Investigating COX-2 and 5-LOX Enzyme-Related Anti-Inflammatory and Antioxidant Activities and Phytochemical Features of Scutellaria salviifolia Benth
Abstract
1. Introduction
2. Results
2.1. Enzyme Inhibition Assays
2.2. Antioxidant Activity Assays
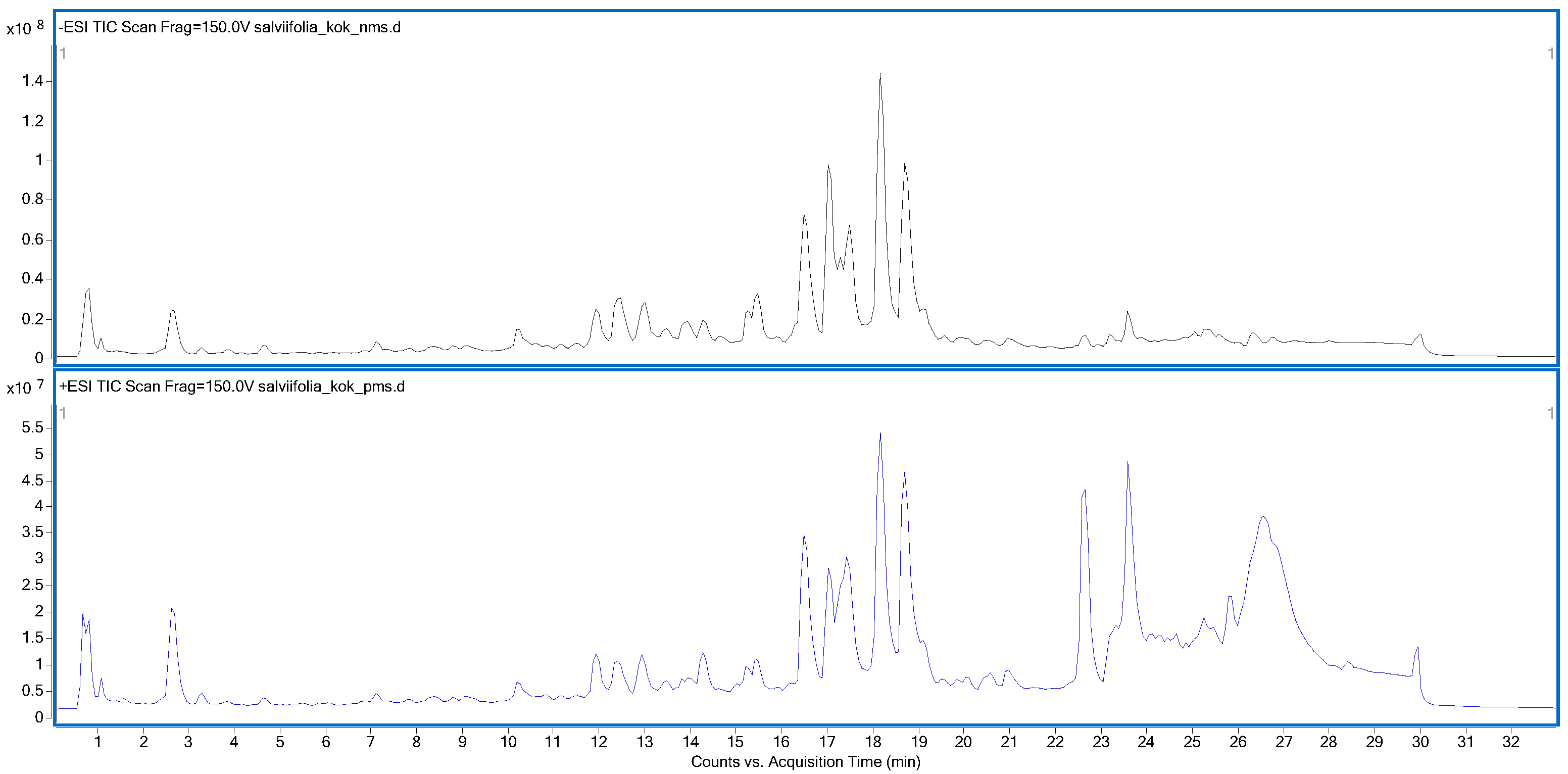
2.3. Phytochemical Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Plant Materials and Extracts
4.3. Enzyme Inhibition Assays
4.3.1. 5-Lipoxygenase (5-LOX) Enzyme Inhibition
4.3.2. Cyclooxygenase-2 (COX-2) Enzyme Inhibition
4.4. Antioxidant Activity Assays
4.4.1. DPPH Radical Scavenging Activity
4.4.2. ABTS Radical Scavenging Activity
4.5. Phytochemical Analysis
Quadrupole Time of Flight Liquid Chromatography/Mass Spectrometry (Q-TOF LC/MS) Analysis Conditions
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-LOX | 5-Lipoxygenase |
| AA | Arachidonic acid |
| ABTS | 2,2-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) |
| BHT | Butylated hydroxytoluene |
| COX | Cyclooxygenase |
| DMSO | Dimethyl sulfoxide |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EGFR | Epidermal growth factor receptor |
| HIF-1 | Hypoxia-inducible factor |
| HPLC | High-performance liquid chromatography |
| IC50 | Half-maximal inhibitory concentration |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| LPS | Lipopolysaccharide |
| LT | Leukotriene |
| LXR | Liver X receptor |
| MAPK | Mitogen-activated protein kinase |
| MeOH | Methanol |
| MIC | Minimum inhibitory concentration |
| NF-κB | Nuclear factor kappa B |
| NO | Nitric oxide |
| PG | Prostaglandin |
| Q-TOF LC/MS | Quadrupole time of flight liquid chromatography/mass spectrometry |
| ROS | Reactive oxygen species |
| S.D. | Standard deviation |
| TIC | Total ion chromatogram |
| TNF | Tumor necrosis factor |
References
- Acar, M.; Taşar, N.; Akbulut, G.B. Anatomical, Micromorphological, Karyological and Biochemical study of Scutellaria orientalis subsp. virens and Scutellaria salviifolia. Kahramanmaraş Sütçü İmam Univ. Tarım Doğa Derg. 2022, 25, 125–136. [Google Scholar] [CrossRef]
- Shang, X.; He, X.; He, X.; Li, M.; Zhang, R.; Fan, P.; Zhang, Q.; Jia, Z. The Genus Scutellaria: An Ethnopharmacological and Phytochemical Review. J. Ethnopharmacol. 2010, 128, 279–313. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, G.; Çiçek, M.; Demirci, B.; Başer, K.H.C. Essential Oil Compositions of Subspecies of Scutellaria brevibracteata Stapf from Turkey. J. Essent. Oil Res. 2019, 31, 255–262. [Google Scholar] [CrossRef]
