Harnessing Transient Expression Systems with Plant Viral Vectors for the Production of Biopharmaceuticals in Nicotiana benthamiana
Abstract
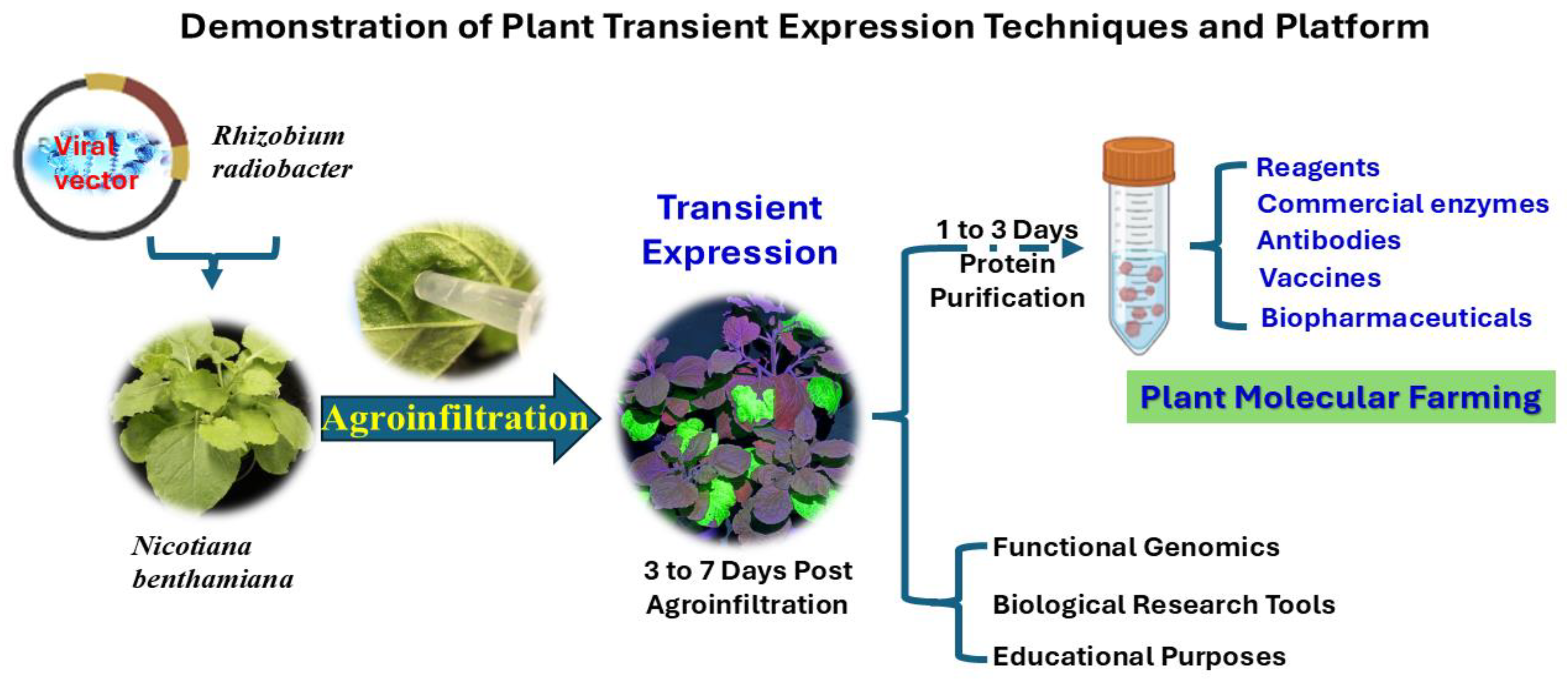
1. Introduction
2. Advancements and Applications of Plant Transient Expression Systems: A Historical Overview
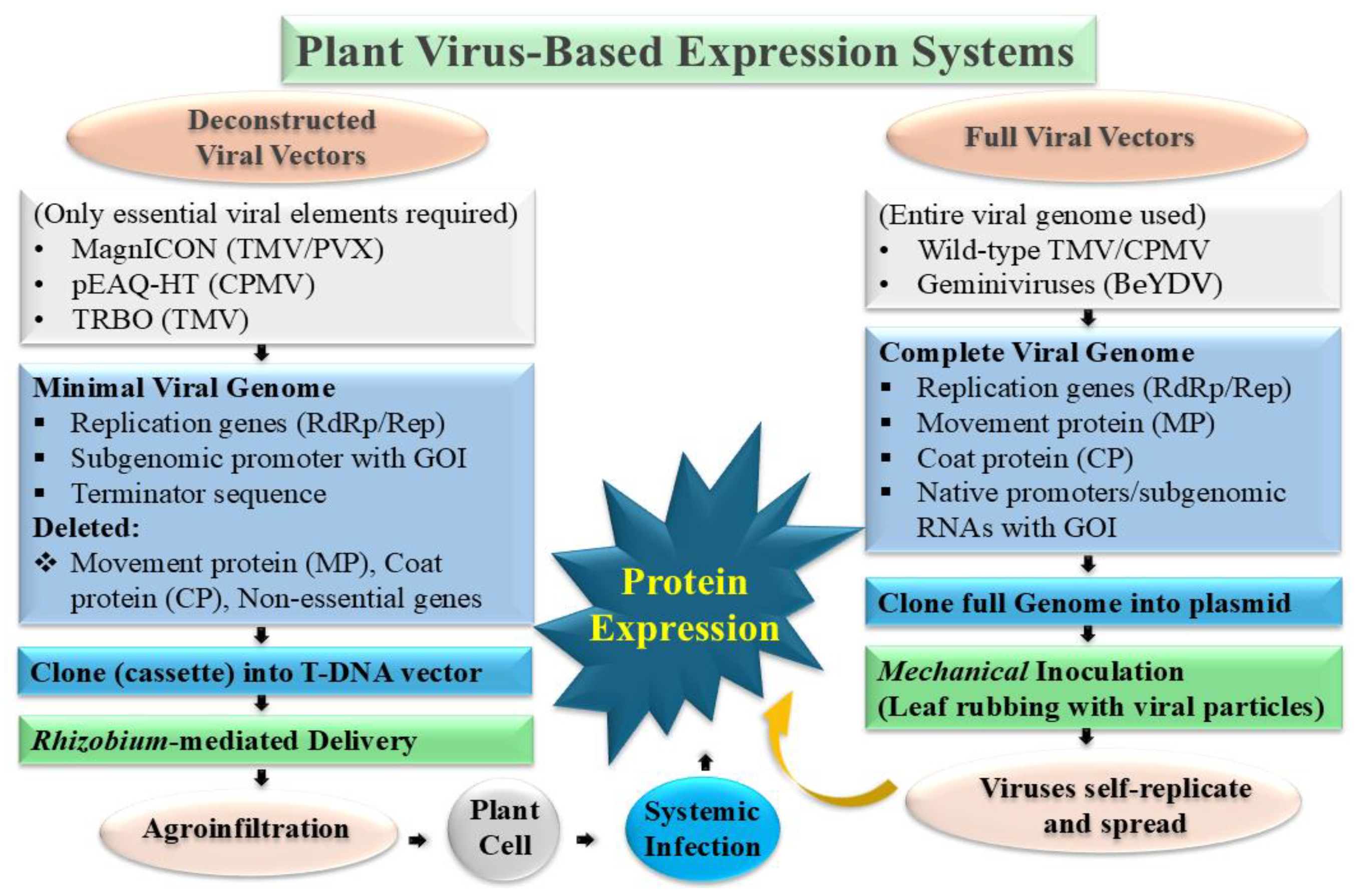
3. Plant Virus-Based Vectors in Transient Protein Expression
| No. | MagnICON® System | BeYDV System |
|---|---|---|
| 1. | The magnICON® agnICON system relies on “deconstructed” viral vectors, employing Rhizobium-mediated systemic delivery for recombinant protein production [84]. | BeYDV, a geminivirus, employs Rhizobium-mediated delivery to plant cells utilizing expression vectors facilitated by the viral replication initiator protein (Rep) to generate high levels of recombinant protein [78]. |
| 2. | Primarily functioning in tobacco, the magnICON® system faces limitations in lettuce and tomatoes due to its reliance on a TMV-based viral vector [85,86]. | BeYDV’s extensive host compatibility enables the efficient production of proteins at elevated levels in numerous dicotyledonous plants [87]. |
| 3. | Transient expression of model proteins like GFP in the magnICON® system yielded 3–5 mg/g FM within one week in N. benthamiana [52]. | Achieved exceptional yields of 3–5 mg/g leaf FW in just 4–5 days, equivalent to roughly 50% of TSP [64,87]. |
| 4. | The transient nature of the magnICON® system, free from stable genetic plant modifications, enables faster and more adaptable production [79]. | The BeYDV system offers flexibility in expressing large gene fragments and has the potential for high-volume production of recombinant proteins [88]. |
| 5. | Supports systemic movement and requires low Rhizobium density, enabling cost-effective agroinfiltration and simpler downstream processing [17]. | Lacks systemic movement, required high Rhizobium density (OD600 = 0.2), leads to higher agroinfiltration costs and more complex purification due to endotoxins [29]. |
4. Comparison of Plant-Based Transient Expression Systems with Other Expression Systems
| Metric | Plant-Based | Mammalian Cells | Bacterial | Yeast/Fungi | Insect Cells | Algal Systems | References |
|---|---|---|---|---|---|---|---|
| Expression Yield (mg/L) | 1.6 g/Kg FW | 3.2 g/L | 1.2 g/L | 1.9 g/L | 0.7 g/L | 1.14 μg/g | [21,103] |
| Yield Notes | High yields via agroinfiltration | HEK293/CHO provide human-like PTMs but lower yields | High yield but may lack proper PTMs | Good yields with some glycosylation | Baculovirus system allows for complex folding | Geminiviral vector allowed for the expression of recombinant proteins in Chlorella vulgaris | [89,103,104,105,106,107,108] |
| Time to Expression (Days) | 3–7 | 2–10 | 1–3 | 2–5 | 3–4 | 2 | [61,79,103,109,110,111,112] |
| Time Notes | Fast with agroinfiltration/viral vectors | Quick with lipid transfection, viral vectors take longer | Very fast due to simple machinery | Moderate speed for plasmid-based systems | Requires baculovirus amplification | Time varies by strain and method | [61,79,103,109,110,111,112] |
| Operating cost | Low | Very high | Low | Medium | High | Low | [113] |
| Cost Notes | Cost-effective, good for scaling | Expensive media and slow growth | Very cheap but lacks PTMs | Moderate cost with better PTMs than bacteria | Costly but allows for complex proteins | Sustainable but optimization needed | [113] |
| Scalability | High | Medium to High | Very High | High | Medium | High | [61,105,114,115,116] |
| Scalability Notes | Easily scalable, needs greenhouse/fields | Bioreactor-based, limits scale | Extremely scalable, used industrially | Optimized bioreactors allow for large-scale use | Limited by baculovirus production | Large-scale photobioreactors possible | [61,105,114,115,116] |
| PTM Capability | Moderate | High | None | Incorrect | High | Moderate | [113,117,118] |
| PTM Notes | Glycoproteins, glycosylation differs from humans | Complex, human-like PTMs | Does not support most eukarotic PTMs | Performs glycosylation but differs from humans | Provides PTMs similar to mammalian cells | Minimal PTMs, unsuitable for complex proteins | [105,119,120,121,122,123] |
| Workflow Complexity | Moderate | High | Low | Moderate | High | Moderate | [124,125,126,127,128,129] |
| Complexity Notes | Requires cultivation and agroinfiltration expertise | Needs sterile conditions and expensive media | Simple, well-established protocols | Requires bioreactor optimization for high yield | Baculovirus production adds steps | Cultivation methods need optimization | [124,125,126,127,128,129] |
5. The Role of N. benthamiana and Glycoengineering for Biopharmaceutical Production
5.1. Enhancing the Biocompatibility of Plant-Derived Pharmaceuticals Through Glycosylation Pathway Modifications in N. benthamiana
5.2. Overcoming Downstream Processing Challenges with N. benthamiana
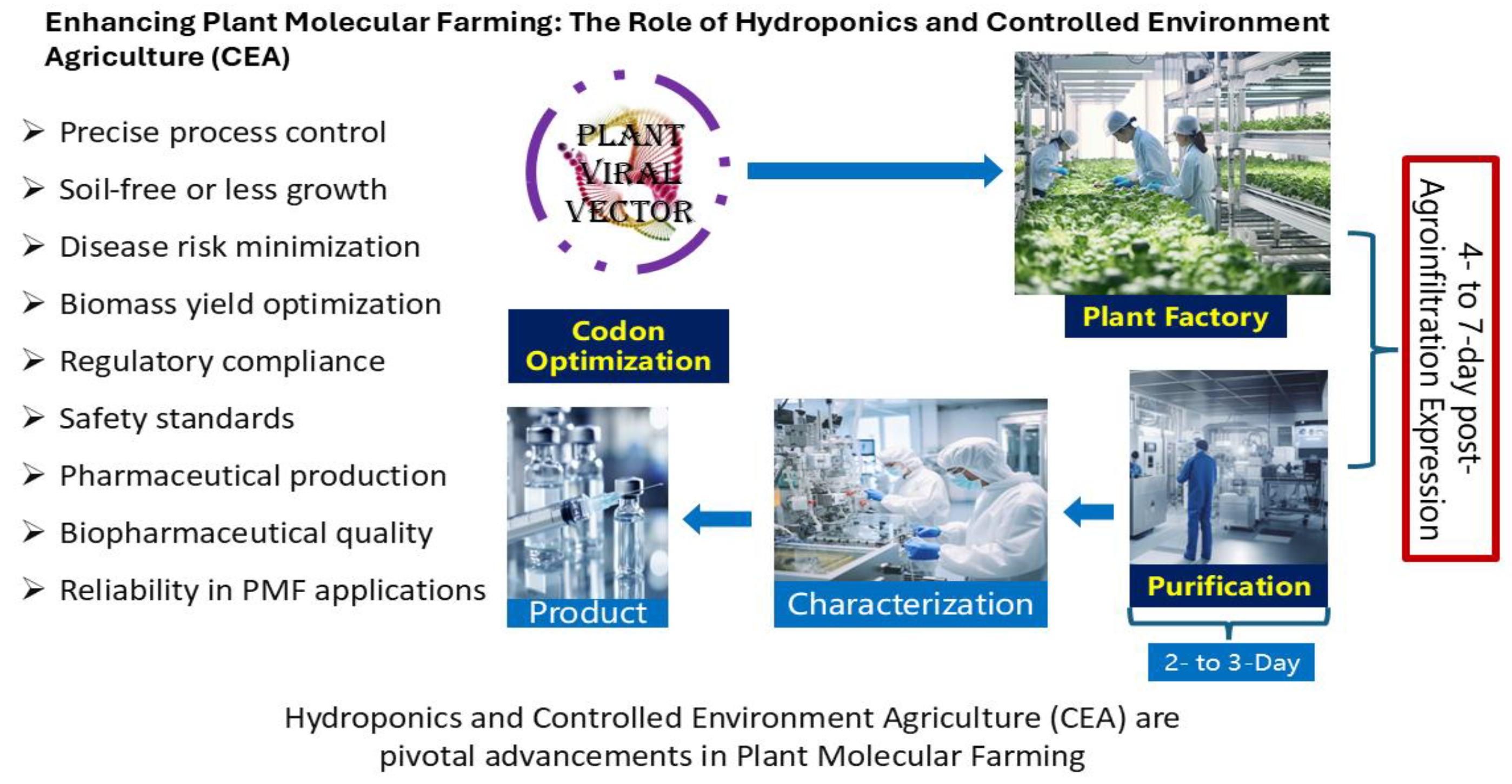
6. Optimizing Plant Molecular Farming Through Hydroponics and Controlled Environment Agriculture (CEA)
7. New Developments in Transient Expression Systems
7.1. Plant Cell Pack (PCP) System for Transient Expression: A Promising Innovation in Plant Biotechnology
7.2. Transient Expression in Hairy Root Cultures
7.3. Bioprinted Plant Cells
7.4. Multi-Host Systems for Complex Protein Production
8. Limitations and Future Challenges
9. Conclusions and Prospectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buyel, J. Product safety aspects of plant molecular farming. Front. Bioeng. Biotechnol. 2023, 11, 1238917. [Google Scholar] [CrossRef] [PubMed]
- Buyel, J. Towards a seamless product and process development workflow for recombinant proteins produced by plant molecular farming. Biotechnol. Adv. 2024, 75, 108403. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Khan, I.; Habibi, P.; Le, M.; Lippert, R.; Hefferon, K. Recent advances in expression and purification strategies for plant made vaccines. Front. Plant Sci. 2023, 14, 1273958. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Aljabali, A.A.; Takova, K.; Minkov, G.; Tambuwala, M.M.; Minkov, I.; Lomonossoff, G.P. Green biologics: Harnessing the power of plants to produce pharmaceuticals. Int. J. Mol. Sci. 2023, 24, 17575. [Google Scholar] [CrossRef]
