Exploring Hirsutism: Epidemiology, Associated Endocrinal Abnormalities, and Societal Challenges in GCC—A Narrative Review
Abstract
1. Introduction
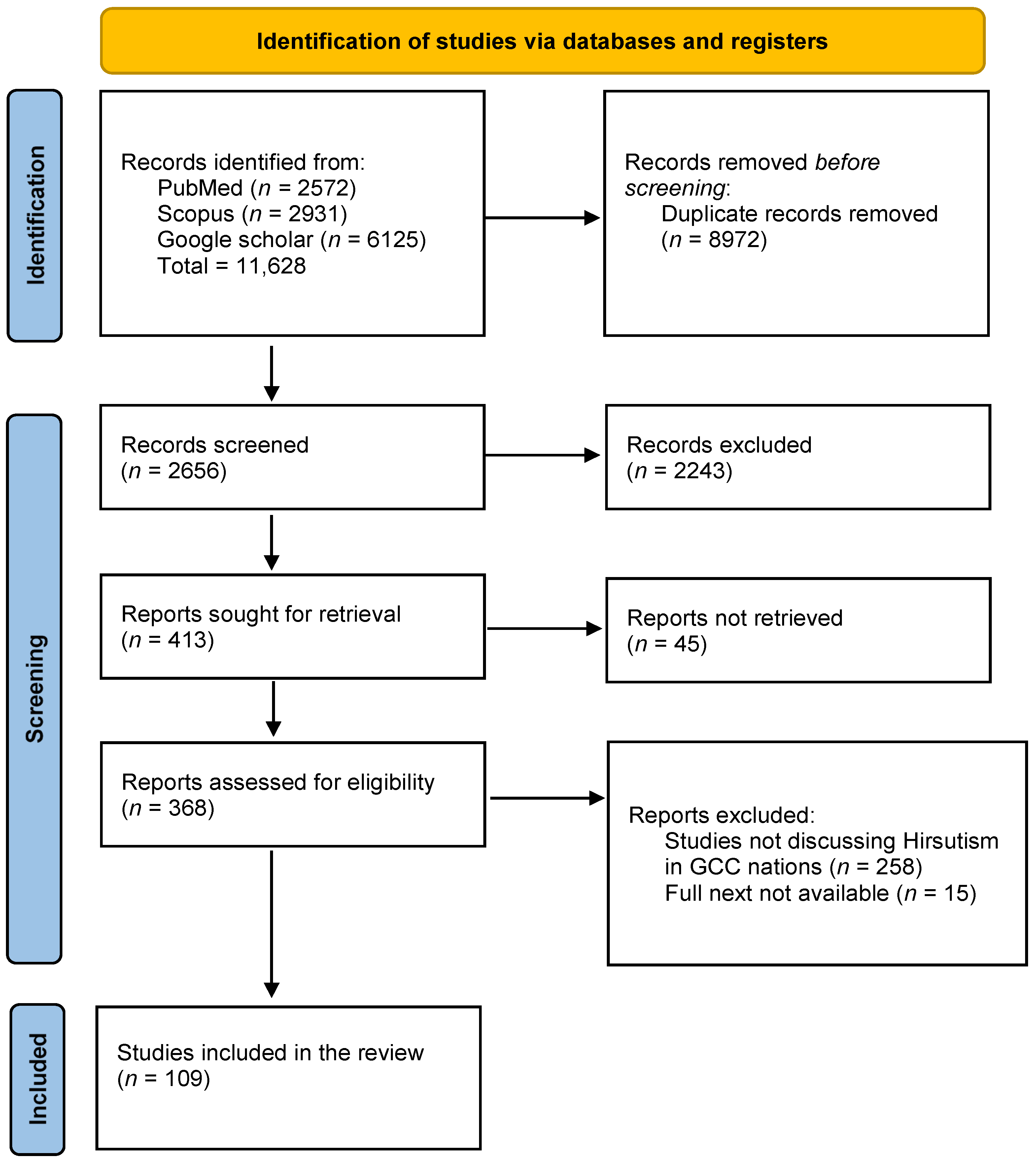
2. Methods
2.1. Search Strategy
2.2. Data Collection
3. Results and Discussion
3.1. Prevalence and Epidemiology
3.2. Cultural and Psychosocial Dimensions
3.3. Pathomechanism of Hirsutism and Molecular Basis
- Adrenal causes
- -
- Congenital adrenal hyperplasia (classic and non-classic)
- -
- Glucocorticoid resistance, cortisone reductase deficiency
- -
- Adrenal adenoma, carcinoma, bilateral macronodular adrenal hyperplasia
- Ovarian causes
- -
- PCOS
- -
- Ovarian tumors: Sertoli–Leydig cell tumors, granulosa-theca cell tumors, hilus cell tumors, hyperthecosis, rarely functioning teratoma, Krukenberg tumors
- Idiopathic Hirsutism and Functional Hyperandrogenism
- Exogenous exposure
- Other endocrine diseases
- -
- Hyperprolactinemia
- -
- Cushing’s disease
- -
- Acromegaly
- -
- Obesity and other insulin resistance syndromes
- Medications (danazol, valproic acid, oxcarbazepine)
- Gestational (luteoma of pregnancy, hyperreactio luteinalis)
3.3.1. Polycystic Ovary Syndrome (PCOS)
3.3.2. ACTH-Dependent Cushing’s Syndrome (Cushing’s Disease)
3.3.3. Androgen-Secreting Tumors
3.3.4. Idiopathic Hyperandrogenism
3.3.5. Obesity-Related Insulin Resistance and Obesity Metabolic Complications
3.3.6. Other Insulin-Resistant Syndromes (IRSs)
3.3.7. Hyperprolactinemia
3.4. Pathomechanism of Hirsutism in GCC
3.5. Screening and Diagnosis
3.6. Management and Treatment Approaches
3.6.1. Pharmacological Treatments
3.6.2. Non-Pharmacological Interventions
3.6.3. Role of Alternative Medicine
3.6.4. Patient Compliance and Challenges
3.7. Public Health and Policy Implications
3.7.1. National Health Strategies
3.7.2. Policy Examples from GCC Countries
3.7.3. Public Health Campaigns
3.7.4. International Collaborations
3.8. Future Directions
3.8.1. Artificial Intelligence and Machine Learning
3.8.2. Patient-Reported Outcome Measures (PROMs)
3.8.3. Regional Research Networks
3.8.4. Educational Materials and Training Programs
Author Contributions
Funding
Conflicts of Interest
Glossary of Genes
| PLGRKT | Plasminogen Receptor with a C-Terminal Lysine |
| ZBTB16 | Zinc Finger and BTB Domain Containing 16 |
| MAPRE1 | Microtubule-Associated Protein RP/EB Family Member 1 |
| THADA | Thyroid Adenoma-Associated Protein |
| ERBB4 | Receptor Protein–Tyrosine Kinase ErbB-4 |
| IRF1/RAD50 | Interferon Regulatory Factor 1/DNA Repair Protein |
| GATA4/NEIL2 | GATA Binding Protein 4/Nei-like DNA Glycosylase 2 |
| FANCC | Fanconi Anemia Complementation Group C |
| TOX3 | TOX High-Mobility Group Box Family Member 3 |
| DENND1A | DENN Domain Containing 1A |
| YAP1 | Yes-Associated Protein 1 |
| ARSD | Arylsulfatase D |
| ARL14EP/FSHB | ARF-like GTPase 14 Effector Protein/Follicle-Stimulating Hormone Subunit Beta |
| SOD2 | Superoxide Dismutase 2 |
| KRR1 | KRR1 Small Subunit Processome Component Homolog |
| ERBB3/RAB5B | Erb-B2 Receptor Tyrosine Kinase 3/Ras-Related Protein Rab-5B |
| C9orf3 | Chromosome 9 Open Reading Frame 3/Aminopeptidase O |
| CYP21A1/CYP21A2 | Cytochrome P450 Family 21 Subfamily A Member 1/2 |
| CYP11 B1 | Cytochrome P450 Family 11 Subfamily B Member 1 |
| HSD3B2 | Hydroxy-delta-5-steroid dehydrogenase, 3 beta-, and Steroid Delta-isomerase 2 |
| USP8 | Ubiquitin-Specific Protease 8 |
| PRKAR1A | Protein Kinase CAMP-Dependent Type I Regulatory Subunit Alpha |
| MEN1 | Multiple Endocrine Neoplasia, Type 1 |
| APC | Adenomatous Polyposis Coli |
| FH | Fumarate Hydratase |
| ARMC5 | Armadillo Repeat Containing 5 |
| GNAS complex | Guanine Nucleotide-Binding Protein, Alpha-Stimulating Activity Polypeptide |
| PRKACA | Protein Kinase CAMP-Activated Catalytic Subunit Alpha |
| PRKACB | Protein Kinase cAMP-Activated Catalytic Subunit Beta |
| PDE11A | Phosphodiesterase 11A |
| PDE8B | Phosphodiesterase 8B |
References
- Lekshmi, S.J.; Pratap, A.; P, A.C.C.; R, L.; A, K.N. A Clinical Approach to Hirsutism. AYUSHDHARA 2022, 9, 99–104. [Google Scholar] [CrossRef]
- Yilmaz, B.; Yildiz, B.O. Endocrinology of Hirsutism: From Androgens to Androgen Excess Disorders. Front. Horm. Res. 2019, 53, 108–119. [Google Scholar] [CrossRef]
