Functional Features of Senescent Cells and Implications for Therapy
Abstract
1. Introduction
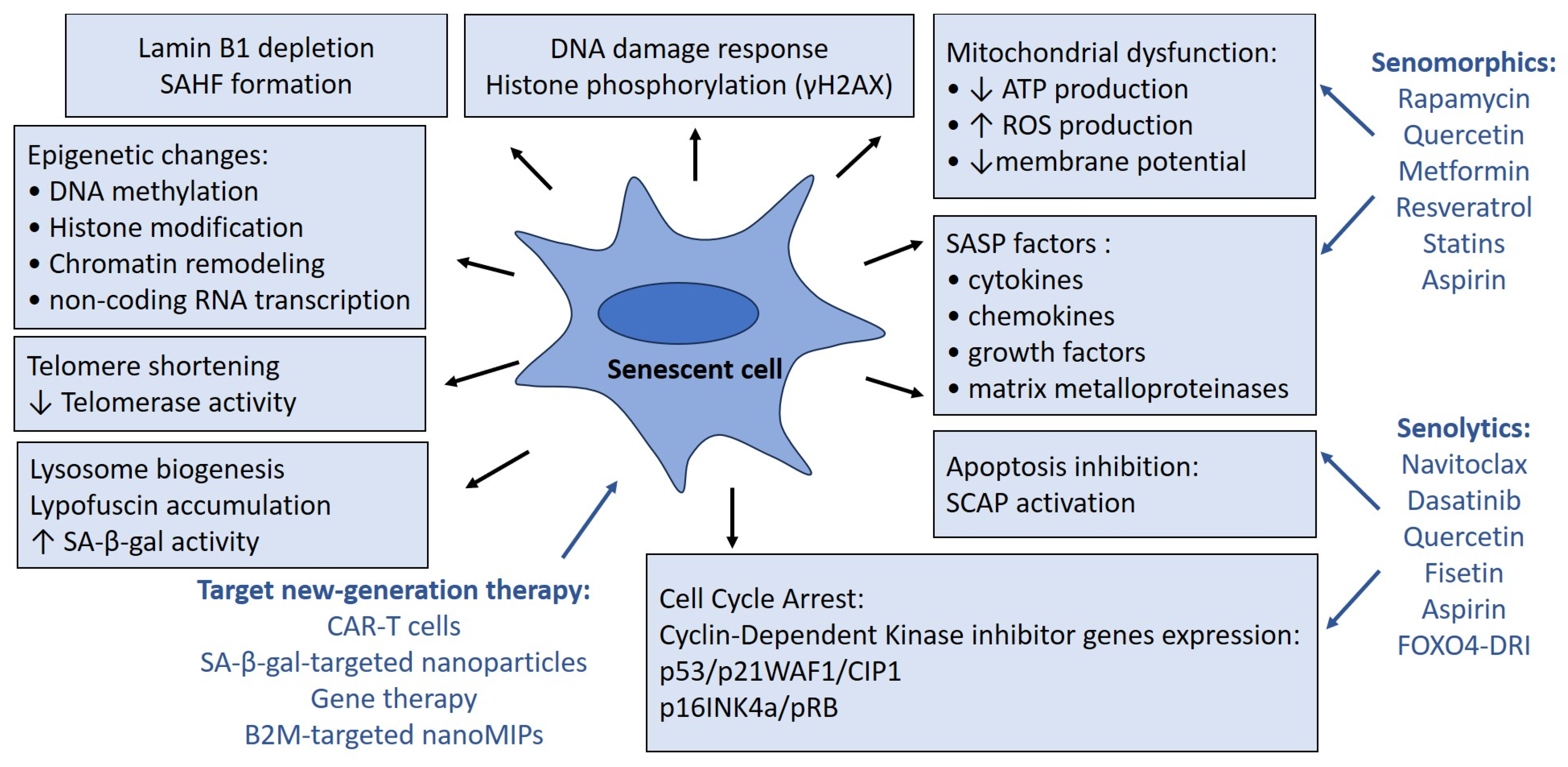
2. Markers of Senescent Cells Dysfunction
2.1. Cell Cycle Arrest
2.2. Lysosome Dysfunction
2.3. Mitochondria Dysfunction
2.4. Senescence-Associated Secretory Phenotype
3. Implications for Anti-Aging Therapy
3.1. Senolytic Therapy
3.2. Senomorphic Therapy
3.3. Clinical Trials of Anti-Aging Agents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Triana-Martínez, F.; Pedraza-Vázquez, G.; Maciel-Barón, L.A.; Königsberg, M. Reflections on the role of senescence during development and aging. Arch. Biochem. Biophys. 2016, 598, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Ogrodnik, M.; Salmonowicz, H.; Gladyshev, V.N. Integrating cellular senescence with the concept of damage accumulation in aging: Relevance for clearance of senescent cells. Aging Cell. 2019, 18, e12841. [Google Scholar] [CrossRef] [PubMed]
- Pangrazzi, L.; Meryk, A. Molecular and Cellular Mechanisms of Immunosenescence: Modulation Through Interventions and Lifestyle Changes. Biology 2024, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Gasek, N.S.; Kuchel, G.A.; Kirkland, J.L.; Xu, M. Strategies for Targeting Senescent Cells in Human Disease. Nat. Aging 2021, 1, 870–879. [Google Scholar] [CrossRef]
- Yamauchi, S.; Takahashi, A. Cellular senescence: Mechanisms and relevance to cancer and aging. J. Biochem. 2025, 177, 163–169. [Google Scholar] [CrossRef]
- Boccardi, V.; Orr, M.E.; Polidori, M.C.; Ruggiero, C.; Mecocci, P. Focus on senescence: Clinical significance and practical applications. J. Intern. Med. 2024, 295, 599–619. [Google Scholar] [CrossRef]
- McHugh, D.; Durán, I.; Gil, J. Senescence as a therapeutic target in cancer and age-related diseases. Nat. Rev. Drug. Discov. 2025, 24, 57–71. [Google Scholar] [CrossRef]
- Kudlova, N.; De Sanctis, J.B.; Hajduch, M. Cellular Senescence: Molecular Targets, Biomarkers, and Senolytic Drugs. Int. J. Mol. Sci. 2022, 23, 4168. [Google Scholar] [CrossRef]
- Shah, J.; Al-Hashimi, A.; Benedetto, M.; Ruchaya, P.J. From bench to bedside: The critical need for standardized senescence detection. Arch. Cardiovasc. Dis. 2025, 118, 205–211. [Google Scholar] [CrossRef]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2021, 288, 56–80. [Google Scholar] [CrossRef]
- Mohamad Kamal, N.S.; Safuan, S.; Shamsuddin, S.; Foroozandeh, P. Aging of the cells: Insight into cellular senescence and detection Methods. Eur. J. Cell. Biol. 2020, 99, 151108. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Epel, E. Stress and telomere shortening: Insights from cellular mechanisms. Ageing Res. Rev. 2022, 73, 101507. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, G.E.; Fountain, A.J.; van Roon, A.M.; Rangan, R.; Das, R.; Collins, K.; Nguyen, T.H.D. Structure of human telomerase holoenzyme with bound telomeric DNA. Nature 2021, 593, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Kamal, S.; Junaid, M.; Ejaz, A.; Bibi, I.; Akash, M.S.H.; Rehman, K. The secrets of telomerase: Retrospective analysis and future prospects. Life Sci. 2020, 257, 118115. [Google Scholar] [CrossRef]
- Gong, P.; Guo, Z.; Wang, S.; Gao, S.; Cao, Q. Histone Phosphorylation in DNA Damage Response. Int. J. Mol. Sci. 2025, 26, 2405. [Google Scholar] [CrossRef]
- Merighi, A.; Gionchiglia, N.; Granato, A.; Lossi, L. The Phosphorylated Form of the Histone H2AX (γH2AX) in the Brain from Embryonic Life to Old Age. Molecules 2021, 26, 7198. [Google Scholar] [CrossRef]
- Zarneshan, S.N.; Fakhri, S.; Bachtel, G.; Bishayee, A. Exploiting pivotal mechanisms behind the senescence-like cell cycle arrest in Cancer. Adv. Protein Chem. Struct. Biol. 2023, 135, 1–19. [Google Scholar] [CrossRef]
- Muthamil, S.; Kim, H.Y.; Jang, H.J.; Lyu, J.H.; Shin, U.C.; Go, Y.; Park, S.H.; Lee, H.G.; Park, J.H. Biomarkers of Cellular Senescence and Aging: Current State-of-the-Art, Challenges and Future Perspectives. Adv. Biol. 2024, 8, e2400079. [Google Scholar] [CrossRef]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Al-Abbasi, F.A.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Uddin, A.; Murtaza, B.N.; et al. Genes and Longevity of Lifespan. Int. J. Mol. Sci. 2022, 23, 1499. [Google Scholar] [CrossRef]
