Therapeutic Potential and Mechanisms of Mesenchymal Stem Cells in Coronary Artery Disease: Narrative Review
Abstract
1. Introduction
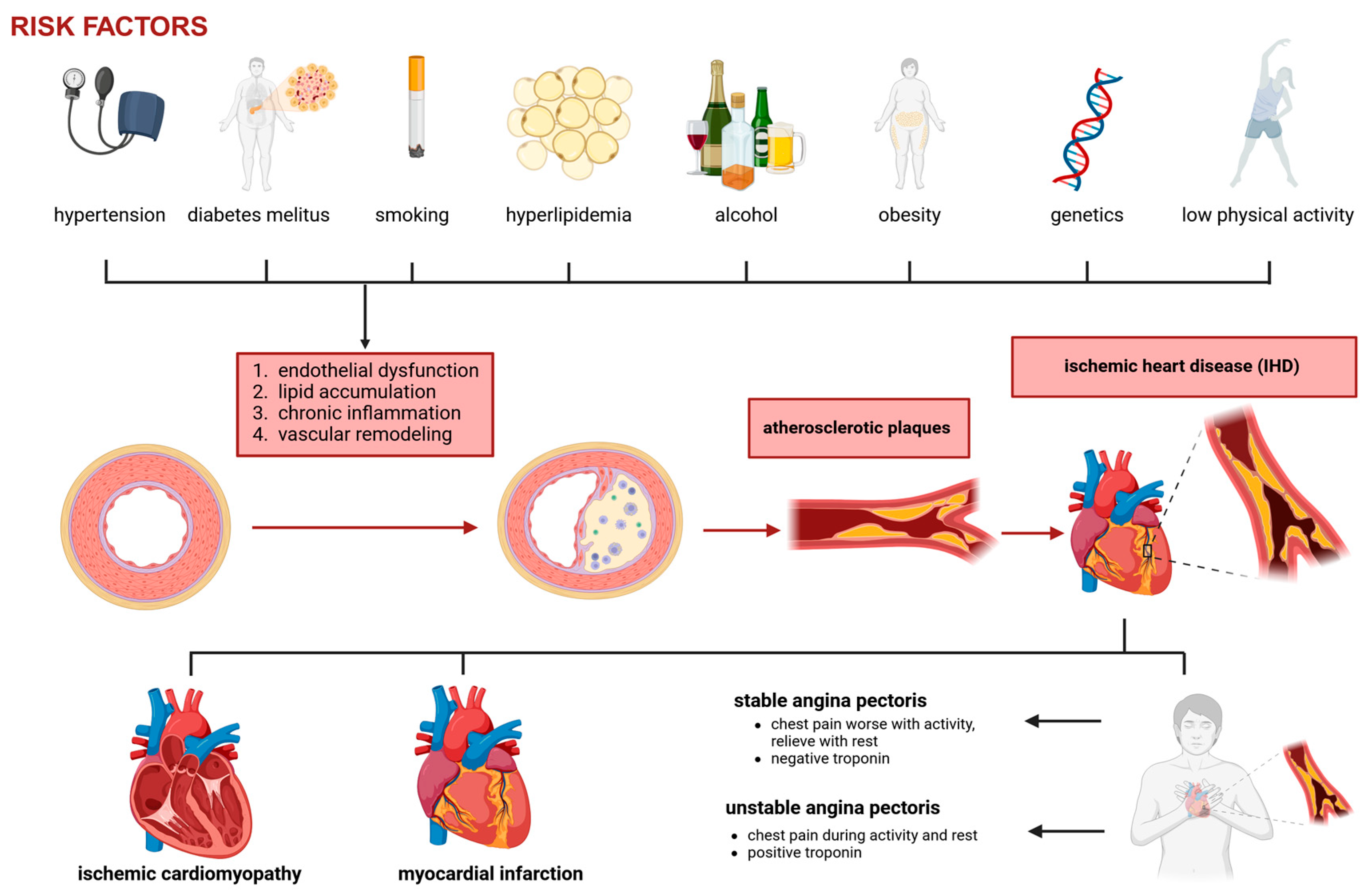
2. Coronary Artery Disease
- Stable angina: Predictable exertional chest pain caused by fixed coronary stenosis leading to supply–demand mismatch in myocardial oxygenation.
- Unstable angina: Increased plaque instability and thrombosis result in worsening ischemic symptoms, often at rest.
- Myocardial infarction (MI): Complete occlusion of a coronary artery leads to myocardial necrosis.
- Ischemic cardiomyopathy: Chronic ischemia contributes to left ventricular dysfunction and heart failure.
