Beware of Sealing Film of Petri Dishes!—Alters the Expression of a Large Number of Genes
Abstract
1. Introduction
2. Results
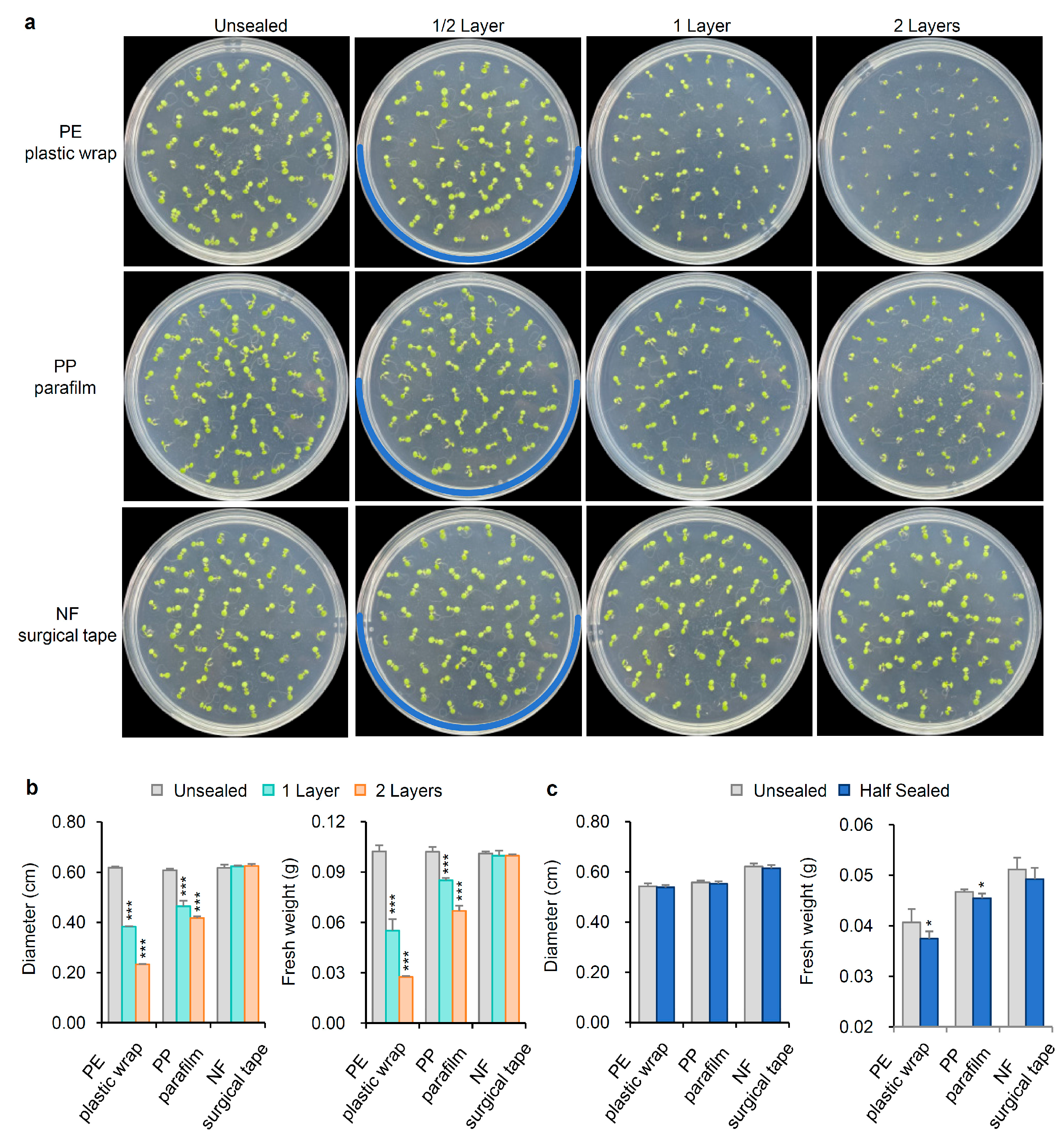
2.1. The Growth of Arabidopsis Thaliana Was Affected by Sealing in Petri Dishes
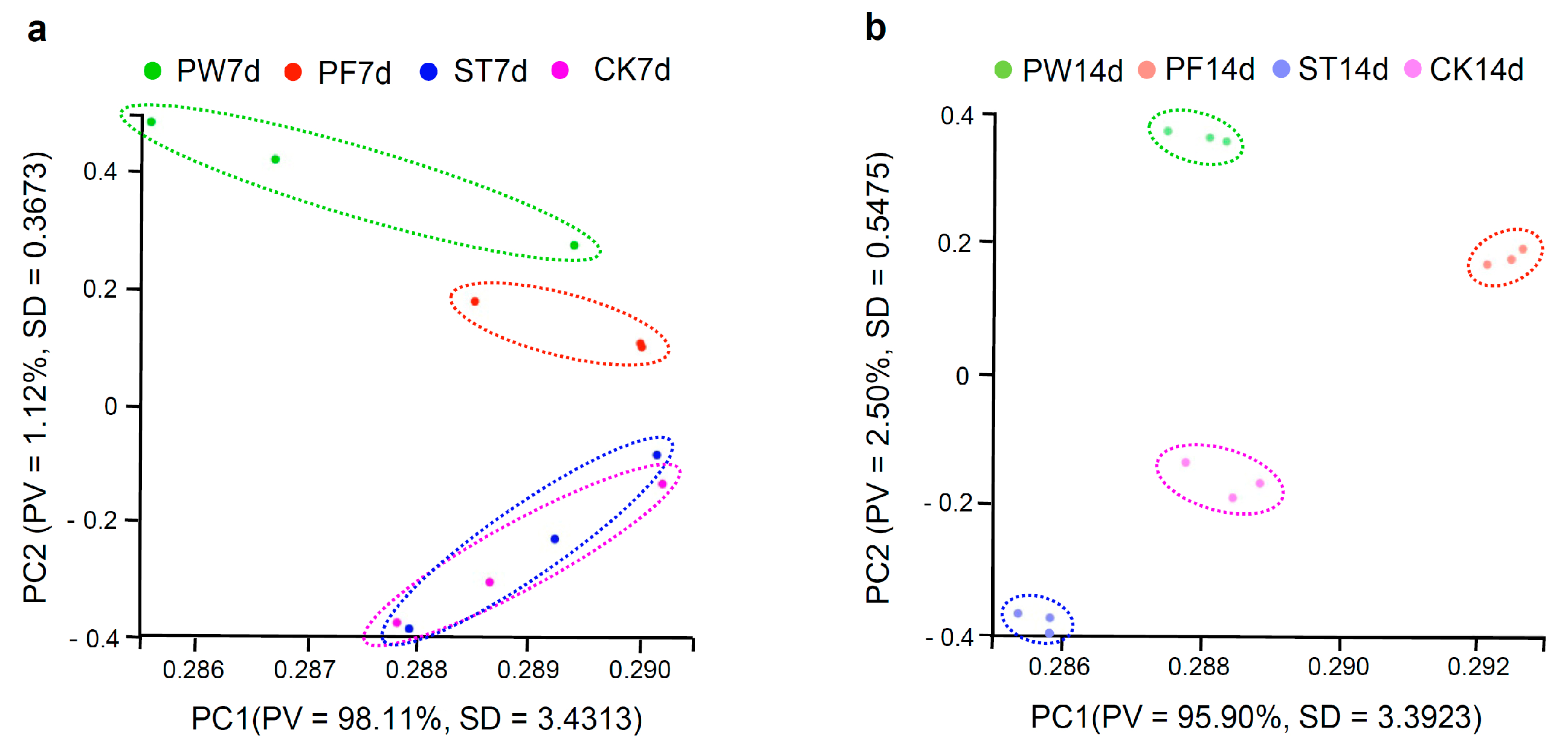
2.2. Transcriptome Analysis
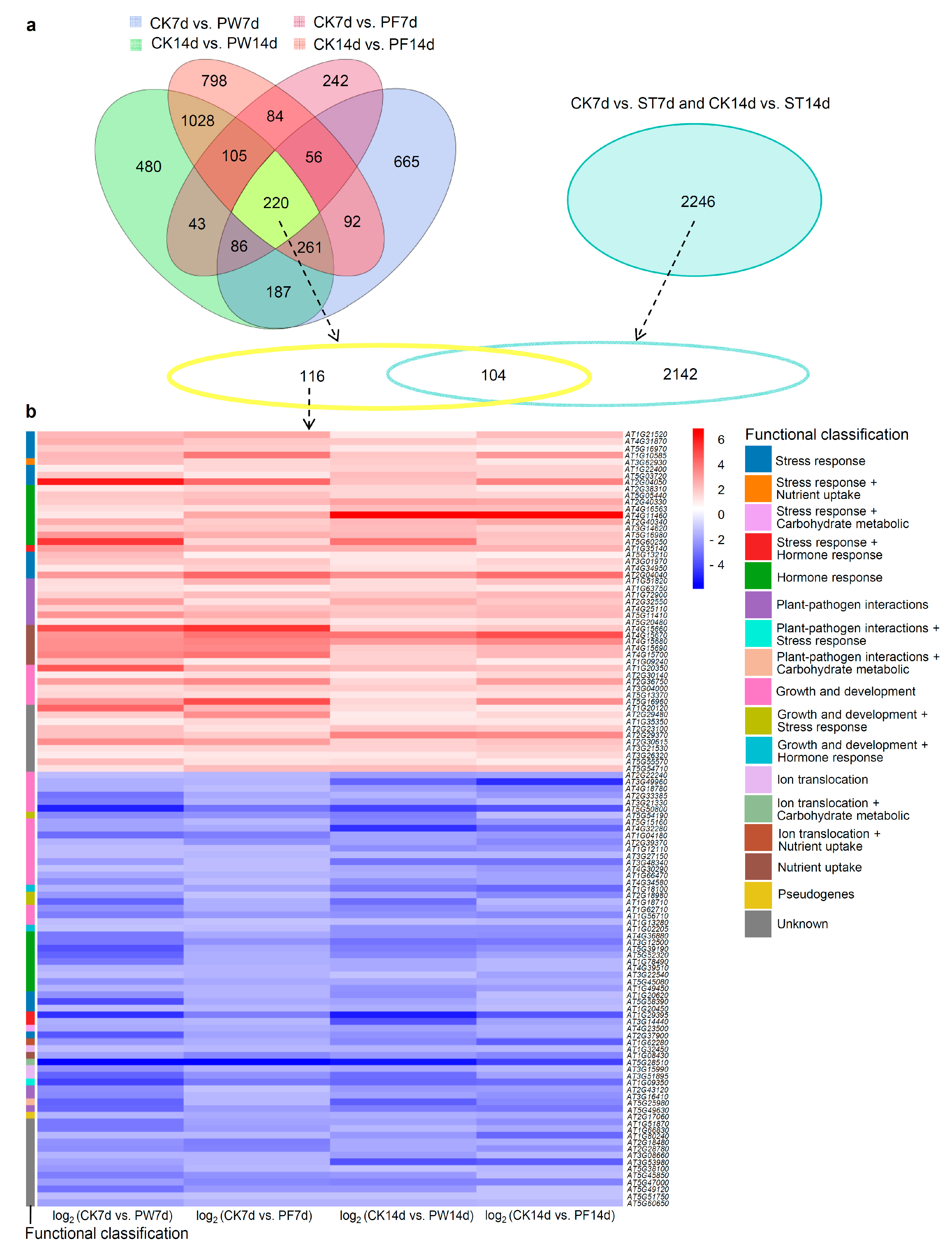
2.3. DEG Analysis
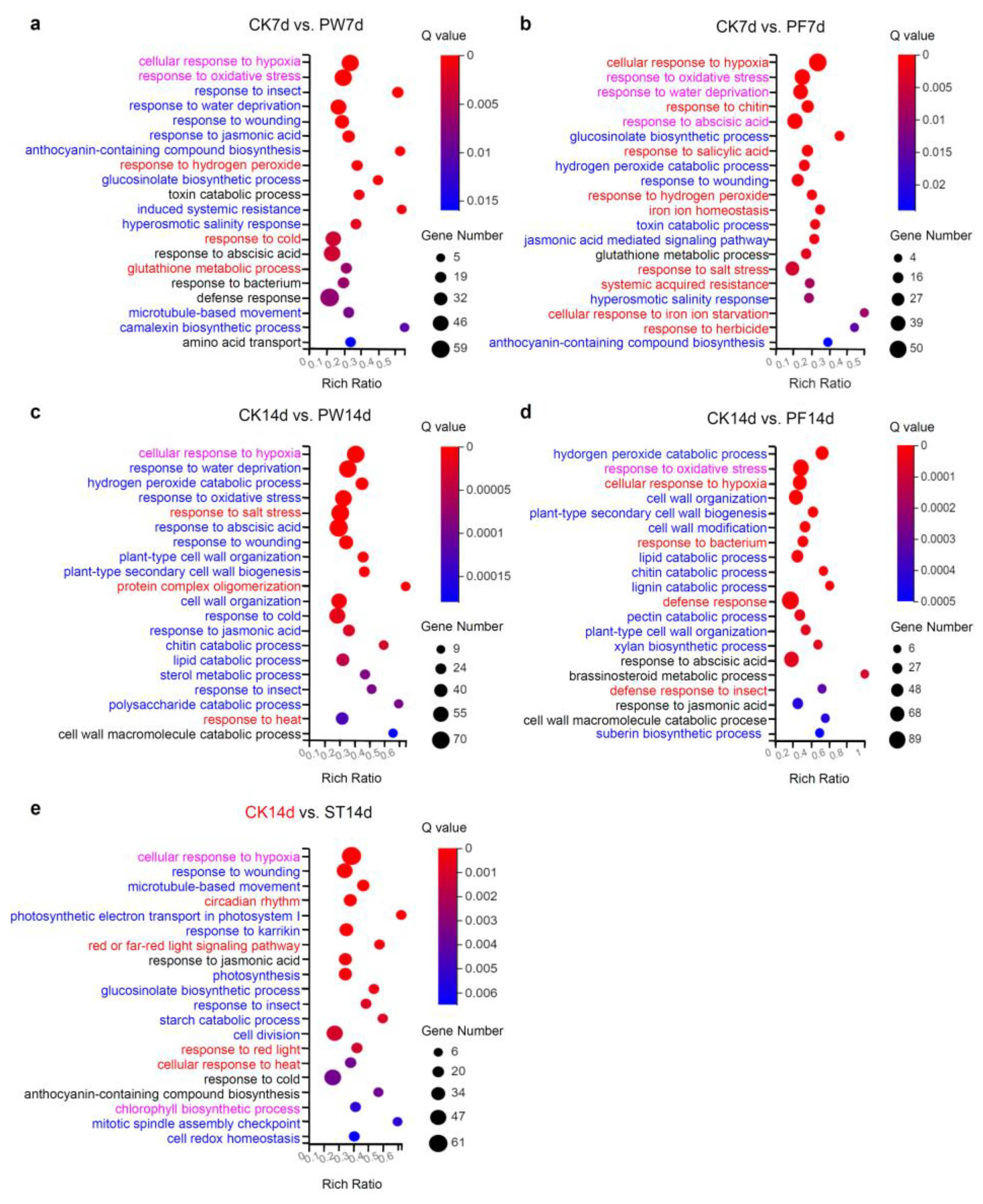
2.4. Enriched Biological Process of DEGs
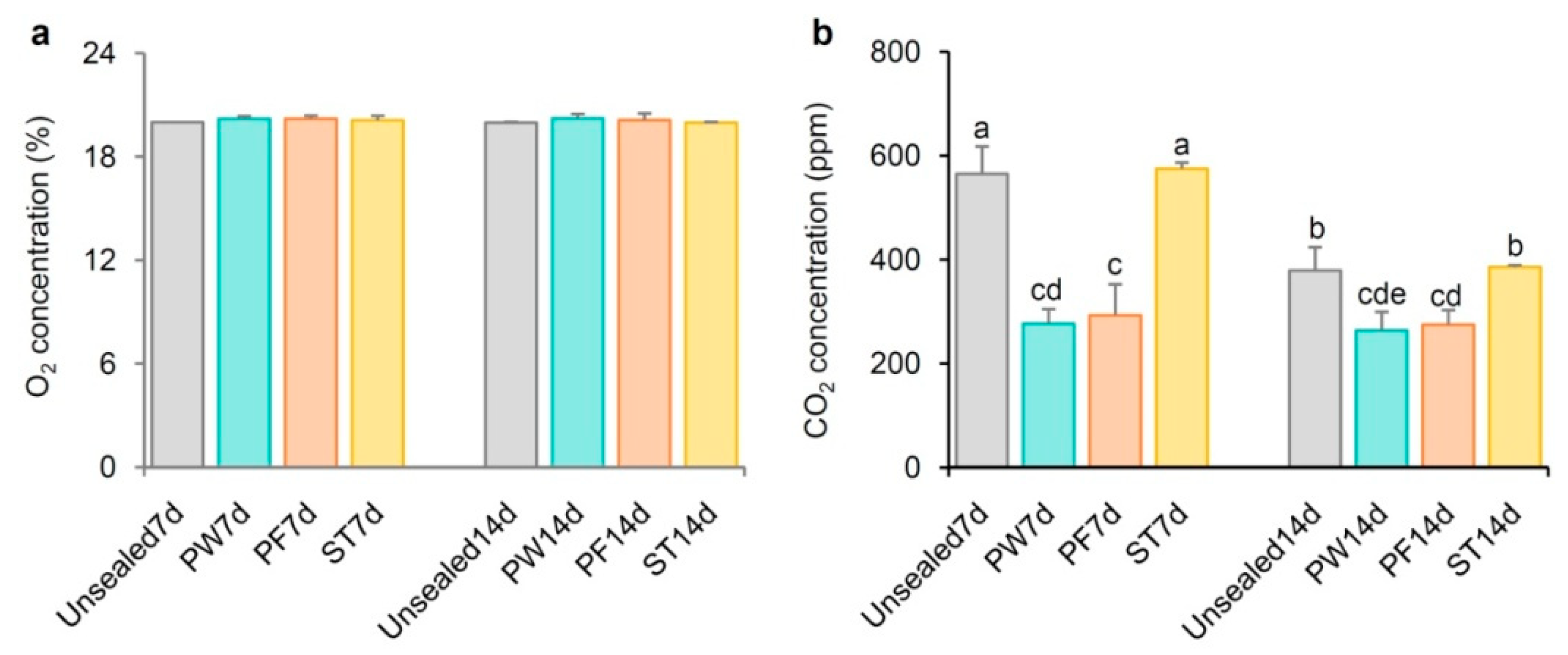
2.5. Different Sealing Methods Affect CO2 but Not O2 Content in Petri Dishes
2.6. Identification of Responsive Genes for Compromised Growth
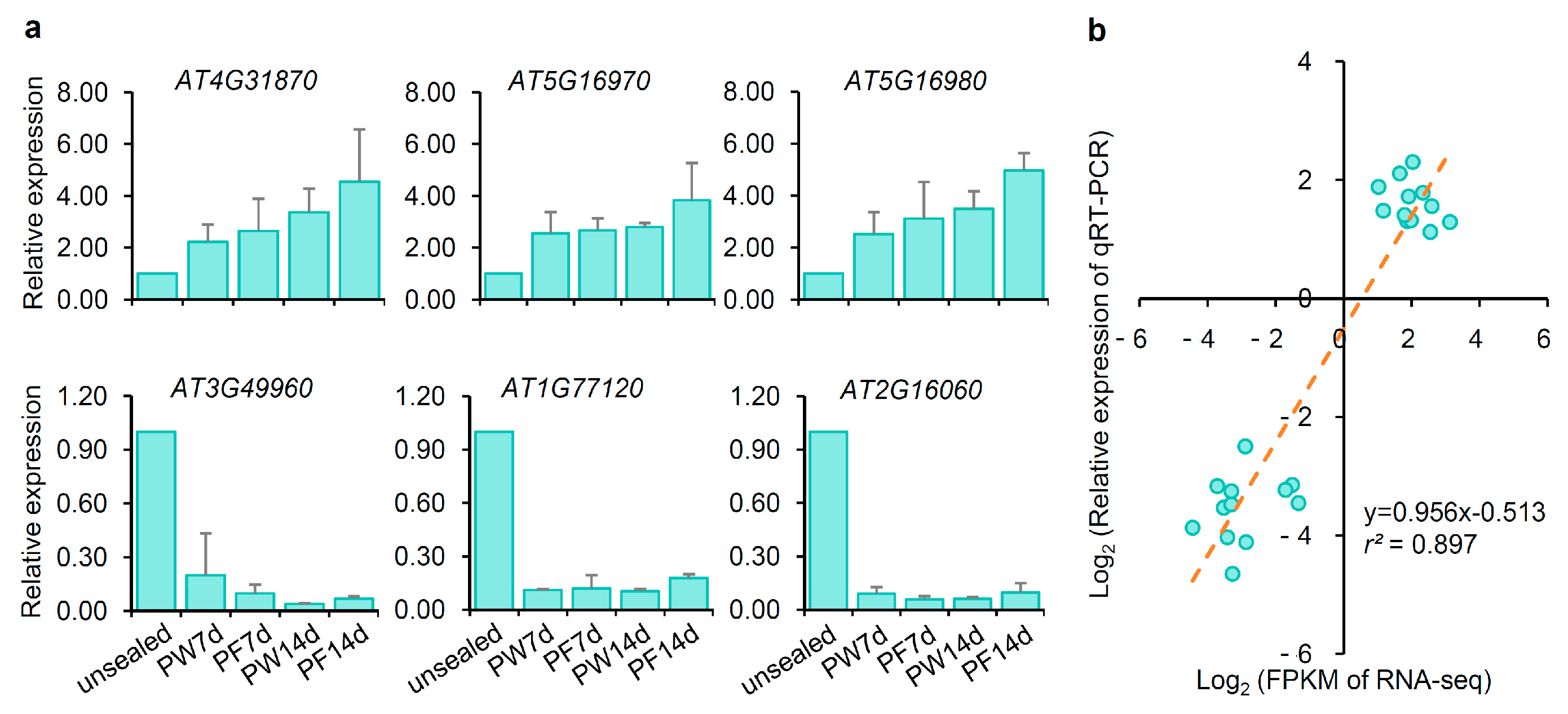
2.7. Transcriptome Data Validated by qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions and Sample Preparation
4.2. RNA Isolation
4.3. RNA Library Preparation and Sequencing
4.4. Functional Annotation
4.5. Gas Content Measurement
4.6. qRT-PCR Detection
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DEGs | differentially expressed genes |
| RNA-seq | transcriptome sequencing |
| PE | polyethylene |
| PP | polyolefin and paraffin wax |
| NF | non-woven fabric and plastic |
| PW | PE plastic wrap |
| PF | PP parafilm |
| ST | NF surgical tape |
| PCA | principal component analysis |
| BP | biological processes |
| MS | Murashige and Skoog |
| qRT-PCR | quantitative real-time PCR |
References
- Meinke, D.W.; Cherry, J.M.; Dean, C.; Rounsley, S.D.; Koornneef, M. Arabidopsis thaliana: A model plant for genome analysis. Science 1998, 282, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Initiative, T.A.G. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef]
- Patel, K.J.; Kao, Y.T.; Llinas, R.J.; Bartel, B. A PEX5 missense allele preferentially disrupts PTS1 cargo import into Arabidopsis peroxisomes. Plant Direct 2019, 3, e00128. [Google Scholar] [CrossRef]
- Jia, Y.; Ding, Y.; Shi, Y.; Zhang, X.; Gong, Z.; Yang, S. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016, 212, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yao, Y.; Yin, K.; Tan, L.; Liu, M.; Hou, J.; Zhang, H.; Liang, R.; Zhang, X.; Yang, H.; et al. ACBP4-WRKY70-RAP2.12 module positively regulates submergence-induced hypoxia response in Arabidopsis thaliana. J. Integr. Plant Biol. 2024, 66, 1052–1067. [Google Scholar] [CrossRef]
- Sujeeth, N.; Mehterov, N.; Gupta, S.; Qureshi, M.K.; Fischer, A.; Proost, S.; Omidbakhshfard, M.A.; Obata, T.; Benina, M.; Staykov, N.; et al. A novel seed plants gene regulates oxidative stress tolerance in Arabidopsis thaliana. Cell. Mol. Life Sci. CMLS 2020, 77, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Luhua, S.; Ciftci-Yilmaz, S.; Harper, J.; Cushman, J.; Mittler, R. Enhanced tolerance to oxidative stress in transgenic Arabidopsis plants expressing proteins of unknown function. Plant Physiol. 2008, 148, 280–292. [Google Scholar] [CrossRef]
- Chang, C.C.; Slesak, I.; Jorda, L.; Sotnikov, A.; Melzer, M.; Miszalski, Z.; Mullineaux, P.M.; Parker, J.E.; Karpinska, B.; Karpinski, S. Arabidopsis chloroplastic glutathione peroxidases play a role in cross talk between photooxidative stress and immune responses. Plant Physiol. 2009, 150, 670–683. [Google Scholar] [CrossRef]
- Lazzarotto, F.; Wahni, K.; Piovesana, M.; Maraschin, F.; Messens, J.; Margis-Pinheiro, M. Arabidopsis APx-R Is a Plastidial Ascorbate-Independent Peroxidase Regulated by Photomorphogenesis. Antioxidants 2021, 10, 65. [Google Scholar] [CrossRef]
- Waszczak, C.; Kerchev, P.I.; Muhlenbock, P.; Hoeberichts, F.A.; Van Der Kelen, K.; Mhamdi, A.; Willems, P.; Denecker, J.; Kumpf, R.P.; Noctor, G.; et al. SHORT-ROOT Deficiency Alleviates the Cell Death Phenotype of the Arabidopsis catalase2 Mutant under Photorespiration-Promoting Conditions. Plant Cell 2016, 28, 1844–1859. [Google Scholar] [CrossRef]
- Yin, L.; Mano, J.; Wang, S.; Tsuji, W.; Tanaka, K. The involvement of lipid peroxide-derived aldehydes in aluminum toxicity of tobacco roots. Plant Physiol. 2010, 152, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.S.; Mano, J. Lipid Peroxide-Derived Short-Chain Carbonyls Mediate Hydrogen Peroxide-Induced and Salt-Induced Programmed Cell Death in Plants. Plant Physiol. 2015, 168, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Inze, A.; Vanderauwera, S.; Hoeberichts, F.A.; Vandorpe, M.; Van Gaever, T.; Van Breusegem, F. A subcellular localization compendium of hydrogen peroxide-induced proteins. Plant Cell Environ. 2012, 35, 308–320. [Google Scholar] [CrossRef]
- Jung, J.Y.; Ahn, J.H.; Schachtman, D.P. CC-type glutaredoxins mediate plant response and signaling under nitrate starvation in Arabidopsis. BMC Plant Biol. 2018, 18, 281. [Google Scholar] [CrossRef]
- Patterson, K.; Walters, L.A.; Cooper, A.M.; Olvera, J.G.; Rosas, M.A.; Rasmusson, A.G.; Escobar, M.A. Nitrate-Regulated Glutaredoxins Control Arabidopsis Primary Root Growth. Plant Physiol. 2016, 170, 989–999. [Google Scholar] [CrossRef]
- Rehman, H.M.; Nawaz, M.A.; Shah, Z.H.; Ludwig-Muller, J.; Chung, G.; Ahmad, M.Q.; Yang, S.H.; Lee, S.I. Comparative genomic and transcriptomic analyses of Family-1 UDP glycosyltransferase in three Brassica species and Arabidopsis indicates stress-responsive regulation. Sci. Rep. 2018, 8, 1875. [Google Scholar] [CrossRef]
- Jin, S.H.; Ma, X.M.; Kojima, M.; Sakakibara, H.; Wang, Y.W.; Hou, B.K. Overexpression of glucosyltransferase UGT85A1 influences trans-zeatin homeostasis and trans-zeatin responses likely through O-glucosylation. Planta 2013, 237, 991–999. [Google Scholar] [CrossRef]
- Kappel, C.; Friedrich, T.; Oberkofler, V.; Jiang, L.; Crawford, T.; Lenhard, M.; Baurle, I. Genomic and epigenomic determinants of heat stress-induced transcriptional memory in Arabidopsis. Genome Biol. 2023, 24, 129. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, T.; Oberkofler, V.; Trindade, I.; Altmann, S.; Brzezinka, K.; Lamke, J.; Gorka, M.; Kappel, C.; Sokolowska, E.; Skirycz, A.; et al. Heteromeric HSFA2/HSFA3 complexes drive transcriptional memory after heat stress in Arabidopsis. Nat. Commun. 2021, 12, 3426. [Google Scholar] [CrossRef]
- Ma, S.; Gong, Q.; Bohnert, H.J. Dissecting salt stress pathways. J. Exp. Bot. 2006, 57, 1097–1107. [Google Scholar] [CrossRef]
- Pri-Tal, O.; Sun, Y.; Dadras, A.; Furst-Jansen, J.M.R.; Zimran, G.; Michaeli, D.; Wijerathna-Yapa, A.; Shpilman, M.; Merilo, E.; Yarmolinsky, D.; et al. Constitutive activation of ABA receptors in Arabidopsis reveals unique regulatory circuitries. New Phytol. 2024, 241, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Aleman, F.; Yazaki, J.; Lee, M.; Takahashi, Y.; Kim, A.Y.; Li, Z.; Kinoshita, T.; Ecker, J.R.; Schroeder, J.I. An ABA-increased interaction of the PYL6 ABA receptor with MYC2 Transcription Factor: A putative link of ABA and JA signaling. Sci. Rep. 2016, 6, 28941. [Google Scholar] [CrossRef]
- Osakabe, Y.; Maruyama, K.; Seki, M.; Satou, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Leucine-rich repeat receptor-like kinase1 is a key membrane-bound regulator of abscisic acid early signaling in Arabidopsis. Plant Cell 2005, 17, 1105–1119. [Google Scholar] [CrossRef]
- Wrzaczek, M.; Brosche, M.; Salojarvi, J.; Kangasjarvi, S.; Idanheimo, N.; Mersmann, S.; Robatzek, S.; Karpinski, S.; Karpinska, B.; Kangasjarvi, J. Transcriptional regulation of the CRK/DUF26 group of receptor-like protein kinases by ozone and plant hormones in Arabidopsis. BMC Plant Biol. 2010, 10, 95. [Google Scholar] [CrossRef]
- Je, J.; Chen, H.; Song, C.; Lim, C.O. Arabidopsis DREB2C modulates ABA biosynthesis during germination. Biochem. Biophys. Res. Commun. 2014, 452, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Hilbert, B.; Dueckershoff, K.; Roitsch, T.; Krischke, M.; Mueller, M.J.; Berger, S. General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell 2008, 20, 768–785. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Park, J.Y.; Ku, S.J.; Ha, Y.M.; Kim, S.; Kim, M.D.; Oh, M.H.; Kim, J. Genome-wide expression profiling of ARABIDOPSIS RESPONSE REGULATOR 7(ARR7) overexpression in cytokinin response. Mol. Genet. Genom. MGG 2007, 277, 115–137. [Google Scholar] [CrossRef]
