SARS-CoV-2 ORF7a Protein Impedes Type I Interferon-Activated JAK/STAT Signaling by Interacting with HNRNPA2B1
Abstract
1. Introduction
2. Results
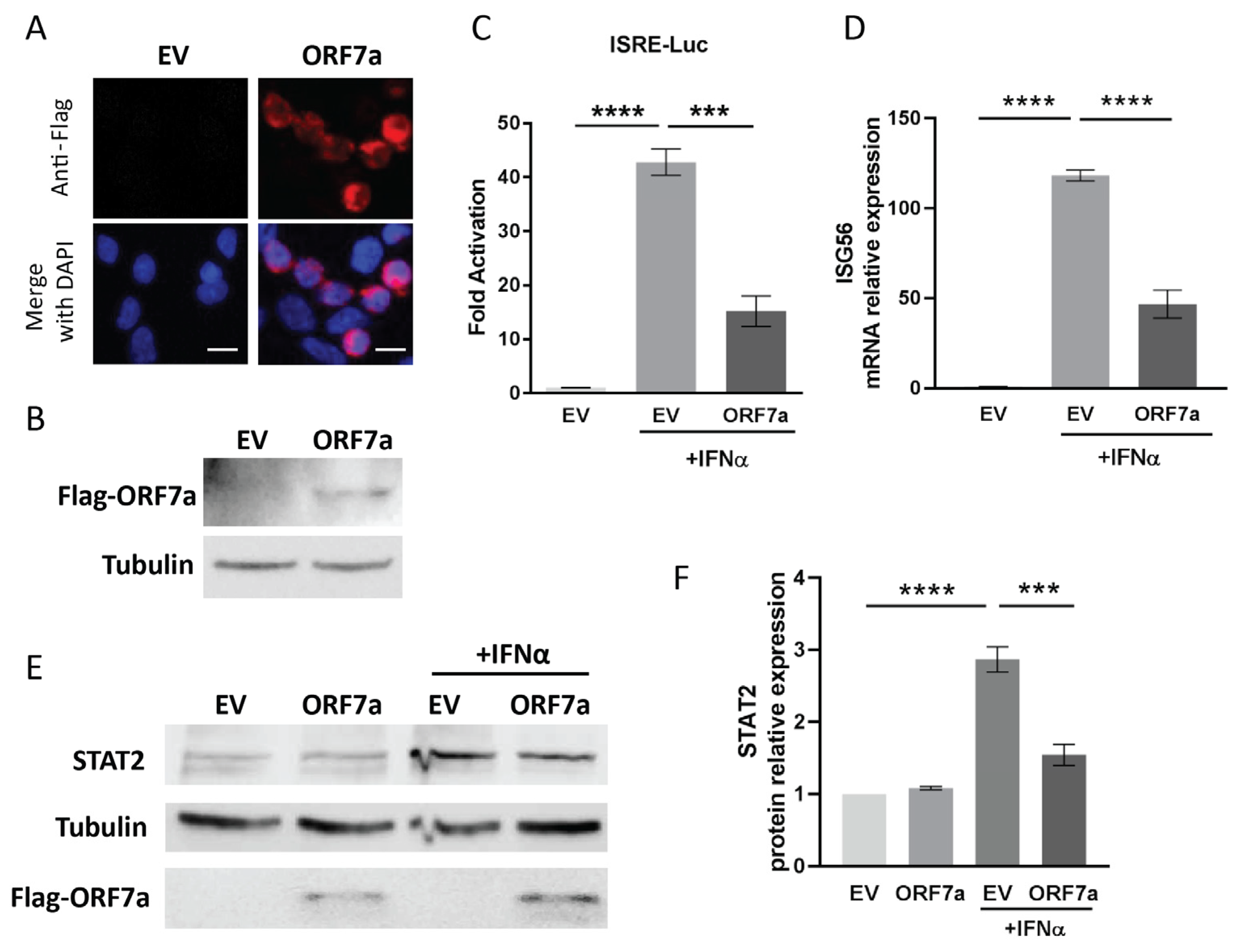
2.1. ORF7a of SARS-CoV-2 Suppresses IFNα-Activated JAK/STAT Signaling
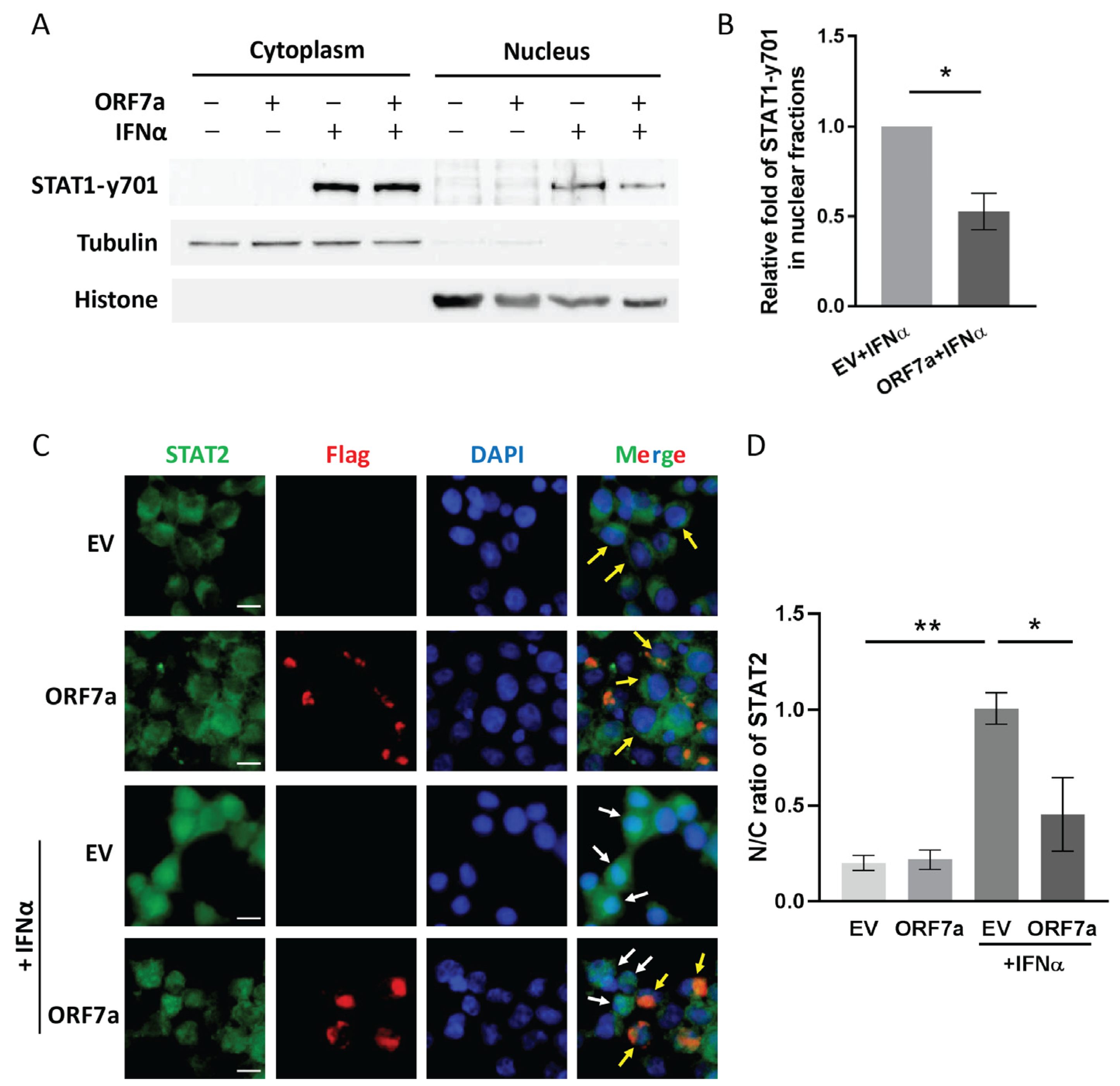
2.2. ORF7a Suppresses IFNα-Induced JAK/STAT Signaling Activation via Blocking Nuclear Translocation of ISGF3
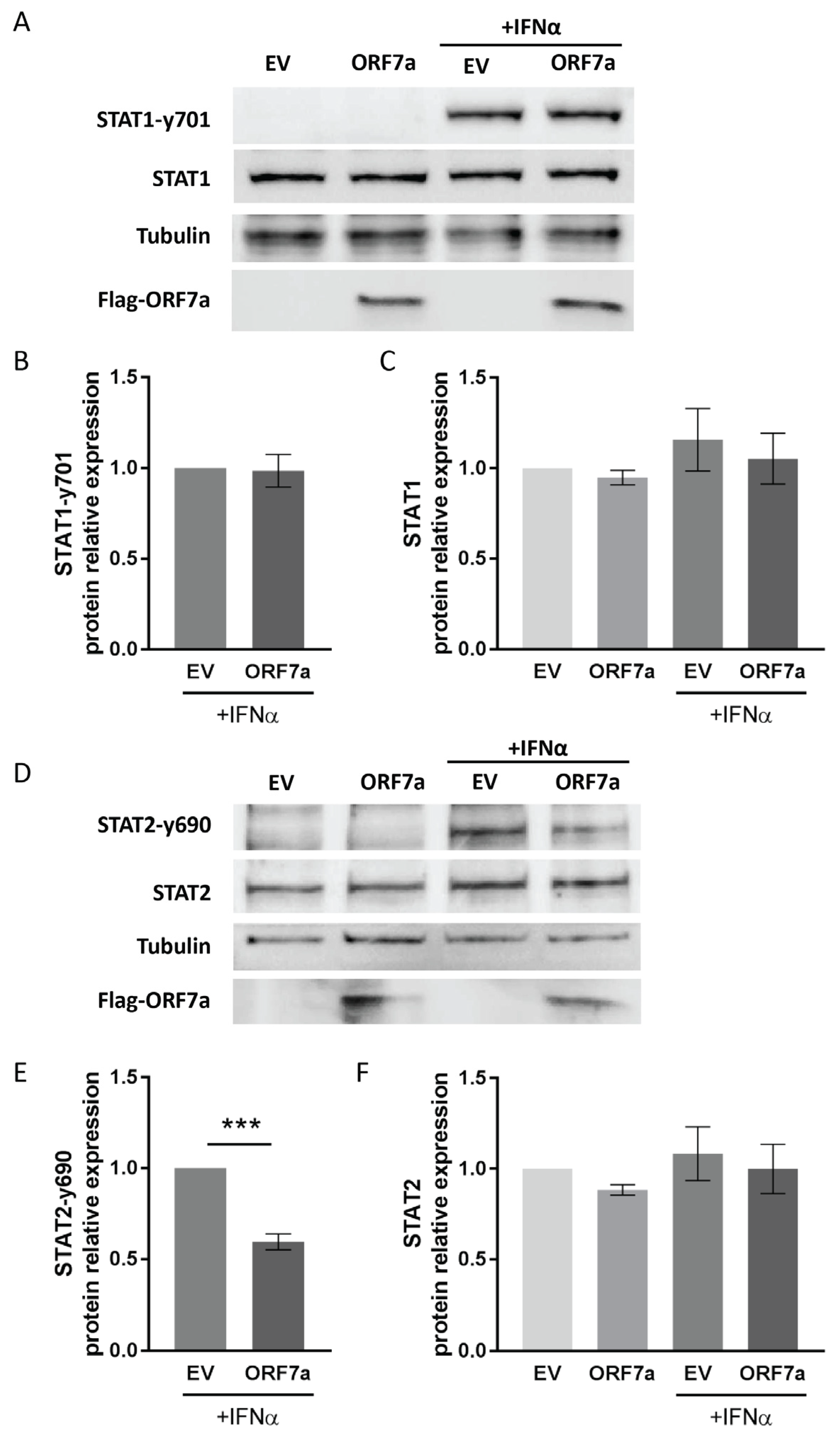
2.3. ORF7a Impairs IFNα-Induced Phosphorylation of STAT2 Instead of STAT1
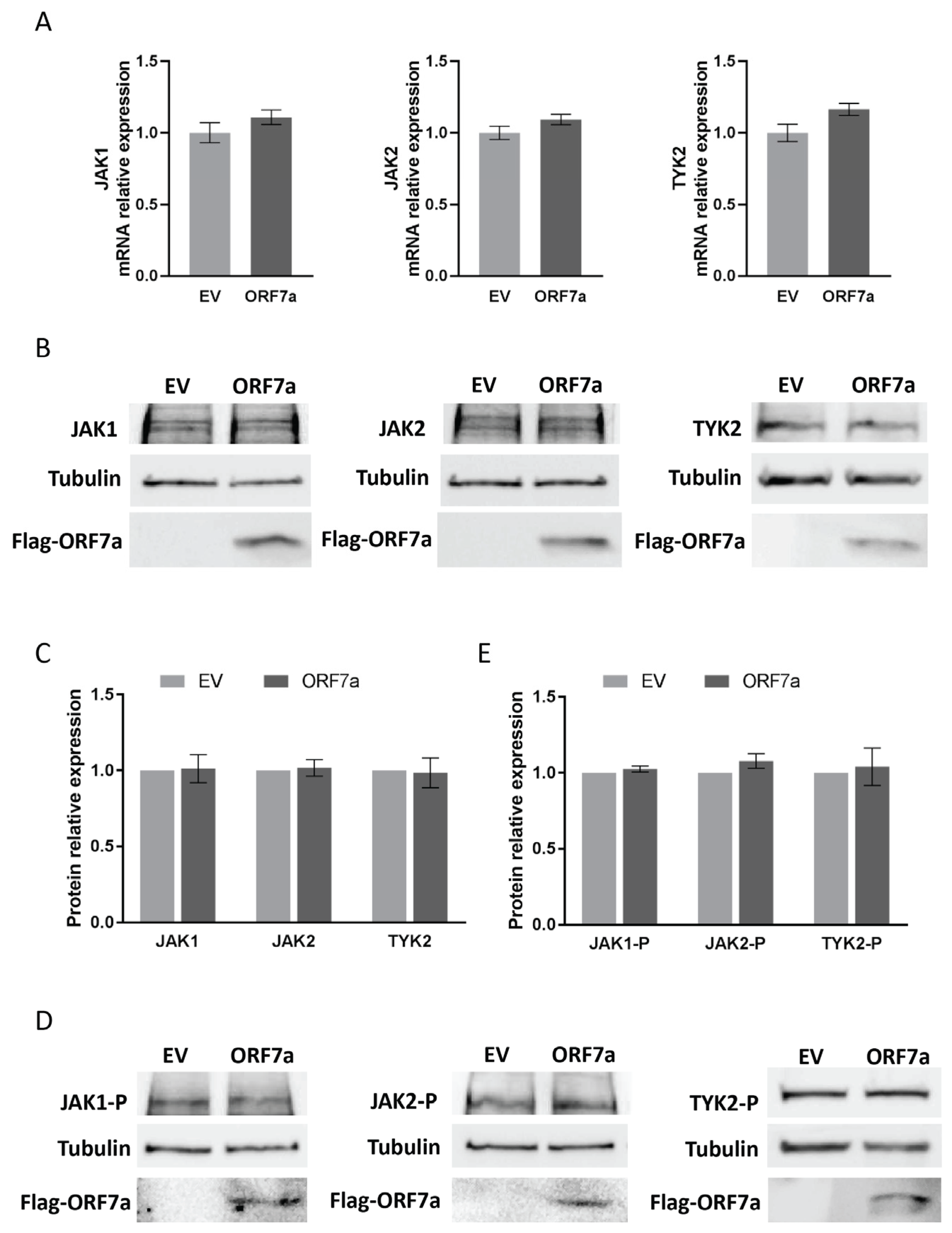
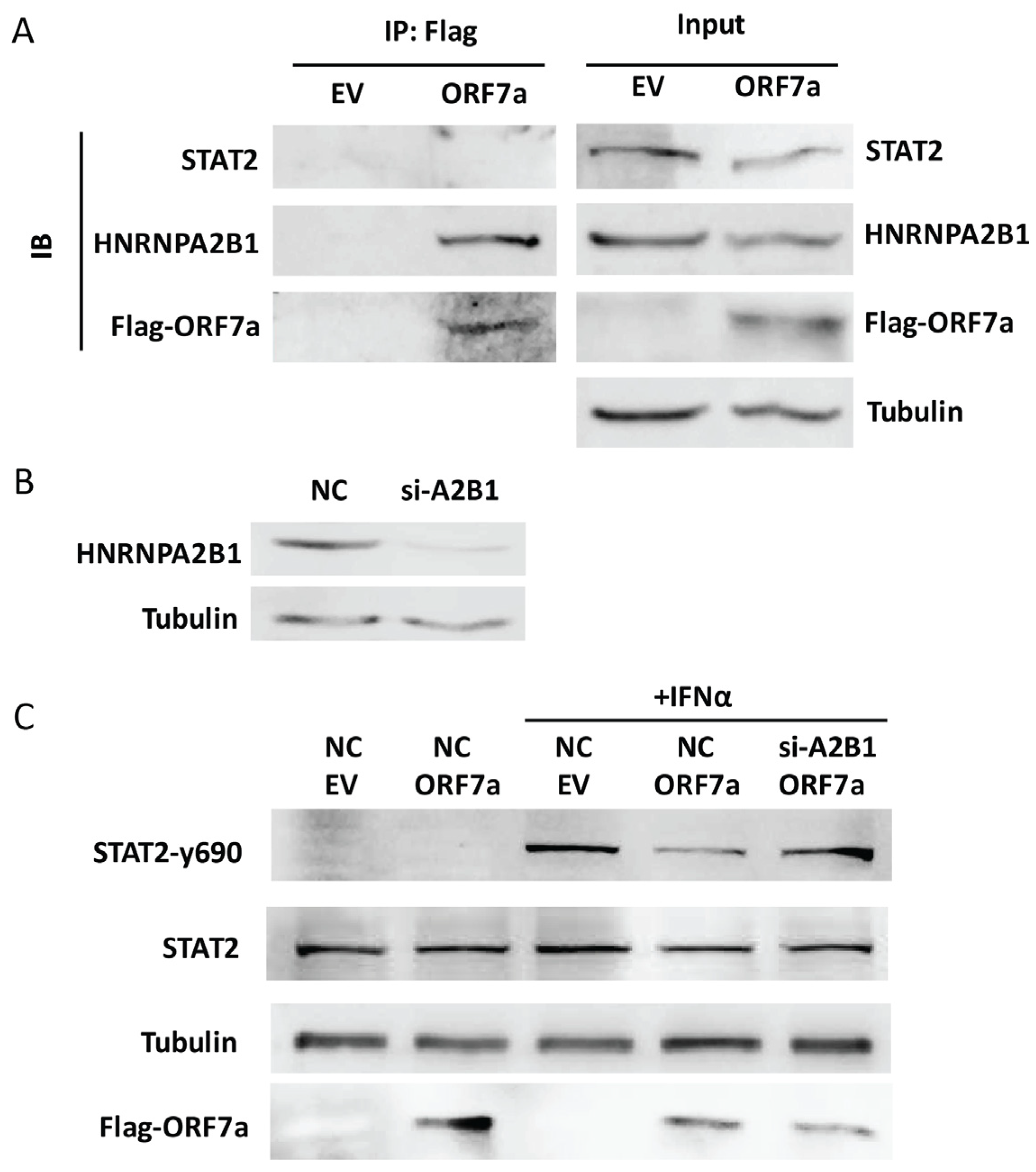
2.4. HNRNPA2B1 Interacts with ORF7a and Is Related to the Inhibitory Effect of ORF7a on STAT2 Phosphorylation
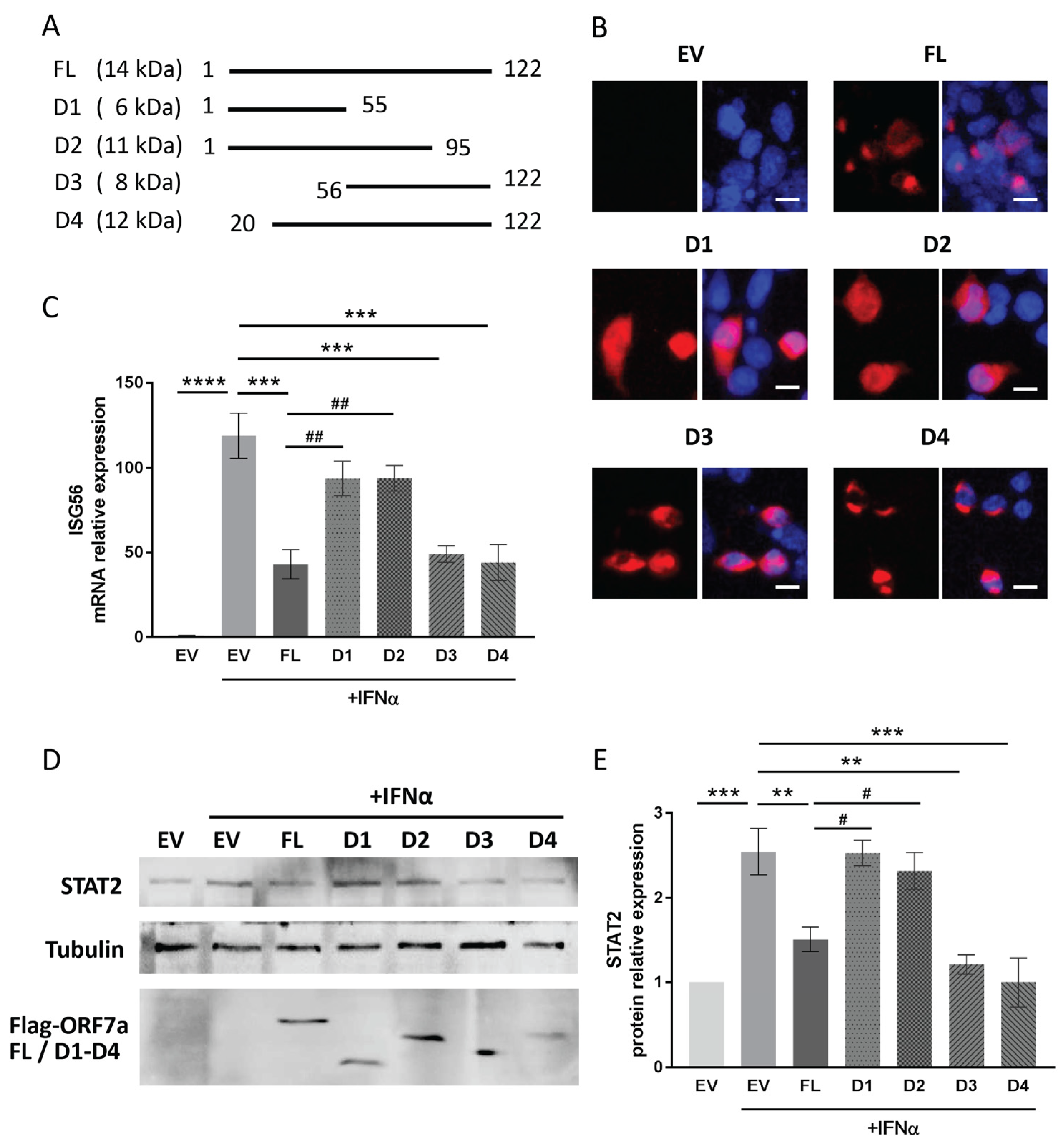
2.5. The C-Terminal Domain of ORF7a (Aa 96–122) Appears to Be Responsible for the IFN Signaling Inhibition
3. Discussion
4. Materials and Methods
4.1. Cells, Plasmids, siRNA and Reagents
4.2. Immunofluorescence Assay
4.3. Luciferase Reporter Assay
4.4. RNA Isolation, Reverse Transcription and Quantitative PCR (RT-qPCR)
4.5. Immunoblotting
4.6. Subcellular Fractionation
4.7. Co-Immunoprecipitation (Co-IP)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Rao, Z. Structural Biology of SARS-CoV-2 and Implications for Therapeutic Development. Nat. Rev. Microbiol. 2021, 19, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Debmalya Barh, K.L. COVID-19 from Bench to Bedside, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 277–281. [Google Scholar]
- González-Navajas, J.M.; Lee, J.; David, M.; Raz, E. Immunomodulatory Functions of Type I Interferons. Nat. Rev. Immunol. 2012, 12, 125–135. [Google Scholar] [CrossRef]
