Unveiling GABA and Serotonin Interactions During Neurodevelopment to Re-Open Adult Critical Periods for Neuropsychiatric Disorders
Abstract
1. Introduction
1.1. The GABAergic System
1.2. The Serotonergic System

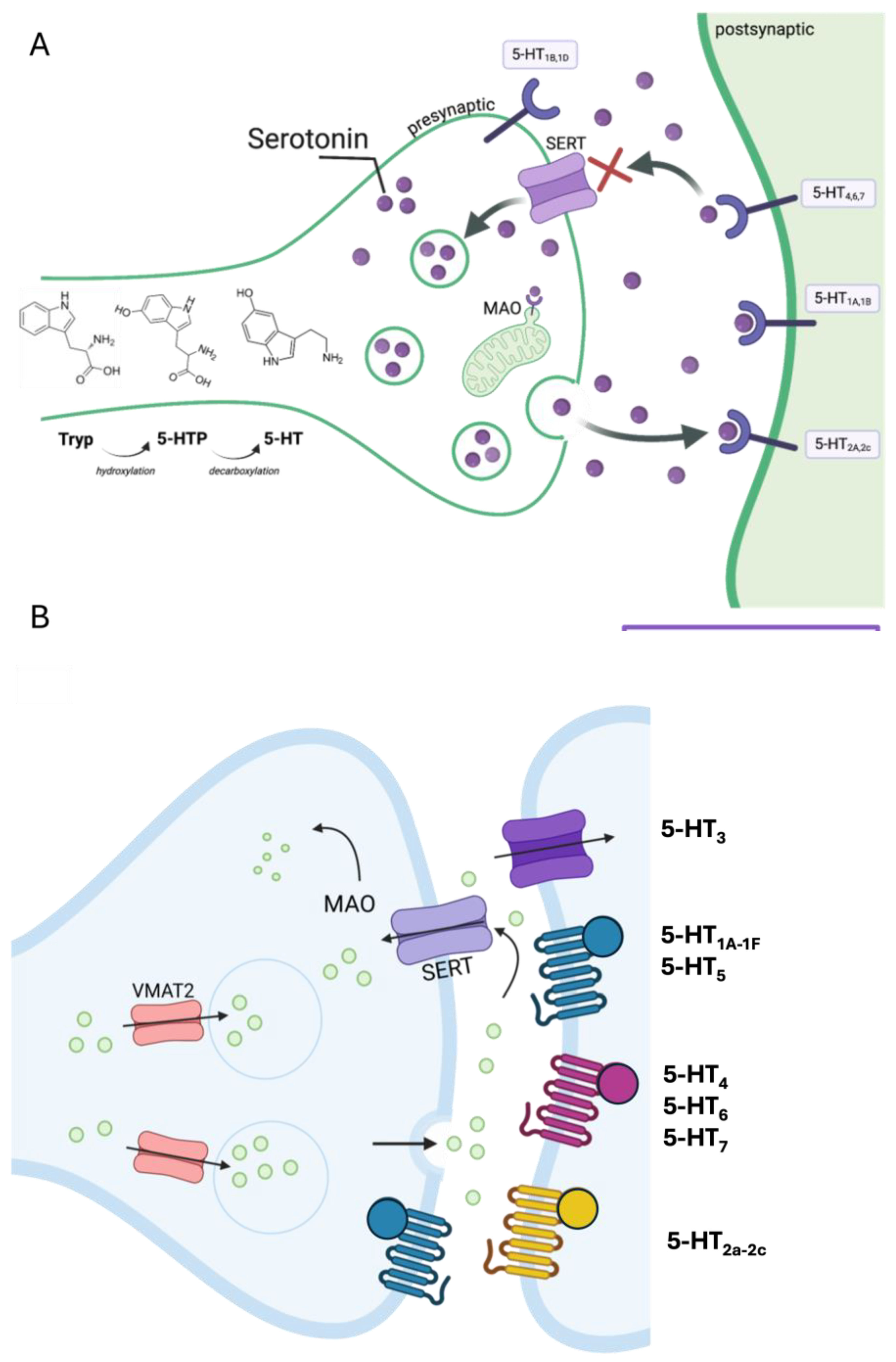
2. GABA and Serotonin as Main Players During Neurodevelopment
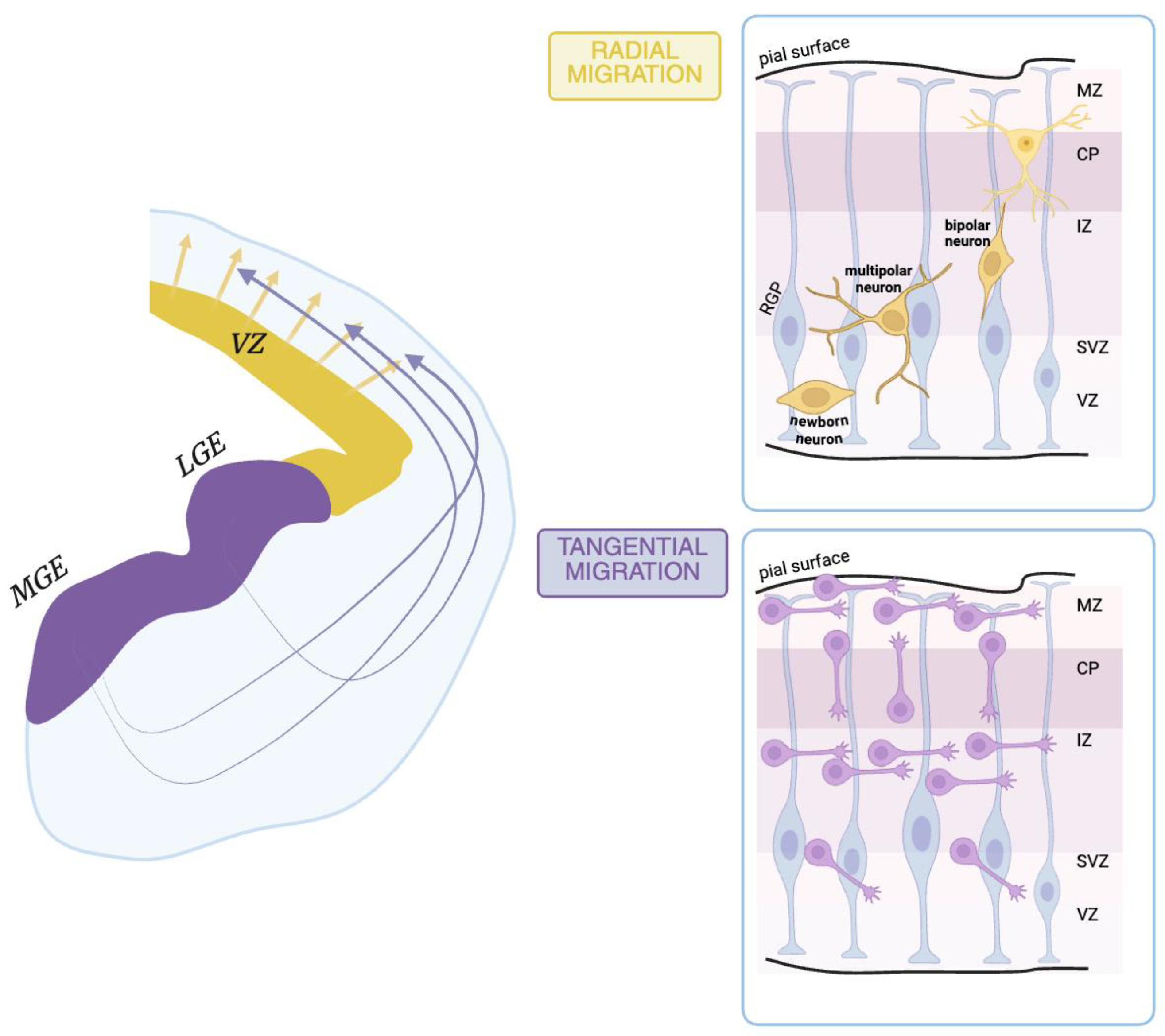
2.1. During Prenatal Life
- In the developing spinal cord
- In the developing cerebellum
2.2. During Postnatal Life
- In somatosensory cortex development
- In visual system development
- In cerebellum development
2.3. During Adult Life
- In the hippocampus
3. Psychedelics to Re-Open Critical Period Windows for Therapeutic Interventions in Neuropsychiatric Disorders
| Drug | Model | Mechanism | Effect | Relevance | References |
|---|---|---|---|---|---|
| MDMA | Adult mice (P96) | Via SERT and 5-HT4 receptors | Activation of oxytocin neurons restores oxytocin; long-term depression in the nucleus accumbens 48 h after a single administration, resulting in an improved score on the social preference task | Social reward learning | Nardou et al. (2019) [218] |
| DOI (5-HT2A/2C agonist) | 129S6/SvEv mice | Via 5-HT2A receptors | Increased expression of genes related to morphogenesis, neuron projection, and synapse structure; facilitated fear extinction | Schizophrenia, depression, hyperactivity disorder, depression, anxiety, and stressor-related disorders | Revenga et al. (2021) [223] |
| LSD, DMT, DOI | Sprague Dawley rats | TrkB, mTOR, and 5-HT2A signaling | Increased dendritic spine density and enhanced neuronal excitability in the cortex, higher spontaneous excitatory postsynaptic current amplitude, and frequency in prefrontal cortical neurons | Psychoplastogens as potential new fast-acting antidepressants, and anxiolytic compounds | Ly et al. (2018) [217] |
| DOI | Male Sprague Dawley rats and (5-HT2A−/−) mice, 129S6/SvEv background, CREB-deficient mouse line, | 5-HT2A receptor via recruitment of CREB | Desouza et al. (2021) [224] | ||
| LSD, ketamine | Cortical cultures from Sprague Dawley rat | AMPA receptor and mTOR activation | Growth of cortical neurons, dendritogenesis, spinogenesis, and synaptogenesis | Implications for central nervous system drug development and neurotherapeutics | Ly et al. (2021) [225] |
| Ketamine, psilocybin | Eight-week-old C57BL/6J mice | Davoudian et al. (2023) [226] | |||
| Psilocybin | Sprague Dawley rats (7–9 weeks) | HT2A receptor (PFC), 5-HT1A (HIP) | Higher expression of genes related to neuroplasticity, and rapid regulation of plasticity-related genes in the prefrontal cortex and the hippocampus in a dose-dependent manner. | Further characterization of both acute and long-term molecular events induced by psilocybin | Jefsen et al. (2021) [230] |
| LSD, psilocybin | humans | Insights into pharmacological modulation of brain function | Singleton et al. (2022) [231] | ||
| LSD | Brain organoids, rats, humans | mTOR pathway | Increased plasticity markers in human brain organoids, enhanced novelty preference in rats, and improved visual memory consolidation and recall in humans | Clarification of the antidepressant and anxiolytic effects of serotonergic psychedelics; the possibility of alleviating and counteracting the cognitive deficits associated with natural or pathological aging | Ornelas et al. (2022) [232] |
| DOI, amphetamine, MK801 (NMDA antagonist) | Male Sprague Dawley rats | Systemic administration of 2,5-dimethoxy-4-iodophenyl-2-aminopropane in rats elicited mixed effects on neuronal firing rates in the medial frontal cortex | Schizophrenia | Wood et al. (2012) [241] |
3.1. Clinical Trials and the Potential of Psychedelic Therapy
3.2. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bystron, I.; Blakemore, C.; Rakic, P. Development of the human cerebral cortex: Boulder Committee revisited. Nat. Rev. Neurosci. 2008, 9, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Marín, O.; Rubenstein, J.L.R. Cell Migration in the Forebrain. Annu. Rev. Neurosci. 2003, 26, 441–483. [Google Scholar] [CrossRef] [PubMed]
- Homem, C.C.F.; Repic, M.; Knoblich, J.A. Proliferation control in neural stem and progenitor cells. Nat. Rev. Neurosci. 2015, 16, 647–659. [Google Scholar] [CrossRef]
- Deidda, G.; Biazzo, M. Gut and Brain: Investigating Physiological and Pathological Interactions Between Microbiota and Brain to Gain New Therapeutic Avenues for Brain Diseases. Front. Neurosci. 2021, 15, 753915. [Google Scholar] [CrossRef]
- Murphy, T.H.; Corbett, D. Plasticity during stroke recovery: From synapse to behaviour. Nat. Rev. Neurosci. 2009, 10, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Gaiarsa, J.-L.; Tyzio, R.; Khazipov, R. GABA: A Pioneer Transmitter That Excites Immature Neurons and Generates Primitive Oscillations. Physiol. Rev. 2007, 87, 1215–1284. [Google Scholar] [CrossRef]
- Deidda, G.; Bozarth, I.F.; Cancedda, L. Modulation of GABAergic transmission in development and neurodevelopmental disorders: Investigating physiology and pathology to gain therapeutic perspectives. Front. Cell. Neurosci. 2014, 8, 119. [Google Scholar] [CrossRef]