- Marsh, Z.; Yang, T.; Nopo-Olazabal, L.; Wu, S.; Ingle, T.; Joshee, N.; Medina-Bolivar, F. Effect of Light, Methyl Jasmonate and Cyclodextrin on Production of Phenolic Compounds in Hairy Root Cultures of Scutellaria lateriflora. Phytochemistry 2014, 107, 50–60. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Gołąb, K.; Gburek, J.; Wysokińska, H.; Matkowski, A. Inhibition of Advanced Glycation End-Product Formation and Antioxidant Activity by Extracts and Polyphenols from Scutellaria alpina L. and S. altissima L. Molecules 2016, 21, 739. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, S.; Kuang, Y.; Hu, Z.-M.; Qiao, X.; Ye, M. A Comprehensive Review on Phytochemistry, Pharmacology, and Flavonoid Biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, F.; Cui, Y.; Zhao, L.; Sun, X.; Tang, W.; Cai, P. An Update on Chinese Herbal Medicines as Adjuvant Treatment of Anticancer Therapeutics. Biosci. Trends 2018, 12, 220–239. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, D.; Wang, N.; Li, S.; Tan, H.-Y.; Feng, Y. Polyphenols of Chinese Skullcap Roots: From Chemical Profiles to Anticancer Effects. RSC Adv. 2019, 9, 25518–25532. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Hu, P.; Ji, J.; Li, X.; Chen, J. Neoclerodane Diterpenoids from Scutellaria barbata with Cytotoxic Activities. Nat. Prod. Res. 2020, 34, 1345–1351. [Google Scholar] [CrossRef]
- Arweiler, N.B.; Pergola, G.; Kuenz, J.; Hellwig, E.; Sculean, A.; Auschill, T.M. Clinical and Antibacterial Effect of an Anti-inflammatory Toothpaste Formulation with Scutellaria baicalensis Extract on Experimental Gingivitis. Clin. Oral Investig. 2011, 15, 909–913. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, J.; Lin, K.; Wang, H.; Liang, Z.; Ren, X.; Huang, L.; Xia, C. Efficacy of Scutellaria baicalensis for the Treatment of Hand, Foot, and Mouth Disease Associated with Encephalitis in Patients Infected with EV71: A Multicenter, Retrospective Analysis. Biomed. Res. Int. 2016, 2016, 5697571. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.K.; Kwak, J.Y.; Choi, G.W.; An, S.M.; Kwak, J.H.; Seo, H.H.; Suh, H.J.; Boo, Y.C. Scutellaria Radix Extract as a Natural UV Protectant for Human Skin. Phytother. Res. 2016, 30, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare of Japan. The Japanese Pharmacopoeia, 13th ed.; Ministry of Health and Welfare of Japan: Tokyo, Japan, 1996.
