Bidirectional Interplay Between Microglia and Mast Cells
Abstract
1. Introduction
2. Microglia Surveillance
3. Mast Cells as Guardians to the Brain
4. Key Signaling Pathways Between Microglia and Mast Cells
4.1. PAR2 Signaling
4.2. Purinergic Signaling
4.3. Histamine Signaling
5. Other Routes for the Activation of Mast Cells and Microglia
5.1. Toll-like Receptors
5.2. Involvement of Inflammasomes
6. Mast Cell-Related Signaling in M1/M2 Polarization
6.1. Microglia Polarization: From M1 to M2 or from M2 to M1
6.2. Mast Cell-Induced Microglial Polarization
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALS | amyotrophic lateral sclerosis |
| BBB | blood–brain barrier |
| C48/80 | compound 48/80 |
| CCL2 | chemokine (C-C motif) ligand 2 |
| CNS | central nervous system |
| Iba1 | calcium-binding adaptor molecule 1 |
| IgE | immunoglobulin E |
| IL | interleukin |
| MAPK | mitogen-activated protein kinase |
| MMP | matrix metalloproteinase |
| MRF-1 | microglial response factor-1 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | nitric oxide |
| NOD | nucleotide-binding and oligomerization domain |
| NLRP3 | NOD-, LRR-, and pyrin domain-containing protein 3 |
| PAR | protease-activated receptor |
| PGE2 | prostaglandin E2 |
| ROS | reactive oxygen species |
| SCF | stem cell factor |
| TGF-β | transforming growth factor β |
| TLR | toll-like receptor |
| TNF | tumor necrosis factor |
References
- Szalay, G.; Martinecz, B.; Lénárt, N.; Környei, Z.; Orsolits, B.; Judák, L.; Császár, E.; Fekete, R.; West, B.L.; Katona, G.; et al. Microglia Protect against Brain Injury and Their Selective Elimination Dysregulates Neuronal Network Activity after Stroke. Nat. Commun. 2016, 7, 11499. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Rehman, S.A.; Subhani, A.; Khan, M.A.; Rahman, Z.; Iqubal, M.K.; Iqubal, A. Mechanism of Microglia-Mediated Neuroinflammation, Associated Cognitive Dysfunction, and Therapeutic Updates in Alzheimer’s Disease. hLife 2025, 3, 64–81. [Google Scholar] [CrossRef]
- Tsai, M.; Grimbaldeston, M.; Galli, S. Mast Cells and Immunoregulation/Immunomodulation. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2000. [Google Scholar]
- Conti, P.; Lauritano, D.; Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; Ronconi, G.; Martinotti, S. Microglia and Mast Cells Generate Proinflammatory Cytokines in the Brain and Worsen Inflammatory State: Suppressor Effect of IL-37. Eur. J. Pharmacol. 2020, 875, 173035. [Google Scholar] [CrossRef] [PubMed]
- Salcman, B.; Affleck, K.; Bulfone-Paus, S. P2X Receptor-Dependent Modulation of Mast Cell and Glial Cell Activities in Neuroinflammation. Cells 2021, 10, 2282. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Dong, H.; Xu, Y.; Zhang, S. Induction of Microglial Activation by Mediators Released from Mast Cells. Cell Physiol. Biochem. 2016, 38, 1520–1531. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, H.; Li, N.; Zhang, S.; Sun, J.; Zhang, S.; Qian, Y. Activated Brain Mast Cells Contribute to Postoperative Cognitive Dysfunction by Evoking Microglia Activation and Neuronal Apoptosis. J. Neuroinflamm. 2016, 13, 127. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, X.; Wang, Y.; Zhou, X.; Qian, Y.; Zhang, S. Suppression of Brain Mast Cells Degranulation Inhibits Microglial Activation and Central Nervous System Inflammation. Mol. Neurobiol. 2017, 54, 997–1007. [Google Scholar] [CrossRef]
