Scaffold Hopping from Dehydrozingerone: Design, Synthesis, and Antifungal Activity of Phenoxyltrifluoromethylpyridines
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. In Vitro and In Vivo Antifungal Activity
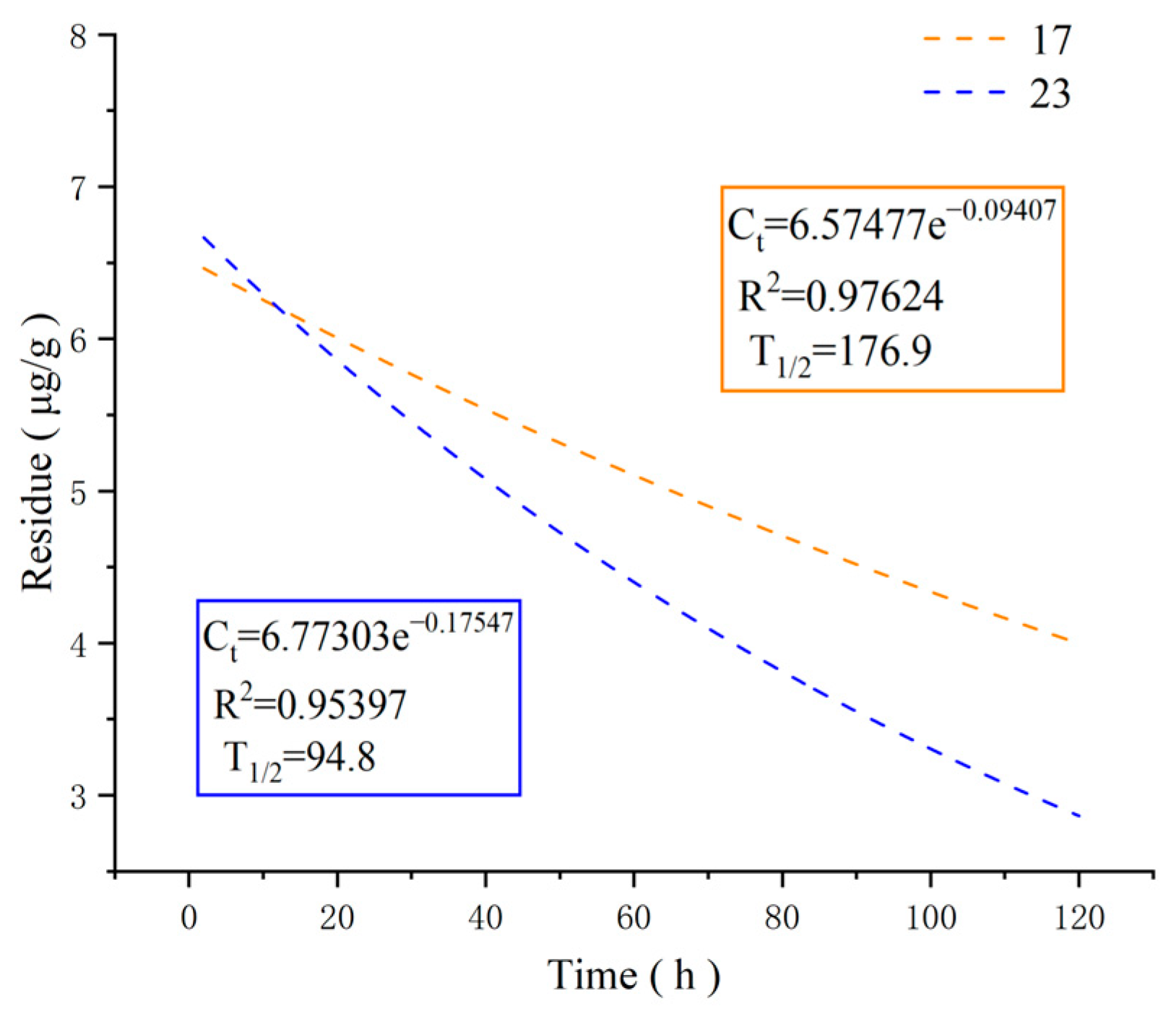
2.3. Degradation of Compounds 17 and 23 in Bananas
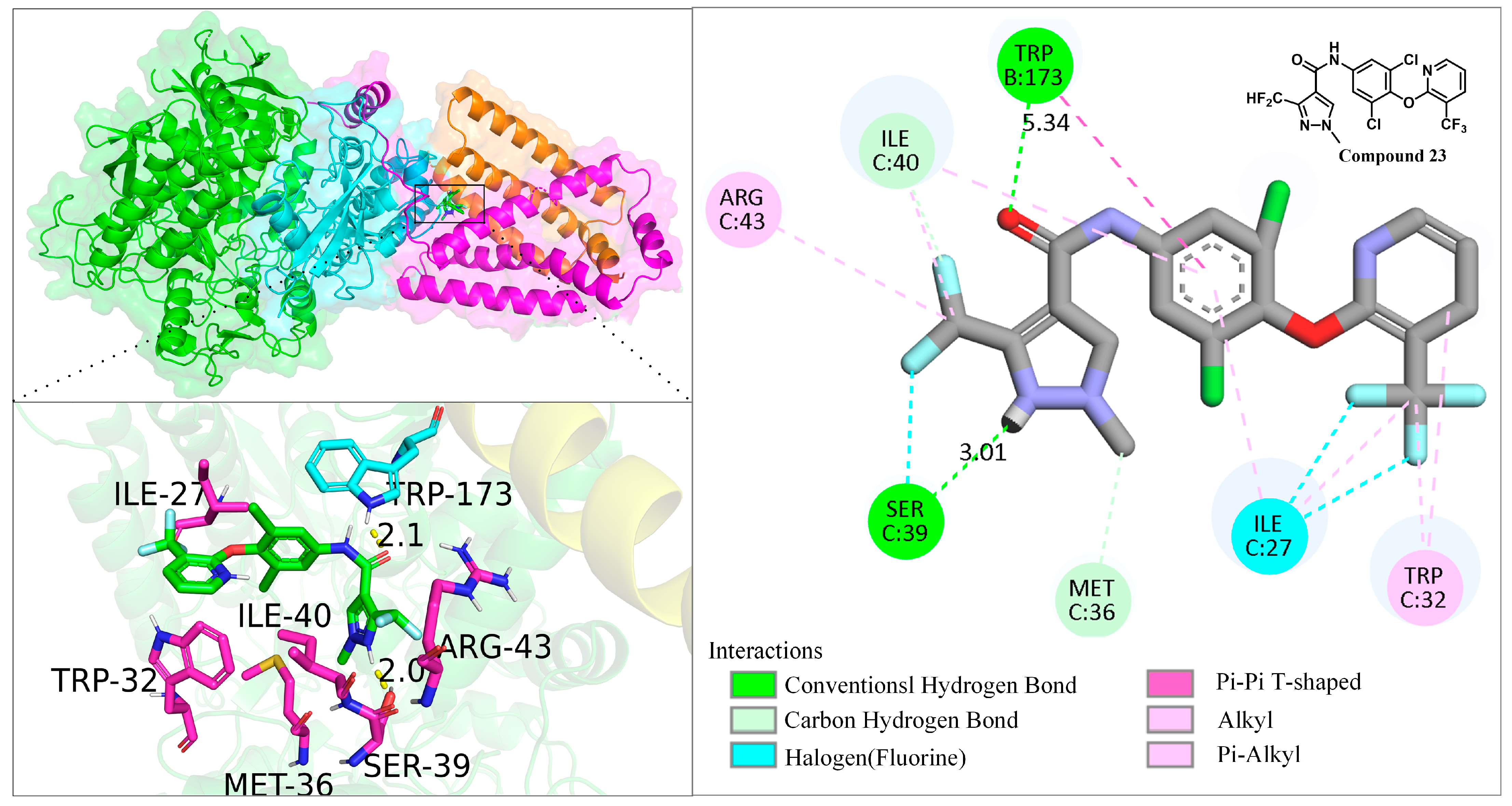
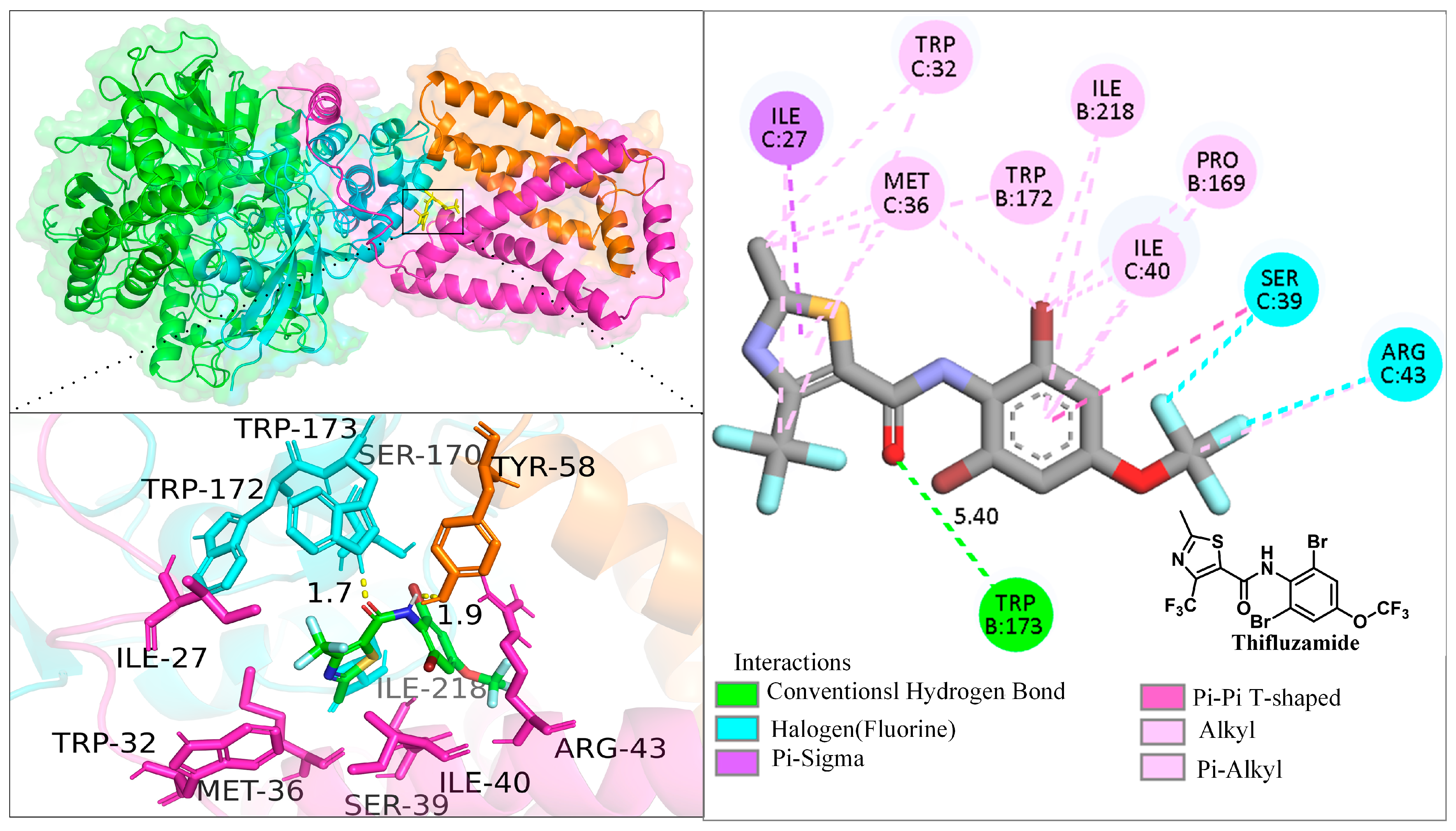
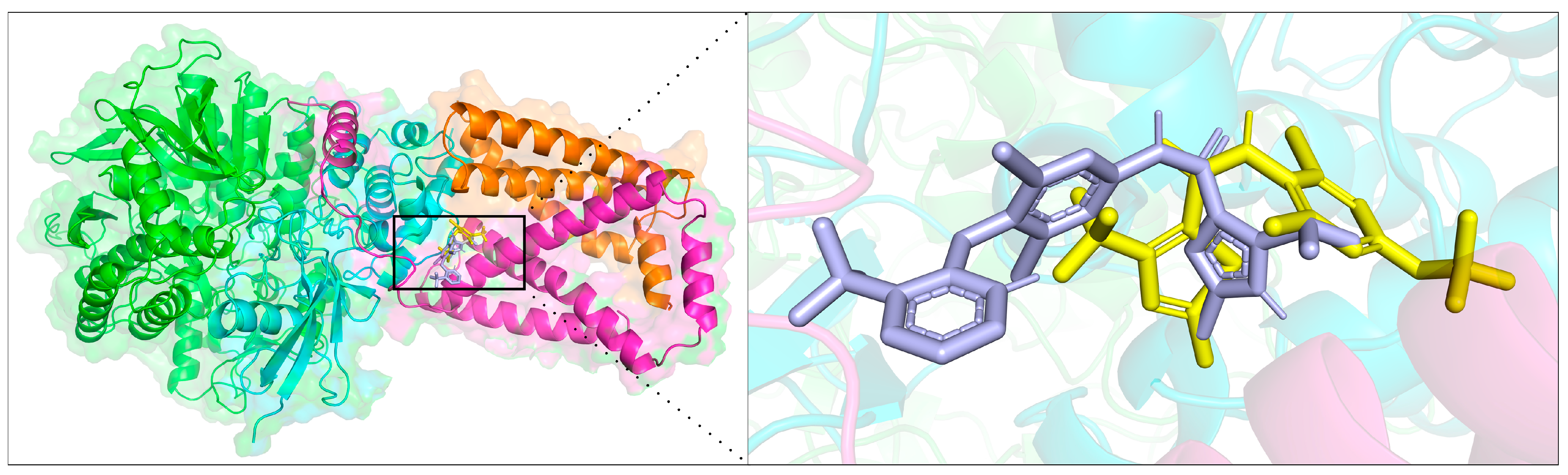
2.4. Molecular Docking
3. Materials and Methods
3.1. General
3.2. Synthesis
3.3. Biological Assays
3.4. Degradation of Compounds 17 and 23 in Bananas [36]
3.5. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stukenbrock, E.; Gurr, S. Address the Growing Urgency of Fungal Disease in Crops. Nature 2023, 617, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Zhang, Y.; Lu, P.; Wu, J.; Li, Q.X.; Song, B. Status and Perspective on Green Pesticide Utilizations and Food Security. Annu. Rev. Food Sci. Technol. 2024, 15, 473–493. [Google Scholar] [CrossRef]
- Fones, H.N.; Bebber, D.P.; Chaloner, T.M.; Kay, W.T.; Steinberg, G.; Gurr, S.J. Threats to Global Food Security from Emerging Fungal and Oomycete Crop Pathogens. Nat. Food 2020, 1, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide Emergence of Resistance to Antifungal Drugs Challenges Human Health and Food Security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef]
- Case, N.T.; Gurr, S.J.; Fisher, M.C.; Blehert, D.S.; Boone, C.; Casadevall, A.; Chowdhary, A.; Cuomo, C.A.; Currie, C.R.; Denning, D.W.; et al. Fungal Impacts on Earth’s Ecosystems. Nature 2025, 638, 49–57. [Google Scholar] [CrossRef]
- Sparks, T.C.; Sparks, J.M.; Duke, S.O. Natural Product-Based Crop Protection Compounds─Origins and Future Prospects. J. Agric. Food Chem. 2023, 71, 2259–2269. [Google Scholar] [CrossRef]