3. Mesenchymal Stem Cells
4. Current Clinical Applications of MSCs in CAD
4.1. Bone-Marrow-Derived Mesenchymal Stem Cell Therapy in CAD
4.2. Adipose-Derived Mesenchymal Stem Cell Therapy in CAD
4.3. Umbilical-Cord-Derived Mesenchymal Stem Cell Therapy in CAD
4.4. Animal Models
5. CD34+ Stem Cells
Comparison to MSC-Based Approaches
6. Administration Strategies of MSC Therapy
6.1. Intravenous Infusion of MSCs
6.2. Intracoronary Injection of MSCs
6.3. Intramyocardial Injection of MSCs
6.4. Subcutaneous Transplantation of MSCs
6.5. Cell Sheet Transplantation of MSCs
6.6. Bioengineered Scaffolds for MSC Delivery
7. Fresh vs. Cryopreserved MSCs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Duggan, J.P.; Peters, A.S.; Trachiotis, G.D.; Antevil, J.L. Epidemiology of Coronary Artery Disease. Surg. Clin. N. Am. 2022, 102, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Theroux, P. Pathophysiology of Coronary Artery Disease. Circulation 2005, 111, 3482–3488. [Google Scholar] [CrossRef] [PubMed]
- Sulava, E.F.; Johnson, J.C. Management of Coronary Artery Disease. Surg. Clin. N. Am. 2022, 102, 449–464. [Google Scholar] [CrossRef]
- Bakinowska, E.; Kiełbowski, K.; Boboryko, D.; Bratborska, A.W.; Olejnik-Wojciechowska, J.; Rusiński, M.; Pawlik, A. The Role of Stem Cells in the Treatment of Cardiovascular Diseases. Int. J. Mol. Sci. 2024, 25, 3901. [Google Scholar] [CrossRef]
- Menshikov, M.; Zubkova, E.; Stafeev, I.; Parfyonova, Y. Autophagy, Mesenchymal Stem Cell Differentiation, and Secretion. Biomedicines 2021, 9, 1178. [Google Scholar] [CrossRef]
- Shao, C.; Wang, J.; Tian, J.; Tang, Y.D. Coronary Artery Disease: From Mechanism to Clinical Practice. In Coronary Artery Disease: Therapeutics and Drug Discovery; Springer: Singapore, 2020; pp. 1–36. [Google Scholar]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Tsai, I.T.; Sun, C.K. Stem Cell Therapy against Ischemic Heart Disease. Int. J. Mol. Sci. 2024, 25, 3778. [Google Scholar] [CrossRef]
- Grimaldi, V.; Mancini, F.P.; Casamassimi, A.; Al-Omran, M.; Zullo, A.; Infante, T.; Napoli, C. Potential benefits of cell therapy in coronary heart disease. J. Cardiol. 2013, 62, 267–276. [Google Scholar] [CrossRef]
- Wang, S.; Cui, J.; Peng, W.; Lu, M. Intracoronary Autologous CD34+ Stem Cell Therapy for Intractable Angina. Cardiology 2010, 117, 140–147. [Google Scholar] [CrossRef]
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Cheng, M.; Lusis, A.J.; Yang, X. Gene Regulatory Networks in Coronary Artery Disease. Curr. Atheroscler. Rep. 2023, 25, 1013–1023. [Google Scholar] [CrossRef]
- Li, D.L.; Kronenberg, M.W. Myocardial Perfusion and Viability Imaging in Coronary Artery Disease: Clinical Value in Diagnosis, Prognosis, and Therapeutic Guidance. Am. J. Med. 2021, 134, 968–975. [Google Scholar] [CrossRef]
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef]
- Ghanta, S.N.; Kattamuri, L.P.V.; Odueke, A.; Mehta, J.L. Molecular Insights into Ischemia—Reperfusion Injury in Coronary Artery Disease: Mechanisms and Therapeutic Implications: A Comprehensive Review. Antioxidants 2025, 14, 213. [Google Scholar] [CrossRef]
- Liu, M.; Gomez, D. Smooth Muscle Cell Phenotypic Diversity. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1715–1723. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Y.; Hu, S.; Chen, Y.; Shen, Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020, 11, 349. [Google Scholar] [CrossRef]
- Mishra, V.K.; Shih, H.H.; Parveen, F.; Lenzen, D.; Ito, E.; Chan, T.F.; Ke, L.Y. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells 2020, 9, 1145. [Google Scholar] [CrossRef]
- Liu, J.; Gao, J.; Liang, Z.; Gao, C.; Niu, Q.; Wu, F.; Zhang, L. Mesenchymal stem cells and their microenvironment. Stem Cell Res. Ther. 2022, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Akinola, E.; Itua, F.I.; Afolayan-Oloye, O.; Okikiade, A.; Oloye, E.A. Therapeutic Use of Stem Cells in the Management of Coronary Artery Disease and Heart Failure; Current Trends, Progress, and Challenges. Cardiol. Angiol. Int. J. 2022, 11, 392–415. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, Y.; Yashiro, R. Therapeutic Strategy of Mesenchymal-Stem-Cell-Derived Extracellular Vesicles as Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 6480. [Google Scholar] [CrossRef]