- Sandhu, J.K.; Kulka, M. Decoding Mast Cell-Microglia Communication in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 1093. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, H.; Wang, F.; Zhang, J. Mast Cell Deficiency Protects Mice from Surgery-Induced Neuroinflammation. Mediat. Inflamm. 2020, 2020, 1921826. [Google Scholar] [CrossRef]
- Dong, H.; Wang, Y.; Zhang, X.; Zhang, X.; Qian, Y.; Ding, H.; Zhang, S. Stabilization of Brain Mast Cells Alleviates LPS-Induced Neuroinflammation by Inhibiting Microglia Activation. Front. Cell Neurosci. 2019, 13, 191. [Google Scholar] [CrossRef]
- Cătălin, B.; Stopper, L.; Bălşeanu, T.-A.; Scheller, A. The in Situ Morphology of Microglia Is Highly Sensitive to the Mode of Tissue Fixation. J. Chem. Neuroanat. 2017, 86, 59–66. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma In Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Sierra, A.; Abiega, O.; Shahraz, A.; Neumann, H. Janus-Faced Microglia: Beneficial and Detrimental Consequences of Microglial Phagocytosis. Front. Cell Neurosci. 2013, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Cornell, J.; Salinas, S.; Huang, H.-Y.; Zhou, M. Microglia Regulation of Synaptic Plasticity and Learning and Memory. Neural Regen. Res. 2022, 17, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Mordelt, A.; de Witte, L.D. Microglia-Mediated Synaptic Pruning as a Key Deficit in Neurodevelopmental Disorders: Hype or Hope? Curr. Opin. Neurobiol. 2023, 79, 102674. [Google Scholar] [CrossRef]
- Weinhard, L.; di Bartolomei, G.; Bolasco, G.; Machado, P.; Schieber, N.L.; Neniskyte, U.; Exiga, M.; Vadisiute, A.; Raggioli, A.; Schertel, A.; et al. Microglia Remodel Synapses by Presynaptic Trogocytosis and Spine Head Filopodia Induction. Nat. Commun. 2018, 9, 1228. [Google Scholar] [CrossRef]
- Xie, M.; Wang, T.; Feng, J.; Ma, D.; Feng, L.; Hao, Y. Roles of Microglia in Synaptogenesis, Synaptic Pruning, and Synaptic Plasticity in Physiological Conditions and Central Nervous System Disorders. Curr. Neuropharmacol. 2025. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Wang, X.; Liu, C.; Zhang, H.-L. Pharmacological Targeting of Microglial Activation: New Therapeutic Approach. Front. Cell Neurosci. 2019, 13, 514. [Google Scholar] [CrossRef]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 Polarization and Metabolic States. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Lauro, C.; Limatola, C. Metabolic Reprograming of Microglia in the Regulation of the Innate Inflammatory Response. Front. Immunol. 2020, 11, 493. [Google Scholar] [CrossRef]
- Sharma, P.; Ping, L. Calcium Ion Influx in Microglial Cells: Physiological and Therapeutic Significance. J. Neurosci. Res. 2014, 92, 409–423. [Google Scholar] [CrossRef]
- Ramírez-Ponce, M.P.; Sola-García, A.; Balseiro-Gómez, S.; Maldonado, M.D.; Acosta, J.; Alés, E.; Flores, J.A. Mast Cell Changes the Phenotype of Microglia via Histamine and ATP. Cell Physiol. Biochem. 2021, 55, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Sperlágh, B.; Illes, P. Purinergic Modulation of Microglial Cell Activation. Purinergic Signal 2007, 3, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in Neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.; Saez-Orellana, F.; Godoy, P.A.; Fuentealba, J. Microglial Activation Modulated by P2X4R in Ischemia and Repercussions in Alzheimer’s Disease. Front. Physiol. 2022, 13, 814999. [Google Scholar] [CrossRef]