- Späth, G.; Loiseleur, O. Chemical Case Studies from Natural Products of Recent Interest in the Crop Protection Industry. Nat. Prod. Rep. 2024, 41, 1915–1938. [Google Scholar] [CrossRef]
- De Bernardi, M.; Vidari, G.; Vita-Finzi, P. Dehydrozingerone from Aframomum Giganteum. Phytochemistry 1976, 15, 1785–1786. [Google Scholar] [CrossRef]
- Hampannavar, G.A.; Karpoormath, R.; Palkar, M.B.; Shaikh, M.S. An Appraisal on Recent Medicinal Perspective of Curcumin Degradant: Dehydrozingerone (DZG). Bioorganic Med. Chem. 2016, 24, 501–520. [Google Scholar] [CrossRef]
- Kumar, V.; Bala, R.; Dhawan, S.; Singh, P.; Karpoormath, R. The Multi-Biological Targeted Role of Dehydrozingerone and Its Analogues. ChemistrySelect 2022, 7, e202201938. [Google Scholar] [CrossRef]
- Agarwal, M.; Walia, S.; Dhingra, S.; Khambay, B.P.S. Insect Growth Inhibition, Antifeedant and Antifungal Activity of Compounds Isolated/Derived from Zingiber officinale Roscoe (Ginger) Rhizomes. Pest Manag. Sci. 2001, 57, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, B.L.; Park, S.J.; Royer, J.E.; Jamie, J.F.; Taylor, P.W.; Jamie, I.M. Systematic Modification of Zingerone Reveals Structural Requirements for Attraction of Jarvis’s Fruit Fly. Sci. Rep. 2019, 9, 19332. [Google Scholar] [CrossRef]
- Jung, K.-S.; Lee, Y.; Ganbat, D.; Park, S.J.; Lee, S.-E. Antifungal and Antimycotoxigenic Activities of a Synthetic Zingerone-Derivative 4-(4-Hydroxy-3-Nitrophenyl)-2-Butanone against Aspergillus flavus and Fusarium graminearum. Appl. Food Res. 2025, 5, 100664. [Google Scholar] [CrossRef]
