Quantitative Fluorescence Imaging of Chemophototherapy Drug Pharmacokinetics Using Laparoscopic SFDI
Abstract
1. Introduction
2. Results
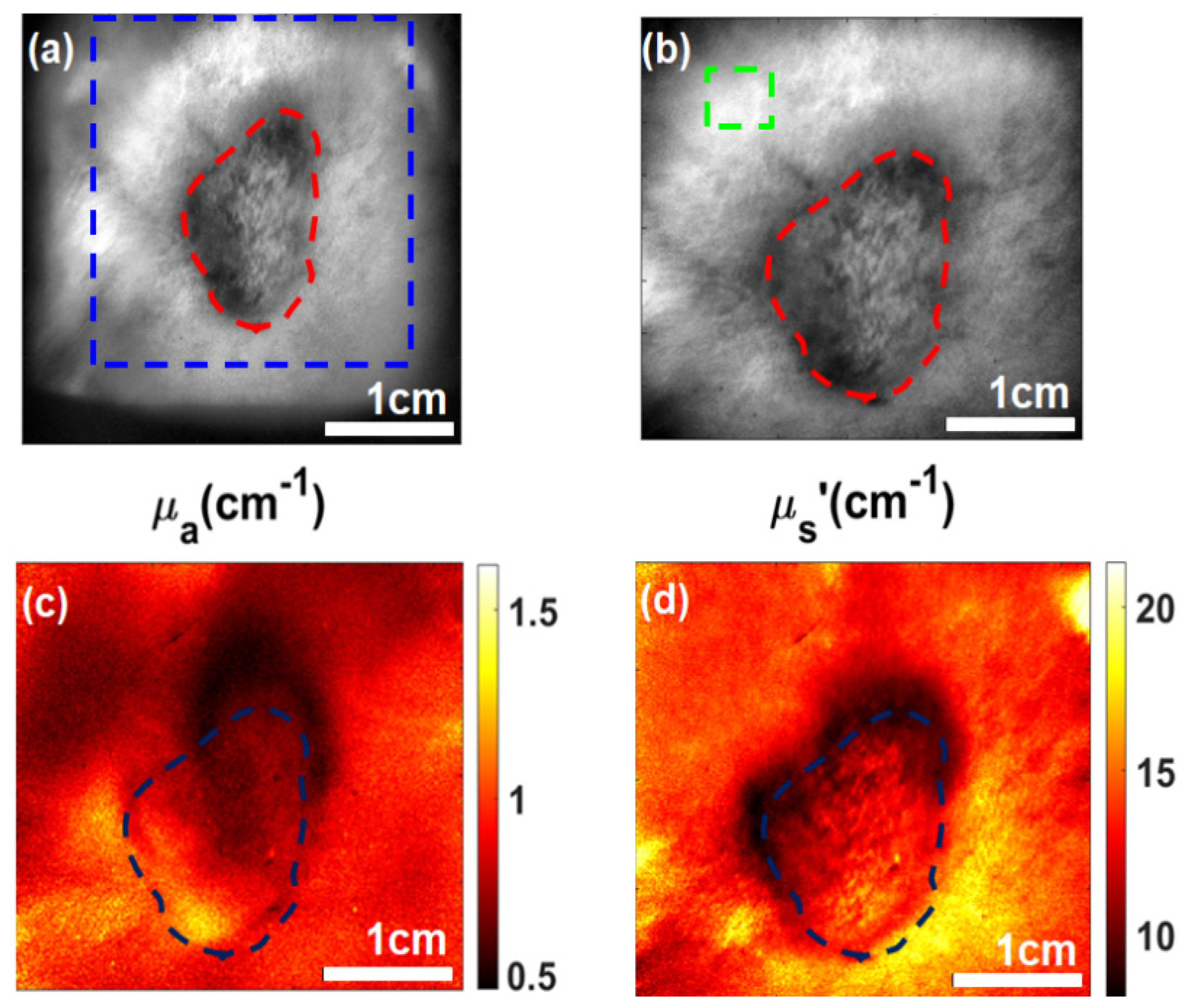
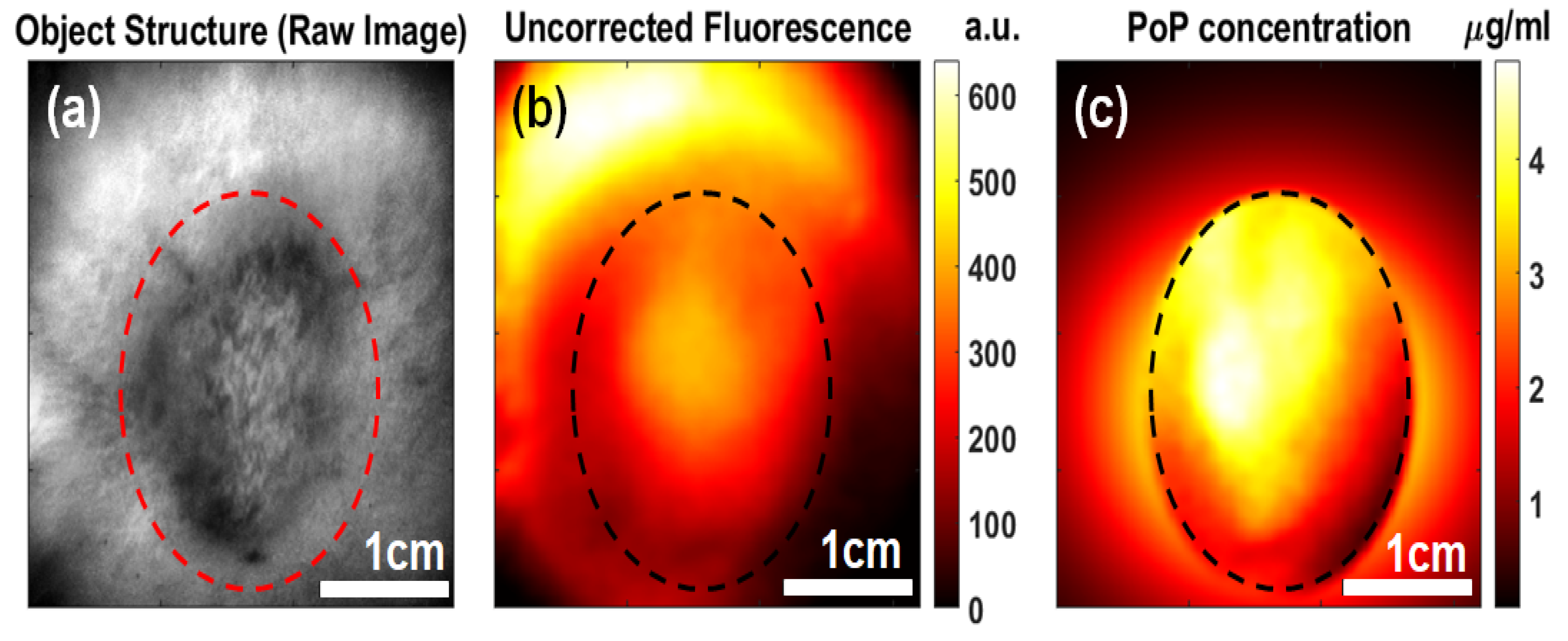
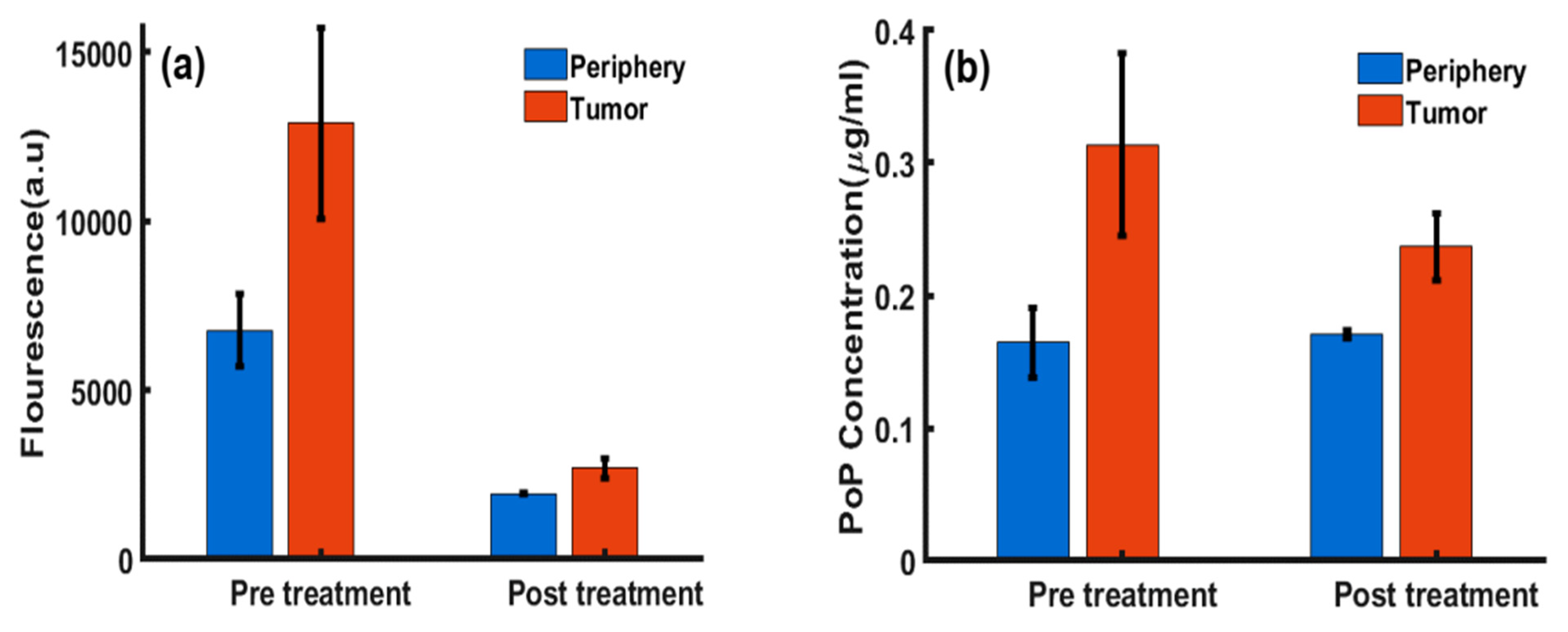
2.1. PoP Fluorescence Concentration Contrast and Photobleaching in Subcutaneous Tumors
2.2. Dox Release in Mouse Carcass
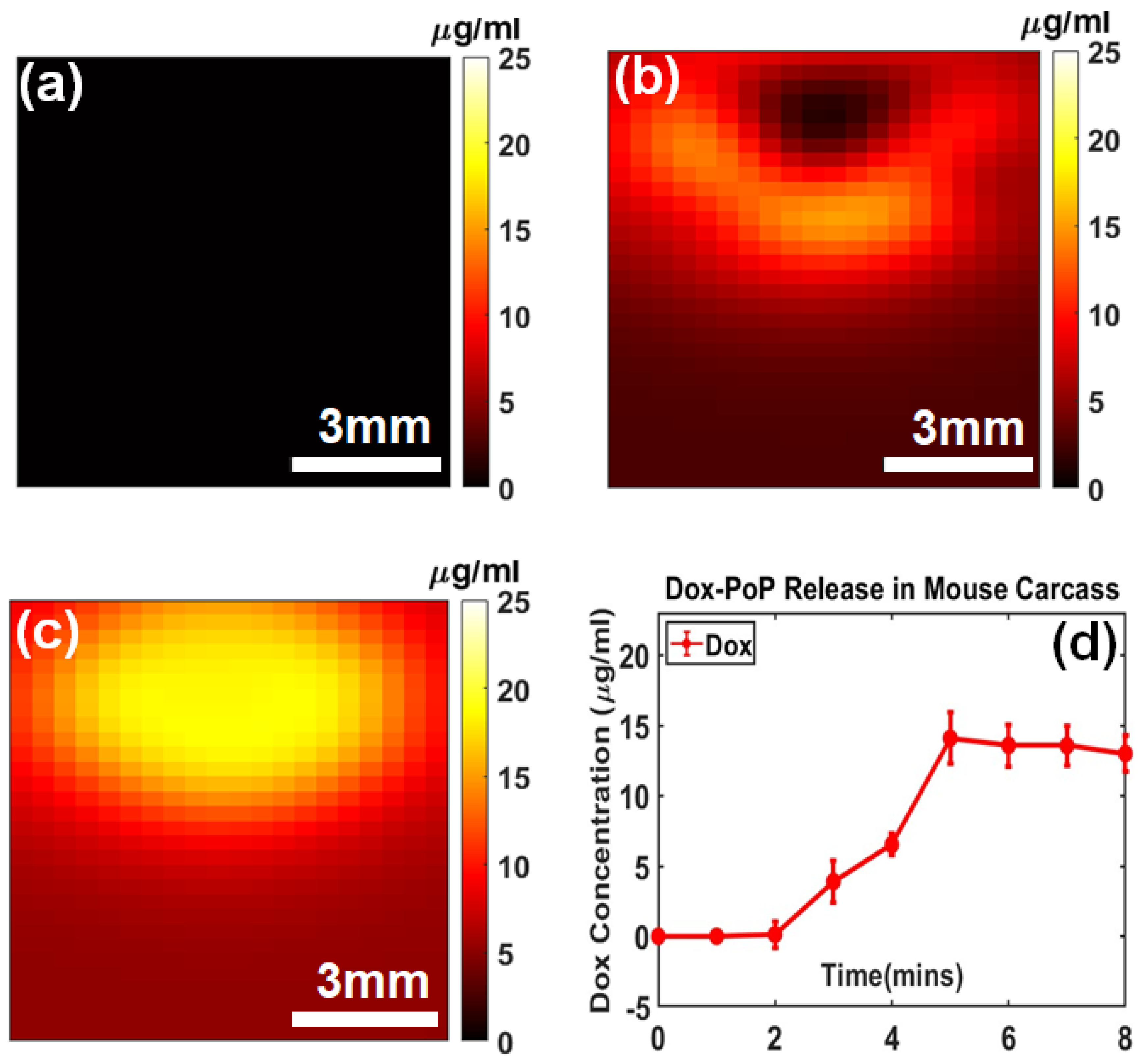
2.2.1. Dox Imaging Contrast
2.2.2. Dox Release Kinetics
2.2.3. Porphyrin Photobleaching in Dox-PoP Liposomes
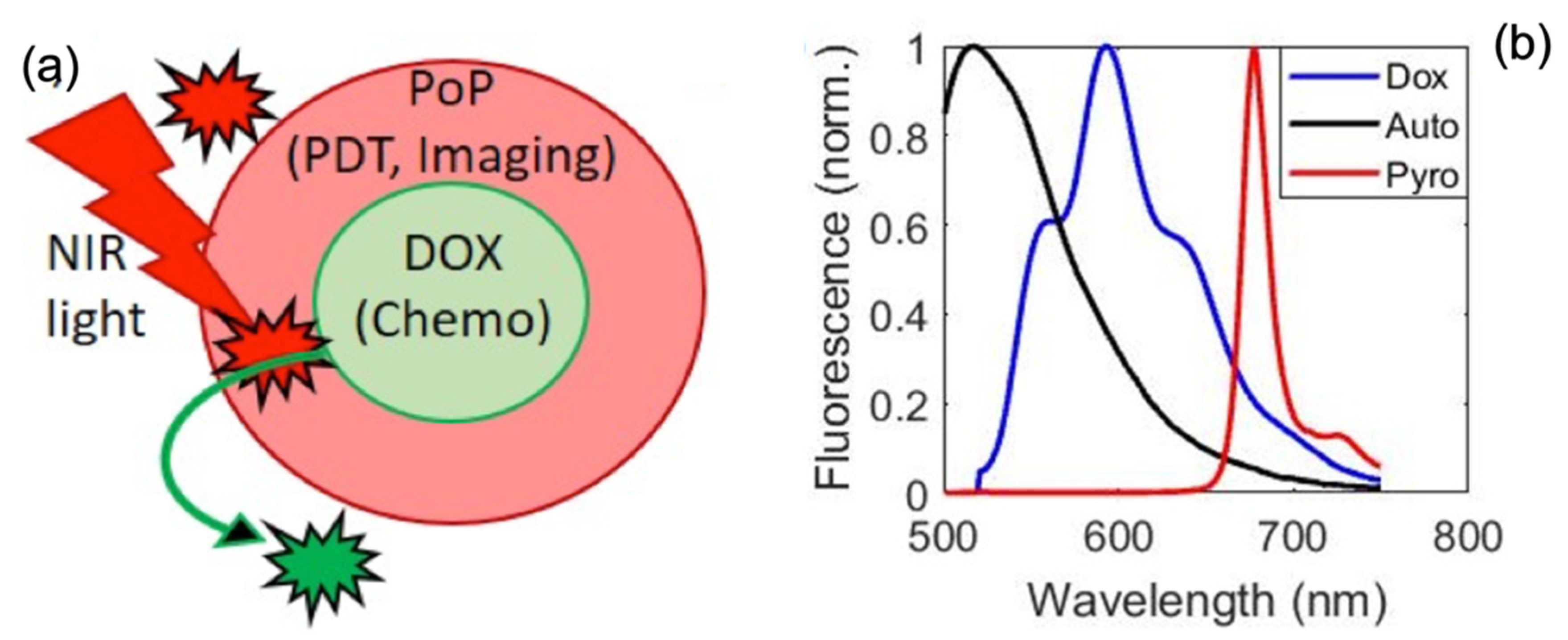
3. Discussion
4. Materials and Methods
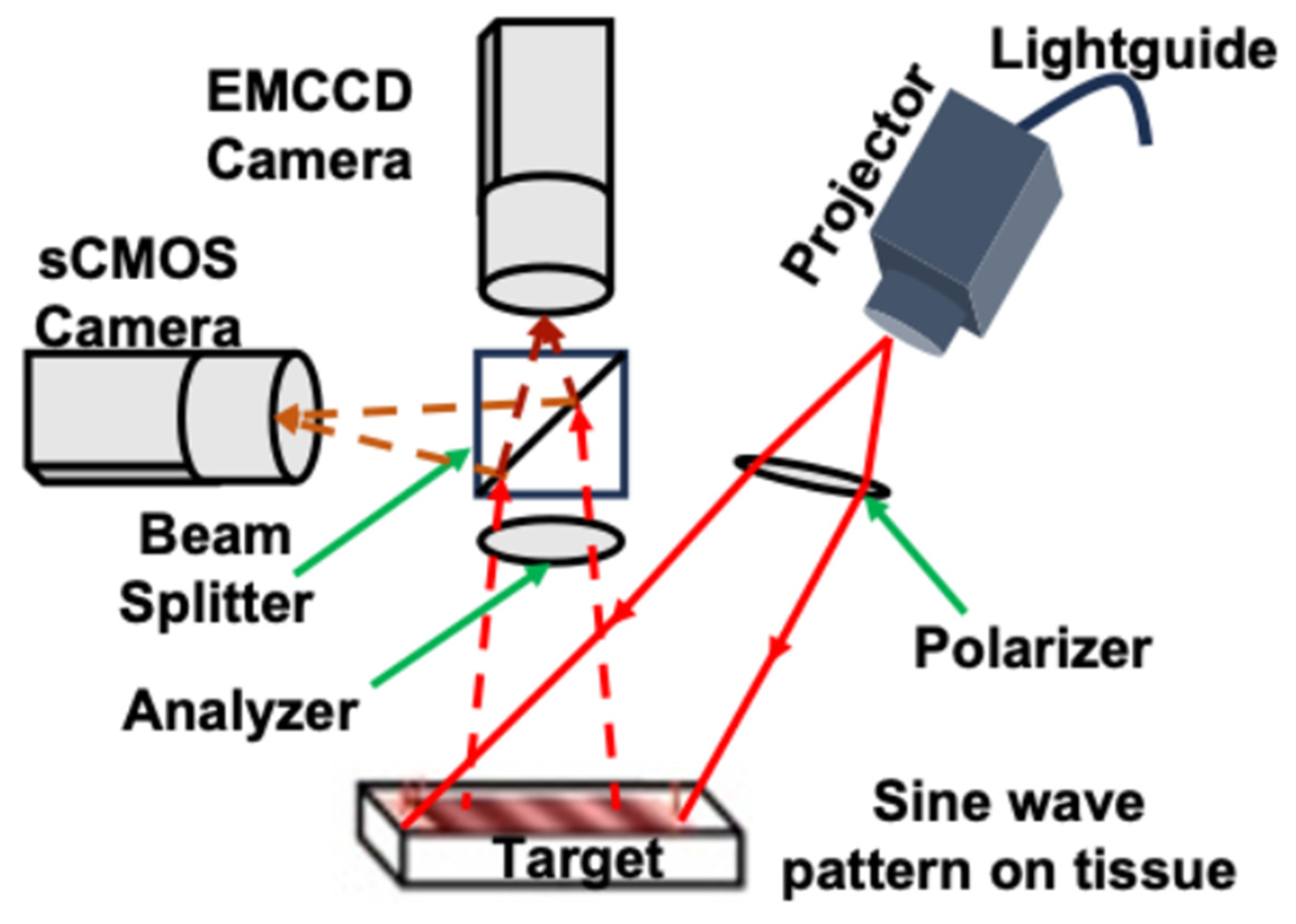
4.1. Wide-Field SFDI System
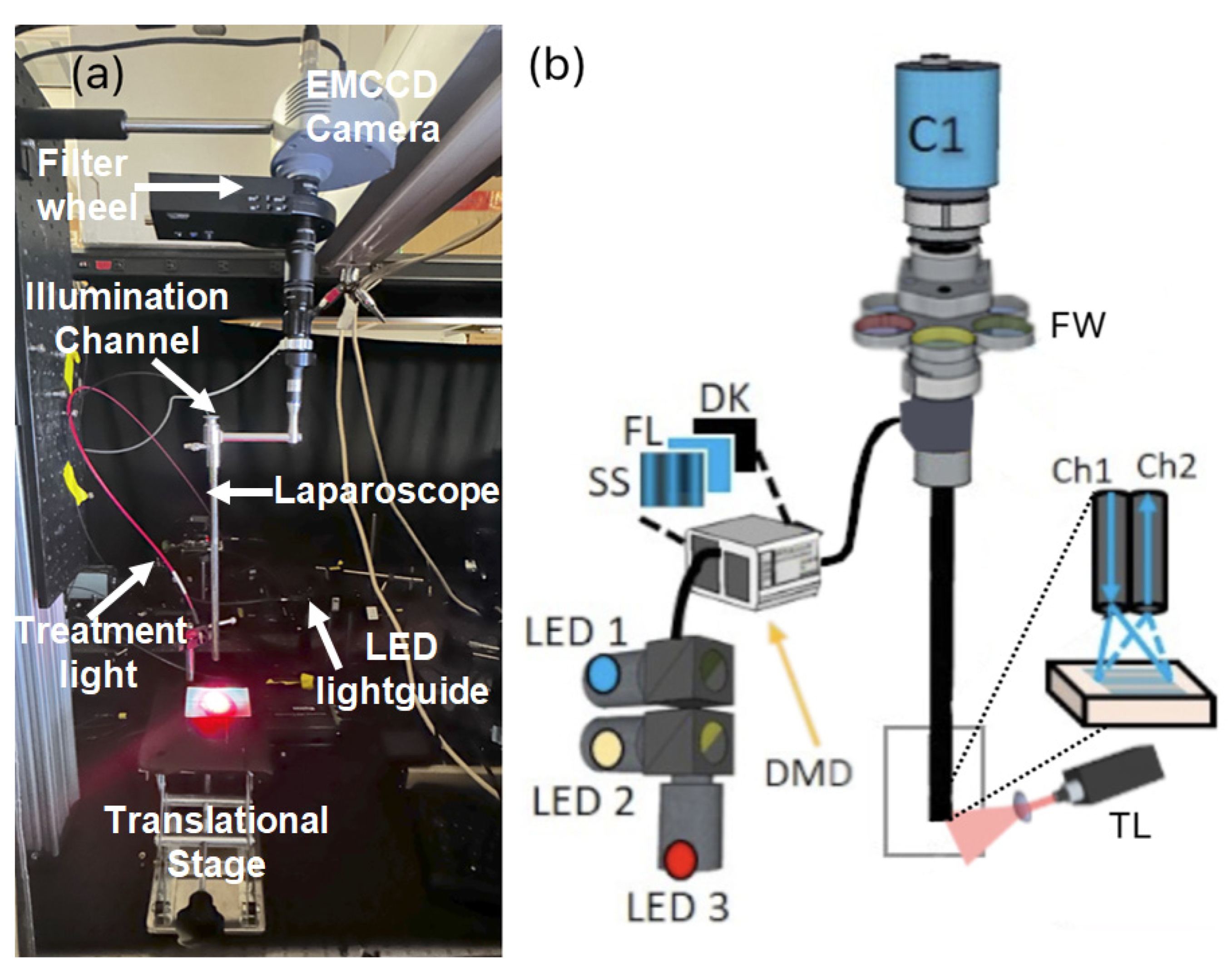
4.2. Laparoscopic SFDI System
4.3. System Calibration
4.4. Subcutaneous Tumor Culture in Mouse Model
4.5. Preparation of Long-Circulating Dox in PoP Liposomes
4.6. Dox Release Quantification and PoP Photobleaching by Laparoscopic SFDI in a Mouse Model
4.7. Attenuation-Compensated Fluorescence Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azaïs, H.; Schmitt, C.; Tardivel, M.; Kerdraon, O.; Stallivieri, A.; Frochot, C.; Betrouni, N.; Collinet, P.; Mordon, S. Assessment of the specificity of a new folate-targeted photosensitizer for peritoneal metastasis of epithelial ovarian cancer to enable intraperitoneal photodynamic therapy. A preclinical study. Photodiagnosis Photodyn. Ther. 2016, 13, 130–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Longmire, M.; Kosaka, N.; Ogawa, M.; Choyke, P.L.; Kobayashi, H. Multicolor in vivo targeted imaging to guide real-time surgery of HER2-positive micrometastases in a two-tumor coincident model of ovarian cancer. Cancer Sci. 2009, 100, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Nishida, T.; Komai, K.; Nishimura, H.; Yakushiji, M. Comparison of CA 125 assays with abdominopelvic computed tomography and transvaginal ultrasound in monitoring of ovarian cancer. Int. J. Gynecol. Obstet. 1996, 54, 251–256. [Google Scholar] [CrossRef]
