Regulation of Renal and Extrarenal Calcitriol Synthesis and Its Clinical Implications
Abstract
1. Introduction
2. Regulation of Circulating Calcitriol, Calcium, and Phosphorus
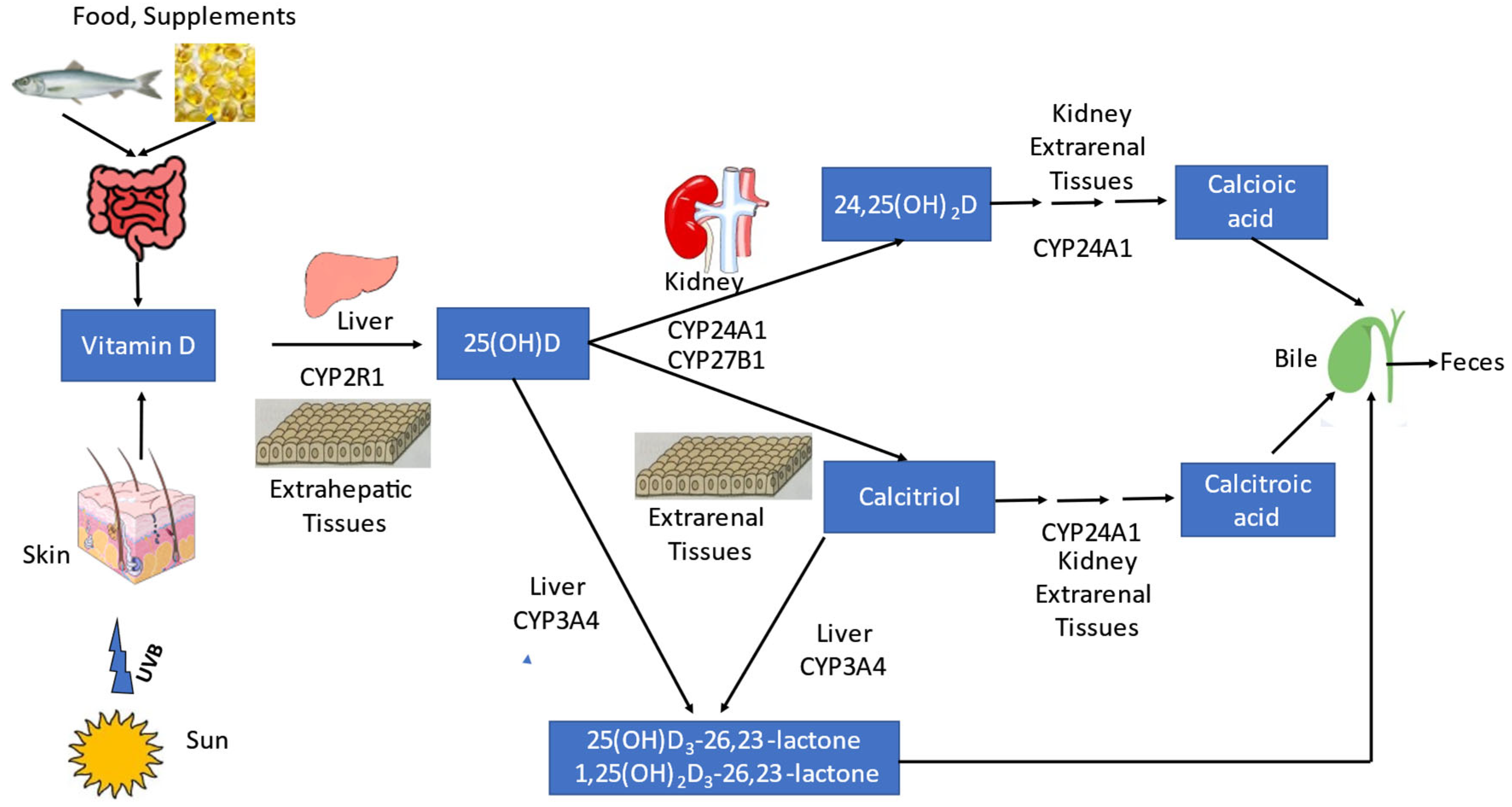
2.1. Calcitriol Metabolism
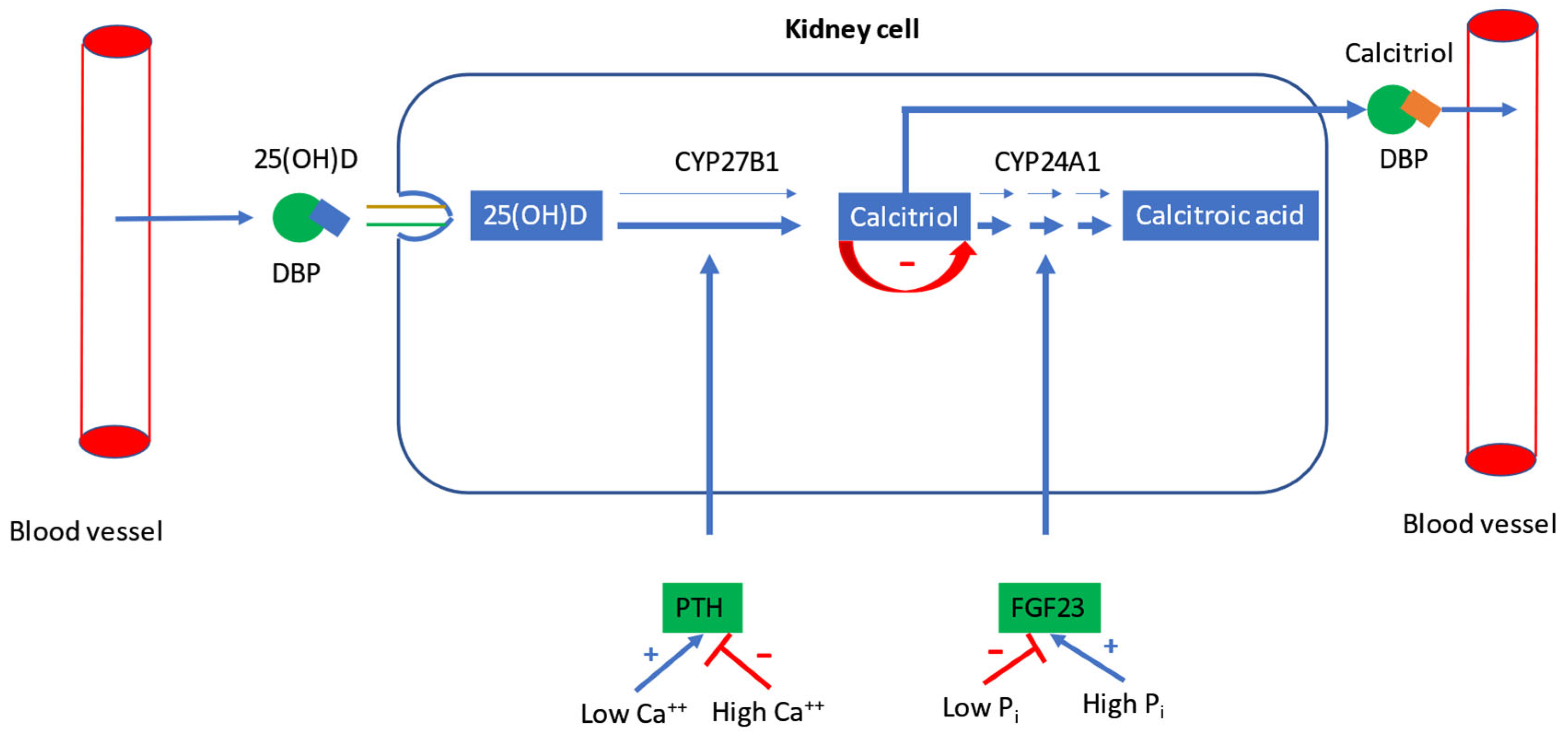
2.2. Regulation of Calcitriol Synthesis
2.3. Regulation of Plasma Calcium and Phosphorus
3. Nutritional Factors Influencing Circulating Calcitriol
3.1. Vitamin D and Calcium Supplementation
3.1.1. Rickets and Osteomalacia
3.1.2. General Population
3.1.3. Obesity
3.2. Phosphorus Supplementation
3.3. Magnesium Intake
4. Mechanical Loading/Unloading and Circulating Calcitriol
4.1. Physical Activity
4.2. Bedrest
5. Pregnancy
6. Diseases
6.1. Chronic Kidney Disease
6.2. Heart Failure
6.2.1. Rickets/Osteomalacia
6.2.2. Non-Osteomalacic Adult Population
7. Genetic Disorders
7.1. Vitamin D-Dependent Rickets
7.2. CYP24A1 Mutations
8. Extrarenal Calcitriol Synthesis and Regulation
9. Clinical Implications
9.1. Vitamin D Supplementation, Rickets/Osteomalacia and Bone Diseases
9.2. Diabetes Mellitus
9.3. Preeclampsia
9.4. Chronic Kidney Disease Treatment
9.5. Heart Failure Treatment
9.6. Acute Respiratory Tract Infection
10. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
Abbreviations
| 25(OH)D | 25-Hydroxyvitamin D |
| 24,25(OH)2D | 24,25-Dihydroxyvitamin D |
| BMI | Body mass index |
| Ca | Calcium |
| CaSR | Calcium-sensing receptor |
| CKD | Chronic kidney disease |
| CYP | Cytochrome P |
| DBP | Vitamin D binding protein |
| GFR | Glomerular filtration rate |
| IFN | Interferon |
| IL | Interleukin |
| IU | International unit |
| FGF | Fibroblast growth factor |
| HF | Herat failure |
| LPS | Lipopolysaccharide |
| P | Phosphorus |
| Pi | Inorganic phosphate |
| PTH | Parathyroid hormone |
| RCT | Randomized controlled trial |
| TNF | Tumor necrosis factor |
| TLR | Toll-like receptor |
| VDR | Vitamin D receptor |
| VDDR | Vitamin D-dependent rickets |
References
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.; Gordon-Thomson, C.; Hoy, A.J.; Balaban, S.; Rybchyn, M.S.; Cole, L.; Su, Y.; Brennan-Speranza, T.C.; Fraser, D.R.; Mason, R.S. Uptake of 25-hydroxyvitamin D by muscle and fat cells. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 232–236. [Google Scholar] [CrossRef]
- Martinaityte, I.; Kamycheva, E.; Didriksen, A.; Jakobsen, J.; Jorde, R. Vitamin D Stored in Fat Tissue During a 5-Year Intervention Affects Serum 25-Hydroxyvitamin D Levels the Following Year. J. Clin. Endocrinol. Metab. 2017, 102, 3731–3738. [Google Scholar] [CrossRef] [PubMed]
- IOM (Institute of Medicine). Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- DeLuca, H. History of the discovery of vitamin D and its active metabolites. BoneKEy Rep. 2014, 3, 479. [Google Scholar] [CrossRef]
- Jarnagin, K.; Brommage, R.; DeLuca, H.F.; Yamada, S.; Takayama, H. 1- but not 24-hydroxylation of vitamin D is required for skeletal mineralization in rats. Am. J. Physiol. 1983, 244, E298–E304. [Google Scholar] [CrossRef]
- Jarnagin, K.; Brommage, R.; DeLuca, H.F.; Yamada, S.; Takayama, H. 1- but not 24-hydroxylation of vitamin D is required for growth and reproduction in rats. Am. J. Physiol. 1983, 244, E290–E297. [Google Scholar] [CrossRef]