- Song, X.; Zhu, X.; Li, T.; Liang, C.; Zhang, M.; Shao, Y.; Tao, J.; Sun, R. Dehydrozingerone Inspired Discovery of Potential Broad-Spectrum Fungicidal Agents as Ergosterol Biosynthesis Inhibitors. J. Agric. Food Chem. 2019, 67, 11354–11363. [Google Scholar] [CrossRef]
- Zhang, M.; Dou, M.; Xia, Y.; Hu, Z.; Zhang, B.; Bai, Y.; Xie, J.; Liu, Q.; Xie, C.; Lu, D.; et al. Photostable 1-Trifluoromethyl Cinnamyl Alcohol Derivatives Designed as Potential Fungicides and Bactericides. J. Agric. Food Chem. 2021, 69, 5435–5445. [Google Scholar] [CrossRef]
- Burriss, A.; Edmunds, A.J.F.; Emery, D.; Hall, R.G.; Jacob, O.; Schaetzer, J. The Importance of Trifluoromethyl Pyridines in Crop Protection. Pest Manag. Sci. 2018, 74, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Dai, A.; Jin, Z.; Chi, Y.R.; Wu, J. Trifluoromethylpyridine: An Important Active Fragment for the Discovery of New Pesticides. J. Agric. Food Chem. 2022, 70, 11019–11030. [Google Scholar] [CrossRef]
- Tsukamoto, M.; Nakamura, T.; Kimura, H.; Nakayama, H. Synthesis and Application of Trifluoromethylpyridines as a Key Structural Motif in Active Agrochemical and Pharmaceutical Ingredients. J. Pestic. Sci. 2021, 46, 125–142. [Google Scholar] [CrossRef]
- Luo, B.; Ning, Y. Comprehensive Overview of Carboxamide Derivatives as Succinate Dehydrogenase Inhibitors. J. Agric. Food Chem. 2022, 70, 957–975. [Google Scholar] [CrossRef]
- Bao, A.-L.; Xie, X.-S.; Wang, D.-Y.; Deng, Z.-Q.; Chen, Y.; Liu, D.; Li, W.-Y.; Tang, X.-R.; Cheng, W.; Yan, Y.-K. Design, Synthesis and Antifungal Activity of Novel Pyrazole-Amide-Isothiazole Derivatives as Succinate Dehydrogenase Inhibitors. Food Chem. 2025, 464, 141465. [Google Scholar] [CrossRef]