- Barnard, E.A. The Molecular Architecture of GABAA Receptors. Pharmacology of GABA and Glycine Neurotransmission. In Handbook of Experimental Pharmacology; Möhler, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 79–99. Available online: http://link.springer.com/10.1007/978-3-642-56833-6_2 (accessed on 18 January 2025).
- Deidda, G.; Crunelli, V.; Di Giovanni, G. 5-HT/GABA interaction in epilepsy. Prog. Brain Res. 2021, 259, 265–286. [Google Scholar]
- Liu, R.; Wang, J.; Liang, S.; Zhang, G.; Yang, X. Role of NKCC1 and KCC2 in Epilepsy: From Expression to Function. Front. Neurol. 2020, 10, 1407. [Google Scholar] [CrossRef]
- Lam, P.; Newland, J.; Faull, R.L.M.; Kwakowsky, A. Cation-Chloride Cotransporters KCC2 and NKCC1 as Therapeutic Targets in Neurological and Neuropsychiatric Disorders. Molecules 2023, 28, 1344. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Khalilov, I.; Kahle, K.T.; Cherubini, E. The GABA Excitatory/Inhibitory Shift in Brain Maturation and Neurological Disorders. Neuroscience 2012, 18, 467–486. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Gao, C.S.; Lou, Q.W.; Chen, Z.; Wang, Y. The diverse role of the raphe 5-HTergic systems in epilepsy. Acta Pharmacol. Sin. 2022, 43, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Mozumder, S.; Bej, A.; Mukherjee, S.; Sengupta, J.; Chattopadhyay, A. Structure, dynamics and lipid interactions of serotonin receptors: Excitements and challenges. Biophys. Rev. 2021, 13, 101–122. [Google Scholar] [CrossRef]
- Bashammakh, S.; Würtele, M.; Kotnik, K.; Abdelilah-Seyfried, S.; Bader, M. Serotonin is required for pharyngeal arch morphogenesis in zebrafish. Sci. Res. 2014, 10, 1–9. [Google Scholar] [CrossRef]
- Reisoli, E.; De Lucchini, S.; Nardi, I.; Ori, M. Serotonin 2B receptor signaling is required for craniofacial morphogenesis and jaw joint formation in Xenopus. Development 2010, 137, 2927–2937. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vitalis, T.; Parnavelas, J.G. The Role of Serotonin in Early Cortical Development. Dev. Neurosci. 2003, 25, 245–256. [Google Scholar] [CrossRef]
- Millan, M.; Marin, P.; Bockaert, J.; Mannourylacour, C. Signaling at G-protein-coupled serotonin receptors: Recent advances and future research directions. Trends Pharmacol. Sci. 2008, 29, 454–464. [Google Scholar] [CrossRef]
- Olivier, B. Serotonin: A never-ending story. Eur. J. Pharmacol. 2015, 753, 2–18. [Google Scholar] [CrossRef]
- Oh, C.-M.; Park, S.; Kim, H. Serotonin as a New Therapeutic Target for Diabetes Mellitus and Obesity. Diabetes Metab. J. 2016, 40, 89–98. [Google Scholar] [CrossRef]
- Yun, H.-M.; Rhim, H. The Serotonin-6 Receptor as a Novel Therapeutic Target. Exp. Neurobiol. 2011, 20, 159–168. [Google Scholar] [CrossRef]
- Matthes, S.; Bader, M. Peripheral Serotonin Synthesis as a New Drug Target. Trends Pharmacol. Sci. 2018, 39, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Menezes, E.C.; Santos, P.R.; Goes, T.C.; Carvalho, V.C.B.; Teixeira-Silva, F.; Stevens, H.E.; Badauê-Passos, D.J. Effects of a rat model of gestational hypothyroidism on forebrain dopaminergic, GABAergic, and serotonergic systems and related behaviors. Behav. Brain Res. 2019, 366, 77–87. [Google Scholar] [CrossRef]
- Sodhi, M.S.K.; Sanders-Bush, E. Serotonin and Brain Development. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2004; pp. 111–174. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0074774204590062 (accessed on 13 November 2024).
- Malave, L.; van Dijk, M.T.; Anacker, C. Early life adversity shapes neural circuit function during sensitive postnatal developmental periods. Transl. Psychiatry 2022, 12, 306. [Google Scholar] [CrossRef]
- Andrade-Talavera, Y.; Pérez-Rodríguez, M.; Prius-Mengual, J.; Rodríguez-Moreno, A. Neuronal and astrocyte determinants of critical periods of plasticity. Trends Neurosci. 2023, 46, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Gibel-Russo, R.; Benacom, D.; Di Nardo, A.A. Non-Cell-Autonomous Factors Implicated in Parvalbumin Interneuron Maturation and Critical Periods. Front. Neural Circuits 2022, 16, 875873. [Google Scholar] [CrossRef]
- Reh, R.K.; Dias, B.G.; Nelson, C.A.; Kaufer, D.; Werker, J.F.; Kolb, B.; Levine, J.D.; Hensch, T.K. Critical period regulation across multiple timescales. Proc. Natl. Acad. Sci. USA 2020, 117, 23242–23251. [Google Scholar] [CrossRef]
- Hensch, T.K.; Bilimoria, P.M. Re-opening Windows: Manipulating Critical Periods for Brain Development. Cerebrum Dana Forum Brain Sci. 2012, 2012, 11. [Google Scholar]
- Koh, W.; Kwak, H.; Cheong, E.; Lee, C.J. GABA tone regulation and its cognitive functions in the brain. Nat. Rev. Neurosci. 2023, 24, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Jaenisch, R.; Sur, M. The role of GABAergic signalling in neurodevelopmental disorders. Nat. Rev. Neurosci. 2021, 22, 290–307. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Roth, F.C.; Draguhn, A. GABA Metabolism and transport: Effects on synaptic efficacy. Neural Plast. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Sallard, E.; Letourneur, D.; Legendre, P. Electrophysiology of ionotropic GABA receptors. Cell. Mol. Life Sci. 2021, 78, 5341–5370. [Google Scholar] [CrossRef]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef]
- Chrysafides, S.M.; Bordes, S.J.; Sharma, S. Physiology, Resting Potential. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK538338/ (accessed on 15 November 2024).