- Chan, J.K.; Monk, B.J.; Cuccia, D.; Pham, H.; Kimel, S.; Gu, M.; Hammer-Wilson, M.J.; Liaw, L.-H.L.; Osann, K.; DiSaia, P.J.; et al. Laparoscopic Photodynamic Diagnosis of Ovarian Cancer Using 5-Aminolevulinic Acid in a Rat Model. Gynecol. Oncol. 2002, 87, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, I.; Celli, J.P.; Evans, C.L.; Abu-Yousif, A.O.; Muzikansky, A.; Pogue, B.W.; Finkelstein, D.; Hasan, T. Synergistic enhancement of carboplatin efficacy with photodynamic therapy in a three-dimensional model for micrometastatic ovarian cancer. Cancer Res. 2010, 70, 9319–9328. [Google Scholar] [CrossRef]
- van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.; van der Zee, A.G.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Rose, P.G.; Faulhaber, P.; Miraldi, F.; Abdul-Karim, F.W. Positive Emission Tomography for Evaluating a Complete Clinical Response in Patients with Ovarian or Peritoneal Carcinoma: Correlation with Second-Look Laparotomy. Gynecol. Oncol. 2001, 82, 17–21. [Google Scholar] [CrossRef]
- Boussios, S.; Pentheroudakis, G.; Katsanos, K.; Pavlidis, N. Systemic Treatment-Induced Gastrointestinal Toxicity: Incidence, Clinical Presentation and Management. Ann Gastroenterol. 2012, 25, 106–118. [Google Scholar]
- Mallidi, S.; Anbil, S.; Bulin, A.-L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef]
- Sunar, U.; Rohrbach, D.; Rigual, N.; Tracy, E.; Keymel, K.; Cooper, M.T.; Baumann, H.; Henderson, B.H. Monitoring photobleaching and hemodynamic responses to HPPH-mediated photodynamic therapy of head and neck cancer: A case report. Opt. Express 2010, 18, 14969–14978. [Google Scholar] [CrossRef]