- Pharmacopoeia Commission, Ministry of Public Health. Pharmacopoeia of the People’s Republic of China. Part 1; Pharmacopoeia Commission, Ministry of Public Health: Beijing, China, 2000. (In Chinese)
- Özçelik, H.; Ay, G.; Öztürk, M. Some Traditional Plants of East and Southeast Anatolia. In Proceedings of the 10th National Symposium on Biology; Atatürk University: Erzurum, Türkiye, 1990; pp. 1–10. [Google Scholar]
- Baytop, T. Therapy with Medicinal Plants in Turkey (Past and Present); Publication of Istanbul University: Istanbul, Türkiye, 1999; p. 312. [Google Scholar]
- Altundag, E.; Ozturk, M. Ethnomedicinal Studies on the Plant Resources of East Anatolia, Turkey. Procedia Soc. Behav. Sci. 2011, 19, 756–777. [Google Scholar] [CrossRef]
- Cakilcioglu, U.; Turkoglu, I. An Ethnobotanical Survey of Medicinal Plants in Sivrice (Elazığ-Turkey). J. Ethnopharmacol. 2010, 132, 165–175. [Google Scholar] [CrossRef]
- Mükemre, M.; Behçet, L.; Cakılcıoğlu, U. Ethnobotanical Study on Medicinal Plants in Villages of Çatak (Van-Turkey). J. Ethnopharmacol. 2015, 166, 361–374. [Google Scholar] [CrossRef]
- Saracoglu, I.; Inoue, M.; Calis, I.; Ogihara, Y. Studies on Constituents with Cytotoxic and Cytostatic Activity of Two Turkish Medicinal Plants, Phlomis armeniaca and Scutellaria salviifolia. Biol. Pharm. Bull. 1995, 18, 1396–1400. [Google Scholar] [CrossRef]
- Şenol, F.; Orhan, İ.; Yilmaz, G.; Çiçek, M.; Şener, B. Acetylcholinesterase, Butyrylcholinesterase, and Tyrosinase Inhibition Studies and Antioxidant Activities of 33 Scutellaria L. Taxa from Turkey. Food Chem. Toxicol. 2010, 48, 781–788. [Google Scholar] [CrossRef]
- Doğan, Z.; Kutluay, V.M.; Saracoglu, I. Bioactivity-Based Phytochemical Studies on Scutellaria salviifolia Benth. Planta Med. 2015, 81, 78. [Google Scholar] [CrossRef]
- Doğan, Z.; Saracoglu, I. Selective Cytotoxic Activity of Scutellaria Species. Proceedings 2017, 1, 1053. [Google Scholar] [CrossRef]
- Zengin, G.; Llorent-Martínez, E.J.; Molina-García, L.; Fernández-de Córdova, M.L.; Aktumsek, A.; Uysal, S.; Rengasamy, K.R.R.; Aumeeruddy, M.Z.; Bahadori, M.B.; Mahomoodally, M.F. Chemical Profile, Antioxidant, and Enzyme Inhibitory Properties of Two Scutellaria Species: S. orientalis L. and S. salviifolia Benth. J. Pharm. Pharmacol. 2019, 71, 270–280. [Google Scholar] [CrossRef]
- Arıtuluk, Z.C.; Koçak, C.Ö.; Renda, G.; Ekizoğlu, M.; Ezer, N. Antimicrobial Activity of Three Scutellaria L. Species from Turkey. Marmara Pharm. J. 2019, 23, 552–558. [Google Scholar] [CrossRef]
- Güven, U.M.; Kayıran, S.D.; Aygül, A.; Nenni, M.; Kırıcı, S. Design of Microemulsion Formulations Loaded with Scutellaria salviifolia Benth, Sideritis libanotica Labill. subsp. linearis (Bentham) Bornm, and Ziziphora clinopodioides Lam. Extracts from Turkey and In Vitro Evaluation of Their Biological Activities. Turk. J. Bot. 2021, 45, 7. [Google Scholar] [CrossRef]
