Endothelial Function and Matrix Metalloproteinase 9 (MMP9) in Women with Polycystic Ovary Syndrome (PCOS)
Abstract
1. Introduction
2. Results
2.1. Baseline Assessment
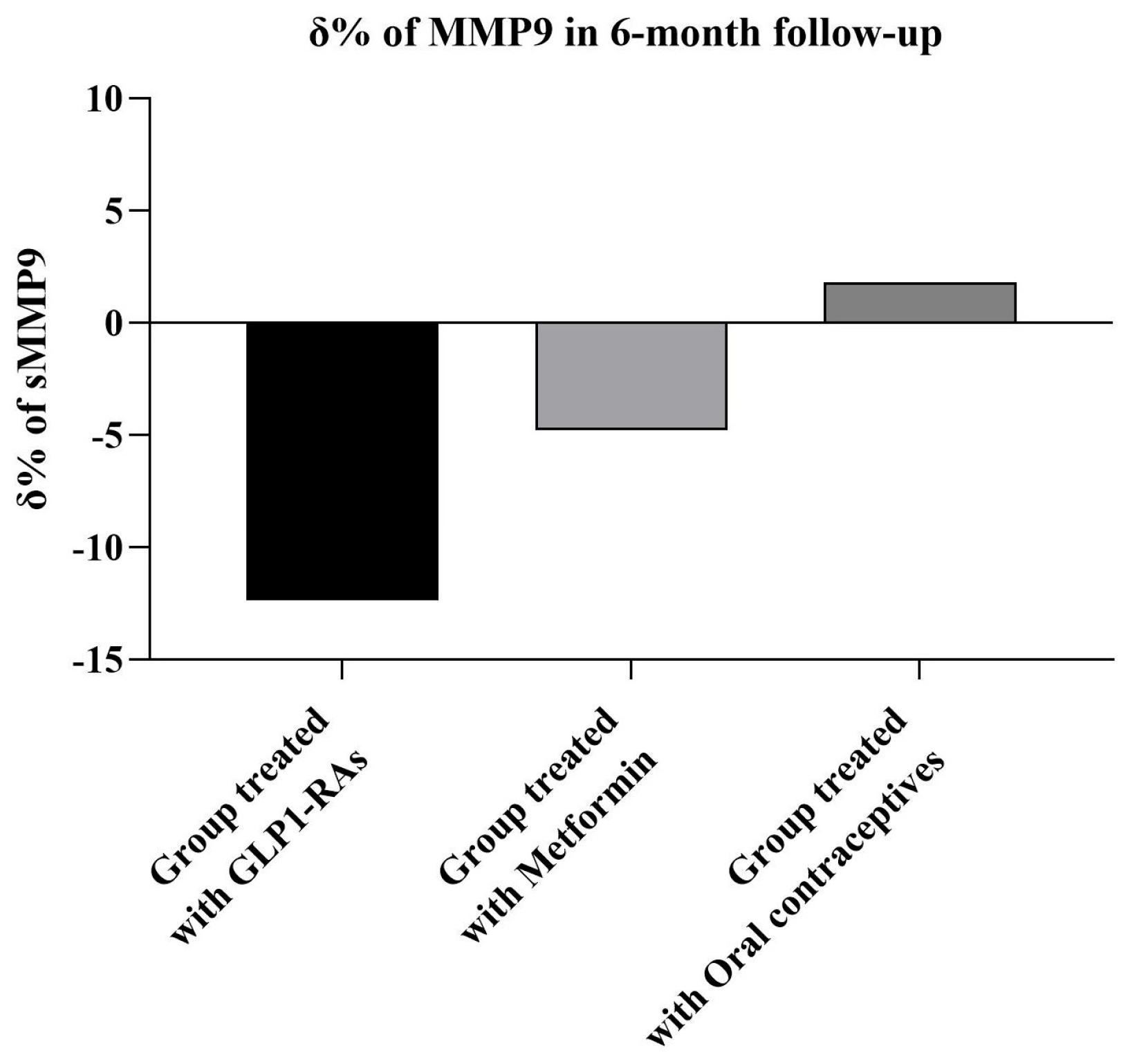
2.2. 6-Month Follow-Up Assessment
3. Discussion
4. Materials and Methods
4.1. Study Population
- GLP1-RAs and particularly liraglutide injection with a starting dosage of 0.6 mg to the dosage of 3 mg per week (for adults with a BMI ≥ 30 kg/m2). GLP1-RAs are a class of incretin-based therapies used primarily for the treatment of type 2 diabetes mellitus and, more recently, obesity [49];
- Metformin dose of 1000 mg per day. Metformin belongs to the class of biguanides, which are oral antihyperglycemic agents, being a first-line treatment for type 2 diabetes mellitus and also used in the treatment of PCOS, a syndrome which is often associated with insulin resistance and hyperinsulinemia [50];
- Oral contraceptives with drospirenone/ethinyl estradiol oral tablet 3 mg/0.02 mg per day for 20 days per month. Drospirenone/ethinyl estradiol is a combined oral contraceptive, being first-line pharmacologic therapy for the management of hyperandrogenic symptoms and menstrual irregularities in women with PCOS not seeking pregnancy [51].