- Schroder, F.; Lisso, J.; Lange, P.; Mussig, C. The extracellular EXO protein mediates cell expansion in Arabidopsis leaves. BMC Plant Biol. 2009, 9, 20. [Google Scholar] [CrossRef]
- Schroder, F.; Lisso, J.; Mussig, C. EXORDIUM-LIKE1 promotes growth during low carbon availability in Arabidopsis. Plant Physiol. 2011, 156, 1620–1630. [Google Scholar] [CrossRef]
- Nicolai, M.; Roncato, M.A.; Canoy, A.S.; Rouquie, D.; Sarda, X.; Freyssinet, G.; Robaglia, C. Large-scale analysis of mRNA translation states during sucrose starvation in arabidopsis cells identifies cell proliferation and chromatin structure as targets of translational control. Plant Physiol. 2006, 141, 663–673. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Q.; Kong, Y.H.; Chen, Y.; Duan, J.Y.; Wu, W.H.; Chen, Y.F. Arabidopsis WRKY45 transcription factor activates PHOSPHATE TRANSPORTER1;1 expression in response to phosphate starvation. Plant Physiol. 2014, 164, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Linn, J.; Ren, M.; Berkowitz, O.; Ding, W.; van der Merwe, M.J.; Whelan, J.; Jost, R. Root Cell-Specific Regulators of Phosphate-Dependent Growth. Plant Physiol. 2017, 174, 1969–1989. [Google Scholar] [CrossRef]
- Li, L.; He, Z.; Pandey, G.K.; Tsuchiya, T.; Luan, S. Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J. Biol. Chem. 2002, 277, 5360–5368. [Google Scholar] [CrossRef]
- Zarattini, M.; Corso, M.; Kadowaki, M.A.; Monclaro, A.; Magri, S.; Milanese, I.; Jolivet, S.; de Godoy, M.O.; Hermans, C.; Fagard, M.; et al. LPMO-oxidized cellulose oligosaccharides evoke immunity in Arabidopsis conferring resistance towards necrotrophic fungus B. cinerea. Commun. Biol. 2021, 4, 727. [Google Scholar] [CrossRef]
- Lopez-Marquez, D.; Del-Espino, A.; Lopez-Pagan, N.; Rodriguez-Negrete, E.A.; Rubio-Somoza, I.; Ruiz-Albert, J.; Bejarano, E.R.; Beuzon, C.R. miR825-5p targets the TIR-NBS-LRR gene MIST1 and down-regulates basal immunity against Pseudomonas syringae in Arabidopsis. J. Exp. Bot. 2021, 72, 7316–7334. [Google Scholar] [CrossRef]
- Meyers, B.C.; Morgante, M.; Michelmore, R.W. TIR-X and TIR-NBS proteins: Two new families related to disease resistance TIR-NBS-LRR proteins encoded in Arabidopsis and other plant genomes. Plant J. Cell Mol. Biol. 2002, 32, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, J.; An, L.; Doerge, R.W.; Chen, Z.J.; Grau, C.R.; Meng, J.; Osborn, T.C. Analysis of gene expression profiles in response to Sclerotinia sclerotiorum in Brassica napus. Planta 2007, 227, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Coll, N.S.; Vercammen, D.; Smidler, A.; Clover, C.; Van Breusegem, F.; Dangl, J.L.; Epple, P. Arabidopsis type I metacaspases control cell death. Science 2010, 330, 1393–1397. [Google Scholar] [CrossRef]
- Liu, C.; Cui, D.; Zhao, J.; Liu, N.; Wang, B.; Liu, J.; Xu, E.; Hu, Z.; Ren, D.; Tang, D.; et al. Two Arabidopsis Receptor-like Cytoplasmic Kinases SZE1 and SZE2 Associate with the ZAR1-ZED1 Complex and Are Required for Effector-Triggered Immunity. Mol. Plant 2019, 12, 967–983. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.M.; He, S.Y.; Xin, X.F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Ehrary, A.; Rosas, M.; Carpinelli, S.; Davalos, O.; Cowling, C.; Fernandez, F.; Escobar, M. Glutaredoxin AtGRXS8 represses transcriptional and developmental responses to nitrate in Arabidopsis thaliana roots. Plant Direct 2020, 4, e00227. [Google Scholar] [CrossRef] [PubMed]
- Walters, L.A.; Escobar, M.A. The AtGRXS3/4/5/7/8 glutaredoxin gene cluster on Arabidopsis thaliana chromosome 4 is coordinately regulated by nitrate and appears to control primary root growth. Plant Signal. Behav. 2016, 11, e1171450. [Google Scholar] [CrossRef]
- Schuler, M.; Rellan-Alvarez, R.; Fink-Straube, C.; Abadia, J.; Bauer, P. Nicotianamine functions in the Phloem-based transport of iron to sink organs, in pollen development and pollen tube growth in Arabidopsis. Plant Cell 2012, 24, 2380–2400. [Google Scholar] [CrossRef]
- Klatte, M.; Schuler, M.; Wirtz, M.; Fink-Straube, C.; Hell, R.; Bauer, P. The analysis of Arabidopsis nicotianamine synthase mutants reveals functions for nicotianamine in seed iron loading and iron deficiency responses. Plant Physiol. 2009, 150, 257–271. [Google Scholar] [CrossRef]
- Wang, Y.; Law, S.R.; Ivanova, A.; van Aken, O.; Kubiszewski-Jakubiak, S.; Uggalla, V.; van der Merwe, M.; Duncan, O.; Narsai, R.; Whelan, J.; et al. The mitochondrial protein import component, TRANSLOCASE OF THE INNER MEMBRANE17-1, plays a role in defining the timing of germination in Arabidopsis. Plant Physiol. 2014, 166, 1420–1435. [Google Scholar] [CrossRef]
- Wang, B.; Jin, S.H.; Hu, H.Q.; Sun, Y.G.; Wang, Y.W.; Han, P.; Hou, B.K. UGT87A2, an Arabidopsis glycosyltransferase, regulates flowering time via FLOWERING LOCUS C. New Phytol. 2012, 194, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, M.; Manrique, S.; Cuesta, C.; Benkova, E.; Novak, O.; Colombo, L. CUP-SHAPED COTYLEDON1 (CUC1) and CUC2 regulate cytokinin homeostasis to determine ovule number in Arabidopsis. J. Exp. Bot. 2018, 69, 5169–5176. [Google Scholar] [CrossRef] [PubMed]
- Oguchi, R.; Hanada, K.; Shimizu, M.; Mishio, M.; Ozaki, H.; Hikosaka, K. Enhanced growth rate under elevated CO2 conditions was observed for transgenic lines of genes identified by intraspecific variation analyses in Arabidopsis thaliana. Plant Mol. Biol. 2022, 110, 333–345. [Google Scholar] [CrossRef]
- Sherp, A.M.; Lee, S.G.; Schraft, E.; Jez, J.M. Modification of auxinic phenoxyalkanoic acid herbicides by the acyl acid amido synthetase GH3.15 from Arabidopsis. J. Biol. Chem. 2018, 293, 17731–17738. [Google Scholar] [CrossRef]
- Sherp, A.M.; Westfall, C.S.; Alvarez, S.; Jez, J.M. Arabidopsis thaliana GH3.15 acyl acid amido synthetase has a highly specific substrate preference for the auxin precursor indole-3-butyric acid. J. Biol. Chem. 2018, 293, 4277–4288. [Google Scholar] [CrossRef]
- Cartagena, J.A.; Matsunaga, S.; Seki, M.; Kurihara, D.; Yokoyama, M.; Shinozaki, K.; Fujimoto, S.; Azumi, Y.; Uchiyama, S.; Fukui, K. The Arabidopsis SDG4 contributes to the regulation of pollen tube growth by methylation of histone H3 lysines 4 and 36 in mature pollen. Dev. Biol. 2008, 315, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.P.; Huang, L.M.; Chen, L.O.; Chan, M.T.; Shaw, J.F. Genome-wide analysis of GDSL-type esterases/lipases in Arabidopsis. Plant Mol. Biol. 2017, 95, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Wagner, U.; Edwards, R.; Dixon, D.P.; Mauch, F. Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol. Biol. 2002, 49, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ribot, C.; Rezzonico, E.; Poirier, Y. Structure and expression profile of the Arabidopsis PHO1 gene family indicates a broad role in inorganic phosphate homeostasis. Plant Physiol. 2004, 135, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Knight, H.; Deyholos, M.; Openshaw, M.R.; Galbraith, D.W.; Warren, G.; Knight, M.R. The sfr6 mutant of Arabidopsis is defective in transcriptional activation via CBF/DREB1 and DREB2 and shows sensitivity to osmotic stress. Plant J. Cell Mol. Biol. 2003, 34, 395–406. [Google Scholar] [CrossRef]
- Peltier, J.B.; Emanuelsson, O.; Kalume, D.E.; Ytterberg, J.; Friso, G.; Rudella, A.; Liberles, D.A.; Soderberg, L.; Roepstorff, P.; von Heijne, G.; et al. Central functions of the lumenal and peripheral thylakoid proteome of Arabidopsis determined by experimentation and genome-wide prediction. Plant Cell 2002, 14, 211–236. [Google Scholar] [CrossRef]
- Fleet, C.M.; Yen, J.Y.; Hill, E.A.; Gillaspy, G.E. Co-suppression of AtMIPS demonstrates cooperation of MIPS1, MIPS2 and MIPS3 in maintaining myo-inositol synthesis. Plant Mol. Biol. 2018, 97, 253–263. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Kim, Y.C.; Lee, J.S.; Kim, D.G.; Lee, J.H. Reduced Expression of PRX2/ATPRX1, PRX8, PRX35, and PRX73 Affects Cell Elongation, Vegetative Growth, and Vasculature Structures in Arabidopsis thaliana. Plants 2022, 11, 3353. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, L.; Carr, P.; Pilling, M.; Gardner, P.; Mansfield, S.D.; Turner, S. Exploiting CELLULOSE SYNTHASE (CESA) Class Specificity to Probe Cellulose Microfibril Biosynthesis. Plant Physiol. 2018, 177, 151–167. [Google Scholar] [CrossRef]
