Relationship Between Oxidative Stress and Cardiovascular Risk in Adolescents in Montenegro
Abstract
1. Introduction
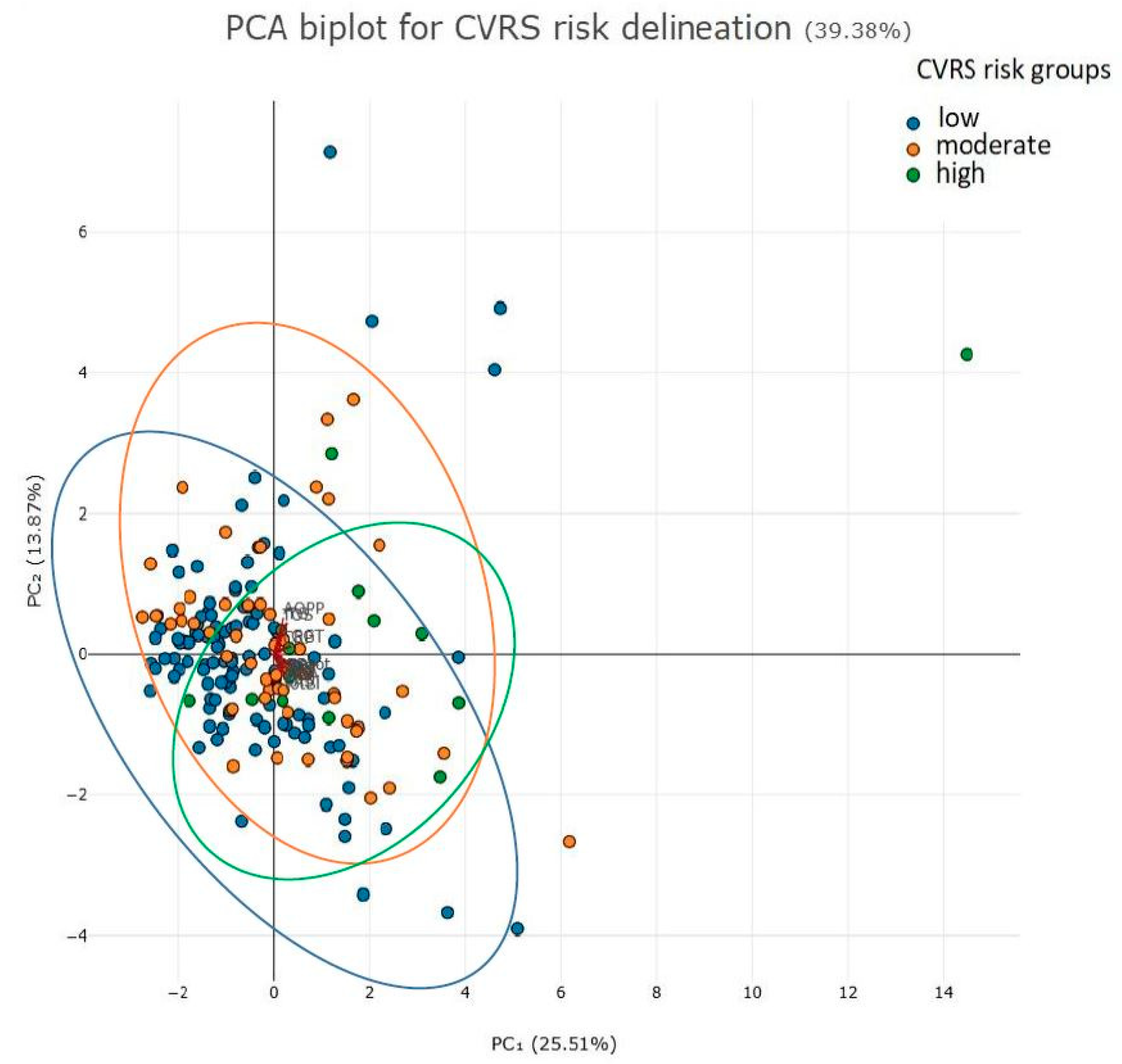
2. Results
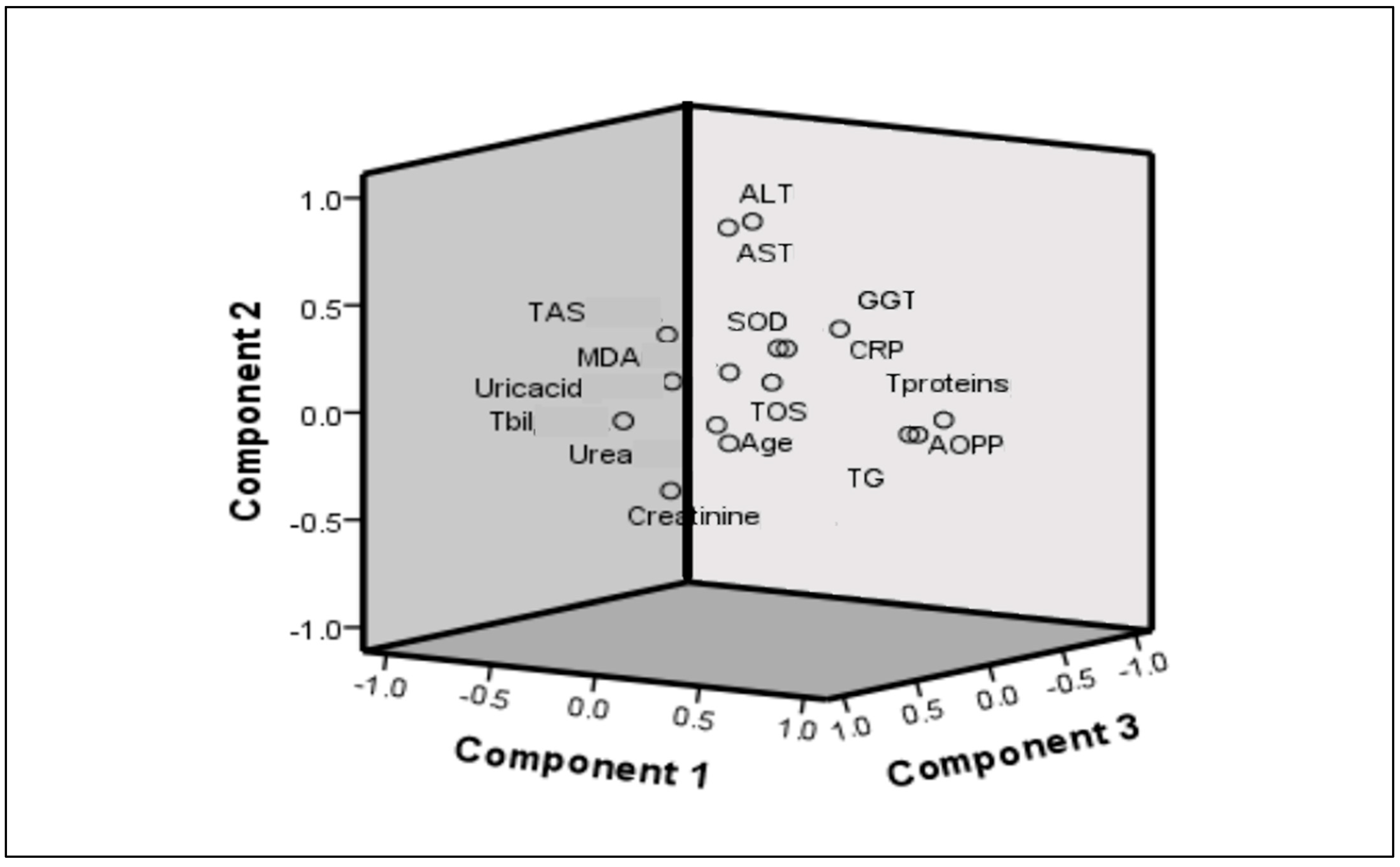
Factorial (Principal Component Analysis)
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Biochemical Analyses
4.3. Oxidative Stress Biomarkers
4.3.1. Superoxide Dismutase (SOD)
4.3.2. Total Antioxidative Status (TAS)
4.3.3. Advanced Oxidation Protein Products (AOPPs)
4.3.4. Malondialdehyde (MDA)
4.3.5. Total Oxidative Status (TOS)
4.3.6. TAS/TOS Ratio
4.4. Calculation of Pro-Oxidant, Antioxidant and Oxy Scores
4.5. Calculation of CVRS
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Xie, Y.; Li, K.; Yuan, R.; Zhang, L. The global burden and risk factors of cardiovascular diseases in adolescent and young adults, 1990–2019. BMC Public Health 2024, 24, 1017. [Google Scholar] [CrossRef]
- Kelly, A.S.; Armstrong, S.C.; Michalsky, M.P.; Fox, C.K. Obesity in Adolescents: A Review. JAMA 2024, 332, 738–748. [Google Scholar] [CrossRef]
- Klisic, A.; Kavaric, N.; Soldatovic, I.; Bjelakovic, B.; Kotur-Stevuljevic, J. Relationship between Cardiovascular Risk Score and Traditional and Nontraditional Cardiometabolic Parameters in Obese Adolescent Girls. J. Med. Biochem. 2016, 35, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Carrasquilla, G.D.; Ängquist, L.; Sørensen, T.I.A.; Kilpeläinen, T.O.; Loos, R.J.F. Child-to-adult body size change and risk of type 2 diabetes and cardiovascular disease. Diabetologia 2024, 67, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Klisic, A.; Radoman Vujačić, I.; Vučković, L.J.; Ninic, A. Total leukocyte count, leukocyte subsets and their indexes in relation to cardiovascular risk in adolescent population. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Corbacho-Alonso, N.; Baldán-Martín, M.; López, J.A.; Rodríguez-Sánchez, E.; Martínez, P.J.; Mourino-Alvarez, L.; Sastre-Oliva, T.; Cabrera, M.; Calvo, E.; Padial, L.R.; et al. Cardiovascular Risk Stratification Based on Oxidative Stress for Early Detection of Pathology. Antioxid. Redox Signal. 2021, 35, 602–617. [Google Scholar] [CrossRef]
- Mitu, O.; Crisan, A.; Redwood, S.; Cazacu-Davidescu, I.E.; Mitu, I.; Costache, I.I.; Onofrei, V.; Miftode, R.S.; Costache, A.D.; Haba, C.M.S.; et al. The Relationship between Cardiovascular Risk Scores and Several Markers of Subclinical Atherosclerosis in an Asymptomatic Population. J. Clin. Med. 2021, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Klisic, A.; Kavaric, N.; Vujcic, S.; Spasojevic-Kalimanovska, V.; Kotur-Stevuljevic, J.; Ninic, A. Total oxidant status and oxidative stress index as indicators of increased Reynolds Risk Score in postmenopausal women. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10126–10133. [Google Scholar] [CrossRef]
- Najman, J.M.; Kisely, S.; Scott, J.G.; Ushula, T.W.; Williams, G.M.; Clavarino, A.M.; McGee, T.R.; Mamun, A.A.; Wang, W.Y.S. Gender differences in cardiovascular disease risk: Adolescence to young adulthood. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 98–106. [Google Scholar] [CrossRef]
- Vekic, J.; Stromsnes, K.; Mazzalai, S.; Zeljkovic, A.; Rizzo, M.; Gambini, J. Oxidative Stress, Atherogenic Dyslipidemia, and Cardiovascular Risk. Biomedicines 2023, 11, 2897. [Google Scholar] [CrossRef]
- Hertiš Petek, T.; Marčun Varda, N. Childhood Cardiovascular Health, Obesity, and Some Related Disorders: Insights into Chronic Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 9706. [Google Scholar] [CrossRef] [PubMed]
- Hertiš Petek, T.; Homšak, E.; Svetej, M.; Marčun Varda, N. Systemic Inflammation and Oxidative Stress in Childhood Obesity: Sex Differences in Adiposity Indices and Cardiovascular Risk. Biomedicines 2024, 13, 58. [Google Scholar] [CrossRef]