4.2. Measurements
4.2.1. Laboratory Measurements
4.2.2. Endothelial Glycocalyx Assessment
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Lancet Regional Health-Europe. Polycystic ovary syndrome: What more can be done for patients? Lancet Reg. Health Eur. 2022, 21, 100524. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef]
- Pililis, S.; Lampsas, S.; Kountouri, A.; Pliouta, L.; Korakas, E.; Livadas, S.; Thymis, J.; Peppa, M.; Kalantaridou, S.; Oikonomou, E.; et al. The Cardiometabolic Risk in Women with Polycystic Ovarian Syndrome (PCOS): From Pathophysiology to Diagnosis and Treatment. Medicina 2024, 60, 1656. [Google Scholar] [CrossRef]
- Tosi, F.; Bonora, E.; Moghetti, P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: A comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum. Reprod. 2017, 32, 2515–2521. [Google Scholar] [CrossRef]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A.E.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. 2022, 27, 105. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef]
- Gall, A.L.; Ruff, M.; Kannan, R.; Cuniasse, P.; Yiotakis, A.; Dive, V.; Rio, M.C.; Basset, P.; Moras, D. Crystal structure of the stromelysin-3 (MMP-11) catalytic domain complexed with a phosphinic inhibitor mimicking the transition-state. J. Mol. Biol. 2001, 307, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Doldi, N.; Grossi, D.; Destefani, A.; Gessi, A.; Ferrari, A. Polycystic ovary syndrome: Evidence for reduced 3 beta-hydroxysteroid dehydrogenase gene expression in human luteinizing granulosa cells. Gynecol. Endocrinol. 2000, 14, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Huet, C.; Monget, P.; Pisselet, C.; Hennequet, C.; Locatelli, A.; Monniaux, D. Chronology of events accompanying follicular atresia in hypophysectomized ewes. Changes in levels of steroidogenic enzymes, connexin 43, insulin-like growth factor II/mannose 6 phosphate receptor, extracellular matrix components, and matrix metalloproteinases. Biol. Reprod. 1998, 58, 175–185. [Google Scholar] [CrossRef]
- McCaffery, F.H.; Leask, R.; Riley, S.C.; Telfer, E.E. Culture of bovine preantral follicles in a serum-free system: Markers for assessment of growth and development. Biol. Reprod. 2000, 63, 267–273. [Google Scholar] [CrossRef]
- Curry, T.E., Jr.; Osteen, K.G. The matrix metalloproteinase system: Changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr. Rev. 2003, 24, 428–465. [Google Scholar] [CrossRef]
- Butler, A.E.; Nandakumar, M.; Sathyapalan, T.; Brennan, E.; Atkin, S.L. Matrix Metalloproteinases, Tissue Inhibitors of Metalloproteinases, and Their Ratios in Women with Polycystic Ovary Syndrome and Healthy Controls. Int. J. Mol. Sci. 2025, 26, 321. [Google Scholar] [CrossRef] [PubMed]