- Mo, W.; Rohrbach, D.J.; Sunar, U. Imaging a photodynamic therapy photosensitizer in vivo with a time-gated fluorescence tomography system. J. Biomed. Opt. 2012, 17, 71306. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.S.; Rohrbach, D.; Sunar, U.; Intes, X. Mesoscopic Fluorescence Tomography of a Photosensitizer (HPPH) 3D Biodistribution in Skin Cancer. Acad. Radiol. 2014, 21, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Staropoli, N.; Ciliberto, D.; Botta, C.; Fiorillo, L.; Grimaldi, A.; Lama, S.; Caraglia, M.; Salvino, A.; Tassone, P.; Tagliaferri, P. Pegylated liposomal doxorubicin in the management of ovarian cancer. Cancer Biol. Ther. 2014, 15, 707–720. [Google Scholar] [CrossRef]
- Sunar, U.; Rohrbach, D.J.; Morgan, J.; Zeitouni, N.; Henderson, B.W. Quantification of PpIX concentration in basal cell carcinoma and squamous cell carcinoma models using spatial frequency domain imaging. Biomed. Opt. Express 2013, 4, 531–537. [Google Scholar] [CrossRef]
- Major, A.L.; Rose, G.; Chapman, C.F.; Hiserodt, J.C.; Tromberg, B.J.; Krasieva, T.B.; Tadir, Y.; Haller, U.; Disaia, P.J.; Berns, M.W. In VivoFluorescence Detection of Ovarian Cancer in the NuTu-19 Epithelial Ovarian Cancer Animal Model Using 5-Aminolevulinic Acid (ALA). Gynecol. Oncol. 1997, 66, 122–132. [Google Scholar] [CrossRef][Green Version]
- Celli, J.P.; Rizvi, I.; Blanden, A.R.; Massodi, I.; Glidden, M.D.; Pogue, B.W.; Hasan, T. An imaging-based platform for high-content, quantitative evaluation of therapeutic response in 3D tumour models. Sci. Rep. 2014, 4, 3751. [Google Scholar] [CrossRef]
- Spring, B.Q.; Sears, R.B.; Zheng, L.Z.; Mai, Z.; Watanabe, R.; Sherwood, M.E.; Schoenfeld, D.A.; Pogue, B.W.; Pereira, S.P.; Villa, E.; et al. A photoactivable multi-inhibitor nanoliposome for tumour control and simultaneous inhibition of treatment escape pathways. Nat. Nanotechnol. 2016, 11, 378–387. [Google Scholar] [CrossRef]