- Blaesse, P.; Airaksinen, M.S.; Rivera, C.; Kaila, K. Cation-Chloride Cotransporters and Neuronal Function. Neuron 2009, 61, 820–838. [Google Scholar] [CrossRef]
- Kahle, K.T.; Deeb, T.Z.; Puskarjov, M.; Silayeva, L.; Liang, B.; Kaila, K.; Moss, S.J. Modulation of neuronal activity by phosphorylation of the K–Cl cotransporter KCC2. Trends Neurosci. 2013, 36, 726–737. [Google Scholar] [CrossRef]
- Rivera, C.; Voipio, J.; Payne, J.A.; Ruusuvuori, E.; Lahtinen, H.; Lamsa, K.; Pirvola, U.; Saarma, M.; Kaila, K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 1999, 397, 251–255. [Google Scholar] [CrossRef]
- Bettler, B.; Kaupmann, K.; Mosbacher, J.; Gassmann, M. Molecular Structure and Physiological Functions of GABAB Receptors. Physiol. Rev. 2004, 84, 835–867. [Google Scholar] [CrossRef]
- Bassetti, D. Keeping the Balance: GABAB Receptors in the Developing Brain and Beyond. Brain Sci. 2022, 12, 419. [Google Scholar] [CrossRef]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A review. J. Vet. Pharmacol Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef]
- Kanova, M.; Kohout, P. Serotonin—Its Synthesis and Roles in the Healthy and the Critically Ill. Int. J. Mol. Sci. 2021, 22, 4837. [Google Scholar] [CrossRef]
- Alvarez, B.D.; Morales, C.A.; Amodeo, D.A. Impact of specific serotonin receptor modulation on behavioral flexibility. Pharmacol. Biochem. Behav. 2021, 209, 173243. [Google Scholar] [CrossRef]
- Pourhamzeh, M.; Moravej, F.G.; Arabi, M.; Shahriari, E.; Mehrabi, S.; Ward, R.; Ahadi, R.; Joghataei, M.T. The Roles of Serotonin in Neuropsychiatric Disorders. Cell. Mol. Neurobiol. 2022, 42, 1671–1692. [Google Scholar] [CrossRef]
- Kranz, G.S.; Kasper, S.; Lanzenberger, R. Reward and the serotonergic system. Neuroscience 2010, 166, 1023–1035. [Google Scholar] [CrossRef]
- Pannu, A.; KGoyal, R. Serotonin and Depression: Scrutiny of New Targets for Future Anti-Depressant Drug Development. Curr. Drug Targets 2023, 24, 816–837. [Google Scholar] [CrossRef]
- Sałaciak, K.; Pytka, K. Biased agonism in drug discovery: Is there a future for biased 5-HT1A receptor agonists in the treatment of neuropsychiatric diseases? Pharmacol. Ther. 2021, 227, 107872. [Google Scholar] [CrossRef]
- Dahlström, A.; Fuxe, K. Localization of monoamines in the lower brain stem. Cell. Mol. Life Sci. 1964, 20, 398–399. [Google Scholar] [CrossRef]
- Perrin, F.E.; Noristani, H.N. Serotonergic mechanisms in spinal cord injury. Exp. Neurol. 2019, 318, 174–191. [Google Scholar] [CrossRef]
- Hornung, J.-P. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 2003, 26, 331–343. [Google Scholar] [CrossRef]
- Vertes, R.P.; Crane, A.M. Distribution, quantification, and morphological characteristics of serotonin-immunoreactive cells of the supralemniscal nucleus (B9) and pontomesencephalic reticular formation in the rat. J. Comp. Neurol. 1997, 378, 411–424. [Google Scholar] [CrossRef]
- Rodríguez, J.J.; Noristani, H.N.; Hoover, W.B.; Linley, S.B.; Vertes, R.P. Serotonergic projections and serotonin receptor expression in the reticular nucleus of the thalamus in the rat. Synapse 2011, 65, 919–928. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Neal, K.B.; Parry, L.J.; Bornstein, J.C. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J. Physiol. 2009, 587, 567–586. [Google Scholar] [CrossRef]
- Gutknecht, L.; Waider, J.; Kraft, S.; Kriegebaum, C.; Holtmann, B.; Reif, A.; Schmitt, A.; Lesch, K.-P. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J. Neural Transm. 2008, 115, 1127–1132. [Google Scholar] [CrossRef]
- Risbrough, V. Behavioral Correlates of Anxiety. In Behavioral Neurobiology of Anxiety and Its Treatment; Stein, M.B., Steckler, T., Eds.; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2009; Volume 2, pp. 205–228. Available online: https://link.springer.com/10.1007/7854_2009_11 (accessed on 17 November 2024).
- Squires, L.N.; Jakubowski, J.A.; Stuart, J.N.; Rubakhin, S.S.; Hatcher, N.G.; Kim, W.-S.; Chen, K.; Shih, J.C.; Seif, I.; Sweedler, J.V. Serotonin Catabolism and the Formation and Fate of 5-Hydroxyindole Thiazolidine Carboxylic Acid. J. Biol. Chem. 2006, 281, 13463–13470. [Google Scholar] [CrossRef]
- Vitalis, T.; Ansorge, M.S.; Dayer, A.G. Serotonin homeostasis and serotonin receptors as actors of cortical construction: Special attention to the 5-HT3A and 5-HT6 receptor subtypes. Front. Cell. Neurosci. 2013, 7, 50204. [Google Scholar] [CrossRef]
- Popova, N.K.; Tsybko, A.S.; Naumenko, V.S. The Implication of 5-HT Receptor Family Members in Aggression, Depression and Suicide: Similarity and Difference. Int. J. Mol. Sci. 2022, 23, 8814. [Google Scholar] [CrossRef]
- Sharp, T.; Barnes, N.M. Central 5-HT receptors and their function; present and future. Neuropharmacology 2020, 177, 108155. [Google Scholar] [CrossRef]
- Liu, R.; Jolas, T.; Aghajanian, G. Serotonin 5-HT2 receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res. 2000, 873, 34–45. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.; Lovatt, D.; Xu, J.; Song, D.; Goldman, S.A.; Nedergaard, M.; Hertz, L.; Peng, L. 5-HT2Breceptors are expressed on astrocytes from brain and in culture and are a chronic target for all five conventional ‘serotonin-specific reuptake inhibitors’. Neuron Glia Biol. 2010, 6, 113–125. [Google Scholar] [CrossRef]
- Moffat, J.J.; Ka, M.; Jung, E.-M.; Kim, W.-Y. Genes and brain malformations associated with abnormal neuron positioning. Mol. Brain 2015, 8, 72. [Google Scholar] [CrossRef]
- Artigas, F. Serotonin receptors involved in antidepressant effects. Pharmacol. Ther. 2013, 137, 119–131. [Google Scholar] [CrossRef]
- Voronova, I.P.; Naumenko, V.S.; Khramova, G.M.; Kozyreva, T.V.; Popova, N.K. Central 5-HT3 receptor-induced hypothermia is associated with reduced metabolic rate and increased heat loss. Neurosci. Lett. 2011, 504, 209–214. [Google Scholar] [CrossRef]
- Faerber, L.; Drechsler, S.; Ladenburger, S.; Gschaidmeier, H.; Fischer, W. The neuronal 5-HT3 receptor network after 20 years of research—Evolving concepts in management of pain and inflammation. Eur. J. Pharmacol. 2007, 560, 1–8. [Google Scholar] [CrossRef]
- Machu, T.K. Therapeutics of 5-HT3 receptor antagonists: Current uses and future directions. Pharmacol. Ther. 2011, 130, 338–347. [Google Scholar] [CrossRef]
- Vidal, R.; Castro, E.; Pilar-Cuellar, F.; Pascual-Brazo, J.; Diaz, A.; Rojo, M.; Linge, R.; Martin, A.; Valdizan, M.E.; Pazos, A. Serotonin 5-HT4 Receptors: A New Strategy for Developing Fast Acting Antidepressants? Curr. Pharm. Des. 2014, 20, 3751–3762. [Google Scholar] [CrossRef]
- Rebholz, H.; Friedman, E.; Castello, J. Alterations of Expression of the Serotonin 5-HT4 Receptor in Brain Disorders. Int. J. Mol. Sci. 2018, 19, 3581. [Google Scholar] [CrossRef]
- Sourbron, J.; Lagae, L. Serotonin receptors in epilepsy: Novel treatment targets? Epilepsia Open. 2022, 7, 231–246. [Google Scholar] [CrossRef]
- Gharedaghi, M.H.; Seyedabadi, M.; Ghia, J.-E.; Dehpour, A.R.; Rahimian, R. The role of different serotonin receptor subtypes in seizure susceptibility. Exp. Brain Res. 2014, 232, 347–367. [Google Scholar] [CrossRef]
- Strac, D.S.; Pivac, N.; Smolders, I.J.; Fogel, W.A.; De Deurwaerdere, P.; Di Giovanni, G. Monoaminergic Mechanisms in Epilepsy May Offer Innovative Therapeutic Opportunity for Monoaminergic Multi-Target Drugs. Front. Neurosci. 2016, 10, 492. [Google Scholar] [CrossRef]
- Karila, L.; Megarbane, B.; Cottencin, O.; Lejoyeux, M. Synthetic Cathinones: A New Public Health Problem. Curr. Neuropharmacol. 2015, 13, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Blattner, K.M.; Canney, D.J.; Pippin, D.A.; Blass, B.E. Pharmacology and Therapeutic Potential of the 5-HT7 Receptor. ACS Chem. Neurosci. 2019, 10, 89–119. [Google Scholar] [CrossRef] [PubMed]
- Glennon, R.A.; Dukat, M.; Grella, B.; Hong, S.S.; Costantino, L.; Teitler, M.; Smith, C.; Egan, C.; Davis, K.; Mattson, M.V. Binding of β-carbolines and related agents at serotonin (5-HT2 and 5-HT1A), dopamine (D2) and benzodiazepine receptors. Drug Alcohol Depend. 2000, 60, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Aoki, N.; Mori, C.; Homma, K.J.; Yamaguchi, S. Molecular biology of serotonergic systems in avian brains. Front. Mol. Neurosci. 2023, 16, 1226645. [Google Scholar] [CrossRef]
- Salinas, R.; Connolly, D.R.; Song, H. Invited Review: Epigenetics in neurodevelopment. Neuropathol. Appl. Neurobiol. 2020, 46, 6–27. [Google Scholar] [CrossRef]
- Hussain, S.I.; Muhammad, N.; Shah, S.A.; Rehman, A.U.; Alam Khan, S.; Saleha, S.; Khan, Y.M.; Muhammad, N.; Khan, S.; Wasif, N. Variants in HCFC1 and MN1 genes causing intellectual disability in two Pakistani families. BMC Med. Genom. 2024, 17, 176. [Google Scholar] [CrossRef]
- Ben-Ari, Y. The Developing Cortex. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 417–426. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780444528919000452 (accessed on 13 November 2024).