- Molnar, V.; Pavelić, E.; Vrdoljak, K.; Čemerin, M.; Klarić, E.; Matišić, V.; Bjelica, R.; Brlek, P.; Kovačić, I.; Tremolada, C.; et al. Mesenchymal Stem Cell Mechanisms of Action and Clinical Effects in Osteoarthritis: A Narrative Review. Genes 2022, 13, 949. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Chu, Q.; Jiang, X.; Xiao, Y. Rebuilding the myocardial microenvironment to enhance mesenchymal stem cells-mediated regeneration in ischemic heart disease. Front. Bioeng. Biotechnol. 2024, 12, 1468833. [Google Scholar] [CrossRef]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.-S.; Ashraf, M.; Xu, M. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 2017, 8, 45200–45212. [Google Scholar] [CrossRef]
- Bhaskara, M.; Anjorin, O.; Wang, M. Mesenchymal Stem Cell-Derived Exosomal microRNAs in Cardiac Regeneration. Cells 2023, 12, 2815. [Google Scholar] [CrossRef]
- Giorgino, R.; Albano, D.; Fusco, S.; Peretti, G.M.; Mangiavini, L.; Messina, C. Knee Osteoarthritis: Epidemiology, Pathogenesis, and Mesenchymal Stem Cells: What Else Is New? An Update. Int. J. Mol. Sci. 2023, 24, 6405. [Google Scholar] [CrossRef]
- Wen, Z.; Mai, Z.; Zhu, X.; Wu, T.; Chen, Y.; Geng, D.; Wang, J. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Xia, Y.; Zhao, H.; Xu, X.; Ma, X.; Tao, L. Stem cell-based therapy in cardiac repair after myocardial infarction: Promise, challenges, and future directions. J. Mol. Cell Cardiol. 2024, 188, 1–14. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Yu, Y.; Li, C.; Zhang, C. Advances in the study of exosomes derived from mesenchymal stem cells and cardiac cells for the treatment of myocardial infarction. Cell Commun. Signal. 2023, 21, 202. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Qi, Z.; Cai, C.; Song, H.; Song, G.; Zhao, X. Improved therapeutic effects on vascular intimal hyperplasia by mesenchymal stem cells expressing MIR155HG that function as a ceRNA for microRNA-205. J. Cell Mol. Med. 2024, 28, e18351. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Bunnell, B.A. Adipose Tissue-Derived Stem Cells: Immunomodulatory Effects and Therapeutic Potential. Physiology 2020, 35, 125–133. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, Z.; Yan, Z.; Ji, N.; Yang, X.; Gao, D.; Hu, L.; Lv, H.; Zhang, J.; Li, M. Mesenchymal Stem Cell Immunomodulation: A Novel Intervention Mechanism in Cardiovascular Disease. Front. Cell Dev. Biol. 2022, 9, 742088. [Google Scholar] [CrossRef]
- Yin, X.; Lin, L.; Fang, F.; Zhang, B.; Shen, C. Mechanisms and Optimization Strategies of Paracrine Exosomes from Mesenchymal Stem Cells in Ischemic Heart Disease. Stem Cells Int. 2023, 2023, 6500831. [Google Scholar] [CrossRef]
- Shi, W.; Xin, Q.; Yuan, R.; Yuan, Y.; Cong, W.; Chen, K. Neovascularization: The Main Mechanism of MSCs in Ischemic Heart Disease Therapy. Front. Cardiovasc. Med. 2021, 8, 633300. [Google Scholar] [CrossRef]
- Heldman, A.W.; DiFede, D.L.; Fishman, J.E.; Zambrano, J.P.; Trachtenberg, B.H.; Karantalis, V.; Mushtaq, M.; Williams, A.R.; Suncion, V.Y.; McNiece, I.K.; et al. Transendocardial Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells for Ischemic Cardiomyopathy. JAMA 2014, 311, 62. [Google Scholar] [CrossRef]
- Mathiasen, A.B.; Qayyum, A.A.; Jørgensen, E.; Helqvist, S.; Fischer-Nielsen, A.; Kofoed, K.F.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: A randomized placebo-controlled trial (MSC-HF trial). Eur. Heart J. 2015, 36, 1744–1753. [Google Scholar] [CrossRef]
- Hare, J.M.; DiFede, D.L.; Rieger, A.C.; Florea, V.; Landin, A.M.; El-Khorazaty, J.; Khan, A.; Mushtaq, M.; Lowery, M.H.; Byrnes, J.J.; et al. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy. J. Am. Coll Cardiol. 2017, 69, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Perin, E.C.; Borow, K.M.; Henry, T.D.; Mendelsohn, F.O.; Miller, L.W.; Swiggum, E.; Adler, E.D.; Chang, D.H.; Fish, R.D.; Bouchard, A.; et al. Randomized Trial of Targeted Transendocardial Mesenchymal Precursor Cell Therapy in Patients with Heart Failure. J. Am. Coll Cardiol. 2023, 81, 849–863. [Google Scholar] [CrossRef]
- Qayyum, A.A.; Mouridsen, M.; Nilsson, B.; Gustafsson, I.; Schou, M.; Nielsen, O.W.; Hove, J.D.; Mathiasen, A.B.; Jørgensen, E.; Helqvist, S.; et al. Danish phase II trial using adipose tissue derived mesenchymal stromal cells for patients with ischaemic heart failure. ESC Heart Fail. 2023, 10, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.A.; Mathiasen, A.B.; Helqvist, S.; Jørgensen, E.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J. Autologous adipose-derived stromal cell treatment for patients with refractory angina (MyStromalCell Trial): 3-years follow-up results. J. Transl. Med. 2019, 17, 360. [Google Scholar] [CrossRef]
