Electrospun Poly(L-lactide-co-ε-caprolactone) Nanofibers with Hydroxyapatite Nanoparticles Mimic Cellular Interplay in Bone Regeneration
Abstract
1. Introduction
2. Results
2.1. Fibers Fabrication
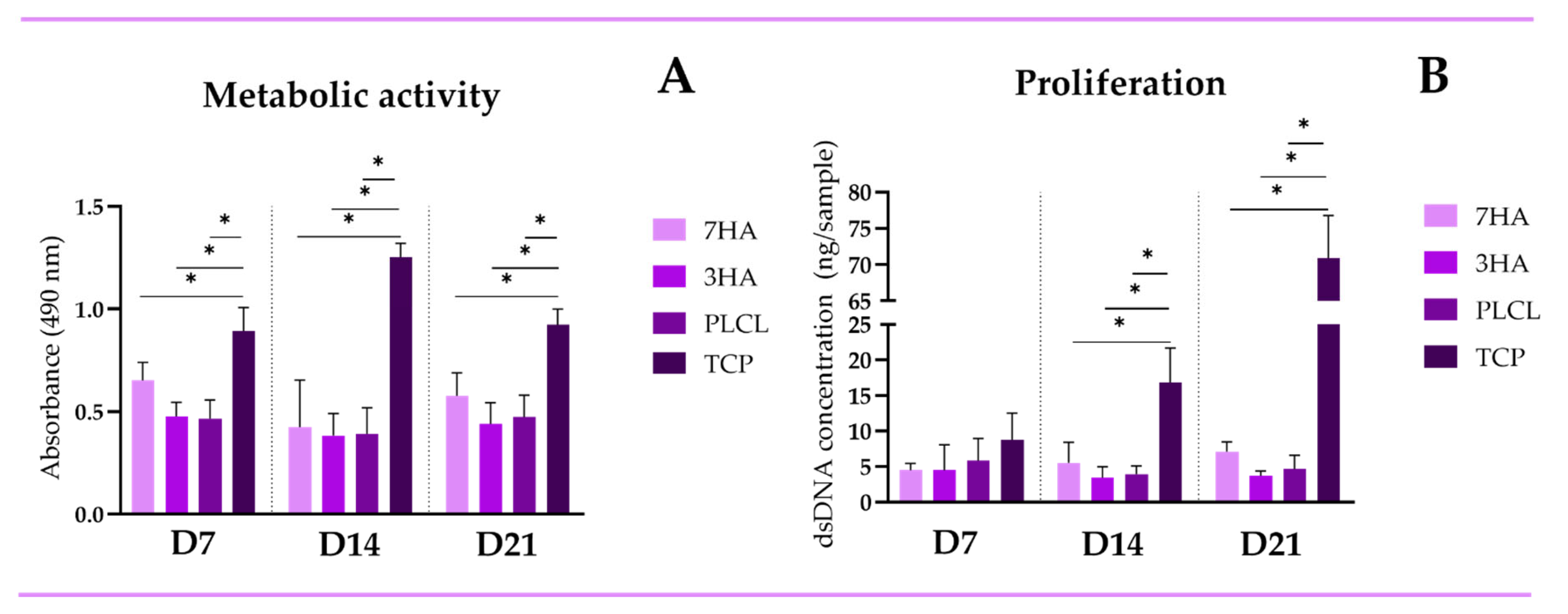
2.2. Metabolic Activity and Proliferation
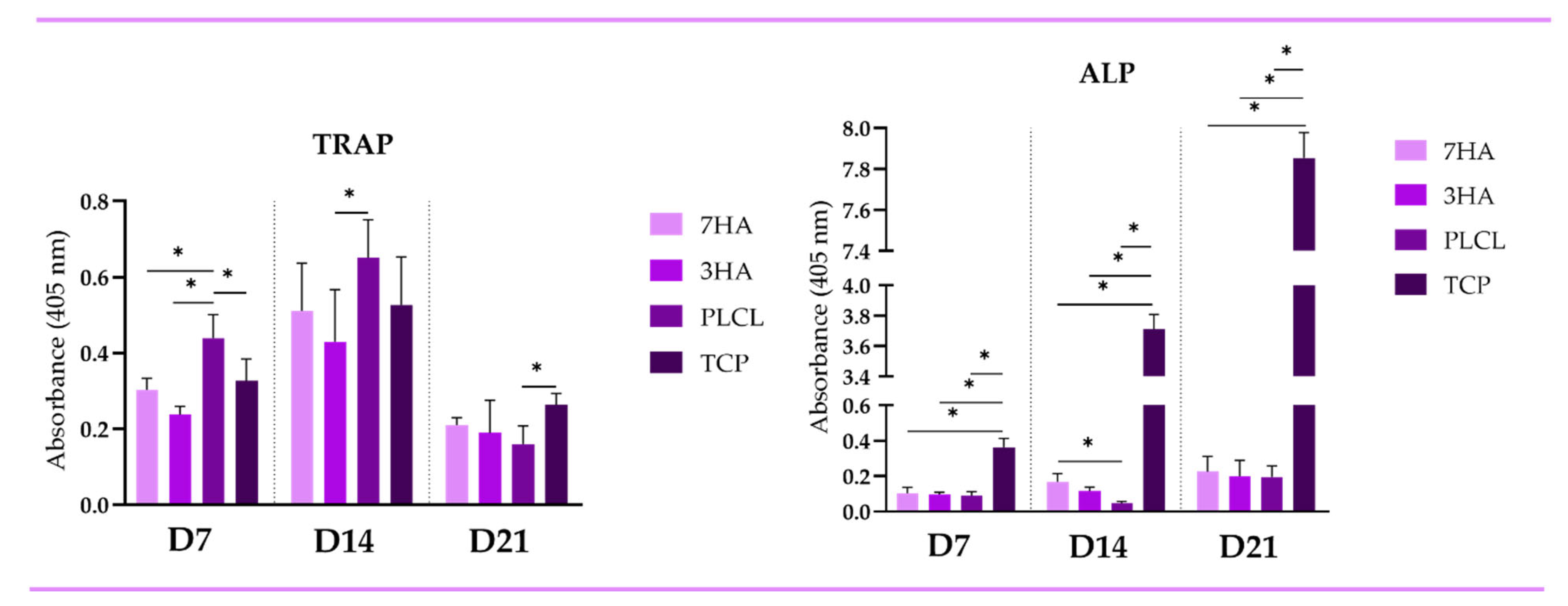
2.3. Enzymatic Activity