- Gleeson, J.G.; Walsh, C.A. Neuronal migration disorders: From genetic diseases to developmental mechanisms. Trends Neurosci. 2000, 23, 352–359. [Google Scholar] [CrossRef]
- Wegiel, J.; Kuchna, I.; Nowicki, K.; Imaki, H.; Wegiel, J.; Marchi, E.; Ma, S.Y.; Chauhan, A.; Chauhan, V.; Bobrowicz, T.W.; et al. The neuropathology of autism: Defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol. 2010, 119, 755–770. [Google Scholar] [CrossRef]
- Parnavelas, J.G. The origin and migration of cortical neurones: New vistas. Trends Neurosci. 2000, 23, 126–131. [Google Scholar] [CrossRef]
- Deidda, G.; Allegra, M.; Cerri, C.; Naskar, S.; Bony, G.; Zunino, G.; Bozzi, Y.; Caleo, M.; Cancedda, L. Early depolarizing GABA controls critical-period plasticity in the rat visual cortex. Nat. Neurosci. 2015, 18, 87–96. [Google Scholar] [CrossRef]
- Greig, L.C.; Woodworth, M.B.; Galazo, M.J.; Padmanabhan, H.; Macklis, J.D. Molecular logic of neocortical projection neuron specification, development and diversity. Nat. Rev. Neurosci. 2013, 14, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Noctor, S.C.; Flint, A.C.; Weissman, T.A.; Dammerman, R.S.; Kriegstein, A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 2001, 409, 714–720. [Google Scholar] [CrossRef]
- Hartfuss, E.; Galli, R.; Heins, N.; Götz, M. Characterization of CNS Precursor Subtypes and Radial Glia. Dev. Biol. 2001, 229, 15–30. [Google Scholar] [CrossRef]
- Deidda, G.; Pierucci, M.; Crunelli, V.; Di Giovanni, G. 5-HT/GABA Interaction in Neurodevelopment and Plasticity. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2021; pp. 287–317. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0079612321000091 (accessed on 21 May 2025).
- Teissier, A.; Soiza-Reilly, M.; Gaspar, P. Refining the Role of 5-HT in Postnatal Development of Brain Circuits. Front. Cell. Neurosci. 2017, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Rood, B.D.; Calizo, L.H.; Piel, D.; Spangler, Z.P.; Campbell, K.; Beck, S.G. Dorsal Raphe Serotonin Neurons in Mice: Immature Hyperexcitability Transitions to Adult State during First Three Postnatal Weeks Suggesting Sensitive Period for Environmental Perturbation. J. Neurosci. 2014, 34, 4809–4821. [Google Scholar] [CrossRef] [PubMed]
- Calizo, L.H.; Akanwa, A.; Ma, X.; Pan, Y.-Z.; Lemos, J.C.; Craige, C.; Heemstra, L.A.; Beck, S.G. Raphe serotonin neurons are not homogenous: Electrophysiological, morphological and neurochemical evidence. Neuropharmacology 2011, 61, 524–543. [Google Scholar] [CrossRef]
- Kiyasova, V.; Bonnavion, P.; Scotto-Lomassese, S.; Fabre, V.; Sahly, I.; Tronche, F.; Deneris, E.; Gaspar, P.; Fernandez, S.P. A Subpopulation of Serotonergic Neurons That Do Not Express the 5-HT1A Autoreceptor. ACS Chem. Neurosci. 2013, 4, 89–95. [Google Scholar] [CrossRef]
- Fernandez, S.P.; Cauli, B.; Cabezas, C.; Muzerelle, A.; Poncer, J.-C.; Gaspar, P. Multiscale single-cell analysis reveals unique phenotypes of raphe 5-HT neurons projecting to the forebrain. Brain Struct. Funct. 2016, 221, 4007–4025. [Google Scholar] [CrossRef]
- Sinenko, S.A.; Kuzmin, A.A.; Skvortsova, E.V.; Ponomartsev, S.V.; Efimova, E.V.; Bader, M.; Alenina, N.; Tomilin, A.N. Tryptophan Hydroxylase-2-Mediated Serotonin Biosynthesis Suppresses Cell Reprogramming into Pluripotent State. Int. J. Mol. Sci. 2023, 24, 4862. [Google Scholar] [CrossRef]
- Antonini, A.; Stryker, M.P. Rapid Remodeling of Axonal Arbors in the Visual Cortex. Science 1993, 260, 1819–1821. [Google Scholar] [CrossRef]
- Chapman, B.; Jacobson, M.D.; Reiter, H.O.; Stryker, M.P. Ocular dominance shift in kitten visual cortex caused by imbalance in retinal electrical activity. Nature 1986, 324, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Crair, M.C.; Gillespie, D.C.; Stryker, M.P. The Role of Visual Experience in the Development of Columns in Cat Visual Cortex. Science 1998, 279, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Erzurumlu, R.S.; Gaspar, P. Development and critical period plasticity of the barrel cortex. Eur. J. Neurosci. 2012, 35, 1540–1553. [Google Scholar] [CrossRef]
- Morishita, H.; Hensch, T.K. Critical period revisited: Impact on vision. Curr. Opin. Neurobiol. 2008, 18, 101–107. [Google Scholar] [CrossRef]
- Luhmann, H.J.; Fukuda, A.; Kilb, W. Control of cortical neuronal migration by glutamate and GABA. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.; Jernigan, T.L. The Basics of Brain Development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef]
- Schmidt, M.J.; Mirnics, K. Neurodevelopment, GABA System Dysfunction, and Schizophrenia. Neuropsychopharmacology 2015, 40, 190–206. [Google Scholar] [CrossRef]
- Cellot, G.; Cherubini, E. Functional role of ambient GABA in refining neuronal circuits early in postnatal development. Front. Neural Circuits 2013, 7, 136. [Google Scholar] [CrossRef]
- Cherubini, E. Generating diversity at GAB Aergic synapses. Trends Neurosci. 2001, 24, 155–162. [Google Scholar] [CrossRef]
- Pouille, F.; Scanziani, M. Enforcement of Temporal Fidelity in Pyramidal Cells by Somatic Feed-Forward Inhibition. Science 2001, 293, 1159–1163. [Google Scholar] [CrossRef]
- Cobb, S.R.; Buhl, E.H.; Halasy, K.; Paulsen, O.; Somogyi, P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 1995, 378, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.; Jedlicka, P.; Luhmann, H.J.; Kilb, W. Interactions between Membrane Resistance, GABA-A Receptor Properties, Bicarbonate Dynamics and Cl−-Transport Shape Activity-Dependent Changes of Intracellular Cl− Concentration. Int. J. Mol. Sci. 2019, 20, 1416. [Google Scholar] [CrossRef] [PubMed]
- Kasyanov, A.M.; Safiulina, V.F.; Voronin, L.L.; Cherubini, E. GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proc. Natl. Acad. Sci. USA 2004, 101, 3967–3972. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, A. Role of Astrocytes in the Maintenance and Modulation of Glutamatergic and GABAergic Neurotransmission. Neurochem. Res. 2003, 28, 347–352. [Google Scholar] [CrossRef]
- Kaneda, M.; Farrant, M.; Cull-Candy, S.G. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J. Physiol. 1995, 485, 419–435. [Google Scholar] [CrossRef]
- Brickley, S.G.; Cull-Candy, S.G.; Farrant, M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J. Physiol. 1996, 497, 753–759. [Google Scholar] [CrossRef]
- Wall, M.J.; Usowicz, M.M. Development of Action Potential-dependent and Independent Spontaneous GABAA Receptor-mediated Currents in Granule Cells of Postnatal Rat Cerebellum. Eur. J. Neurosci. 1997, 9, 533–548. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, W.; Díez-Sampedro, A.; Richerson, G.B. Nonvesicular Inhibitory Neurotransmission via Reversal of the GABA Transporter GAT-1. Neuron 2007, 56, 851–865. [Google Scholar] [CrossRef]
- Attwell, D.; Barbour, B.; Szatkowski, M. Nonvesicular release of neurotransmitter. Neuron 1993, 11, 401–407. [Google Scholar] [CrossRef]
- Bragina, L.; Marchionni, I.; Omrani, A.; Cozzi, A.; Pellegrini-Giampietro, D.E.; Cherubini, E.; Conti, F. GAT-1 regulates both tonic and phasic GABAA receptor-mediated inhibition in the cerebral cortex. J. Neurochem. 2008, 105, 1781–1793. [Google Scholar] [CrossRef]
- Farrant, M.; Nusser, Z. Variations on an inhibitory theme: Phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 2005, 6, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Barbin, G.; Pollard, H.; Gaïarsa, J.; Ben-Ari, Y. Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neurosci. Lett. 1993, 152, 150–154. [Google Scholar] [CrossRef]
- Wang, D.D.; Kriegstein, A.R. GABA Regulates Excitatory Synapse Formation in the Neocortex via NMDA Receptor Activation. J. Neurosci. 2008, 28, 5547–5558. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, Z.; Ning, G.; Guo, Y.; Ali, R.; Macdonald, R.L.; De Blas, A.L.; Luscher, B.; Chen, G. γ-Aminobutyric Acid Type A (GABAA) Receptor α Subunits Play a Direct Role in Synaptic Versus Extrasynaptic Targeting. J. Biol. Chem. 2012, 287, 27417–27430. [Google Scholar] [CrossRef]