- Low, Z.Y.; Zabidi, N.Z.; Yip, A.J.W.; Puniyamurti, A.; Chow, V.T.K.; Lal, S.K. SARS-CoV-2 Non-Structural Proteins and Their Roles in Host Immune Evasion. Viruses 2022, 14, 1991. [Google Scholar] [CrossRef]
- Minkoff, J.M.; tenOever, B. Innate Immune Evasion Strategies of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 178–194. [Google Scholar] [CrossRef]
- Redondo, N.; Zaldívar-López, S.; Garrido, J.J.; Montoya, M. SARS-CoV-2 Accessory Proteins in Viral Pathogenesis: Knowns and Unknowns. Front. Immunol. 2021, 12, 708264. [Google Scholar] [CrossRef]
- García-García, T.; Fernández-Rodríguez, R.; Redondo, N.; de Lucas-Rius, A.; Zaldívar-López, S.; López-Ayllón, B.D.; Suárez-Cárdenas, J.M.; Jiménez-Marín, A.; Montoya, M.; Garrido, J.J. Impairment of Antiviral Immune Response and Disruption of Cellular Functions by SARS-CoV-2 ORF7a and ORF7b. IScience 2022, 25, 105444. [Google Scholar] [CrossRef]
- Kumar, A.; Ishida, R.; Strilets, T.; Cole, J.; Lopez-Orozco, J.; Fayad, N.; Felix-Lopez, A.; Elaish, M.; Evseev, D.; Magor, K.E.; et al. SARS-CoV-2 Nonstructural Protein 1 Inhibits the Interferon Response by Causing Depletion of Key Host Signaling Factors. J. Virol. 2021, 95, e0026621. [Google Scholar] [CrossRef]
- Lei, X.B.; Dong, X.J.; Ma, R.Y.; Wang, W.J.; Xiao, X.; Tian, Z.Q.; Wang, C.H.; Wang, Y.; Li, L.; Ren, L.L.; et al. Activation and Evasion of Type I Interferon Responses by SARS-CoV-2. Nat. Commun. 2020, 11, 3810. [Google Scholar] [CrossRef]
- Li, J.Y.; Liao, C.H.; Wang, Q.; Tan, Y.J.; Luo, R.; Qiu, Y.; Ge, X.Y. The ORF6, ORF8 and Nucleocapsid Proteins of SARS-CoV-2 Inhibit Type I Interferon Signaling Pathway. Virus Res. 2020, 286, 198074. [Google Scholar] [CrossRef]