- Wang, S.; Hüttmann, G.; Scholzen, T.; Zhang, Z.; Vogel, A.; Hasan, T.; Rahmanzadeh, R. A light-controlled switch after dual targeting of proliferating tumor cells via the membrane receptor EGFR and the nuclear protein Ki-67. Sci. Rep. 2016, 6, 27032. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Celli, J.P.; Rizvi, I.; Mai, Z.; Spring, B.Q.; Yun, S.H.; Hasan, T. In vivo high-resolution fluorescence microendoscopy for ovarian cancer detection and treatment monitoring. Br. J. Cancer 2009, 101, 2015–2022. [Google Scholar] [CrossRef][Green Version]
- Azaïs, H.; Queniat, G.; Bonner, C.; Kerdraon, O.; Tardivel, M.; Jetpisbayeva, G.; Frochot, C.; Betrouni, N.; Collinet, P.; Mordon, S. Fischer 344 Rat: A Preclinical Model for Epithelial Ovarian Cancer Folate-Targeted Therapy. Int. J. Gynecol. Cancer 2015, 25, 1194–1200. [Google Scholar]
- Guyon, L.; Ascencio, M.; Collinet, P.; Mordon, S. Photodiagnosis and photodynamic therapy of peritoneal metastasis of ovarian cancer. Photodiagnosis Photodyn. Ther. 2012, 9, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-D.; Huang, L. Pharmacokinetics and Biodistribution of Nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef]
- Kress, J.; Rohrbach, D.J.; Carter, K.A.; Luo, D.; Poon, C.; Aygun-Sunar, S.; Shao, S.; Lele, S.; Lovell, J.F.; Sunar, U. A dual-channel endoscope for quantitative imaging, monitoring, and triggering of doxorubicin release from liposomes in living mice. Sci. Rep. 2017, 7, 15578. [Google Scholar] [CrossRef]
- Rohrbach, D.J.; Carter, K.A.; Luo, D.; Shao, S.; Aygun-Sunar, S.; Lovell, J.F.; Sunar, U. Fluence rate-dependent kinetics of light-triggered liposomal doxorubicin assessed by quantitative fluorescence-based endoscopic probe. bioRxiv 2024. [Google Scholar] [CrossRef]