- Walter, H. Pyrazole Carboxamide Fungicides Inhibiting Succinate Dehydrogenase. In Bioactive Heterocyclic Compound Classes; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 175–193. [Google Scholar] [CrossRef]
- Huang, Y.H.; Wei, G.; Wang, W.J.; Liu, Z.; Yin, M.X.; Guo, W.M.; Zhu, X.L.; Yang, G.F. Structure-Based Discovery of New Succinate Dehydrogenase Inhibitors Via Scaffold Hopping Strategy. J. Agric. Food Chem. 2023, 71, 18292–18300. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, T.; An, X.; Ma, H.; Wang, M. Design, Synthesis, Antifungal Activity and Molecular Docking of Novel Pyrazole-4-Carboxamides Containing Tertiary Alcohol and Difluoromethyl Moiety as Potential Succinate Dehydrogenase Inhibitors. Pest Manag. Sci. 2024, 80, 2032–2041. [Google Scholar] [CrossRef]
- Sun, N.-B.; Min, L.-J.; Sun, X.-P.; Zhai, Z.-W.; Bajsa-Hirschel, J.; Wei, Z.-C.; Hua, X.-W.; Cantrell, C.L.; Xu, H.; Duke, S.O.; et al. Novel Pyrazole Acyl(thio)urea Derivatives Containing a Biphenyl Scaffold as Potential Succinate Dehydrogenase Inhibitors: Design, Synthesis, Fungicidal Activity and SAR. J. Agric. Food Chem. 2024, 72, 2512–2525. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, A.; Qiu, L.; Chen, M.; Lu, A.; Li, G.; Yang, C.; Xue, W. Expedient Discovery for Novel Antifungal Leads Targeting Succinate Dehydrogenase: Pyrazole-4-Formylhydrazide Derivatives Bearing a Diphenyl Ether Fragment. J. Agric. Food Chem. 2020, 68, 14426–14437. [Google Scholar] [CrossRef]
- Sauter, H.; Steglich, W.; Anke, T. Strobilurins: Evolution of a New Class of Active Substances. Angew. Chem. Int. Ed. 1999, 38, 1328–1349. [Google Scholar] [CrossRef]
- Lamberth, C. Ring Closure and Ring Opening as Useful Scaffold Hopping Tools in Agrochemistry. J. Agric. Food Chem. 2023, 71, 18133–18140. [Google Scholar] [CrossRef]
