zurich, the University of Zurich and the Cantonal Ethical Committee of Zurich, Number 2024-00691 (approval date: 17 May 2024).
Informed Consent Statement
Written informed consent for publication was obtained from each participating patients that their data could be analyzed in an encrypted form (General consent of the University Hospital of Zurich).
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to the encryption of the dataguaranteed to all participants.
Conflicts of Interest
P.F., P.C. and C.G. have no COI to declare. P.S.-G. has received honoraria for lecturing and advisory board participation from Buehlmann Labs, Euroimmun, Thermo Fisher, and RUWAG and study support from all these companies. The present study has not been directly from these companies neither had they any input on the manuscript writing and editing.
References
- Biedermann, T.; Winther, L.; Till, S.J.; Panzner, P.; Knulst, A.; Valovirta, E. Birch pollen allergy in Europe. Allergy 2019, 74, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Kurganskiy, A.; Creer, S.; De Vere, N.; Griffith, G.W.; Osborne, N.J.; Wheeler, B.W.; McInnes, R.N.; Clewlow, Y.; Barber, A.; Brennan, G.L.; et al. Predicting the severity of the grass pollen season and the effect of climate change in Northwest Europe. Sci. Adv. 2021, 7, eabd7658. [Google Scholar] [CrossRef] [PubMed]
- Casale, T.B.; Pedersen, S.; Rodriguez Del Rio, P.; Liu, A.H.; Demoly, P.; Price, D. The Role of Aeroallergen Sensitization Testing in Asthma Management. J. Allergy Clin. Immunol. Pract. 2020, 8, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa-Mineoka, R.; Katoh, N. Atopic Dermatitis: Identification and Management of Complicating Factors. Int. J. Mol. Sci. 2020, 21, 2671. [Google Scholar] [CrossRef]
- Jalbert, I.; Golebiowski, B. Environmental aeroallergens and allergic rhino-conjunctivitis. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 476–481. [Google Scholar] [CrossRef]
- Traidl-Hoffmann, C.; Afghani, J.; Akdis, C.A.; Akdis, M.; Aydin, H.; Bärenfaller, K.; Behrendt, H.; Bieber, T.; Bigliardi, P.; Bigliardi-Qi, M.; et al. Navigating the evolving landscape of atopic dermatitis: Challenges and future opportunities: The 4th Davos declaration. Allergy 2024, 79, 2605–2624. [Google Scholar] [CrossRef]
- D’Amato, G.; Vitale, C.; Sanduzzi, A.; Molino, A.; Vatrella, A.; D’Amato, M. Allergenic pollen and pollen allergy in Europe. Allergy 2007, 62, 976–990. [Google Scholar] [CrossRef]
- Burbach, G.J.; Heinzerling, L.M.; Edenharter, G.; Bachert, C.; Bindslev-Jensen, C.; Bonini, S.; Bousquet, J.; Bousquet-Rouanet, L.; Bousquet, P.J.; Bresciani, M.; et al. GA(2)LEN skin test study II: Clinical relevance of inhalant allergen sensitizations in Europe. Allergy 2009, 64, 1507–1515. [Google Scholar] [CrossRef]
- Bielory, L.; Lyons, K.; Goldberg, R. Climate change and allergic disease. Curr. Allergy Asthma Rep. 2012, 12, 485–494. [Google Scholar] [CrossRef]
- Lorenz, A.R.; Lüttkopf, D.; May, S.; Scheurer, S.; Vieths, S. The principle of homologous groups in regulatory affairs of allergen products—A proposal. Int. Arch. Allergy Immunol. 2009, 148, 1–17. [Google Scholar] [CrossRef]