- Dorf, N.; Maciejczyk, M. Skin senescence-from basic research to clinical practice. Front. Med. 2024, 11, 1484345. [Google Scholar] [CrossRef]
- Bulbiankova, D.; Díaz-Puertas, R.; Álvarez-Martínez, F.J.; Herranz-López, M.; Barrajón-Catalán, E.; Micol, V. Hallmarks and Biomarkers of Skin Senescence: An Updated Review of Skin Senotherapeutics. Antioxidants 2023, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Piechota, M.; Sunderland, P.; Wysocka, A.; Nalberczak, M.; Sliwinska, M.A.; Radwanska, K.; Sikora, E. Is senescence-associated β-galactosidase a marker of neuronal senescence? Oncotarget 2016, 7, 81099–81109. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Yoon, G.; Kang, H.T. A comparative analysis of the cell biology of senescence and aging. Cell. Mol. Life Sci. 2009, 66, 2503–2524. [Google Scholar] [CrossRef] [PubMed]
- Renteln, M. Toward Systemic Lipofuscin Removal. Rejuvenation Res. 2024, 27, 171–179. [Google Scholar] [CrossRef]
- Moreno, T.M.; Nieto-Torres, J.L.; Kumsta, C. Monitoring Autophagy in Human Aging: Key Cell Models and Insights. Front. Biosci. (Landmark Ed.) 2025, 30, 27091. [Google Scholar] [CrossRef]
- Dellorusso, P.V.; Proven, M.A.; Calero-Nieto, F.J.; Wang, X.; Mitchell, C.A.; Hartmann, F.; Amouzgar, M.; Favaro, P.; DeVilbiss, A.; Swann, J.W.; et al. Autophagy counters inflammation-driven glycolytic impairment in aging hematopoietic stem cells. Cell. Stem. Cell. 2024, 31, 1020–1037.e9. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, R.; Liu, M.; Zhang, Y.; Jia, B.; Ruan, J.; Shen, J.; Zhang, Y.; Liu, M.; Wang, T. Targeting autophagy in atherosclerosis: Advances and therapeutic potential of natural bioactive compounds from herbal medicines and natural products. Biomed. Pharmacother. 2022, 155, 113712. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Guo, Y.; Guan, T.; Shafiq, K.; Yu, Q.; Jiao, X.; Na, D.; Li, M.; Zhang, G.; Kong, J. Mitochondrial dysfunction in aging. Ageing Res. Rev. 2023, 88, 101955. [Google Scholar] [CrossRef]
- Rossi, C.; Macchi, C.; D’Alonzo, C.; Venturin, M.; Ruscica, M.; Corsini, A.; Battaglia, C.; Bellosta, S. Simvastatin ameliorates senescence-induced mitochondrial dysfunction in vascular smooth muscle cells. Atherosclerosis 2025, 403, 119176. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Zhao, L.; Long, J.; Feng, Z.; Su, J.; Gao, F.; Liu, J. Mitochondria as a sensor, a central hub and a biological clock in psychological stress-accelerated aging. Ageing Res. Rev. 2024, 93, 102145. [Google Scholar] [CrossRef] [PubMed]
- Vaurs, M.; Dolu, E.B.; Decottignies, A. Mitochondria and telomeres: Hand in glove. Biogerontology 2024, 25, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Lingner, J. Impact of oxidative stress on telomere biology. Differentiation 2018, 99, 21–27. [Google Scholar] [CrossRef]
- Zhang, Y.; Unnikrishnan, A.; Deepa, S.S.; Liu, Y.; Li, Y.; Ikeno, Y.; Sosnowska, D.; Van Remmen, H.; Richardson, A. A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1-/- mice is correlated to increased cellular senescence. Redox. Biol. 2017, 11, 30–37. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Liang, R.; Huang, Y.; Tang, Q. Aging through the lens of mitochondrial DNA mutations and inheritance paradoxes. Biogerontology 2025, 26, 33. [Google Scholar] [CrossRef]
- Quan, T.; Li, R.; Gao, T. Role of Mitochondrial Dynamics in Skin Homeostasis: An Update. Int. J. Mol. Sci. 2025, 26, 1803. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Coelho-Junior, H.J.; Picca, A. Mitochondrial pathways and sarcopenia in the geroscience era. J. Nutr. Health Aging 2024, 28, 100397. [Google Scholar] [CrossRef]
- Picca, A.; Lozanoska-Ochser, B.; Calvani, R.; Coelho-Júnior, H.J.; Leewenburgh, C.; Marzetti, E. Inflammatory, mitochondrial, and senescence-related markers: Underlying biological pathways of muscle aging and new therapeutic targets. Exp. Gerontol. 2023, 178, 112204. [Google Scholar] [CrossRef]
- Martini, H.; Passos, J.F. Cellular senescence: All roads lead to mitochondria. FEBS J. 2023, 290, 1186–1202. [Google Scholar] [CrossRef]