- Hertiš Petek, T.; Petek, T.; Močnik, M.; Marčun Varda, N. Systemic Inflammation, Oxidative Stress and Cardiovascular Health in Children and Adolescents: A Systematic Review. Antioxidants 2022, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Vukicevic, P.; Klisic, A.; Neskovic, V.; Babic, L.; Mikic, A.; Bogavac-Stanojevic, N.; Matkovic, M.; Milićević, V.; Aleksic, N.; Kotur-Stevuljevic, J. Oxidative Stress in Patients before and after On-Pump and Off-Pump Coronary Artery Bypass Grafting: Relationship with Syntax Score. Oxidative Med. Cell. Longev. 2021, 2021, 3315951. [Google Scholar] [CrossRef]
- Elmas, B.; Karacan, M.; Dervişoğlu, P.; Kösecik, M.; İşgüven, Ş.P.; Bal, C. Dynamic thiol/disulphide homeostasis as a novel indicator of oxidative stress in obese children and its relationship with inflammatory-cardiovascular markers. Anatol. J. Cardiol. 2017, 18, 361–369. [Google Scholar] [CrossRef]
- Romuk, E.; Wojciechowska, C.; Jacheć, W.; Zemła-Woszek, A.; Momot, A.; Buczkowska, M.; Rozentryt, P. Malondialdehyde and Uric Acid as Predictors of Adverse Outcome in Patients with Chronic Heart Failure. Oxidative Med. Cell. Longev. 2019, 2019, 9246138. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, S.; Vania, A.; Caroli, M.; Orlando, A.; Lieti, G.; Parati, G.; Giussani, M. Non-Pharmacological Treatment for Cardiovascular Risk Prevention in Children and Adolescents with Obesity. Nutrients 2024, 16, 2497. [Google Scholar] [CrossRef]
- de Miranda, J.A.; Cunha, W.R.; Lovisi, J.C.M.; Moreira Lanna, C.M.; Pinheiro, L.C.; Lacchini, R.; Tanus-Santos, J.E.; de Almeida Belo, V. Oxidative stress and obesity are associated with endothelial dysfunction and subclinical atherosclerosis in adolescents. Clin. Biochem. 2025, 136, 110889. [Google Scholar] [CrossRef]