- Kim, W.C.; Kim, J.Y.; Ko, J.H.; Kang, H.; Han, K.H. Identification of direct targets of transcription factor MYB46 provides insights into the transcriptional regulation of secondary wall biosynthesis. Plant Mol. Biol. 2014, 85, 589–599. [Google Scholar] [CrossRef]
- Szymanski, D.B. Breaking the WAVE complex: The point of Arabidopsis trichomes. Curr. Opin. Plant Biol. 2005, 8, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Yang, H.; Ma, L.; Sun, N.; Yu, H.; Liu, T.; Gao, Y.; Gu, H.; Chen, Z.; Wada, M.; et al. A genome-wide analysis of blue-light regulation of Arabidopsis transcription factor gene expression during seedling development. Plant Physiol. 2003, 133, 1480–1493. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Shen, Y.; Toledo-Ortiz, G.; Kikis, E.A.; Johannesson, H.; Hwang, Y.S.; Quail, P.H. Functional profiling reveals that only a small number of phytochrome-regulated early-response genes in Arabidopsis are necessary for optimal deetiolation. Plant Cell 2006, 18, 2157–2171. [Google Scholar] [CrossRef]
- Isoda, R.; Palmai, Z.; Yoshinari, A.; Chen, L.Q.; Tama, F.; Frommer, W.B.; Nakamura, M. SWEET13 transport of sucrose, but not gibberellin, restores male fertility in Arabidopsis sweet13;14. Proc. Natl. Acad. Sci. USA 2022, 119, e2207558119. [Google Scholar] [CrossRef]
- Weigle, A.T.; Shukla, D. The Arabidopsis AtSWEET13 transporter discriminates sugars by selective facial and positional substrate recognition. Commun. Biol. 2024, 7, 764. [Google Scholar] [CrossRef]
- Liang, M.; Gu, D.; Lie, Z.; Yang, Y.; Lu, L.; Dai, G.; Peng, T.; Deng, L.; Zheng, F.; Liu, X. Regulation of chlorophyll biosynthesis by light-dependent acetylation of NADPH:protochlorophyll oxidoreductase A in Arabidopsis. Plant Sci. Int. J. Exp. Plant Biol. 2023, 330, 111641. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.H.; Couto, D.; Santos, M.A.; Freitas, S.; Lourenco, T.; Dias, E.; Huguet, S.; Marques da Silva, J.; Tavares, R.M.; Bejarano, E.R.; et al. SUMO E3 ligase SIZ1 connects sumoylation and reactive oxygen species homeostasis processes in Arabidopsis. Plant Physiol. 2022, 189, 934–954. [Google Scholar] [CrossRef]
- Mara, C.D.; Huang, T.; Irish, V.F. The Arabidopsis floral homeotic proteins APETALA3 and PISTILLATA negatively regulate the BANQUO genes implicated in light signaling. Plant Cell 2010, 22, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Torii, K.; Araki, T.; Endo, M. Importance of epidermal clocks for regulation of hypocotyl elongation through PIF4 and IAA29. Plant Signal. Behav. 2016, 11, e1143999. [Google Scholar] [CrossRef][Green Version]
- Lee, K.; Seo, P.J. Coordination of matrix attachment and ATP-dependent chromatin remodeling regulate auxin biosynthesis and Arabidopsis hypocotyl elongation. PLoS ONE 2017, 12, e0181804. [Google Scholar] [CrossRef]
- Chen, Q.; Dai, X.; De-Paoli, H.; Cheng, Y.; Takebayashi, Y.; Kasahara, H.; Kamiya, Y.; Zhao, Y. Auxin overproduction in shoots cannot rescue auxin deficiencies in Arabidopsis roots. Plant Cell Physiol. 2014, 55, 1072–1079. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutierrez, R.A. Nitrate Transport, Sensing, and Responses in Plants. Mol. Plant 2016, 9, 837–856. [Google Scholar] [CrossRef] [PubMed]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K.; et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Audenaert, D.; Parizot, B.; Moller, B.K.; Njo, M.F.; De Rybel, B.; De Rop, G.; Van Isterdael, G.; Mahonen, A.P.; Vanneste, S.; et al. Root Cap-Derived Auxin Pre-patterns the Longitudinal Axis of the Arabidopsis Root. Curr. Biol. CB 2015, 25, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Sexauer, M.; Bhasin, H.; Schon, M.; Roitsch, E.; Wall, C.; Herzog, U.; Markmann, K. A micro RNA mediates shoot control of root branching. Nat. Commun. 2023, 14, 8083. [Google Scholar] [CrossRef]
- Xu, P.; Fang, S.; Chen, H.; Cai, W. The brassinosteroid-responsive xyloglucan endotransglucosylase/hydrolase 19 (XTH19) and XTH23 genes are involved in lateral root development under salt stress in Arabidopsis. Plant J. Cell Mol. Biol. 2020, 104, 59–75. [Google Scholar] [CrossRef]
- Pitaksaringkarn, W.; Matsuoka, K.; Asahina, M.; Miura, K.; Sage-Ono, K.; Ono, M.; Yokoyama, R.; Nishitani, K.; Ishii, T.; Iwai, H.; et al. XTH20 and XTH19 regulated by ANAC071 under auxin flow are involved in cell proliferation in incised Arabidopsis inflorescence stems. Plant J. Cell Mol. Biol. 2014, 80, 604–614. [Google Scholar] [CrossRef]
- Howing, T.; Dann, M.; Muller, B.; Helm, M.; Scholz, S.; Schneitz, K.; Hammes, U.Z.; Gietl, C. The role of KDEL-tailed cysteine endopeptidases of Arabidopsis (AtCEP2 and AtCEP1) in root development. PLoS ONE 2018, 13, e0209407. [Google Scholar] [CrossRef]
- Hierl, G.; Howing, T.; Isono, E.; Lottspeich, F.; Gietl, C. Ex vivo processing for maturation of Arabidopsis KDEL-tailed cysteine endopeptidase 2 (AtCEP2) pro-enzyme and its storage in endoplasmic reticulum derived organelles. Plant Mol. Biol. 2014, 84, 605–620. [Google Scholar] [CrossRef][Green Version]
- Han, X.; Zhang, M.; Yang, M.; Hu, Y. Arabidopsis JAZ Proteins Interact with and Suppress RHD6 Transcription Factor to Regulate Jasmonate-Stimulated Root Hair Development. Plant Cell 2020, 32, 1049–1062. [Google Scholar] [CrossRef]
- Gonzalez-Perez, E.; Ortega-Amaro, M.A.; Salazar-Badillo, F.B.; Bautista, E.; Douterlungne, D.; Jimenez-Bremont, J.F. The Arabidopsis-Trichoderma interaction reveals that the fungal growth medium is an important factor in plant growth induction. Sci. Rep. 2018, 8, 16427. [Google Scholar] [CrossRef] [PubMed]
- Kf de Campos, M.; Schaaf, G. The regulation of cell polarity by lipid transfer proteins of the SEC14 family. Curr. Opin. Plant Biol. 2017, 40, 158–168. [Google Scholar] [CrossRef]
- Grierson, C.S.; Roberts, K.; Feldmann, K.A.; Dolan, L. The COW1 locus of arabidopsis acts after RHD2, and in parallel with RHD3 and TIP1, to determine the shape, rate of elongation, and number of root hairs produced from each site of hair formation. Plant Physiol. 1997, 115, 981–990. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barros-Galvao, T.; Dave, A.; Cole, A.; Harvey, D.; Langer, S.; Larson, T.R.; Vaistij, F.E.; Graham, I.A. cis-12-Oxo-phytodienoic acid represses Arabidopsis seed germination in shade conditions. J. Exp. Bot. 2019, 70, 5919–5927. [Google Scholar] [CrossRef]
- Jia, L.; Xu, W.; Li, W.; Ye, N.; Liu, R.; Shi, L.; Bin Rahman, A.N.; Fan, M.; Zhang, J. Class III peroxidases are activated in proanthocyanidin-deficient Arabidopsis thaliana seeds. Ann. Bot. 2013, 111, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Oono, Y.; Seki, M.; Satou, M.; Iida, K.; Akiyama, K.; Sakurai, T.; Fujita, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Monitoring expression profiles of Arabidopsis genes during cold acclimation and deacclimation using DNA microarrays. Funct. Integr. Genom. 2006, 6, 212–234. [Google Scholar] [CrossRef]
- Jemmat, A.M.; Ranocha, P.; Le Ru, A.; Neel, M.; Jauneau, A.; Raggi, S.; Ferrari, S.; Burlat, V.; Dunand, C. Coordination of five class III peroxidase-encoding genes for early germination events of Arabidopsis thaliana. Plant Sci. Int. J. Exp. Plant Biol. 2020, 298, 110565. [Google Scholar] [CrossRef]
- Renard, J.; Ninoles, R.; Martinez-Almonacid, I.; Gayubas, B.; Mateos-Fernandez, R.; Bissoli, G.; Bueso, E.; Serrano, R.; Gadea, J. Identification of novel seed longevity genes related to oxidative stress and seed coat by genome-wide association studies and reverse genetics. Plant Cell Environ. 2020, 43, 2523–2539. [Google Scholar] [CrossRef]
- Marquis, V.; Smirnova, E.; Graindorge, S.; Delcros, P.; Villette, C.; Zumsteg, J.; Heintz, D.; Heitz, T. Broad-spectrum stress tolerance conferred by suppressing jasmonate signaling attenuation in Arabidopsis JASMONIC ACID OXIDASE mutants. Plant J. Cell Mol. Biol. 2022, 109, 856–872. [Google Scholar] [CrossRef]