- Allain, A.-E.; Meyrand, P.; Branchereau, P. Ontogenic Changes of the Spinal GABAergic Cell Population Are Controlled by the Serotonin (5-HT) System: Implication of 5-HT1Receptor Family. J. Neurosci. 2005, 25, 8714–8724. [Google Scholar] [CrossRef]
- Martin, E.; Cazenave, W.; Allain, A.-E.; Cattaert, D.; Branchereau, P. Implication of 5-HT in the Dysregulation of Chloride Homeostasis in Prenatal Spinal Motoneurons from the G93A Mouse Model of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 21, 1107. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, D.C. A 2020 view of tension-based cortical morphogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 32868–32879. [Google Scholar] [CrossRef]
- Strahlendorf, J.; Lee, M.; Strahlendorf, H. Modulatory role of serotonin on GABA-elicited inhibition of cerebellar Purkinje cells. Neuroscience 1989, 30, 117–125. [Google Scholar] [CrossRef]
- Oostland, M.; Sellmeijer, J.; van Hooft, J.A. Transient expression of functional serotonin 5-HT3 receptors by glutamatergic granule cells in the early postnatal mouse cerebellum. J. Physiol. 2011, 589, 4837–4846. [Google Scholar] [CrossRef]
- Oostland, M.; Buijink, M.R.; van Hooft, J.A. Serotonergic control of Purkinje cell maturation and climbing fibre elimination by 5-HT3 receptors in the juvenile mouse cerebellum. J. Physiol. 2013, 591, 1793–1807. [Google Scholar] [CrossRef]
- Oostland, M.; Buijink, M.R.; Teunisse, G.M.; von Oerthel, L.; Smidt, M.P.; van Hooft, J.A. Distinct Temporal Expression of 5-HT1A and 5-HT2A Receptors on Cerebellar Granule Cells in Mice. Cerebellum 2014, 13, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Kish, S.J.; Furukawa, Y.; Chang, L.-J.; Tong, J.; Ginovart, N.; Wilson, A.; Houle, S.; Meyer, J.H. Regional distribution of serotonin transporter protein in postmortem human brain. Nucl. Med. Biol. 2005, 32, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Yamasaki, M.; Tanaka, K.F.; Watanabe, M. Compartmentalized Input–Output Organization of Lugaro Cells in the Cerebellar Cortex. Neuroscience 2021, 462, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Dieudonné, S.; Dumoulin, A. Serotonin-Driven Long-Range Inhibitory Connections in the Cerebellar Cortex. J. Neurosci. 2000, 20, 1837–1848. [Google Scholar] [CrossRef]
- Berardi, N.; Pizzorusso, T.; Maffei, L. Critical periods during sensory development. Curr. Opin. Neurobiol. 2000, 10, 138–145. [Google Scholar] [CrossRef]
- Levelt, C.N.; Hübener, M. Critical-Period Plasticity in the Visual Cortex. Annu. Rev. Neurosci. 2012, 35, 309–330. [Google Scholar] [CrossRef]
- McQuail, J.A.; Frazier, C.J.; Bizon, J.L. Molecular aspects of age-related cognitive decline: The role of GABA signaling. Trends Mol. Med. 2015, 21, 450–460. [Google Scholar] [CrossRef]
- Dierssen, M. Top ten discoveries of the year: Neurodevelopmental disorders. Free Neuropathol. 2020, 1, 13. [Google Scholar]
- Sherr, E.H. Neurodevelopmental Disorders, Causes, and Consequences. In Genomics, Circuits, and Pathways in Clinical Neuropsychiatry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 587–599. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128001059000366 (accessed on 8 March 2025).
- Knudsen, E.I. Sensitive Periods in the Development of the Brain and Behavior. J. Cogn. Neurosci. 2004, 16, 1412–1425. [Google Scholar] [CrossRef]
- Hensch, T.K.; Fagiolini, M. Excitatory–Inhibitory Balance and Critical Period Plasticity in Developing Visual Cortex. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2005; pp. 115–124. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0079612304470095 (accessed on 4 April 2025).
- Lavenex, P.; Banta Lavenex, P. Building hippocampal circuits to learn and remember: Insights into the development of human memory. Behav. Brain Res. 2013, 254, 8–21. [Google Scholar] [CrossRef]
- Chubakov, A.R.; Gromova, E.A.; Konovalov, G.V.; Sarkisova, E.F.; Chumasov, E.I. The effects of serotonin on the morpho-functional development of rat cerebral neocortex in tissue culture. Brain Res. 1986, 369, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Saitow, F.; Nagano, M.; Suzuki, H. Developmental Changes in Serotonergic Modulation of GABAergic Synaptic Transmission and Postsynaptic GABAA Receptor Composition in the Cerebellar Nuclei. Cerebellum 2018, 17, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Lee, C.J.; Schroeder, A.; Kim, Y.S.; Jung, S.H.; Kim, J.S.; Kim, D.Y.; Son, E.J.; Han, H.C.; Kil Hong, S.; et al. Excitatory Actions of GABA in the Suprachiasmatic Nucleus. J. Neurosci. 2008, 28, 5450–5459. [Google Scholar] [CrossRef]
- Ojeda, J.; Ávila, A. Early Actions of Neurotransmitters During Cortex Development and Maturation of Reprogrammed Neurons. Front. Synaptic Neurosci. 2019, 11, 33. [Google Scholar] [CrossRef]
- Li, H.; Crair, M.C. How do barrels form in somatosensory cortex? Ann. N. Y. Acad. Sci. 2011, 1225, 119–129. [Google Scholar] [CrossRef]
- Woolsey, T.A.; Van Der Loos, H. The structural organization of layer IV in the somatosensory region (S I) of mouse cerebral cortex. Brain Res. 1970, 17, 205–242. [Google Scholar] [CrossRef] [PubMed]
- Erzurumlu, R.S.; Kind, P.C. Neural activity: Sculptor of ‘barrels’ in the neocortex. Trends Neurosci. 2001, 24, 589–595. [Google Scholar] [CrossRef]
- Miceli, S.; Kasri, N.N.; Joosten, J.; Huang, C.; Kepser, L.; Proville, R.; Selten, M.M.; van Eijs, F.; Azarfar, A.; Homberg, J.R.; et al. Reduced Inhibition within Layer IV of Sert Knockout Rat Barrel Cortex is Associated with Faster Sensory Integration. Cereb. Cortex 2017, 27, 933–949. [Google Scholar] [CrossRef]
- Cases, O.; Vitalis, T.; Seif, I.; De Maeyer, E.; Sotelo, C.; Gaspar, P. Lack of Barrels in the Somatosensory Cortex of Monoamine Oxidase A–Deficient Mice: Role of a Serotonin Excess during the Critical Period. Neuron 1996, 16, 297–307. [Google Scholar] [CrossRef]
- Rebsam, A.; Seif, I.; Gaspar, P. Refinement of thalamocortical arbors and emergence of barrel domains in the primary somatosensory cortex: A study of normal and monoamine oxidase a knock-out mice. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 8541–8552. [Google Scholar] [CrossRef]
- Akhmetshina, D.; Zakharov, A.; Vinokurova, D.; Nasretdinov, A.; Valeeva, G.; Khazipov, R. The serotonin reuptake inhibitor citalopram suppresses activity in the neonatal rat barrel cortex in vivo. Brain Res. Bull. 2016, 124, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Persico, A.M.; Mengual, E.; Moessner, R.; Hall, S.F.; Revay, R.S.; Sora, I.; Arellano, J.; DeFelipe, J.; Giménez-Amaya, J.M.; Conciatori, M.; et al. Barrel Pattern Formation Requires Serotonin Uptake by Thalamocortical Afferents, and Not Vesicular Monoamine Release. J. Neurosci. 2001, 21, 6862–6873. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yoon, B.-E.; Berglund, K.; Oh, S.-J.; Park, H.; Shin, H.-S.; Augustine, G.J.; Lee, C.J. Channel-Mediated Tonic GABA Release from Glia. Science 2010, 330, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Naskar, S.; Narducci, R.; Balzani, E.; Cwetsch, A.W.; Tucci, V.; Cancedda, L. The development of synaptic transmission is time-locked to early social behaviors in rats. Nat. Commun. 2019, 10, 1195. [Google Scholar] [CrossRef]
- Berry, K.P.; Nedivi, E. Experience-Dependent Structural Plasticity in the Visual System. Annu. Rev. Vis. Sci. 2016, 2, 17–35. [Google Scholar] [CrossRef]
- Hofer, S.B.; Mrsic-Flogel, T.D.; Bonhoeffer, T.; Hübener, M. Lifelong learning: Ocular dominance plasticity in mouse visual cortex. Curr. Opin. Neurobiol. 2006, 16, 451–459. [Google Scholar] [CrossRef]
- Hubel, D.H.; Wiesel, T.N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. 1970, 206, 419–436. [Google Scholar] [CrossRef]
- Antonini, A.; Fagiolini, M.; Stryker, M.P. Anatomical Correlates of Functional Plasticity in Mouse Visual Cortex. J. Neurosci. 1999, 19, 4388–4406. [Google Scholar] [CrossRef]
- Kirkwood, A.; Lee, H.-K.; Bear, M.F. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature 1995, 375, 328–331. [Google Scholar] [CrossRef]