- Papapoulos, S.E.; Clemens, T.L.; Fraher, L.J.; Gleed, J.; O’Riordan, J.L. Metabolites of vitamin D in human vitamin-D deficiency: Effect of vitamin D3 or 1,25-dihydroxycholecalciferol. Lancet 1980, 316, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Ernst, J.B.; Becker, T.; Dreier, J.; Knabbe, C.; Gummert, J.F.; Kuhn, J. Measurement of Circulating 1,25-Dihydroxyvitamin D: Comparison of an Automated Method with a Liquid Chromatography Tandem Mass Spectrometry Method. Int. J. Anal. Chem. 2016, 2016, 8501435. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.K.; Lloyd-Jones, D.M.; Thadhani, R.I.; Shaw, A.C.; Deraska, D.J.; Kitch, B.T.; Vamvakas, E.C.; Dick, I.M.; Prince, R.L.; Finkelstein, J.S. Hypovitaminosis D in medical inpatients. N. Engl. J. Med. 1998, 338, 777–783. [Google Scholar] [CrossRef]
- Docio, S.; Riancho, J.A.; Pérez, A.; Olmos, J.M.; Amado, J.A.; González-Macías, J. Seasonal deficiency of vitamin D in children: A potential target for osteoporosis-preventing strategies? J. Bone Miner. Res. 1998, 13, 544–548. [Google Scholar] [CrossRef]
- Stanbury, S.W.; Mawer, E.B. The metabolism of a physiological dose of radioactive cholecalciferol (vitamin D3) to its hydroxylated metabolites in man. Clin. Sci. 1980, 58, 523–535. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Production, Metabolism and Mechanisms of Action. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2021. [Google Scholar]
- Jones, G.; Prosser, D.E.; Kaufmann, M. The activating enzymes of vitamin D metabolism (25- and 1a-hydroxylases). In Fieldman and Pike’s Vitamin D, 5th ed.; Hewison, M., Bouillon, R., Giovanucci, E., Goltzman, D., Meyer, M., Welsh, J., Eds.; Academic Press: Oxford, UK, 2024; Chapter 4; pp. 47–69. [Google Scholar]
- Hewison, M.; Bouillon, R.; Giovanucci, E.; Goltzman, D.; Meyer, M.; Welsh, J. (Eds.) Relevant lab values in adults and children. In Fieldman and Pike’s Vitamin D, 5th ed.; Academic Press: Oxford, UK, 2024; p. li. [Google Scholar]
- Zittermann, A.; Ernst, J.B. Calciotropic and phosphaturic hormones in heart failure. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 971–979. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Razzaque, M.S. Vitamin D and Phosphate Interactions in Health and Disease. Adv. Exp. Med. Biol. 2022, 1362, 37–46. [Google Scholar] [CrossRef]
- Tashiro, K.; Abe, T.; Oue, N.; Yasui, W.; Ryoji, M. Characterization of vitamin D-mediated induction of the CYP 24 transcription. Mol. Cell. Endocrinol. 2004, 226, 27–32. [Google Scholar] [CrossRef]
- Aberger, S.; Schreiber, N.; Pilz, S.; Eller, K.; Rosenkranz, A.R.; Kirsch, A.H. Targeting Calcitriol Metabolism in Acute Vitamin D Toxicity-A Comprehensive Review and Clinical Insight. Int. J. Mol. Sci. 2024, 25, 10003. [Google Scholar] [CrossRef]
- St-Arnaud, R.; Jones, G. CYP24A1: Structure, function, and physiological role. In Fieldman and Pike’s Vitamin D, 5th ed.; Hewison, M., Bouillon, R., Giovanucci, E., Goltzman, D., Meyer, M., Welsh, J., Eds.; Academic Press: Oxford, UK, 2024; Chapter 5; pp. 71–83. [Google Scholar]
- Kärkkäinen, M.U.; Wiersma, J.W.; Lamberg-Allardt, C.J. Postprandial parathyroid hormone response to four calcium-rich foodstuffs. Am. J. Clin. Nutr. 1997, 65, 1726–1730. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; US National Academy of Sciences: Washington, DC, USA, 1997. [Google Scholar]
- U.S. Department of Agriculture, Department of Health and Human Services. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Appendix E-2.1: Usual Intake Distributions, 2007–2010, by Age/Gender Groups. 2015. Available online: https://health.gov/dietaryguidelines/2015-binder/meeting2/docs/refMaterials/Usual_Intake_072013.pdf (accessed on 20 May 2025).