- Carter, K.A.; Shao, S.; Hoopes, M.I.; Luo, D.; Ahsan, B.; Grigoryants, V.M.; Song, W.; Huang, H.; Zhang, G.; Pandey, R.K.; et al. Porphyrin–phospholipid liposomes permeabilized by near-infrared light. Nat. Commun. 2014, 5, 3546. [Google Scholar] [CrossRef]
- Luo, D.; Carter, K.A.; Razi, A.; Geng, J.; Shao, S.; Lin, C.; Ortega, J.; Lovell, J.F. Porphyrin-phospholipid liposomes with tunable leakiness. J. Control. Release 2015, 220, 484–494. [Google Scholar] [CrossRef]
- Venugopal, V.; Park, M.; Ashitate, Y.; Neacsu, F.; Kettenring, F.; Frangioni, J.V.; Gangadharan, S.P.; Gioux, S. Design and characterization of an optimized simultaneous color and near-infrared fluorescence rigid endoscopic imaging system. J. Biomed. Opt. 2013, 18, 1. [Google Scholar] [CrossRef]
- Palmer, G.M.; Boruta, R.J.; Viglianti, B.L.; Lan, L.; Spasojevic, I.; Dewhirst, M.W. Non-invasive monitoring of intra-tumor drug concentration and therapeutic response using optical spectroscopy. J. Control. Release 2010, 142, 457–464. [Google Scholar] [CrossRef][Green Version]
- Bogaards, A.; Sterenborg, H.J.C.M.; Wilson, B.C. In vivo quantification of fluorescent molecular markers in real-time: A review to evaluate the performance of five existing methods. Photodiagnosis Photodyn. Ther. 2007, 4, 170–178. [Google Scholar] [CrossRef]
- Yang, B.; Sharma, M.; Tunnell, J.W. Attenuation-corrected fluorescence extraction for image-guided surgery in spatial frequency domain. J. Biomed. Opt. 2013, 18, 80503. [Google Scholar] [CrossRef] [PubMed]