- Perin, E.C.; Sanz-Ruiz, R.; Sánchez, P.L.; Lasso, J.; Pérez-Cano, R.; Alonso-Farto, J.C.; Pérez-David, E.; Fernández-Santos, M.E.; Serruys, P.W.; Duckers, H.J.; et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am. Heart J. 2014, 168, 88–95.e2. [Google Scholar] [CrossRef]
- Kastrup, J.; Haack-Sørensen, M.; Juhl, M.; Harary Søndergaard, R.; Follin, B.; Drozd Lund, L.; Johansen, E.M.; Qayyum, A.A.; Mathiasen, A.B.; Jørgensen, E.; et al. Cryopreserved Off-the-Shelf Allogeneic Adipose-Derived Stromal Cells for Therapy in Patients with Ischemic Heart Disease and Heart Failure—A Safety Study. Stem Cells Transl. Med. 2017, 6, 1963–1971. [Google Scholar] [CrossRef]
- Houtgraaf, J.H.; den Dekker, W.K.; van Dalen, B.M.; Springeling, T.; de Jong, R.; van Geuns, R.J.; Geleijnse, M.L.; Fernandez-Aviles, F.; Zijlsta, F.; Serruys, P.W.; et al. First Experience in Humans Using Adipose Tissue–Derived Regenerative Cells in the Treatment of Patients with ST-Segment Elevation Myocardial Infarction. J Am Coll Cardiol. 2012, 59, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.A.; van Klarenbosch, B.; Frljak, S.; Cerar, A.; Poglajen, G.; Traxler-Weidenauer, D.; Nadrowski, P.; Paitazoglou, C.; Vrtovec, B.; Bergmann, M.W.; et al. Effect of allogeneic adipose tissue-derived mesenchymal stromal cell treatment in chronic ischaemic heart failure with reduced ejection fraction—The SCIENCE trial. Eur. J. Heart Fail. 2023, 25, 576–587. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Lu, G.-L.; Huang, B.-X.; Wang, D.-W.; Shao, Y.; Lu, M.-J. Hypoxic Preconditioning Enhances Cellular Viability and Pro-angiogenic Paracrine Activity: The Roles of VEGF-A and SDF-1a in Rat Adipose Stem Cells. Front. Cell Dev. Biol. 2020, 8, 580131. [Google Scholar] [CrossRef]
- Mytsyk, M.; Cerino, G.; Reid, G.; Sole, L.G.; Eckstein, F.S.; Santer, D.; Marsano, A. Long-Term Severe In Vitro Hypoxia Exposure Enhances the Vascularization Potential of Human Adipose Tissue-Derived Stromal Vascular Fraction Cell Engineered Tissues. Int. J. Mol. Sci. 2021, 22, 7920. [Google Scholar] [CrossRef]
- He, J.; Cai, Y.; Luo, L.M.; Liu, H.B. Hypoxic adipose mesenchymal stem cells derived conditioned medium protects myocardial infarct in rat. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4397–4406. [Google Scholar] [PubMed]
- Li, X.; Hu, Y.-D.; Guo, Y.; Chen, Y.; Guo, D.-X.; Zhou, H.-L.; Zhang, F.-L.; Zhao, Q.-N. Safety and Efficacy of Intracoronary Human Umbilical Cord-Derived Mesenchymal Stem Cell Treatment for Very Old Patients with Coronary Chronic Total Occlusion. Curr. Pharm. Des. 2015, 21, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Jiao, F.; Zhang, X.; Feng, Y.; Liu, Y.; Liang, D.; Yan, X.; Zhang, H. Human Umbilical Cord Mesenchymal Stem Cells Inhibit Coronary Artery Injury in Mice with Lactobacillus casei Wall Extract-Induced Kawasaki Disease. Sains Malays. 2022, 51, 577–583. [Google Scholar] [CrossRef]
- Koutela, A.; Loudos, G.; Rouchota, M.; Kletsas, D.; Karameris, A.; Vilaras, G.; Zografos, G.C.; Myoteri, D.; Dougenis, D.; Papalois, A.E. Mesenchymal Stem Cell Transplantation Has a Regenerative Effect in Ischemic Myocardium: An Experimental Rat Model Evaluated by SPECT-CT Assessment. Diagnostics 2024, 14, 401. [Google Scholar] [CrossRef]
- Sepehri, M.; Rabbani, S.; Ai, J.; Bahrami, N.; Ghanbari, H.; Namini, M.S.; Pazoki-Toroudi, H. Therapeutic potential of exosomes derived from human endometrial mesenchymal stem cells for heart tissue regeneration after myocardial infarction. Regen. Ther. 2025, 28, 451–461. [Google Scholar] [CrossRef]
- Aggarwal, R.; Potel, K.N.; Shao, A.; So, S.W.; Swingen, C.; Reyes, C.P.; Rose, R.; Wright, C.; Stone, L.L.H.; McFalls, E.O.; et al. An Adjuvant Stem Cell Patch with Coronary Artery Bypass Graft Surgery Improves Diastolic Recovery in Porcine Hibernating Myocardium. Int. J. Mol. Sci. 2023, 24, 5475. [Google Scholar] [CrossRef]
- Henry, T.D.; Bairey Merz, C.N.; Wei, J.; Corban, M.T.; Quesada, O.; Joung, S.; Kotynski, C.L.; Wang, J.; Lewis, M.; Schumacher, A.M.; et al. Autologous CD34+ Stem Cell Therapy Increases Coronary Flow Reserve and Reduces Angina in Patients With Coronary Microvascular Dysfunction. Circ. Cardiovasc. Interv. 2022, 15, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Giri, J.; Galipeau, J. Mesenchymal stromal cell therapeutic potency is dependent upon viability, route of delivery, and immune match. Blood Adv. 2020, 4, 1987–1997. [Google Scholar] [CrossRef]
- Preda, M.B.; Lupan, A.; Neculachi, C.A.; Leti, L.I.; Fenyo, I.M.; Popescu, S.; Rusu, E.G.; Marinescu, C.I.; Simionescu, M.; Burlacu, A. Evidence of mesenchymal stromal cell adaptation to local microenvironment following subcutaneous transplantation. J. Cell Mol. Med. 2020, 24, 10889–10897. [Google Scholar] [CrossRef]