- Hoy, M.K.; Goldman, J.D. Calcium intake of the U.S. population: What We Eat in America, NHANES 2009–2010. In FSRG Dietary Data Briefs [Internet]; United States Department of Agriculture (USDA): Beltsville, MD, USA, 2014; Dietary Data Brief No. 13. [Google Scholar] [PubMed]
- Kruse, K. Pathophysiology of calcium metabolism in children with vitamin D-deficiency rickets. J. Pediatr. 1995, 126 Pt 1, 736–741. [Google Scholar] [CrossRef]
- Zittermann, A. The Biphasic Effect of Vitamin D on the Musculoskeletal and Cardiovascular System. Int. J. Endocrinol. 2017, 2017, 3206240. [Google Scholar] [CrossRef]
- Rios-Leyvraz, M.; Thacher, T.D.; Dabas, A.; Elsedfy, H.H.; Baroncelli, G.I.; Cashman, K.D. Serum 25-hydroxyvitamin D threshold and risk of rickets in young children: A systematic review and individual participant data meta-analysis to inform the development of dietary requirements for vitamin D. Eur. J. Nutr. 2024, 63, 673–695. [Google Scholar] [CrossRef]
- Pettifor, J.M.; Thandrayen, K.; Thacher, T.D. Vitamin D deficiency and nutritional rickets in infants and children. In Fieldman and Pike’s Vitamin D, 5th ed.; Hewison, M., Bouillon, R., Giovanucci, E., Goltzman, D., Meyer, M., Welsh, J., Eds.; Academic Press: Oxford, UK, 2024; Chapter 63; pp. 241–279. [Google Scholar]
- Fischer, P.R.; Sempos, C.T.; Pettifor, J.M.; Fraser, D.R.; Munns, C.F.; Durazo-Arvizu, R.A.; Thacher, T.D. Serum 1,25-dihydroxyvitamin D levels in the diagnosis and pathogenesis of nutritional rickets—A multivariable re-analysis of a case-control study. Am. J. Clin. Nutr. 2023, 117, 998–1004. [Google Scholar] [CrossRef]
- Hafner, D.; Leifheit-Nestler, M.; Grund, A.; Schnabel, D. Rickets guidance: Part I—Diagnostic workup. Pediatr. Nephrol. 2022, 37, 2013–2036. [Google Scholar] [CrossRef] [PubMed]
- Audran, M.; Renier, J.C.; Jallet, P.; Bidet, M.; Basle, M.F.; Seret, P. Serum concentrations of 1,25-dihydroxyvitamin D in cases of osteomalacia, parathyroid dysfunction and idiopathic hypercalciuria. Rev. Rhum. Mal. Osteoartic. 1987, 54, 163–169. [Google Scholar] [PubMed]
- Mosekilde, L.; Melsen, F.; Hessov, I.; Christensen, M.S.; Lund, B.J.; Lund, B.I.; Sørensenet, O.H. Low serum levels of 1.25-dihydroxyvitamin D and histomorphometric evidence of osteomalacia after jejunoileal bypass for obesity. Gut 1980, 21, 624–631. [Google Scholar] [CrossRef]
- Stanbury, S.W.; Taylor, C.M.; Lumb, G.A.; Mawer, E.B.; Berry, J.; Hann, J.; Wallace, J. Formation of vitamin D metabolites following correction of human vitamin D deficiency: Observations in patients with nutritional osteomalacia. Miner. Electrolyte Metab. 1981, 5, 212–227. [Google Scholar]
- Zittermann, A.; Ernst, J.B.; Birschmann, I.; Dittrich, M. Effect of Vitamin D or Activated Vitamin D on Circulating 1,25-Dihydroxyvitamin D Concentrations: A Systematic Review and Metaanalysis of randomized Controlled Trials. Clin. Chem. 2015, 61, 1484–1494. [Google Scholar] [CrossRef]
- Moslehi, N.; Shab-Bidar, S.; Mirmiran, P.; Hosseinpanah, F.; Azizi, F. Determinants of parathyroid hormone response to vitamin D supplementation: A systematic review and meta-analysis of randomized controlled trials. Br. J. Nutr. 2015, 114, 1360–1374. [Google Scholar] [CrossRef]
- Zittermann, A.; Berthold, H.K.; Pilz, S. The effect of vitamin D on fibroblast growth factor 23: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2021, 75, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Steingrimsdottir, L.; Gunnarsson, O.; Indridason, O.S.; Franzson, L.; Sigurdsson, G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005, 294, 2336–2341. [Google Scholar] [CrossRef]
- Konradsen, S.; Ag, H.; Lindberg, F.; Hexeberg, S.; Jorde, R. Serum 1,25-dihydroxy vitamin D is inversely associated with body mass index. Eur. J. Nutr. 2008, 47, 87–91. [Google Scholar] [CrossRef]
- Zittermann, A.; Ernst, J.B.; Gummert, J.F.; Börgermann, J. Vitamin D supplementation, body weight and human serum 25-hydroxyvitamin D response: A systematic review. Eur. J. Nutr. 2014, 53, 367–374. [Google Scholar] [CrossRef]
- Elkhwanky, M.S.; Kummu, O.; Piltonen, T.T.; Laru, J.; Morin-Papunen, L.; Mutikainen, M.; Tavi, P.; Hakkola, J. Obesity Represses CYP2R1, the Vitamin D 25-Hydroxylase, in the Liver and Extrahepatic Tissues. JBMR Plus 2020, 4, e10397. [Google Scholar] [CrossRef]
- Rajakumar, K.; Fernstrom, J.D.; Holick, M.F.; Janosky, J.E.; Greenspan, S.L. Vitamin D status and response to Vitamin D(3) in obese vs. non-obese African American children. Obes. (Silver Spring) 2008, 16, 90–95. [Google Scholar] [CrossRef]
- Wood, A.D.; Secombes, K.R.; Thies, F.; Aucott, L.S.; Black, A.J.; Reid, D.M.; Mavroeidi, A.; Simpson, W.G.; Fraser, W.D.; Macdonald, H.M. A parallel group double-blind RCT of vitamin D3 assessing physical function: Is the biochemical response to treatment affected by overweight and obesity? Osteoporos. Int. 2014, 25, 305–315. [Google Scholar] [CrossRef]
- Bhagatwala, J.; Zhu, H.; Parikh, S.J.; Guo, D.H.; Kotak, I.; Huang, Y.; Havens, R.; Pham, M.; Afari, E.; Kim, S.; et al. Dose and time responses of vitamin D biomarkers to monthly vitamin D3 supplementation in overweight/obese African Americans with suboptimal vitamin d status: A placebo controlled randomized clinical trial. BMC Obes. 2015, 2, 27. [Google Scholar] [CrossRef]
- Camozzi, V.; Frigo, A.C.; Zaninotto, M.; Sanguin, F.; Plebani, M.; Boscaro, M.; Schiavon, L.; Luisetto, G. 25-Hydroxycholecalciferol response to single oral cholecalciferol loading in the normal weight, overweight, and obese. Osteoporos. Int. 2016, 27, 2593–2602. [Google Scholar] [CrossRef]
- Das, L.; Sachdeva, N.; Holick, M.F.; Devnani, M.; Dutta, P.; Marwaha, R.K. Impact of BMI on serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D with calcifediol supplementation in young adults: A longitudinal study. Endocrine 2024, 86, 391–399. [Google Scholar] [CrossRef]
- Ferrari, S.L.; Bonjour, J.P.; Rizzoli, R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J. Clin. Endocrinol. Metab. 2005, 90, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Antoniucci, D.M.; Yamashita, T.; Portale, A.A. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J. Clin. Endocrinol. Metab. 2006, 91, 3144–3149. [Google Scholar] [CrossRef]
- Burnett, S.M.; Gunawardene, S.C.; Bringhurst, F.R.; Jüppner, H.; Lee, H.; Finkelstein, J.S. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J. Bone Miner. Res. 2006, 21, 1187–1196. [Google Scholar] [CrossRef]
