Silver Nanoparticles as a Novel Tissue Preservative: A Comparative Study with 10% Neutral Buffered Formalin
Abstract
1. Introduction
2. Results
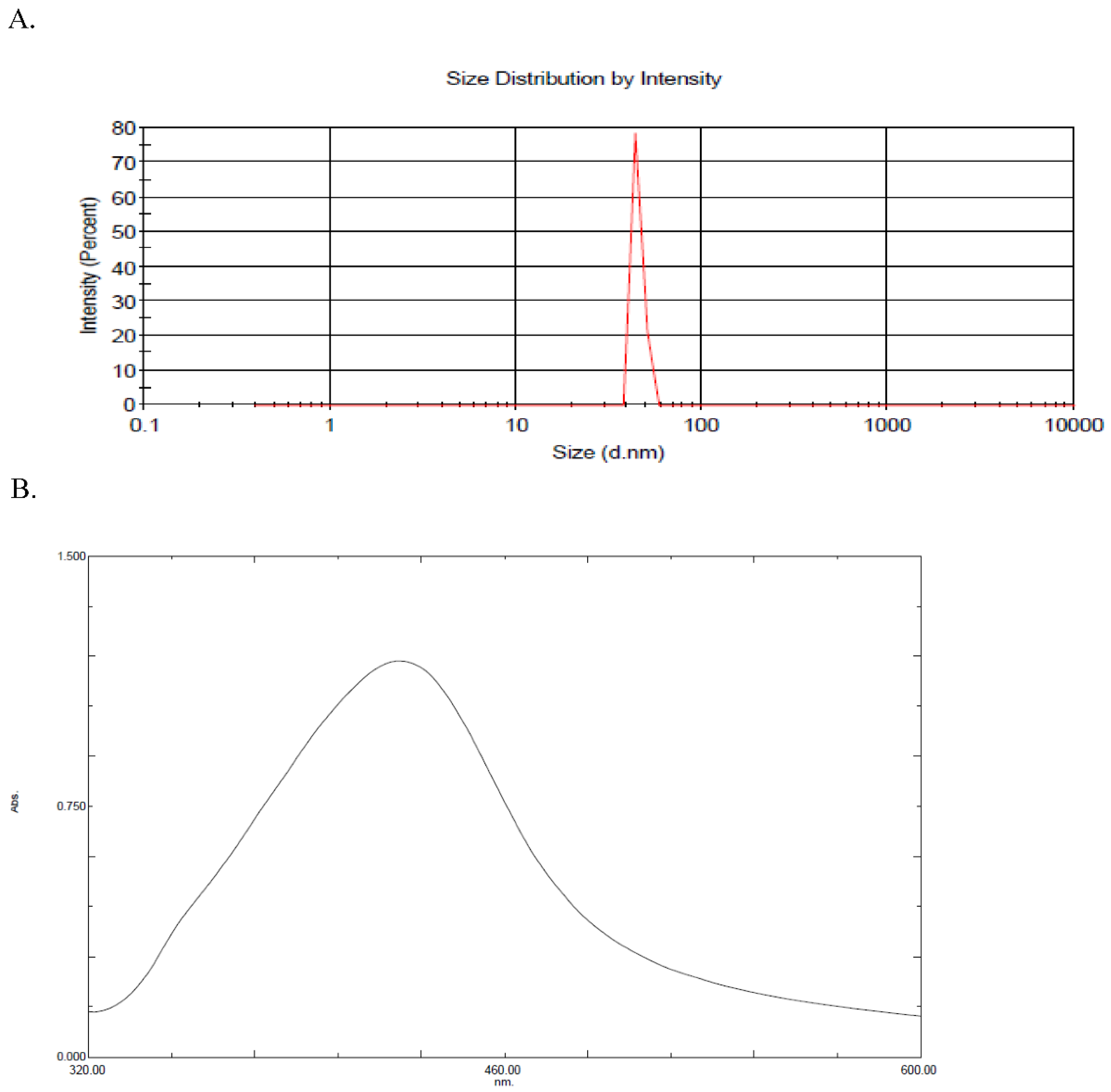
2.1. Silver Synthesis and Characterization