- Klisic, A.; Kotur-Stevuljevic, J.; Gluscevic, S.; Sahin, S.B.; Mercantepe, F. Biochemical markers and carotid intima-media thickness in relation to cardiovascular risk in young women. Sci. Rep. 2024, 14, 24776. [Google Scholar] [CrossRef]
- Cojocaru, K.-A.; Luchian, I.; Goriuc, A.; Antoci, L.-M.; Ciobanu, C.-G.; Popescu, R.; Vlad, C.-E.; Blaj, M.; Foia, L.G. Mitochondrial Dysfunction, Oxidative Stress, and Therapeutic Strategies in Diabetes, Obesity, and Cardiovascular Disease. Antioxidants 2023, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Xu, C.; An, P.; Luo, Y.; Jiao, L.; Luo, J.; Li, Y. Mitochondrial Dysfunction and Therapeutic Perspectives in Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 16053. [Google Scholar] [CrossRef]
- Čolak, E.; Pap, D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J. Med. Biochem. 2021, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, Z.; Proctor, G.; Moskowitz, S.; Liebman, S.E.; Rogers, T.; Lucia, M.S.; Li, J.; Levi, M. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J. Biol. Chem. 2005, 280, 32317–32325. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, X.; Lv, L.; Wang, C.; Ye, Z.; Li, S.; Liu, Q.; Lou, T.; Liu, X. Correlation between Serum Lipid Levels and Measured Glomerular Filtration Rate in Chinese Patients with Chronic Kidney Disease. PLoS ONE 2016, 11, e0163767. [Google Scholar] [CrossRef] [PubMed]
- Klisic, A.; Kavaric, N.; Ninic, A. Serum cystatin C levels are associated with triglycerides/high-density lipoprotein cholesterol ratio in adolescent girls ages between 16–19 years old. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10680–10686. [Google Scholar] [CrossRef] [PubMed]
- Rowicka, G.; Dyląg, H.; Ambroszkiewicz, J.; Riahi, A.; Weker, H.; Chełchowska, M. Total Oxidant and Antioxidant Status in Prepubertal Children with Obesity. Oxidative Med. Cell. Longev. 2017, 2017, 5621989. [Google Scholar] [CrossRef]