- Gao, L.R.; Chen, Y.; Zhang, N.K.; Yang, X.L.; Liu, H.L.; Wang, Z.G.; Yan, X.Y.; Wang, Y.; Zhu, Z.M.; Li, T.C.; et al. Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: Double-blind, randomized controlled trial. BMC Med. 2015, 13, 162. [Google Scholar] [CrossRef]
- Hsiao, L.C.; Lin, Y.N.; Shyu, W.C.; Ho, M.; Lu, C.R.; Chang, S.S.; Wang, Y.-C.; Chen, J.-Y.; Lu, S.-Y.; Wu, M.-Y.; et al. First-in-human pilot trial of combined intracoronary and intravenous mesenchymal stem cell therapy in acute myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 961920. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, F.; Ma, W.; Chen, B.; Zhou, F.; Xu, Z.; Zhang, Y.; Zhang, D.; Zhu, T.; Wang, L.; et al. A Novel Approach to Transplanting Bone Marrow Stem Cells to Repair Human Myocardial Infarction: Delivery via a Noninfarct-relative Artery. Cardiovasc. Ther. 2010, 28, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, S.F.; van Ramshorst, J.; Hoogslag, G.E.; Boden, H.; Velders, M.A.; Cannegieter, S.C.; Roelofs, H.; Al Younis, I.; Dibbets-Schneider, P.; Fibbe, W.E.; et al. Intramyocardial Injection of Autologous Bone Marrow-Derived Ex Vivo Expanded Mesenchymal Stem Cells in Acute Myocardial Infarction Patients is Feasible and Safe up to 5 Years of Follow-up. J. Cardiovasc. Transl. Res. 2013, 6, 816–825. [Google Scholar] [CrossRef]
- Haack-Sorensen, M.; Bindslev, L.; Mortensen, S.; Friis, T.; Kastrup, J. The influence of freezing and storage on the characteristics and functions of human mesenchymal stromal cells isolated for clinical use. Cytotherapy 2007, 9, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef]
- Kalou, Y.; Al-Khani, A.M.; Haider, K.H. Bone Marrow Mesenchymal Stem Cells for Heart Failure Treatment: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2023, 32, 870–880. [Google Scholar] [CrossRef]
- Poomani, M.S.; Mariappan, I.; Perumal, R.; Regurajan, R.; Muthan, K.; Subramanian, V. Mesenchymal Stem Cell (MSCs) Therapy for Ischemic Heart Disease: A Promising Frontier. Glob. Heart 2022, 17, 19. [Google Scholar] [CrossRef]
- Ni, H.; Zhao, Y.; Ji, Y.; Shen, J.; Xiang, M.; Xie, Y. Adipose-derived stem cells contribute to cardiovascular remodeling. Aging 2019, 11, 11756–11769. [Google Scholar] [CrossRef]
- Lee, H.; Cho, H.J.; Han, Y.; Lee, S.H. Mid- to long-term efficacy and safety of stem cell therapy for acute myocardial infarction: A systematic review and meta-analysis. Stem Cell Res. Ther. 2024, 15, 290. [Google Scholar] [CrossRef]
- Giugni, F.R.; Giugni Mde, O.V.; Pinesi, H.T.; Habrum, F.C.; Laranjeira, L.N.; Sady, E.R.R.; Suzumura, E.A.; Gowdak, L.H.W.; Krieger, J.E. Safety and Efficacy of Adipose-Derived Mesenchymal Stem Cell Therapy for Ischemic Heart Disease: A Systematic Review. Arq. Bras. Cardiol. 2024, 121, e20230830. [Google Scholar] [CrossRef]
- Gorjipour, F.; Gohari, L.H.; Hajimiresmaiel, S.J.; Janani, L.; Moradi, Y.; Pazoki-toroudi, H. Amniotic Membrane-Derived Mesenchymal Stem Cells for Heart Failure: A Systematic Review and Meta-Analysis of the Published Preclinical Studies. Med. J. Islam. Repub. Iran. 2021, 35, 187. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xie, Y.; Wang, B. Genetically modified mesenchymal stromal cells: A cell-based therapy offering more efficient repair after myocardial infarction. Stem Cell Res. Ther. 2024, 15, 323. [Google Scholar] [CrossRef]
- Mackie, A.R.; Losordo, D.W. CD34-positive stem cells: In the treatment of heart and vascular disease in human beings. Tex. Heart Inst. J. 2011, 38, 474–485. [Google Scholar]
- Marvasti, T.B.; Alibhai, F.J.; Weisel, R.D.; Li, R.K. CD34+ Stem Cells: Promising Roles in Cardiac Repair and Regeneration. Can. J. Cardiol. 2019, 35, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Fan, T.; Gao, S.; Jin, Y.; Zhang, M.; Ono, M. Application of mesenchymal stem cell sheet to treatment of ischemic heart disease. Stem Cell Res. Ther. 2021, 12, 384. [Google Scholar] [CrossRef]
- Hodonsky, C.; Mundada, L.; Wang, S.; Witt, R.; Raff, G.; Kaushal, S.; Si, M.-S. Effects of scaffold material used in cardiovascular surgery on mesenchymal stem cells and cardiac progenitor cells. Ann. Thorac. Surg. 2015, 99, 605–611. [Google Scholar] [CrossRef]
- Dave, C.; Mei, S.H.; McRae, A.; Hum, C.; Sullivan, K.J.; Champagne, J.; Ramsay, T.; McIntyre, L. Comparison of freshly cultured versus cryopreserved mesenchymal stem cells in animal models of inflammation: A pre-clinical systematic review. Elife 2022, 11, e75053. [Google Scholar] [CrossRef] [PubMed]