- Maienfisch, P.; Lamberth, C. Introduction to Recent Highlights in Bioisosteric Replacements and Scaffold Hopping in Crop Protection Research. J. Agric. Food Chem. 2023, 71, 18169–18170. [Google Scholar] [CrossRef]
- Kwan, E.E.; Zeng, Y.; Besser, H.A.; Jacobsen, E.N. Concerted Nucleophilic Aromatic Substitutions. Nat. Chem. 2018, 10, 917–923. [Google Scholar] [CrossRef]
- Frederique, B.; Dragos, H. Molecular Similarity and Property Similarity. Curr. Top. Med. Chem. 2004, 4, 589–600. [Google Scholar] [CrossRef]
- López-Pérez, K.; Avellaneda-Tamayo, J.F.; Chen, L.; López-López, E.; Juárez-Mercado, K.E.; Medina-Franco, J.L.; Miranda-Quintana, R.A. Molecular Similarity: Theory, Applications, and Perspectives. Artif. Intell. Chem. 2024, 2, 100077. [Google Scholar] [CrossRef]
- Gijsen, H.J.; Berthelot, D.; Zaja, M.; Brône, B.; Geuens, I.; Mercken, M. Analogues of Morphanthridine and the Tear Gas Dibenz[b,f][1,4]Oxazepine (CR) as Extremely Potent Activators of the Human Transient Receptor Potential Ankyrin 1 (TRPA1) Channel. J. Med. Chem. 2010, 53, 7011–7020. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, B.; Zheng, S.; Zhao, K.; Wang, K.; Sun, R. Design, Synthesis, and Nematocidal Evaluation of Waltherione a Derivatives: Leveraging a Structural Simplification Strategy. Int. J. Mol. Sci. 2024, 25, 9209. [Google Scholar] [CrossRef]
- Li, J.-L.; Liu, X.-Y.; Xie, J.-T.; Di, Y.-L.; Zhu, F.-X. A Comparison of Different Estimation Methods for Fungicide EC50 and EC95 Values. J. Phytopathol. 2015, 163, 239–244. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, X.; Zhou, Y.; Li, Q.; Hu, Z.; Li, T.; Tao, J.; Dou, M.; Zhang, M.; Shao, Y.; et al. Discovery of N-Aryl-Pyridine-4-Ones as Novel Potential Agrochemical Fungicides and Bactericides. J. Agric. Food Chem. 2019, 67, 13904–13913. [Google Scholar] [CrossRef]