- Kinoshita, T.; Nishimura, M.; Hara-Nishimura, I. Homologues of a vacuolar processing enzyme that are expressed in different organs in Arabidopsis thaliana. Plant Mol. Biol. 1995, 29, 81–89. [Google Scholar] [CrossRef]
- Shimada, T.; Yamada, K.; Kataoka, M.; Nakaune, S.; Koumoto, Y.; Kuroyanagi, M.; Tabata, S.; Kato, T.; Shinozaki, K.; Seki, M.; et al. Vacuolar processing enzymes are essential for proper processing of seed storage proteins in Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 32292–32299. [Google Scholar] [CrossRef] [PubMed]
- Estornell, L.H.; Landberg, K.; Cierlik, I.; Sundberg, E. SHI/STY Genes Affect Pre- and Post-meiotic Anther Processes in Auxin Sensing Domains in Arabidopsis. Front. Plant Sci. 2018, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Otto, M.; Naumann, C.; Brandt, W.; Wasternack, C.; Hause, B. Activity Regulation by Heteromerization of Arabidopsis Allene Oxide Cyclase Family Members. Plants 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Bourdenx, B.; Bernard, A.; Domergue, F.; Pascal, S.; Leger, A.; Roby, D.; Pervent, M.; Vile, D.; Haslam, R.P.; Napier, J.A.; et al. Overexpression of Arabidopsis ECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses. Plant Physiol. 2011, 156, 29–45. [Google Scholar] [CrossRef]
- Aarts, M.G.; Keijzer, C.J.; Stiekema, W.J.; Pereira, A. Molecular characterization of the CER1 gene of arabidopsis involved in epicuticular wax biosynthesis and pollen fertility. Plant Cell 1995, 7, 2115–2127. [Google Scholar] [CrossRef]
- Ogawa, M.; Hanada, A.; Yamauchi, Y.; Kuwahara, A.; Kamiya, Y.; Yamaguchi, S. Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 2003, 15, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- Dinolfo, M.I.; Castanares, E.; Stenglein, S.A. Resistance of Fusarium poae in Arabidopsis leaves requires mainly functional JA and ET signaling pathways. Fungal Biol. 2017, 121, 841–848. [Google Scholar] [CrossRef]
- De Paepe, A.; Vuylsteke, M.; Van Hummelen, P.; Zabeau, M.; Van Der Straeten, D. Transcriptional profiling by cDNA-AFLP and microarray analysis reveals novel insights into the early response to ethylene in Arabidopsis. Plant J. Cell Mol. Biol. 2004, 39, 537–559. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Y.; Yang, P.; Zhao, H.; Jenks, M.A.; Lu, S.; Yang, X. The Arabidopsis cytochrome P450 enzyme CYP96A4 is involved in the wound-induced biosynthesis of cuticular wax and cutin monomers. Plant J. Cell Mol. Biol. 2024, 118, 1619–1634. [Google Scholar] [CrossRef]
- Goda, H.; Sawa, S.; Asami, T.; Fujioka, S.; Shimada, Y.; Yoshida, S. Comprehensive comparison of auxin-regulated and brassinosteroid-regulated genes in Arabidopsis. Plant Physiol. 2004, 134, 1555–1573. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Wang, Y.; Zhou, G.; Liu, S.; Li, D.; Adnan; Hussain, S.; Ahmed, S.; Zhang, C.; et al. AIW1 and AIW2, two ABA-induced WD40 repeat-containing transcription repressors function redundantly to regulate ABA and salt responses in Arabidopsis. J. Plant Interact. 2020, 15, 196–206. [Google Scholar] [CrossRef]
- Huang, K.C.; Lin, W.C.; Cheng, W.H. Salt hypersensitive mutant 9, a nucleolar APUM23 protein, is essential for salt sensitivity in association with the ABA signaling pathway in Arabidopsis. BMC Plant Biol. 2018, 18, 40. [Google Scholar] [CrossRef]
- Jing, Y.; Shi, L.; Li, X.; Zheng, H.; Gao, J.; Wang, M.; He, L.; Zhang, W. OXS2 is Required for Salt Tolerance Mainly through Associating with Salt Inducible Genes, CA1 and Araport11, in Arabidopsis. Sci. Rep. 2019, 9, 20341. [Google Scholar] [CrossRef] [PubMed]
- Okawa, K.; Nakayama, K.; Kakizaki, T.; Yamashita, T.; Inaba, T. Identification and characterization of Cor413im proteins as novel components of the chloroplast inner envelope. Plant Cell Environ. 2008, 31, 1470–1483. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Xia, J.Q.; Wu, J.; Han, Y.; Zhang, G.Q.; Zhao, P.X.; Xiang, C.B. Root-derived long-distance signals trigger ABA synthesis and enhance drought resistance in Arabidopsis. J. Genet. Genom. 2024, 51, 749–761. [Google Scholar] [CrossRef]
- Zou, J.J.; Li, X.D.; Ratnasekera, D.; Wang, C.; Liu, W.X.; Song, L.F.; Zhang, W.Z.; Wu, W.H. Arabidopsis CALCIUM-DEPENDENT PROTEIN KINASE8 and CATALASE3 Function in Abscisic Acid-Mediated Signaling and H2O2 Homeostasis in Stomatal Guard Cells under Drought Stress. Plant Cell 2015, 27, 1445–1460. [Google Scholar] [CrossRef]
- Valerio, L.; De Meyer, M.; Penel, C.; Dunand, C. Expression analysis of the Arabidopsis peroxidase multigenic family. Phytochemistry 2004, 65, 1331–1342. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Mittler, R. The water-water cycle is essential for chloroplast protection in the absence of stress. J. Biol. Chem. 2003, 278, 38921–38925. [Google Scholar] [CrossRef]
- Welinder, K.G.; Justesen, A.F.; Kjaersgard, I.V.; Jensen, R.B.; Rasmussen, S.K.; Jespersen, H.M.; Duroux, L. Structural diversity and transcription of class III peroxidases from Arabidopsis thaliana. Eur. J. Biochem. 2002, 269, 6063–6081. [Google Scholar] [CrossRef]
- Kovacs, D.; Kalmar, E.; Torok, Z.; Tompa, P. Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiol. 2008, 147, 381–390. [Google Scholar] [CrossRef]
- Li, J.Y.; Fu, Y.L.; Pike, S.M.; Bao, J.; Tian, W.; Zhang, Y.; Chen, C.Z.; Li, H.M.; Huang, J.; Li, L.G.; et al. The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell 2010, 22, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, T.; Sari, N.; Misaki, R.; Fujiyama, K. Biochemical characterization of Arabidopsis clade F polygalacturonase shows a substrate preference toward oligogalacturonic acids. J. Biosci. Bioeng. 2022, 133, 1–7. [Google Scholar] [CrossRef]
- Gong, Q.; Li, P.; Ma, S.; Indu Rupassara, S.; Bohnert, H.J. Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2005, 44, 826–839. [Google Scholar] [CrossRef]
- Negi, J.; Matsuda, O.; Nagasawa, T.; Oba, Y.; Takahashi, H.; Kawai-Yamada, M.; Uchimiya, H.; Hashimoto, M.; Iba, K. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 2008, 452, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Cubero-Font, P.; Maierhofer, T.; Jaslan, J.; Rosales, M.A.; Espartero, J.; Diaz-Rueda, P.; Muller, H.M.; Hurter, A.L.; Al-Rasheid, K.A.; Marten, I.; et al. Silent S-Type Anion Channel Subunit SLAH1 Gates SLAH3 Open for Chloride Root-to-Shoot Translocation. Curr. Biol. CB 2016, 26, 2213–2220. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q.; Wang, X.; Zhang, J.; Ismail, A.M.; Zhang, Z. Nitrogen form-mediated ethylene signal regulates root-to-shoot K+ translocation via NRT1.5. Plant Cell Environ. 2021, 44, 3576–3588. [Google Scholar] [CrossRef]
- Sena, F.; Kunze, R. The K+ transporter NPF7.3/NRT1.5 and the proton pump AHA2 contribute to K+ transport in Arabidopsis thaliana under K+ and NO3− deficiency. Front. Plant Sci. 2023, 14, 1287843. [Google Scholar] [CrossRef]
- Hoekenga, O.A.; Maron, L.G.; Pineros, M.A.; Cancado, G.M.; Shaff, J.; Kobayashi, Y.; Ryan, P.R.; Dong, B.; Delhaize, E.; Sasaki, T.; et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 9738–9743. [Google Scholar] [CrossRef] [PubMed]
- Armengaud, P.; Breitling, R.; Amtmann, A. The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant Physiol. 2004, 136, 2556–2576. [Google Scholar] [CrossRef]
- Xu, Z.; Escamilla-Trevino, L.; Zeng, L.; Lalgondar, M.; Bevan, D.; Winkel, B.; Mohamed, A.; Cheng, C.L.; Shih, M.C.; Poulton, J.; et al. Functional genomic analysis of Arabidopsis thaliana glycoside hydrolase family 1. Plant Mol. Biol. 2004, 55, 343–367. [Google Scholar] [CrossRef]
- Ding, G.; Lei, G.J.; Yamaji, N.; Yokosho, K.; Mitani-Ueno, N.; Huang, S.; Ma, J.F. Vascular Cambium-Localized AtSPDT Mediates Xylem-to-Phloem Transfer of Phosphorus for Its Preferential Distribution in Arabidopsis. Mol. Plant 2020, 13, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.J.; Wang, Z.; Wirtz, M.; Hell, R.; Oliver, D.J.; Xiang, C.B. SULTR3;1 is a chloroplast-localized sulfate transporter in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2013, 73, 607–616. [Google Scholar] [CrossRef] [PubMed]