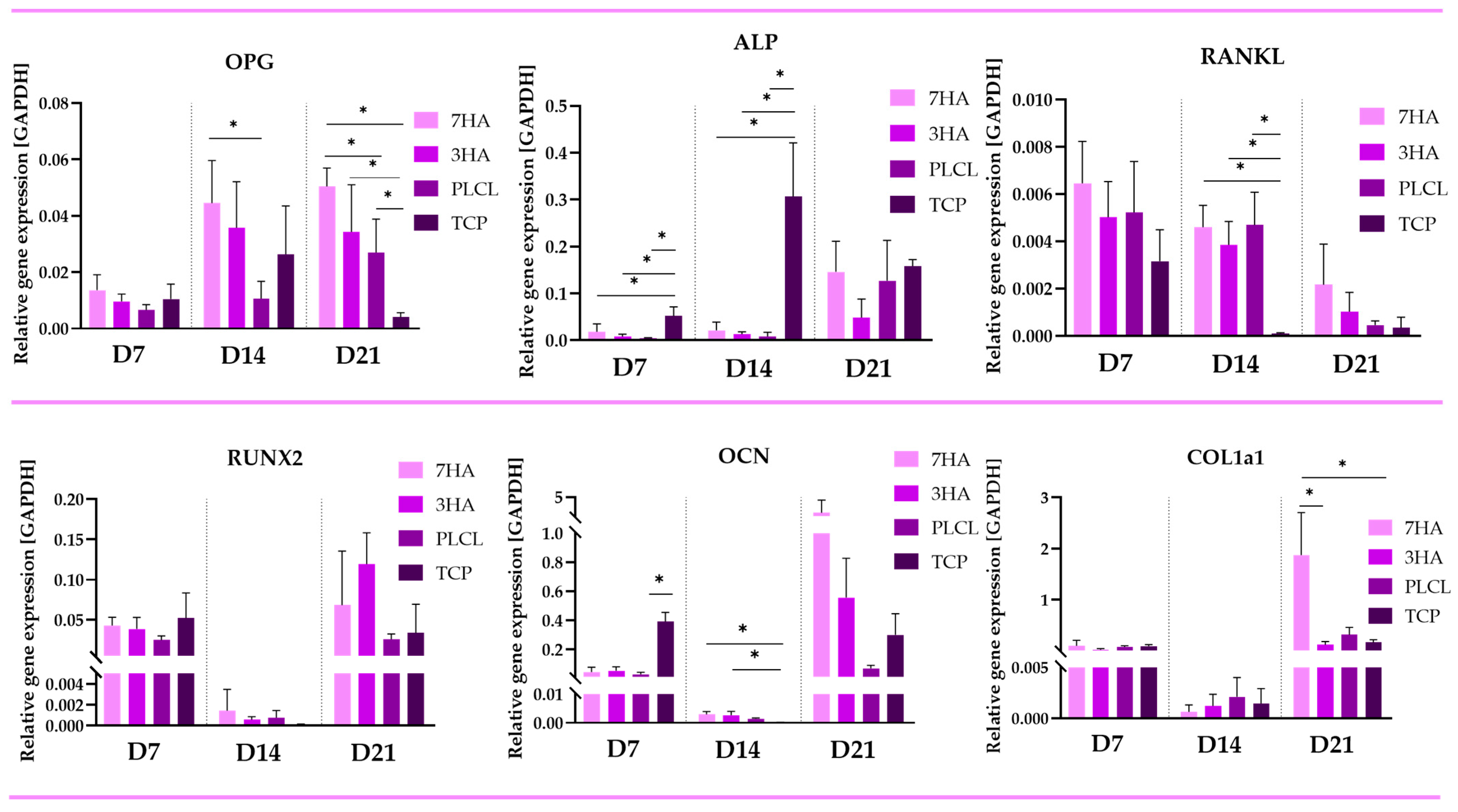
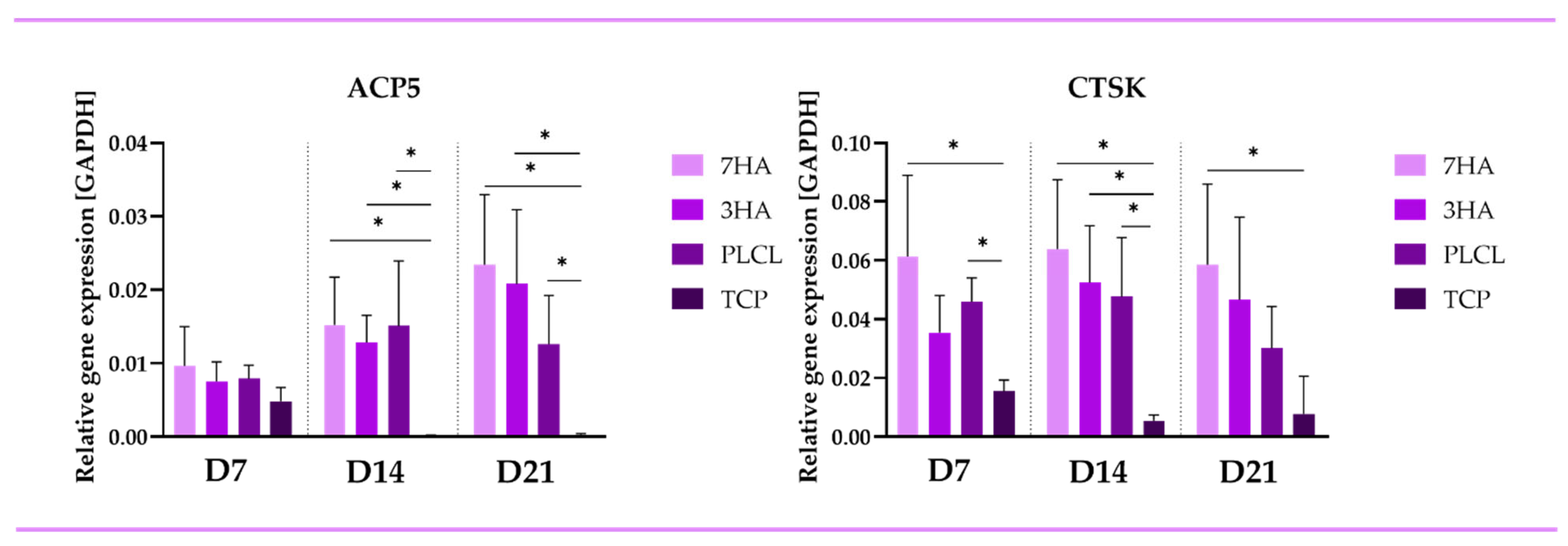
2.4. Quantitative PCR
2.4.1. Osteoblastic Markers
2.4.2. Osteoclastic Markers
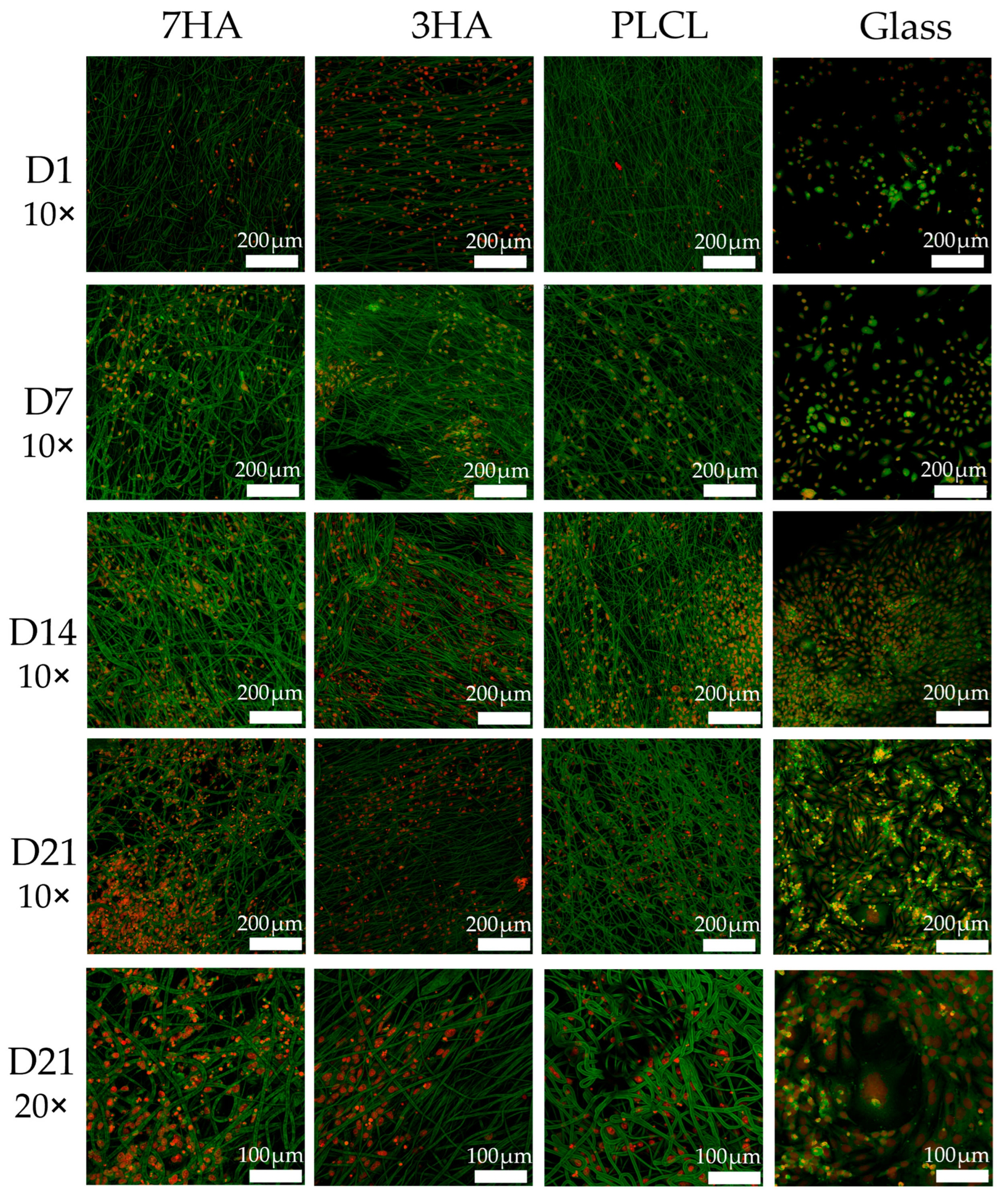
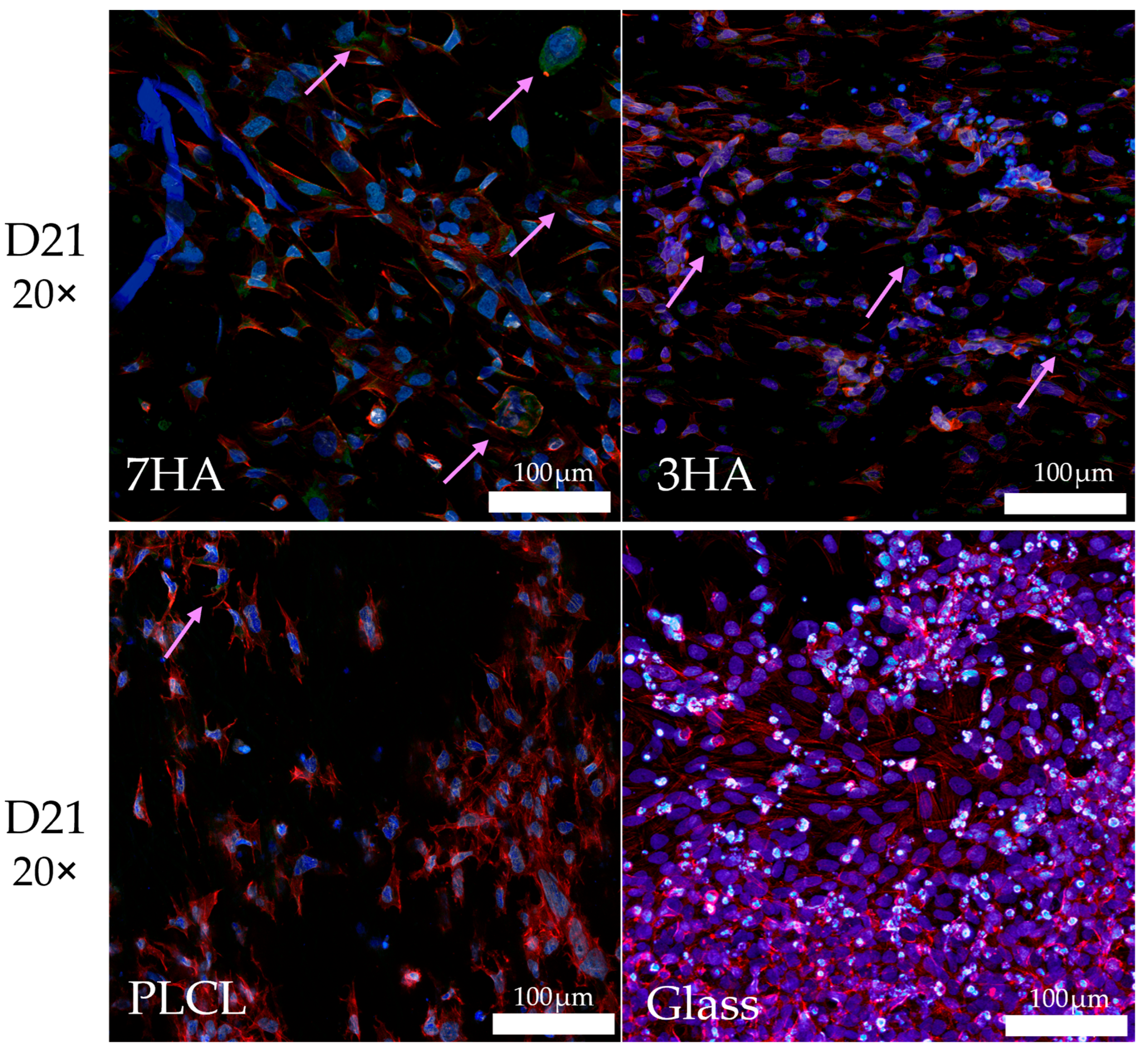
2.5. Cell Visualization
2.6. Collagen Type I Staining
3. Discussion
4. Methods
4.1. Scaffold Preparation
4.2. Scanning Electron Microscopy (SEM)
4.3. Cell Culture
- THP-1 Cells: Human THP-1 acute monocytic leukemia cells (ATCC, Manassas, VA USA) were grown in RPMI medium 1640 (Sigma Aldrich, St. Louis, MO, USA), supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco, Grand Island, NY, USA; 10270-106) and 1% penicillin/streptomycin (P/S; Life Technologies, Carlsbad, CA, USA). The cells were cultured at 37 °C with 5% CO2.
- Saos-2 Cells: Saos-2 cells (ATCC, Manassas, VA, USA) were cultured in McCoy’s 5A medium (Merck, Darmstadt, Germany; M9309) supplemented with 15% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA; 10270-106) and 1% P/S The cells were maintained in a humidified atmosphere with 5% CO2 at 37 °C.