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018, 36, 1136–1145. [Google Scholar] [CrossRef]
- Leader, B.; Baca, Q.J.; Golan, D.E. Protein therapeutics: A summary and pharmacological classification. Nat. Rev. Drug Discov. 2008, 7, 21–39. [Google Scholar] [CrossRef]
- Pham, P.V. Medical biotechnology: Techniques and applications. In Omics Technologies and Bio-Engineering: Towards Improving Quality of Life; Barh, V.K., Azevedo, V., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 1, pp. 449–469. [Google Scholar]
- Batta, A.; Kalra, B.S.; Khirasaria, R. Trends in FDA drug approvals over last 2 decades: An observational study. JFMPC 2020, 9, 105–114. [Google Scholar]
- de La Torre, B.G.; Albericio, F. The pharmaceutical industry in 2020. An analysis of FDA drug approvals from the perspective of molecules. Molecules 2021, 26, 627. [Google Scholar] [CrossRef]
- Venkataraman, S.; Hefferon, K. Application of plant viruses in biotechnology, medicine, and human health. Viruses 2021, 13, 1697. [Google Scholar] [CrossRef]
- Eidenberger, L.; Kogelmann, B.; Steinkellner, H. Plant-based biopharmaceutical engineering. Nat. Rev. Bioeng. 2023, 1, 426–439. [Google Scholar] [CrossRef]
- Cabedo Díaz, P.; Covarrubias, M.P.; Handford, M. Verifying plasmid constructs via transient Agrobacterium tumefaciens–mediated plant transformation in Nicotiana benthamiana. In Agrobacterium: Methods in Molecular Biology; Klein, C.S., Ed.; Humana: New York, NY, USA, 2025; Volume 2911, pp. 21–36. [Google Scholar]
- Bally, J.; Jung, H.; Mortimer, C.; Naim, F.; Philips, J.G.; Hellens, R.; Bombarely, A.; Goodin, M.M.; Waterhouse, P.M. The rise and rise of Nicotiana benthamiana: A plant for all reasons. Annu. Rev. Phytopathol. 2018, 56, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Singh, A.; Singh, A.K.; Dalmay, T.; Chakraborty, S. Tobacco RNA-dependent RNA polymerase 1 affects the expression of defence-related genes in Nicotiana benthamiana upon Tomato leaf curl Gujarat virus infection. Planta 2020, 252, 11. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Shirai, R.; Suzuki, T.; Matsushita, Y.; Sasaki, N. Enhanced virus infection in Nicotiana benthamiana transiently overexpressing MDP92, encoding a tobacco MYB transcription factor. JGPP 2025, 91, 69–82. [Google Scholar] [CrossRef]
- England, C.; TrejoMartinez, J.; PerezSanchez, P.; Karki, U.; Xu, J. Plants as biofactories for therapeutic proteins and antiviral compounds to combat COVID-19. Life 2023, 13, 617. [Google Scholar] [CrossRef]
- Chen, Q.; Lai, H.; Hurtado, J.; Stahnke, J.; Leuzinger, K.; Dent, M. Agroinfiltration as an effective and scalable strategy of gene delivery for production of pharmaceutical proteins. Adv. Tech. Biol. Med. 2013, 1, 103. [Google Scholar] [CrossRef]
- Kapila, J.; De Rycke, R.; Van Montagu, M.; Angenon, G. An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci. 1997, 122, 101–108. [Google Scholar] [CrossRef]
- Gleba, Y.; Marillonnet, S.; Klimyuk, V. Engineering viral expression vectors for plants: The ‘full virus’ and the ‘deconstructed virus’ strategies. Curr. Opin. Plant Biol. 2004, 7, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Naseri, Z.; Khezri, G.; Davarpanah, S.J.; Ofoghi, H. Virus-based vectors: A new approach for the production of recombinant proteins. Appl. Biotechnol. Rep. 2019, 6, 6–14. [Google Scholar] [CrossRef]
- Jadhav, R.R.; Khare, D. Green biotherapeutics: Overcoming challenges in plant-based expression platforms. Plant Biotechnol. Rep. 2024, 18, 465–486. [Google Scholar] [CrossRef]
- Tran, H.H. Developing a Plant Virus-Based Expression System for the Expression Of Vaccines against Porcine Reproductive and Respiratory Syndrome Virus. Ph.D. Thesis, The University of Western Ontario, London, ON, Canada, 2017. [Google Scholar]
- Gleba, Y.; Klimyuk, V.; Marillonnet, S. Magnifection—A new platform for expressing recombinant vaccines in plants. Vaccine 2005, 23, 2042–2048. [Google Scholar] [CrossRef]
- Sainsbury, F.; Thuenemann, E.C.; Lomonossoff, G.P. pEAQ: Versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol. J. 2009, 7, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Peyret, H.; Lomonossoff, G.P. The pEAQ vector series: The easy and quick way to produce recombinant proteins in plants. Plant Mol. Biol. 2013, 83, 51–58. [Google Scholar] [CrossRef]
- Peyret, H.; Brown, J.K.M.; Lomonossoff, G.P. Lomonossoff. Improving plant transient expression through the rational design of synthetic 5′ and 3′ untranslated regions. Plant Methods 2019, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Rozov, S.; Deineko, E. Recombinant VLP vaccines synthesized in plant expression systems: Current updates and prospects. Mol. Biol. 2024, 58, 402–418. [Google Scholar] [CrossRef]
- Mardanova, E.S.; Vasyagin, E.A.; Ravin, N.V. Virus-like particles produced in plants: A promising platform for recombinant vaccine development. Plants 2024, 13, 3564. [Google Scholar] [CrossRef]
- Diamos, A.G.; Mason, H.S. Modifying the replication of geminiviral vectors reduces cell death and enhances expression of biopharmaceutical proteins in Nicotiana benthamiana leaves. Front. Plant Sci. 2019, 9, 1974. [Google Scholar] [CrossRef]
- Rattanapisit, K.; Shanmugaraj, B.; Manopwisedjaroen, S.; Purwono, P.B.; Siriwattananon, K.; Khorattanakulchai, N.; Hanittinan, O.; Boonyayothin, W.; Thitithanyanont, A.; Smith, D.R.; et al. Rapid production of SARS-CoV-2 receptor binding domain (RBD) and spike specific monoclonal antibody CR3022 in Nicotiana benthamiana. Sci. Rep. 2020, 10, 17698. [Google Scholar] [CrossRef]
- Egelkrout, E.; Rajan, V.; Howard, J.A. Overproduction of recombinant proteins in plants. Plant Sci. 2012, 184, 83–101. [Google Scholar] [CrossRef]
- Nerkar, G.; Suresha, G.; Ram, B.; Appunu, C. Key Challenges in Developing Products from Transgenic Plants. In Advances in Plant Transgenics: Methods and Applications; Sathishkumar, R., Kumar, S.R., Hema, J., Baskar, V., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2019; pp. 315–331. [Google Scholar]