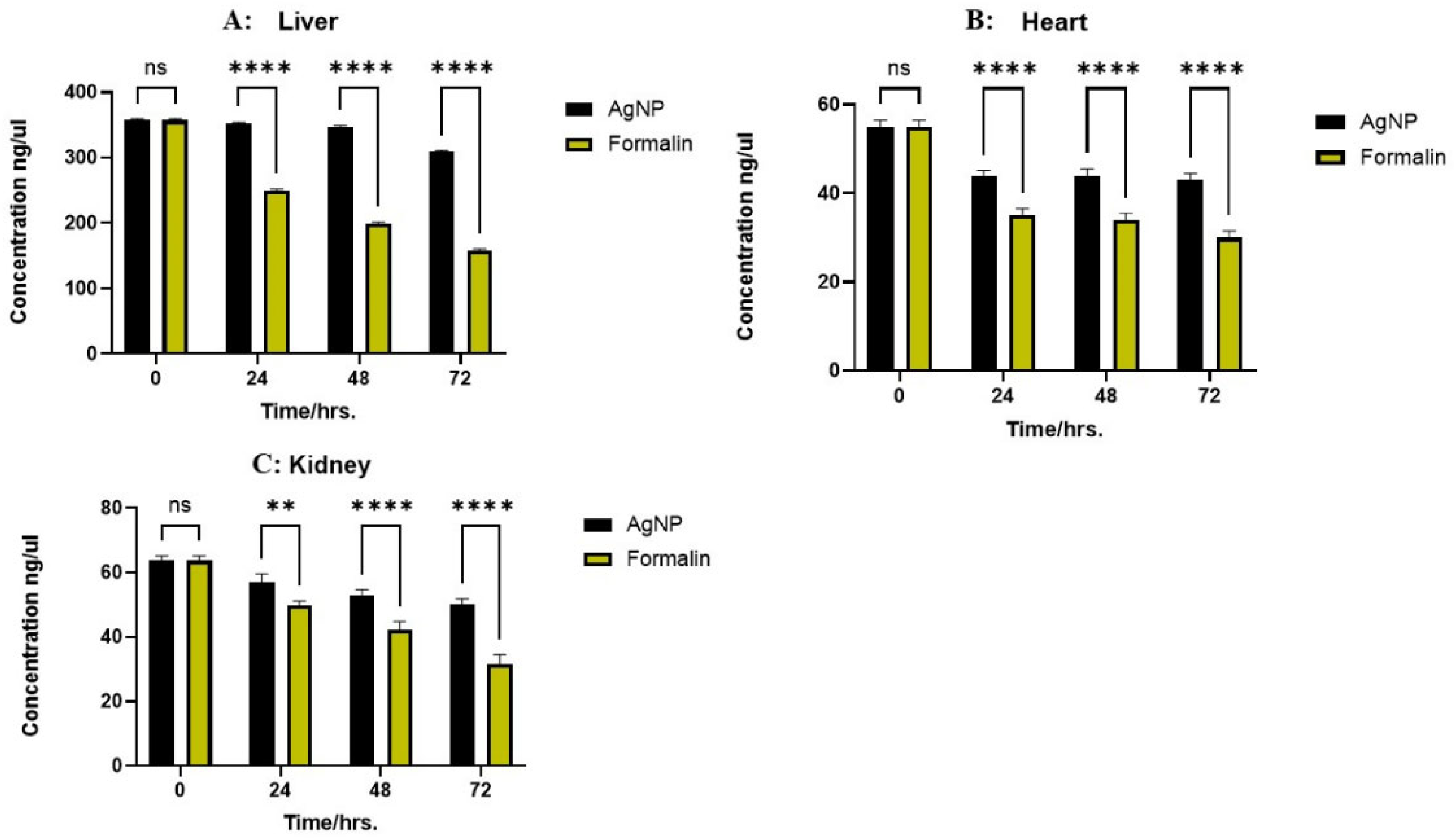
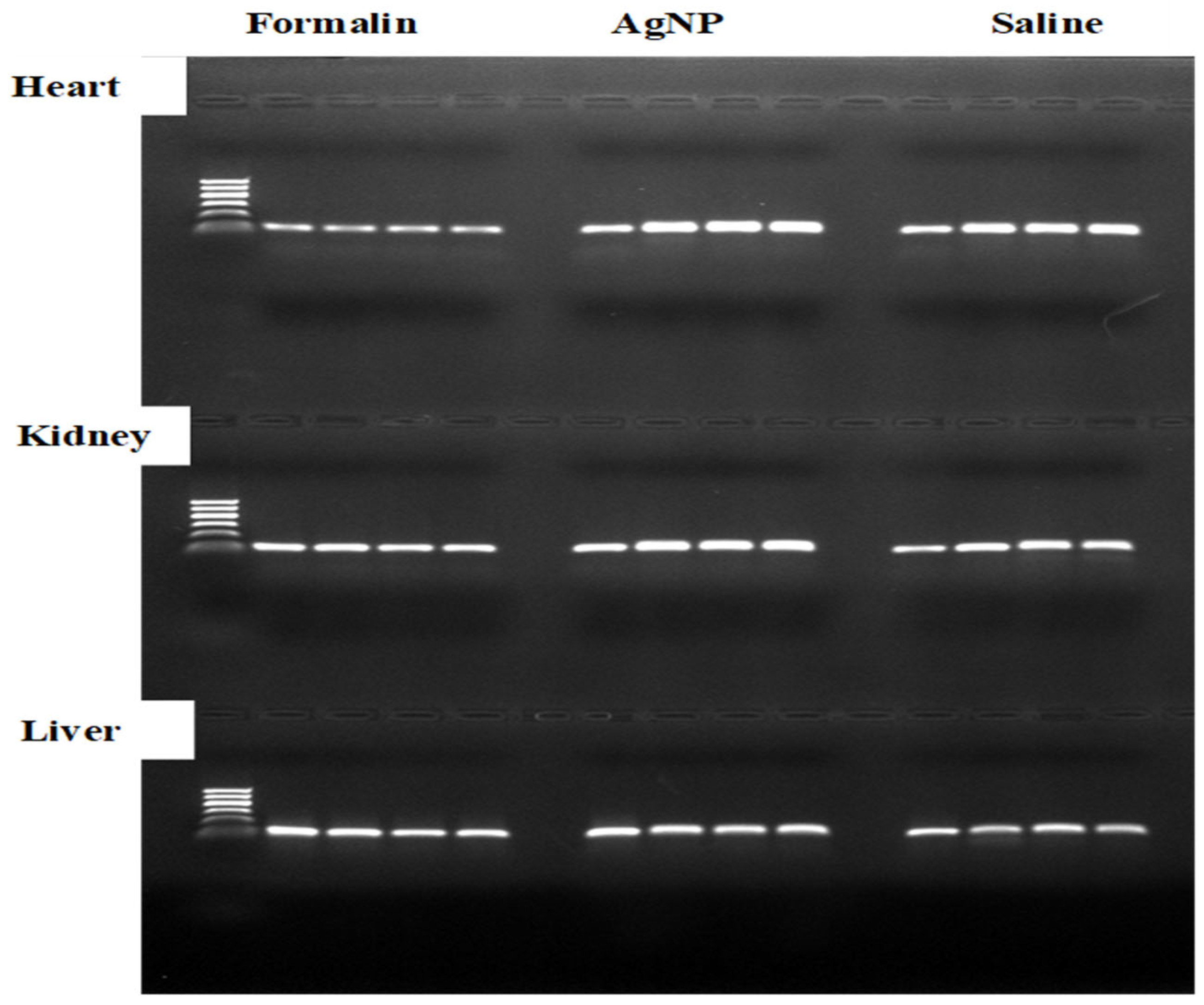
2.2. DNA Concentration and Purity
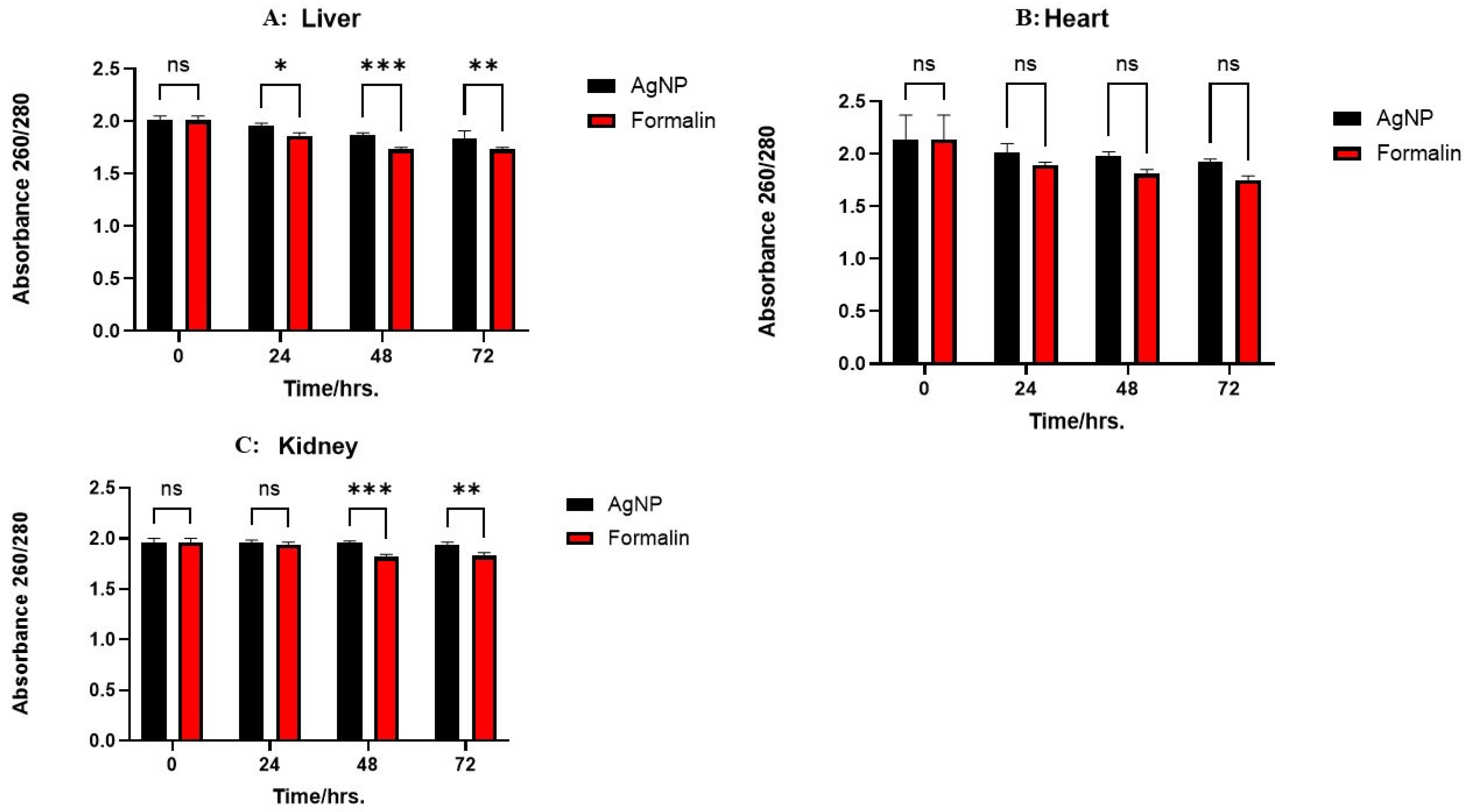