- La Mantia, J.; Unda, F.; Douglas, C.J.; Mansfield, S.D.; Hamelin, R. Overexpression of AtGolS3 and CsRFS in poplar enhances ROS tolerance and represses defense response to leaf rust disease. Tree Physiol. 2018, 38, 457–470. [Google Scholar] [CrossRef]
- Taji, T.; Ohsumi, C.; Iuchi, S.; Seki, M.; Kasuga, M.; Kobayashi, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2002, 29, 417–426. [Google Scholar] [CrossRef]
- Zhang, B.; Tremousaygue, D.; Denance, N.; van Esse, H.P.; Horger, A.C.; Dabos, P.; Goffner, D.; Thomma, B.P.; van der Hoorn, R.A.; Tuominen, H. PIRIN2 stabilizes cysteine protease XCP2 and increases susceptibility to the vascular pathogen Ralstonia solanacearum in Arabidopsis. Plant J. Cell Mol. Biol. 2014, 79, 1009–1019. [Google Scholar] [CrossRef]
- Rebolledo-Prudencio, O.G.; Estrada-Rivera, M.; Dautt-Castro, M.; Arteaga-Vazquez, M.A.; Arenas-Huertero, C.; Rosendo-Vargas, M.M.; Jin, H.L.; Casas-Flores, S. The small RNA-mediated gene silencing machinery is required in Arabidopsis for stimulation of growth, systemic disease resistance, and suppression of the nitrile-specifier gene by. Plant J. 2022, 109, 873–890. [Google Scholar] [CrossRef] [PubMed]
- Widemann, E.; Bruinsma, K.; Walshe-Roussel, B.; Rioja, C.; Arbona, V.; Saha, R.K.; Letwin, D.; Zhurov, V.; Gomez-Cadenas, A.; Bernards, M.A.; et al. Multiple indole glucosinolates and myrosinases defend Arabidopsis against Tetranychus urticae herbivory. Plant Physiol. 2021, 187, 116–132. [Google Scholar] [CrossRef]
- Barth, C.; Jander, G. Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. Plant J. Cell Mol. Biol. 2006, 46, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.K.; Rupinikrishna, K.; Akhil, V.S.; Vashisth, N.; Phani, V.; Pankaj; Sirohi, A.; Chinnusamy, V. CRISPR/Cas9-induced knockout of an amino acid permease gene (AAP6) reduced Arabidopsis thaliana susceptibility to Meloidogyne incognita. BMC Plant Biol. 2024, 24, 515. [Google Scholar] [CrossRef]
- Marella, H.H.; Nielsen, E.; Schachtman, D.P.; Taylor, C.G. The amino acid permeases AAP3 and AAP6 are involved in root-knot nematode parasitism of Arabidopsis. Mol. Plant Microbe Interact. 2013, 26, 44–54. [Google Scholar] [CrossRef]
- Meyers, B.C.; Kozik, A.; Griego, A.; Kuang, H.; Michelmore, R.W. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 2003, 15, 809–834. [Google Scholar] [CrossRef] [PubMed]
- Dievart, A.; Clark, S.E. Using mutant alleles to determine the structure and function of leucine-rich repeat receptor-like kinases. Curr. Opin. Plant Biol. 2003, 6, 507–516. [Google Scholar] [CrossRef]
- Gao, Y.; Badejo, A.A.; Sawa, Y.; Ishikawa, T. Analysis of two L-Galactono-1,4-lactone-responsive genes with complementary expression during the development of Arabidopsis thaliana. Plant Cell Physiol. 2012, 53, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Klepek, Y.S.; Geiger, D.; Stadler, R.; Klebl, F.; Landouar-Arsivaud, L.; Lemoine, R.; Hedrich, R.; Sauer, N. Arabidopsis POLYOL TRANSPORTER5, a new member of the monosaccharide transporter-like superfamily, mediates H+-Symport of numerous substrates, including myo-inositol, glycerol, and ribose. Plant Cell 2005, 17, 204–218. [Google Scholar] [CrossRef]
- Jagadeeswaran, G.; Li, Y.F.; Sunkar, R. Redox signaling mediates the expression of a sulfate-deprivation-inducible microRNA395 in Arabidopsis. Plant J. Cell Mol. Biol. 2014, 77, 85–96. [Google Scholar] [CrossRef]
- Frei dit Frey, N.; Muller, P.; Jammes, F.; Kizis, D.; Leung, J.; Perrot-Rechenmann, C.; Bianchi, M.W. The RNA binding protein Tudor-SN is essential for stress tolerance and stabilizes levels of stress-responsive mRNAs encoding secreted proteins in Arabidopsis. Plant Cell 2010, 22, 1575–1591. [Google Scholar] [CrossRef]
- Wang, B.; Li, M.; Yuan, Y.; Liu, S. Genome-Wide Comprehensive Analysis of the SABATH Gene Family in Arabidopsis and Rice. Evol. Bioinform. Online 2019, 15, 1176934319860864. [Google Scholar] [CrossRef]
- Lee, J.; He, K.; Stolc, V.; Lee, H.; Figueroa, P.; Gao, Y.; Tongprasit, W.; Zhao, H.; Lee, I.; Deng, X.W. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 2007, 19, 731–749. [Google Scholar] [CrossRef] [PubMed]
- K, M.J.; Laxmi, A. DUF581 is plant specific FCS-like zinc finger involved in protein-protein interaction. PLoS ONE 2014, 9, e99074. [Google Scholar] [CrossRef]
- Rautengarten, C.; Steinhauser, D.; Bussis, D.; Stintzi, A.; Schaller, A.; Kopka, J.; Altmann, T. Inferring hypotheses on functional relationships of genes: Analysis of the Arabidopsis thaliana subtilase gene family. PLoS Comput. Biol. 2005, 1, e40. [Google Scholar] [CrossRef]
- Bui, L.T.; Giuntoli, B.; Kosmacz, M.; Parlanti, S.; Licausi, F. Constitutively expressed ERF-VII transcription factors redundantly activate the core anaerobic response in Arabidopsis thaliana. Plant Sci. Int. J. Exp. Plant Biol. 2015, 236, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Pucciariello, C.; Perata, P. New insights into reactive oxygen species and nitric oxide signalling under low oxygen in plants. Plant Cell Environ. 2017, 40, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Burssens, S.; Himanen, K.; van de Cotte, B.; Beeckman, T.; Van Montagu, M.; Inze, D.; Verbruggen, N. Expression of cell cycle regulatory genes and morphological alterations in response to salt stress in Arabidopsis thaliana. Planta 2000, 211, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Granier, C.; Inze, D.; Tardieu, F. Spatial distribution of cell division rate can be deduced from that of p34(cdc2) kinase activity in maize leaves grown at contrasting temperatures and soil water conditions. Plant Physiol. 2000, 124, 1393–1402. [Google Scholar] [CrossRef]
- Vernoux, T.; Wilson, R.C.; Seeley, K.A.; Reichheld, J.P.; Muroy, S.; Brown, S.; Maughan, S.C.; Cobbett, C.S.; Van Montagu, M.; Inze, D.; et al. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 2000, 12, 97–110. [Google Scholar] [CrossRef]
- May, M. Review article. Glutathione homeostasis in plants: Implications for environmental sensing and plant development. J. Exp. Bot. 1998, 49, 649–667. [Google Scholar] [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef]
- Zolla, G.; Heimer, Y.M.; Barak, S. Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. J. Exp. Bot. 2010, 61, 211–224. [Google Scholar] [CrossRef]
- Blomster, T.; Salojarvi, J.; Sipari, N.; Brosche, M.; Ahlfors, R.; Keinanen, M.; Overmyer, K.; Kangasjarvi, J. Apoplastic reactive oxygen species transiently decrease auxin signaling and cause stress-induced morphogenic response in Arabidopsis. Plant Physiol. 2011, 157, 1866–1883. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium. Gene Ontology Consortium: Going forward. Nucleic Acids Res. 2015, 43, D1049–D1056. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Segura Broncano, L.; Pukacz, K.R.; Reichel-Deland, V.; Schluter, U.; Triesch, S.; Weber, A.P.M. Photorespiration is the solution, not the problem. J. Plant Physiol. 2023, 282, 153928. [Google Scholar] [CrossRef] [PubMed]
- Bowes, G.; Ogren, W.L.; Hageman, R.H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem. Biophys. Res. Commun. 1971, 45, 716–722. [Google Scholar] [CrossRef]
- Levey, M.; Timm, S.; Mettler-Altmann, T.; Luca Borghi, G.; Koczor, M.; Arrivault, S.; Pm Weber, A.; Bauwe, H.; Gowik, U.; Westhoff, P. Efficient 2-phosphoglycolate degradation is required to maintain carbon assimilation and allocation in the C4 plant Flaveria bidentis. J. Exp. Bot. 2019, 70, 575–587. [Google Scholar] [CrossRef]
- Flugel, F.; Timm, S.; Arrivault, S.; Florian, A.; Stitt, M.; Fernie, A.R.; Bauwe, H. The Photorespiratory Metabolite 2-Phosphoglycolate Regulates Photosynthesis and Starch Accumulation in Arabidopsis. Plant Cell 2017, 29, 2537–2551. [Google Scholar] [CrossRef]
- Queval, G.; Issakidis-Bourguet, E.; Hoeberichts, F.A.; Vandorpe, M.; Gakiere, B.; Vanacker, H.; Miginiac-Maslow, M.; Van Breusegem, F.; Noctor, G. Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J. Cell Mol. Biol. 2007, 52, 640–657. [Google Scholar] [CrossRef]