- Unluhizarci, K.; Kaltsas, G.; Kelestimur, F. Non Polycystic Ovary Syndrome-Related Endocrine Disorders Associated with Hirsutism. Eur. J. Clin. Investig. 2012, 42, 86–94. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Carmina, E.; Dewailly, D.; Gambineri, A.; Kelestimur, F.; Moghetti, P.; Pugeat, M.; Qiao, J.; Wijeyaratne, C.N.; Witchel, S.F.; et al. Epidemiology, Diagnosis and Management of Hirsutism: A Consensus Statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum. Reprod. Update 2012, 18, 146–170. [Google Scholar] [CrossRef]
- Ahmadi, A.; Akbarzadeh, M.; Mohammadi, F.; Akbari, M.; Jafari, B.; Tolide-Ie, H.R. Anthropometric Characteristics and Dietary Pattern of Women with Polycystic Ovary Syndrome. Indian. J. Endocrinol. Metab. 2013, 17, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Al-Noaimi, H. The State of Sexuality: Formation and Regulation of Sexual Norms in the Persian Gulf. Hawwa 2018, 16, 205–235. [Google Scholar] [CrossRef]
- Aldosari, M.H.; Saleh, A.; Kutubi, A.; Al-Ruhaimi, A.F.; Bashawri, Y.; Althubyani, A.K.; Saleh, R.; Khalil, H. An Evaluation of the Management of Hirsutism in Public versus Private Outpatient Departments in Saudi Arabia. J. Dermatol. Dermatol. Surg. 2023, 27, 1. [Google Scholar] [CrossRef]
- Al-Ruhaily, A.D.; Malabu, U.H.; Sulimani, R.A. Hirsutism in Saudi Females of Reproductive Age: A Hospital-Based Study. Ann. Saudi Med. 2008, 28, 28–32. [Google Scholar] [CrossRef][Green Version]
- Alsous, M.M.; Ali, A.A.; Al-Azzam, S.I.; Abdel Jalil, M.H.; Al-Obaidi, H.J.; Al-abbadi, E.I.; Hussain, Z.K.; Jirjees, F.J. Knowledge and Awareness about Human Papillomavirus Infection and Its Vaccination among Women in Arab Communities. Sci. Rep. 2021, 11, 786. [Google Scholar] [CrossRef]
- Khan, S.; Woolhead, G. Perspectives on Cervical Cancer Screening among Educated Muslim Women in Dubai (the UAE): A Qualitative Study. BMC Women’s Health 2015, 15, 90. [Google Scholar] [CrossRef]
- Al-Kuwari, M.G.; Al Abdulla, S.; Abdulla, M.; Mohammed, A.M.; Haj Bakri, A.; Shaikhan, F.; Buhaddoud, H. Qualitative Focus Group Study Examining Perceptions of the Community’s Important Health Issues, Health Care Needs and Perceived Barriers to Access Among Arabic Speaking Primary Care Clients in the State of Qatar. J. Multidiscip. Healthc. 2021, 14, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Spritzer, P.M.; Marchesan, L.B.; Santos, B.R.; Fighera, T.M. Hirsutism, Normal Androgens and Diagnosis of PCOS. Diagnostics 2022, 12, 1922. [Google Scholar] [CrossRef]
- Alam, Z.; Alseari, S.; Alameemi, M.; Alzaabi, M.; Alkhoori, R.; Östlundh, L.; Melhem, O.; Abdalla, M.A.; Al-Rifai, R.H. Prevalence of Polycystic Ovary Syndrome among Infertile Women in the Gulf Cooperation Council (GCC) Countries: A Systematic Review and Meta-Analysis. Heliyon 2024, 10, e40603. [Google Scholar] [CrossRef]
- Shaman, A.A.; Mukhtar, H.B.; Mirghani, H.O. Risk Factors Associated with Metabolic Syndrome and Cardiovascular Disease among Women with Polycystic Ovary Syndrome in Tabuk, Saudi Arabia. Electron. Physician 2017, 9, 5697–5704. [Google Scholar] [CrossRef] [PubMed]
- Mirza, F.G.; Tahlak, M.A.; Hazari, K.; Khamis, A.H.; Atiomo, W. Prevalence of Polycystic Ovary Syndrome amongst Females Aged between 15 and 45 Years at a Major Women’s Hospital in Dubai, United Arab Emirates. Int. J. Environ. Res. Public Health 2023, 20, 5717. [Google Scholar] [CrossRef]
- Alamri, A.S.; Alhomrani, M.; Alsanie, W.F.; Almuqbil, M.; Alqarni, K.M.; Alshehri, S.M.; Abdulaziz, O.; Salih, M.M.; Raafat, B.M.; Alamri, A.; et al. Role of Polycystic Ovarian Syndrome in Developing Psychological Burden in Saudi Arabian Females: A Case Control Study. Front. Public Health 2022, 10, 999813. [Google Scholar] [CrossRef]
- Albogami, S.S.; Albassam, W.B.; Alghamdi, E.G.; Alabdullatif, A.; Alajlan, Z.A.; AlAwad, S.I.; Hamd, Z.Y. Prevalence of Polycystic Ovary Syndrome by Ultrasound and It’s Relation with Endometrial Hyperplasic and Depression. J. Radiat. Res. Appl. Sci. 2023, 16, 100637. [Google Scholar] [CrossRef]
- Alozaibi, N. Patient and Professional Perceptions of Metabolic Syndrome and Its Management: A Qualitative Study in the United Arab Emirates. Available online: https://eprints.nottingham.ac.uk/12884/ (accessed on 24 January 2025).