- Canale, P.; Borghini, A. Mitochondrial microRNAs: New Emerging Players in Vascular Senescence and Atherosclerotic Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 6620. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell. Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef] [PubMed]
- Princilly, J.; Veerabhadrappa, B.; Rao, N.N.; Dyavaiah, M. Cellular senescence in aging: Molecular basis, implications and therapeutic interventions. Adv. Protein Chem. Struct. Biol. 2023, 136, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Luo, Y.; Yuan, Z.; Tian, Y.; Jin, T.; Xu, F. Cellular senescence and SASP in tumor progression and therapeutic opportunities. Mol. Cancer 2024, 23, 181. [Google Scholar] [CrossRef]
- Rana, K.S.; Marwah, M.K.; Raja, F.N.S.; Dias, I.; Hindalekar, Y.S.; Al Tahan, M.A.; Brown, J.E.; Bellary, S. The influence of senescent associated secretory phenotype on glucose homeostasis in C2C12 muscle cells: Insights into potential p38 inhibitor interventions. J. Recept. Signal. Transduct. Res. 2025, 45, 118–127. [Google Scholar] [CrossRef]
- Zhao, S.; Qiao, Z.; Pfeifer, R.; Pape, H.C.; Mao, K.; Tang, H.; Meng, B.; Chen, S.; Liu, H. Modulation of Fracture Healing by Senescence-Associated Secretory Phenotype (SASP): A Narrative Review of the Current Literature. Eur. J. Med. Res. 2024, 29, 38. [Google Scholar] [CrossRef]
- Shreeya, T.; Ansari, M.S.; Kumar, P.; Saifi, M.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I. Senescence: A DNA Damage Response and Its Role in Aging and Neurodegenerative Diseases. Front. Aging 2023, 4, 1292053. [Google Scholar] [CrossRef]
- Cho, S.; Hwang, E.S. Status of mTOR activity may phenotypically differentiate senescence and quiescence. Mol. Cells 2012, 33, 597–604. [Google Scholar] [CrossRef]
- Diwan, B.; Yadav, R.; Goyal, R.; Sharma, R. Sustained exposure to high glucose induces differential expression of cellular senescence markers in murine macrophages but impairs immunosurveillance response to senescent cells secretome. Biogerontology 2024, 25, 627–647. [Google Scholar] [CrossRef]
- Khavinson, V.; Linkova, N.; Dyatlova, A.; Kantemirova, R.; Kozlov, K. Senescence-Associated Secretory Phenotype of Cardiovascular System Cells and Inflammaging: Perspectives of Peptide Regulation. Cells 2022, 12, 106. [Google Scholar] [CrossRef]
- Schlett, J.S.; Mettang, M.; Skaf, A.; Schweizer, P.; Errerd, A.; Mulugeta, E.A.; Hein, T.M.; Tsesmelis, K.; Tsesmelis, M.; Büttner, U.F.G.; et al. NF-κB is a critical mediator of post-mitotic senescence in oligodendrocytes and subsequent white matter loss. Mol. Neurodegener. 2023, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Rattanaprukskul, K.; Xia, X.J.; Jiang, M.; Albuquerque-Souza, E.; Bandyopadhyay, D.; Sahingur, S.E. Molecular Signatures of Senescence in Periodontitis: Clinical Insights. J. Dent. Res. 2024, 103, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Dominic, A.; Banerjee, P.; Hamilton, D.J.; Le, N.T.; Abe, J.I. Time-dependent replicative senescence vs. disturbed flow-induced pre-mature aging in atherosclerosis. Redox. Biol. 2020, 37, 101614. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Däbritz, J.H.M.; Zhao, Z.; Yu, Y.; Dörr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-associated reprogramming promotes cancer stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef]
- Malaquin, N.; Rodier, F. Dynamic and scalable assessment of the senescence-associated secretory phenotype (SASP). Methods Cell. Biol. 2024, 181, 181–195. [Google Scholar] [CrossRef]
- Suryadevara, V.; Hudgins, A.D.; Rajesh, A.; Pappalardo, A.; Karpova, A.; Dey, A.K.; Hertzel, A.; Agudelo, A.; Rocha, A.; Soygur, B.; et al. SenNet recommendations for detecting senescent cells in different tissues. Nat. Rev. Mol. Cell. Biol. 2024, 25, 1001–1023. [Google Scholar] [CrossRef]
- Lavarti, R.; Alvarez-Diaz, T.; Marti, K.; Kar, P.; Raju, R.P. The context-dependent effect of cellular senescence: From embryogenesis and wound healing to aging. Ageing Res. Rev. 2025, 109, 102760. [Google Scholar] [CrossRef]