- Sid-Otmane, C.; Perrault, L.P.; Ly, H.Q. Mesenchymal stem cell mediates cardiac repair through autocrine, paracrine and endocrine axes. J. Transl. Med. 2020, 18, 336. [Google Scholar] [CrossRef]
- Whaley, D.; Damyar, K.; Witek, R.P.; Mendoza, A.; Alexander, M.; Lakey, J.R. Cryopreservation: An Overview of Principles and Cell-Specific Considerations. Cell Transpl. 2021, 30, 0963689721999617. [Google Scholar] [CrossRef]
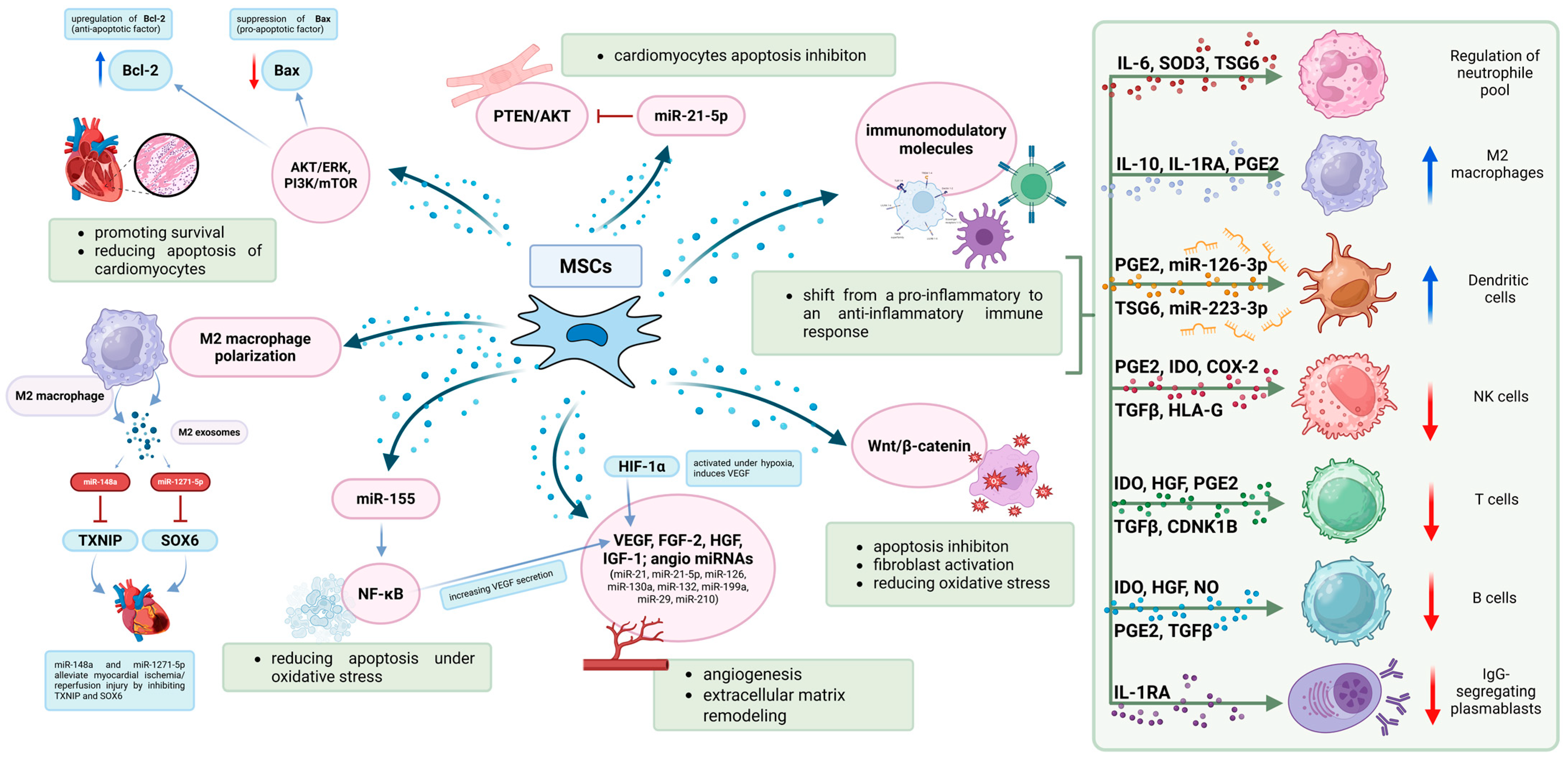
| No. | Author(s) | Year | Study Title | Study Type | MSC Source | Delivery Method | Results | Limitations |
|---|---|---|---|---|---|---|---|---|
| [11] | Wang et al. | 2010 | Intracoronary Autologous CD34+ Stem Cell Therapy for Intractable Angina | Clinical | Bone Marrow | Intracoronary Injection | Reduction in the frequency of angina episodes per week at 3 and 6 months post-infusion; improvement in nitroglycerine usage, exercise time, CCS class, and myocardial perfusion | Small sample size; single-center recruitment; possible placebo effect of intracoronary infusion alone |
| [29] | Gong et al. | 2017 | Mesenchymal Stem Cells Release Exosomes that Transfer Mirnas to Endothelial Cells and Promote Angiogenesis | Preclinical | MSCs line C3H10T1/2 cells purchased from ATCC (Manassas, VA, USA) | - | Conditioned medium increased tube formation and angiogenesis; exosomes mediated miR transfer to HUVECs; pro-angiogenic effects depend on miR cargo | Complex composition of exosomes; comparisons with simple medium/BSA may be inadequate |
| [32] | Wen et al. | 2020 | Mesenchymal Stem Cell-derived Exosomes Ameliorate Cardiomyocyte Apoptosis in Hypoxic Conditions Through MicroRNA144 by Targeting the PTEN/AKT Pathway | Preclinical | Bone Marrow | - | MSC-derived exosomes reduce apoptosis in hypoxia via miR-144/PTEN/AKT pathway; cardioprotective effect independent of differentiation | Tested at one time point only; limited generalizability to other hypoxic conditions |
| [35] | Bai et al. | 2024 | Improved Therapeutic Effects on Vascular Intimal Hyperplasia by Mesenchymal Stem Cells expressing MIR155HG that Function as a ceRNA for MicroRNA-205 | Preclinical | Cyagen Biosciences Inc. (Shanghai, China) | - | MIR155HG improved MSCs viability and migration; acted as sponge for miR-205; enhanced anti-apoptotic and pro-angiogenic function | Further research on MIR155HG needed before clinical application |
| [40] | Heldman et al. | 2014 | Transendocardial Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells for Ischemic Cardiomyopathy | Clinical | Bone Marrow | Transendocardial Injection | MSCs were associated with decreasing scar fraction and increasing viable myocardial mass, suggesting true myocardial regeneration; MSCs improved the Minnesota Living With Heart Failure score | Small sample size; not powered to draw efficacy comparisons; multiple comparisons limit conclusions |
| [41] | Mathiasen et al. | 2015 | Bone Marrow-derived Mesenchymal Stromal Cell Treatment in Patients with Severe Ischaemic Heart Failure: A Randomized Placebo-controlled Trial (MSC-HF trial) | Clinical | Bone Marrow | Intramyocardial Injection | Significant improvements in LV function (LVESV, LVEF, SV); LV mass and wall thickness improved in treated patients | Adverse events during procedure; underpowered SAE analysis; limited MRI eligibility |
| [42] | Hare et al. | 2017 | Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy | Clinical | Bone Marrow | Transendocardial Injection | Improvements in EF, 6MWT, MLHFQ; allo-MSCs improved endothelial function, TNF-a suppression, NYHA class, MACE, hospitalization rates | No placebo group; patient loss; small sample size limits efficacy interpretation |
| [43] | Perin et al. | 2023 | Randomized Trial of Targeted Transendocardial Mesenchymal Precursor Cell Therapy in Patients with Heart Failure | Clinical | Bone Marrow | Transendocardial Injection | No change in nonfatal hospitalization; significant reduction in TTFE for MI or stroke after 30 months | Endpoints may not capture full benefit/mechanism of MPCs |
| [44] | Qayyum et al. | 2023 | Danish Phase II Trial using Adipose Tissue Derived Mesenchymal Stromal Cells for Patients with Ischaemic Heart Failure | Clinical | Adipose Tissue | Intramyocardial Injection | No significant change in LV volumes or LVEF; improved quality-of-life and symptoms in ASC group | Safe but no myocardial or clinical improvement |