- Begum, G.S.; Almashaikhi, N.A.T.; Albalushi, M.Y.; Alsalehi, H.M.; Alazawi, R.S.; Goud, B.K.M.; Dube, R. Prevalence of Polycystic Ovary Syndrome (PCOS) and Its Associated Risk Factors among Medical Students in Two Countries. Int. J. Environ. Res. Public Health 2024, 21, 1165. [Google Scholar] [CrossRef]
- Al Hammadi, H.; Reilly, J. Prevalence of Obesity among School-Age Children and Adolescents in the Gulf Cooperation Council (GCC) States: A Systematic Review. BMC Obes. 2019, 6, 3. [Google Scholar] [CrossRef]
- Musaiger, A.O.; Al-Mannai, M.; Tayyem, R.; Al-Lalla, O.; Ali, E.Y.H.; Kalam, F.; Benhamed, M.M.; Saghir, S.; Halahleh, I.; Djoudi, Z.; et al. Prevalence of Overweight and Obesity among Adolescents in Seven Arab Countries: A Cross-Cultural Study. J. Obes. 2012, 2012, 981390. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Mohammed, H.S.; Elsaid, N.M.A.B.; Salim, A.A.; Fathy, E.G.; Hasaneen, N.M. Risk Factors for Polycystic Ovary Syndrome among Women of Reproductive Age in Egypt: A Case Control Study. Afr. J. Reprod. Health 2023, 27, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.; Al-Jefout, M.; Alsafar, H.; Kirtley, S.; Lindgren, C.M.; Missmer, S.A.; Becker, C.M.; Zondervan, K.T.; Rahmioglu, N. Prevalence of Common Gynecological Conditions in the Middle East: Systematic Review and Meta-Analysis. Front. Reprod. Health 2021, 3, 661360. [Google Scholar] [CrossRef]
- Ben-Omran, T.; Al Ghanim, K.; Yavarna, T.; El Akoum, M.; Samara, M.; Chandra, P.; Al-Dewik, N. Effects of Consanguinity in a Cohort of Subjects with Certain Genetic Disorders in Qatar. Mol. Genet. Genom. Med. 2020, 8, e1051. [Google Scholar] [CrossRef]
- Kronfol, N.M. Access and Barriers to Health Care Delivery in Arab Countries: A Review. Easter Mediterr. Health J. 2012, 18, 1239–1246. [Google Scholar] [CrossRef]
- Almazrou, S.H.; Alfaifi, S.I.; Alfaifi, S.H.; Hakami, L.E.; Al-Aqeel, S.A. Barriers to and Facilitators of Adherence to Clinical Practice Guidelines in the Middle East and North Africa Region: A Systematic Review. Healthcare 2020, 8, 564. [Google Scholar] [CrossRef]
- Alyahya, T.; Zakaria, O.M.; AlAlwan, A.; AlMaghlouth, M.; Alkhars, H.; AlAlwan, M. Local Community View of Aesthetic Surgery: Results of a Cross-Sectional Survey. Cureus 2022, 14, e33078. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Baumann, S.L. Understanding Shame as an Obstacle: Toward a Global Perspective. Nurs. Sci. Q. 2021, 34, 196–201. [Google Scholar] [CrossRef]
- Adis Medical Writers. Identify Underlying Cause of Hirsutism and Individualize Treatment as Required. Drugs Ther. Perspect. 2014, 30, 417–421. [Google Scholar] [CrossRef]
- Hafsi, W.; Kaur, J. Hirsutism. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Shalowitz, M.U.; Isacco, A.; Barquin, N.; Clark-Kauffman, E.; Delger, P.; Nelson, D.; Quinn, A.; Wagenaar, K.A. Community-Based Participatory Research: A Review of the Literature with Strategies for Community Engagement. J. Dev. Behav. Pediatr. 2009, 30, 350–361. [Google Scholar] [CrossRef]
- Qualitative Methods in Community-Based Participatory Research: Coming of Age—Lauren Clark, William Ventres. 2016. Available online: https://journals.sagepub.com/doi/full/10.1177/1049732315617445 (accessed on 27 January 2025).