- Sigrist, M.; Tang, M.; Beaulieu, M.; Espino-Hernandez, G.; Er, L.; Djurdjev, O.; Levin, A. Responsiveness of FGF-23 and mineral metabolism to altered dietary phosphate intake in chronic kidney disease (CKD): Results of a randomized trial. Nephrol. Dial. Transplant. 2013, 28, 161–169. [Google Scholar] [CrossRef]
- Gerber, J.S.; Arroyo, E.M.P.; Pastor, J.; Correia, M.; Rudloff, S.; Moe, O.W.; Egli-Spichtig, D.; Mohebbi, N.; Wagner, C.A. Controlled dietary phosphate loading in healthy young men elevates plasma phosphate and FGF23 levels. Pflugers Arch. 2025, 477, 495–508. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in vitamin D activation and function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Zittermann, A.; Zelzer, S.; Herrmann, M.; Kleber, M.; Maerz, W.; Pilz, S. Association between magnesium and vitamin D status in adults with high prevalence of vitamin D deficiency and insufficiency. Eur. J. Nutr. 2024, 64, 48. [Google Scholar] [CrossRef]
- Cheung, M.M.; Dall, R.D.; Shewokis, P.A.; Altasan, A.; Volpe, S.L.; Amori, R.; Singh, H.; Sukumar, D. The effect of combined magnesium and vitamin D supplementation on vitamin D status, systemic inflammation, and blood pressure: A randomized double-blinded controlled trial. Nutrition 2022, 99–100, 111674. [Google Scholar] [CrossRef]
- Dai, Q.; Zhu, X.; Manson, J.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence vitamin D status and metabolism: Results from a randomized trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258. [Google Scholar] [CrossRef]
- Soong, R.Y.; Low, C.E.; Ong, V.; Sim, I.; Lee, C.; Lee, F.; Chew, L.; Yau, C.E.; Lee, A.R.Y.B.; Chen, M.Z. Exercise Interventions for Depression, Anxiety, and Quality of Life in Older Adults with Cancer: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2025, 8, e2457859. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Redington, A.; George, K.P.; Hopman, M.T.E.; Jones, H. Association of Exercise Preconditioning with Immediate Cardioprotection: A Review. JAMA Cardiol. 2018, 3, 169–176. [Google Scholar] [CrossRef]
- Mazza, O.; Nielsen, J.; Mathiesen, J.; Højme, D.; Lundby, C.; Hostrup, M.; Thomassen, M.; Plomgaard, P.; Gejl, K.D.; Ørtenblad, N. Effects of 8 Weeks of Moderate- or High-Volume Strength Training on Sarcoplasmic Reticulum Ca2+ Handling in Elite Female and Male Rowers. Scand. J. Med. Sci. Sports 2025, 35, e70017. [Google Scholar] [CrossRef]
- Gaffney-Stomberg, E.; Nakayama, A.T.; Lutz, L.J.; McClung, J.P.; O’Brien, K.O.; Staab, J.S. Load carriage exercise increases calcium absorption and retention in healthy young women. J. Bone Miner. Res. 2024, 39, 39–49. [Google Scholar] [CrossRef]
- Zittermann, A.; Schleithoff, S.S.; Tenderich, G.; Berthold, H.K.; Körfer, R.; Stehle, P. Low vitamin D status: A contributing factor in the pathogenesis of congestive heart failure? J. Am. Coll. Cardiol. 2003, 41, 105–112. [Google Scholar] [CrossRef]
- Montain, S.J.; Cheuvront, S.N.; Lukaski, H.C. Sweat mineral-element responses during 7 h of exercise-heat stress. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Sabatschus, O.; Jantzen, S.; Platen, P.; Danz, A.; Dimitriou, T.; Scheld, K.; Klein, K.; Stehle, P. Exercise-trained young men have higher calcium absorption rates and plasma calcitriol levels compared with age-matched sedentary controls. Calcif. Tissue Int. 2000, 67, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Nemoseck, T.; Kern, M. The effects of high-impact and resistance exercise on urinary calcium excretion. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 162–171. [Google Scholar] [CrossRef]
- Dent, E.; Daly, R.M.; Hoogendijk, E.O.; Scott, D. Exercise to Prevent and Manage Frailty and Fragility Fractures. Curr. Osteoporos. Rep. 2023, 21, 205–215. [Google Scholar] [CrossRef]
- O’Bryan, S.J.; Giuliano, C.; Woessner, M.N.; Vogrin, S.; Smith, C.; Duque, G.; Levinger, I. Progressive Resistance Training for Concomitant Increases in Muscle Strength and Bone Mineral Density in Older Adults: A Systematic Review and Meta-Analysis. Sports Med. 2022, 52, 1939–1960. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, M.; Yi, L.; Li, F.; Feng, L.; Ji, T.; Zang, Y.; Qiu, J. Effects of 8-week High-Intensity Interval Training and Moderate-Intensity Continuous Training on Bone Metabolism in Sedentary Young Females. J. Exerc. Sci. Fit. 2022, 20, 77–83. [Google Scholar] [CrossRef]
- De-la-O, A.; Jurado-Fasoli, L.; Castillo, M.J.; Gutiérrez, Á.; Amaro-Gahete, F.J. Effect of Exercise Training on 1,25(OH)2D Levels: The FIT-AGEING Randomized Controlled Trial. Sports Health 2022, 14, 518–526. [Google Scholar] [CrossRef]
- Nikniaz, L.; Ghojazadeh, M.; Nateghian, H.; Nikniaz, Z.; Farhangi, M.A.; Pourmanaf, H. The interaction effect of aerobic exercise and vitamin D supplementation on inflammatory factors, anti-inflammatory proteins, and lung function in male smokers: A randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2021, 13, 102. [Google Scholar] [CrossRef]
- Tajima, O.; Ashizawa, N.; Ishii, T.; Amagai, H.; Mashimo, T.; Liu, L.J.; Saitoh, S.; Tokuyama, K.; Suzuki, M. Interaction of the effects between vitamin D receptor polymorphism and exercise training on bone metabolism. J. Appl. Physiol. (1985) 2000, 88, 1271–1276. [Google Scholar] [CrossRef]
- Zorbas, Y.G.; Kakuris, K.K.; Deogenov, V.A.; Yerullis, K.B. Phosphate homeostasis in healthy subjects during prolonged periodic and continuous hypokinesia. Clin. Biochem. 2007, 40, 460–466. [Google Scholar] [CrossRef]
- Barry, D.W.; Hansen, K.C.; van Pelt, R.E.; Witten, M.; Wolfe, P.; Kohrt, W.M. Acute calcium ingestion attenuates exercise-induced disruption of calcium homeostasis. Med. Sci. Sports Exerc. 2011, 43, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Coombs, C.V.; Greeves, J.P.; Young, C.D.; Irving, A.S.; Eisenhauer, A.; Kolevica, A.; Heuser, A.; Tang, J.C.Y.; Fraser, W.D.; O’Leary, T.J. The effect of calcium supplementation on bone calcium balance and calcium and bone metabolism during load carriage in women: A randomised controlled crossover trial. J. Bone Miner. Res. 2025, 40, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Zorbas, Y.G.; Petrov, K.L.; Kakurin, V.J.; Kuznetsov, N.A.; Charapakhin, K.P.; Alexeyev, I.D.; Denogradov, S.D. Calcium supplementation effect on calcium balance in endurance-trained athletes during prolonged hypokinesia and ambulatory conditions. Biol. Trace Elem. Res. 2000, 73, 231–250. [Google Scholar] [CrossRef]