- Takahata, T. Development of ocular dominance columns across rodents and other species: Revisiting the concept of critical period plasticity. Front. Neural Circuits 2024, 18, 1402700. [Google Scholar] [CrossRef]
- Heimel, J.A.; van Versendaal, D.; Levelt, C.N. The Role of GABAergic Inhibition in Ocular Dominance Plasticity. Neural Plast. 2011, 2011, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, L.; Putignano, E.; Sale, A.; Viegi, A.; Berardi, N.; Maffei, L. Acceleration of Visual System Development by Environmental Enrichment. J. Neurosci. 2004, 24, 4840–4848. [Google Scholar] [CrossRef] [PubMed]
- Fagiolini, M.; Pizzorusso, T.; Berardi, N.; Domenici, L.; Maffei, L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: Dark rearing and monocular deprivation. Vis. Res. 1994, 34, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Gianfranceschi, L.; Siciliano, R.; Walls, J.; Morales, B.; Kirkwood, A.; Huang, Z.J.; Tonegawa, S.; Maffei, L. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc. Natl. Acad. Sci. USA 2003, 100, 12486–12491. [Google Scholar] [CrossRef]
- Gu, Q.; Singer, W. Involvement of Serotonin in Developmental Plasticity of Kitten Visual Cortex. Eur. J. Neurosci. 1995, 7, 1146–1153. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Q.; Cynader, M.S. Blockade of serotonin-2C receptors by mesulergine reduces ocular dominance plasticity in kitten visual cortex. Exp. Brain Res. 1997, 114, 321–328. [Google Scholar] [CrossRef]
- Edagawa, Y.; Saito, H.; Abe, K. Endogenous Serotonin Contributes to a Developmental Decrease in Long-Term Potentiation in the Rat Visual Cortex. J. Neurosci. 2001, 21, 1532–1537. [Google Scholar] [CrossRef]
- Vetencourt, J.F.M.; Sale, A.; Viegi, A.; Baroncelli, L.; De Pasquale, R.; O’Leary, O.F.; Castrén, E.; Maffei, L. The Antidepressant Fluoxetine Restores Plasticity in the Adult Visual Cortex. Science 2008, 320, 385–388. [Google Scholar] [CrossRef]
- Rosenzweig, M.R.; Bennett, E.L.; Hebert, M.; Morimoto, H. Social grouping cannot account for cerebral effects of enriched environments. Brain Res. 1978, 153, 563–576. [Google Scholar] [CrossRef]
- Nithianantharajah, J.; Hannan, A.J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 2006, 7, 697–709. [Google Scholar] [CrossRef]
- Sale, A.M.; Vetencourt, J.F.M.; Medini, P.; Cenni, M.C.; Baroncelli, L.; De Pasquale, R.; Maffei, L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat. Neurosci. 2007, 10, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef] [PubMed]
- Fryer, R.H.; Kaplan, D.R.; Feinstein, S.C.; Radeke, M.J.; Grayson, D.R.; Kromer, L.F. Developmental and mature expression of full-length and truncated TrkB, receptors in the rat forebrain. J. Comp. Neurol. 1996, 374, 21–40. [Google Scholar] [CrossRef]
- Hu, H.; Gan, J.; Jonas, P. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science 2014, 345, 1255263. [Google Scholar] [CrossRef]
- Fagiolini, M.; Fritschy, J.-M.; Löw, K.; Möhler, H.; Rudolph, U.; Hensch, T.K. Specific GABA A Circuits for Visual Cortical Plasticity. Science 2004, 303, 1681–1683. [Google Scholar] [CrossRef] [PubMed]
- Cannarozzo, C.; Rubiolo, A.; Casarotto, P.; Castrén, E. Ketamine and its metabolite 2 R,6 R-hydroxynorketamine promote ocular dominance plasticity and release tropomyosin-related kinase B from inhibitory control without reducing perineuronal nets enwrapping parvalbumin interneurons. Eur. J. Neurosci. 2023, 57, 940–950. [Google Scholar] [CrossRef]
- Brunello, C.A.; Cannarozzo, C.; Castrén, E. Rethinking the role of TRKB in the action of antidepressants and psychedelics. Trends Neurosci. 2024, 47, 865–874. [Google Scholar] [CrossRef]
- Saitow, F.; Murano, M.; Suzuki, H. Modulatory Effects of Serotonin on GABAergic Synaptic Transmission and Membrane Properties in the Deep Cerebellar Nuclei. J. Neurophysiol. 2009, 101, 1361–1374. [Google Scholar] [CrossRef][Green Version]
- Hart, G. The role of asparagine-linked oligosaccharides in cellular recognition by thymic lymphocytes. Effects of tunicamycin on the mixed lymphocyte reaction. J. Biol. Chem. 1982, 257, 151–158. [Google Scholar] [CrossRef]
- Pulli, E.P.; Kumpulainen, V.; Kasurinen, J.H.; Korja, R.; Merisaari, H.; Karlsson, L.; Parkkola, R.; Saunavaara, J.; Lähdesmäki, T.; Scheinin, N.M.; et al. Prenatal exposures and infant brain: Review of magnetic resonance imaging studies and a population description analysis. Hum. Brain Mapp. 2019, 40, 1987–2000. [Google Scholar] [CrossRef]
- Lövdén, M.; Wenger, E.; Mårtensson, J.; Lindenberger, U.; Bäckman, L. Structural brain plasticity in adult learning and development. Neurosci. Biobehav. Rev. 2013, 37, 2296–2310. [Google Scholar] [CrossRef]
- Rohlfs Domínguez, P. Promoting our understanding of neural plasticity by exploring developmental plasticity in early and adult life. Brain Res. Bull. 2014, 107, 31–36. [Google Scholar] [CrossRef]
- Bonfanti, L.; La Rosa, C.; Ghibaudi, M.; Sherwood, C.C. Adult neurogenesis and “immature” neurons in mammals: An evolutionary trade-off in plasticity? Brain Struct. Funct. 2023, 229, 1775–1793. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.; Morrone, M.C.; Bex, P.; Lozama, A.; Sabel, B.A. Harnessing brain plasticity to improve binocular vision in amblyopia: An evidence-based update. Eur. J. Ophthalmol. 2024, 34, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Aimone, J.B.; Li, Y.; Lee, S.W.; Clemenson, G.D.; Deng, W.; Gage, F.H. Regulation and Function of Adult Neurogenesis: From Genes to Cognition. Physiol. Rev. 2014, 94, 991–1026. [Google Scholar] [CrossRef]
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146, dev156059. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, A.; García-Verdugo, J.M. Neurogenesis in Adult Subventricular Zone. J. Neurosci. 2002, 22, 629–634. [Google Scholar] [CrossRef]
- Lois, C.; Alvarez-Buylla, A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc. Natl. Acad. Sci. USA 1993, 90, 2074–2077. [Google Scholar] [CrossRef]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef]
- Jurkowski, M.P.; Bettio, L.; KWoo, E.; Patten, A.; Yau, S.Y.; Gil-Mohapel, J. Beyond the Hippocampus and the SVZ: Adult Neurogenesis Throughout the Brain. Front. Cell Neurosci. 2020, 14, 576444. [Google Scholar] [CrossRef]
- Schweyer, K.; Rüschoff-Steiner, C.; Arias-Carrión, O.; Oertel, W.H.; Rösler, T.W.; Höglinger, G.U. Neuronal precursor cells with dopaminergic commitment in the rostral migratory stream of the mouse. Sci. Rep. 2019, 9, 13359. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, N.S.; Park, E.H.; Hen, R.; Fenton, A.A. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus 2012, 22, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- Kee, N.; Teixeira, C.M.; Wang, A.H.; Frankland, P.W. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 2007, 10, 355–362. [Google Scholar] [CrossRef]
- Garthe, A.; Roeder, I.; Kempermann, G. Mice in an enriched environment learn more flexibly because of adult hippocampal neurogenesis. Hippocampus 2016, 26, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Leal-Galicia, P.; Chávez-Hernández, M.E.; Mata, F.; Mata-Luévanos, J.; Rodríguez-Serrano, L.M.; Tapia-De-Jesús, A.; Buenrostro-Jáuregui, M.H. Adult Neurogenesis: A Story Ranging from Controversial New Neurogenic Areas and Human Adult Neurogenesis to Molecular Regulation. Int. J. Mol. Sci. 2021, 22, 11489. [Google Scholar] [CrossRef]
- Tong, C.K.; Chen, J.; Cebrián-Silla, A.; Mirzadeh, Z.; Obernier, K.; Guinto, C.D.; Tecott, L.H.; García-Verdugo, J.M.; Kriegstein, A.; Alvarez-Buylla, A. Axonal Control of the Adult Neural Stem Cell Niche. Cell Stem Cell 2014, 14, 500–511. [Google Scholar] [CrossRef]
- Sachs, B.D.; Caron, M.G. Chronic Fluoxetine Increases Extra-Hippocampal Neurogenesis in Adult Mice. Int. J. Neuropsychopharmacol. 2015, 18, pyu029. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Haydar, T.F.; Bordey, A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 2005, 8, 1179–1187. [Google Scholar] [CrossRef]