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.F.; Vesa, C.M.; Behl, T.; Bungau, A.F.; et al. Oxidative stress—Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed. Pharmacother. 2022, 152, 113238. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Girotti, M.J.; Khan, N.; McLellan, B.A. Early measurement of systemic lipid peroxidation products in the plasma of major blunt trauma patients. J. Trauma Acute Care Surg. 1991, 31, 32–35. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Veglia, F.; Cighetti, G.; De Franceschi, M.; Zingaro, L.; Boccotti, L.; Tremoli, E.; Cavalca, V. Age- and gender-related oxidative status determined in healthy subjects by means of OXY-SCORE, a potential new comprehensive index. Biomarkers 2006, 11, 562–573. [Google Scholar] [CrossRef] [PubMed]
- McMahan, C.A.; Gidding, S.S.; Fayad, Z.A.; Zieske, A.W.; Malcom, G.T.; Tracy, R.E.; Strong, J.P.; McGill, H.C., Jr. Risk scores predict atherosclerotic lesions in young people. Arch. Intern. Med. 2005, 165, 883–890. [Google Scholar] [CrossRef] [PubMed]
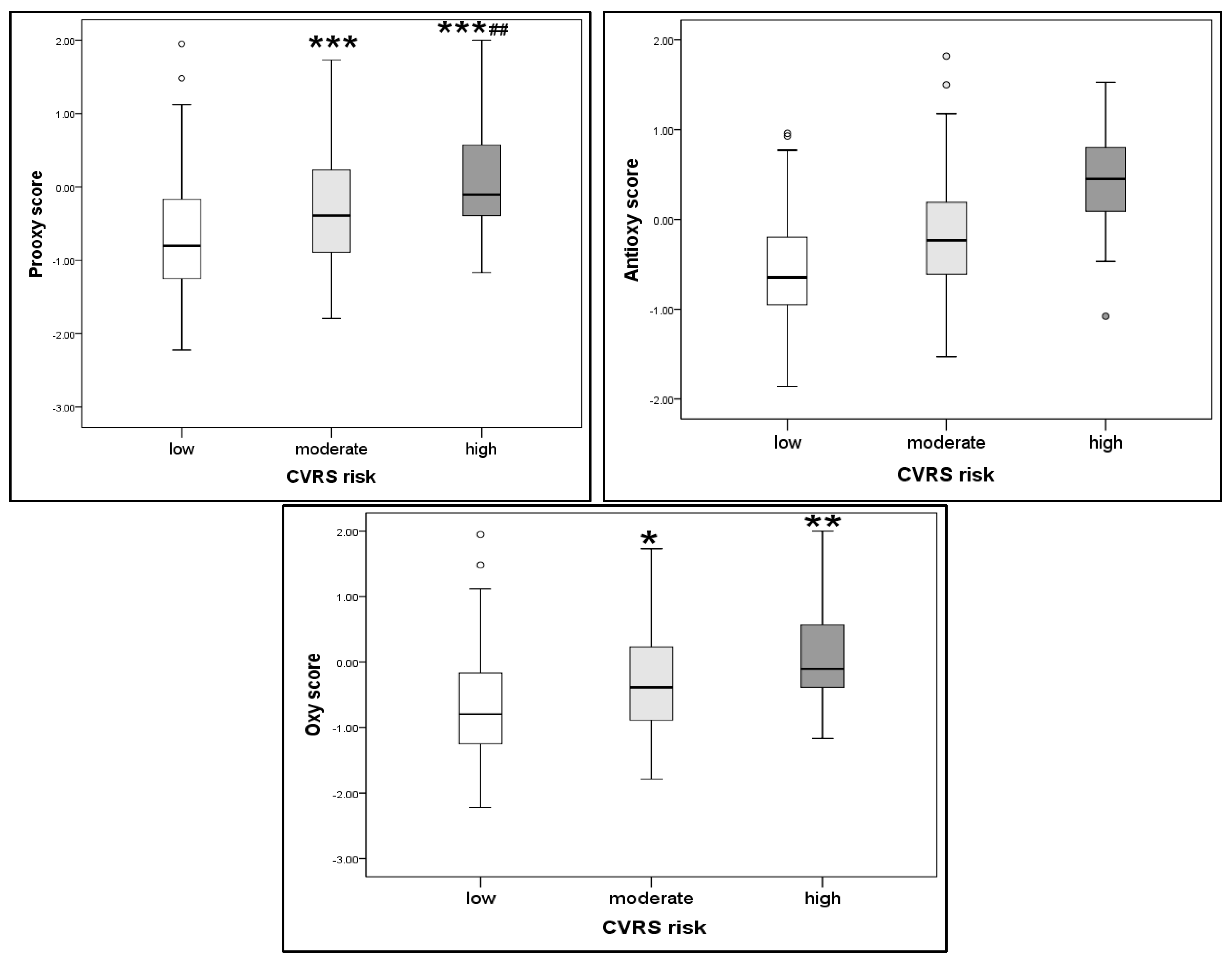
| Parameter | CVRS | p | ||
|---|---|---|---|---|
| Low Risk (−2–1) n = 114 | Moderate Risk (2–4) n = 54 | High Risk (≥5) n = 14 | ||
| Age (years) | 16 (16–17) | 17 (16–18) | 17 (16–18) | 0.070 |
| Gender, m/f: n (%) | 21/93 (18.4/81.6) | 13/41 (24.1/75.9) | 10/4 (71.4/28.6) | 19.1, <0.001 |
| Smoking status no/yes | 106/8 (93.0/7.0) | 49/5 (90.7/9.3) | 10/4 (71.4/28.6) | 6.8, 0.034 |
| BMI (kg/m2) | 22.1 (20.6–24.4) | 23.3 (22.0–26.1) *** | 27.0 (25.5–31.0) ***, # | <0.001 |
| WC (cm) | 72 (68–78) | 76 (71–83) ** | 89 (75–100) ***, ## | <0.001 |
| SBP (mmHg) | 118 (114–125) | 134 (130–137) *** | 137 (134–144) ***, # | <0.001 |
| DBP (mmHg) | 74 (68–78) | 82 (78–89) *** | 81 (77–85) *** | <0.001 |