- Xia, H.J.; Cao, Z.G.; Xie, X.P.; Zhang, X.W.; Chen, J.Y.C.; Wang, H.L.; Menachery, V.D.; Rajsbaum, R.; Shi, P.Y. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 2020, 33, 108234. [Google Scholar] [CrossRef]
- Mann, M.M.; Hsieh, M.K.; Tang, J.D.; Hart, W.S.; Lazzara, M.J.; Klauda, J.B.; Berger, B.W. Understanding How Transmembrane Domains Regulate Interactions between Human BST-2 and the SARS-CoV-2 Accessory Protein ORF7a. Biochim. Et Biophys. Acta Biomembr. 2023, 1865, 184174. [Google Scholar] [CrossRef] [PubMed]
- Hagelauer, E.; Lotke, R.; Kmiec, D.; Hu, D.; Hohner, M.; Stopper, S.; Nchioua, R.; Kirchhoff, F.; Sauter, D.; Schindler, M. Tetherin Restricts SARS-CoV-2 Despite the Presence of Multiple Viral Antagonists. Viruses 2023, 15, 2364. [Google Scholar] [CrossRef] [PubMed]
- Arshad, N.; Laurent-Rolle, M.; Ahmed, W.S.; Hsu, J.C.; Mitchell, S.M.; Pawlak, J.; Sengupta, D.; Biswas, K.H.; Cresswell, P. SARS-CoV-2 Accessory Proteins ORF7a and ORF3a Use Distinct Mechanisms to Down-regulate MHC-I Surface Expression. Proc. Natl. Acad. Sci. USA 2023, 120, e2208525120. [Google Scholar] [CrossRef]
- Hou, P.; Wang, X.; Wang, H.; Wang, T.; Yu, Z.; Xu, C.; Zhao, Y.; Wang, W.; Zhao, Y.; Chu, F.; et al. The ORF7a Protein of SARS-CoV-2 Initiates Autophagy and Limits Autophagosome-lysosome Fusion via Degradation of SNAP29 to Promote Virus Replication. Autophagy 2023, 19, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fu, Y.; Huang, Y.; Zeng, F.; Rao, J.; Xiao, X.; Sun, X.; Jin, H.; Li, J.; Yang, J.; et al. Ubiquitination of SARS-CoV-2 ORF7a Prevents Cell Death Induced by Recruiting BclXL to Activate ER Stress. Microbiol. Spectr. 2022, 10, e0150922. [Google Scholar] [CrossRef]
- Nishitsuji, H.; Iwahori, S.; Ohmori, M.; Shimotohno, K.; Murata, T. Ubiquitination of SARS-CoV-2 NSP6 and ORF7a Facilitates NF-κB Activation. MBio 2022, 13, e0097122. [Google Scholar] [CrossRef]