- Doğan, Z.; Kutluay, V.M.; Genc, Y.; Saracoglu, I. Interactions Between Phenolic Constituents of Scutellaria salviifolia and Key Targets Associated with Inflammation: Network Pharmacology, Molecular Docking Analysis and In Vitro Assays. J. Biomol. Struct. Dyn. 2023, 41, 1281–1294. [Google Scholar] [CrossRef]
- Maleki, S.; Akaberi, T.; Emami, S.A.; Akaberi, M. Diterpenes of Scutellaria spp.: Phytochemistry and pharmacology. Phytochemistry 2022, 201, 113285. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lin, J.; Xu, W.; Cai, Q.; Shen, A.; Hong, Z.; Peng, J. Scutellaria barbata D. Don Inhibits Tumor Angiogenesis via Suppression of Hedgehog Pathway in a Mouse Model of Colorectal Cancer. Int. J. Mol. Sci. 2012, 13, 9419–9430. [Google Scholar] [CrossRef]
- Brock, C.; Whitehouse, J.; Tewfik, I.; Towell, T. American Skullcap (Scutellaria lateriflora): A Randomised, Double-Blind Placebo-Controlled Crossover Study of Its Effects on Mood in Healthy Volunteers. Phytother. Res. 2014, 28, 692–698. [Google Scholar] [CrossRef]
- Zhang, Z.; Lian, X.-Y.; Li, S.; Stringer, J.L. Characterization of Chemical Ingredients and Anticonvulsant Activity of American Skullcap (Scutellaria lateriflora). Phytomedicine 2009, 16, 485–493. [Google Scholar] [CrossRef]
- Bazzaz, B.F.; Khayat, M.H.; Emami, S.A.; Asili, J.; Sahebkar, A.; Neishabory, E.J. Antioxidant and Antimicrobial Activity of Methanol, Dichloromethane, and Ethyl Acetate Extracts of Scutellaria litwinowii. ScienceAsia 2011, 37, 327–334. [Google Scholar] [CrossRef]
- Lu, Y.; Joerger, R.; Wu, C. Study of the Chemical Composition and Antimicrobial Activities of Ethanolic Extracts from Roots of Scutellaria baicalensis Georgi. J. Agric. Food Chem. 2011, 59, 10934–10942. [Google Scholar] [CrossRef]
- Deng, Y.X.; Shi, Q.-Z.; Chen, B.; Zhang, X.-J.; Liu, S.-Z.; Qiu, X.M. Comparative Pharmacokinetics of Baicalin in Normal and Type 2 Diabetic Rats after Oral Administration of the Radix Scutellariae Extract. Fitoterapia 2012, 83, 1435–1442. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Xiao, D. A Review of Phytochemistry and Antitumor Activity of a Valuable Medicinal Species: Scutellaria barbata. J. Med. Plants Res. 2012, 6, 4259–4275. [Google Scholar] [CrossRef]
- Ma, W.; Liu, T.; Ogaji, O.D.; Lia, J.; Du, K.; Chang, Y. Recent advances in Scutellariae radix: A comprehensive review on ethnobotanical uses, processing, phytochemistry, pharmacological effects, quality control and influence factors of biosynthesis. Heliyon 2024, 10, e36146. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qiu, Y.; Tian, M.; Wang, L.; Gao, K.; Yang, X.; Jiang, Z. Flavonoids from Scutellaria baicalensis: Promising Alternatives for Enhancing Swine Production and Health. Int. J. Mol. Sci. 2025, 26, 3703. [Google Scholar] [CrossRef] [PubMed]