2.3. Effect of Preservation Time on Nucleic Acid and Protein Integrity
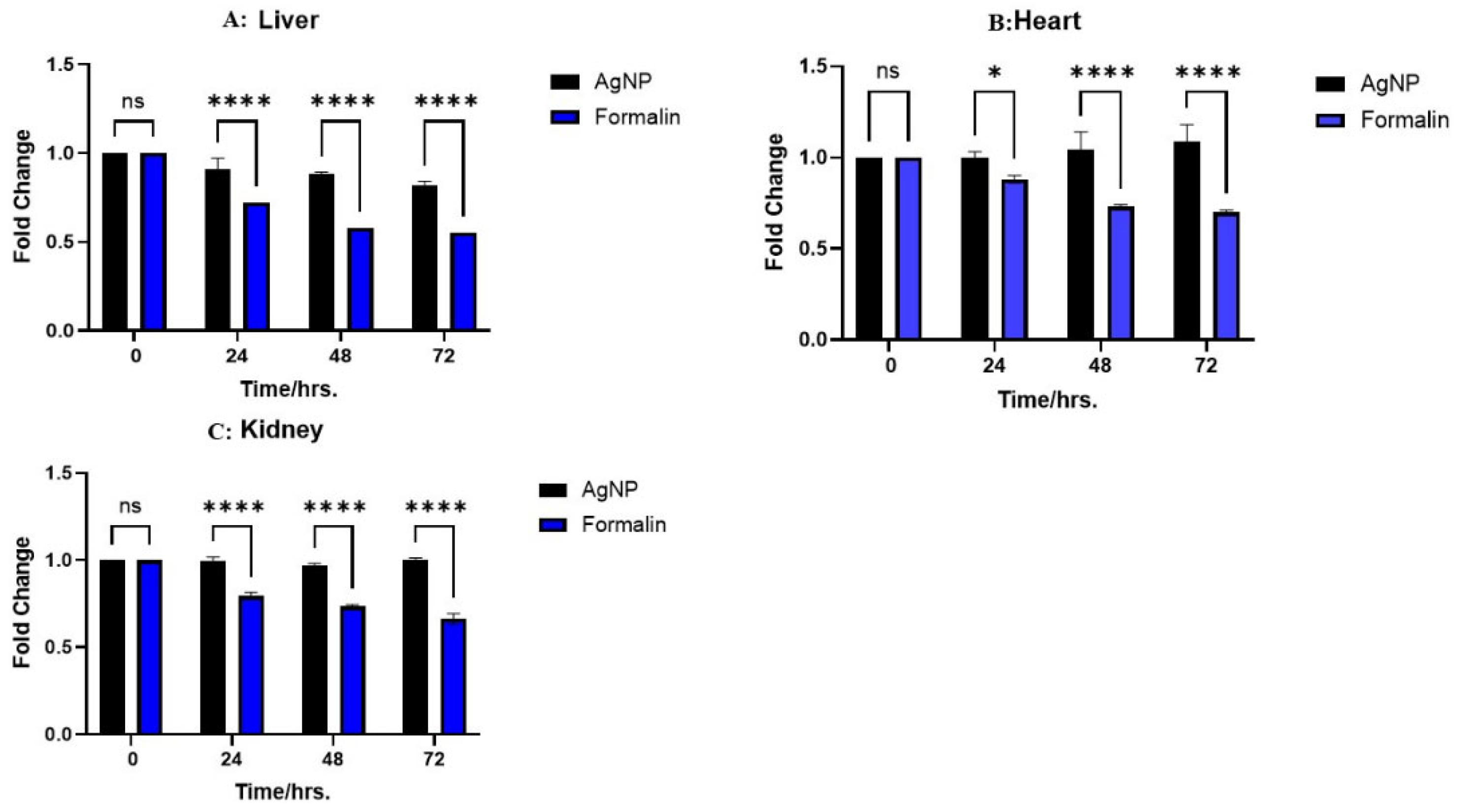