- Neher, J.J.; Cunningham, C. Priming Microglia for Innate Immune Memory in the Brain. Trends Immunol. 2019, 40, 358–374. [Google Scholar] [CrossRef]
- Norden, D.M.; Muccigrosso, M.M.; Godbout, J.P. Microglial Priming and Enhanced Reactivity to Secondary Insult in Aging, and Traumatic CNS Injury, and Neurodegenerative Disease. Neuropharmacology 2015, 96 Pt A, 29–41. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast Cell Secretory Granules: Armed for Battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Silver, R.; Curley, J.P. Mast Cells on the Mind: New Insights and Opportunities. Trends Neurosci. 2013, 36, 513–521. [Google Scholar] [CrossRef]
- Lauritano, D.; Mastrangelo, F.; D’Ovidio, C.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Frydas, I.; Kritas, S.K.; Trimarchi, M.; Carinci, F.; et al. Activation of Mast Cells by Neuropeptides: The Role of Pro-Inflammatory and Anti-Inflammatory Cytokines. Int. J. Mol. Sci. 2023, 24, 4811. [Google Scholar] [CrossRef]
- Conti, P.; Caraffa, A.; Kritas, S.; Ronconi, G.; Lessiani, G.; Toniato, E.; Theoharides, T. Mast Cell, pro-Inflammatory and Anti-Inflammatory: Jekyll and Hyde, the Story Continues. J. Biol. Regul. Homeost. Agents 2017, 31, 263–267. [Google Scholar] [PubMed]
- Conti, P.; D’Ovidio, C.; Conti, C.; Gallenga, C.E.; Lauritano, D.; Caraffa, A.; Kritas, S.K.; Ronconi, G. Progression in Migraine: Role of Mast Cells and pro-Inflammatory and Anti-Inflammatory Cytokines. Eur. J. Pharmacol. 2019, 844, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.H. Mast Cell Tryptases and Chymases in Inflammation and Host Defense. Immunol. Rev. 2007, 217, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Ronconi, G.; Lauritano, D.; Mastrangelo, F.; Caraffa, A.; Gallenga, C.E.; Frydas, I.; Kritas, S.K.; Carinci, F.; Gaudelli, F.; et al. Impact of TNF and IL-33 Cytokines on Mast Cells in Neuroinflammation. Int. J. Mol. Sci. 2024, 25, 3248. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, X.; Qian, Y. Mast Cells and Neuroinflammation. Med. Sci. Monit. Basic Res. 2014, 20, 200–206. [Google Scholar] [CrossRef]
- Pietrzak, A.; Wierzbicki, M.; Wiktorska, M.; Brzezińska-Błaszczyk, E. Surface TLR2 and TLR4 Expression on Mature Rat Mast Cells Can Be Affected by Some Bacterial Components and Proinflammatory Cytokines. Mediat. Inflamm. 2011, 2011, 427473. [Google Scholar] [CrossRef]
- Pang, X.; Letourneau, R.; Rozniecki, J.J.; Wang, L.; Theoharides, T.C. Definitive Characterization of Rat Hypothalamic Mast Cells. Neuroscience 1996, 73, 889–902. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Twahir, A.; Kempuraj, D. Mast Cells in the Autonomic Nervous System and Potential Role in Disorders with Dysautonomia and Neuroinflammation. Ann. Allergy Asthma Immunol. 2024, 132, 440–454. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Yang, H.; Hu, G.; He, S. Mast Cell Tryptase Induces Microglia Activation via Protease-Activated Receptor 2 Signaling. Cell. Physiol. Biochem. 2012, 29, 931–940. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I.; Conti, P. Mast Cells May Regulate The Anti-Inflammatory Activity of IL-37. Int. J. Mol. Sci. 2019, 20, 3701. [Google Scholar] [CrossRef]