- Kiconco, S.; Tay, C.T.; Rassie, K.L.; Azziz, R.; Teede, H.J.; Joham, A.E. Where Are We in Understanding the Natural History of Polycystic Ovary Syndrome? A Systematic Review of Longitudinal Cohort Studies. Hum. Reprod. 2022, 37, 1255–1273. [Google Scholar] [CrossRef]
- Nott, M.; Schmidt, D.; Thomas, M.; Reilly, K.; Saksena, T.; Kennedy, J.; Hawke, C.; Christian, B. Collaborations between Health Services and Educational Institutions to Develop Research Capacity in Health Services and Health Service Staff: A Systematic Scoping Review. BMC Health Serv. Res. 2024, 24, 1363. [Google Scholar] [CrossRef]
- Mathis, A. Reducing Disparities by Improving Access to and Use of Preventive Care. Fla. Public Health Rev. 2010, 7, 13. [Google Scholar]
- Malhotra, K.; Pan, C.; Davitadze, M.; Kempegowda, P. Identifying the Challenges and Opportunities of PCOS Awareness Month by Analysing Its Global Digital Impact. Front. Endocrinol. 2023, 14, 1109141. [Google Scholar] [CrossRef]
- Thomas, J.; Al Marzooqi, F.H.; Tahboub-Schulte, S.; Furber, S.W. Changing Physical Appearance Preferences in the United Arab Emirates. Ment. Health Relig. Cult. 2014, 17, 594–600. [Google Scholar] [CrossRef]
- Pate, C. The Story Plot of Living the Embarrassment of Hirsutism. Arch. Psychiatr. Nurs. 2013, 27, 156–157. [Google Scholar] [CrossRef]
- Mironica, A.; Popescu, C.A.; George, D.; Tegzeșiu, A.M.; Gherman, C.D. Social Media Influence on Body Image and Cosmetic Surgery Considerations: A Systematic Review. Cureus 2024, 16, e65626. [Google Scholar] [CrossRef] [PubMed]
- Zaitoun, B.; Al Kubaisi, A.; AlQattan, N.; Alassouli, Y.; Mohammad, A.; Alameeri, H.; Mohammed, G. Polycystic Ovarian Syndrome Awareness among Females in the UAE: A Cross-Sectional Study. BMC Women’s Health 2023, 23, 181. [Google Scholar] [CrossRef]
- Edwin, M.; Vergara, M. A Study on Consumers’ Brand Preferences Relating to Specific Cosmetic Products among Omani Women. Saudi J. Bus. Manag. Stud. 2020, 5, 418–427. [Google Scholar] [CrossRef]
- Calogero, R.M.; Boroughs, M.; Thompson, J.K. The Impact of Western Beauty Ideals on the Lives of Women: A Sociocultural Perspective. In The Body Beautiful: Evolutionary and Sociocultural Perspectives; Swami, V., Furnham, A., Eds.; Palgrave Macmillan: London, UK, 2007; pp. 259–298. ISBN 978-0-230-59688-7. [Google Scholar]
- Rahman, S.A.A.; Othman, N.; Mohamad, M.A. Traditional Medicine Treatment in Addressing the Issue of Women’s Stress and Depression: A Policy Implementation Perspective. J. Sci. Technol. Innov. Policy 2022, 8, 1–5. [Google Scholar] [CrossRef]
- Nortje, G.; Oladeji, B.; Gureje, O.; Seedat, S. Effectiveness of Traditional Healers in Treating Mental Disorders: A Systematic Review. Lancet Psychiatry 2016, 3, 154–170. [Google Scholar] [CrossRef]
- Awad, A.; Al-Shaye, D. Public Awareness, Patterns of Use and Attitudes toward Natural Health Products in Kuwait: A Cross-Sectional Survey. BMC Complement. Altern. Med. 2014, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Jiotsa, B.; Naccache, B.; Duval, M.; Rocher, B.; Grall-Bronnec, M. Social Media Use and Body Image Disorders: Association between Frequency of Comparing One’s Own Physical Appearance to That of People Being Followed on Social Media and Body Dissatisfaction and Drive for Thinness. Int. J. Environ. Res. Public Health 2021, 18, 2880. [Google Scholar] [CrossRef]
- Thomas, S.; Kotian, S. A Systematic Review and Research Agenda on the Influence of the Media and Celebrities on Body Image. Int. J. Manag. Technol. Soc. Sci. 2023, 8, 156–174. [Google Scholar] [CrossRef]
- Alkheyr, Z.; Murad, M.; Das, P.; Aljenaee, K.; Kamel, C.; Hajji, S.A.; Flood, J.; Atkin, S.L.; Ali, K.F. Self-Esteem and Body Image Satisfaction in Women with PCOS in the Middle East: Cross-Sectional Social Media Study. PLoS ONE 2024, 19, e0301707. [Google Scholar] [CrossRef]
- Fernandes, T.; Nettleship, H.; Pinto, L. Judging a Book by Its Cover? The Role of Unconventional Appearance on Social Media Influencers Effectiveness. J. Retail. Consum. Serv. 2022, 66, 102917. [Google Scholar] [CrossRef]
- Health, F.B.W. Ministry of Health Saudi Arabia. Available online: https://www.moh.gov.sa/en/Pages/Default.aspx (accessed on 2 February 2025).
- Dewani, D.; Karwade, P.; Mahajan, K.S. The Invisible Struggle: The Psychosocial Aspects of Polycystic Ovary Syndrome. Cureus 2023, 15, e51321. [Google Scholar] [CrossRef] [PubMed]
- Nyblade, L.; Stockton, M.A.; Giger, K.; Bond, V.; Ekstrand, M.L.; Lean, R.M.; Mitchell, E.M.H.; Nelson, L.R.E.; Sapag, J.C.; Siraprapasiri, T.; et al. Stigma in Health Facilities: Why It Matters and How We Can Change It. BMC Med. 2019, 17, 25. [Google Scholar] [CrossRef]
- Schoenberg, N.E.; Swanson, M. Rural Religious Leaders’ Perspectives on Their Communities’ Health Priorities and Health. South Med. J. 2017, 110, 447–451. [Google Scholar] [CrossRef]