- Şener, K. Investigation of the effects of Lavandula officinalis Mill., Melissa officinalis L., Mentha piperita L., Salvia officinalis L. and Scutellaria orientalis L. Species Belonging to Lamiaceae Family on Anti-Inflammatory Pathway Enzymes Lipoxygenase (LOX) and Cyclooxygenase (COX). Master’s Thesis, Department of Biology, Institute of Science, Gazi University, Ankara, Türkiye, 2021. [Google Scholar]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic Inflammation and Oxidative Stress as a Major Cause of Age-Related Diseases and Cancer. Recent. Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Hoferová, Z.; Falk, M. Brief Story on Prostaglandins, Inhibitors of Their Synthesis, Hematopoiesis, and Acute Radiation Syndrome. Molecules 2019, 24, 4019. [Google Scholar] [CrossRef]
- Fu, J.-Y.; Masferrer, J.; Seibert, K.; Raz, A.; Needleman, P. The Induction and Suppression of Prostaglandin H2 Synthase (Cyclooxygenase) in Human Monocytes. J. Biol. Chem. 1990, 265, 16737–16740. [Google Scholar] [CrossRef]
- Hawkey, C.J. COX-2 Chronology. Gut 2005, 54, 1509–1514. [Google Scholar] [CrossRef]
- De Vries, E. Imaging of Cyclooxygenase-2 (COX-2) Expression: Potential Use in Diagnosis and Drug Evaluation. Curr. Pharm. Des. 2006, 12, 3847–3856. [Google Scholar] [CrossRef]
- Wisastra, R.; Dekker, F.J. Inflammation, Cancer, and Oxidative Lipoxygenase Activity Are Intimately Linked. Cancers 2014, 6, 1500–1521. [Google Scholar] [CrossRef]
- Kazani, S.; Planaguma, A.; Ono, E.; Bonini, M.; Zahid, M.; Marigowda, G.; Wechsler, M.E.; Levy, B.D.; Israel, E. Exhaled Breath Condensate Eicosanoid Levels Associate with Asthma and Its Severity. J. Allergy Clin. Immunol. 2013, 132, 547–553. [Google Scholar] [CrossRef]
- Dixit, N.; Wu, D.J.; Belgacem, Y.H.; Borodinsky, L.N.; Gershwin, M.E.; Adamopoulos, I.E. Leukotriene B4 Activates Intracellular Calcium and Augments Human Osteoclastogenesis. Arthritis Res. Ther. 2014, 16, 496. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Lin, T.-H.; Wu, M.-Y.; Chiu, Y.-C.; Tang, C.-H.; Hour, M.-J.; Liou, H.-C.; Tu, H.-J.; Yang, R.-S.; Fu, W.-M. 5-Lipoxygenase Inhibitors Attenuate TNF-α-Induced Inflammation in Human Synovial Fibroblasts. PLoS ONE 2014, 9, e107890. [Google Scholar] [CrossRef] [PubMed]
- Bouchareychas, L.; Grössinger, E.M.; Kang, M.; Qiu, H.; Adamopoulos, I.E. Critical Role of LTB4/BLT1 in IL-23-Induced Synovial Inflammation and Osteoclastogenesis via NF-κB. J. Immunol. 2017, 198, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and Human Diseases. Clin. Chim. Acta 2014, 436, 332–347. [Google Scholar] [CrossRef]
- Chatterjee, S. Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–58. [Google Scholar] [CrossRef]