- Ogrodnik, M.; Carlos Acosta, J.; Adams, P.D.; d’Adda di Fagagna, F.; Baker, D.J.; Bishop, C.L.; Chandra, T.; Collado, M.; Gil, J.; Gorgoulis, V.; et al. Guidelines for minimal information on cellular senescence experimentation in vivo. Cell 2024, 187, 4150–4175. [Google Scholar] [CrossRef]
- Pandey, S.N.; Afzal, M.; Uikey, J.; Ganesan, S.; Mishra, S.; Bansal, P.; Kazmi, I.; Alzarea, S.I.; Almalk, W.H.; Goyal, K.; et al. ATM and p53 in aging and cancer: A double-edged sword in genomic integrity. Biogerontology 2025, 26, 102. [Google Scholar] [CrossRef]
- Santos-Sousa, D.C.; da Rosa, S.; Filippi-Chiela, E. Molecular signatures of cellular senescence in cancer: A critical review of prognostic implications and therapeutic opportunities. Mech. Ageing Dev. 2025, 225, 112052. [Google Scholar] [CrossRef]
- Yu, G.T.; Gomez, P.T.; Prata, L.G.; Lehman, J.S.; Tchkonia, T.; Kirkland, J.L.; Meves, A.; Wyles, S.P. Clinicopathological and cellular senescence biomarkers in chronic stalled wounds. Int. J. Dermatol. 2024, 63, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Suda, M.; Tchkonia, T.; Kirkland, J.L.; Minamino, T. Targeting senescent cells for the treatment of age-associated diseases. J. Biochem. 2025, 177, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.E.; Zhou, Z. Senescent cells as a target for anti-aging interventions: From senolytics to immune therapies. J. Transl. Int. Med. 2025, 13, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Olivieri, A.; Manzione, L. Dasatinib: A new step in molecular target therapy. Ann. Oncol. 2007, 18, vi42–vi46. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food. Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell. 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Rattanaprukskul, K.; Xia, X.J.; Hysa, M.; Jiang, M.; Hung, M.; Suslavich, S.F.; Sahingur, S.E. Dasatinib and Quercetin Limit Gingival Senescence, Inflammation, and Bone Loss. J. Dent. Res. 2025, 104, 419–427. [Google Scholar] [CrossRef]
- Dungan, C.M.; Murach, K.A.; Zdunek, C.J.; Tang, Z.J.; Nolt, G.L.; Brightwell, C.R.; Hettinger, Z.; Englund, D.A.; Liu, Z.; Fry, C.S.; et al. Deletion of SA β-Gal+ cells using senolytics improves muscle regeneration in old mice. Aging Cell. 2022, 21, e13528. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, T.; Tang, X.; Wen, J.; Zhang, Y.; Zhang, J.; Wang, S. Increased cellular senescence in doxorubicin-induced murine ovarian injury: Effect of senolytics. Geroscience 2023, 45, 1775–1790. [Google Scholar] [CrossRef]
- Luo, Q.T.; Ye, Y.C.; Guo, W.M.; Zhu, Q.; Wang, S.S.; Li, N.; Wang, L.; Cheng, C.S.; Fan, G. Senolytic Treatment Improve Small Intestine Regeneration in Aging. Aging Dis. 2024, 15, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, A.; Kellogg D3rd Justice, J.; Goros, M.; Gelfond, J.; Pascual, R.; Hashmi, S.; Masternak, M.; Prata, L.; LeBrasseur, N.; Limper, A.; et al. Senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis: Results of a phase I, single-blind, single-center, randomized, placebo-controlled pilot trial on feasibility and tolerability. EBioMedicine 2023, 90, 104481. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.L.; Iloputaife, I.; Baldyga, K.; Norling, A.M.; Boulougoura, A.; Vichos, T.; Tchkonia, T.; Deisinger, A.; Pirtskhalava, T.; Kirkland, J.L.; et al. A pilot study of senolytics to improve cognition and mobility in older adults at risk for Alzheimer’s disease. EBioMedicine 2025, 113, 105612. [Google Scholar] [CrossRef]
- Farr, J.N.; Atkinson, E.J.; Achenbach, S.J.; Volkman, T.L.; Tweed, A.J.; Vos, S.J.; Ruan, M.; Sfeir, J.; Drake, M.T.; Saul, D.; et al. Effects of intermittent senolytic therapy on bone metabolism in postmenopausal women: A phase 2 randomized controlled trial. Nat. Med. 2024, 30, 2605–2612. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Takaya, K.; Asou, T.; Kishi, K. Fisetin, a potential skin rejuvenation drug that eliminates senescent cells in the dermis. Biogerontology 2024, 25, 161–175. [Google Scholar] [CrossRef]
- Huard, C.A.; Gao, X.; Dey Hazra, M.E.; Dey Hazra, R.O.; Lebsock, K.; Easley, J.T.; Millett, P.J.; Huard, J. Effects of Fisetin Treatment on Cellular Senescence of Various Tissues and Organs of Old Sheep. Antioxidants 2023, 12, 1646. [Google Scholar] [CrossRef]