- Rosta, J.; Jancsó, G.; Dux, M. Activation of Proteinase-Activated Receptor-2 (PAR-2) Induces Meningeal Vasodilatation and Modulates Nociceptor Function. Neuropeptides 2009, 43, 438–439. [Google Scholar]
- Park, G.H.; Jeon, S.J.; Ko, H.M.; Ryu, J.R.; Lee, J.M.; Kim, H.-Y.; Han, S.-H.; Kang, Y.S.; Park, S.H.; Shin, C.Y.; et al. Activation of Microglial Cells via Protease-Activated Receptor 2 Mediates Neuronal Cell Death in Cultured Rat Primary Neuron. Nitric Oxide 2010, 22, 18–29. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, X.; Zhou, S.; Chen, Q.; Zhu, X.; Ma, X.; He, X.; Tian, M.; Shi, X. Role of Mast Cell Activation in Inducing Microglial Cells to Release Neurotrophin. J. Neurosci. Res. 2010, 88, 1348–1354. [Google Scholar] [CrossRef]
- Ocak, U.; Eser, P.; Huang, L.; Xu, W.; Zuo, Y.; Li, P.; Gamdzyk, M.; Zuo, G.; Mo, J.; Zhang, G.; et al. Inhibition of Mast Cell Tryptase Attenuates Neuroinflammation via PAR-2/P38/NFκB Pathway Following Asphyxial Cardiac Arrest in Rats. J. Neuroinflamm. 2020, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, S.; Xu, L.; Yang, H.; He, S. Activation of Protease-Activated Receptor 2-Mediated Signaling by Mast Cell Tryptase Modulates Cytokine Production in Primary Cultured Astrocytes. Mediat. Inflamm. 2013, 2013, 140812. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Giusti, P. Mast Cells, Glia and Neuroinflammation: Partners in Crime? Immunology 2014, 141, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Alam, A.; Chen, Q.; Eusman, M.A.; Pal, A.; Eguchi, S.; Wu, L.; Ma, D. The Role of Microglia in the Pathobiology of Neuropathic Pain Development: What Do We Know? Br. J. Anaesth. 2017, 118, 504–516. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate Immune Activation in Neurodegenerative Disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef]
- Vázquez-Villoldo, N.; Domercq, M.; Martín, A.; Llop, J.; Gómez-Vallejo, V.; Matute, C. P2X4 Receptors Control the Fate and Survival of Activated Microglia. Glia 2014, 62, 171–184. [Google Scholar] [CrossRef]
- Calovi, S.; Mut-Arbona, P.; Sperlágh, B. Microglia and the Purinergic Signaling System. Neuroscience 2019, 405, 137–147. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Pankratov, Y.; Lalo, U.; Nedergaard, M. P2X Receptors in Neuroglia. Wiley Interdiscip. Rev. Membr. Transp. Signal 2012, 1, 151–161. [Google Scholar] [CrossRef]
- Bulanova, E.; Bulfone-Paus, S. P2 Receptor-Mediated Signaling in Mast Cell Biology. Purinergic Signal 2010, 6, 3–17. [Google Scholar] [CrossRef]
- Kolosowska, N.; Keuters, M.H.; Wojciechowski, S.; Keksa-Goldsteine, V.; Laine, M.; Malm, T.; Goldsteins, G.; Koistinaho, J.; Dhungana, H. Peripheral Administration of IL-13 Induces Anti-Inflammatory Microglial/Macrophage Responses and Provides Neuroprotection in Ischemic Stroke. Neurotherapeutics 2019, 16, 1304–1319. [Google Scholar] [CrossRef]
- West, P.K.; Viengkhou, B.; Campbell, I.L.; Hofer, M.J. Microglia Responses to Interleukin-6 and Type I Interferons in Neuroinflammatory Disease. Glia 2019, 67, 1821–1841. [Google Scholar] [CrossRef]