- Yang, V.X.D.; Muller, P.J.; Herman, P.; Wilson, B.C. A multispectral fluorescence imaging system: Design and initial clinical tests in intra-operative Photofrin-photodynamic therapy of brain tumors. Lasers Surg. Med. 2003, 32, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Baran, T.M.; Foster, T.H. Recovery of intrinsic fluorescence from single-point interstitial measurements for quantification of doxorubicin concentration. Lasers Surg. Med. 2013, 45, 542–550. [Google Scholar] [CrossRef]
- Kim, A.; Khurana, M.; Moriyama, Y.; Wilson, B.C. Quantification of in vivo fluorescence decoupled from the effects of tissue optical properties using fiber-optic spectroscopy measurements. J. Biomed. Opt. 2010, 15, 67006. [Google Scholar] [CrossRef]
- Zhadin, N.N.; Alfano, R.R. Correction of the Internal Absorption Effect in Fluorescence Emission and Excitation Spectra from Absorbing and Highly Scattering Media: Theory and Experiment. J. Biomed. Opt. April. 1998, 3, 171-86. [Google Scholar] [CrossRef]
- Sheng, C.; Pogue, B.W.; Wang, E.; Hutchins, J.E.; Hoopes, P.J. Assessment of Photosensitizer Dosimetry and Tissue Damage Assay for Photodynamic Therapy in Advanced-stage Tumors. Photochem. Photobiol. 2004, 79, 520–526. [Google Scholar]
- Applegate, M.B.; Karrobi, K.; Angelo, J.P.; Austin, W.M.; Tabassum, S.M.; Aguénounon, E.; Tilbury, K.; Saager, R.B.; Gioux, S.; Roblyer, D.M. OpenSFDI: An open-source guide for constructing a spatial frequency domain imaging system. J. Biomed. Opt. 2020, 25, 16002. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Carter, K.A.; Razi, A.; Geng, J.; Shao, S.; Giraldo, D.; Sunar, U.; Ortega, J.; Lovell, J.F. Doxorubicin encapsulated in stealth liposomes conferred with light-triggered drug release. Biomaterials 2016, 75, 193–202. [Google Scholar] [CrossRef]
- Bellnier, D.A.; Henderson, B.W.; Pandey, R.K.; Potter, W.R.; Dougherty, T.J. Murine pharmacokinetics and antitumor efficacy of the photodynamic sensitizer 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a. J. Photochem. Photobiol. B 1993, 20, 55–61. [Google Scholar] [CrossRef]