- Cheng, D.; Lin, S.; Liu, G.; Zhou, Y.; Zhang, Z. Residue and Distribution of Fosthiazate in Cucumber (Cucumis sativus L.) and Dietary Intake Risk Assessment for Various Populations. Int. J. Environ. Anal. Chem. 2021, 103, 6804–6815. [Google Scholar] [CrossRef]
- Huang, L.S.; Sun, G.; Cobessi, D.; Wang, A.C.; Shen, J.T.; Tung, E.Y.; Anderson, V.E.; Berry, E.A. 3-Nitropropionic Acid Is a Suicide Inhibitor of Mitochondrial Respiration That, upon Oxidation by Complex II, Forms a Covalent Adduct with a Catalytic Base Arginine in the Active Site of the Enzyme. J. Biol. Chem. 2006, 281, 5965–5972. [Google Scholar] [CrossRef]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational Protein–Ligand Docking and Virtual Drug Screening with the Autodock Suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef]



| Con. a | Compound | Inhibition Rate ± SD (%) | |||||
|---|---|---|---|---|---|---|---|
| R. s b | P. o | C. m | F. g | B. c | C. s | ||
| 50 | 1 | 93.5 ± 0.7 | 79.1 ± 2.4 | 86.1 ± 0.7 | 76.8 ± 1.5 | 90.7 ± 1.8 | 55.7 ± 3.5 |
| 50 | 2 | 67.3 ± 2.4 | 28.6 ± 3.1 | 47.1 ± 4.1 | 10.7 ± 7.7 | 33.2 ± 3.5 | 23.8 ± 0.7 |
| 50 | 3 | 86.2 ± 1.1 | 33.6 ± 3.7 | 68.4 ± 7.9 | 58.4 ± 10.6 | 53.1 ± 14.2 | 42.9 ± 2.1 |
| 50 | 4 | 79.1 ± 0.0 | 59.7 ± 1.4 | 81.8 ± 0.6 | 39.1 ± 1.2 | 15.6 ± 2.1 | 46.3 ± 4.1 |
| 50 | 5 | 86.4 ± 0.6 | 48.1 ± 2.0 | 84.9 ± 2.8 | 44.7 ± 3.4 | 48.3 ± 6.7 | 51.4 ± 0.7 |
| 50 | 6 | 74.0 ± 1.0 | 25.7 ± 5.5 | 74.3 ± 2.8 | 28.1 ± 1.4 | 39.6 ± 1.4 | 32.4 ± 2.2 |
| 50 | 7 | 78.8 ± 0.7 | 30.3 ± 1.8 | 72.5 ± 7.0 | 53.7 ± 6.1 | 17.6 ± 4.5 | 37.3 ± 4.2 |