- Widholm, J.M.; Ogren, W.L. Photorespiratory-induced senescence of plants under conditions of low carbon dioxide. Proc. Natl. Acad. Sci. USA 1969, 63, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Schurmann, P.; Buchanan, B.B. The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antioxid. Redox Signal. 2008, 10, 1235–1274. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Van den Ende, W.; Valluru, R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Considine, M.J.; Foyer, C.H. Redox regulation of plant development. Antioxid. Redox Signal. 2014, 21, 1305–1326. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Lopez-Delgado, H.; Dat, J.F.; Scott, I.M. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant. 2006, 100, 241–254. [Google Scholar] [CrossRef]
- Xue, S.; Hu, H.; Ries, A.; Merilo, E.; Kollist, H.; Schroeder, J.I. Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J. 2011, 30, 1645–1658. [Google Scholar] [CrossRef]
- Gibbs, D.J.; Lee, S.C.; Isa, N.M.; Gramuglia, S.; Fukao, T.; Bassel, G.W.; Correia, C.S.; Corbineau, F.; Theodoulou, F.L.; Bailey-Serres, J.; et al. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 2011, 479, 415–418. [Google Scholar] [CrossRef]
- Licausi, F.; Kosmacz, M.; Weits, D.A.; Giuntoli, B.; Giorgi, F.M.; Voesenek, L.A.; Perata, P.; van Dongen, J.T. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 2011, 479, 419–422. [Google Scholar] [CrossRef]
- Gonzali, S.; Loreti, E.; Cardarelli, F.; Novi, G.; Parlanti, S.; Pucciariello, C.; Bassolino, L.; Banti, V.; Licausi, F.; Perata, P. Universal stress protein HRU1 mediates ROS homeostasis under anoxia. Nat. Plants 2015, 1, 15151. [Google Scholar] [CrossRef] [PubMed]
- Weits, D.A.; Giuntoli, B.; Kosmacz, M.; Parlanti, S.; Hubberten, H.M.; Riegler, H.; Hoefgen, R.; Perata, P.; van Dongen, J.T.; Licausi, F. Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat. Commun. 2014, 5, 3425. [Google Scholar] [CrossRef]
- Giuntoli, B.; Lee, S.C.; Licausi, F.; Kosmacz, M.; Oosumi, T.; van Dongen, J.T.; Bailey-Serres, J.; Perata, P. A trihelix DNA binding protein counterbalances hypoxia-responsive transcriptional activation in Arabidopsis. PLoS Biol. 2014, 12, e1001950. [Google Scholar] [CrossRef]
- Hoeren, F.U.; Dolferus, R.; Wu, Y.; Peacock, W.J.; Dennis, E.S. Evidence for a role for AtMYB2 in the induction of the Arabidopsis alcohol dehydrogenase gene (ADH1) by low oxygen. Genetics 1998, 149, 479–490. [Google Scholar] [CrossRef]
- Ellis, M.H.; Dennis, E.S.; Peacock, W.J. Arabidopsis roots and shoots have different mechanisms for hypoxic stress tolerance. Plant Physiol. 1999, 119, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Iwai, H. Virtual issue: Cell wall functions in plant growth and environmental responses. J. Plant Res. 2021, 134, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Gigli-Bisceglia, N.; Engelsdorf, T.; Hamann, T. Plant cell wall integrity maintenance in model plants and crop species-relevant cell wall components and underlying guiding principles. Cell. Mol. Life Sci. 2020, 77, 2049–2077. [Google Scholar] [CrossRef] [PubMed]
- Polko, J.K.; Kieber, J.J. The Regulation of Cellulose Biosynthesis in Plants. Plant Cell 2019, 31, 282–296. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Structure and growth of plant cell walls. Nat. reviews. Mol. Cell Biol. 2024, 25, 340–358. [Google Scholar] [CrossRef]
- Lin, C.; Choi, H.S.; Cho, H.T. Root hair-specific EXPANSIN A7 is required for root hair elongation in Arabidopsis. Mol. Cells 2011, 31, 393–397. [Google Scholar] [CrossRef]
- Abbasi, A.; Malekpour, M.; Sobhanverdi, S. The Arabidopsis expansin gene (AtEXPA18) is capable to ameliorate drought stress tolerance in transgenic tobacco plants. Mol. Biol. Rep. 2021, 48, 5913–5922. [Google Scholar] [CrossRef]
- Wieczorek, K.; Grundler, F.M. Expanding nematode-induced syncytia: The role of expansins. Plant Signal. Behav. 2006, 1, 223–224. [Google Scholar] [CrossRef]
- Brown, D.; Wightman, R.; Zhang, Z.; Gomez, L.D.; Atanassov, I.; Bukowski, J.P.; Tryfona, T.; McQueen-Mason, S.J.; Dupree, P.; Turner, S. Arabidopsis genes IRREGULAR XYLEM (IRX15) and IRX15L encode DUF579-containing proteins that are essential for normal xylan deposition in the secondary cell wall. Plant J. Cell Mol. Biol. 2011, 66, 401–413. [Google Scholar] [CrossRef]
- Jensen, J.K.; Kim, H.; Cocuron, J.C.; Orler, R.; Ralph, J.; Wilkerson, C.G. The DUF579 domain containing proteins IRX15 and IRX15-L affect xylan synthesis in Arabidopsis. Plant J. Cell Mol. Biol. 2011, 66, 387–400. [Google Scholar] [CrossRef]
- Ren, Y.; Hansen, S.F.; Ebert, B.; Lau, J.; Scheller, H.V. Site-directed mutagenesis of IRX9, IRX9L and IRX14 proteins involved in xylan biosynthesis: Glycosyltransferase activity is not required for IRX9 function in Arabidopsis. PLoS ONE 2014, 9, e105014. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Kaida, R. Functions of xyloglucan in plant cells. Mol. Plant 2011, 4, 17–24. [Google Scholar] [CrossRef]
- Behar, H.; Graham, S.W.; Brumer, H. Comprehensive cross-genome survey and phylogeny of glycoside hydrolase family 16 members reveals the evolutionary origin of EG16 and XTH proteins in plant lineages. Plant J. Cell Mol. Biol. 2018, 95, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Behar, H.; Tamura, K.; Wagner, E.R.; Cosgrove, D.J.; Brumer, H. Conservation of endo-glucanase 16 (EG16) activity across highly divergent plant lineages. Biochem. J. 2021, 478, 3063–3078. [Google Scholar] [CrossRef]
- Hrmova, M.; Stratilova, B.; Stratilova, E. Broad Specific Xyloglucan:Xyloglucosyl Transferases Are Formidable Players in the Re-Modelling of Plant Cell Wall Structures. Int. J. Mol. Sci. 2022, 23, 1656. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, S.; Shabala, S.; Jian, T.; Zhang, W. Plants Grown in Parafilm-Wrapped Petri Dishes Are Stressed and Possess Altered Gene Expression Profile. Front. Plant Sci. 2019, 10, 637. [Google Scholar] [CrossRef]
- Ma, Y.; Yao, L.; Zhang, L.; Su, A.; Wang, R.; Song, W.; Li, Z.; Zhao, J. Genome-wide association analysis of chilling-tolerant germination in a new maize association mapping panel. Food Energy Secur. 2022, 12, e445. [Google Scholar] [CrossRef]


| Catalog | DEGs | Up | Down |
|---|---|---|---|
| CK7d vs. PW7d | 1814 | 643 | 1171 |
| CK7d vs. PF7d | 1083 | 635 | 448 |
| CK7d vs. ST7d | 2 | 2 | 0 |
| CK14d vs. PW14d | 2644 | 592 | 1818 |
| CK14d vs. PF14d | 2410 | 644 | 2000 |
| CK14d vs. ST14d | 2246 | 751 | 1495 |
| CK7d vs. PW7d and CK7d vs. PF7d | 609 | 274 | 335 |
| CK14d vs. PW14d and CK14d vs. PF14d | 1614 | 347 | 1267 |
| CK7d vs. PW7d and CK7d vs. PF7d and CK14d vs. PW14d and CK14d vs. PF14d | 220 | 73 | 134 |
| Gene ID | Other Name | Description | Functional Classification | Representative Reference | log2 (CK7d vs. PW7d) | log2 (CK7d vs. PF7d) | log2 (CK14d vs. PW14d) | log2 (CK14d vs. PF14d) |
|---|---|---|---|---|---|---|---|---|
| AT1G21520 | hypothetical protein | Stress response (oxidative stress) | [6,7] | 2.28 | 2.74 | 1.23 | 2.22 | |
| AT4G31870 | ATGPX7 | Glutathione peroxidase | Stress response (oxidative stress) | [8,9,10] | 2.55 | 1.98 | 1.91 | 1.65 |
| AT5G16970 | AER | A 2-alkenal reductase (EC 1.3.1.74) | Stress response (oxidative stress) | [11,12] | 1.87 | 1.79 | 1.17 | 1.03 |
| AT1G10585 | Basic helix-loop-helix (bHLH) DNA-binding superfamily protein | Stress response (oxidative stress and wounding stress) | [13] | 2.31 | 3.82 | 1.94 | 3.05 | |
| AT3G62930 | ROXY17/AtGRXS6 | Member of the CC-type glutaredoxin (ROXY) family | Stress response (oxidative stress) + Nutrient uptake (nitrate) | [14,15] | 2.22 | 1.65 | 1.84 | 1.24 |
| AT1G22400 | UGT85A1 | UDP-Glycosyltransferase superfamily protein | Stress response (heat) | [16,17] | 1.04 | 1.58 | 1.71 | 1.72 |
| AT5G03720 | HSFA3 | Member of heat stress transcription factor (Hsf) family | Stress response (heat) | [18,19] | 2 | 1.18 | 2.2 | 1.72 |
| AT2G04050 | MATE efflux family protein | Stress response (Salt) | [20] | 5.94 | 4.15 | 2.12 | 3.51 | |
| AT2G38310 | PYL4 | Member of the PYR (pyrabactinresistance)/PYL(PYR1-like)/RCAR (regulatory components of ABA receptor) family proteins | Hormone response (abscisic acid) | [21] | 1.25 | 1.59 | 1.57 | 1.16 |
| AT5G05440 | PYL5 | Member of the PYR (pyrabactinresistance)/PYL(PYR1-like)/RCAR (regulatory components of ABA receptor) family proteins | Hormone response (abscisic acid) | [21] | 1.93 | 2.07 | 1.63 | 1.59 |