- Hinojosa, A.E.; Garcia-Bueno, B.; Leza, J.C.; Madrigal, J.L. CCL2/MCP-1 Modulation of Microglial Activation and Proliferation. J. Neuroinflamm. 2011, 8, 77. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Ceruti, S.; Bramanti, P.; Abbracchio, M.P. Purinergic Signalling in Inflammation of the Central Nervous System. Trends Neurosci. 2009, 32, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Carthy, E.; Ellender, T. Histamine, Neuroinflammation and Neurodevelopment: A Review. Front. Neurosci. 2021, 15, 680214. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Niimi, M.; Yamamoto, Y.; Kawamura, T.; Morimoto-Ishizuka, T.; Sawada, M.; Takemori, H.; Yamatodani, A. Histamine Production by Cultured Microglial Cells of the Mouse. Neurosci. Lett. 2001, 305, 181–184. [Google Scholar] [CrossRef]
- Rocha, S.M.; Saraiva, T.; Cristóvão, A.C.; Ferreira, R.; Santos, T.; Esteves, M.; Saraiva, C.; Je, G.; Cortes, L.; Valero, J.; et al. Histamine Induces Microglia Activation and Dopaminergic Neuronal Toxicity via H1 Receptor Activation. J. Neuroinflamm. 2016, 13, 137. [Google Scholar] [CrossRef]
- Lenz, K.M.; Pickett, L.A.; Wright, C.L.; Davis, K.T.; Joshi, A.; McCarthy, M.M. Mast Cells in the Developing Brain Determine Adult Sexual Behavior. J. Neurosci. 2018, 38, 8044–8059. [Google Scholar] [CrossRef]
- Zhu, J.; Qu, C.; Lu, X.; Zhang, S. Activation of Microglia by Histamine and Substance P. Cell Physiol. Biochem. 2014, 34, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, B.; Harris, O.A.; Luo, J. TGF-β Signaling in Microglia: A Key Regulator of Development, Homeostasis and Reactivity. Biomedicines 2024, 12, 2468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Zhang, Y.; Qu, C.; Zhou, X.; Zhang, S. Histamine Induces Microglia Activation and the Release of Proinflammatory Mediators in Rat Brain Via H(1)R or H(4)R. J. Neuroimmune Pharmacol. 2020, 15, 280–291. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, W.; Zeng, X.; Hu, G.; Zhang, H.; He, S.; Zhang, S. Histamine Induces Upregulated Expression of Histamine Receptors and Increases Release of Inflammatory Mediators from Microglia. Mol. Neurobiol. 2014, 49, 1487–1500. [Google Scholar] [CrossRef]
- Fang, Q.; Xicoy, H.; Shen, J.; Luchetti, S.; Dai, D.; Zhou, P.; Qi, X.-R.; Martens, G.J.M.; Huitinga, I.; Swaab, D.F.; et al. Histamine-4 Receptor Antagonist Ameliorates Parkinson-like Pathology in the Striatum. Brain Behav. Immun. 2021, 92, 127–138. [Google Scholar] [CrossRef]
- Frick, L.; Rapanelli, M.; Abbasi, E.; Ohtsu, H.; Pittenger, C. Histamine Regulation of Microglia: Gene-Environment Interaction in the Regulation of Central Nervous System Inflammation. Brain Behav. Immun. 2016, 57, 326–337. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. Neuroinflammation, Mast Cells, and Glia: Dangerous Liaisons. Neuroscientist 2017, 23, 478–498. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Brown, M.A. Innate Immunity in the Central Nervous System. J. Clin. Investig. 2012, 122, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- De Nardo, D. Toll-like Receptors: Activation, Signalling and Transcriptional Modulation. Cytokine 2015, 74, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Fiebich, B.L.; Batista, C.R.A.; Saliba, S.W.; Yousif, N.M.; de Oliveira, A.C.P. Role of Microglia TLRs in Neurodegeneration. Front. Cell Neurosci. 2018, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Labzin, L.I.; Heneka, M.T.; Latz, E. Innate Immunity and Neurodegeneration. Annu. Rev. Med. 2018, 69, 437–449. [Google Scholar] [CrossRef]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in Neuroinflammatory and Neurodegenerative Diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef] [PubMed]