- Giangregorio, L.; Blimkie, C.J. Skeletal adaptations to alterations in weight-bearing activity: A comparison of models of disuse osteoporosis. Sports Med. 2002, 32, 459–476. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.D.; Spector, E.R.; Evans, H.J.; Sibonga, J.D. Skeletal responses to space flight and the bed rest analog: A review. J. Musculoskelet. Neuronal Interact. 2007, 7, 33–47. [Google Scholar] [PubMed]
- Zerwekh, J.E.; Ruml, L.A.; Gottschalk, F.; Pak, C.Y. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J. Bone Miner. Res. 1998, 13, 1594–1601. [Google Scholar] [CrossRef]
- McGrath, E.R.; Frings-Meuthen, P.; Sibonga, J.; Heer, M.; Clement, G.R.; Mulder, E.; Smith, S.M.; Zwart, S.R. Bone metabolism during strict head-down tilt bed rest and exposure to elevated levels of ambient CO2. NPJ Microgravity 2022, 8, 57. [Google Scholar] [CrossRef]
- van der Wiel, H.E.; Lips, P.; Nauta, J.; Netelenbos, J.C.; Hazenberg, G.J. Biochemical parameters of bone turnover during ten days of bed rest and subsequent mobilization. Bone Miner. 1991, 13, 123–129. [Google Scholar] [CrossRef]
- Smith, S.M.; Zwart, S.R.; Heer, M.A.; Baecker, N.; Evans, H.J.; Feiveson, A.H.; Shackelford, L.C.; Leblanc, A.D. Effects of artificial gravity during bed rest on bone metabolism in humans. J. Appl. Physiol. (1985) 2009, 107, 47–53. [Google Scholar] [CrossRef]
- Zwart, S.R.; Hargens, A.R.; Lee, S.M.; Macias, B.R.; Watenpaugh, D.E.; Tse, K.; Smith, S.M. Lower body negative pressure treadmill exercise as a countermeasure for bed rest-induced bone loss in female identical twins. Bone 2007, 40, 529–537. [Google Scholar] [CrossRef]
- Morgan, J.L.; Zwart, S.R.; Heer, M.; Ploutz-Snyder, R.; Ericson, K.; Smith, S.M. Bone metabolism and nutritional status during 30-day head-down-tilt bed rest. J. Appl. Physiol. (1985) 2012, 113, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Scheld, K.; Zittermann, A.; Heer, M.; Herzog, B.; Mika, C.; Drummer, C.; Stehle, P. Nitrogen metabolism and bone metabolism markers in healthy adults during 16 weeks of bed rest. Clin. Chem. 2001, 47, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Halloran, B.P.; Bikle, D.D.; Wronski, T.J.; Globus, R.K.; Levens, M.J.; Morey-Holton, E. The role of 1,25-dihydroxyvitamin D in the inhibition of bone formation induced by skeletal unloading. Endocrinology 1986, 118, 948–954. [Google Scholar] [CrossRef]
- Austermann, K.; Baecker, N.; Zwart, S.R.; Fimmers, R.; Frippiat, J.P.; Stehle, P.; Smith, S.M.; Heer, M. Antioxidant Supplementation Does Not Affect Bone Turnover Markers During 60 Days of 6 Head-Down Tilt Bed Rest: Results from an Exploratory bedridden older patients. Age Ageing 2008, 37, 25–31. [Google Scholar] [CrossRef]
- Frings-Meuthen, P.; Bernhardt, G.; Buehlmeier, J.; Baecker, N.; May, F.; Heer, M. The negative effect of unloading exceeds the bone-sparing effect of alkaline supplementation: A bed rest study. Osteoporos. Int. 2019, 30, 431–439. [Google Scholar] [CrossRef]
- Björkman, M.; Sorva, A.; Risteli, J.; Tilvis, R. Vitamin D supplementation has minor effects on parathyroid hormone and bone turnover markers in vitamin D-deficient bedridden older patients. Age Ageing 2008, 37, 25–31. [Google Scholar] [CrossRef][Green Version]
- Linossier, M.T.; Amirova, L.E.; Thomas, M.; Normand, M.; Bareille, M.P.; Gauquelin-Koch, G.; Beck, A.; Costes-Salon, M.C.; Bonneau, C.; Gharib, C.; et al. Effects of short-term dry immersion on bone remodeling markers, insulin and adipokines. PLoS ONE 2017, 12, e0182970. [Google Scholar] [CrossRef]
- Sato, T.; Yamamoto, H.; Sawada, N.; Nashiki, K.; Tsuji, M.; Nikawa, T.; Arai, H.; Morita, K.; Taketani, Y.; Takeda, E. Immobilization decreases duodenal calcium absorption through a 1,25-dihydroxyvitamin D-dependent pathway. J. Bone Miner. Metab. 2006, 24, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Ortiz, A.; Avila, E.; Durand-Carbajal, M.; Díaz, L. Regulation of calcitriol biosynthesis and activity: Focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients 2015, 7, 443–480. [Google Scholar] [CrossRef]
- Zittermann, A.; Schlüter, A.; Hötzel, D. Effect of gravidity and lactation on calcium metabolism and urinary hydroxyproline excretion. A longitudinal study. In Nutritional Aspects of Osteoporosis ’94; Burckhardt, P., Heaney, R.P., Eds.; Challenges of Modern Medicine; Ares-Serono Symposia: Rome, Italy, 1995; Volume 7, pp. 87–96. [Google Scholar]
- Papapetrou, P.D. The interrelationship of serum 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D in pregnancy at term: A meta-analysis. Hormones 2010, 9, 136–144. [Google Scholar] [CrossRef]
- Ryan, B.A.; Kovacs, C.S. The role of vitamin D physiology in regulating calcium and bone metabolism in mother and child: Pregnancy, lactation, postweaning, fetus, and neonate. In Fieldman and Pike’s Vitamin D, 5th ed.; Hewison, M., Bouillon, R., Giovanucci, E., Goltzman, D., Meyer, M., Welsh, J., Eds.; Academic Press: Oxford, UK, 2024; Chapter 33; pp. 693–758. [Google Scholar]
- Pérez-López, F.R.; Pilz, S.; Chedraui, P. Vitamin D supplementation during pregnancy: An overview. Curr. Opin. Obstet. Gynecol. 2020, 32, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, K.; Kamaluddeen, M.; Hasan, S.U.; Al-Awad, E.; Finch, R.A.; Akierman, A.R. Ionized calcium levels in umbilical cord blood of women with preeclampsia and normotensive pregnancies. J. Matern. Fetal Neonatal Med. 2012, 25, 203–205. [Google Scholar] [CrossRef]
- Gebreyohannes, R.D.; Abdella, A.; Ayele, W.; Eke, A.C. Association of dietary calcium intake, total and ionized serum calcium levels with preeclampsia in Ethiopia. BMC Pregnancy Childbirth 2021, 21, 532. [Google Scholar] [CrossRef]
- Taufield, P.A.; Ales, K.L.; Resnick, L.M.; Druzin, M.L.; Gertner, J.M.; Laragh, J.H. Hypocalciuria in preeclampsia. N. Engl. J. Med. 1987, 316, 715–718. [Google Scholar] [CrossRef] [PubMed]
- August, P.; Marcaccio, B.; Gertner, J.M.; Druzin, M.L.; Resnick, L.M.; Laragh, J.H. Abnormal 1,25-dihydroxyvitamin D metabolism in preeclampsia. Am. J. Obstet. Gynecol. 1992, 166, 1295–1299. [Google Scholar] [CrossRef]
- Seely, E.W.; Wood, R.J.; Brown, E.M.; Graves, S.W. Lower serum ionized calcium and abnormal calciotropic hormone levels in preeclampsia. J. Clin. Endocrinol. Metab. 1992, 74, 1436–1440. [Google Scholar] [CrossRef]
- Halhali, A.; Tovar, A.R.; Torres, N.; Bourges, H.; Garabedian, M.; Larrea, F. Preeclampsia is associated with low circulating levels of insulin-like growth factor I and 1,25-dihydroxyvitamin D in maternal and umbilical cord compartments. J. Clin. Endocrinol. Metab. 2000, 85, 1828–1833. [Google Scholar] [CrossRef]