- Kutluay, V.M.; Genc, Y.; Doğan, Z.; Inoue, M.; Saracoglu, I. Nuclear Receptor Agonist Activity Studies on Some Plantago Species and Scutellaria salviifolia Benth.: A Particular Focus on Liver X Receptor Alpha and Retinoid X Receptor Alpha Connected with the Inflammation Process. J. Res. Pharm. 2022, 26, 272–278. [Google Scholar] [CrossRef]
- Doğan, Z.; Ishiuchi, K.; Makino, T.; Saracoglu, I. Alpha-Pyrone Glycosides from Scutellaria salviifolia Benth. Turk. J. Chem. 2019, 43, 972–981. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Tatipamula, V.B.; Kukavica, B. Phenolic Compounds as Antidiabetic, Anti-Inflammatory, and Anticancer Agents and Improvement of Their Bioavailability by Liposomes. Cell Biochem. Funct. 2021, 39, 926–944. [Google Scholar] [CrossRef]
- Liu, W.; Cui, X.; Zhong, Y.; Ma, R.; Liu, B.; Xia, Y. Phenolic Metabolites as Therapeutic in Inflammation and Neoplasms: Molecular Pathways Explaining Their Efficacy. Pharmacol. Res. 2023, 193, 106812. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, Y.; Zhang, C.; Zhao, B.; Wang, Y. The pharmacological efficacy of baicalin in inflammatory diseases. Int. J. Mol. Sci. 2023, 24, 9317. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.H.; Doroudi, A. Review of the Antioxidant Potential of Flavonoids as a Subgroup of Polyphenols and Partial Substitute for Synthetic Antioxidants. Avicenna J. Phytomed. 2023, 13, 354–376. [Google Scholar] [CrossRef]
- Burnett, B.P.; Jia, Q.; Zhao, Y.; Levy, R.M. A Medicinal Extract of Scutellaria baicalensis and Acacia catechu Acts as a Dual Inhibitor of Cyclooxygenase and 5-Lipoxygenase to Reduce Inflammation. J. Med. Food 2007, 10, 442–451. [Google Scholar] [CrossRef]
- Doğan, Z.; Telli, G.; Tel, B.C.; Saracoglu, I. Scutellaria brevibracteata Stapf and Active Principles with Anti-Inflammatory Effects through Regulation of NF-κB/COX-2/iNOS Pathways. Fitoterapia 2022, 158, 105159. [Google Scholar] [CrossRef]
- Clarke, G.; Ting, K.N.; Wiart, C.; Fry, J. High Correlation of DPPH Radical Scavenging, Ferric Reducing Activity Potential and Total Phenolics Content Indicates Redundancy in Use of All Three Assays to Screen for Antioxidant Activity of Extracts of Plants from the Malaysian Rainforest. Antioxidants 2013, 2, 1–10. [Google Scholar] [CrossRef]
- Chun, S.S.; Vattem, D.A.; Lin, Y.T.; Shetty, K. Phenolic Antioxidants from Clonal Oregano (Origanum vulgare) with Antimicrobial Activity against Helicobacter pylori. Process Biochem. 2005, 40, 809–816. [Google Scholar] [CrossRef]
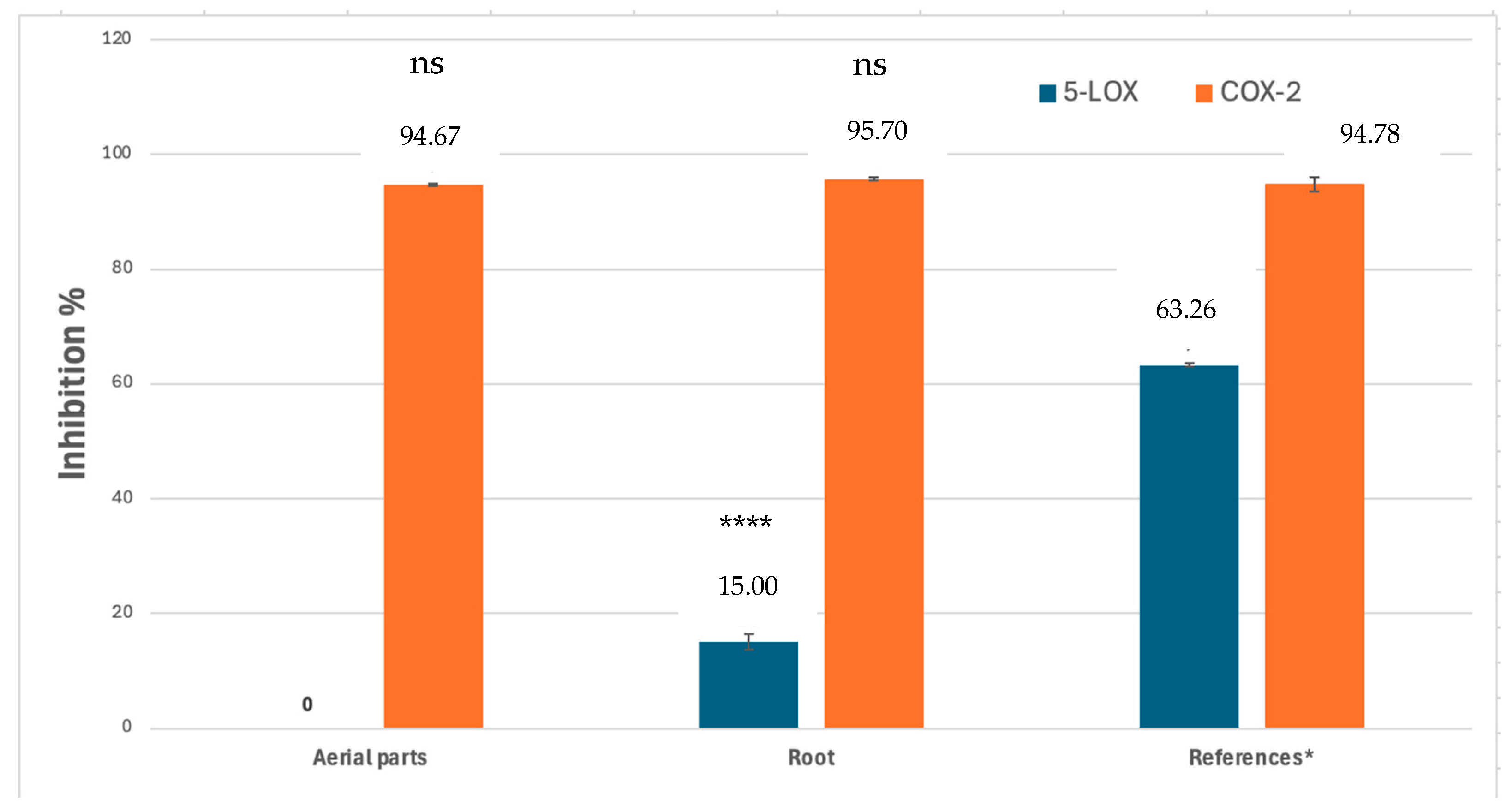

| Extracts and References | ABTS Radical Scavenging Activity% ± S.D. a (IC50: μg/mL) 2 mg/mL b | DPPH Radical Scavenging Activity% ± S.D. a (IC50: μg/mL) 2 mg/mL b |
|---|---|---|
| S. salviifolia aerial part extract | 89.2 ± 0.03 ns (IC50: 28.77 ± 0.20) | 86.8 ± 0.28 * (IC50: 40.7 ± 1.15) |