- Ranasinghe, B.; Balasuriya, A.; Wijeyaratne, C.; Fernando, N. The Impact of Peer-Led Support Groups on Health-Related Quality of Life, Coping Skills and Depressive Symptomatology for Women with PCOS. Psychol. Health Med. 2021, 28, 564–573. [Google Scholar] [CrossRef]
- Davies, S. Hirsutism and Quality of Life. In Handbook of Disease Burdens and Quality of Life Measures; Preedy, V.R., Watson, R.R., Eds.; Springer: New York, NY, USA, 2010; pp. 1825–1837. ISBN 978-0-387-78665-0. [Google Scholar]
- Dong, J.; Rees, D.A. Polycystic Ovary Syndrome: Pathophysiology and Therapeutic Opportunities. BMJ Med. 2023, 2, e000548. [Google Scholar] [CrossRef]
- Dube, R.; Bambani, T.; Saif, S.; Hashmi, N.; Patni, M.A.M.F.; Kedia, N.R. The Prevalence of Gestational Diabetes Mellitus in Polycystic Ovary Disease—A Systematic Review, Meta-Analysis, and Exploration of Associated Risk Factors. Diabetology 2024, 5, 430–446. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, J.; Zhao, H.; He, B. Effect of Sex Hormone-Binding Globulin on Polycystic Ovary Syndrome: Mechanisms, Manifestations, Genetics, and Treatment. Int. J. Women’s Health 2022, 14, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Davison, S.L.; Bell, R. Androgen Physiology. Semin. Reprod. Med. 2006, 24, 71–77. [Google Scholar] [CrossRef]
- Burger, H.G. Androgen Production in Women. Fertil. Steril. 2002, 77 (Suppl. S4), S3–S5. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Handelsman, D.J. Role of Androgens in the Ovary. Mol. Cell Endocrinol. 2018, 465, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Bahutair, S.N.M.; Dube, R.; Kuruba, M.G.B.; Salama, R.A.A.; Patni, M.A.M.F.; Kar, S.S.; Kar, R. Molecular Basis of Hydatidiform Moles—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 8739. [Google Scholar] [CrossRef]
- Narasimhan, M.L.; Khattab, A. Genetics of Congenital Adrenal Hyperplasia and Genotype-Phenotype Correlation. Fertil. Steril. 2019, 111, 24–29. [Google Scholar] [CrossRef]
- Day, F.; Karaderi, T.; Jones, M.R.; Meun, C.; He, C.; Drong, A.; Kraft, P.; Lin, N.; Huang, H.; Broer, L.; et al. Large-Scale Genome-Wide Meta-Analysis of Polycystic Ovary Syndrome Suggests Shared Genetic Architecture for Different Diagnosis Criteria. PLoS Genet. 2018, 14, e1007813. [Google Scholar] [CrossRef]
- Dundar, A.; Bayramov, R.; Onal, M.G.; Akkus, M.; Dogan, M.E.; Kenanoglu, S.; Cerrah Gunes, M.; Kazimli, U.; Ozbek, M.N.; Ercan, O.; et al. The Molecular Basis and Genotype-Phenotype Correlations of Congenital Adrenal Hyperplasia (CAH) in Anatolian Population. Mol. Biol. Rep. 2019, 46, 3677–3690. [Google Scholar] [CrossRef]
- Takayasu, S.; Kageyama, K.; Daimon, M. Advances in Molecular Pathophysiology and Targeted Therapy for Cushing’s Disease. Cancers 2023, 15, 496. [Google Scholar] [CrossRef]
- Vaduva, P.; Bonnet, F.; Bertherat, J. Molecular Basis of Primary Aldosteronism and Adrenal Cushing Syndrome. J. Endocr. Soc. 2020, 4, bvaa075. [Google Scholar] [CrossRef] [PubMed]
- Kamilaris, C.D.C.; Stratakis, C.A.; Hannah-Shmouni, F. Molecular Genetic and Genomic Alterations in Cushing’s Syndrome and Primary Aldosteronism. Front. Endocrinol. 2021, 12, 632543. [Google Scholar] [CrossRef] [PubMed]
- Unluhizarci, K.; Hacioglu, A.; Taheri, S.; Karaca, Z.; Kelestimur, F. Idiopathic Hirsutism: Is It Really Idiopathic or Is It Misnomer? World J. Clin. Cases 2023, 11, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Torchen, L.C.; Wu, M.; Thompson, B.; Beaudouin, A. Mechanisms of Adrenal Hyperandrogenism in Polycystic Ovary Syndrome. Reproduction 2025, 169, e250091. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Alshaikh, M.K.; Filippidis, F.T.; Al-Omar, H.A.; Rawaf, S.; Majeed, A.; Salmasi, A.-M. The Ticking Time Bomb in Lifestyle-Related Diseases among Women in the Gulf Cooperation Council Countries; Review of Systematic Reviews. BMC Public Health 2017, 17, 536. [Google Scholar] [CrossRef]
- Al Thani, M.; Al Thani, A.A.; Al-Chetachi, W.; Al Malki, B.; Khalifa, S.A.H.; Bakri, A.H.; Hwalla, N.; Naja, F.; Nasreddine, L. Adherence to the Qatar Dietary Guidelines: A Cross-Sectional Study of the Gaps, Determinants and Association with Cardiometabolic Risk amongst Adults. BMC Public Health 2018, 18, 503. [Google Scholar] [CrossRef]
- Guy, G.W.; Nunn, A.V.W.; Thomas, L.E.; Bell, J.D. Obesity, Diabetes and Longevity in the Gulf: Is There a Gulf Metabolic Syndrome? Int. J. Diabetes Mellit. 2009, 1, 43–54. [Google Scholar] [CrossRef][Green Version]