- Halhali, A.; Díaz, L.; Avila, E.; Ariza, A.C.; Garabédian, M.; Larrea, F. Decreased fractional urinary calcium excretion and serum 1,25-dihydroxyvitamin D and IGF-I levels in preeclampsia. J. Steroid Biochem. Mol. Biol. 2007, 103, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Dalmar, A.; Raff, H.; Chauhan, S.P.; Singh, M.; Siddiqui, D.S. Serum 25-hydroxyvitamin D, calcium, and calcium-regulating hormones in preeclamptics and controls during first day postpartum. Endocrine 2015, 48, 287–292. [Google Scholar] [CrossRef]
- Zhang, D.; Zeng, J.; Miao, X.; Liu, H.; Ge, L.; Huang, W.; Jiao, J.; Ye, D. Glucocorticoid exposure induces preeclampsia via dampening 1,25-dihydroxyvitamin D(3). Hypertens. Res. 2018, 41, 104–111. [Google Scholar] [CrossRef]
- Isakova, T.; Wahl, P.; Vargas, G.S. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2012, 79, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Gutierrez, O.; Shah, A. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J. Am. Soc. Nephrol. 2008, 19, 615–623. [Google Scholar] [CrossRef]
- Andress, D.L. Vitamin D in chronic kidney disease: A systemic role for selective vitamin D receptor activation. Kidney Int. 2006, 69, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Drüeke, T.B. Hyperparathyroidism in Chronic Kidney Disease. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2025. [Google Scholar] [PubMed]
- Pavik, I.; Jaeger, P.; Ebner, L.; Wagner, C.A.; Petzold, K.; Spichtig, D.; Poster, D.; Wüthrich, R.P.; Russmann, S.; Serra, A.L. Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: A sequence suggested from a cross-sectional study. Nephrol. Dial. Transplant. 2013, 28, 352–359. [Google Scholar] [CrossRef]
- Rhee, H.; Yang, J.Y.; Jung, W.J.; Shin, M.J.; Yang, B.Y.; Song, S.H.; Kwak, I.S.; Seong, E.Y. Significance of residual renal function for phosphate control in chronic hemodialysis patients. Kidney Res. Clin. Pract. 2014, 33, 58–64. [Google Scholar] [CrossRef]
- Petkovich, M.; Jones, G. CYP24A1 and kidney disease. Curr. Opin. Nephrol. Hypertens. 2011, 20, 337–344. [Google Scholar] [CrossRef]
- Bosworth, C.R.; Levin, G.; Robinson-Cohen, C.; Hoofnagle, A.N.; Ruzinski, J.; Young, B.; Schwartz, S.M.; Himmelfarb, J.; Kestenbaum, B.; de Boer, I.H. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int. 2012, 82, 693–700. [Google Scholar] [CrossRef]
- de Boer, I.H.; Sachs, M.C.; Chonchol, M.; Himmelfarb, J.; Hoofnagle, A.N.; Ix, J.H.; Kremsdorf, R.A.; Lin, Y.S.; Mehrotra, R.; Robinson-Cohen, C.; et al. Estimated GFR and circulating 24,25-dihydroxyvitamin D3 concentration: A participant-level analysis of 5 cohort studies and clinical trials. Am. J. Kidney Dis. 2014, 64, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, R.; Schuh, A.; Heine, G.H.; Brandenburg, V. FGF23 in Cardiovascular Disease: Innocent Bystander or Active Mediator? Front. Endocrinol. 2018, 9, 351. [Google Scholar] [CrossRef]
- Elidrissy, A.T.; Munawarah, M.; Alharbi, K.M. Hypocalcemic rachitic cardiomyopathy in infants. J. Saudi Heart Assoc. 2013, 25, 25–33. [Google Scholar] [CrossRef]
- Avery, P.G.; Arnold, I.R.; Hubner, P.J.; Iqbal, S.J. Cardiac failure secondary to hypocalcaemia of nutritional osteomalacia. Eur. Heart J. 1992, 13, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.S.C.; Polcwiartek, C.; Søgaard, P.; Mortensen, R.N.; Davidsen, L.; Aldahl, M.; Eriksen, M.A.; Kragholm, K.; Torp-Pedersen, C.; Hansen, S.M. The Association Between Serum Calcium Levels and Short-Term Mortality in Patients with Chronic Heart Failure. Am. J. Med. 2019, 132, 200–208.e1. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Gona, P.; Benjamin, E.J.; Wang, T.J.; Aragam, J.; D’Agostino, R.B.; Kannel, W.B.; Vasan, R.S. Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Eur. J. Heart Fail. 2010, 12, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Morshuis, M.; Kuhn, J.; Pilz, S.; Ernst, J.B.; Oezpeker, C.; Dreier, J.; Knabbe, C.; Gummert, J.F.; Milting, H. Vitamin D metabolites and fibroblast growth factor-23 in patients with left ventricular assist device implants: Association with stroke and mortality risk. Eur. J. Nutr. 2016, 55, 305–313. [Google Scholar] [CrossRef]
- Zittermann, A.; Schleithoff, S.S.; Götting, C.; Dronow, O.; Fuchs, U.; Kuhn, J.; Kleesiek, K.; Tenderich, G.; Koerfer, R. Poor outcome in end-stage heart failure patients with low circulating calcitriol levels. Eur. J. Heart Fail. 2008, 10, 321–327. [Google Scholar] [CrossRef]
- Zittermann, A.; Fuchs, U.; Kuhn, J.; Dreier, J.; Schulz, U.; Gummert, J.F.; Börgermann, J. Parameters of mineral metabolism predict midterm clinical outcome in end-stage heart failure patients. Scand. Cardiovasc. J. 2011, 45, 342–348. [Google Scholar] [CrossRef]
- Zittermann, A.; Schleithoff, S.S.; Götting, C.; Fuchs, U.; Kuhn, J.; Kleesiek, K.; Tenderich, G.; Koerfer, R. Calcitriol deficiency and 1-year mortality in cardiac transplant recipients. Transplantation 2009, 87, 118–124. [Google Scholar] [CrossRef]
- Zittermann, A.; Zelzer, S.; Herrmann, M.; Gummert, J.F.; Kleber, M.; Trummer, C.; Theiler-Schwetz, V.; Keppel, M.H.; Maerz, W.; Pilz, S. Determinants of circulating calcitriol in cardiovascular disease. J. Steroid Biochem. Mol. Biol. 2024, 241, 106528. [Google Scholar] [CrossRef]
- Glorieux, F.H.; St-Arnaud, R. Vitamin D hydroxylationedeficient rickets, type 1A. In Fieldman and Pike’s Vitamin D, 5th ed.; Hewison, M., Bouillon, R., Giovanucci, E., Goltzman, D., Meyer, M., Welsh, J., Eds.; Academic Press: Oxford, UK, 2024; Chapter 664; pp. 327–340. [Google Scholar]
- Cuervo, C.; Abitbol, C.L.; Zilleruelo, G.E.; Freundlich, M. Fibroblast growth factor-23 and renin-angiotensin system levels in vitamin-D-dependent rickets type I. Pediatr. Nephrol. 2016, 31, 1189–1193. [Google Scholar] [CrossRef]
- Roizen, J.D.; Levine, M.A. The role of genetic variation in CYP2R1, the principal vitamin D 25-hydroxylase, and CYP3A4 in vitamin D homeostasis. In Fieldman and Pike’s Vitamin D, 5th ed.; Hewison, M., Bouillon, R., Giovanucci, E., Goltzman, D., Meyer, M., Welsh, J., Eds.; Academic Press: Oxford, UK, 2024; Chapter 67; pp. 341–358. [Google Scholar]
- Malloy, P.J.; Tiosano, D.; Feldman, D. Hereditary 1,25-dihydroxyvitamin D resistant rickets (VDDR-2A). In Fieldman and Pike’s Vitamin D, 5th ed.; Hewison, M., Bouillon, R., Giovanucci, E., Goltzman, D., Meyer, M., Welsh, J., Eds.; Academic Press: Oxford, UK, 2024; Chapter 68; pp. 359–400. [Google Scholar]