| [45] | Qayyum et al. | 2019 | Autologous Adipose-Derived Stromal Cell Treatment for Patients with Refractory Angina (Mystromalcell Trial): 3-Year Follow-Up Results | Clinical | Adipose Tissue | Intramyocardial Injection | Improved cardiac symptoms in ASC group; exercise capacity unchanged; deterioration observed in placebo group | No significant difference between ASC and placebo groups |
| [46] | Perin et al. | 2014 | Adipose-Derived Regenerative Cells in Patients with Ischemic Cardiomyopathy: The PRECISE Trial | Clinical | Adipose Tissue | Transendocardial Injection | Metabolic equivalents and MVO2 preserved in ADRC group; improved LV mass and wall motion; reduced ischemia up to 18 months | Did not reduce scar size or increase LVEF; small sample; baseline MRI/SPECT variability |
| [47] | Kastrup et al. | 2017 | Cryopreserved Off-the-Shelf Allogeneic Adipose-Derived Stromal Cells for Therapy in Patients with Ischemic Heart Disease and Heart Failure—A Safety Study | Clinical | Adipose Tissue | Intramyocardial Injection | Improved LV pump function and 6MWT; no procedure-related complications | Used DMSO; no control group; underpowered study |
| [48] | Houtgraaf et al. | 2012 | First Experience in Humans Using Adipose Tissue–Derived Regenerative Cells in the Treatment of Patients with ST-Segment Elevation Myocardial Infarction | Clinical | Adipose Tissue | Intracoronary Injection | Safe ADRC infusion; improved cardiac function; reduced scar formation | Small sample; bleeding events during liposuction in 2 patients |
| [49] | Qayyum et al. | 2023 | Effect of Allogeneic Adipose Tissue-Derived Mesenchymal Stromal Cell Treatment in Chronic Ischaemic Heart Failure—the SCIENCE Trial | Clinical | Adipose Tissue | Intramyocardial Injection | Safe over 3 years; no significant changes in LVESV, LVEF, or functional markers | Possibly insufficient dose or retention; small adverse events noted |
| [50] | Zhao et al. | 2020 | Hypoxic Preconditioning Enhances Cellular Viability and Pro-angiogenic Paracrine Activity: The Roles of VEGF-A and SDF-1a in Rat Adipose Stem Cells | Preclinical | Adipose Tissue | - | Improved protection under hypoxia; upregulation of VEGF-A and SDF-1a pathways | Variable differentiation/survival; optimal hypoxia exposure remains unclear |
| [51] | Mytsyk et al. | 2021 | Long-Term Severe In Vitro Hypoxia Exposure Enhances the Vascularization Potential of Human Adipose Tissue-Derived Stromal Vascular Fraction | Preclinical | Adipose Tissue | - | Increased VEGF release and vessel density after hypoxic exposure | High variability; low dividing/apoptotic cell counts; implantation challenges |
| [52] | He et al. | 2015 | Hypoxic Adipose Mesenchymal Stem Cells Derived Conditioned Medium Protects Myocardial Infarct in Rat | Experimental | Adipose Tissue | - | HypoCM increased VEGF, HGF, SDF-1; improved cardiomyocyte survival and infarct healing | ADMSCs identity debated; oxygen tension regulation is crucial but unclear |
| [53] | Li et al. | 2015 | Safety and Efficacy of Intracoronary Human Umbilical Cord-Derived Mesenchymal Stem Cell Treatment for Very Old Patients with Coronary Chronic Total Occlusion | Clinical | Umbilical Cord | Intracoronary Injection | No major cardiac events in 24 months; reduced infarct size; increased LVEF | Small sample (15 patients) |
| [54] | Guo et al. | 2022 | Human Umbilical Cord Mesenchymal Stem Cells Inhibit Coronary Artery Injury in Mice with Lactobacillus casei Wall Extract-Induced Kawasaki Disease | Experimental | Umbilical Cord | - | Reduced coronary artery damage in Kawasaki disease model; improved pathology | Short experiment; poor KD model simulation; dose-dependency unstudied |
| [55] | Koutela et al. | 2024 | MSC Transplantation has a Regenerative Effect in Ischemic Myocardium: SPECT-CT Assesment | Experimental | Adipose Tissue | - | Regeneration of ischemic myocardium confirmed by SPECT/CT, histology, and immunohistochemistry | Limited to female donors and male recipients; short monitoring period |
| [56] | Sepehri et al. | 2025 | Therapeutic Potential of Exosomes Derived from Human Endometrial Mscs for Heart Tissue Regeneration after myocardial infarction | Experimental | Endometrium | - | Exosomes reduced fibrosis and inflammation; improved cardiac function post-infarction | Low survival and retention of exosomes; mild and short-term effect |
| [57] | Aggarwal et al. | 2023 | An Adjuvant Stem Cell Patch with CABG Surgery Improves Diastolic Recovery in Porcine Hibernating Myocardium | Experimental | Bone Marrow | - | MSC patch improved diastolic function, increased PGC1α, reduced inflammation and fibrosis | Juvenile animal model; not representative of advanced atherosclerosis; small CABG+MSC group |