- Nikolov, Z.L.; Regan, J.T.; Dickey, L.F.; Woodard, S.L. Purification of monoclonal antibodies from plants. In Process Scale Purification of Antibodies, 2nd ed.; Gottschalk, U., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 631–653. [Google Scholar]
- Szeto, W.; Hamer, D.; Carlson, P.; Thomas, C., Jr. Cloning of cauliflower mosaic virus (CLMV) DNA in Escherichia coli. Science 1977, 196, 210–212. [Google Scholar] [CrossRef]
- Klein, T.M.; Wolf, E.D.; Wu, R.; Sanford, J.C. High-velocity microprojectiles for delivering nucleic acids into living cells. Nature 1987, 327, 70–73. [Google Scholar] [CrossRef]
- Marcotte, W.R., Jr.; Bayley, C.C.; Quatrano, R.S. Regulation of a wheat promoter by abscisic acid in rice protoplasts. Nature 1988, 335, 454–457. [Google Scholar] [CrossRef]
- Gronenborn, B.; Matzeit, V. Plant gene vectors and genetic transformation: Plant viruses as vectors. In Molecular Biology of Plant Nuclear Genes; Daniell, H., Streatfield, S.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 69–100. [Google Scholar]
- Takamatsu, N.; Ishikawa, M.; Meshi, T.; Okada, Y. Expression of bacterial chloramphenicol acetyltransferase gene in tobacco plants mediated by TMV-RNA. EMBO J. 1987, 6, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.L.; Joshi, V.; Ow, D. BSMV genome mediated expression of a foreign gene in dicot and monocot plant cells. EMBO J. 1990, 9, 2663–2669. [Google Scholar] [CrossRef] [PubMed]
- Donson, J.; Kearney, C.; Hilf, M.A.; Dawson, W. Systemic expression of a bacterial gene by a tobacco mosaic virus-based vector. Proc. Natl. Acad. Sci. USA 1991, 88, 7204–7208. [Google Scholar] [CrossRef]
- Tang, X.; Frederick, R.D.; Zhou, J.; Halterman, D.A.; Jia, Y.; Martin, G.B. Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science 1996, 274, 2060–2063. [Google Scholar] [CrossRef]
- Schöb, H.; Kunz, C.; Meins Jr, F. Silencing of transgenes introduced into leaves by agroinfiltration: A simple, rapid method for investigating sequence requirements for gene silencing. Mol. Gen. Genet. 1997, 256, 581–585. [Google Scholar] [CrossRef]
- Giritch, A.; Marillonnet, S.; Engler, C.; van Eldik, G.; Botterman, J.; Klimyuk, V.; Gleba, Y. Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc. Natl. Acad. Sci. USA 2006, 103, 14701–14706. [Google Scholar] [CrossRef]
- Marillonnet, S.; Giritch, A.; Gils, M.; Kandzia, R.; Klimyuk, V.; Gleba, Y. In planta engineering of viral RNA replicons: Efficient assembly by recombination of DNA modules delivered by Agrobacterium. Proc. Natl. Acad. Sci. USA 2004, 101, 6852–6857. [Google Scholar] [CrossRef]
- Erokhina, T.; Ryabukhina, E.V.; Lyapina, I.S.; Ryazantsev, D.Y.; Zavriev, S.K.; Morozov, S.Y. Promising Biotechnological Applications of the Artificial Derivatives Designed and Constructed from Plant microRNA Genes. Plants 2025, 14, 325. [Google Scholar] [CrossRef]
- Komarova, T.; Skulachev, M.; Zvereva, A.; Schwartz, A.; Dorokhov, Y.L.; Atabekov, J. New viral vector for efficient production of target proteins in plants. Biochemistry 2006, 71, 846–850. [Google Scholar] [CrossRef]
- Merwaiss, F.; Lozano-Sanchez, E.; Zulaica, J.; Rusu, L.; Vazquez-Vilar, M.; Orzáez, D.; Rodrigo, G.; Geller, R.; Daròs, J.A. Plant virus-derived nanoparticles decorated with genetically encoded SARS-CoV-2 nanobodies display enhanced neutralizing activity. Plant Biotechnol. J. 2024, 22, 876–891. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, F.; Lomonossoff, G.P. Extremely high-level and rapid transient protein production in plants without the use of viral replication. Plant Physiol. 2008, 148, 1212–1218. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Q.; Hjelm, B.; Arntzen, C.; Mason, H. A DNA replicon system for rapid high-level production of virus-like particles in plants. Biotechnol. Bioeng. 2009, 103, 706–714. [Google Scholar] [CrossRef]
- Mor, T.S.; Moon, Y.S.; Palmer, K.E.; Mason, H.S. Geminivirus vectors for high-level expression of foreign proteins in plant cells. Biotechnol. Bioeng. 2003, 81, 430–437. [Google Scholar] [CrossRef]
- García Pérez, E. Development of a Copper Sensor and Geminivirus-Based Processors for Engineering Synthetic Gene Circuits in Plants. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, December 2024. [Google Scholar]
- Marillonnet, S.; Thoeringer, C.; Kandzia, R.; Klimyuk, V.; Gleba, Y. Systemic Agrobacterium tumefaciens–mediated transfection of viral replicons for efficient transient expression in plants. Nat. Biotechnol. 2005, 23, 718–723. [Google Scholar] [CrossRef]
- Fox, J.L. First plant-made biologic approved. Nat. Biotechnol. 2012, 30, 472–473. [Google Scholar] [CrossRef]
- Zeitlin, L.; Pettitt, J.; Scully, C.; Bohorova, N.; Kim, D.; Pauly, M.; Hiatt, A.; Ngo, L.; Steinkellner, H.; Whaley, K.J. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc. Natl. Acad. Sci. USA 2011, 108, 20690–20694. [Google Scholar] [CrossRef] [PubMed]
- Phoolcharoen, W.; Dye, J.M.; Kilbourne, J.; Piensook, K.; Pratt, W.D.; Arntzen, C.J.; Chen, Q.; Mason, H.S.; Herbst-Kralovetz, M.M. A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge. Proc. Natl. Acad. Sci. USA 2011, 108, 20695–20700. [Google Scholar] [CrossRef]
- Arntzen, C. Plant-made pharmaceuticals: From ‘Edible Vaccines’ to Ebola therapeutics. Plant Biotechnol. J. 2015, 13, 1013. [Google Scholar] [CrossRef]
- Dhama, K.; Natesan, S.; Iqbal Yatoo, M.; Patel, S.K.; Tiwari, R.; Saxena, S.K.; Harapan, H. Plant-based vaccines and antibodies to combat COVID-19: Current status and prospects. Hum. Vaccines Immunother. 2020, 16, 2913–2920. [Google Scholar] [CrossRef]
- Hager, K.J.; Pérez Marc, G.; Gobeil, P.; Diaz, R.S.; Heizer, G.; Llapur, C.; Makarkov, A.I.; Vasconcellos, E.; Pillet, S.; Riera, F. Efficacy and safety of a recombinant plant-based adjuvanted COVID-19 vaccine. N. Engl. J. Med. 2022, 386, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Benvenuto, E.; Broer, I.; D’Aoust, M.-A.; Hitzeroth, I.; Hundleby, P.; Menassa, R.; Oksman-Caldentey, K.-M.; Peyret, H.; Salgueiro, S.; Saxena, P. Plant molecular farming in the wake of the closure of Medicago Inc. Nat. Biotechnol. 2023, 41, 893–894. [Google Scholar] [CrossRef]