- Cetani, F.; Cappellani, D.; Brancatella, A.; Jones, G.; Marcocci, C. Infantile hypercalcemia and CYP24A1 mutations. In Fieldman and Pike’s Vitamin D, 5th ed.; Hewison, M., Bouillon, R., Giovanucci, E., Goltzman, D., Meyer, M., Welsh, J., Eds.; Academic Press: Oxford, UK, 2024; Chapter 69; pp. 401–410. [Google Scholar]
- Marcocci, C.; Cetani, F. Clinical practice. Primary hyperparathyroidism. N. Engl. J. Med. 2011, 365, 2389–2397. [Google Scholar] [CrossRef]
- Bennin, D.; Hartery, S.A.; Kirby, B.J.; Maekawa, A.S.; St-Arnaud, R.; Kovacs, C.S. Loss of 24-hydroxylated catabolism increases calcitriol and fibroblast growth factor 23 and alters calcium and phosphate metabolism in fetal mice. JBMR Plus 2024, 8, ziae012. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Rafison, B.; Witzel, S.; Reyes, R.E.; Shieh, A.; Chun, R.; Zavala, K.; Hewison, M.; Liu, P.T. Regulation of the extrarenal CYP27B1-hydroxylase. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 22–27. [Google Scholar] [CrossRef]
- Heaney, R.P. Vitamin D in health and disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M.; Adams, J.S. Regulation of extra-renal synthesis of 1,25(OH)2D. In Fieldman and Pike’s Vitamin D, 5th ed.; Hewison, M., Bouillon, R., Giovanucci, E., Goltzman, D., Meyer, M., Welsh, J., Eds.; Academic Press: Oxford, UK, 2024; Chapter 9; pp. 155–189. [Google Scholar]
- Somjen, D.; Weisman, Y.; Kohen, F.; Gayer, B.; Limor, R.; Sharon, O.; Jaccard, N.; Knoll, E.; Stern, N. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation 2005, 111, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Chanakul, A.; Zhang, M.Y.; Louw, A.; Armbrecht, H.J.; Miller, W.L.; Portale, A.A.; Parwad, F. FGF23 regulates CYP27B1 transcription in the kidney and in extra-renal tissues. PLoS ONE 2013, 8, e72816. [Google Scholar] [CrossRef]
- Uday, S.; Kongjonaj, A.; Aguiar, M.; Tulchinsky, T.; Högler, W. Variations in infant and childhood vitamin D supplementation programmes across Europe and factors influencing adherence. Endocr. Connect. 2017, 6, 667–675. [Google Scholar] [CrossRef]
- Thacher, T.D.; Pludowski, P.; Shaw, N.J.; Mughal, M.Z.; Munns, C.F.; Högler, W. Nutritional rickets in immigrant and refugee children. Public Health Rev. 2016, 37, 3. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.; Gamble, G.D.; Reid, I.R. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: A trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014, 2, 307–320. [Google Scholar] [CrossRef]
- Uday, S.; Högler, W. Growth plate histology, bone histomorphometry, and radiologic features of nutritional rickets and osteomalacia. In Fieldman and Pike’s Vitamin D, 5th ed.; Hewison, M., Bouillon, R., Giovanucci, E., Goltzman, D., Meyer, M., Welsh, J., Eds.; Academic Press: Oxford, UK, 2024; Chapter 62; pp. 223–240. [Google Scholar]
- Frost, H.M. From Wolff’s law to the Utah paradigm: Insights about bone physiology and its clinical applications. Anat. Rec. 2001, 262, 398–419. [Google Scholar] [CrossRef]
- Avenell, A.; Mak, J.C.; O’Connell, D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst. Rev. 2014, 2014, CD000227. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Arlot, M.E.; Duboeuf, F.; Brun, J.; Crouzet, B.; Arnaud, S.; Delmas, P.D.; Meunier, P.J. Vitamin D3 and calcium to prevent hip fractures in elderly women. N. Engl. J. Med. 1992, 327, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, P.; Weisnagel, S.J.; Caron, A.Z.; Julien, A.S.; Morisset, A.S.; Carreau, A.M.; Poirier, J.; Tchernof, A.; Robitaille, J.; Bergeron, J.; et al. Effects of 6-month vitamin D supplementation on insulin sensitivity and secretion: A randomised, placebo-controlled trial. Eur. J. Endocrinol. 2019, 181, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Grande, J.P.; Roche, P.C.; Kumar, R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am. J. Physiol. 1994, 267, E356–E360. [Google Scholar] [CrossRef]
- Zeitz, U.; Weber, K.; Soegiarto, D.W.; Wolf, E.; Balling, R.; Erben, R.G. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003, 17, 509–511. [Google Scholar] [CrossRef]
- Barbarawi, M.; Zayed, Y.; Barbarawi, O.; Bala, A.; Alabdouh, A.; Gakhal, I.; Rizk, F.; Alkasasbeh, M.; Bachuwa, G.; Manson, J.E. Effect of Vitamin D Supplementation on the Incidence of Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2020, 105, dgaa335. [Google Scholar] [CrossRef]
- Jaiswal, V.; Joshi, A.; Jha, M.; Hanif, M.; Arora, A.; Gupta, S.; Shah, M.; Deb, N.; Peng Ang, S.; Aujla, S.; et al. Association between calcium supplementation and gestational hypertension, and preeclampsia: A Meta-analysis of 26 randomized controlled trials. Curr. Probl. Cardiol. 2024, 49, 102217. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, Q.; Liu, M.; Wei, Y. Effectiveness of calcium supplementation in the prevention of gestational hypertension: A systematic review and meta-analysis of randomised controlled trials. Pregnancy Hypertens. 2024, 38, 101174. [Google Scholar] [CrossRef]
- Chen, W.Y.; Sun, S.F. Clinical efficacy of low-dose aspirin combined with calcium in preventing preeclampsia: A systematic review and meta-analysis. Medicine 2023, 102, e34620. [Google Scholar] [CrossRef]
- Moghib, K.; Ghanm, T.I.; Abunamoos, A.; Rajabi, M.; Moawad, S.M.; Mohsen, A.; Kasem, S.; Elsayed, K.; Sayed, M.; Dawoud, A.I.; et al. Efficacy of vitamin D supplementation on the incidence of preeclampsia: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2024, 24, 852. [Google Scholar] [CrossRef]
- Pettifor, J.M. Recent advances in pediatric metabolic bone disease: The consequences of altered phosphate homeostasis in renal insufficiency and hypophosphatemic vitamin D-resistant rickets. Bone Miner. 1990, 9, 199–214. [Google Scholar] [CrossRef]
- Stremke, E.R.; Hill Gallant, K.M. Intestinal Phosphorus Absorption in Chronic Kidney Disease. Nutrients 2018, 10, 1364. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.A.; Pedraza, F.; Lenz, O.; Isakova, T. Phosphate control in end-stage renal disease: Barriers and opportunities. Nephrol. Dial. Transplant. 2013, 28, 2961–2968. [Google Scholar] [CrossRef]