- Duncan, W.C. The human corpus luteum: Remodelling during luteolysis and maternal recognition of pregnancy. Rev. Reprod. 2000, 5, 12–17. [Google Scholar] [CrossRef]
- Duncan, W.C.; McNeilly, A.S.; Illingworth, P.J. The effect of luteal “rescue” on the expression and localization of matrix metalloproteinases and their tissue inhibitors in the human corpus luteum. J. Clin. Endocrinol. Metab. 1998, 83, 2470–2478. [Google Scholar] [CrossRef][Green Version]
- Bode, W.; Fernandez-Catalan, C.; Tschesche, H.; Grams, F.; Nagase, H.; Maskos, K. Structural properties of matrix metalloproteinases. Cell. Mol. Life. Sci. 1999, 55, 639–652. [Google Scholar] [CrossRef]
- Shalev, E.; Goldman, S.; Ben-Shlomo, I. The balance between MMP-9 and MMP-2 and their tissue inhibitor (TIMP)-1 in luteinized granulosa cells: Comparison between women with PCOS and normal ovulatory women. Mol. Hum. Reprod. 2001, 7, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Qi, J.; Xue, X.; Li, X.; Liao, Y.; Sun, Y.; Tao, Y.; Yin, H.; Liu, W.; Li, S.; et al. Follicular free fatty acid metabolic signatures and their effects on oocyte competence in non-obese PCOS patients. Reproduction 2022, 164, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Qu, H.; Yang, L.; Shou, L. Effects of GLP1RAs on pregnancy rate and menstrual cyclicity in women with polycystic ovary syndrome: A meta-analysis and systematic review. BMC Endocr. Disord. 2023, 23, 245. [Google Scholar] [CrossRef]
- Prentza, V.; Pavlidis, G.; Ikonomidis, I.; Pililis, S.; Lampsas, S.; Kountouri, A.; Pliouta, L.; Korakas, E.; Thymis, J.; Palaiodimou, L.; et al. Antidiabetic Treatment and Prevention of Ischemic Stroke: A Systematic Review. J. Clin. Med. 2024, 13, 5786. [Google Scholar] [CrossRef]
- Martin, K.A.; Anderson, R.R.; Chang, R.J.; Ehrmann, D.A.; Lobo, R.A.; Murad, M.H.; Pugeat, M.M.; Rosenfield, R.L. Evaluation and Treatment of Hirsutism in Premenopausal Women: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1233–1257. [Google Scholar] [CrossRef]
- Holm Nielsen, S.; Jonasson, L.; Kalogeropoulos, K.; Karsdal, M.A.; Reese-Petersen, A.L.; Auf dem Keller, U.; Genovese, F.; Nilsson, J.; Goncalves, I. Exploring the role of extracellular matrix proteins to develop biomarkers of plaque vulnerability and outcome. J. Intern. Med. 2020, 287, 493–513. [Google Scholar] [CrossRef] [PubMed]
- Falk, E.; Nakano, M.; Bentzon, J.F.; Finn, A.V.; Virmani, R. Update on acute coronary syndromes: The pathologists’ view. Eur. Heart J. 2013, 34, 719–728. [Google Scholar] [CrossRef]
- Loftus, I.M.; Naylor, A.R.; Goodall, S.; Crowther, M.; Jones, L.; Bell, P.R.; Thompson, M.M. Increased matrix metalloproteinase-9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke 2000, 31, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Magid, R.; Murphy, T.J.; Galis, Z.S. Expression of matrix metalloproteinase-9 in endothelial cells is differentially regulated by shear stress. Role of c-Myc. J. Biol. Chem. 2003, 278, 32994–32999. [Google Scholar] [CrossRef]