| AT2G40330 | PYL6 | Member of the PYR (pyrabactinresistance)/PYL(PYR1-like)/RCAR (regulatory components of ABA receptor) family proteins | Hormone response (abscisic acid) | [22] | 1.8 | 2.32 | 2.82 | 2.65 |
| AT4G16563 | Eukaryotic aspartyl protease family protein | Hormone response (abscisic acid) | [23] | 1.51 | 1.4 | 2.16 | 2.2 | |
| AT4G11460 | CRK30 | Cysteine-rich receptor-like protein kinase | Hormone response (abscisic acid) | [24] | 1.23 | 2.68 | 6.7 | 6.45 |
| AT2G40340 | DREB2C | Member of the DREB subfamily A-2 of ERF/AP2 transcription factor family | Hormone response (abscisic acid) | [25] | 2.57 | 1.82 | 2.3 | 2.65 |
| AT3G14620 | CYP72A8 | Putative cytochrome P450 | Hormone response (phytoprostanes) | [26] | 1.68 | 1.94 | 2.15 | 1.77 |
| AT5G16980 | Zinc-binding dehydrogenase family protein | Hormone response (phytoprostanes) | [26] | 3.13 | 2.59 | 2.33 | 2.03 | |
| AT5G60250 | Zinc finger (C3HC4-type RING finger) family protein | Hormone response (cytokinin) | [27] | 5.25 | 1.6 | 3.91 | 1.95 | |
| AT1G35140 | EXL1 | Exordium like 1; Hypoxia response unknown protein 46 | Stress response (carbon starvation) + Hormone response (brassinolide) | [28,29] | 2.78 | 2.43 | 2.4 | 2.43 |
| AT5G13210 | Uncharacterized conserved protein UCP015417 | Stress response (sucrose starvation) | [30] | 2.24 | 1.01 | 1.2 | 1 | |
| AT3G01970 | WRKY45 | Member of WRKY Transcription Factor | Stress response (phosphate starvation) | [31] | 1.98 | 2.02 | 1.28 | 1.73 |
| AT4G34950 | MFS1 | Major facilitator superfamily protein | Stress response (phosphate starvation) | [32] | 1.28 | 1.68 | 1.29 | 1.52 |
| AT2G04040 | ATDTX1 | A detoxifying efflux carrier for plant-derived antibiotics and other toxic compounds | Stress response (heavy metal, Cadmium) | [33] | 3.04 | 4.23 | 3.32 | 4.08 |
| AT1G51820 | SIF4 | Leucine-rich repeat protein kinase family protein | Plant-pathogen interactions | [34] | 1.41 | 2.51 | 1.48 | 2.04 |
| AT1G63750 | MiR825-5p target proposed as a phasiRNA producing locus | Plant-pathogen interactions | [35] | 1.35 | 1.05 | 1.44 | 1.19 | |
| AT1G72900 | TN7 | Toll-Interleukin-Resistance (TIR) domain-containing protein | Plant-pathogen interactions | [36] | 1.7 | 2.17 | 2.02 | 2.3 |
| AT2G32550 | NOT9C | Rcd1-like protein | Plant-pathogen interactions | [37] | 2.9 | 1.3 | 2.57 | 1.94 |
| AT4G25110 | AtMC2 | A type I metacaspase | Plant-pathogen interactions | [38] | 1.77 | 2.13 | 2.02 | 2.23 |
| AT5G11410 | SZE2 | Similar to receptor like kinase but does not appear to have kinase activity (pseudokinase) | Plant-pathogen interactions | [39] | 3.19 | 2.52 | 2.12 | 2.32 |
| AT5G20480 | EFR | A predicted leucine-rich repeat receptor kinase (LRR-RLK) | Plant-pathogen interactions | [40] | 1.17 | 1.82 | 1.25 | 1.29 |
| AT4G15660 | GRXS8;ROXY15 | A member of the CC-type glutaredoxin (ROXY) family | Nutrient uptake (soil nitrate) | [41] | 4.74 | 5.41 | 1.71 | 2 |
| AT4G15670 | GRXS7;ROXY14 | A member of the CC-type glutaredoxin (ROXY) family | Nutrient uptake (soil nitrate) | [42] | 3.23 | 3.88 | 3.91 | 4.76 |
| AT4G15680 | GRXS4;ROXY13 | A member of the CC-type glutaredoxin (ROXY) family | Nutrient uptake (soil nitrate) | [42] | 3.36 | 3.5 | 3.05 | 3.44 |
| AT4G15690 | GRXS5;ROXY12 | A member of the CC-type glutaredoxin (ROXY) family | Nutrient uptake (soil nitrate) | [42] | 3.34 | 3.46 | 1.81 | 2.3 |
| AT4G15700 | GRXS3;ROXY11 | A member of the CC-type glutaredoxin (ROXY) family | Nutrient uptake (soil nitrate) | [42] | 3.58 | 3.97 | 1.71 | 2.37 |
| AT1G09240 | NAS3 | A nicotianamine synthase | Nutrient uptake (iron) | [43,44] | 2.05 | 1.07 | 1.66 | 1.14 |
| AT1G20350 | TIM17-1 | Mitochondrial inner membrane translocase | Growth and development (seed germination) | [45] | 4.5 | 2.57 | 2.25 | 2.03 |
| AT2G30140 | UGT87A2 | A putative glycosyltransferase | Growth and development (flowering time) | [46] | 1.07 | 1.52 | 1.07 | 1.6 |
| AT2G36750 | UGT73C1 | UDP-glucosyl transferase 73CC1 | Growth and development (pistils and siliques elongation) | [47] | 2.91 | 3.48 | 1.65 | 2.92 |
| AT3G04000 | CHLADR | An aldehyde reductase | Growth and development (seedlings growth) | [48] | 1.52 | 1.81 | 1.84 | 1.62 |
| AT5G13370 | GH3.15 | IBA—specific acyl acid amido synthetase, which conjugates glutamine to IBA | Growth and development (primary root and lateral root growth) | [49,50] | 1.53 | 1.52 | 1.19 | 1.3 |
| AT5G16960 | Zinc-binding dehydrogenase family protein | Growth and development (pollen tube growth) | [51] | 3.05 | 4.85 | 1.6 | 3.33 | |
| AT1G20120 | AtGELP3 | GDSL-motif esterase/acyltransferase/lipase | Unknown | [52] | 4.35 | 2.37 | 1.48 | 1.16 |
| AT2G29480 | ATGSTU2 | Glutathione transferase belonging to the tau class of GSTs | unknown | [53] | 1.85 | 3.31 | 1.07 | 1.56 |
| AT1G35350 | PHO1;H8 | EXS (ERD1/XPR1/SYG1) family protein | Unknown | [54] | 1.04 | 1.53 | 1.2 | 1.26 |
| AT2G23100 | Cysteine/Histidine-rich C1 domain family protein | Unknown | [55] | 2.11 | 1.37 | 2.04 | 1.84 | |
| AT2G29370 | NAD(P)-binding Rossmann-fold superfamily protein | Unknown | none | 2.11 | 1.8 | 3.6 | 3.39 | |
| AT2G30615 | F-box/LRR protein | Unknown | none | 3.35 | 2.96 | 1.73 | 2.09 | |
| AT3G21530 | DNAse I-like superfamily protein | Unknown | none | 1.29 | 1.67 | 1.67 | 1.8 | |
| AT3G26320 | CYP71B36 | Putative cytochrome P450 | Unknown | none | 1.11 | 1.22 | 1.27 | 1.56 |
| AT5G55570 | Transmembrane protein | Unknown | [56] | 2.16 | 1.31 | 1.45 | 1.04 | |
| AT5G54710 | Ankyrin repeat family protein | Unknown | none | 1.38 | 2.25 | 2.23 | 2.03 |
| Gene ID | Other Name | Description | Functional Classification | Representative Reference | log2 (CK7d vs. PW7d) | log2 (CK7d vs. PF7d) | log2 (CK14d vs. PW14d) | log2 (CK14d vs. PF14d) |
|---|---|---|---|---|---|---|---|---|
| AT2G22240 | ATMIPS2 | Myo-inositol-1-phosphate synthase isoform 2 | Growth and development (vegetative growth) | [57] | −1.09 | −1.29 | −1.85 | −1.69 |
| AT3G49960 | PRX35 | Class III peroxidase genes | Growth and development (vegetative growth) | [58] | −1.51 | −1.33 | −3.27 | −4.44 |
| AT4G18780 | CESA8/IRX1 | A member of the cellulose synthase family | Growth and development (vegetative growth) | [59,60] | −1.51 | −1.08 | −1.86 | −2.32 |
| AT2G33385 | ARPC2B | Actin-related protein C2B | Growth and development (cell morphogenesis) | [61] | −2.79 | −1.67 | −1.32 | −1.98 |
| AT3G21330 | Basic helix-loop-helix (bHLH) DNA-binding superfamily protein | Growth and development (seedling development) | [62,63] | −2.65 | −1.42 | −1.91 | −1.23 | |
| AT5G50800 | SWEET13 | Member of the SWEET sucrose efflux transporter family proteins | Growth and development (anthers, seeds and seedlings development) | [64,65] | −4.66 | −2.87 | −4 | −3.56 |
| AT5G54190 | PORA | Light-dependent NADPH:protochlorophyllide oxidoreductase A | Growth and development (photomorphogenesis) + Stress response (oxidative stress) | [66,67] | −2.33 | −2.15 | −2.6 | −1.06 |
| AT5G15160 | BNQ2 | A atypical non-DNA binding basic helix-loop-helix (bHLH) proteins | Growth and development (hypocotyl elongation, petal differentiation and flowering time) | [68] | −1.71 | −1.24 | −2.31 | −1.96 |
| AT4G32280 | IAA29 | Indole-3-acetic acid inducible 29 protein | Growth and development (hypocotyl growth) | [69] | −1.79 | −1.64 | −4.31 | −3.14 |
| AT1G04180 | YUC9 | Flavin monooxygenase | Growth and development (hypocotyl and root growth) | [70,71] | −3.02 | −2.58 | −1.72 | −1.94 |
| AT1G12110 | NRT1.1/CHL1 | A dual-affinity nitrate transporter. | Growth and development (lateral root development) | [72,73] | −1.15 | −1.16 | −2.17 | −2 |
| AT2G39370 | MAKR4 | A member of the MAKR (MEMBRANE-ASSOCIATED KINASE REGULATOR) gene family | Growth and development (lateral roots formation) | [74] | −1.32 | −2.14 | −1.7 | −2.35 |
| AT3G27150 | HOLT | Target gene of MIR2111-5p | Growth and development (lateral root initiation) | [75] | −1.17 | −1.22 | −1.26 | −1.26 |
| AT4G30290 | XTH19 | A xyloglucan endotransglucosylase/hydrolase | Growth and development (lateral root development) | [76,77] | −1.05 | −1.1 | −1.58 | −1.92 |