| Glucose (mmoL/L) | 4.7 (4.5–4.9) | 4.7 (4.5–4.9) | 4.9 (4.8–5.3) *,# | 0.040 |
| Urea (mmol/L) | 3.8 (3.3–4.6) | 3.7 (3.3–4.3) | 3.8 (3.2–4.4) | 0.620 |
| Creatinine (µmol/L) | 60 (53–71) | 62 (57–67) | 69 (62–80) **, # | 0.006 |
| Uric acid (µmol/L) | 237 (212–288) | 255 (218–294) | 276 (231–331) | 0.175 |
| Total proteins (g/L) | 77 (74–79) | 78 (75–81) | 78 (76–82) | 0.224 |
| hsCRP (mg/L) | 0.40 (0.30–0.90) | 0.70 (0.40–1.50) * | 1.10 (0.40–4.80) **, # | 0.013 |
| TC (mmol/L) | 4.01 (3.59–4.44) | 4.09 (3.56–4.44) | 3.84 (3.40–4.79) | 0.729 |
| HDL-c (mmol/L) | 1.45 (1.32–1.68) | 1.48 (1.20–1.73) | 1.26 (1.02–1.44) *, # | 0.033 |
| LDL-c (mmol/L) | 2.13 (1.80–2.54) | 2.06 (1.84–2.53) | 2.22 (1.81–2.77) | 0.514 |
| TG (mmol/L) | 0.73 (0.57–0.88) | 0.80 (0.67–1.01) * | 0.73 (0.62–1.16) | 0.037 |
| TG/HDL-c ratio (mmol/L) | 0.455 (0.366–0.568) | 0.537 (0.375–0.791) * | 0.608 (0.559–0.780) * | 0.014 |
| Parameter | CVRS | p | ||
|---|---|---|---|---|
| Low Risk (−2–1) n = 114 | Moderate Risk (2–4) n = 54 | High Risk (≥5) n = 14 | ||
| SOD (U/L) | 141 (131–146) | 139 (28–146) | 139 (137–142) | 0.757 |
| TAS (μmol/L) | 1183 (1103–1241) | 1176 (1111–1248) | 1219 (1181–1265) | 0.256 |
| TOS (μmol/L) | 6.5 (4.9–9.9) | 7.8 (6.6–11.1) * | 11.0 (5.8–12.2) * | 0.005 |
| TAS/TOS | 176 (114–242) | 142 (109–188) * | 109 (98–196) * | 0.015 |
| AOPP (μmol/L) | 12.7 (10.4–15.6) | 12.7 (11.6–16.8) | 15.5 (13.7–17.3) * | 0.065 |
| MDA (μmol/L) | 2.56 (1.92–3.04) | 2.89 (2.48–3.29) ** | 3.33 (2.89–4.00) ***, # | <0.001 |
| Parameter | CVRS (ρ, p) |
|---|---|
| TAS (μmol/L) | ns |
| TOS (μmol/L) | 0.246, 0.001 |
| SOD (U/L) | ns |
| AOPP (μmol/L) | 0.231, 0.002 |
| TAS/TOS | −0.208, 0.005 |
| MDA (μmol/L) | 0.339, <0.001 |
| Factor | Variables | Factor Loadings | Variability Percentage (Total 41%) |
|---|---|---|---|
| Factor 1: Pro-oxidant-dyslipidemia-related factor | AOPP (µmol/L) | 0.864 | 16 |