4.4. Cell Seeding
4.5. Metabolic Activity
4.6. Proliferation Analysis
4.7. ALP and TRAP Activity
4.8. qPCR
4.9. Dioc/Pi Staining
4.10. Collagen Type I Staining
4.11. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HANPs | Hydroxyapatite nanoparticles |
| ALP | Alkaline phosphatase |
| ACP5 | Tartrate-resistant acid phosphatase type 5 |
| P/S | Penicillin/Streptomycin |
| TCP | Tissue culture polystyrene |
| HA | Hydroxyapatite |
| OCN | Osteocalcin |
| TRAP | Tartrate-resistant acid phosphatase |
| RANKL | Receptor activator of nuclear factor κB ligand |
| ECM | Extracellular matrix |
| DNA | Deoxyribonucleic acid |
| qPCR | Quantitative polymerase chain reaction |
| PLA | Poly(lactic acid) |
| PCL | Poly(ε-caprolactone) |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (cell viability assay) |
| RNA | Ribonucleic acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| PLCL | poly(L-lactide-co-ε-caprolactone) |
| RPMI | Roswell Park Memorial Institute medium |
References
- Šromová, V.; Sobola, D.; Kaspar, P. A Brief Review of Bone Cell Function and Importance. Cells 2023, 12, 2576. [Google Scholar] [CrossRef] [PubMed]
- ElHawary, H.; Baradaran, A.; Abi-Rafeh, J.; Vorstenbosch, J.; Xu, L.; Efanov, J.I. Bone Healing and Inflammation: Principles of Fracture and Repair. Semin. Plast. Surg. 2021, 35, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Steffi, C.; Shi, Z.; Kong, C.H.; Wang, W. Modulation of Osteoclast Interactions with Orthopaedic Biomaterials. J. Funct. Biomater. 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Khosla, S. Fracture Healing in the Setting of Endocrine Diseases, Aging, and Cellular Senescence. Endocr. Rev. 2022, 43, 984–1002. [Google Scholar] [CrossRef]
- Nashi, N.; Nashi, N.; Kagda, F.H.; Kagda, F.H. Current concepts of bone grafting in trauma surgery. J. Clin. Orthop. Trauma. 2023, 43, 102231. [Google Scholar] [CrossRef]
- Chang, L.-C. Comparison of Clinical Parameters in Dental Implant Therapy between Implant Site Development Using Porcine- and Bovine-Derived Xenografts. Technologies 2021, 9, 72. [Google Scholar] [CrossRef]
- Carvalho, P.H.d.A.; Ciaramicolo, N.d.O.; Júnior, O.F.; Pereira-Filho, V.A. Clinical and laboratorial outcomes of xenogeneic biomaterials: Literature review. Front. Oral. Maxillofac. Med. 2023, 5, 8. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Abril-García, D.; Carrillo-Galvez, A.B.; Zurita, F.; Martín-Morales, N.; O’valle, F.; Padial-Molina, M. Maxillary sinus floor augmentation comparing bovine versus porcine bone xenografts mixed with autogenous bone graft. A split-mouth randomized controlled trial. Clin. Oral. Implant. Res. 2022, 33, 524–536. [Google Scholar] [CrossRef]
- Steijvers, E.; Ghei, A.; Xia, Z. Manufacturing artificial bone allografts: A perspective. Biomater. Transl. 2022, 3, 65–80. [Google Scholar] [CrossRef]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef]
- Xie, H.; Ruan, S.; Zhao, M.; Long, J.; Ma, X.; Guo, J.; Lin, X. Preparation and characterization of 3D hydroxyapatite/collagen scaffolds and its application in bone regeneration with bone morphogenetic protein-2. RSC Adv. 2023, 13, 23010–23020. [Google Scholar] [CrossRef] [PubMed]
- Noohi, P.; Mahdavi, S.S.; Abdekhodaie, M.J.; Nekoofar, M.H.; Baradaran-Rafii, A. Photoreactive Hydrogels Based on Type I Collagen Extracted from Different Sources as Scaffolds for Tissue Engineering Applications: A Comparative Study. Materialia 2023, 27, 101651. [Google Scholar] [CrossRef]
- Hoque, M.E.; Nuge, T.; Tshai, K.Y.; Nordin, N.; Prasad, V. Gelatin Based Scaffolds For Tissue Engineering—A review. Polym. Res. J. 2015, 9, 15–32. [Google Scholar]
- Liu, X.; Ma, P.X. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials 2009, 30, 4094–4103. [Google Scholar] [CrossRef]
- Wu, E.; Huang, L.; Shen, Y.; Wei, Z.; Li, Y.; Wang, J.; Chen, Z. Application of gelatin-based composites in bone tissue engineering. Heliyon 2024, 10, e36258. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2017, 3, 129–138. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Khan, R.H.; Suman, R. 3D printing applications in bone tissue engineering. J. Clin. Orthop. Trauma. 2020, 11, S118–S124. [Google Scholar] [CrossRef]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen Tissue Engineering: Development of Novel Biomaterials and Applications. Pediatr. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef]