- Bonnekoh, H.; Scheffel, J.; Kambe, N.; Krause, K. The Role of Mast Cells in Autoinflammation. Immunol. Rev. 2018, 282, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Nambayan, R.J.T.; Sandin, S.I.; Quint, D.A.; Satyadi, D.M.; de Alba, E. The Inflammasome Adapter ASC Assembles into Filaments with Integral Participation of Its Two Death Domains, PYD and CARD. J. Biol. Chem. 2019, 294, 439–452. [Google Scholar] [CrossRef]
- Mencarelli, A.; Bist, P.; Choi, H.W.; Khameneh, H.J.; Mortellaro, A.; Abraham, S.N. Anaphylactic Degranulation by Mast Cells Requires the Mobilization of Inflammasome Components. Nat. Immunol. 2024, 25, 693–702. [Google Scholar] [CrossRef]
- Baroja-Mazo, A.; Martín-Sánchez, F.; Gomez, A.I.; Martínez, C.M.; Amores-Iniesta, J.; Compan, V.; Barberà-Cremades, M.; Yagüe, J.; Ruiz-Ortiz, E.; Antón, J.; et al. The NLRP3 Inflammasome Is Released as a Particulate Danger Signal That Amplifies the Inflammatory Response. Nat. Immunol. 2014, 15, 738–748. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Xiao, Y.; Zhang, W.; Wu, S.; Qin, T.; Yue, Y.; Qian, W.; Li, L. NLRP3 Inflammasome and Inflammatory Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4063562. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Liao, B.; Zhao, W.; Beers, D.R.; Henkel, J.S.; Appel, S.H. Transformation from a Neuroprotective to a Neurotoxic Microglial Phenotype in a Mouse Model of ALS. Exp. Neurol. 2012, 237, 147–152. [Google Scholar] [CrossRef]
- Song, G.J.; Suk, K. Pharmacological Modulation of Functional Phenotypes of Microglia in Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 139. [Google Scholar] [CrossRef]
- Fan, Z.; Brooks, D.J.; Okello, A.; Edison, P. An Early and Late Peak in Microglial Activation in Alzheimer’s Disease Trajectory. Brain 2017, 140, 792–803. [Google Scholar] [CrossRef]
- Ren, C.; Li, D.; Zhou, Q.; Hu, X. Mitochondria-Targeted TPP-MoS(2) with Dual Enzyme Activity Provides Efficient Neuroprotection through M1/M2 Microglial Polarization in an Alzheimer’s Disease Model. Biomaterials 2020, 232, 119752. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Beers, D.R.; Henkel, J.S.; Zhang, W.; Urushitani, M.; Julien, J.-P.; Appel, S.H. Extracellular Mutant SOD1 Induces Microglial-Mediated Motoneuron Injury. Glia 2010, 58, 231–243. [Google Scholar] [CrossRef]
- Huang, C.; Tong, J.; Bi, F.; Zhou, H.; Xia, X.-G. Mutant TDP-43 in Motor Neurons Promotes the Onset and Progression of ALS in Rats. J. Clin. Investig. 2012, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sun, D.; Feng, J.; Tan, W.; Fang, X.; Zhao, M.; Zhao, X.; Pu, Y.; Huang, A.; Xiang, Z.; et al. MSX3 Switches Microglia Polarization and Protects from Inflammation-Induced Demyelination. J. Neurosci. 2015, 35, 6350–6365. [Google Scholar] [CrossRef]