- Timilsina, U.; Umthong, S.; Ivey, E.B.; Waxman, B.; Stavrou, S. SARS-CoV-2 ORF7a Potently Inhibits the Antiviral Effect of The Host Factor SERINC5. Nat. Commun. 2022, 13, 2935. [Google Scholar] [CrossRef]
- Cao, Z.G.; Xia, H.J.; Rajsbaum, R.; Xia, X.Z.; Wang, H.L.; Shi, P.Y. Ubiquitination of SARS-CoV-2 ORF7a Promotes Antagonism of Interferon Response. Cell. Mol. Immunol. 2021, 18, 746–748. [Google Scholar] [CrossRef]
- Miorin, L.; Kehrer, T.; Sanchez-Aparicio, M.T.; Zhang, K.; Cohen, P.; Patel, R.S.; Cupic, A.; Makio, T.; Mei, M.H.; Moreno, E.; et al. SARS-CoV-2 Orf6 Hijacks Nup98 to Block STAT Nuclear Import and Antagonize Interferon Signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 28344–28354. [Google Scholar] [CrossRef]
- Wang, R.; Yang, X.; Chang, M.; Xue, Z.; Wang, W.; Bai, L.; Zhao, S.; Liu, E. ORF3a Protein of Severe Acute Respiratory Syndrome Coronavirus 2 Inhibits Interferon-Activated Janus Kinase/Signal Transducer and Activator of Transcription Signaling via Elevating Suppressor of Cytokine Signaling 1. Front. Microbiol. 2021, 12, 752597. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, S.L. The Roles of hnRNP A2/B1 in RNA Biology and Disease. Wiley Interdiscip. Rev. RNA 2021, 12, e1612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Flavell, R.A.; Li, H.B. hnRNPA2B1: A Nuclear DNA Sensor in Antiviral Immunity. Cell Res. 2019, 29, 879–880. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.H.; Wan, Q.Y.; Chen, S.; Chen, Y.; Wang, P.H.; Yao, X.; He, M.L. Attenuating Innate Immunity and Facilitating β-coronavirus Infection by NSP1 of SARS-CoV-2 through Specific Redistributing hnRNP A2/B1 Cellular Localization. Signal Transduct. Target. Ther. 2021, 6, 371. [Google Scholar] [CrossRef] [PubMed]