- Lopes, M.S.; Jardini, A.; Filho, R.M. Poly (Lactic Acid) Production for Tissue Engineering Applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E. Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties. e-Polymers 2020, 20, 571–599. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The Application of Polycaprolactone in Three-Dimensional Printing Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ding, J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus. 2012, 2, 366–377. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Zhang, C.; Salick, M.R.; Cordie, T.M.; Ellingham, T.; Dan, Y.; Turng, L.-S. Incorporation of poly(ethylene glycol) grafted cellulose nanocrystals in poly(lactic acid) electrospun nanocomposite fibers as potential scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 49, 463–471. [Google Scholar] [CrossRef]
- Leal, F.; Nirwan, V.; Gonçalves, A.M.; Panitschewski, N.; Filová, E.; Fahmi, A.; Costa, P.F. Bio-inspired nanoporous scaffold: Electrospun hybrid fibers based on self-assembled block copolymer mineralized with inorganic nanoparticles for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, 1054–1067. [Google Scholar] [CrossRef]
- de Souza, J.R.; Cardoso, L.M.; de Toledo, P.T.A.; Rahimnejad, M.; Kito, L.T.; Thim, G.P.; Campos, T.M.B.; Borges, A.L.S.; Bottino, M.C. Biodegradable electrospun poly(L-lactide-co-ε-caprolactone)/polyethylene glycol/bioactive glass composite scaffold for bone tissue engineering. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2024, 112, e35406. [Google Scholar] [CrossRef]
- Ciobanu, P.; Panuta, A.; Radu, I.; Forna, N.; Arcana, S.; Tudor, R.; Covaciu, A.; Niculescu, V.; Poroch, V.; Puha, B. Treatment of Bone Defects Resulted after Excision of Enchondroma of the Hand in 15 Patients, Comparing the Techniques of Autologous Bone Graft, Injectable Bone Substitute and Spontaneous Healing. Appl. Sci. 2022, 12, 1300. [Google Scholar] [CrossRef]
- Jain, G.; Blaauw, D.; Chang, S. A Comparative Study of Two Bone Graft Substitutes—InterOss® Collagen and OCS-B Collagen®. J. Funct. Biomater. 2022, 13, 28. [Google Scholar] [CrossRef]
- Kim, J.-S.; Jang, T.-S.; Kim, S.-Y.; Lee, W.-P. Octacalcium Phosphate Bone Substitute (Bontree®): From Basic Research to Clinical Case Study. Appl. Sci. 2021, 11, 7921. [Google Scholar] [CrossRef]
- Ortega-Oller, I.; Padial-Molina, M.; Galindo-Moreno, P.; O’valle, F.; Jódar-Reyes, A.B.; Peula-García, J.M. Bone Regeneration from PLGA Micro-Nanoparticles. BioMed Res. Int. 2015, 2015, 415289. [Google Scholar] [CrossRef] [PubMed]
- Karbowniczek, J.E.; Kaniuk, Ł.; Berniak, K.; Gruszczyński, A.; Stachewicz, U. Enhanced Cells Anchoring to Electrospun Hybrid Scaffolds With PHBV and HA Particles for Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 632029. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Mobasheri, H.; Azami, M.; Faridi-Majidi, R. Osteoconductive and electroactive carbon nanofibers/hydroxyapatite nanocomposite tailored for bone tissue engineering: In vitro and in vivo studies. Sci. Rep. 2020, 10, 14853. [Google Scholar] [CrossRef]
- Lim, D.-J. Bone Mineralization in Electrospun-Based Bone Tissue Engineering. Polymers 2022, 14, 2123. [Google Scholar] [CrossRef]
- Song, H.; Zhang, Y.; Zhang, Z.; Xiong, S.; Ma, X.; Li, Y. Hydroxyapatite/NELL-1 Nanoparticles Electrospun Fibers for Osteoinduction in Bone Tissue Engineering Application. Int. J. Nanomed. 2021, 16, 4321–4332. [Google Scholar] [CrossRef]
- Kaur, K.; Das, S.; Ghosh, S. Regulation of Human Osteoblast-to-Osteocyte Differentiation by Direct-Write 3D Microperiodic Hydroxyapatite Scaffolds. ACS Omega 2019, 4, 1504–1515. [Google Scholar] [CrossRef]
- Anjum, S.; Arya, D.K.; Saeed, M.; Ali, D.; Athar, M.S.; Yulin, W.; Alarifi, S.; Wu, X.; Rajinikanth, P.; Ao, Q. Multifunctional electrospun nanofibrous scaffold enriched with alendronate and hydroxyapatite for balancing osteogenic and osteoclast activity to promote bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1302594. [Google Scholar] [CrossRef]
- Hedvičáková, V.; Žižková, R.; Buzgo, M.; Vištejnová, L.; Klein, P.; Hovořáková, M.; Bartoš, M.; Steklíková, K.; Luňáčková, J.; Šebová, E.; et al. The Gradual Release of Alendronate for the Treatment of Critical Bone Defects in Osteoporotic and Control Rats. Int. J. Nanomed. 2023, 18, 541–560. [Google Scholar] [CrossRef]
- Stastna, E.; Castkova, K.; Rahel, J. Influence of Hydroxyapatite Nanoparticles and Surface Plasma Treatment on Bioactivity of Polycaprolactone Nanofibers. Polymers 2020, 12, 1877. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Zein, S.H.S.; Othman, M.R.; Jansen, J.A. Hydroxyapatite nanoparticles: Electrospinning and calcination of hydroxyapatite/polyvinyl butyral nanofibers and growth kinetics. J. Biomed. Mater. Res. Part. A 2013, 101A, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Meesuk, L.; Suwanprateeb, J.; Thammarakcharoen, F.; Tantrawatpan, C.; Kheolamai, P.; Palang, I.; Tantikanlayaporn, D.; Manochantr, S. Osteogenic differentiation and proliferation potentials of human bone marrow and umbilical cord-derived mesenchymal stem cells on the 3D-printed hydroxyapatite scaffolds. Sci. Rep. 2022, 12, 19509. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, J.; Song, W.-X.; Tang, N.; Luo, J.; Deng, Z.-L.; Sharff, K.A.; He, G.; Bi, Y.; He, B.-C.; et al. Osteogenic BMPs promote tumor growth of human osteosarcomas that harbor differentiation defects. Mod. Pathol. 2008, 88, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Nabil, H.; Kummu, O.; Lehenkari, P.; Rysä, J.; Risteli, J.; Hakkola, J.; Hukkanen, J. Rifampicin induces the bone form of alkaline phosphatase in humans. Basic. Clin. Pharmacol. Toxicol. 2022, 130, 81–94. [Google Scholar] [CrossRef]