- Aryanpour, R.; Pasbakhsh, P.; Zibara, K.; Namjoo, Z.; Beigi Boroujeni, F.; Shahbeigi, S.; Kashani, I.R.; Beyer, C.; Zendehdel, A. Progesterone Therapy Induces an M1 to M2 Switch in Microglia Phenotype and Suppresses NLRP3 Inflammasome in a Cuprizone-Induced Demyelination Mouse Model. Int. Immunopharmacol. 2017, 51, 131–139. [Google Scholar] [CrossRef]
- Lan, X.; Han, X.; Li, Q.; Yang, Q.-W.; Wang, J. Modulators of Microglial Activation and Polarization after Intracerebral Haemorrhage. Nat. Rev. Neurol. 2017, 13, 420–433. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, H.; Xie, Q.; Shen, J. GAS6/AXL Signaling Promotes M2 Microglia Efferocytosis to Alleviate Neuroinflammation in Sepsis-Associated Encephalopathy. Cell Death Discov. 2025, 11, 268. [Google Scholar] [CrossRef]
- He, Y.; Gao, Y.; Zhang, Q.; Zhou, G.; Cao, F.; Yao, S. IL-4 Switches Microglia/Macrophage M1/M2 Polarization and Alleviates Neurological Damage by Modulating the JAK1/STAT6 Pathway Following ICH. Neuroscience 2020, 437, 161–171. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Chen, G.-W.; Chen, Y.-C.; Shen, C.-K.; Lu, D.-Y.; Yang, L.-Y.; Chen, J.-H.; Yeh, W.-L. Regulatory Effects of Quercetin on M1/M2 Macrophage Polarization and Oxidative/Antioxidative Balance. Nutrients 2021, 14, 67. [Google Scholar] [CrossRef]
- Zhang, J.; Buller, B.A.; Zhang, Z.G.; Zhang, Y.; Lu, M.; Rosene, D.L.; Medalla, M.; Moore, T.L.; Chopp, M. Exosomes Derived from Bone Marrow Mesenchymal Stromal Cells Promote Remyelination and Reduce Neuroinflammation in the Demyelinating Central Nervous System. Exp. Neurol. 2022, 347, 113895. [Google Scholar] [CrossRef]
- Pennington, D.; Thomas, P.; Lopez, A.; Gold, W. Transforming Growth Factor-Beta Production by Dog Mastocytoma Cells. Storage and Release from Mast Cell Granules. Chest 1991, 99 (Suppl. 3), 66S. [Google Scholar] [CrossRef] [PubMed]
- Burd, P.R.; Thompson, W.C.; Max, E.E.; Mills, F.C. Activated Mast Cells Produce Interleukin 13. J. Exp. Med. 1995, 181, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Apolloni, S.; Fabbrizio, P.; Amadio, S.; Volonté, C. Actions of the Antihistaminergic Clemastine on Presymptomatic SOD1-G93A Mice Ameliorate ALS Disease Progression. J. Neuroinflamm. 2016, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.D.; Abdalla, S.; Eissa, N.; Akour, A.; Jha, N.K.; Ojha, S.; Sadek, B. Targeting Microglia in Neuroinflammation: H3 Receptor Antagonists as a Novel Therapeutic Approach for Alzheimer’s Disease, Parkinson’s Disease, and Autism Spectrum Disorder. Pharmaceuticals 2024, 17, 831. [Google Scholar] [CrossRef]
- Marquardt, D.L.; Gruber, H.E.; Wasserman, S.I. Adenosine Release from Stimulated Mast Cells. Proc. Natl. Acad. Sci. USA 1984, 81, 6192–6196. [Google Scholar] [CrossRef]
- Janks, L.; Sharma, C.V.R.; Egan, T.M. A Central Role for P2X7 Receptors in Human Microglia. J. Neuroinflamm. 2018, 15, 325. [Google Scholar] [CrossRef]
- Du, R.-H.; Sun, H.-B.; Hu, Z.-L.; Lu, M.; Ding, J.-H.; Hu, G. Kir6.1/K-ATP Channel Modulates Microglia Phenotypes: Implication in Parkinson’s Disease. Cell Death Dis. 2018, 9, 404. [Google Scholar] [CrossRef]
- Haynes, S.E.; Hollopeter, G.; Yang, G.; Kurpius, D.; Dailey, M.E.; Gan, W.-B.; Julius, D. The P2Y12 Receptor Regulates Microglial Activation by Extracellular Nucleotides. Nat. Neurosci. 2006, 9, 1512–1519. [Google Scholar] [CrossRef]