- Georgakoudi, I.; Nichols, M.G.; Foster, T.H. The Mechanism of Photofrin Photobleaching and Its Consequences for Photodynamic Dosimetry. Photochem. Photobiol. 1997, 65, 135–144. [Google Scholar] [CrossRef]
- Sheng, C.; Jack Hoopes, P.; Hasan, T.; Pogue, B.W. Photobleaching-based Dosimetry Predicts Deposited Dose in ALA-PpIX PDT of Rodent Esophagus. Photochem. Photobiol. 2007, 83, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Patterson, M.S.; Lilge, L. Implicit and explicit dosimetry in photodynamic therapy: A New paradigm. Lasers Med. Sci. 1997, 12, 182–199. [Google Scholar] [CrossRef]
- Zhou, X.; Pogue, B.W.; Chen, B.; Demidenko, E.; Joshi, R.; Hoopes, J.; Hasan, T. Pretreatment photosensitizer dosimetry reduces variation in tumor response. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1211–1220. [Google Scholar] [CrossRef]
- Angelo, J.P.; van de Giessen, M.; Gioux, S. Real-time endoscopic optical properties imaging. Biomed. Opt. Express 2017, 8, 5113–5126. [Google Scholar] [CrossRef]
- van de Giessen, M.; Angelo, J.P.; Gioux, S. Real-time, profile-corrected single snapshot imaging of optical properties. Biomed. Opt. Express 2015, 6, 4051–4062. [Google Scholar] [CrossRef] [PubMed]
- Angelo, J.; Vargas, C.R.; Lee, B.T.; Bigio, I.J.; Gioux, S. Ultrafast optical property map generation using lookup tables. J. Biomed. Opt. 2016, 21, 110501. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, D.J.; Bevilacqua, F.; Durkin, A.J.; Ayers, F.R.; Tromberg, B.J. Quantitation and mapping of tissue optical properties using modulated imaging. J. Biomed. Opt. 2009, 14, 024012. [Google Scholar] [CrossRef]
- Kress, J.; Rohrbach, D.J.; Carter, K.A.; Luo, D.; Shao, S.; Lele, S.; Lovell, J.F.; Sunar, U. Quantitative imaging of light-triggered doxorubicin release. Biomed. Opt. Express 2015, 6, 3546–3555. [Google Scholar] [CrossRef]