| 50 | 8 | 76.8 ± 1.5 | 21.1 ± 3.3 | 65.4 ± 3.6 | 35.5 ± 3.4 | 37.9 ± 10.2 | 26.4 ± 3.0 |
| 50 | 9 | 91.6 ± 0.0 | 73.8 ± 0.7 | 87.0 ± 0.0 | 67.9 ± 2.5 | 95.8 ± 1.2 | 66.7 ± 0.7 |
| 50 | 10 | 27.2 ± 1.7 | 8.4 ± 4.6 | 13.3 ± 2.3 | 26.1 ± 3.5 | 13.25 ± 1.0 | 6.21 ± 2.1 |
| 50 | 11 | 80.2 ± 1.7 | 27.5 ± 5.2 | 70.7 ± 4.5 | 37.8 ± 23.8 | 43.0 ± 1.5 | 29.4 ± 7.1 |
| 50 | 12 | 86.5 ± 3.7 | 84.2 ± 6.1 | 79.8 ± 6.9 | 78.6 ± 1.5 | 87.3 ± 3.5 | 52.8 ± 3.3 |
| 10 | 13 | 40.1 ± 1.7 | 25.3 ± 0.7 | 6.5 ± 2.4 | 6.3 ± 1.1 | 14.0 ± 4.0 | −0.5 ± 0.7 |
| 10 | 14 | 59.0 ± 1.2 | 33.9 ± 3.5 | 10.2 ± 0.7 | 15.1 ± 3.4 | 5.8 ± 6.6 | 9.7 ± 1.4 |
| 10 | 15 | 15.0 ± 0.7 | 5.6 ± 2.6 | 13.4 ± 3.5 | −2.2 ± 1.7 | 30.3 ± 0.7 | 4.2 ± 2.7 |
| 10 | 16 | 60.8 ± 1.3 | 57.4 ± 4.6 | 59.6 ± 6.2 | 31.0 ± 1.8 | 49.2 ± 6.8 | 16.2 ± 1.4 |
| 10 | 17 | 76.8 ± 1.1 | 60.3 ± 0.6 | 75.1 ± 0.7 | 63.0 ± 1.3 | 79.6 ± 1.4 | 48.5 ± 1.4 |
| 10 | 18 | 31.3 ± 1.1 | 0.0 ± 0.5 | 20.7 ± 1.5 | 23.0 ± 9.0 | 8.5 ± 1.9 | 12.5 ± 1.5 |
| 10 | 19 | 1.8 ± 3.4 | 11.5 ± 0.0 | 19.7 ± 2.5 | 7.1 ± 1.2 | −9.7 ± 3.3 | 9.8 ± 5.9 |
| 10 | 20 | 8.0 ± 3.5 | 14.7 ± 0.7 | 7.9 ± 4.7 | 5.4 ± 3.5 | −13.5 ± 2.6 | 9.4 ± 2.2 |
| 10 | 21 | 7.8 ± 4.7 | 2.7 ± 1.6 | 13.1 ± 5.0 | 4.8 ± 1.8 | −18.9 ± 10.1 | 5.8 ± 1.4 |
| 10 | 22 | 37.1 ± 2.4 | 25.8 ± 2.1 | 40.3 ± 1.1 | 26.4 ± 2.2 | 14.5 ± 2.2 | 25.0 ± 3.4 |
| 10 | 23 | 70.5 ± 1.3 | 54.1 ± 2.7 | 81.1 ± 3.1 | 58.6 ± 3.1 | 36.8 ± 11.3 | 42.4 ± 2.5 |
| 50 | pyrimethanil | 84.0 ± 1.3 | 65.9 ± 1.1 | 75.2 ± 14.3 | 39.9 ± 2.8 | 100.0 ± 0.0 | 26.1 ± 1.6 |
| 10 | azoxystrobin | 58.4 ± 0.6 | 46.0 ± 1.3 | 54.2 ± 2.1 | 47.6 ± 3.4 | - c | 39.0 ± 0.8 |
| Compound | R s b | P. o | C. m | F. g | B. c | C. s |
|---|---|---|---|---|---|---|