- Malberg, J.E.; Eisch, A.J.; Nestler, E.J.; Duman, R.S. Chronic Antidepressant Treatment Increases Neurogenesis in Adult Rat Hippocampus. J. Neurosci. 2000, 20, 9104–9110. [Google Scholar] [CrossRef]
- Yohn, C.N.; Shifman, S.; Garino, A.; Diethorn, E.; Bokka, L.; Ashamalla, S.A.; Samuels, B.A. Fluoxetine effects on behavior and adult hippocampal neurogenesis in female C57BL/6J mice across the estrous cycle. Psychopharmacology 2020, 237, 1281–1290. [Google Scholar] [CrossRef]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of Hippocampal Neurogenesis for the Behavioral Effects of Antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [PubMed]
- David, D.J.; Samuels, B.A.; Rainer, Q.; Wang, J.-W.; Marsteller, D.; Mendez, I.; Drew, M.; Craig, D.A.; Guiard, B.P.; Guilloux, J.-P.; et al. Neurogenesis-Dependent and -Independent Effects of Fluoxetine in an Animal Model of Anxiety/Depression. Neuron 2009, 62, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Mateus-Pinheiro, A.; Patrício, P.; Bessa, J.M.; Sousa, N.; Pinto, L. Cell genesis and dendritic plasticity: A neuroplastic pas de deux in the onset and remission from depression. Mol. Psychiatry 2013, 18, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Olivas-Cano, I.; Rodriguez-Andreu, J.; Blasco-Ibañez, J.; Crespo, C.; Nácher, J.; Varea, E. Fluoxetine increased adult neurogenesis is mediated by 5-HT3 receptor. Neurosci. Lett. 2023, 795, 137027. [Google Scholar] [CrossRef]
- Ciranna, L. Serotonin as a Modulator of Glutamate- and GABA-Mediated Neurotransmission: Implications in Physiological Functions and in Pathology. Curr. Neuropharmacol. 2006, 4, 101–114. [Google Scholar] [CrossRef]
- Koyama, S.; Matsumoto, N.; Kubo, C.; Akaike, N. Presynaptic 5-HT3 receptor-mediated modulation of synaptic GABA release in the mechanically dissociated rat amygdala neurons. J. Physiol. 2000, 529, 373–383. [Google Scholar] [CrossRef]
- Muñoz, M.D.; de la Fuente, N.; Sánchez-Capelo, A. TGF-β/Smad3 Signalling Modulates GABA Neurotransmission: Implications in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 590. [Google Scholar] [CrossRef]
- Miguelez, C.; Morera-Herreras, T.; Torrecilla, M.; Ruiz-Ortega, J.A.; Ugedo, L. Interaction between the 5-HT system and the basal ganglia: Functional implication and therapeutic perspective in Parkinson’s disease. Front. Neural Circuits 2014, 8, 21. [Google Scholar] [CrossRef]
- Nimgampalle, M.; Chakravarthy, H.; Sharma, S.; Shree, S.; Bhat, A.R.; Pradeepkiran, J.A.; Devanathan, V. Neurotransmitter systems in the etiology of major neurological disorders: Emerging insights and therapeutic implications. Ageing Res. Rev. 2023, 89, 101994. [Google Scholar] [CrossRef]
- Herlenius, E.; Lagercrantz, H. Neurotransmitters and neuromodulators during early human development. Early Hum. Dev. 2001, 65, 21–37. [Google Scholar] [CrossRef]
- Sanchez-Vives, M.V.; Barbero-Castillo, A.; Perez-Zabalza, M.; Reig, R. GABAB receptors: Modulation of thalamocortical dynamics and synaptic plasticity. Neuroscience 2021, 456, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.K.; Naumenko, V.S. Neuronal and behavioral plasticity: The role of serotonin and BDNF systems tandem. Expert Opin. Ther. Targets 2019, 23, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Franco, J.M.; Voss, P.; Thomas, M.E.; De Villers-Sidani, E. Critical Periods of Brain Development. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 75–88. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780444641502000095 (accessed on 8 March 2025).
- Nardou, R.; Sawyer, E.; Song, Y.J.; Wilkinson, M.; Padovan-Hernandez, Y.; de Deus, J.L.; Wright, N.; Lama, C.; Faltin, S.; Goff, L.A.; et al. Psychedelics reopen the social reward learning critical period. Nature 2023, 618, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Ballentine, G.; Friedman, S.F.; Bzdok, D. Trips and neurotransmitters: Discovering principled patterns across 6850 hallucinogenic experiences. Sci. Adv. 2022, 8, eabl6989. [Google Scholar] [CrossRef]
- Liechti, M.E. Modern Clinical Research on LSD. Neuropsychopharmacology 2017, 42, 2114–2127. [Google Scholar] [CrossRef]
- Su, T.-P.; Hayashi, T.; Vaupel, D.B. When the Endogenous Hallucinogenic Trace AmineN,N-Dimethyltryptamine Meets the Sigma-1 Receptor. Sci. Signal. 2009, 2, pe12. [Google Scholar] [CrossRef]
- Nabizadeh, F.; Sheykhlou, S.; Mahmoodi, S.; Khalili, E.; Zafari, R.; Hosseini, H. Neuroimaging Findings of Psychosis in Alzheimer’s Disease: A Systematic Review. Brain Behav. 2025, 15, e70205. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Zarandi, S.S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- Nardou, R.; Lewis, E.M.; Rothhaas, R.; Xu, R.; Yang, A.; Boyden, E.; Dölen, G. Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature 2019, 569, 116–120. [Google Scholar] [CrossRef]
- Dölen, G.; Darvishzadeh, A.; Huang, K.W.; Malenka, R.C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 2013, 501, 179–184. [Google Scholar] [CrossRef]
- Simmler, L.; Buser, T.; Donzelli, M.; Schramm, Y.; Dieu, L.; Huwyler, J.; Chaboz, S.; Hoener, M.; Liechti, M. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.G.; Gudelsky, G.A. 3,4-Methylenedioxymethamphetamine (MDMA) enhances the release of acetylcholine by 5-HT4 and D1 receptor mechanisms in the rat prefrontal cortex. Synapse 2005, 58, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, F.H.; Ismail, S.; Khadijah, N.M.J. Cerebrospinal Fluid Serotonin level as Biomarker for Neurotoxicity after 3,4-Methylenedioxymethamphetamine (MDMA). Res. J. Pharm. Technol. 2022, 15, 3796–3801. [Google Scholar] [CrossRef]
- Revenga, M.d.l.F.; Zhu, B.; Guevara, C.A.; Naler, L.B.; Saunders, J.M.; Zhou, Z.; Toneatti, R.; Sierra, S.; Wolstenholme, J.T.; Beardsley, P.M.; et al. Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep. 2021, 37, 109836. [Google Scholar] [CrossRef] [PubMed]
- Desouza, L.A.; Benekareddy, M.; Fanibunda, S.E.; Mohammad, F.; Janakiraman, B.; Ghai, U.; Gur, T.; Blendy, J.A.; Vaidya, V.A. The Hallucinogenic Serotonin2A Receptor Agonist, 2,5-Dimethoxy-4-Iodoamphetamine, Promotes cAMP Response Element Binding Protein-Dependent Gene Expression of Specific Plasticity-Associated Genes in the Rodent Neocortex. Front. Mol. Neurosci. 2021, 14, 790213. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Vargas, M.V.; Duim, W.C.; Grodzki, A.C.G.; Lein, P.J.; Olson, D.E. Transient Stimulation with Psychoplastogens Is Sufficient to Initiate Neuronal Growth. ACS Pharmacol. Transl. Sci. 2021, 4, 452–460. [Google Scholar] [CrossRef]
- Davoudian, P.A.; Shao, L.-X.; Kwan, A.C. Shared and Distinct Brain Regions Targeted for Immediate Early Gene Expression by Ketamine and Psilocybin. ACS Chem. Neurosci. 2023, 14, 468–480. [Google Scholar] [CrossRef]
- Tregub, P.P.; Komleva, Y.K.; Kukla, M.V.; Averchuk, A.S.; Vetchinova, A.S.; Rozanova, N.A.; Illarioshkin, S.N.; Salmina, A.B. Brain Plasticity and Cell Competition: Immediate Early Genes Are the Focus. Cells 2025, 14, 143. [Google Scholar] [CrossRef]
- Matveychuk, D.; Thomas, R.K.; Swainson, J.; Khullar, A.; MacKay, M.-A.; Baker, G.B.; Dursun, S.M. Ketamine as an antidepressant: Overview of its mechanisms of action and potential predictive biomarkers. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320916657. [Google Scholar] [CrossRef]
- Prouzeau, D.; Conejero, I.; Voyvodic, P.L.; Becamel, C.; Abbar, M.; Lopez-Castroman, J. Psilocybin Efficacy and Mechanisms of Action in Major Depressive Disorder: A Review. Curr. Psychiatry Rep. 2022, 24, 573–581. [Google Scholar] [CrossRef]
- Jefsen, O.H.; Elfving, B.; Wegener, G.; Müller, H.K. Transcriptional regulation in the rat prefrontal cortex and hippocampus after a single administration of psilocybin. J. Psychopharmacol. 2021, 35, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Singleton, S.P.; Luppi, A.I.; Carhart-Harris, R.L.; Cruzat, J.; Roseman, L.; Nutt, D.J.; Deco, G.; Kringelbach, M.L.; Stamatakis, E.A.; Kuceyeski, A. Receptor-informed network control theory links LSD and psilocybin to a flattening of the brain’s control energy landscape. Nat. Commun. 2022, 13, 5812. [Google Scholar] [CrossRef]