- Salama, R.A.A.; Patni, M.A.M.F.; Ba-Hutair, S.N.M.; Wadid, N.A.; Akikwala, M.S. Exploring Novel Treatment Modalities for Type 1 Diabetes Mellitus: Potential and Prospects. Healthcare 2024, 12, 1485. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Olza, J.; Gil, Á.; Aguilera, C.M. Chapter 1—Oxidative Stress and Inflammation in Obesity and Metabolic Syndrome. In Obesity; del Moral, A.M., Aguilera García, C.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–15. ISBN 978-0-12-812504-5. [Google Scholar]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose Tissue Inflammation and Metabolic Dysfunction in Obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Sulaiman, M.A.; Al-Farsi, Y.M.; Al-Khaduri, M.M.; Saleh, J.; Waly, M.I. Polycystic Ovarian Syndrome Is Linked to Increased Oxidative Stress in Omani Women. Int. J. Womens Health 2018, 10, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Angelidi, A.M.; Filippaios, A.; Mantzoros, C.S. Severe insulin resistance syndromes. J. Clin. Investig. 2021, 131, e142245. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Chanoine, J.-P. Consanguineous Marriages and Endocrine Diseases in Arab Societies. Pediatr. Endocrinol. Rev. 2017, 15, 159–164. [Google Scholar] [CrossRef] [PubMed]
- López-Alarcón, M.; Perichart-Perera, O.; Flores-Huerta, S.; Inda-Icaza, P.; Rodríguez-Cruz, M.; Armenta-Álvarez, A.; Bram-Falcón, M.T.; Mayorga-Ochoa, M. Excessive Refined Carbohydrates and Scarce Micronutrients Intakes Increase Inflammatory Mediators and Insulin Resistance in Prepubertal and Pubertal Obese Children Independently of Obesity. Mediat. Inflamm. 2014, 2014, 849031. [Google Scholar] [CrossRef]
- Manta, A.; Paschou, S.A.; Isari, G.; Mavroeidi, I.; Kalantaridou, S.; Peppa, M. Glycemic Index and Glycemic Load Estimates in the Dietary Approach of Polycystic Ovary Syndrome. Nutrients 2023, 15, 3483. [Google Scholar] [CrossRef]
- Shele, G.; Genkil, J.; Speelman, D. A Systematic Review of the Effects of Exercise on Hormones in Women with Polycystic Ovary Syndrome. J. Funct. Morphol. Kinesiol. 2020, 5, 35. [Google Scholar] [CrossRef]
- Balkrishna, A.; Rana, M.; Mishra, S.; Srivastava, D.; Bhardwaj, R.; Singh, S.; Rajput, S.K.; Arya, V. Incredible Combination of Lifestyle Modification and Herbal Remedies for Polycystic Ovarian Syndrome Management. Evid.-Based Complement. Altern. Med. 2023, 2023, 3705508. [Google Scholar] [CrossRef]
- Mohammadi, F.; Kohan, S.; Mostafavi, F.; Gholami, A. The Stigma of Reproductive Health Services Utilization by Unmarried Women. Iran. Red. Crescent Med. J. 2016, 18, e24231. [Google Scholar] [CrossRef]
- Lumezi, B.G.; Berisha, V.L.; Pupovci, H.L.; Goçi, A.; Hajrushi, A.B. Grading of Hirsutism Based on the Ferriman-Gallwey Scoring System in Kosovar Women. Postepy Dermatol. Alergol. 2018, 35, 631–635. [Google Scholar] [CrossRef]
- Aljenaee, K.; Ghanbar, M.; Ali, S. MON-221 Recalibrating the Modified Ferriman-Gallwey Score in Middle Eastern Women: Population Study. J. Endocr. Soc. 2019, 3, MON-221. [Google Scholar] [CrossRef]
- Sharma, A.; Welt, C.K. Practical Approach to Hyperandrogenism in Women. Med. Clin. N. Am. 2021, 105, 1099–1116. [Google Scholar] [CrossRef] [PubMed]
- Matheson, E.; Bain, J. Hirsutism in Women. AFP 2019, 100, 168–175. [Google Scholar]
- Bryan, C.J.; Allen, M.H.; Thomsen, C.J.; May, A.M.; Baker, J.C.; Harris, J.A.; Bryan, A.O.; Russell, W.A. The PRImary Care Screening Methods (PRISM) Study: Rationale and Design Considerations. Contemp. Clin. Trials 2019, 84, 105823. [Google Scholar] [CrossRef] [PubMed]
- Bohsas, H.; Alibrahim, H.; Swed, S.; Abouainain, Y.; Aljabali, A.; Kazan, L.; Jabban, Y.K.E.; Mehmood, Q.; Sawaf, B.; Eissa, N.; et al. Prevalence and Knowledge of Polycystic Ovary Syndrome (PCOS) and Health-Related Practices among Women of Syria: A Cross-Sectional Study. J. Psychosom. Obstet. Gynecol. 2024, 45. in press. [Google Scholar] [CrossRef] [PubMed]
- Almarri, M.A.; Haber, M.; Lootah, R.A.; Hallast, P.; Al Turki, S.; Martin, H.C.; Xue, Y.; Tyler-Smith, C. The Genomic History of the Middle East. Cell 2021, 184, 4612–4625.e14. [Google Scholar] [CrossRef]
- Dhoot, R.; Humphrey, J.M.; O’Meara, P.; Gardner, A.; McDonald, C.J.; Ogot, K.; Antani, S.; Abuya, J.; Kohli, M. Implementing a Mobile Diagnostic Unit to Increase Access to Imaging and Laboratory Services in Western Kenya. BMJ Glob. Health 2018, 3, e000947. [Google Scholar] [CrossRef]
- Purabi, N.S. Digital Revolution in Healthcare: Potential Tool for Achieving Health Equity in Bangladesh. Int. J. Hum. Health Sci. 2019, 3, 201–206. [Google Scholar] [CrossRef]
- Alotaibi, M.; Shaman, A.A. Enhancing Polycystic Ovarian Syndrome Awareness Using Private Social Network. Mhealth 2020, 6, 33. [Google Scholar] [CrossRef]
- Hohl, A.; Ronsoni, M.F.; Oliveira, M. de Hirsutism: Diagnosis and Treatment. Arq. Bras. Endocrinol. Metabol. 2014, 58, 97–107. [Google Scholar] [CrossRef]
- Kerscher, M.; Reuther, T.; Krueger, N.; Buntrock, H. Effects of an Oral Contraceptive Containing Chlormadinone Acetate and Ethinylestradiol on Hair and Skin Quality in Women Wishing to Use Hormonal Contraception. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 601–608. [Google Scholar] [CrossRef]
- Markovski, M.; Hall, J.; Jin, M.; Laubscher, T.; Regier, L. Approach to the Management of Idiopathic Hirsutism. Can. Fam. Physician 2012, 58, 173–177. [Google Scholar] [PubMed]