- Trivedi, S.; Srivastava, K.; Gupta, A.; Saluja, T.S.; Kumar, S.; Mehrotra, D.; Singh, S.K. A quantitative method to determine osteogenic differentiation aptness of scaffold. J. Oral. Biol. Craniofacial Res. 2020, 10, 158–160. [Google Scholar] [CrossRef]
- Sato, M.; Saitoh, I.; Kiyokawa, Y.; Iwase, Y.; Kubota, N.; Ibano, N.; Noguchi, H.; Yamasaki, Y.; Inada, E. Tissue-Nonspecific Alkaline Phosphatase, a Possible Mediator of Cell Maturation: Towards a New Paradigm. Cells 2021, 10, 3338. [Google Scholar] [CrossRef]
- Shimasaki, M.; Ichiseki, T.; Ueda, S.; Hirata, H.; Kawahara, N.; Ueda, Y. Mesenchymal Stem Cells Preconditioned with Hypoxia and Dexamethasone Promote Osteoblast Differentiation Under Stress Conditions. Int. J. Med. Sci. 2024, 21, 1511–1517. [Google Scholar] [CrossRef]
- Grundt, A.; Grafe, I.A.; Liegibel, U.; Sommer, U.; Nawroth, P.; Kasperk, C. Direct effects of osteoprotegerin on human bone cell metabolism. Biochem. Biophys. Res. Commun. 2009, 389, 550–555. [Google Scholar] [CrossRef]
- Shetty, S.; Paul, T.V.; Kapoor, N.; Bondu, J.D.; Thomas, N. Bone turnover markers: Emerging tool in the management of osteoporosis. Indian. J. Endocrinol. Metab. 2016, 20, 846–852. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.; Kim, I.; Seong, S.; Koh, J.-T.; Kim, N. The ATF3–OPG Axis Contributes to Bone Formation by Regulating the Differentiation of Osteoclasts, Osteoblasts, and Adipocytes. Int. J. Mol. Sci. 2022, 23, 3500. [Google Scholar] [CrossRef]
- Yu, H.; de Vos, P.; Ren, Y. Overexpression of osteoprotegerin promotes preosteoblast differentiation to mature osteoblasts. Angle Orthod. 2011, 81, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Baker, S.; Eisman, J.; Gardiner, E. Changing RANKL/OPG mRNA expression in differentiating murine primary osteoblasts. J. Endocrinol. 2001, 170, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Troen, B.R. The Regulation of Cathepsin K Gene Expression. Ann. N.Y. Acad. Sci. 2006, 1068, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Corisdeo, S.; Gyda, M.; Zaidi, M.; Moonga, B.S.; Troen, B.R. New Insights into the Regulation of Cathepsin K Gene Expression by Osteoprotegerin Ligand. Biochem. Biophys. Res. Commun. 2001, 285, 335–339. [Google Scholar] [CrossRef]
- Hsu, H.; Lacey, D.L.; Dunstan, C.R.; Solovyev, I.; Colombero, A.; Timms, E.; Tan, H.-L.; Elliott, G.; Kelley, M.J.; Sarosi, I.; et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. USA 1999, 96, 3540–3545. [Google Scholar] [CrossRef]
- Pang, M.; Martinez, A.F.; Jacobs, J.; Balkan, W.; Troen, B.R. RANK ligand and interferon gamma differentially regulate cathepsin gene expression in pre-osteoclastic cells. Biochem. Biophys. Res. Commun. 2005, 328, 756–763. [Google Scholar] [CrossRef]
- Shalhoub, V.; Faust, J.; Boyle, W.J.; Dunstan, C.R.; Kelley, M.; Kaufman, S.; Lacey, D.L. Osteoprotegerin and osteoprotegerin ligand effects on osteoclast formation from human peripheral blood mononuclear cell precursors. J. Cell. Biochem. 1999, 72, 251–261. [Google Scholar] [CrossRef]
- Fuller, K.; Lawrence, K.M.; Ross, J.L.; Grabowska, U.B.; Shiroo, M.; Samuelsson, B.; Chambers, T.J. Cathepsin K inhibitors prevent matrix-derived growth factor degradation by human osteoclasts. Bone 2008, 42, 200–211. [Google Scholar] [CrossRef]
- Zenger, S.; Hollberg, K.; Ljusberg, J.; Norgård, M.; Ek-Rylander, B.; Kiviranta, R.; Andersson, G. Proteolytic processing and polarized secretion of tartrate-resistant acid phosphatase is altered in a subpopulation of metaphyseal osteoclasts in cathepsin K-deficient mice. Bone 2007, 41, 820–832. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Huang, Z.; Chen, X.; Zhang, B. The roles of osteoprotegerin in cancer, far beyond a bone player. Cell Death Discov. 2022, 8, 252. [Google Scholar] [CrossRef]
- Yun, T.J.; Tallquist, M.D.; Aicher, A.; Rafferty, K.L.; Marshall, A.J.; Moon, J.J.; Ewings, M.K.; Mohaupt, M.; Herring, S.W.; Clark, E.A. Osteoprotegerin, a Crucial Regulator of Bone Metabolism, Also Regulates B Cell Development and Function1. J. Immunol. 2001, 166, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Jimi, E.; Kokabu, S.; Matsubara, T.; Nakatomi, C.; Matsuo, K.; Watanabe, S. NF-κB acts as a multifunctional modulator in bone invasion by oral squamous cell carcinoma. Oral. Sci. Int. 2016, 13, 1–6. [Google Scholar] [CrossRef]