- Gough, P.J.; Gomez, I.G.; Wille, P.T.; Raines, E.W. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J. Clin. Investig. 2006, 116, 59–69. [Google Scholar] [CrossRef]
- Choi, E.T.; Collins, E.T.; Marine, L.A.; Uberti, M.G.; Uchida, H.; Leidenfrost, J.E.; Khan, M.F.; Boc, K.P.; Abendschein, D.R.; Parks, W.C. Matrix metalloproteinase-9 modulation by resident arterial cells is responsible for injury-induced accelerated atherosclerotic plaque development in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1020–1025. [Google Scholar] [CrossRef]
- Sylus, A.M.; Nandeesha, H.; Chitra, T. Matrix metalloproteinase-9 increases and Interleukin-10 reduces with increase in body mass index in polycystic ovary syndrome: A cross-sectional study. Int. J. Reprod. Biomed. 2020, 18, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Lin, C.P.; Wu, H.M.; Chu, P.H. Endothelial dysfunction in subfertile women with polycystic ovary syndrome. Reprod. Biomed. Online 2023, 46, 391–398. [Google Scholar] [CrossRef]
- Dambala, K.; Paschou, S.A.; Michopoulos, A.; Siasos, G.; Goulis, D.G.; Vavilis, D.; Tarlatzis, B.C. Biomarkers of Endothelial Dysfunction in Women With Polycystic Ovary Syndrome. Angiology 2019, 70, 797–801. [Google Scholar] [CrossRef]
- Oncul, M.; Albayrak, M.; Sozer, V.; Karakus, B.; Gelisgen, R.; Karatas, S.; Simsek, G.; Uzun, H. Polycystic ovary syndrome and endothelial dysfunction: A potential role for soluble lectin-like oxidized low density lipoprotein receptor-1. Reprod. Biol. 2020, 20, 396–401. [Google Scholar] [CrossRef]
- Tsigkou, V.; Oikonomou, E.; Anastasiou, A.; Lampsas, S.; Zakynthinos, G.E.; Kalogeras, K.; Katsioupa, M.; Kapsali, M.; Kourampi, I.; Pesiridis, T.; et al. Molecular Mechanisms and Therapeutic Implications of Endothelial Dysfunction in Patients with Heart Failure. Int. J. Mol. Sci. 2023, 24, 4321. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhu, M.; Zhang, M.; Jia, X.; Cheng, X.; Ding, S.; Zhu, Q. Amelioration of compound 4,4’-diphenylmethane-bis(methyl)carbamate on high mobility group box1-mediated inflammation and oxidant stress responses in human umbilical vein endothelial cells via RAGE/ERK1/2/NF-kappaB pathway. Int. Immunopharmacol. 2013, 15, 206–216. [Google Scholar] [CrossRef]
- Tarkun, I.; Arslan, B.C.; Canturk, Z.; Turemen, E.; Sahin, T.; Duman, C. Endothelial dysfunction in young women with polycystic ovary syndrome: Relationship with insulin resistance and low-grade chronic inflammation. J. Clin. Endocrinol. Metab. 2004, 89, 5592–5596. [Google Scholar] [CrossRef] [PubMed]