| [58] | Henry et al. | 2022 | Autologous CD34+ Stem Cell Therapy Increases Coronary Flow Reserve and Reduces Angina in Coronary Microvascular Dysfunction | Clinical | - | Intracoronary Injection | Improved coronary flow reserve, reduced angina, improved CCS class and quality of life; no serious adverse events | No control group; small sample; variation in CD34+ delivery; no dose-response observed |
| [59] | Giri et al. | 2020 | Mesenchymal stromal cell therapeutic potency is dependent upon viability, route of delivery, and immune match | Experimental | Bone Marrow | - | Subcutaneous/intraperitoneal MSCs effective; heat-inactivated or thawed MSCs lost efficacy; immune match allowed redosing | Cryoinjury may reduce MSC function post-thaw; human translation affected |
| [60] | Preda et al. | 2020 | Evidence of mesenchymal stromal cell adaptation to local microenvironment following subcutaneous transplantation | Experimental | Bone Marrow | Subcutaneous Transplantation | MSC aggregates stimulated angiogenesis and protective factors via hypoxia signaling; inflammation noted with high-dose | Cytokine elevation likely reflects host immune response, not MSC effect |
| [61] | Gao et al. | 2015 | Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: Double-blind, randomized controlled trial | Clinical | Wharton’s Jelly (Umbilical Cord) | Intracoronary Injection | Reduced infarct size; improved function and perfusion; prevented adverse LV remodeling | Mechanisms not explored; CE-MRI not universally available; PET used |
| [62] | Hsiao et al. | 2022 | First-in-human pilot trial of combined intracoronary and intravenous mesenchymal stem cell therapy in acute myocardial infarction | Clinical | Umbilical Cord | Intracoronary and Intravenous Injections | Improved LVEF and wall motion; NT-proBNP decreased; no major adverse events | Small sample; no placebo group; no immunological marker analysis |
| [63] | Yang et al. | 2010 | A Novel Approach to Transplanting Bone Marrow Stem Cells to Repair Human Myocardial Infarction: Delivery via a Noninfarct-relative Artery | Clinical | Bone Marrow | Intracoronary Injection | Improved cardiac function and perfusion 6 months post-treatment; safe and feasible | Small sample; benefits may overlap with PCI effects |
| [64] | Rodrigo et al. | 2013 | Intramyocardial Injection of Autologous Bone Marrow-Derived Ex Vivo Expanded Mesenchymal Stem Cells in Acute Myocardial Infarction Patients is Feasible and Safe up to 5 Years of Follow-up | Clinical | Bone Marrow | Intramyocardial Injection | A 5-year event-free survival comparable to controls; improved LV function at 12 months; safe and feasible | Small sample; nonrandomized control; underpowered to detect LV treatment effect |
| [65] | Haack-Sorensen et al. | 2007 | The influence of freezing and storage on the characteristics and functions of human mesenchymal stromal cells isolated for clinical use | Preclinical | Bone Marrow | - | Proliferation/differentiation capacities unchanged after freezing; comparable to fresh MSCs | MSC cultures are morphologically heterogeneous; no well-defined marker for BM-derived MSCs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, T.; Mešić, J.; Meretzki, S.; Bronshtein, T.; Brlek, P.; Kivity, V.; Pancholy, S.B.; Petrović, M.; Primorac, D. Therapeutic Potential and Mechanisms of Mesenchymal Stem Cells in Coronary Artery Disease: Narrative Review. Int. J. Mol. Sci. 2025, 26, 5414. https://doi.org/10.3390/ijms26115414
Patel T, Mešić J, Meretzki S, Bronshtein T, Brlek P, Kivity V, Pancholy SB, Petrović M, Primorac D. Therapeutic Potential and Mechanisms of Mesenchymal Stem Cells in Coronary Artery Disease: Narrative Review. International Journal of Molecular Sciences. 2025; 26(11):5414. https://doi.org/10.3390/ijms26115414
Chicago/Turabian StylePatel, Tejas, Jana Mešić, Shai Meretzki, Tomer Bronshtein, Petar Brlek, Vered Kivity, Samir B. Pancholy, Matko Petrović, and Dragan Primorac. 2025. "Therapeutic Potential and Mechanisms of Mesenchymal Stem Cells in Coronary Artery Disease: Narrative Review" International Journal of Molecular Sciences 26, no. 11: 5414. https://doi.org/10.3390/ijms26115414
APA StylePatel, T., Mešić, J., Meretzki, S., Bronshtein, T., Brlek, P., Kivity, V., Pancholy, S. B., Petrović, M., & Primorac, D. (2025). Therapeutic Potential and Mechanisms of Mesenchymal Stem Cells in Coronary Artery Disease: Narrative Review. International Journal of Molecular Sciences, 26(11), 5414. https://doi.org/10.3390/ijms26115414