- Brown, J.C.; Ma, C.; Shi, Q.; Niedzwiecki, D.; Zemla, T.; Couture, F.; Kuebler, P.; Kumar, P.; Hopkins, J.O.; Tan, B.; et al. Association between Physical Activity and the Time Course of Cancer Recurrence in Stage III Colon Cancer. Br. J. Sports Med. 2023, 57, 965–971. [Google Scholar] [CrossRef]
- Soldat-Stanković, V.; Popović-Pejičić, S.; Stanković, S.; Prtina, A.; Malešević, G.; Bjekić-Macut, J.; Livadas, S.; Ognjanović, S.; Mastorakos, G.; Micić, D.; et al. The Effect of Metformin and Myoinositol on Metabolic Outcomes in Women with Polycystic Ovary Syndrome: Role of Body Mass and Adiponectin in a Randomized Controlled Trial. J. Endocrinol. Investig. 2022, 45, 583–595. [Google Scholar] [CrossRef]
- Amiri, L.; Galadari, H.; Al Mugaddam, F.; Souid, A.K.; Stip, E.; Javaid, S.F. Perception of Cosmetic Procedures among Middle Eastern Youth. J. Clin. Aesthet. Dermatol. 2021, 14, E74–E83. [Google Scholar]
- Alizadeh, K.; Elzanie, A. Plastic Surgery in the Elderly. In Surgical Decision Making in Geriatrics; Latifi, R., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 317–328. ISBN 978-3-030-47962-6. [Google Scholar]
- Richards, R.N.; McKenzie, M.A.; Meharg, G.E. Electroepilation (Electrolysis) in Hirsutism: 35,000 Hours’ Experience on the Face and Neck. J. Am. Acad. Dermatol. 1986, 15, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Alharethy, S.E. Trends and Demographic Characteristics of Saudi Cosmetic Surgery Patients. Saudi Med. J. 2017, 38, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A. How Can Traditional and Complementary Medicines Be Integrated into Health Care in Saudi Arabia? The Perspectives of Health Professionals and Policymakers. Ph.D. Thesis, Liverpool John Moores University, Liverpool, UK, 2020. [Google Scholar]
- Grant, P. Spearmint Herbal Tea Has Significant Anti-Androgen Effects in Polycystic Ovarian Syndrome. A Randomized Control. Trial. Phytother. Res. 2010, 24, 186–188. [Google Scholar] [CrossRef]
- Akdoğan, M.; Tamer, M.N.; Cüre, E.; Cüre, M.C.; Köroğlu, B.K.; Delibaş, N. Effect of Spearmint (Mentha Spicata Labiatae) Teas on Androgen Levels in Women with Hirsutism. Phytother. Res. 2007, 21, 444–447. [Google Scholar] [CrossRef]
- Kebede, E.B.; Tan, J.; Iftikhar, S.; Abu Lebdeh, H.S.; Duggirala, M.K.; Ghosh, A.K.; Croghan, I.T.; Jenkins, S.M.; Mahapatra, S.; Bauer, B.A.; et al. Complementary and Alternative Medicine Use by Patients From the Gulf Region Seen in the International Practice of a Tertiary Care Medical Center. Glob. Adv. Health Med. 2021, 10, 21649561211010129. [Google Scholar] [CrossRef]
- Dar-Odeh, N.; Abu-Hammad, O. Herbal Remedies Use in Arab Societies. In Handbook of Healthcare in the Arab World; Laher, I., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–21. ISBN 978-3-319-74365-3. [Google Scholar]
- Sahin, Y.; Kelestimur, F. Medical Treatment Regimens of Hirsutism. Reprod. Biomed. Online 2004, 8, 538–546. [Google Scholar] [CrossRef]
- Khoja, T.; Rawaf, S.; Qidwai, W.; Rawaf, D.; Nanji, K.; Hamad, A. Health Care in Gulf Cooperation Council Countries: A Review of Challenges and Opportunities. Cureus 2017, 9, e1586. [Google Scholar] [CrossRef] [PubMed]
- Ezeh, U.; Huang, A.; Landay, M.; Azziz, R. Long-Term Response of Hirsutism and Other Hyperandrogenic Symptoms to Combination Therapy in Polycystic Ovary Syndrome. J. Womens Health 2018, 27, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Somani, N.; Turvy, D. Hirsutism: An Evidence-Based Treatment Update. Am. J. Clin. Dermatol. 2014, 15, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Elyamani, R.; Naja, S.; Al-Dahshan, A.; Hamoud, H.; Bougmiza, M.I.; Alkubaisi, N. Mental Health Literacy in Arab States of the Gulf Cooperation Council: A Systematic Review. PLoS ONE 2021, 16, e0245156. [Google Scholar] [CrossRef]
- Fadhil, I.; Ali, R.; Al-Raisi, S.S.; Bin Belaila, B.A.; Galadari, S.; Javed, A.; Sulaiman, K.; Saeed, K.; Arifeen, S. Review of National Healthcare Systems in the Gulf Cooperation Council Countries for Noncommunicable Diseases Management. Oman Med. J. 2022, 37, e370. [Google Scholar] [CrossRef]
- Marshall, J.C.; Dunaif, A. All Women With PCOS Should Be Treated For Insulin Resistance. Fertil. Steril. 2012, 97, 18–22. [Google Scholar] [CrossRef]
- Agrawal, N.K. Management of Hirsutism. Indian. J. Endocrinol. Metab. 2013, 17, S77–S82. [Google Scholar] [CrossRef]
- Health Sector Transformation Program. Available online: https://www.vision2030.gov.sa/en/explore/programs/health-sector-transformation-program?utm_source=chatgpt.com (accessed on 2 February 2025).
- National Strategy for Wellbeing 2031|The Official Portal of the UAE Government. Available online: https://u.ae/en/about-the-uae/strategies-initiatives-and-awards/strategies-plans-and-visions/social-affairs/national-strategy-for-wellbeing-2031?utm_source=chatgpt.com (accessed on 2 February 2025).
- BC23. Available online: https://www.adphc.gov.ae/Media-Center/News/BC23 (accessed on 2 February 2025).