| 1 | 8.08 | 24.06 | 17.27 | 31.70 | 21.96 | - c |
| 16 | 7.28 | 14.98 | 12.08 | 22.14 | 4.17 | 52.19 |
| 17 | 2.88 | 5.96 | 4.32 | 6.23 | 2.99 | 9.09 |
| 23 | 4.24 | 8.92 | 3.20 | 44.40 | 18.43 | 14.33 |
| azoxystrobin | <0.01 | 12.21 | 5.09 | 16.54 | - | 31.20 |
| Compound | Concentration (μg/mL) | Protective Effect | |
|---|---|---|---|
| Disease Index | Control Efficacy (%) | ||
| 17 | 200 | 46.67 ± 1.56 b | 46.24 ± 2.34 b |
| 23 | 200 | 42.22 ± 0.91 b | 48.45 ± 1.46 b |
| azoxystrobin | 200 | 24.44 ± 0.32 c | 70.27 ± 0.67 a |
| CK | - | 82.22 ± 0.54 a | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nan, X.; Wang, K.; Sun, X.; Hu, Z.; Sun, R. Scaffold Hopping from Dehydrozingerone: Design, Synthesis, and Antifungal Activity of Phenoxyltrifluoromethylpyridines. Int. J. Mol. Sci. 2025, 26, 5345. https://doi.org/10.3390/ijms26115345
Nan X, Wang K, Sun X, Hu Z, Sun R. Scaffold Hopping from Dehydrozingerone: Design, Synthesis, and Antifungal Activity of Phenoxyltrifluoromethylpyridines. International Journal of Molecular Sciences. 2025; 26(11):5345. https://doi.org/10.3390/ijms26115345
Chicago/Turabian StyleNan, Xiaohui, Kaifeng Wang, Xinru Sun, Zhan Hu, and Ranfeng Sun. 2025. "Scaffold Hopping from Dehydrozingerone: Design, Synthesis, and Antifungal Activity of Phenoxyltrifluoromethylpyridines" International Journal of Molecular Sciences 26, no. 11: 5345. https://doi.org/10.3390/ijms26115345
APA StyleNan, X., Wang, K., Sun, X., Hu, Z., & Sun, R. (2025). Scaffold Hopping from Dehydrozingerone: Design, Synthesis, and Antifungal Activity of Phenoxyltrifluoromethylpyridines. International Journal of Molecular Sciences, 26(11), 5345. https://doi.org/10.3390/ijms26115345