- Christakou, C.; Economou, F.; Livadas, S.; Piperi, C.; Adamopoulos, C.; Marinakis, E.; Jdiamanti-Kandarakis, E. Strong and positive association of endothelin-1 with AGEs in PCOS: A causal relationship or a bystander? Hormones 2011, 10, 292–297. [Google Scholar] [CrossRef]
- Abraham Gnanadass, S.; Divakar Prabhu, Y.; Valsala Gopalakrishnan, A. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): An update. Arch. Gynecol. Obstet. 2021, 303, 631–643. [Google Scholar] [CrossRef]
- Wekker, V.; van Dammen, L.; Koning, A.; Heida, K.Y.; Painter, R.C.; Limpens, J.; Laven, J.S.E.; Roeters van Lennep, J.E.; Roseboom, T.J.; Hoek, A. Long-term cardiometabolic disease risk in women with PCOS: A systematic review and meta-analysis. Hum. Reprod. Update 2020, 26, 942–960. [Google Scholar] [CrossRef]
- Oikonomou, E.; Tsaplaris, P.; Anastasiou, A.; Xenou, M.; Lampsas, S.; Siasos, G.; Pantelidis, P.; Theofilis, P.; Tsatsaragkou, A.; Katsarou, O.; et al. Interleukin-1 in Coronary Artery Disease. Curr. Top. Med. Chem. 2022, 22, 2368–2389. [Google Scholar] [CrossRef]
- Glintborg, D.; Sidelmann, J.J.; Altinok, M.L.; Mumm, H.; Andersen, M. Increased thrombin generation in women with polycystic ovary syndrome: A pilot study on the effect of metformin and oral contraceptives. Metabolism 2015, 64, 1272–1278. [Google Scholar] [CrossRef]
- Manzoor, S.; Ganie, M.A.; Amin, S.; Shah, Z.A.; Bhat, I.A.; Yousuf, S.D.; Jeelani, H.; Kawa, I.A.; Fatima, Q.; Rashid, F. Oral contraceptive use increases risk of inflammatory and coagulatory disorders in women with Polycystic Ovarian Syndrome: An observational study. Sci. Rep. 2019, 9, 10182. [Google Scholar] [CrossRef]
- Ryan, D.H.; Lingvay, I.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Kahn, S.E.; Kushner, R.F.; Marso, S.; Plutzky, J.; Brown-Frandsen, K.; et al. Semaglutide Effects on Cardiovascular Outcomes in People With Overweight or Obesity (SELECT) rationale and design. Am. Heart J. 2020, 229, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Frossing, S.; Nylander, M.; Kistorp, C.; Skouby, S.O.; Faber, J. Effect of liraglutide on atrial natriuretic peptide, adrenomedullin, and copeptin in PCOS. Endocr. Connect. 2018, 7, 115–123. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, L.; He, W.; Mi, Y. The Effect of Oral Antidiabetic Drugs on Improving the Endocrine and Metabolic States in Women with Polycystic Ovary Syndrome: A Systematic Review and Network Meta-analysis. Drugs 2022, 82, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E.; Cirillo, F.; Catellani, C.; Dauriz, M.; Lazzeroni, P.; Sartori, C.; Moghetti, P. Current treatment for polycystic ovary syndrome: Focus on adolescence. Minerva Pediatr. 2020, 72, 288–311. [Google Scholar] [CrossRef]