- Hayn, M.; Hirschenberger, M.; Koepke, L.; Nchioua, R.; Straub, J.H.; Klute, S.; Hunszinger, V.; Zech, F.; Bozzo, C.P.; Aftab, W.; et al. Systematic Functional Analysis of SARS-CoV-2 Proteins Uncovers Viral Innate Immune Antagonists and Remaining Vulnerabilities. Cell Rep. 2021, 35, 109126. [Google Scholar] [CrossRef]
- Hsu, J.C.C.; Laurent-Rolle, M.; Pawlak, J.B.; Wilen, C.B.; Cresswell, P. Translational Shutdown and Evasion of The Innate Immune Response by SARS-CoV-2 NSP14 Protein. Proc. Natl. Acad. Sci. USA 2021, 118, e2101161118. [Google Scholar] [CrossRef]
- Oh, S.J.; Shin, O.S. SARS-CoV-2 Nucleocapsid Protein Targets RIG-I-Like Receptor Pathways to Inhibit the Induction of Interferon Response. Cells 2021, 10, 530. [Google Scholar] [CrossRef]
- Wu, Y.X.; Ma, L.; Zhuang, Z.; Cai, S.H.; Zhao, Z.Y.; Zhou, L.L.; Zhang, J.; Wang, P.H.; Zhao, J.C.; Cui, J. Main protease of SARS-CoV-2 serves as a bifunctional molecule in restricting type I interferon antiviral signaling. Signal Transduct. Target. Ther. 2020, 5, 221. [Google Scholar] [CrossRef]
- Gordon, H.; Ajamian, L.; Valiente-Echeverrìa, F.; Lévesque, K.; Rigby, W.F.; Mouland, A.J. Depletion of hnRNP A2/B1 Overrides the Nuclear Retention of the HIV-1 Genomic RNA. RNA Biol. 2013, 10, 1714–1725. [Google Scholar] [CrossRef]
- Wang, L.; Wen, M.Y.; Cao, X.T. Nuclear hnRNPA2B1 initiates and amplifies the innate immune response to DNA viruses. Science 2019, 365, 656. [Google Scholar] [CrossRef]
- Zuo, D.; Chen, Y.; Cai, J.P.; Yuan, H.Y.; Wu, J.Q.; Yin, Y.; Xie, J.W.; Lin, J.M.; Luo, J.; Feng, Y.; et al. A hnRNPA2B1 Agonist Effectively Inhibits HBV and SARS-CoV-2 Omicron in Vivo. Protein Cell 2023, 14, 37–50. [Google Scholar]