| TG (mmol/L) | 0.784 | ||
| TOS (µmol/L) | 0.782 | ||
| Factor 2: Hepatic steatosis-related factor | ALT (U/L) | 0.841 | 13 |
| AST (U/L) | 0.800 | ||
| Factor 3: Antioxidant-renal function-related factor | Uric acid (µmol/L) | 0.694 | 12 |
| Creatinine (µmol/L) | 0.631 | ||
| TAS (µmol/L) | 0.631 |
| Variables | B (SE) | Wald Coefficient | OR (95% CI) | p |
|---|---|---|---|---|
| Factor 1 | 0.408 (0.337) | 1.461 | 1.503 (0.776–2.912) | 0.227 |
| Factor 2 | 0.073 (0.478) | 0.024 | 1.076 (0.422–2.744) | 0.878 |
| Factor 3 | 1.034 (0.528) | 3.839 | 2.810 (1.000–7.900) | 0.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klisic, A.; Bozovic, M.; Ostanek, B.; Marc, J.; Karakasis, P.; Mercantepe, F.; Kotur-Stevuljevic, J. Relationship Between Oxidative Stress and Cardiovascular Risk in Adolescents in Montenegro. Int. J. Mol. Sci. 2025, 26, 7650. https://doi.org/10.3390/ijms26157650
Klisic A, Bozovic M, Ostanek B, Marc J, Karakasis P, Mercantepe F, Kotur-Stevuljevic J. Relationship Between Oxidative Stress and Cardiovascular Risk in Adolescents in Montenegro. International Journal of Molecular Sciences. 2025; 26(15):7650. https://doi.org/10.3390/ijms26157650
Chicago/Turabian StyleKlisic, Aleksandra, Marija Bozovic, Barbara Ostanek, Janja Marc, Paschalis Karakasis, Filiz Mercantepe, and Jelena Kotur-Stevuljevic. 2025. "Relationship Between Oxidative Stress and Cardiovascular Risk in Adolescents in Montenegro" International Journal of Molecular Sciences 26, no. 15: 7650. https://doi.org/10.3390/ijms26157650
APA StyleKlisic, A., Bozovic, M., Ostanek, B., Marc, J., Karakasis, P., Mercantepe, F., & Kotur-Stevuljevic, J. (2025). Relationship Between Oxidative Stress and Cardiovascular Risk in Adolescents in Montenegro. International Journal of Molecular Sciences, 26(15), 7650. https://doi.org/10.3390/ijms26157650