- Liu, B.; Su, L.; Loo, S.J.; Gao, Y.; Khin, E.; Kong, X.; Dalan, R.; Su, X.; Lee, K.O.; Ma, J.; et al. Matrix metallopeptidase 9 contributes to the beginning of plaque and is a potential biomarker for the early identification of atherosclerosis in asymptomatic patients with diabetes. Front. Endocrinol. 2024, 15, 1369369. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.; Xenou, M.; Zakynthinos, G.E.; Tsaplaris, P.; Lampsas, S.; Bletsa, E.; Gialamas, I.; Kalogeras, K.; Goliopoulou, A.; Gounaridi, M.I.; et al. Novel Approaches to the Management of Diabetes Mellitus in Patients with Coronary Artery Disease. Curr. Pharm. Des. 2023, 29, 1844–1862. [Google Scholar] [CrossRef]
- Gajewska, B.; Sliwinska-Mosson, M. Association of MMP-2 and MMP-9 Polymorphisms with Diabetes and Pathogenesis of Diabetic Complications. Int. J. Mol. Sci. 2022, 23, 10571. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Maselli, D.B.; Camilleri, M. Effects of GLP-1 and Its Analogs on Gastric Physiology in Diabetes Mellitus and Obesity. Adv. Exp. Med. Biol. 2021, 1307, 171–192. [Google Scholar] [CrossRef]
- Yerevanian, A.; Soukas, A.A. Metformin: Mechanisms in Human Obesity and Weight Loss. Curr. Obes. Rep. 2019, 8, 156–164. [Google Scholar] [CrossRef]
- Li, L.; Zhang, R.; Zeng, J.; Ke, H.; Peng, X.; Huang, L.; Zhang, H.; Chen, Z.; Li, T.T.; Tan, Q.; et al. Effectiveness and safety assessment of drospirenone/ethinyl estradiol tablet in treatment of PCOS patients: A single center, prospective, observational study. BMC Womens Health 2020, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Resneck, J.S., Jr. Revisions to the Declaration of Helsinki on Its 60th Anniversary: A Modernized Set of Ethical Principles to Promote and Ensure Respect for Participants in a Rapidly Innovating Medical Research Ecosystem. JAMA 2025, 333, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Khalili, D.; Khayamzadeh, M.; Kohansal, K.; Ahanchi, N.S.; Hasheminia, M.; Hadaegh, F.; Tohidi, M.; Azizi, F.; Habibi-Moeini, A.S. Are HOMA-IR and HOMA-B good predictors for diabetes and pre-diabetes subtypes? BMC Endocr. Disord. 2023, 23, 39. [Google Scholar] [CrossRef]
- Jivrajani, S.J.; Bhad Patil, W.A. Effect of Low Intensity Laser Therapy (LILT) on MMP-9 expression in gingival crevicular fluid and rate of orthodontic tooth movement in patients undergoing canine retraction: A randomized controlled trial. Int. Orthod. 2020, 18, 330–339. [Google Scholar] [CrossRef]
- Lekakis, J.; Abraham, P.; Balbarini, A.; Blann, A.; Boulanger, C.M.; Cockcroft, J.; Cosentino, F.; Deanfield, J.; Gallino, A.; Ikonomidis, I.; et al. Methods for evaluating endothelial function: A position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 775–789. [Google Scholar] [CrossRef] [PubMed]