| S. salviifolia root extract | 88.6 ± 0.88 ns (IC50: 31.75 ± 0.15) | 87.9 ± 0.36 ns (IC50: 40.1 ± 0.81) |
| References | 89.5 ± 0.26 (IC50: 12.33 ± 0.26) c | 87.4 ± 0.32 (IC50: 3.26 ± 0.09) d |
| No. | RT (min) | Precursor Ion (m/z) | Product Ion (m/z) | Ionization Mode | Proposed Compound | Reference/Database | Herba | Root |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.95 | 133.0147 | 115.0036, 89.0238, 71.0135 | [M-H]− | Malic acid | L2/Metlin | ✓ | ✓ |
| 2 | 1.08 | 191.0194 | 111.0088, 87.0086, 57.0342 | [M-H]− | Citric acid | L1/Metlin | ✓ | |
| 3 | 2.69 | 303.0766 | 141.0199, 129.0195, 113.0241 | [M-H]− | Pentahydroxyflavanone | X/Metlin | ✓ | ✓ |
| 4 | 4.16 | 341.0891 | 179.0361, 161.0249, 133.0295 | [M-H]− | Caffeoyl hexoside 1 | L1/Metlin | ✓ | |
| 5 | 4.70 | 341.0877 | 161.0245, 135.0449, 93.0347 | [M-H]− | Caffeoyl hexoside 2 | L1/Metlin | ✓ | |
| 6 | 5.44 | 341.0887 | 179.0357, 161.0247, 135.0448 | [M-H]− | Caffeoyl hexoside 3 | L1/Metlin | ✓ | ✓ |
| 7 | 5.82 | 165.0548 | 147.0442, 119.0491, 91.0543 | [M+H]+ | p-Coumaric | Metlin and PubChem | ✓ | |
| 8 | 6.24 | 179.0349 | 135.0448 | [M-H]− | Caffeic acid | LY/Metlin | ✓ | |
| 9 | 6.37 | 325.0930 | 205.0505, 163.0398, 145.0296 | [M-H]− | Coumaric acid-O-hexoside 1 | L1 | ✓ | |
| 10 | 6.91 | 355.1049 | 217.0509, 193.0506, 175.0401 | [M-H]− | Ferulic acid-O-hexoside | L1 | ✓ | |
| 11 | 7.10 | 475.1816 | 329.1245, 311.1120, 161.0452 | [M-H]− | Phenylethanoid glycoside | L1 | ✓ | |
| 12 | 7.25 | 325.0931 | 205.0503, 163.0398, 145.0296 | [M-H]− | Coumaric acid-O-hexoside 2 | L1 | ✓ | |
| 13 | 8.45 | 355.1037 | 295.0824, 265.0720, 235.0612 | [M-H]− | Ferulic acid-8-C-hexoside | L1 | ✓ | |
| 14 | 8.85 | 163.0402 | 119.0497, 93.0348, 65.0391 | [M-H]− | m-Coumaric | Metlin and PubChem | ✓ | |
| 15 | 10.18 | 563.1419 | 443.0994, 383.0784, 353.0681 | [M-H]− | Apigenin-C-hexoside-C-pentoside | L1 | ✓ | ✓ |
| 16 | 10.45 | 477.0674 | 301.0350 | [M-H]− | Quercetin glucuronide isomer 1 | Metlin | ✓ | ✓ |
| 17 | 11.80 | 477.0677 | 301.0359 | [M-H]− | Quercetin glucuronide isomer 2 | Metlin | ✓ | ✓ |
| 18 | 11.93 | 547.1457 | 457.1136, 367.0826, 337.0720 | [M-H]− | Chrysin-6-C-glucoside-8-C-arabinoside | L1 | ✓ | |
| 19 | 12.46 | 461.0829 | 285.0437 | [M-H]− | Flavonoid-O-glucuronide | L1 | ✓ | ✓ |
| 20 | 12.87 | 547.1458 | 457.1142, 427.1036, 367.0827, 337.0721 | [M-H]− | Chrysin-6-C-arabinoside-8-C-glucoside | A/Metlin | ✓ | |
| 21 | 12.93 | 431.0980 | 269.0467, 225.0563, 161.0249 | [M-H]− | Apigenin-O-hexoside | L1 | ✓ | ✓ |
| 22 | 13.07 | 623.1985 | 461.1662, 269.0459, 179.0368, 161.0250 | [M-H]− | Verbascoside | A/Metlin and PubChem | ✓ | ✓ |
| 23 | 13.88 | 461.0796 | 285.0433, 113.0240 | [M-H]− | Scutellarin | L1/PubChem | ✓ | ✓ |
| 24 | 14.27 | 621.1621 | 445.1241, 283.0660, 268.0416 | [M-H]− | Wogonin-O-glucuronide-O-hexoside | L1 | ✓ | |