- Gordon, A.N.; Fleagle, J.T.; Guthrie, D.; Parkin, D.E.; Gore, M.E.; Lacave, A.J. Recurrent Epithelial Ovarian Carcinoma: A Randomized Phase III Study of Pegylated Liposomal Doxorubicin Versus Topotecan. J. Clin. Oncol. 2001, 19, 3312–3322. [Google Scholar] [CrossRef]
- Gardner, C.M.; Jacques, S.L.; Welch, A.J. Fluorescence spectroscopy of tissue: Recovery of intrinsic fluorescence from measured fluorescence. Appl. Opt. 1996, 35, 1780–1792. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmmed, R.; Kluiszo, E.; Aygun-Sunar, S.; Willadsen, M.; Kutscher, H.L.; Lovell, J.F.; Sunar, U. Quantitative Fluorescence Imaging of Chemophototherapy Drug Pharmacokinetics Using Laparoscopic SFDI. Int. J. Mol. Sci. 2025, 26, 5571. https://doi.org/10.3390/ijms26125571
Ahmmed R, Kluiszo E, Aygun-Sunar S, Willadsen M, Kutscher HL, Lovell JF, Sunar U. Quantitative Fluorescence Imaging of Chemophototherapy Drug Pharmacokinetics Using Laparoscopic SFDI. International Journal of Molecular Sciences. 2025; 26(12):5571. https://doi.org/10.3390/ijms26125571
Chicago/Turabian StyleAhmmed, Rasel, Elias Kluiszo, Semra Aygun-Sunar, Matthew Willadsen, Hilliard L. Kutscher, Jonathan F. Lovell, and Ulas Sunar. 2025. "Quantitative Fluorescence Imaging of Chemophototherapy Drug Pharmacokinetics Using Laparoscopic SFDI" International Journal of Molecular Sciences 26, no. 12: 5571. https://doi.org/10.3390/ijms26125571
APA StyleAhmmed, R., Kluiszo, E., Aygun-Sunar, S., Willadsen, M., Kutscher, H. L., Lovell, J. F., & Sunar, U. (2025). Quantitative Fluorescence Imaging of Chemophototherapy Drug Pharmacokinetics Using Laparoscopic SFDI. International Journal of Molecular Sciences, 26(12), 5571. https://doi.org/10.3390/ijms26125571