- Kim, S.G.; Sung, J.Y.; Kang, Y.J.; Choi, H.C. Fisetin alleviates cellular senescence through PTEN mediated inhibition of PKCδ-NOX1 pathway in vascular smooth muscle cells. Arch. Gerontol. Geriatr. 2023, 108, 104927. [Google Scholar] [CrossRef]
- Wissler Gerdes, E.O.; Misra, A.; Netto, J.M.E.; Tchkonia, T.; Kirkland, J.L. Strategies for late phase preclinical and early clinical trials of senolytics. Mech. Ageing Dev. 2021, 200, 111591. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Fuhrmann-Stroissnigg, H.; Dai, H.M.; Ling, Y.Y.; Stout, M.B.; Pirtskhalava, T.; Giorgadze, N.; Johnson, K.O.; Giles, C.B.; et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016, 15, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; He, S.; Wang, H.; Li, J.; Liu, Y.; Liu, S. Targeting Cellular Senescence in Aging and Age-Related Diseases: Challenges, Considerations, and the Emerging Role of Senolytic and Senomorphic Therapies. Aging Dis. 2024, 15, 2554–2594. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Kim, N.Y.; Kim, Y.; Baek, D.J.; Park, T.J.; Kang, H.Y. Senolytic Targeting of Anti-Apoptotic Bcl Family Increases Cell Death in UV-Irradiated Senescent Melanocytes: Search for Senolytics. Exp. Dermatol. 2025, 34, e70037. [Google Scholar] [CrossRef] [PubMed]
- Csik, B.; Nyúl-Tóth, Á.; Gulej, R.; Patai, R.; Kiss, T.; Delfavero, J.; Nagaraja, R.Y.; Balasubramanian, P.; Shanmugarama, S.; Ungvari, A.; et al. Senescent Endothelial Cells in Cerebral Microcirculation Are Key Drivers of Age-Related Blood-Brain Barrier Disruption, Microvascular Rarefaction, and Neurovascular Coupling Impairment in Mice. Aging Cell. 2025, e70048, Epub ahead of print. [Google Scholar] [CrossRef]
- Mohamad Anuar, N.N.; Nor Hisam, N.S.; Liew, S.L.; Ugusman, A. Clinical Review: Navitoclax as a Pro-Apoptotic and Anti-Fibrotic Agent. Front. Pharmacol. 2020, 11, 564108. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T.; Zhu, Y.; Niedernhofer, L.J.; Robbins, P.D. The Clinical Potential of Senolytic Drugs. J. Am. Geriatr. Soc. 2017, 65, 2297–2301. [Google Scholar] [CrossRef] [PubMed]
- Jeayeng, S.; Kwanthongdee, J.; Jittreeprasert, R.; Runganantchai, K.; Naksavasdi, K.; Rirkkrai, R.; Wongcharoenthavorn, V.; Mahikul, W.; Chatsirisupachai, A. Natural products as promising therapeutics for fine particulate matter-induced skin damage: A review of pre-clinical studies on skin inflammation and barrier dysfunction. PeerJ 2025, 13, e19316. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, X.; Han, J. Quercetin Regulates the Polarization of Microglia through the NRF2/HO1 Pathway and Mitigates Alzheimer’s Disease. Actas. Esp. Psiquiatr. 2024, 52, 786–799. [Google Scholar] [CrossRef]
- Wiciński, M.; Erdmann, J.; Nowacka, A.; Kuźmiński, O.; Michalak, K.; Janowski, K.; Ohla, J.; Biernaciak, A.; Szambelan, M.; Zabrzyński, J. Natural Phytochemicals as SIRT Activators-Focus on Potential Biochemical Mechanisms. Nutrients 2023, 15, 3578. [Google Scholar] [CrossRef]
- Wei, H.K.; Qi, J.J.; Wang, Y.Q.; Qu, H.X.; Yan, C.X.; Li, T.T.; Wang, Y.; Sun, H.; Sun, B.X.; Liang, S. Fisetin alleviates oxidative stress and promotes porcine early embryonic development via activation of the NRF2-ARE signalling pathway. Anim. Biosci. 2025, 38, 1160–1174. [Google Scholar] [CrossRef]
- Fatima, R.; Soni, P.; Sharma, M.; Prasher, P.; Kaverikana, R.; Mangalpady, S.S.; Sharifi-Rad, J.; Calina, D. Fisetin as a chemoprotective and chemotherapeutic agent: Mechanistic insights and future directions in cancer therapy. Med. Oncol. 2025, 42, 104. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hu, D.N.; Lin, H.W.; Yang, W.E.; Hsieh, Y.H.; Chien, H.W.; Yang, S.F. Fisetin induces apoptosis through mitochondrial apoptosis pathway in human uveal melanoma cells. Environ. Toxicol. 2018, 33, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Dutta Gupta, S.; Pan, C.H. Recent update on discovery and development of Hsp90 inhibitors as senolytic agents. Int. J. Biol. Macromol. 2020, 161, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Orea-Soufi, A.; Paik, J.; Bragança, J.; Donlon, T.A.; Willcox, B.J.; Link, W. FOXO transcription factors as therapeutic targets in human diseases. Trends. Pharmacol. Sci. 2022, 43, 1070–1084. [Google Scholar] [CrossRef]