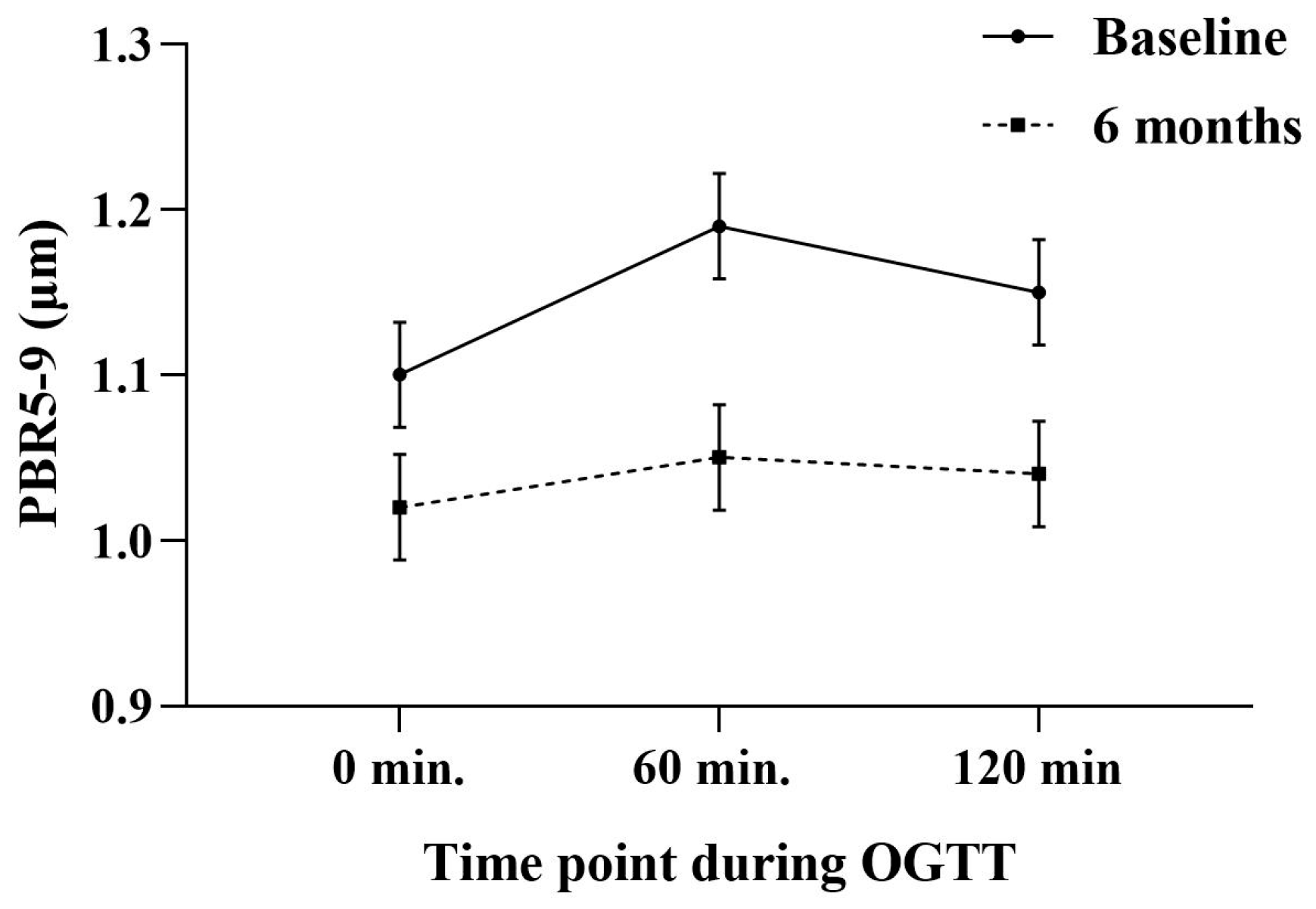

| PBR5–9 (μm) | Glucose (mg/dL) | |
|---|---|---|
| 0 min | 1.10 ± 0.1 | 83 ± 12 |
| 60 min | 1.19 ± 0.1 § | 112 ± 16 § |
| 120 min | 1.15 ± 0.1 * | 96 ± 14 * |
| All Participants (n = 40) | GLP1-RA Group (n = 10) | Metformin Group (n = 20) | Oral Contraceptive Pills (n = 10) | |
|---|---|---|---|---|
| BMI (kg/m2) | ||||
| Baseline | 33.8 ± 9.1 | 34.1 ± 8.9 | 33.7 ± 9.2 | 33.7 ± 9 |
| 6 months | 32.4 ± 8.9 † | 32.6 ± 9 † | 32.3 ± 8.8 † | 32.4 ± 8.8 † |
| Homa Index | ||||
| Baseline | 5.3 ± 1.8 | 5.4 ± 1.7 | 5.2 ± 1.9 | 5.4 ± 1.7 |
| 6 months | 2.9 ± 0.9 ††† | 2.8 ± 0.8 ††† | 2.9 ± 0.9 †† | 3 ± 1 † |
| Matsuda Index | ||||
| Baseline | 7 ± 1.9 | 6.8 ± 2.2 | 7.2 ± 1.6 | 6.9 ± 2 |
| 6 months | 9.1 ± 2.1 †† | 9.8 ± 2.5 †† | 9 ± 2 †† | 8.7 ± 1.9 † |
| MMP9 (ng/mL) | ||||
| Baseline | 210 ± 45 | 220 ± 59 | 203 ± 48 | 188 ± 29 |
| 6 months | 178 ± 40 † | 170 ± 51 † | 180 ± 45 †,* | 189 ± 30 ** |
| PBR5–9 (μm) | ||||
| Baseline | 1.10 ± 0.1 | 1.10 ± 0.1 | 1.10 ± 0.1 | 1.09 ± 0.1 |
| 6 months | 1.02 ± 0.1 † | 1.03 ± 0.1 † | 1.02 ± 0.1 † | 1.03 ± 0.1 † |
| Testosterone (ng/dL) | ||||
| Baseline | 44.2 ± 11 | 44.9 ± 10 | 45 ± 13 | 43.1 ± 8 |
| 6 months | 39.1 ± 9 † | 38.7 ± 9 | 38.5 ± 9 | 39.9 ± 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambadiari, V.; Pililis, S.; Lampsas, S.; Kountouri, A.; Thymis, J.; Pliouta, L.; Peppa, M.; Kalantaridou, S.; Oikonomou, E.; Siasos, G.; et al. Endothelial Function and Matrix Metalloproteinase 9 (MMP9) in Women with Polycystic Ovary Syndrome (PCOS). Int. J. Mol. Sci. 2025, 26, 5488. https://doi.org/10.3390/ijms26125488
Lambadiari V, Pililis S, Lampsas S, Kountouri A, Thymis J, Pliouta L, Peppa M, Kalantaridou S, Oikonomou E, Siasos G, et al. Endothelial Function and Matrix Metalloproteinase 9 (MMP9) in Women with Polycystic Ovary Syndrome (PCOS). International Journal of Molecular Sciences. 2025; 26(12):5488. https://doi.org/10.3390/ijms26125488
Chicago/Turabian StyleLambadiari, Vaia, Sotirios Pililis, Stamatios Lampsas, Aikaterini Kountouri, John Thymis, Loukia Pliouta, Melpomeni Peppa, Sophia Kalantaridou, Evangelos Oikonomou, Gerasimos Siasos, and et al. 2025. "Endothelial Function and Matrix Metalloproteinase 9 (MMP9) in Women with Polycystic Ovary Syndrome (PCOS)" International Journal of Molecular Sciences 26, no. 12: 5488. https://doi.org/10.3390/ijms26125488
APA StyleLambadiari, V., Pililis, S., Lampsas, S., Kountouri, A., Thymis, J., Pliouta, L., Peppa, M., Kalantaridou, S., Oikonomou, E., Siasos, G., & Ikonomidis, I. (2025). Endothelial Function and Matrix Metalloproteinase 9 (MMP9) in Women with Polycystic Ovary Syndrome (PCOS). International Journal of Molecular Sciences, 26(12), 5488. https://doi.org/10.3390/ijms26125488