- Chung, Y.H.; Church, D.; Koellhoffer, E.C.; Osota, E.; Shukla, S.; Rybicki, E.P.; Pokorski, J.K.; Steinmetz, N.F. Integrating plant molecular farming and materials research for next-generation vaccines. Nat. Rev. Mater. 2022, 7, 372–388. [Google Scholar] [CrossRef] [PubMed]
- Nosaki, S.; Hoshikawa, K.; Ezura, H.; Miura, K. Transient protein expression systems in plants and their applications. Plant Biotechnol. 2021, 38, 297–304. [Google Scholar] [CrossRef]
- Castells-Graells, R.; Lomonossoff, G.P. Plant-based production can result in covalent cross-linking of proteins. Plant Biotechnol. J. 2021, 19, 1095. [Google Scholar] [CrossRef] [PubMed]
- Lindbo, J.A. TRBO: A high-efficiency tobacco mosaic virus RNA-based overexpression vector. Plant Physiol. 2007, 145, 1232–1240. [Google Scholar] [CrossRef]
- Diamos, A.G.; Hunter, J.G.; Pardhe, M.D.; Rosenthal, S.H.; Sun, H.; Foster, B.C.; DiPalma, M.P.; Chen, Q.; Mason, H.S. High level production of monoclonal antibodies using an optimized plant expression system. Front. Bioeng. Biotechnol. 2020, 7, 472. [Google Scholar] [CrossRef] [PubMed]
- Pantazica, A.M.; van Eerde, A.; Dobrica, M.O.; Caras, I.; Ionescu, I.; Costache, A.; Tucureanu, C.; Steen, H.; Lazar, C.; Heldal, I. The “humanized” N-glycosylation pathway in CRISPR/Cas9-edited Nicotiana benthamiana significantly enhances the immunogenicity of a S/preS1 Hepatitis B Virus antigen and the virus-neutralizing antibody response in vaccinated mice. Plant Biotechnol. J. 2023, 21, 1176–1190. [Google Scholar] [CrossRef]
- Strasser, R. Engineering of human-type O-glycosylation in Nicotiana benthamiana plants. Bioengineered 2013, 4, 191–196. [Google Scholar] [CrossRef]
- Coates, R.J.; Scofield, S.; Young, M.T. Incorporation of regulatory DNA elements within a viral vector improves recombinant protein expression in plants. Sci. Rep. 2024, 14, 28865. [Google Scholar] [CrossRef]
- Hefferon, K. Plant virus expression vectors: A powerhouse for global health. Biomedicines 2017, 5, 44. [Google Scholar] [CrossRef]
- Fujiuchi, N.; Matoba, N.; Fujiwara, K.; Matsuda, R. Effects of lighting conditions on Agrobacterium-mediated transient expression of recombinant hemagglutinin in detached Nicotiana benthamiana leaves inoculated with a deconstructed viral vector. Plant Cell Tiss. Organ Cult. 2021, 145, 679–688. [Google Scholar] [CrossRef]
- Gleba, Y.; Klimyuk, V.; Marillonnet, S. Viral vectors for the expression of proteins in plants. Curr. Opin. Biotechnol. 2007, 18, 134–141. [Google Scholar] [CrossRef]
- Shcherbakov, D.N.; Isaeva, A.A.; Mustaev, E.A. Treatment of Ebola Virus Disease: From Serotherapy to the Use of Monoclonal Antibodies. Antibodies 2025, 14, 22. [Google Scholar] [CrossRef]
- Xie, D.; Cao, L.; Guo, M.; Wang, L.; Zhang, X.; Huang, S. Study on the Recombinant Human Interferon α1b, α2b, and Gamma Transient Expression and in Vitro Activities in Tobacco. J. Interferon Cytokine Res. 2024, 44, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Keshvari, T.; Melnik, S.; Sun, L.; Niazi, A.; Aram, F.; Moghadam, A.; Kogelmann, B.; Wozniak-Knopp, G.; Kallolimath, S.; Ramezani, A. Efficient expression of functionally active aflibercept with designed N-glycans. Antibodies 2024, 13, 29. [Google Scholar] [CrossRef]
- Modarresi, M.; Javaran, M.J.; Shams-Bakhsh, M.; Zeinali, S.; Behdani, M.; Mirzaee, M. Transient expression of anti-VEFGR2 nanobody in Nicotiana tabacum and N. benthamiana. 3 Biotech 2018, 8, 484. [Google Scholar] [CrossRef] [PubMed]
- Kallolimath, S.; Palt, R.; Föderl-Höbenreich, E.; Sun, L.; Chen, Q.; Pruckner, F.; Eidenberger, L.; Strasser, R.; Zatloukal, K.; Steinkellner, H. Glyco engineered pentameric SARS-CoV-2 IgMs show superior activities compared to IgG1 orthologues. Front. Immunol. 2023, 14, 1147960. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, I.; Maes, C.; Joye, J.; Jacobs, B.; Jarczowski, F.; Diessner, A.; Janssens, Y.; Waerlop, G.; Tamminen, K.; Heinimäki, S. A randomized, double-blind, placebo-controlled, dose-escalating phase I trial to evaluate safety and immunogenicity of a plant-produced, bivalent, recombinant norovirus-like particle vaccine. Front. Immunol. 2022, 13, 1021500. [Google Scholar] [CrossRef]
- Tusé, D.; Malm, M.; Tamminen, K.; Diessner, A.; Thieme, F.; Jarczowski, F.; Blazevic, V.; Klimyuk, V. Safety and immunogenicity studies in animal models support clinical development of a bivalent norovirus-like particle vaccine produced in plants. Vaccine 2022, 40, 977–987. [Google Scholar] [CrossRef]
- Chen, Q.; He, J.; Phoolcharoen, W.; Mason, H.S. Geminiviral vectors based on bean yellow dwarf virus for production of vaccine antigens and monoclonal antibodies in plants. Hum. Vaccines 2011, 7, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hoshikawa, K.; Ezura, K.; Okazawa, R.; Fujita, S.; Takaoka, M.; Mason, H.S.; Ezura, H.; Miura, K. Improvement of the transient expression system for production of recombinant proteins in plants. Sci. Rep. 2018, 8, 4755. [Google Scholar] [CrossRef]
- Ceballo, Y.; López, A.; González, C.E.; Ramos, O.; Andújar, I.; Martínez, R.U.; Hernández, A. Transient production of receptor-binding domain of SARS-CoV-2 in Nicotiana benthamiana plants induces specific antibodies in immunized mice. Mol. Biol. Rep. 2022, 49, 6113–6123. [Google Scholar] [CrossRef]
- Bulaon, C.J.I.; Khorattanakulchai, N.; Rattanapisit, K.; Sun, H.; Pisuttinusart, N.; Strasser, R.; Tanaka, S.; Soon-Shiong, P.; Phoolcharoen, W. Antitumor effect of plant-produced anti-CTLA-4 monoclonal antibody in a murine model of colon cancer. Front. Plant Sci. 2023, 14, 1149455. [Google Scholar] [CrossRef]
- Rattanapisit, K.; Bulaon, C.J.I.; Strasser, R.; Sun, H.; Phoolcharoen, W. In vitro and in vivo studies of plant-produced Atezolizumab as a potential immunotherapeutic antibody. Sci. Rep. 2023, 13, 14146. [Google Scholar] [CrossRef]