- Pawge, G.; Khatik, G.L. p53 regulated senescence mechanism and role of its modulators in age-related disorders. Biochem. Pharmacol. 2021, 190, 114651. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Y.; He, B.; Wu, D.; Hua, J.; Qian, H.; Zhang, J. DNA nanoparticles targeting FOXO4 selectively eliminate cigarette smoke-induced senescent lung fibroblasts. Nanoscale Adv. 2023, 5, 5965–5973. [Google Scholar] [CrossRef]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132. [Google Scholar] [CrossRef]
- Patel, K.K.; Tariveranmoshabad, M.; Kadu, S.; Shobaki, N.; June, C. From concept to cure: The evolution of CAR-T cell therapy. Mol. Ther. 2025, 33, 2123–2140. [Google Scholar] [CrossRef]
- García-Fleitas, J.; García-Fernández, A.; Martí-Centelles, V.; Sancenón, F.; Bernardos, A.; Martínez-Máñez, R. Chemical Strategies for the Detection and Elimination of Senescent Cells. Acc. Chem. Res. 2024, 57, 1238–1253. [Google Scholar] [CrossRef]
- Dhokia, V.; Albati, A.; Smith, H.; Thomas, G.; Macip, S. A second generation of senotherapies: The development of targeted senolytics, senoblockers and senoreversers for healthy. Ageing Biochem. Soc. Trans. 2024, 52, 1661–1671. [Google Scholar] [CrossRef]
- Fossel, M.; Bean, J.; Khera, N.; Kolonin, M.G. A Unified Model of Age-Related Cardiovascular Disease. Biology 2022, 11, 1768. [Google Scholar] [CrossRef] [PubMed]
- Lagoumtzi, S.M.; Chondrogianni, N. Senolytics and senomorphics: Natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic. Biol. Med. 2021, 171, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Selvarani, R.; Mohammed, S.; Richardson, A. Effect of rapamycin on aging and age-related diseases-past and future. Geroscience 2021, 43, 1135–1158. [Google Scholar] [CrossRef] [PubMed]
- An, J.Y.; Kerns, K.A.; Ouellette, A.; Robinson, L.; Morris, H.D.; Kaczorowski, C.; Park, S.I.; Mekvanich, T.; Kang, A.; McLean, J.S.; et al. Rapamycin rejuvenates oral health in aging mice. eLife 2020, 9, e54318. [Google Scholar] [CrossRef]
- Ham, D.J.; Börsch, A.; Chojnowska, K.; Lin, S.; Leuchtmann, A.B.; Ham, A.S.; Thürkauf, M.; Delezie, J.; Furrer, R.; Burri, D.; et al. Distinct and additive effects of calorie restriction and rapamycin in aging skeletal muscle. Nat. Commun. 2022, 13, 2025. [Google Scholar] [CrossRef]
- Ceschi, A.; Heistermann, E.; Gros, S.; Reichert, C.; Kupferschmidt, H.; Banner, N.R.; Krähenbühl, S.; Taegtmeyer, A.B. Acute sirolimus overdose: A multicenter case series. PLoS ONE. 2015, 10, e0128033. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Nemati, M.; Zandvakili, R.; Jafarzadeh, A. Modulation of M1 and M2 macrophage polarization by metformin: Implications for inflammatory diseases and malignant tumors. Int. Immunopharmacol. 2025, 151, 114345. [Google Scholar] [CrossRef]
- Chen, S.; Gan, D.; Lin, S.; Zhong, Y.; Chen, M.; Zou, X.; Shao, Z.; Xiao, G. Metformin in aging and aging-related diseases: Clinical applications and relevant mechanisms. Theranostics 2022, 12, 2722–2740. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, X.; Liu, N.; Ma, S.; Zhang, H.; Zhang, Z.; Yang, K.; Jiang, M.; Zheng, Z.; Qiao, Y.; et al. Metformin decelerates aging clock in male monkeys. Cell 2024, 187, 6358–6378.e29. [Google Scholar] [CrossRef]
- Della Vedova, L.; Baron, G.; Morazzoni, P.; Aldini, G.; Gado, F. The Potential of Polyphenols in Modulating the Cellular Senescence Process: Implications and Mechanism of Action. Pharmaceuticals 2025, 18, 138. [Google Scholar] [CrossRef]
- Liu, H.M.; Cheng, M.Y.; Xun, M.H.; Zhao, Z.W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef] [PubMed]
- Holub, A.; Mousa, S.; Abdolahi, A.; Godugu, K.; Tu, X.M.; Brenna, J.T.; Block, R.C. The effects of aspirin and N-3 fatty acids on telomerase activity in adults with diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Kim, J.; Field, K.; Reid, C.; Chatzistamou, I.; Shim, M. Aspirin ameliorates the long-term adverse effects of doxorubicin through suppression of cellular senescence. FASEB Bioadv. 2019, 1, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.L.; Lawrence, I.; Hoffman, M.; Elgindi, D.; Nadhan, K.; Potnis, M.; Jin, A.; Sershon, C.; Binnebose, R.; Lorenzini, A.; et al. Topical rapamycin reduces markers of senescence and aging in human skin: An exploratory, prospective, randomized trial. Geroscience 2019, 41, 861–869. [Google Scholar] [CrossRef]