- Li, Y.-P.; Chen, W. Characterization of mouse cathepsin K gene, the gene promoter, and the gene expression. J. Bone Miner. Res. 1999, 14, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X.; Li, G.; Chong, Y.; Zhang, J.; Guo, X.; Li, B.; Bi, Z. Osteoclast regulation of osteoblasts via RANK-RANKL reverse signal transduction in vitro. Mol. Med. Rep. 2017, 16, 3994–4000. [Google Scholar] [CrossRef]
- Schoppet, M.; Preissner, K.T.; Hofbauer, L.C. RANK ligand and osteoprotegerin: Paracrine regulators of bone metabolism and vascular function. Arter. Thromb. Vasc. Biol. 2002, 22, 549–553. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, X.; Tao, Y.; Ping, Z.; Wang, L.; Shi, J.; Wu, X.; Zhang, W.; Yang, H.; Nie, Z.; et al. Strontium inhibits titanium particle-induced osteoclast activation and chronic inflammation via suppression of NF-κB pathway. Sci. Rep. 2016, 6, 36251. [Google Scholar] [CrossRef]
- Owen, R.; Reilly, G.C. In vitro Models of Bone Remodelling and Associated Disorders. Front. Bioeng. Biotechnol. 2018, 6, 134. [Google Scholar] [CrossRef]
- Kumari, S.; Katiyar, S.; Darshna; Anand, A.; Singh, D.; Singh, B.N.; Mallick, S.P.; Mishra, A.; Srivastava, P. Design strategies for composite matrix and multifunctional polymeric scaffolds with enhanced bioactivity for bone tissue engineering. Front. Chem. 2022, 10, 1051678. [Google Scholar] [CrossRef]
- Liang, W.; Ding, P.; Li, G.; Lu, E.; Zhao, Z. Hydroxyapatite nanoparticles facilitate osteoblast differentiation and bone formation within sagittal suture during expansion in rats. Drug Des. Devel. Ther. 2021, 15, 905–917. [Google Scholar] [CrossRef]
- Jolly, J.J.; Chin, K.-Y.; Farhana, M.F.N.; Alias, E.; Chua, K.H.; Hasan, W.N.W.; Ima-Nirwana, S. Optimization of the static human osteoblast/osteoclast co-culture system. Iran. J. Med. Sci. 2018, 43, 208–213. [Google Scholar]
- Lynch, M.P.; Stein, J.L.; Stein, G.S.; Lian, J.B. The influence of type I collagen on the development and maintenance of the osteoblast phenotype in primary and passaged rat calvarial osteoblasts: Modification of expression of genes supporting cell growth, adhesion, and extracellular matrix mineralization. Exp. Cell Res. 1995, 216, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of Osteoblast Differentiation by Runx2. In Osteoimmunology; Choi, Y., Ed.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2009; Volume 658. [Google Scholar] [CrossRef]
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteocalcin: Skeletal and extra-skeletal effects. J. Cell. Physiol. 2013, 228, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, J.; Thomas, B.; Hoarau-Véchot, J.; Odeh, T.; Robay, A.; Chidiac, O.; Dargham, S.R.; Turjoman, R.; Halama, A.; Fakhro, K.; et al. Circulating microparticles in acute diabetic Charcot foot exhibit a high content of inflammatory cytokines, and support monocyte-to-osteoclast cell induction. Sci. Rep. 2017, 7, 16450. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Si, Y.; Xu, G.; Chen, X.M.; Xiong, H.; Lai, L.; Zheng, Y.Q.; Zhang, Z.G. High-dose PMA with RANKL and MCSF induces THP-1 cell differentiation into human functional osteoclasts in vitro. Mol. Med. Rep. 2017, 16, 8380–8384. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šebová, E.; Leal, F.; Klusáček Rampichová, M.; Nirwan, V.P.; Fahmi, A.; Costa, P.F.; Filová, E. Electrospun Poly(L-lactide-co-ε-caprolactone) Nanofibers with Hydroxyapatite Nanoparticles Mimic Cellular Interplay in Bone Regeneration. Int. J. Mol. Sci. 2025, 26, 5383. https://doi.org/10.3390/ijms26115383
Šebová E, Leal F, Klusáček Rampichová M, Nirwan VP, Fahmi A, Costa PF, Filová E. Electrospun Poly(L-lactide-co-ε-caprolactone) Nanofibers with Hydroxyapatite Nanoparticles Mimic Cellular Interplay in Bone Regeneration. International Journal of Molecular Sciences. 2025; 26(11):5383. https://doi.org/10.3390/ijms26115383
Chicago/Turabian StyleŠebová, Eva, Filipa Leal, Michala Klusáček Rampichová, Viraj P. Nirwan, Amir Fahmi, Pedro F. Costa, and Eva Filová. 2025. "Electrospun Poly(L-lactide-co-ε-caprolactone) Nanofibers with Hydroxyapatite Nanoparticles Mimic Cellular Interplay in Bone Regeneration" International Journal of Molecular Sciences 26, no. 11: 5383. https://doi.org/10.3390/ijms26115383
APA StyleŠebová, E., Leal, F., Klusáček Rampichová, M., Nirwan, V. P., Fahmi, A., Costa, P. F., & Filová, E. (2025). Electrospun Poly(L-lactide-co-ε-caprolactone) Nanofibers with Hydroxyapatite Nanoparticles Mimic Cellular Interplay in Bone Regeneration. International Journal of Molecular Sciences, 26(11), 5383. https://doi.org/10.3390/ijms26115383