| AT3G48340 | CEP2 | KDEL-tailed cysteine endopeptidase | Growth and development (lateral root growth) | [78,79] | −1.8 | −1.06 | −2.81 | −2.84 |
| AT1G66470 | RHD6 | Basic helix loop helix transcription factor | Growth and development (root hair initiation) | [80,81] | −1.6 | −1.34 | −1.52 | −1.71 |
| AT4G34580 | COW1/AtSFH1 | A phosphatidylinositol transfer protein | Growth and development (root hair growth) | [82,83] | −2.19 | −1.1 | −2.15 | −1.79 |
| AT1G18100 | MFT | A member of the FT and TFL1 family of phosphatidylethanolamine-binding proteins | Growth and development (seed germination) + Hormone response (abscisic acid) | [84] | −1.36 | −1.71 | −2.74 | −3.1 |
| AT2G18980 | PRX16 | Class III peroxidase cell wall-targeted protein | Growth and development (seed germination) + Stress response (salt) | [85,86,87] | −1.99 | −1.01 | −1.6 | −2.38 |
| AT1G18710 | ATMYB47 | Member of the R2R3 factor gene family | Growth and development (seed longevity) + Stress response (drought) | [88,89] | −3.25 | −1.45 | −2.97 | −1.23 |
| AT1G62710 | AtAEP2; β-VPE | A vacuolar processing enzyme | Growth and development (seed proteins maturation) | [90,91] | −2.11 | −1.46 | −2.39 | −1.62 |
| AT1G56710 | PGL1 | Pectin lyase-like superfamily protein | Growth and development (anther morphogenesis) | [92] | −2.58 | −2 | −2.11 | −2.33 |
| AT1G13280 | AOC4 | An allene oxide cyclase | Growth and development (male fertility) | [93] | −1.07 | −1.04 | −1.14 | −1.25 |
| AT1G02205 | CER1 | An aldehyde decarbonylase | Growth and development (male fertility) + Hormone response (abscisic acid) | [94,95] | −1.04 | −1.14 | −2.06 | −2.08 |
| AT4G36880 | AtCP1 | Cysteine proteinase1 | Hormone response (gibberellin) | [96] | −2.76 | −1.56 | −2.13 | −2.2 |
| AT3G12500 | PR3 | A basic chitinase | Hormone response (ethylene/jasmonic acid) | [97] | −2.71 | −2.12 | −2.85 | −2.77 |
| AT5G39190 | GER2 | Germin-like protein | Hormone response (ethylene) | [98] | −3.6 | −1.65 | −2.63 | −2.26 |
| AT5G52320 | CYP96A4 | Cytochrome P450 enzyme | Hormone response (jasmonic acid) | [99] | −3.25 | −1.5 | −2.34 | −1.69 |
| AT1G78490 | CYP708A3 | Member of CYP708A family | Hormone response (brassinolide) | [100] | −2.76 | −1.48 | −1.6 | −1.19 |
| AT4G39510 | CYP96A12 | Member of CYP96A | Hormone response (brassinolide) | [100] | −1.39 | −1.4 | −1.55 | −1.3 |
| AT3G22540 | Hypothetical protein | Hormone response (cytokinin) | [27] | −1.09 | −1.33 | −1.04 | −1.73 | |
| AT5G45080 | PP2-A6 | Phloem protein 2-A6 | Hormone response (cytokinin) | [27] | −2.08 | −1.56 | −1.6 | −1.52 |
| AT1G49450 | AIW2 | Transducin/WD40 repeat-like superfamily protein | Hormone response (abscisic acid) | [101] | −1.38 | −1.38 | −2.12 | −1.87 |
| AT1G29395 | COR413IM1;COR414-TM1 | Integral membrane protein in the inner envelope of chloroplasts | Hormone response (abscisic acid) + Stress response (drought and cold) | [102,103,104] | −4.33 | −2.55 | −4.79 | −3.41 |
| AT3G14440 | ATNCED3 | Encodes 9-cis-epoxycarotenoid dioxygenase | Hormone response (abscisic acid) + Stress response (drought and salt) | [105] | −1.51 | −1.29 | −3.59 | −1.69 |
| AT1G20620 | ATCAT3 | Catalase | Stress response (oxidative stress) | [106] | −2.12 | −1.34 | −2.2 | −1.16 |
| AT5G58390 | AtP44/AtPrx67 | Peroxidase superfamily protein | Stress response (oxidative stress) | [107,108,109] | −3.67 | −1.65 | −1.77 | −1.23 |
| AT1G20450 | ERD10 | Encodes a gene induced by low temperature and dehydration | Stress response (salt, drought, and cold) | [110] | −1.11 | −1.25 | −1.49 | −1.17 |
| AT2G37900 | Major facilitator superfamily protein | Stress response (heavy metal, Cadmium) | [111] | −3.46 | −1.79 | −2.08 | −1.67 | |
| AT4G23500 | PGF12 | Pectin lyase-like superfamily protein | Stress response (salt) + Carbohydrate metabolic | [112,113] | −1.66 | −1.44 | −1.7 | −1.52 |
| AT1G62280 | SLAH1 | A protein with ten predicted transmembrane helices | Ion translocation + Nutrient uptake (potassium, chloride, malate, fumarate, and succinate) | [114,115] | −2 | −1.45 | −2.32 | −3.36 |
| AT1G32450 | NRT1.5;NPF7.3 | Transmembrane nitrate transporter | Ion translocation (potassium and nitrate) | [116,117] | −1.15 | −1.19 | −1.36 | −1.25 |
| AT1G08430 | ALMT1 | Al-activated malate efflux transporter | Nutrient uptake (aluminum-activated malate) | [118] | −1.76 | −2.35 | −1.94 | −2.29 |
| AT5G28510 | BGLU24 | Beta glucosidase 24 | Ion translocation (potassium) + Carbohydrate metabolic | [119,120] | −5.6 | −5.61 | −4.92 | −3.91 |
| AT3G15990 | SPDT | Vascular cambium-localized sulfate transporter | Ion translocation (phosphorus) | [121] | −2.07 | −1.42 | −1.6 | −1.2 |
| AT3G51895 | SULTR3;1 | Encodes a chloroplast-localized sulfate transporter | Ion translocation (sulfate) | [122] | −3.19 | −2.14 | −2.95 | −1.73 |
| AT1G09350 | ATGOLS3 | Predicted to encode a galactinol synthase | Plant-pathogen interactions + Stress response (cold) | [123,124] | −3.94 | −2.76 | −3.04 | −3.02 |
| AT2G43120 | PIRIN2 | A member of the functionally diverse cupin protein superfamily | Plant-pathogen interactions | [125] | −2.24 | −1.02 | −1.96 | −1.9 |
| AT3G16410 | NSP4 | Encodes a nitrile-specifier protein NSP4 | Plant-pathogen interactions | [126] | −2.4 | −1.24 | −1.7 | −2.08 |
| AT5G25980 | TGG2 | Myrosinase (thioglucosideglucohydrolase) gene | Plant-pathogen interactions + Carbohydrate metabolic | [127,128] | −3.19 | −1.19 | −3.29 | −2.39 |
| AT5G49630 | AAP6 | A high affinity amino acid transporter | Plant-pathogen interactions | [129,130] | −3.12 | −1.89 | −2.64 | −2.73 |
| AT2G17060 | Disease resistance protein (TIR-NBS-LRR class) family | Pseudogenes | [131] | −1.35 | −1.57 | −1.47 | −1.6 | |
| AT1G51870 | Protein kinase family protein | unknown | none | −2.67 | −1.71 | −1.71 | −2.24 | |
| AT1G66830 | Leucine-rich repeat protein kinase family protein | unknown | [132] | −2.66 | −1.89 | −1.27 | −1.66 | |
| AT1G80240 | DGR1 | DUF642 gene | unknown | [133] | −1.29 | −1.21 | −2.03 | −3.04 |
| AT2G18480 | AtPLT3 | Polyol transporter | unknown | [134] | −2.05 | −2.54 | −1.62 | −1.52 |
| AT2G28780 | P-hydroxybenzoic acid efflux pump subunit | unknown | [135] | −2.27 | −2.42 | −1.65 | −2.11 | |
| AT3G08660 | Phototropicresponsive NPH3 family protein | unknown | none | −1.37 | −1.34 | −1.91 | −1.25 | |
| AT3G53980 | Bifunctional inhibitor/lipid-transfer protein/seed storage 2S albumin superfamily protein | unknown | [136] | −1.74 | −1.37 | −3.59 | −3.35 | |
| AT5G38100 | SABATH family methyltransferase | unknown | [137] | −1.99 | −1.29 | −1.04 | −1.26 | |
| AT5G45850 | Hypothetical protein (DUF688) | unknown | [138] | −2.62 | −2.16 | −1.92 | −1.17 | |
| AT5G47000 | AtP43/AtPrx65 | Peroxidase superfamily protein | unknown | [107,109] | −1.83 | −2.36 | −2.43 | −2.42 |
| AT5G49120 | DUF581 family protein, putative (DUF581) | unknown | [139] | −2.85 | −1.99 | −1.98 | −1.02 | |
| AT5G51750 | SBT1.3 | Subtilase 1.3 | unknown | [140] | −1.07 | −1.01 | −1.01 | −1.03 |
| AT5G60650 | Proline-rich receptor-like kinase | unknown | none | −1.76 | −1.38 | −1.42 | −1.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Li, F.; Wang, X.; Sun, Q.; Wang, R.; Zhao, J. Beware of Sealing Film of Petri Dishes!—Alters the Expression of a Large Number of Genes. Int. J. Mol. Sci. 2025, 26, 5484. https://doi.org/10.3390/ijms26125484
Ma Y, Li F, Wang X, Sun Q, Wang R, Zhao J. Beware of Sealing Film of Petri Dishes!—Alters the Expression of a Large Number of Genes. International Journal of Molecular Sciences. 2025; 26(12):5484. https://doi.org/10.3390/ijms26125484
Chicago/Turabian StyleMa, Yun, Fang Li, Xuyang Wang, Qingpeng Sun, Ronghuan Wang, and Jiuran Zhao. 2025. "Beware of Sealing Film of Petri Dishes!—Alters the Expression of a Large Number of Genes" International Journal of Molecular Sciences 26, no. 12: 5484. https://doi.org/10.3390/ijms26125484
APA StyleMa, Y., Li, F., Wang, X., Sun, Q., Wang, R., & Zhao, J. (2025). Beware of Sealing Film of Petri Dishes!—Alters the Expression of a Large Number of Genes. International Journal of Molecular Sciences, 26(12), 5484. https://doi.org/10.3390/ijms26125484