- Miguel-Álvarez, M.; Santos-Lozano, A.; Sanchis-Gomar, F.; Fiuza-Luces, C.; Pareja-Galeano, H.; Garatachea, N.; Lucia, A. Non-Steroidal Anti-Inflammatory Drugs as a Treatment for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Treatment Effect. Drugs Aging 2015, 32, 139–147. [Google Scholar] [CrossRef]
- Becker, C.; Jick, S.S.; Meier, C.R. NSAID Use and Risk of Parkinson Disease: A Population-Based Case-Control Study. Eur. J. Neurol. 2011, 18, 1336–1342. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, B.; Yang, L.-E.; Zhang, C. Platycodigenin as Potential Drug Candidate for Alzheimer’s Disease via Modulating Microglial Polarization and Neurite Regeneration. Molecules 2019, 24, 3207. [Google Scholar] [CrossRef]
- Apolloni, S.; Amadio, S.; Parisi, C.; Matteucci, A.; Potenza, R.L.; Armida, M.; Popoli, P.; D’Ambrosi, N.; Volonté, C. Spinal Cord Pathology Is Ameliorated by P2X7 Antagonism in a SOD1-Mutant Mouse Model of Amyotrophic Lateral Sclerosis. Dis. Model. Mech. 2014, 7, 1101–1109. [Google Scholar] [CrossRef]

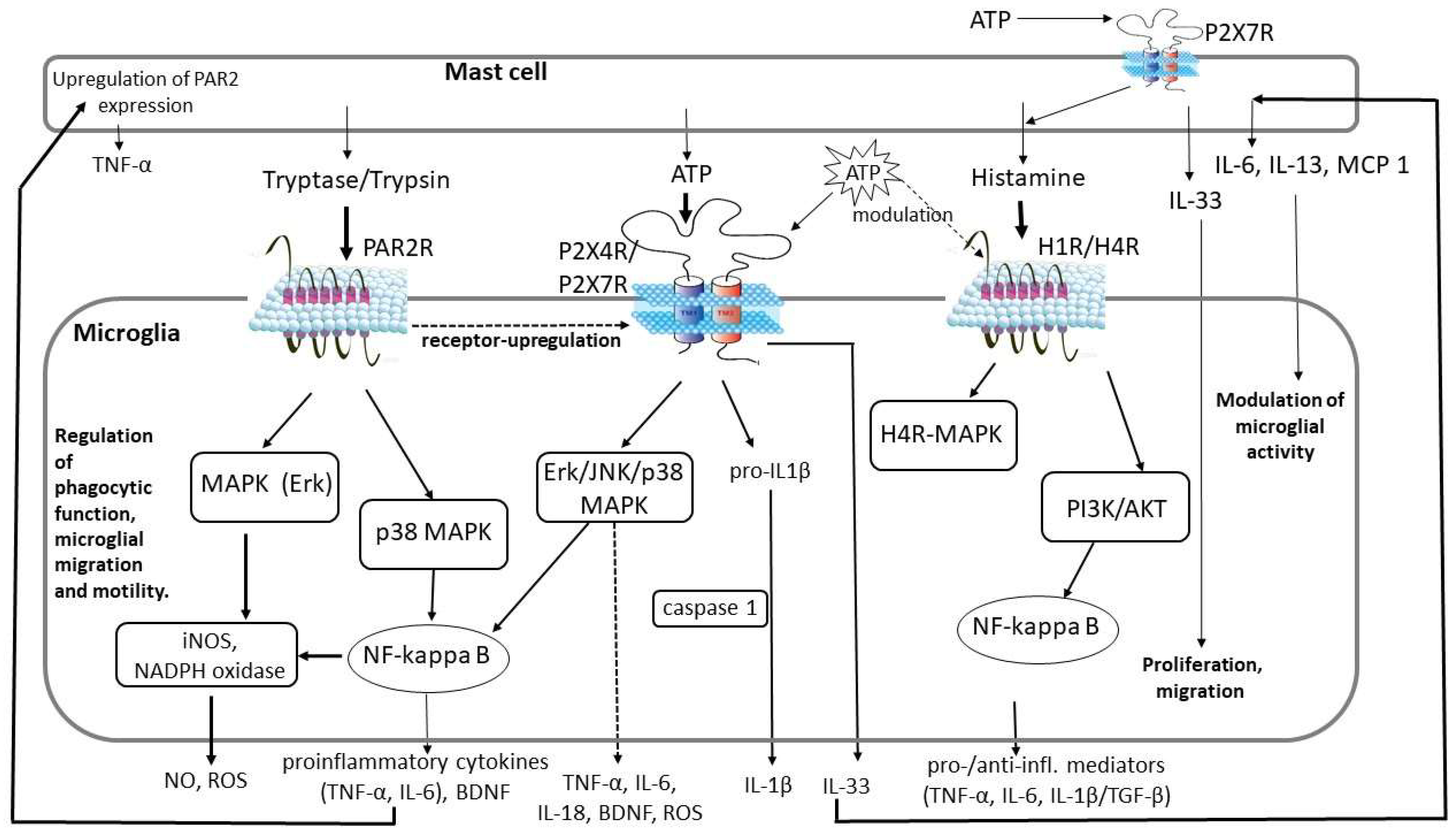
| Factors Released from Activated Mast Cells | Microglial Receptor | Effect on Microglia | Role |
|---|---|---|---|
| Trypsin, tryptase | PAR2 receptor | Release of TNF-α, IL-6, NO, ROS, BDNF. Upregulation of P2X4 receptor. | Microglia-derived IL-6 and TNF-α: upregulation of PAR2 expression in mast cells resulting in mast cell activation and TNF-α release. IL-6: neurogenesis, gliogenesis, cell survival, myelination/demyelination. |
| ATP | P2X4/7 receptor | Release of TNF-α, IL-6, IL-1β, IL-18, CCL2, MRF-1, ROS. Release of IL-33 from preactivated microglia. | IL-33 induces the secretion of IL-6, IL-13, and MCP1 from mast cells, which could affect microglial activity. Microglia migration to the site of injury. Proliferation, phagocytosis. Degradation of ATP by microglial ectonucleotidases. |
| Histamine | H1 and H4 receptor | Release of both pro- (TNF-α, IL-6, IL-1β) and anti-inflammatory (TGF-β, IL-10) cytokines. | Regulation of microglial migration and motility. Microglial phagocytosis (modulated by ATP). Normal microglial functioning. |
| PAMP/DAMP/NAMP | TLR2/TLR4 | The release of IL-6 and CCL5 modulates TLR2/4 expression of mast cells. | Cytokine/chemokine release, which induces pro-inflammatory response in microglia and the recruitment of immune cells to the site of injury. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakatos, S.; Rosta, J. Bidirectional Interplay Between Microglia and Mast Cells. Int. J. Mol. Sci. 2025, 26, 7556. https://doi.org/10.3390/ijms26157556
Lakatos S, Rosta J. Bidirectional Interplay Between Microglia and Mast Cells. International Journal of Molecular Sciences. 2025; 26(15):7556. https://doi.org/10.3390/ijms26157556
Chicago/Turabian StyleLakatos, Szandra, and Judit Rosta. 2025. "Bidirectional Interplay Between Microglia and Mast Cells" International Journal of Molecular Sciences 26, no. 15: 7556. https://doi.org/10.3390/ijms26157556
APA StyleLakatos, S., & Rosta, J. (2025). Bidirectional Interplay Between Microglia and Mast Cells. International Journal of Molecular Sciences, 26(15), 7556. https://doi.org/10.3390/ijms26157556