- Andersson, K.; Lidholm, J. Characteristics and immunobiology of grass pollen allergens. Int. Arch. Allergy Immunol. 2003, 130, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27, 1–250. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, L.; Valenta, R.; Acevedo, N.; Zakzuk, J. Are the Terms Major and Minor Allergens Useful for Precision Allergology? Front. Immunol. 2021, 12, 651500. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Ospina, M.B.; Sideri, K.; Vliagoftis, H. Retrospective analysis of aeroallergen’s sensitization patterns in Edmonton, Canada. Allergy Asthma Clin. Immunol. 2019, 15, 6. [Google Scholar] [CrossRef]
- Navarro-Locsin, C.G.; Lim-Jurado, M. Aeroallergen sensitization and associated comorbid diseases of an adult Filipino population with allergic rhinitis. Asia Pac. Allergy 2018, 8, e25. [Google Scholar] [CrossRef]
- Ozkaya, E.; Sogut, A.; Küçükkoç, M.; Eres, M.; Acemoglu, H.; Yuksel, H.; Murat, N. Sensitization pattern of inhalant allergens in children with asthma who are living different altitudes in Turkey. Int. J. Biometeorol. 2015, 59, 1685–1690. [Google Scholar] [CrossRef]
- Douladiris, N.; Savvatianos, S.; Roumpedaki, I.; Skevaki, C.; Mitsias, D.; Papadopoulos, N.G. A molecular diagnostic algorithm to guide pollen immunotherapy in southern Europe: Towards component-resolved management of allergic diseases. Int. Arch. Allergy Immunol. 2013, 162, 163–172. [Google Scholar] [CrossRef]
- Jutel, M.; Jaeger, L.; Suck, R.; Meyer, H.; Fiebig, H.; Cromwell, O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J. Allergy Clin. Immunol. 2005, 116, 608–613. [Google Scholar] [CrossRef]
- Schmitz, R.; Ellert, U.; Kalcklösch, M.; Dahm, S.; Thamm, M. Patterns of sensitization to inhalant and food allergens—Findings from the German Health Interview and Examination Survey for Children and Adolescents. Int. Arch. Allergy Immunol. 2013, 162, 263–270. [Google Scholar] [CrossRef]
- Panzner, P.; Vachová, M.; Vítovcová, P.; Brodská, P.; Vlas, T. A comprehensive analysis of middle-European molecular sensitization profiles to pollen allergens. Int. Arch. Allergy Immunol. 2014, 164, 74–82. [Google Scholar] [CrossRef]
- Darsow, U.; Brockow, K.; Pfab, F.; Jakob, T.; Petersson, C.J.; Borres, M.P.; Ring, J.; Behrendt, H.; Huss-Marp, J. Allergens. Heterogeneity of molecular sensitization profiles in grass pollen allergy–implications for immunotherapy? Clin. Exp. Allergy 2014, 44, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.E.; Monasterolo, G.; Monasterolo, S. Measurement of IgE antibodies against purified grass-pollen allergens (Phl p 1, 2, 3, 4, 5, 6, 7, 11, and 12) in sera of patients allergic to grass pollen. Allergy 2001, 56, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, P.J.; Chinn, S.; Janson, C.; Kogevinas, M.; Burney, P.; Jarvis, D. Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European Community Respiratory Health Survey, I. Allergy 2007, 62, 301–309. [Google Scholar] [CrossRef]
- Newson, R.B.; Van Ree, R.; Forsberg, B.; Janson, C.; Lötvall, J.; Dahlén, S.E.; Toskala, E.M.; Baelum, J.; Brożek, G.M.; Kasper, L.; et al. Geographical variation in the prevalence of sensitization to common aeroallergens in adults: The GA(2) LEN survey. Allergy 2014, 69, 643–651. [Google Scholar] [CrossRef]
- Cipriani, F.; Mastrorilli, C.; Tripodi, S.; Ricci, G.; Perna, S.; Panetta, V.; Asero, R.; Dondi, A.; Bianchi, A.; Maiello, N.; et al. Diagnostic relevance of IgE sensitization profiles to eight recombinant Phleum pratense molecules. Allergy 2018, 73, 673–682. [Google Scholar] [CrossRef]