- Ornelas, I.M.; Cini, F.A.; Wießner, I.; Marcos, E.; Araújo, D.B.; Goto-Silva, L.; Nascimento, J.; Silva, S.R.; Costa, M.N.; Falchi, M.; et al. Nootropic effects of LSD: Behavioral, molecular and computational evidence. Exp. Neurol. 2022, 356, 114148. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Dames, S.S.; Foldi, C.J.; Shultz, S.R. Psychedelics for acquired brain injury: A review of molecular mechanisms and therapeutic potential. Mol. Psychiatry 2024, 29, 671–685. [Google Scholar] [CrossRef]
- Kwan, A.C.; Olson, D.E.; Preller, K.H.; Roth, B.L. The neural basis of psychedelic action. Nat. Neurosci. 2022, 25, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Miner, L.A.H.; Backstrom, J.R.; Sanders-Bush, E.; Sesack, S.R. Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience 2003, 116, 107–117. [Google Scholar] [CrossRef]
- Willins, D.L.; Deutch, A.Y.; Roth, B.L. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse 1997, 27, 79–82. [Google Scholar] [CrossRef]
- Aghajanian, G.; Marek, G. Serotonin Induces Excitatory Postsynaptic Potentials in Apical Dendrites of Neocortical Pyramidal Cells. Neuropharmacology 1997, 36, 589–599. [Google Scholar] [CrossRef]
- Martin, D.A.; Nichols, C.D. Psychedelics Recruit Multiple Cellular Types and Produce Complex Transcriptional Responses Within the Brain. EBioMedicine 2016, 11, 262–277. [Google Scholar] [CrossRef]
- Savalia, N.K.; Shao, L.-X.; Kwan, A.C. A Dendrite-Focused Framework for Understanding the Actions of Ketamine and Psychedelics. Trends Neurosci. 2021, 44, 260–275. [Google Scholar] [CrossRef]
- Avesar, D.; Gulledge, A.T. Selective serotonergic excitation of callosal projection neurons. Front. Neural Circuits 2012, 6, 23489. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Kim, Y.; Moghaddam, B. Disruption of Prefrontal Cortex Large Scale Neuronal Activity by Different Classes of Psychotomimetic Drugs. J. Neurosci. 2012, 32, 3022–3031. [Google Scholar] [CrossRef] [PubMed]
- Gee, A.; Dazzan, P.; Grace, A.A.; Modinos, G. Corticolimbic circuitry as a druggable target in schizophrenia spectrum disorders: A narrative review. Transl. Psychiatry 2025, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.L.; Kuypers, K.P.C.; Müller, F.; Reckweg, J.; Tse, D.H.Y.; Toennes, S.W.; Hutten, N.R.P.W.; Jansen, J.F.A.; Stiers, P.; Feilding, A.; et al. Me, myself, bye: Regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology 2020, 45, 2003–2011. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Nutt, D. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef]
- Inserra, A.; De Gregorio, D.; Gobbi, G. Psychedelics in Psychiatry: Neuroplastic, Immunomodulatory, and Neurotransmitter Mechanisms. Nader M, editor. Pharmacol. Rev. 2021, 73, 202–277. [Google Scholar] [CrossRef]
- Inserra, A. Hypothesis: The Psychedelic Ayahuasca Heals Traumatic Memories via a Sigma 1 Receptor-Mediated Epigenetic-Mnemonic Process. Front. Pharmacol. 2018, 9, 330. [Google Scholar] [CrossRef]
- Fitzgerald, P.J. Many Drugs of Abuse May Be Acutely Transformed to Dopamine, Norepinephrine and Epinephrine In Vivo. Int. J. Mol. Sci. 2021, 22, 10706. [Google Scholar] [CrossRef]
- De Gregorio, D.; Posa, L.; Ochoa-Sanchez, R.; McLaughlin, R.; Maione, S.; Comai, S.; Gobbi, G. The hallucinogen d-lysergic diethylamide (LSD) decreases dopamine firing activity through 5-HT 1A, D 2 and TAAR 1 receptors. Pharmacol. Res. 2016, 113, 81–91. [Google Scholar] [CrossRef]
- Wojtas, A.; Bysiek, A.; Wawrzczak-Bargiela, A.; Szych, Z.; Majcher-Maślanka, I.; Herian, M.; Maćkowiak, M.; Gołembiowska, K. Effect of Psilocybin and Ketamine on Brain Neurotransmitters, Glutamate Receptors, DNA and Rat Behavior. Int. J. Mol. Sci. 2022, 23, 6713. [Google Scholar] [CrossRef]
- Fortunato, J.J.; Réus, G.Z.; Kirsch, T.R.; Stringari, R.B.; Stertz, L.; Kapczinski, F.; Pinto, J.P.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; et al. Acute harmine administration induces antidepressive-like effects and increases BDNF levels in the rat hippocampus. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Wang, J.; Li, N.; Li, G.; Ma, H.; Zhao, Y.; Li, Y. Sigma-1 Receptors in Depression: Mechanism and Therapeutic Development. Front. Pharmacol. 2022, 13, 925879. [Google Scholar] [CrossRef] [PubMed]
- Dakic, V.; Nascimento, J.M.; Sartore, R.C.; Maciel, R.d.M.; de Araujo, D.B.; Ribeiro, S.; Martins-De-Souza, D.; Rehen, S.K. Short term changes in the proteome of human cerebral organoids induced by 5-MeO-DMT. Sci. Rep. 2017, 7, 12863. [Google Scholar] [CrossRef] [PubMed]
- Lima Da Cruz, R.V.; Moulin, T.C.; Petiz, L.L.; Leão, R.N. A Single Dose of 5-MeO-DMT Stimulates Cell Proliferation, Neuronal Survivability, Morphological and Functional Changes in Adult Mice Ventral Dentate Gyrus. Front. Mol. Neurosci. 2018, 11, 312. [Google Scholar] [CrossRef]
- Morales-Garcia, J.A.; Calleja-Conde, J.; Lopez-Moreno, J.A.; Alonso-Gil, S.; Sanz-SanCristobal, M.; Riba, J.; Perez-Castillo, A. N,N-dimethyltryptamine compound found in the hallucinogenic tea ayahuasca, regulates adult neurogenesis in vitro and in vivo. Transl. Psychiatry 2020, 10, 331. [Google Scholar] [CrossRef]
- Heal, D.; Smith, S.; Belouin, S.; Henningfield, J. Psychedelics: Threshold of a Therapeutic Revolution. Neuropharmacology 2023, 236, 109610. [Google Scholar] [CrossRef]
- Rhee, T.G.; Davoudian, P.A.; Sanacora, G.; Wilkinson, S.T. Psychedelic renaissance: Revitalized potential therapies for psychiatric disorders. Drug Discov. Today 2023, 28, 103818. [Google Scholar] [CrossRef]
- Hogea, L.; Tabugan, D.C.; Costea, I.; Albai, O.; Nussbaum, L.; Cojocaru, A.; Corsaro, L.; Anghel, T. The Therapeutic Potential of Psychedelics in Treating Substance Use Disorders: A Review of Clinical Trials. Medicina 2025, 61, 278. [Google Scholar] [CrossRef]
- Koslowski, M.; Johnson, M.W.; Gründer, G.; Betzler, F. Novel Treatment Approaches for Substance Use Disorders: Therapeutic Use of Psychedelics and the Role of Psychotherapy. Curr. Addict. Rep. 2021, 9, 48–58. [Google Scholar] [CrossRef]
- Perkins, D.; Sarris, J.; Rossell, S.; Bonomo, Y.; Forbes, D.; Davey, C.; Hoyer, D.; Loo, C.; Murray, G.; Hood, S.; et al. Medicinal psychedelics for mental health and addiction: Advancing research of an emerging paradigm. Aust. N. Z. J. Psychiatry 2021, 55, 1127–1133. [Google Scholar] [CrossRef]
- Brown, T.K. Ibogaine in the Treatment of Substance Dependence. Curr. Drug Abus. Rev. 2013, 6, 3–16. [Google Scholar] [CrossRef]
- Rocha, J.M.; Reis, J.A.S.; Rossi, G.N.; Bouso, J.C.; Hallak, J.E.C.; dos Santos, R.G. Guidelines for Establishing Safety in Ayahuasca and Ibogaine Administration in Clinical Settings. Psychoactives 2023, 2, 373–386. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beretta, E.; Cuboni, G.; Deidda, G. Unveiling GABA and Serotonin Interactions During Neurodevelopment to Re-Open Adult Critical Periods for Neuropsychiatric Disorders. Int. J. Mol. Sci. 2025, 26, 5508. https://doi.org/10.3390/ijms26125508
Beretta E, Cuboni G, Deidda G. Unveiling GABA and Serotonin Interactions During Neurodevelopment to Re-Open Adult Critical Periods for Neuropsychiatric Disorders. International Journal of Molecular Sciences. 2025; 26(12):5508. https://doi.org/10.3390/ijms26125508
Chicago/Turabian StyleBeretta, Emanuela, Gianmarco Cuboni, and Gabriele Deidda. 2025. "Unveiling GABA and Serotonin Interactions During Neurodevelopment to Re-Open Adult Critical Periods for Neuropsychiatric Disorders" International Journal of Molecular Sciences 26, no. 12: 5508. https://doi.org/10.3390/ijms26125508
APA StyleBeretta, E., Cuboni, G., & Deidda, G. (2025). Unveiling GABA and Serotonin Interactions During Neurodevelopment to Re-Open Adult Critical Periods for Neuropsychiatric Disorders. International Journal of Molecular Sciences, 26(12), 5508. https://doi.org/10.3390/ijms26125508