- Halloran, B.P.; Schaefer, P.; Lifschitz, M.; Levens, M.; Goldsmith, R.S. Plasma vitamin D metabolite concentrations in chronic renal failure: Effect of oral administration of 25-hydroxyvitamin D3. J. Clin. Endocrinol. Metab. 1984, 59, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.; Aspray, T.J.; Schoenmakers, I. Vitamin D Supplementation for Patients with Chronic Kidney Disease: A Systematic Review and Meta-analyses of Trials Investigating the Response to Supplementation and an Overview of Guidelines. Calcif. Tissue Int. 2021, 109, 157–178. [Google Scholar] [CrossRef]
- Zittermann, A.; Ernst, J.B.; Pilz, S.; Dreier, J.; Kuhn, J.; Knabbe, C.; Gummert, J.F.; Morshuis, M.; Milting, H. Calciotropic and Phosphaturic Hormones in End-Stage Heart Failure Patients Supported by a Left-Ventricular Assist Device. PLoS ONE 2016, 11, e0164459. [Google Scholar] [CrossRef]
- Zhao, J.D.; Jia, J.J.; Dong, P.S.; Zhao, D.; Yang, X.M.; Li, D.L.; Zhang, H.F. Effect of vitamin D on ventricular remodelling in heart failure: A meta-analysis of randomised controlled trials. BMJ Open 2018, 8, e020545. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.; Fu, J.; Min, Z. Meta-analysis of vitamin D supplementation in the treatment of chronic heart failure. Scand. Cardiovasc. J. 2019, 53, 110–116. [Google Scholar] [CrossRef]
- Djoussé, L.; Cook, N.R.; Kim, E.; Bodar, V.; Walter, J.; Bubes, V.; Luttmann-Gibson, H.; Mora, S.; Joseph, J.; Lee, I.M.; et al. VITAL Research Group Supplementation with Vitamin D and Omega-3 Fatty Acids and Incidence of Heart Failure Hospitalization: VITAL-Heart Failure. Circulation 2020, 141, 784–786. [Google Scholar] [CrossRef]
- Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Pilz, S. Long-term supplementation with 3200 to 4000 IU of vitamin D daily and adverse events: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2023, 62, 1833–1844. [Google Scholar] [CrossRef]
- Zittermann, A.; Ernst, J.B.; Prokop, S.; Fuchs, U.; Dreier, J.; Kuhn, J.; Knabbe, C.; Börgermann, J.; Berthold, H.K.; Pilz, S.; et al. Effects of Vitamin D Supplementation on Renin and Aldosterone Concentrations in Patients with Advanced Heart Failure: The EVITA Trial. Int. J. Endocrinol. 2018, 2018, 5015417. [Google Scholar] [CrossRef]
- Yuan, S.; Baron, J.A.; Michaëlsson, K.; Larsson, S.C. Serum calcium and 25-hydroxyvitamin d in relation to longevity, cardiovascular disease and cancer: A mendelian randomization study. NPJ Genom. Med. 2021, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Camargo, C.A., Jr.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Kümmel, L.S.; Krumbein, H.; Fragkou, P.C.; Hünerbein, B.L.; Reiter, R.; Papathanasiou, K.A.; Thölken, C.; Weiss, S.T.; Renz, H.; Skevaki, C. Vitamin D supplementation for the treatment of COVID-19: A systematic review and meta-analysis of randomized controlled trials. Front. Immunol. 2022, 13, 1023903. [Google Scholar] [CrossRef]
- Leong, T.D.; Blose, N.; Mabetha, D.; Kredo, T. Comment on Hosseini et al. Effects of Vitamin D Supplementation on COVID-19 Related Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2134. Nutrients 2023, 15, 59. [Google Scholar] [CrossRef]
- Autier, P.; Doi, G.; Mullie, P.; Vankrunkelsven, P.; D’Ecclesiis, O.; Gandini, S. Vitamin D, acute respiratory infections, and COVID-19: The curse of small-size randomised trials. A critical review with meta-analysis of randomised trials. PLoS ONE 2025, 20, e0303316. [Google Scholar] [CrossRef]
- Piatek, K.; Kutner, A.; Cacsire Castillo-Tong, D.; Manhardt, T.; Kupper, N.; Nowak, U.; Chodyński, M.; Marcinkowska, E.; Kallay, E.; Schepelmann, M. Vitamin D Analogs Regulate the Vitamin D System and Cell Viability in Ovarian Cancer Cells. Int. J. Mol. Sci. 2021, 23, 172. [Google Scholar] [CrossRef]
- Tang, X. Therapeutic Applications of Vitamin D. Front. Biosci. (Landmark Ed.) 2025, 30, 37218. [Google Scholar] [CrossRef]
| 25(OH)D | 24,25(OH)2D | Calcitriol | Serum Calcium | Serum Phosphate | PTH | FGF23 | |
|---|---|---|---|---|---|---|---|
| Nutritional rickets/osteomalacia | ↓↓↓ | ↓↓↓ | varies | N, ↓ | N, ↓ | ↑↑↑ | N, ↓ |
| Vitamin D insufficiency | ↓ | ↓ | varies | N | N | N, ↑ | N |
| Calcium supplement | N | N | ↓ | N | N | N, ↓ | N |
| Dietary phosphate load | N | N | ↓ | N | N | N, ↑ | N, ↑ |
| Hypokinesia | ↓ | ↓ | ↓ to ↓↓ | N | N | N | N |
| Bedrest | ↓ to ↓↓ | ? | ↓↓ | N | N | N, ↓ | N |
| Physical activity | N to ↑ | N to ↑ | ↑ | N | N | N | N |
| Pregnancy | ↓ | ↓ | ↑↑ | ↓ | N | N, ↓ | ↑ |
| Chronic kidney disease stages 1–3 | ↓ | ↓ | ↓ | N | N | ↑ | ↑ |
| Chronic kidney disease stages 4–5 | ↓ to ↓↓ | ↓ to ↓↓ | ↓↓ | N, ↓ | ↑ | ↑↑ | ↑↑ |
| End-stage renal disease/hemodialysis | ↓ to ↓↓ | ↓ to ↓↓ | ↓↓↓ | N, ↓ | ↑↑ | ↑↑↑ | ↑↑↑ |
| End-stage heart failure | ↓ to ↓↓ | ? | ↓↓ to ↓↓↓ | N, ↓ | ↑ | ↑↑↑ | ↑↑↑ |
| Vitamin D-dependent rickets type 1A | ↓ to ↓↓ | N | ↓↓ | ↓ to ↓↓ | N, ↓ | ↑↑↑ | N, ↓ |
| Vitamin D-dependent rickets type 1B | N | ↓ | varies | ↓ to ↓↓ | N, ↓ | ↑↑↑ | N |
| Vitamin D-dependent rickets type 2A | ↓↓ | ? | ↑↑ | ↓ to ↓↓ | N, ↓ | ↑↑↑ | N, ↓ |
| Vitamin D-dependent rickets type 2B | N | ? | ↑↑ | ↓ to ↓↓ | N, ↓ | ↑↑↑ | N |
| Vitamin D-dependent rickets type 3 | N | N? | ↓ to ↓↓↓ | ↓ to ↓↓ | ↓ | ↑↑↑ | ? |
| CYP24A1 mutations | ↓↓ | ↓ to ↓↓↓ | ↑ to ↑↑↑ | ↑ to ↑↑ | N, ↓ | N | ↑↑ |
| Granulomatous diseases | N | ? | ↑ to ↑↑↑ | ↑ to ↑↑ | ↑ | ↓ | ? |
| CYP2R1 Activity | CYP27B1 Activity |
|---|---|
| - | Kidney |
| Thyroid gland | Thyroid gland |
| Pancreas | Pancreas |
| Bone marrow | Bone marrow |
| Prostate | Appendix |
| Retina | Retina |
| Pituitary gland | Adrenal gland |
| Thymus | Thymus |
| Lymph nodes | Lymph nodes |
| Liver | Liver |
| Skin | Skin |
| Testes | Testes |
| Fat tissue | Fat tissue |
| Placenta, vagina, uterus | Cardiomyocytes, vascular smooth muscle cells, and vascular endothelial cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zittermann, A. Regulation of Renal and Extrarenal Calcitriol Synthesis and Its Clinical Implications. Int. J. Mol. Sci. 2025, 26, 5570. https://doi.org/10.3390/ijms26125570
Zittermann A. Regulation of Renal and Extrarenal Calcitriol Synthesis and Its Clinical Implications. International Journal of Molecular Sciences. 2025; 26(12):5570. https://doi.org/10.3390/ijms26125570
Chicago/Turabian StyleZittermann, Armin. 2025. "Regulation of Renal and Extrarenal Calcitriol Synthesis and Its Clinical Implications" International Journal of Molecular Sciences 26, no. 12: 5570. https://doi.org/10.3390/ijms26125570
APA StyleZittermann, A. (2025). Regulation of Renal and Extrarenal Calcitriol Synthesis and Its Clinical Implications. International Journal of Molecular Sciences, 26(12), 5570. https://doi.org/10.3390/ijms26125570