- Hatzler, L.; Panetta, V.; Lau, S.; Wagner, P.; Bergmann, R.L.; Illi, S.; Bergmann, K.E.; Keil, T.; Hofmaier, S.; Rohrbach, A.; et al. Molecular spreading and predictive value of preclinical IgE response to Phleum pratense in children with hay fever. J. Allergy Clin. Immunol. 2012, 130, 894–901.e5. [Google Scholar] [CrossRef]
- Xu, C.; Chai, D.; Zheng, P.; Qiu, R.; Pan, X.; Zhang, Y. The Sensitization Differences of Pollen Allergen Components in Patients with Asthma and/or Rhinitis in Southern China. Int. Arch. Allergy Immunol. 2024, 185, 821–826. [Google Scholar] [CrossRef]
- Matricardi, P.M. Allergen-specific immunoprophylaxis: Toward secondary prevention of allergic rhinitis? Pediatr. Allergy Immunol. 2014, 25, 15–18. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Dramburg, S.; Potapova, E.; Skevaki, C.; Renz, H. Molecular diagnosis for allergen immunotherapy. J. Allergy Clin. Immunol. 2019, 143, 831–843. [Google Scholar] [CrossRef]
- Westman, M.; Åberg, K.; Apostolovic, D.; Lupinek, C.; Gattinger, P.; Mittermann, I.; Andersson, N.; Melén, E.; Bergström, A.; Antó, J.M.; et al. Sensitization to grass pollen allergen molecules in a birth cohort-natural Phl p 4 as an early indicator of grass pollen allergy. J. Allergy Clin. Immunol. 2020, 145, 1174–1181.e6. [Google Scholar] [CrossRef]
- Mendy, A.; Zeldin, D.C. Phl p 4: An early indicator of grass pollen allergy? J. Allergy Clin. Immunol. 2020, 145, 1556–1557. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, X.; Ni, B.; Song, Z. Atopy in chronic urticaria: An important yet overlooked issue. Front. Immunol. 2024, 15, 1279976. [Google Scholar] [CrossRef]
- Cakmak, M.E.; Yegit, O.O.; Öztop, N. A Case-Control Study Comparing the General Characteristics of Patients with Symptomatic Dermographism and Chronic Spontaneous Urticaria: Is Atopy a Risk Factor for Symptomatic Dermographism? Int. Arch. Allergy Immunol. 2024, 185, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, W.; Sestak-Greinecker, G.; Braunsteiner, T.; Wantke, F.; Wöhrl, S. Molecular sensitization patterns in animal allergy: Relationship with clinical relevance and pet ownership. Allergy 2021, 76, 3687–3696. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Yang, M.S.; Borres, M.P.; Andersson, M.; Lee, S.M.; Lee, S.P. The association between specific IgE antibodies to component allergens and allergic symptoms on dog and cat exposure among Korean pet exhibition participants. World Allergy Organ. J. 2022, 15, 100709. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, J.; Lee, J.Y.; Lee, S.C.; Sim, D.W.; Shin, J.U.; Park, C.O.; Lee, J.H.; Lee, K.H.; Jeong, K.Y.; et al. Sensitization to various minor house dust mite allergens is greater in patients with atopic dermatitis than in those with respiratory allergic disease. Clin. Exp. Allergy 2018, 48, 1050–1058. [Google Scholar] [CrossRef]
- Walsemann, T.; Böttger, M.; Traidl, S.; Schwager, C.; Gülsen, A.; Freimooser, S.; Roesner, L.M.; Werfel, T.; Jappe, U. Specific IgE against the house dust mite allergens Der p 5, 20 and 21 influences the phenotype and severity of atopic diseases. Allergy 2023, 78, 731–742. [Google Scholar] [CrossRef]
- Bousquet, J.; Heinzerling, L.; Bachert, C.; Papadopoulos, N.G.; Bousquet, P.J.; Burney, P.G.; Canonica, G.W.; Carlsen, K.H.; Cox, L.; Haahtela, T.; et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy 2012, 67, 18–24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).