- Krittanai, S.; Rattanapisit, K.; Bulaon, C.J.I.; Pitaksajjakul, P.; Keadsanti, S.; Ramasoota, P.; Strasser, R.; Phoolcharoen, W. Nicotiana benthamiana as a potential source for producing anti-dengue virus D54 neutralizing therapeutic antibody. Biotechnol. Rep. 2024, 42, e00844. [Google Scholar] [CrossRef]
- Klimyuk, V.; Pogue, G.; Herz, S.; Butler, J.; Haydon, H. Production of recombinant antigens and antibodies in Nicotiana benthamiana using ‘magnifection’ technology: GMP-compliant facilities for small- and large-scale manufacturing. In Plant Viral Vectors; Gleba, Y., Klimyuk, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 375, pp. 127–154. [Google Scholar]
- Hoshikawa, K.; Fujita, S.; Renhu, N.; Ezura, K.; Yamamoto, T.; Nonaka, S.; Ezura, H.; Miura, K. Efficient transient protein expression in tomato cultivars and wild species using agroinfiltration-mediated high expression system. Plant Cell Rep. 2019, 38, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; He, J.; Engle, M.; Diamond, M.S.; Chen, Q. Robust production of virus-like particles and monoclonal antibodies with geminiviral replicon vectors in lettuce. Plant Biotechnol. J. 2012, 10, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Diamos, A.G.; Mason, H.S. High-level expression and enrichment of norovirus virus-like particles in plants using modified geminiviral vectors. Protein Expr. Purif. 2018, 151, 86–92. [Google Scholar] [CrossRef]
- Regnard, G.L.; Halley-Stott, R.P.; Tanzer, F.L.; Hitzeroth, I.I.; Rybicki, E.P. High level protein expression in plants through the use of a novel autonomously replicating geminivirus shuttle vector. Plant Biotechnol. J. 2010, 1, 38–46. [Google Scholar] [CrossRef]
- Leuzinger, K.; Dent, M.; Hurtado, J.; Stahnke, J.; Lai, H.; Zhou, X.; Chen, Q. Efficient agroinfiltration of plants for high-level transient expression of recombinant proteins. J. Vis. Exp. 2013, 77, 50521. [Google Scholar]
- Nandi, S.; Kwong, A.T.; Holtz, B.R.; Erwin, R.L.; Marcel, S.; McDonald, K.A. Techno-economic analysis of a transient plant-based platform for monoclonal antibody production. mAbs 2016, 8, 1456–1466. [Google Scholar] [CrossRef]
- Fernández, F.J.; Gómez, S.; Vega, M.C. High-throughput protein production in yeast. In High-Throughput Protein Production and Purification: Methods and Protocols; Chen, Y.W., Ed.; Springer: New York, NY, USA, 2019; pp. 69–91. [Google Scholar]
- Tominaga, M.; Shima, Y.; Nozaki, K.; Ito, Y.; Someda, M.; Shoya, Y.; Hashii, N.; Obata, C.; Matsumoto-Kitano, M.; Suematsu, K. Designing strong inducible synthetic promoters in yeasts. Nat. Commun. 2024, 15, 10653. [Google Scholar] [CrossRef]
- Moon, K.-B.; Park, J.-S.; Park, Y.-I.; Song, I.-J.; Lee, H.-J.; Cho, H.S.; Jeon, J.-H.; Kim, H.S. Development of systems for the production of plant-derived biopharmaceuticals. Plants 2019, 9, 30. [Google Scholar] [CrossRef]
- Pogue, G.P.; Vojdani, F.; Palmer, K.E.; Hiatt, E.; Hume, S.; Phelps, J.; Long, L.; Bohorova, N.; Kim, D.; Pauly, M.; et al. Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotechnol. J. 2010, 8, 638–654. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A. Recent advances in recombinant production of soluble proteins in E. coli. Microb. Cell Factories 2025, 24, 21. [Google Scholar] [CrossRef]
- dos Santos, P.B.; Léo, P.; de Souza Oliveira, R.P.; Stephano, M.A. Mammalian cell culture technology. In Pharmaceutical Biotechnology; Galdino, A.S., Meneguello, L., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 195–223. [Google Scholar]
- Mukherjee, S.; Malik, P.; Mukherjee, T.K. Mammalian Cells, Tissues and Organ Culture: Applications. In Practical Approach to Mammalian Cell and Organ Culture; Lim, Y.B., Ed.; Springer Nature: Singapore, 2023; pp. 837–915. [Google Scholar]
- Ceaglio, N.; Oggero, M. Development and Production of Protein-Based Biotherapeutics in Mammalian Cells. In Topics in Medicinal Chemistry; Springer: Cham, Switzerland, 2024; Volume 42, pp. 1–58. [Google Scholar]
- Joshi, M.; Verma, P.; Mago, P.; Agrawal, Y.; Gunwal, I.; Khurana, S.; Tanwar, J.; Yadav, U.; Yadav, A. Recombinant protein expression in mammalian cells. In Fundamentals of Recombinant Protein Production, Purification and Characterization; Yadav, D., Guldhe, A., Kudanga, T., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 79–102. [Google Scholar]
- Zitzmann, J.; Sprick, G.; Weidner, T.; Schreiber, C.; Czermak, P. Process optimization for recombinant protein expression in insect cells. In New Insights into Cell Culture Technology, 1st ed.; Gowder, S.J.T., Ed.; InTech: Rijeka, Croatia, 2017; pp. 43–97. [Google Scholar]
- Cox, M.M. Innovations in the insect cell expression system for industrial recombinant vaccine antigen production. Vaccines 2021, 9, 1504. [Google Scholar] [CrossRef]
- Ma, K.; Deng, L.; Wu, H.; Fan, J. Towards green biomanufacturing of high-value recombinant proteins using promising cell factory: Chlamydomonas reinhardtii chloroplast. Bioresour. Bioprocess. 2022, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Malla, A.; Rosales-Mendoza, S.; Phoolcharoen, W.; Vimolmangkang, S. Efficient transient expression of recombinant proteins using DNA viral vectors in freshwater microalgal species. Front. Plant Sci. 2021, 12, 650820. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, Y.-G.; Lee, G.M. CHO cells in biotechnology for production of recombinant proteins: Current state and further potential. Appl. Microbiol. Biotechnol. 2012, 93, 917–930. [Google Scholar] [CrossRef]
- O’Flaherty, R.; Bergin, A.; Flampouri, E.; Mota, L.M.; Obaidi, I.; Quigley, A.; Xie, Y.; Butler, M. Mammalian cell culture for production of recombinant proteins: A review of the critical steps in their biomanufacturing. Biotechnol. Adv. 2020, 43, 107552. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.K.; Magoola, M. Advances in Escherichia coli-based therapeutic protein expression: Mammalian conversion, continuous manufacturing, and cell-free production. Biologics 2023, 3, 380–401. [Google Scholar] [CrossRef]