- Farr, J.N.; Monroe, D.G.; Atkinson, E.J.; Froemming, M.N.; Ruan, M.; LeBrasseur, N.K.; Khosla, S. Characterization of Human Senescent Cell Biomarkers for Clinical Trials. Aging Cell. 2025, 24, e14489. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Do, K.T.; Kim, J.E.; Cleary, J.M.; Parikh, A.R.; Yeku, O.O.; Xiong, N.; Weekes, C.D.; Veneris, J.; Ahronian, L.G.; et al. Phase I/II Study of Combined BCL-XL and MEK Inhibition with Navitoclax and Trametinib in KRAS or NRAS Mutant Advanced Solid Tumors. Clin. Cancer Res. 2024, 30, 1739–1749. [Google Scholar] [CrossRef]
- Pullarkat, V.A.; Lacayo, N.J.; Jabbour, E.; Rubnitz, J.E.; Bajel, A.; Laetsch, T.W.; Leonard, J.; Colace, S.I.; Khaw, S.L.; Fleming, S.A.; et al. Venetoclax and Navitoclax in Combination with Chemotherapy in Patients with Relapsed or Refractory Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma. Cancer Discov. 2021, 11, 1440–1453. [Google Scholar] [CrossRef]
- Liu, B.; Peng, Z.; Zhang, H.; Zhang, N.; Liu, Z.; Xia, Z.; Huang, S.; Luo, P.; Cheng, Q. Regulation of cellular senescence in tumor progression and therapeutic targeting: Mechanisms and pathways. Mol. Cancer 2025, 24, 106. [Google Scholar] [CrossRef]
| Organs and Tissues | SASP Factors | Other Senescent Markers |
|---|---|---|
| Adipose tissue | IL-6, TNF-α, IL-1β, IL-1α, MCP-1, IL-8, MMP 3, 12 | p16, p21, p53, γH2AX, SA-β-gal, LMNB1 |
| Skin | IL-6, TNF-α, IL-1β, IL-1α, TGF-β, MCP-1, IL-8, MMP 1, 3, 9 | p16, p21, γH2AX, SA-β-gal, lipofuscin, LMNB1, telomere length |
| Cardiovascular system | IL-6, TNF-α, IL-1β, IL-1α, TGF-β, MCP-1, MMP 3, 9, 12 | p16, p21, p53, γH2AX, SA-β-gal, telomere length |
| Bone marrow | IL-6, TNF-α, IL-1β, IL-1α, TGF-β, MCP-1, MMP 9, 12, ICAM-1, IL-17a, IFN-γ, VEGF | p16, p21, p53, SA-β-gal |
| Central nervous system | IL-6, TNF-α, IL-1β, IL-1α, TGF-β, MCP-1, IL-8, MMP 3, 12, TIMP | p16, p21, p53, γH2AX, SA-β-gal, LMNB1, BCL-2 |
| Kidneys | IL-6, TNF-α, IL-1β, TGF-β, MCP-1, MMP 1, 12 | p16, p21, γH2AX, SA-β-gal, telomere length |
| Liver | IL-6, TNF-α, IL-1α, TGF-β, MCP-1, MMP 1, 3 | p16, p21, p53, γH2AX, SA-β-gal |
| Lungs | IL-6, TNF-α, IL-1β, IL-1α, IL-8, TGF-β, MMP 12, VEGF | p16, p21, p53, γH2AX, SA-β-gal |
| Pancreas | IL-6, TNF-α, IL-1β, IL-1α, TGF-β, ICAM-1 | p16, p21, p53, γH2AX, SA-β-gal |
| Ovary | IL-6, IL-1β, IL-1α, TGF-β, MCP-1, IL-8, TIMP | p16, p21, γH2AX, SA-β-gal, lipofuscin, BCL-2 |
| Bone tissue | IL-6, TNF-α, IL-1β, IL-1α, TGF-β, MCP-1, IL-8, MMP 1, 3, 9, 12, ICAM-1, IL-17a, IFN-γ, VEGF, TIMP | p16, p21, p53, LMNB1, BCL-2 |
| Senolytic Molecule | Molecular Targets | Effects |
|---|---|---|
| Dasatinib | Primary target: SCAP inhibition (tyrosine kinases, ephrin receptors) [65,86] | ↓ SA-β-gal+ cells in models of induced senescence of BM-MSC, adipocyte progenitors, human endothelial cells, human gingival keratinocytes, in skeletal myocytes of old C57BL/6 mice, and in ovarian cells of doxorubicin-treated C57BL/6 mice [67,68,69,70]. ↓ expression of cell cycle inhibitors p16 and p21 in jejunum epithelial cell of old C57BL/6 mice and in ovarian cells of doxorubicin-treated C57BL/6 mice [70,71]. |
| Quercetin | SCAP inhibition (PI3K/AKT, BCL-2/BCL-xL, MDM2, TP53/P21) [66,86] MAPK pathway inhibition [87] Cyclooxygenase inhibition [87] Nrf2/HO1 activation [88] SIRT1 activation [89] | |
| Fisetin | NF-κB and PTEN-PKCδ-NOX1 pathway downregulation [79] Nrf2 pathways activation [90] MAPK pathway inhibition [91] PI3K/AKT pathway activation [91] BCL-2 protein family inhibition [92] SIRT1 activation [89] | ↓ expression of cell cycle inhibitors p16 and p21 in ovarian cells of doxorubicin-treated C57BL/6 mice [70]. ↓ SA-β-gal+ cells in murine and human fibroblasts, astrocytes, microglial cells in old sheep [76,77,78]. |
| Navitoclax | BCL-2 protein family inhibition [81] | ↓ number of senescent bone marrow hematopoietic stem cells and myoblasts in mice; in HUVECs, IMR-90 and MEF cell lines; in UV-irradiated senescent melanocytes; in brain endothelial cells in a model of accelerated aging in mice [81,82,83,84]. |