- Foster, C.S.P.; Bull, R.A.; Tedla, N.; Santiago, F.; Agapiou, D.; Adhikari, A.; Walker, G.J.; Shrestha, L.B.; Van Hal, S.J.; Kim, K.W.; et al. Persistence of a Frameshifting Deletion in SARS-CoV-2 ORF7a for the Duration of a Major Outbreak. Viruses 2023, 15, 522. [Google Scholar] [CrossRef] [PubMed]
- Holland, L.A.; Kaelin, E.A.; Maqsood, R.; Estifanos, B.; Wu, L.I.; Varsani, A.; Halden, R.U.; Hogue, B.G.; Scotch, M.; Lima, E.S. An 81-Nucleotide Deletion in SARS-CoV-2 ORF7a Identified from Sentinel Surveillance in Arizona (January to March 2020). J. Virol. 2020, 94, e00711–e00720. [Google Scholar] [CrossRef]
- Lucas, S.; Jones, M.S.; Kothari, S.; Madlambayan, A.; Ngo, C.; Chan, C.V.; Goraichuk, I.V. A 336-nucleotide In-frame Deletion in ORF7a Gene of SARS-CoV-2 Identified In Genomic Surveillance by Next-generation Sequencing. J. Clin. Virol. 2022, 148, 105105. [Google Scholar] [CrossRef]
- Nemudryi, A.; Nemudraia, A.; Wiegand, T.; Nichols, J.; Snyder, D.T.; Hedges, J.F.; Cicha, C.; Lee, H.; Vanderwood, K.K.; Bimczok, D.; et al. SARS-CoV-2 Genomic Surveillance Identifies Naturally Occurring Truncation of ORF7a That Limits Immune Suppression. Cell Rep. 2021, 35, 109197. [Google Scholar] [CrossRef]
- Panzera, Y.; Ramos, N.; Frabasile, S.; Calleros, L.; Marandino, A.; Tomás, G.; Techera, C.; Grecco, S.; Fuques, E.; Goñi, N.; et al. A Deletion in SARS-CoV-2 ORF7 Identified in COVID-19 outbreak in Uruguay. Transbound. Emerg. Dis. 2021, 68, 3075–3082. [Google Scholar] [CrossRef]
- Rahman, M.; Rahim, R.; Hasan, A.; Kawai, N.; Moonga, L.C.; Sugi, T.; Hayashida, K.; Yamagishi, J. SARS-CoV-2 Genome Sequences, Including One with an ORF7a Deletion, Obtained from Patients in Bangladesh. Microbiol. Resour. Ann. 2021, 10, e0076421. [Google Scholar] [CrossRef]