- Fiebig, D.; Bogen, J.P.; Carrara, S.C.; Deweid, L.; Zielonka, S.; Grzeschik, J.; Hock, B.; Kolmar, H. Streamlining the transition from yeast surface display of antibody fragment immune libraries to the production as IgG format in mammalian cells. Front. Bioeng. Biotechnol. 2022, 10, 794389. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, D.L. Baculovirus–insect cell expression systems. Methods Enzymol. 2009, 463, 191–222. [Google Scholar]
- Baldi, L.; Hacker, D.L.; Adam, M.; Wurm, F.M. Recombinant protein production by large-scale transient gene expression in mammalian cells: State of the art and future perspectives. Biotechnol. Lett. 2007, 29, 677–684. [Google Scholar] [CrossRef]
- Sahdev, S.; Khattar, S.K.; Saini, K.S. Production of active eukaryotic proteins through bacterial expression systems: A review of the existing biotechnology strategies. Mol. Cell. Biochem. 2008, 307, 249–264. [Google Scholar] [CrossRef]
- Cregg, J.M.; Tolstorukov, I.; Kusari, A.; Sunga, J.; Madden, K.; Chappell, T. Expression in the yeast Pichia pastoris. Methods Enzymol. 2009, 463, 169–189. [Google Scholar]
- Shen, X.; Pitol, A.K.; Bachmann, V.; Hacker, D.L.; Baldi, L.; Wurm, F.M. A simple plasmid-based transient gene expression method using High Five cells. J. Biotechnol. 2015, 216, 67–75. [Google Scholar] [CrossRef]
- Siddiqui, A.; Wei, Z.; Boehm, M.; Ahmad, N. Engineering microalgae through chloroplast transformation to produce high-value industrial products. Biotechnol. Appl. Biochem. 2020, 67, 30–40. [Google Scholar] [CrossRef]
- Gecchele, E.; Merlin, M.; Brozzetti, A.; Falorni, A.; Pezzotti, M.; Avesani, L. A comparative analysis of recombinant protein expression in different biofactories: Bacteria, insect cells and plant systems. J. Vis. Exp. 2015, 97, 52459. [Google Scholar] [CrossRef]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef] [PubMed]
- de Grahl, I.; Rout, S.S.; Maple-Grødem, J.; Reumann, S. Development of a constitutive and an auto-inducible high-yield expression system for recombinant protein production in the microalga Nannochloropsis oceanica. Appl. Microbiol. Biotechnol. 2020, 104, 8747–8760. [Google Scholar] [CrossRef]
- Yan, N.; Fan, C.; Chen, Y.; Hu, Z. The potential for microalgae as bioreactors to produce pharmaceuticals. Int. J. Mol. Sci. 2016, 17, 962. [Google Scholar] [CrossRef]
- Ahmad, N.; Mukhtar, Z. Green factories: Plastids for the production of foreign proteins at high levels. Gene Ther. Mol. Biol. 2013, 15, 14–29. [Google Scholar]
- Daskalova, S.M.; Radder, J.E.; Cichacz, Z.A.; Olsen, S.H.; Tsaprailis, G.; Mason, H.; Lopez, L.C. Engineering of N. benthamiana L. plants for production of N-acetylgalactosamine-glycosylated proteins-towards development of a plant-based platform for production of protein therapeutics with mucin type O-glycosylation. BMC Biotechnol. 2010, 10, 62. [Google Scholar]
- Tokmakov, A.A.; Kurotani, A.; Takagi, T.; Toyama, M.; Shirouzu, M.; Fukami, Y.; Yokoyama, S. Multiple post-translational modifications affect heterologous protein synthesis. J. Biol. Chem. 2012, 287, 27106–27116. [Google Scholar] [CrossRef] [PubMed]
- De Pourcq, K.; De Schutter, K.; Callewaert, N. Engineering of glycosylation in yeast and other fungi: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 87, 1617–1631. [Google Scholar] [CrossRef]
- Geisler, C.; Mabashi-Asazuma, H.; Jarvis, D.L. An overview and history of glyco-engineering in insect expression systems. In Glyco-Engineering: Methods and Protocols; Castilho, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 1321, pp. 131–152. [Google Scholar]
- Banerjee, A.; Ward, V. Production of recombinant and therapeutic proteins in microalgae. Curr. Opin. Biotechnol. 2022, 78, 102784. [Google Scholar] [CrossRef]
- Kaur, M.; Manchanda, P.; Kalia, A.; Ahmed, F.K.; Nepovimova, E.; Kuca, K.; Abd-Elsalam, K.A. Agroinfiltration mediated scalable transient gene expression in genome edited crop plants. Int. J. Mol. Sci. 2021, 22, 10882. [Google Scholar] [CrossRef]
- Gutiérrez-Granados, S.; Cervera, L.; Kamen, A.A.; Gòdia, F. Advancements in mammalian cell transient gene expression (TGE) technology for accelerated production of biologics. Crit. Rev. Biotechnol. 2018, 38, 918–940. [Google Scholar] [CrossRef]
- Khow, O.; Suntrarachun, S. Strategies for production of active eukaryotic proteins in bacterial expression system. Asian Pac. J. Trop. Biomed. 2012, 2, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Vandermies, M.; Fickers, P. Bioreactor-scale strategies for the production of recombinant protein in the yeast Yarrowia lipolytica. Microorganisms 2019, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Suppmann, S. A new single-step protocol for rapid baculovirus-driven protein production in insect cells. BMC Biotechnol. 2017, 17, 83. [Google Scholar] [CrossRef]
- Hammel, A.; Neupert, J.; Bock, R. Optimized transgene expression in the red alga Porphyridium purpureum and efficient recombinant protein secretion into the culture medium. Plant Mol. Biol. 2024, 114, 18. [Google Scholar] [CrossRef] [PubMed]
- Kurotani, K.-i.; Hirakawa, H.; Shirasawa, K.; Tanizawa, Y.; Nakamura, Y.; Isobe, S.; Notaguchi, M. Genome sequence and analysis of Nicotiana benthamiana, the model plant for interactions between organisms. Plant Cell Physiol. 2023, 64, 248–257. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Tung, J.; Zhang, X.; Liu, D.; Deng, Y.; Tian, Z.; Chen, H.; Wang, T.; Yin, W. High-quality assembled and annotated genomes of Nicotiana tabacum and Nicotiana benthamiana reveal chromosome evolution and changes in defense arsenals. Mol. Plant. 2024, 17, 423–437. [Google Scholar] [CrossRef]
- Nausch, H.; Mikschofsky, H.; Koslowski, R.; Meyer, U.; Broer, I.; Huckauf, J. High-level transient expression of ER-targeted human interleukin 6 in Nicotiana benthamiana. PLoS ONE 2012, 7, e48938. [Google Scholar] [CrossRef]
- Park, S.H.; Ji, K.-Y.; Kim, H.M.; Ma, S.H.; Park, S.Y.; Do, J.H.; Oh, D.-B.; Kang, H.S.; Shim, J.S.; Joung, Y.H. Optimization of the human colorectal carcinoma antigen GA733-2 production in tobacco plants. Plant Biotechnol. Rep. 2021, 15, 55–67. [Google Scholar] [CrossRef]