| 25 | 14.60 | 445.0781 | 269.0489 | [M-H]− | Apigenin-O-glucuronide | L1 | ✓ | ✓ |
| 26 | 15.48 | 475.0972 | 299.0603, 284.0367 | [M-H]− | Methoxylated flavonoid-O-glucuronide | L1 | ✓ | ✓ |
| 27 | 15.82 | 609.1266 | 323.0771, 285.0411, 161.0247 | [M-H]− | Kaempferol 3-(6″-caffeoylglucoside) | Metlin | ✓ | |
| 28 | 16.28 | 365.0049 | 285.0446, 241.0536, 213.0581 | [M-H]− | Scutellarein derivative | L1 | ✓ | ✓ |
| 29 | 16.55 | 447.0933 | 271.0601, 123.0072 | [M+H]+ | Baicalin | Metlin and PubChem | ✓ | ✓ |
| 30 | 16.55 | 891.1861 | 445.0865, 269.0495, 175.0262 | [M-H]− | Apigenin-O-glucuronide (dimer) 1 | L1 | ✓ | |
| 31 | 17.02 | 447.1026 | 271.0600, 175.0261, 113.0242 | [M-H]− | Naringenin-7-O-β-D-Glucuronide | Metlin | ✓ | |
| 32 | 17.11 | 623.1406 | 303.0724, 285.0405 | [M-H]− | Luteolin 7-(6″-ferulylglucoside) | Metlin | ✓ | |
| 33 | 17.49 | 891.1878 | 445.0867, 269.0493, 175.0284 | [M-H]− | Apigenin-O-glucuronide (dimer) 2 | L1 | ✓ | |
| 34 | 17.82 | 285.0411 | 133.0297, 175.0273 | [M-H]− | Luteolin | L2/Metlin | ✓ | ✓ |
| 35 | 18.11 | 459.1016 | 283.0617 | [M-H]− | Wogonin derivative | L1 | ✓ | ✓ |
| 36 | 18.63 | 459.1031 | 283.0607, 268.0371 | [M-H]− | Wogonin-O-glucuronide | L1 | ✓ | ✓ |
| 37 | 18.96 | 489.1149 | 313.0715, 175.0248 | [M-H]− | Dimethyl flavonoid-O-glucuronide | L1 | ✓ | ✓ |
| 38 | 20.44 | 271.0615 | 253.0510, 169.0135, 123.0079 | [M+H]+ | Norwogonin | A/PubChem | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metkin, G.; Süntar, İ.; Şenol Deniz, F.S.; Tugay, O.; Demiralp, M.; Pittalà, V. Investigating COX-2 and 5-LOX Enzyme-Related Anti-Inflammatory and Antioxidant Activities and Phytochemical Features of Scutellaria salviifolia Benth. Int. J. Mol. Sci. 2025, 26, 5608. https://doi.org/10.3390/ijms26125608
Metkin G, Süntar İ, Şenol Deniz FS, Tugay O, Demiralp M, Pittalà V. Investigating COX-2 and 5-LOX Enzyme-Related Anti-Inflammatory and Antioxidant Activities and Phytochemical Features of Scutellaria salviifolia Benth. International Journal of Molecular Sciences. 2025; 26(12):5608. https://doi.org/10.3390/ijms26125608
Chicago/Turabian StyleMetkin, Gülsüm, İpek Süntar, Fatma Sezer Şenol Deniz, Osman Tugay, Mustafa Demiralp, and Valeria Pittalà. 2025. "Investigating COX-2 and 5-LOX Enzyme-Related Anti-Inflammatory and Antioxidant Activities and Phytochemical Features of Scutellaria salviifolia Benth" International Journal of Molecular Sciences 26, no. 12: 5608. https://doi.org/10.3390/ijms26125608
APA StyleMetkin, G., Süntar, İ., Şenol Deniz, F. S., Tugay, O., Demiralp, M., & Pittalà, V. (2025). Investigating COX-2 and 5-LOX Enzyme-Related Anti-Inflammatory and Antioxidant Activities and Phytochemical Features of Scutellaria salviifolia Benth. International Journal of Molecular Sciences, 26(12), 5608. https://doi.org/10.3390/ijms26125608