- Tse, H.; Wong, S.C.Y.; Lp, K.F.; Cheng, V.C.C.; To, K.K.W.; Lung, D.C.; Choi, G.K.Y. Genome Sequences of Three SARS-CoV-2 ORF7a Deletion Variants Obtained from Patients in Hong Kong. Microbiol. Resour. Ann. 2021, 10, e00251-21. [Google Scholar] [CrossRef]
- Addetia, A.; Xie, H.; Roychoudhury, P.; Shrestha, L.; Loprieno, M.; Huang, M.L.; Jerome, K.R.; Greninger, A.L. Identification of Multiple Large Deletions in ORF7a Resulting in In-frame Gene Fusions in Clinical SARS-CoV-2 Isolates. J. Clin. Virol. 2020, 129, 104523. [Google Scholar] [CrossRef]
- Simas, M.C.D.; Costa, S.M.; Gomes, P.D.F.C.; da Cruz, N.V.G.; Correa, I.A.; de Souza, M.R.M.; Dornelas-Ribeiro, M.; Nogueira, T.L.S.; dos Santos, C.G.M.; Hoffmann, L.; et al. Evaluation of SARS-CoV-2 ORF7a Deletions from COVID-19-Positive Individuals and Its Impact on Virus Spread in Cell Culture. Viruses 2023, 15, 801. [Google Scholar] [CrossRef]
- Pyke, A.T.; Nair, N.; van den Hurk, A.F.; Burtonclay, P.; Nguyen, S.; Barcelon, J.; Kistler, C.; Schlebusch, S.; McMahon, J.; Moore, F. Replication Kinetics of B.1.351 and B.1.1.7 SARS-CoV-2 Variants of Concern Including Assessment of a B.1.1.7 Mutant Carrying a Defective ORF7a Gene. Viruses 2021, 13, 1087. [Google Scholar] [CrossRef]
- Wang, R.; Nan, Y.C.; Yu, Y.; Zhang, Y.J. Porcine Reproductive and Respiratory Syndrome Virus Nsp1β Inhibits Interferon-Activated JAK/STAT Signal Transduction by Inducing Karyopherin-α1 Degradation. J. Virol. 2013, 87, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Nan, Y.C.; Yu, Y.; Yang, Z.Q.; Zhang, Y.J. Variable Interference with Interferon Signal Transduction by Different Strains of Porcine Reproductive and Respiratory Syndrome Virus. Vet. Microbiol. 2013, 166, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Protein Name | Protein Score | Coverage (%) | Peptides | Unique Peptides |