| Senomorphic Molecule | Molecular Targets | Effects |
|---|---|---|
| Rapamycin | Primary target: mTOR pathway [103] | ↑ lifespan in mice; ↓ cataract development, ↓ age-related muscle loss, ↑ periodontal bone regeneration [103,104,105]. |
| Quercetin | NF-κB, JNK, ERK, JAK-STAT, mTOR pathway downregulation [111] | ↓ SASP and SA-β-gal activity [67]. |
| Metformin | AMPK-dependent pathways, NF-κB, JAK-STAT, mTOR pathway downregulation [107] | ↑ lifespan of C. elegans and mice [108]; restoration of tissue metabolism and improvement of clinical parameters in patients with age-associated disorders including diabetes mellitus, cardiovascular diseases, neurodegenerative diseases, degenerative musculoskeletal diseases, obesity [108]; ↓ senescence biomarkers in monkeys, neuroprotective effect, tendency to rejuvenation of multidimensional aging clock [109]. |
| Resveratrol | SIRT1 activation [110] NF-κB pathway inhibition [110] | ↓ SASP and ROS production [110]. |
| Simvastatin | HMG-CoA reductase inhibition [30] | ↓ SASP and ROS production, ↑ mitochondrial respiration in aging cells [30]. |
| Geroprotective Agents | Clinical Trial | Age-Associated Conditions | Study Results |
|---|---|---|---|
| Rapamycin | NCT03103893 | Dermal thickness and senescence | Clinical improvement in skin appearance, improvement in histological appearance of skin tissue, histological markers of aging, increase in collagen VII [114] (phase II) |
| NCT05414292 | Muscle mass during physical training in healthy individuals aged 50–90 years | N/A (recruiting healthy male volunteers) | |
| NCT04200911 | Cognitive functions in early Alzheimer’s disease | N/A (early phase I) | |
| Dasatinib + Quercetin | NCT02848131 | Chronic kidney disease | Reduction in SASP, p16, and p21 expression in patients with diabetic kidney disease in combination with Dasatanib [72] (phase II) |
| Dasatinib + Quercetin + Fisetin | NCT04313634 | Bone resorption/bone formation markers in elderly women | Reduction in bone resorption in postmenopausal women in combination with Quercetin and Fisetin [75,115] (phase II) |
| Fisetin | NCT04210986 | Osteoarthritis-related articular cartilage degeneration | N/A (phase II) |
| NCT03325322 | Chronic kidney disease | N/A (phase I) | |
| Navitoclax | NCT02079740 | Advanced or metastatic solid tumors | MAPK pathway inhibition, reductions in KRAS/NRAS mutation levels [116] (phase II) |
| NCT03181126 | Relapsed/refractory acute lymphoblastic leukemia or relapsed/refractory lymphoblastic lymphoma | Complete remission (60% patients) [117] | |
| NCT06156774 | Sarcopenia and simplified geriatric assessment in lymphoma patients | N/A (observational study) | |
| CAR-T cell therapy | NCT04300998 | Older patients with hematologic malignancies | N/A (observational study) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirichenko, T.V.; Markina, Y.V.; Markin, A.M.; Vasilyev, V.S.; Hua, H.; Li, D.; Woo, A.Y.-H.; Deev, R.V.; Eremin, I.I.; Kotenko, K.V. Functional Features of Senescent Cells and Implications for Therapy. Int. J. Mol. Sci. 2025, 26, 5390. https://doi.org/10.3390/ijms26115390
Kirichenko TV, Markina YV, Markin AM, Vasilyev VS, Hua H, Li D, Woo AY-H, Deev RV, Eremin II, Kotenko KV. Functional Features of Senescent Cells and Implications for Therapy. International Journal of Molecular Sciences. 2025; 26(11):5390. https://doi.org/10.3390/ijms26115390
Chicago/Turabian StyleKirichenko, Tatiana V., Yuliya V. Markina, Alexander M. Markin, Vyacheslav S. Vasilyev, Huiming Hua, Dahong Li, Anthony Yiu-Ho Woo, Roman V. Deev, Ilya I. Eremin, and Konstantin V. Kotenko. 2025. "Functional Features of Senescent Cells and Implications for Therapy" International Journal of Molecular Sciences 26, no. 11: 5390. https://doi.org/10.3390/ijms26115390
APA StyleKirichenko, T. V., Markina, Y. V., Markin, A. M., Vasilyev, V. S., Hua, H., Li, D., Woo, A. Y.-H., Deev, R. V., Eremin, I. I., & Kotenko, K. V. (2025). Functional Features of Senescent Cells and Implications for Therapy. International Journal of Molecular Sciences, 26(11), 5390. https://doi.org/10.3390/ijms26115390