- Golubova, D.; Tansley, C.; Su, H.; Patron, N. Engineering Nicotiana benthamiana as a platform for natural product biosynthesis. Curr. Opin. Plant Biol. 2024, 81, 102611. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Li, Z. Transgene-free genome editing in Nicotiana benthamiana with CRISPR/Cas9 delivered by a rhabdovirus vector. In Plant Genome Engineering: Methods and Protocols; Yang, B., Harwood, W., Que, Q., Eds.; Springer: New York, NY, USA, 2023; pp. 173–185. [Google Scholar]
- Shaaltiel, Y.; Tekoah, Y. Plant specific N-glycans do not have proven adverse effects in humans. Nat. Biotechnol. 2016, 34, 706–708. [Google Scholar] [CrossRef]
- Jansing, J.; Sack, M.; Augustine, S.M.; Fischer, R.; Bortesi, L. CRISPR/Cas9-mediated knockout of six glycosyltransferase genes in Nicotiana benthamiana for the production of recombinant proteins lacking β-1, 2-xylose and core α-1, 3-fucose. Plant Biotechnol. J. 2019, 17, 350–361. [Google Scholar] [CrossRef]
- Larsen, J.S.; Karlsson, R.T.G.; Tian, W.; Schulz, M.A.; Matthes, A.; Clausen, H.; Petersen, B.L.; Yang, Z. Engineering mammalian cells to produce plant-specific N-glycosylation on proteins. Glycobiology 2020, 30, 528–538. [Google Scholar] [CrossRef]
- Kogelmann, B.; Melnik, S.; Bogner, M.; Kallolimath, S.; Stöger, E.; Sun, L.; Strasser, R.; D’Aoust, M.A.; Lavoie, P.O.; Saxena, P.; et al. A genome-edited N. benthamiana line for industrial-scale production of recombinant glycoproteins with targeted N-glycosylation. Biotechnol. J. 2024, 19, 2300323. [Google Scholar]
- Eidenberger, L.; Eminger, F.; Castilho, A.; Steinkellner, H. Comparative analysis of plant transient expression vectors for targeted N-glycosylation. Front. Bioeng. Biotechnol. 2022, 10, 1073455. [Google Scholar] [CrossRef] [PubMed]
- Knödler, M.; Buyel, J.F. Extraction and purification of malaria vaccine candidate CLCT produced by transient expression in Nicotiana benthamiana plants. Discov. Chem. Eng. 2023, 3, 14. [Google Scholar] [CrossRef]
- Zhou, N.; Thilakarathna, W.W.; He, Q.S.; Rupasinghe, H.V. A review: Depolymerization of lignin to generate high-value bio-products: Opportunities, challenges, and prospects. Front. Energy Res. 2022, 9, 758744. [Google Scholar] [CrossRef]
- Gimenes, N.C.; Silveira, E.; Tambourgi, E.B. An overview of proteases: Production, downstream processes and industrial applications. Sep. Purif. Rev. 2021, 50, 223–243. [Google Scholar] [CrossRef]
- Weiss, J.; Mannweiler, S.; Salminen, H. Precision processing for value-added fats and oils. Annu. Rev. Food Sci. Technol. 2025, 16, 39–61. [Google Scholar] [CrossRef]
- Menzel, S.; Holland, T.; Boes, A.; Spiegel, H.; Fischer, R.; Buyel, J.F. Downstream processing of a plant-derived malaria transmission-blocking vaccine candidate. Protein Expr. Purif. 2018, 152, 122–130. [Google Scholar] [CrossRef]
- Martin, R.; Liu, F.; Staskawicz, B. Isolation of Protein Complexes from Tobacco Leaves by a Two-Step Tandem Affinity Purification. Curr. Protoc. 2022, 2, e572. [Google Scholar] [CrossRef]
- Opdensteinen, P.; Lobanov, A.; Buyel, J.F. A combined pH and temperature precipitation step facilitates the purification of tobacco-derived recombinant proteins that are sensitive to extremes of either parameter. Biotechnol. J. 2021, 16, 2000340. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.; Hahn-Löbmann, S.; Rosche, F.; Buchholz, M.; Giritch, A.; Gleba, Y. Simple purification of Nicotiana benthamiana-produced recombinant colicins: High-yield recovery of purified proteins with minimum alkaloid content supports the suitability of the host for manufacturing food additives. Int. J. Mol. Sci. 2017, 19, 95. [Google Scholar] [CrossRef] [PubMed]
- Opdensteinen, P.; Clodt, J.I.; Müschen, C.R.; Filiz, V.; Buyel, J.F. A combined ultrafiltration/diafiltration step facilitates the purification of cyanovirin-N from transgenic tobacco extracts. Front. Bioeng. Biotechnol. 2019, 6, 206. [Google Scholar] [CrossRef]
- Faye, L.; Grünwald-Gruber, C.; Vezina, L.-P.; Gomord, V.; Morel, B. A fast and easy one-step purification strategy for plant-made antibodies using Protein A magnetic beads. Front. Plant Sci. 2024, 14, 1276148. [Google Scholar] [CrossRef]
- Su, H.; Van Eerde, A.; Rimstad, E.; Bock, R.; Branza-Nichita, N.; Yakovlev, I.A.; Clarke, J.L. Plant-made vaccines against viral diseases in humans and farm animals. Front. Plant Sci. 2023, 14, 1170815. [Google Scholar] [CrossRef] [PubMed]
- Holtz, B.R.; Berquist, B.R.; Bennett, L.D.; Kommineni, V.J.; Munigunti, R.K.; White, E.L.; Wilkerson, D.C.; Wong, K.Y.I.; Ly, L.H.; Marcel, S. Commercial-scale biotherapeutics manufacturing facility for plant-made pharmaceuticals. Plant Biotechnol. J. 2015, 13, 1180–1190. [Google Scholar] [CrossRef]
- Ojo, M.O.; Zahid, A. Deep learning in controlled environment agriculture: A review of recent advancements, challenges and prospects. Sensors 2022, 22, 7965. [Google Scholar] [CrossRef]
- Rathor, A.S.; Choudhury, S.; Sharma, A.; Nautiyal, P.; Shah, G. Empowering vertical farming through IoT and AI-Driven technologies: A comprehensive review. Heliyon 2024, 10, e34998. [Google Scholar] [CrossRef]
- Rademacher, T.; Sack, M.; Blessing, D.; Fischer, R.; Holland, T.; Buyel, J. Plant cell packs: A scalable platform for recombinant protein production and metabolic engineering. Plant Biotechnol. J. 2019, 17, 1560–1566. [Google Scholar] [CrossRef]
- Gengenbach, B.B.; Opdensteinen, P.; Buyel, J.F. Robot cookies–plant cell packs as an automated high-throughput screening platform based on transient expression. Front. Bioeng. Biotechnol. 2020, 8, 393. [Google Scholar] [CrossRef]
- Poborilova, Z.; Plchova, H.; Cerovska, N.; Gunter, C.J.; Hitzeroth, I.I.; Rybicki, E.P.; Moravec, T. Transient protein expression in tobacco BY-2 plant cell packs using single and multi-cassette replicating vectors. Plant Cell Rep. 2020, 39, 1115–1127. [Google Scholar] [CrossRef]