|---|---|---|---|---|
| MYH10 | 785 | 12 | 21 | 17 |
| HSP90AB1 | 574 | 26 | 16 | 7 |
| HSPA8 | 427 | 17 | 8 | 6 |
| MYL6 | 423 | 40 | 6 | 6 |
| HNRNPA2B1 | 342 | 22 | 6 | 6 |
| VCP | 328 | 10 | 7 | 7 |
| RPS3 | 254 | 21 | 5 | 5 |
| CANX | 197 | 11 | 7 | 7 |
| HSP90B1 | 197 | 8 | 6 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Li, C.; Tang, T.; Luo, C.; Lu, S.; Lyu, N.; Li, Y.; Wang, R. SARS-CoV-2 ORF7a Protein Impedes Type I Interferon-Activated JAK/STAT Signaling by Interacting with HNRNPA2B1. Int. J. Mol. Sci. 2025, 26, 5536. https://doi.org/10.3390/ijms26125536
Wen Y, Li C, Tang T, Luo C, Lu S, Lyu N, Li Y, Wang R. SARS-CoV-2 ORF7a Protein Impedes Type I Interferon-Activated JAK/STAT Signaling by Interacting with HNRNPA2B1. International Journal of Molecular Sciences. 2025; 26(12):5536. https://doi.org/10.3390/ijms26125536
Chicago/Turabian StyleWen, Yujie, Chaochao Li, Tian Tang, Chao Luo, Shan Lu, Na Lyu, Yongxi Li, and Rong Wang. 2025. "SARS-CoV-2 ORF7a Protein Impedes Type I Interferon-Activated JAK/STAT Signaling by Interacting with HNRNPA2B1" International Journal of Molecular Sciences 26, no. 12: 5536. https://doi.org/10.3390/ijms26125536
APA StyleWen, Y., Li, C., Tang, T., Luo, C., Lu, S., Lyu, N., Li, Y., & Wang, R. (2025). SARS-CoV-2 ORF7a Protein Impedes Type I Interferon-Activated JAK/STAT Signaling by Interacting with HNRNPA2B1. International Journal of Molecular Sciences, 26(12), 5536. https://doi.org/10.3390/ijms26125536