- Al-Shehri, O. The Impact of Community-Based Awareness Campaigns on the Early Breast Examination among Women at King Faisal University. Open J. Prev. Med. 2015, 5, 400–408. [Google Scholar] [CrossRef][Green Version]
- emhj Regional Expert Meeting on Policy Action for Healthy Diets, with a Focus on the Gulf Cooperation Council Countries 1. Available online: http://www.emro.who.int/emhj-volume-29-2023/volume-29-issue-12/regional-expert-meeting-on-policy-action-for-healthy-diets-with-a-focus-on-the-gulf-cooperation-council-countries1.html (accessed on 2 February 2025).
- Verma, P.; Maan, P.; Gautam, R.; Arora, T. Unveiling the Role of Artificial Intelligence (AI) in Polycystic Ovary Syndrome (PCOS) Diagnosis: A Comprehensive Review. Reprod. Sci. 2024, 31, 2901–2915. [Google Scholar] [CrossRef]
- Makhni, E.C.; Hennekes, M.E. The Use of Patient-Reported Outcome Measures in Clinical Practice and Clinical Decision Making. J. Am. Acad. Orthop. Surg. 2023, 31, 1059–1066. [Google Scholar] [CrossRef]
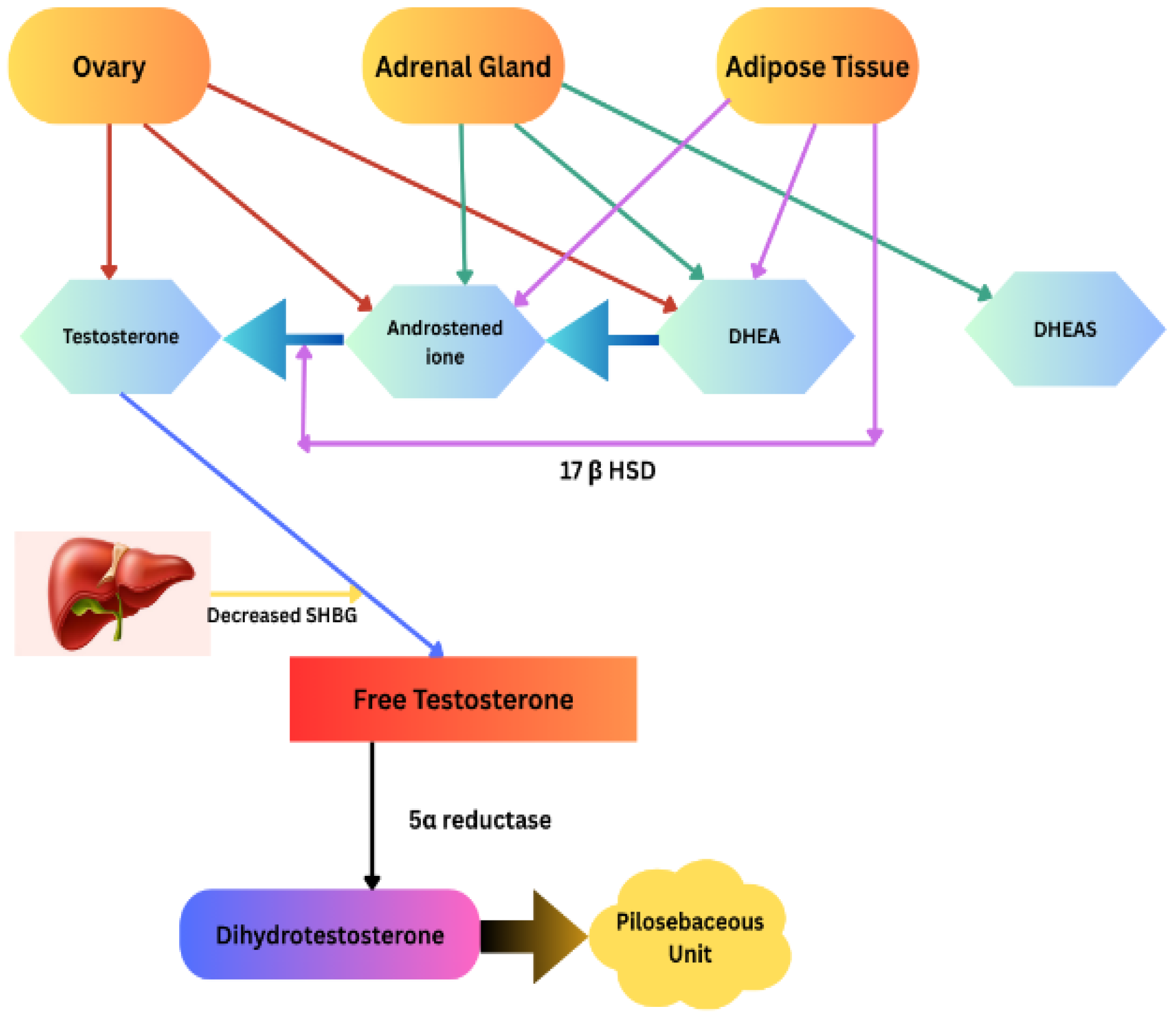
| Disorder | Location in Chromosomes | Identified Genes [Reference] | Possible Mechanism |
|---|---|---|---|
| PCOS | 2, 5, 8, 9, 11, 12, 16, 20, and 23 | Loci near PLGRKT, ZBTB16, MAPRE1, THADA, ERBB4, IRF1/RAD50, GATA4/NEIL2, FANCC, TOX3, DENND1A, YAP1, ARSD, ARL14EP/FSHB, SOD2, KRR1, ERBB3/RAB5B, and C9orf3 [64]. | Identified variants were associated with hyperandrogenism, gonadotropin regulation, and testosterone levels. THADA, FSHβ, and IRF1/RAD50 loci are associated with testosterone levels or regulation. DENND1A is associated with hyperandrogenism. SOD2, ERBB3/RAB5, TOX3, and C9orf3 are associated with hyperandrogenism. |
| CAH | 1, 6, and 8 | CYP21A2, CYP11B1, and HSD3B2—associated with 21-hydroxylase, 11-beta-hydroxylase, and 3-beta-hydroxysteroid dehydrogenase enzyme deficiencies. CYP21A2 and CYP21A1 (pseudogene) mutations [63,65]. | Due to a large gene deletion and conversion. A total of 32 variants of CYP21A2, 9 variants of CYP11B1, and 6 variants of HSD3B2. The mutations comprise promoter region mutations, intronic mutations, frameshift mutations, and single base pair missense mutations. |
| ACTH-dependent Cushing’s syndrome (Cushing’s Disease) | 15, pseudo- genes in 2 and 8 | Somatic mutations in the ubiquitin-specific protease 8 (USP8) gene increase the activity of the enzyme [66]. | Dysregulation of ACTH synthesis and secretion caused by corticotroph tumors. Excessive deubiquitination of epidermal growth factor receptor (EGFR) tyrosine kinase disturbs its degradation [1]. EGFR expression, the overexpression of cyclin E (cell-cycle regulator), and low expression levels of the tumor protein p27 (cell-cycle inhibitor) are seen. |
| Cushing syndrome (endo genous ACTH-independent or exogenous) | 1, 2, 5, 11, 16, 17, and 20 | Bilateral hyperplasia due to PRKAR1A germline-inactivating mutations and macronodular hyperplasia by germline-inactivating mutations of MEN1, APC, FH, and ARMC5 [67]. Others include GNAS, PRKACB, PDE11A, and PDE8B [68]. | These mutations affect the cAMP/PKA/MAPK and Wnt signaling systems for the presentations. Increased production of ACTH leads to hyperandrogenism due to excessive production by the adrenal gland. |
| Adrenal adenoma | 19 | PRKACA somatic-activating mutations [67]. | |
| Idiopathic hirsutism | Local androgen synthesis by the pilosebaceous unit [69]. | Higher expression of steroid sulfatase and 17-beta hydroxysteroid dehydrogenase mRNA in skin. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patni, M.A.; Dube, R.; Kar, S.S.; George, B.T.; Kuruba, M.G.B.; Srinivasamurthy, S.K.; Elamin, A.A.E. Exploring Hirsutism: Epidemiology, Associated Endocrinal Abnormalities, and Societal Challenges in GCC—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 5575. https://doi.org/10.3390/ijms26125575
Patni MA, Dube R, Kar SS, George BT, Kuruba MGB, Srinivasamurthy SK, Elamin AAE. Exploring Hirsutism: Epidemiology, Associated Endocrinal Abnormalities, and Societal Challenges in GCC—A Narrative Review. International Journal of Molecular Sciences. 2025; 26(12):5575. https://doi.org/10.3390/ijms26125575
Chicago/Turabian StylePatni, Mohamed Anas, Rajani Dube, Subhranshu Sekhar Kar, Biji Thomas George, Manjunatha Goud Bellary Kuruba, Suresh Kumar Srinivasamurthy, and Abdalla Ahmed Eldaw Elamin. 2025. "Exploring Hirsutism: Epidemiology, Associated Endocrinal Abnormalities, and Societal Challenges in GCC—A Narrative Review" International Journal of Molecular Sciences 26, no. 12: 5575. https://doi.org/10.3390/ijms26125575
APA StylePatni, M. A., Dube, R., Kar, S. S., George, B. T., Kuruba, M. G. B., Srinivasamurthy, S. K., & Elamin, A. A. E. (2025). Exploring Hirsutism: Epidemiology, Associated Endocrinal Abnormalities, and Societal Challenges in GCC—A Narrative Review. International Journal of Molecular Sciences, 26(12), 5575. https://doi.org/10.3390/ijms26125575