- Skarjinskaia, M.; Ruby, K.; Araujo, A.; Taylor, K.; Gopalasamy-Raju, V.; Musiychuk, K.; Chichester, J.A.; Palmer, G.A.; de la Rosa, P.; Mett, V. Hairy roots as a vaccine production and delivery system. In Biotechnology of Hairy Root Systems; Doran, P.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 134, pp. 115–134. [Google Scholar]
- Ron, M.; Kajala, K.; Pauluzzi, G.; Wang, D.; Reynoso, M.A.; Zumstein, K.; Garcha, J.; Winte, S.; Masson, H.; Inagaki, S. Hairy root transformation using Agrobacterium rhizogenes as a tool for exploring cell type-specific gene expression and function using tomato as a model. Plant Physiol. 2014, 166, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.; Gemeda, H.B.; McNulty, M.J.; McDonald, K.A.; Nandi, S.; Knipe, J.M. Immobilization of transgenic plant cells towards bioprinting for production of a recombinant biodefense agent. Biotechnol. J. 2021, 16, 2100133. [Google Scholar] [CrossRef] [PubMed]
- Beyer, H.M.; Ramírez, V. Integrating bioprinting and optogenetic technologies for precision plant tissue engineering. Curr. Opin. Biotechnol. 2024, 89, 103193. [Google Scholar] [CrossRef]
- Ortega, C.; Prieto, D.; Abreu, C.; Oppezzo, P.; Correa, A. Multi-compartment and multi-host vector suite for recombinant protein expression and purification. Front. Microbiol. 2018, 9, 1384. [Google Scholar] [CrossRef] [PubMed]
- Bleckmann, M.; Schürig, M.; Endres, M.; Samuels, A.; Gebauer, D.; Konisch, N.; van den Heuvel, J. Identifying parameters to improve the reproducibility of transient gene expression in High Five cells. PLoS ONE 2019, 14, e0217878. [Google Scholar] [CrossRef]
- Grosse-Holz, F.; Madeira, L.; Zahid, M.A.; Songer, M.; Kourelis, J.; Fesenko, M.; Ninck, S.; Kaschani, F.; Kaiser, M.; van der Hoorn, R.A. Three unrelated protease inhibitors enhance accumulation of pharmaceutical recombinant proteins in Nicotiana benthamiana. Plant Biotechnol. J. 2018, 16, 1797–1810. [Google Scholar] [CrossRef]
- Beritza, K.; Watts, E.C.; van der Hoorn, R.A. Improving transient protein expression in agroinfiltrated Nicotiana benthamiana. New Phytol. 2024, 243, 846–850. [Google Scholar] [CrossRef]
- Strasser, R. Plant glycoengineering for designing next-generation vaccines and therapeutic proteins. Biotechnol. Adv. 2023, 67, 108197. [Google Scholar] [CrossRef]
- Buyel, J.F.; Twyman, R.M.; Fischer, R. Very-large-scale production of antibodies in plants: The biologization of manufacturing. Biotechnol. Adv. 2017, 35, 458–465. [Google Scholar] [CrossRef]
- Buyel, J.F. Plant molecular farming–integration and exploitation of side streams to achieve sustainable biomanufacturing. Front. Plant Sci. 2019, 9, 1893. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, F.; Lomonossoff, G.P. Transient expressions of synthetic biology in plants. Curr. Opin. Plant Biol. 2014, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bibik, J.D.; Bryson, A.E.; Hamberger, B. Compartmentalized terpenoid production in plants using Agrobacterium-mediated transient expression. In Synthetic Biology: Methods and Protocols; Braman, J.C., Ed.; Springer: New York, NY, USA, 2024; Volume 2760, pp. 21–34. [Google Scholar]
- Singh, A.A.; Pillay, P.; Naicker, P.; Alexandre, K.; Malatji, K.; Mach, L.; Steinkellner, H.; Vorster, J.; Chikwamba, R.; Tsekoa, T.L. Transient proteolysis reduction of Nicotiana benthamiana-produced CAP256 broadly neutralizing antibodies using CRISPR/Cas9. Front. Plant Sci. 2022, 13, 953654. [Google Scholar] [CrossRef]

| Company Name | Country | Product | Vector System Used | Website/Reference |
|---|---|---|---|---|
| Icon Genetics (Denka) | Germany | Various biologics | magnICON® system | https://www.icongenetics.com/ |
| Kentucky BioProcessing (KBP) | USA | Vaccines, antibodies | TMV, other vectors, magnICON® system | https://kbio.com/ |
| Leaf Expression Systems | UK | Proteins, antibodies, enzymes | Hypertrans® Expression System | https://www.leafexpressionsystems.com/ |
| PlantForm Corporation | Canada | Antibody drugs, biosimilars | vivoXPRESS® | https://www.plantformcorp.com/ |
| Cape Biologix | South Africa | Therapeutic proteins | PtX™ viral system | https://capebiologix.com/ |
| Baiya Phytopharm | Thailand | Vaccines, antibodies, growth factors | BaiyaPharming™ | https://baiyaphytopharm.com/ |
| Nomad BioSciences | Germany | Antibacterial and antiviral biologics | magnICON® and NOMADIC™ platforms | https://www.nomadbioscience.com/ |
| Bioapplications | South Korea | Vaccines | magnICON® system | https://www.bioapplications.global/ (accessed on 18 May 2025) |
| Cirsium Biosciences | USA | AAV vectors | TMV, other vectors, magnICON® system | https://cirsiumbio.com/ |
| * iBio Inc. | USA | Vaccines, therapeutic proteins | Hypertrans® Expression System | https://ir.ibioinc.com/ |
| * Medicago | Canada | Vaccines (e.g., COVID-19 vaccine) | vivoXPRESS® | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akher, S.A.; Wang, K.Y.; Hall, K.; Hunpatin, O.S.; Shan, M.; Zhang, Z.; Guo, Y. Harnessing Transient Expression Systems with Plant Viral Vectors for the Production of Biopharmaceuticals in Nicotiana benthamiana. Int. J. Mol. Sci. 2025, 26, 5510. https://doi.org/10.3390/ijms26125510
Akher SA, Wang KY, Hall K, Hunpatin OS, Shan M, Zhang Z, Guo Y. Harnessing Transient Expression Systems with Plant Viral Vectors for the Production of Biopharmaceuticals in Nicotiana benthamiana. International Journal of Molecular Sciences. 2025; 26(12):5510. https://doi.org/10.3390/ijms26125510
Chicago/Turabian StyleAkher, Sayed Abdul, Kevin Yueju Wang, Kylie Hall, Oluwaseyi Setonji Hunpatin, Muhammad Shan, Zenglin Zhang, and Yongfeng Guo. 2025. "Harnessing Transient Expression Systems with Plant Viral Vectors for the Production of Biopharmaceuticals in Nicotiana benthamiana" International Journal of Molecular Sciences 26, no. 12: 5510. https://doi.org/10.3390/ijms26125510
APA StyleAkher, S. A., Wang, K. Y., Hall, K., Hunpatin, O. S., Shan, M., Zhang, Z., & Guo, Y. (2025). Harnessing Transient Expression Systems with Plant Viral Vectors for the Production of Biopharmaceuticals in Nicotiana benthamiana. International Journal of Molecular Sciences, 26(12), 5510. https://doi.org/10.3390/ijms26125510