2.4. Preserving RNA Concentration and Quality: Tissue-Specific Responses to Different Treatments
2.5. GAPDH Expression Determination in Multiple Preservatives
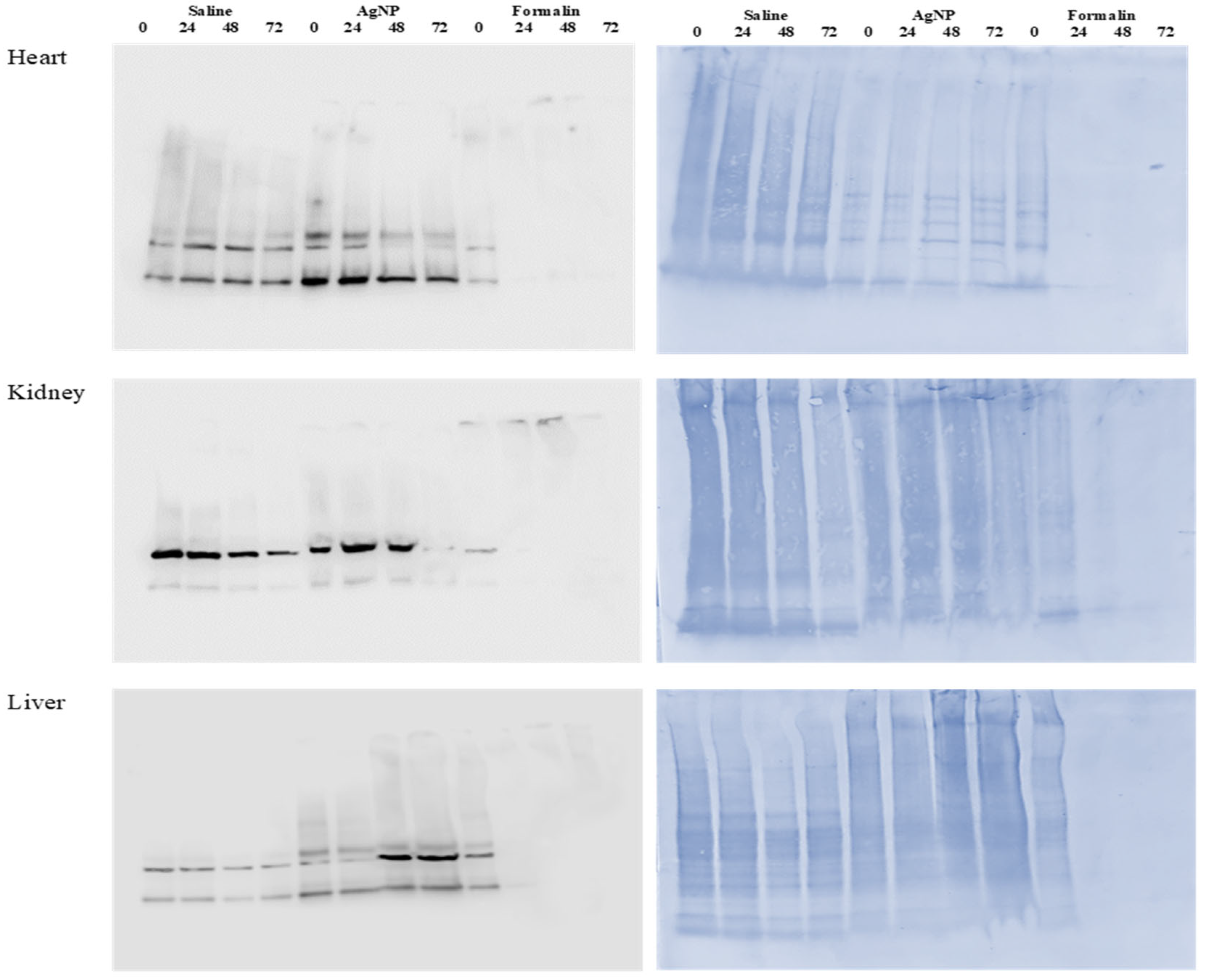
2.6. Protein Extraction and Analysis
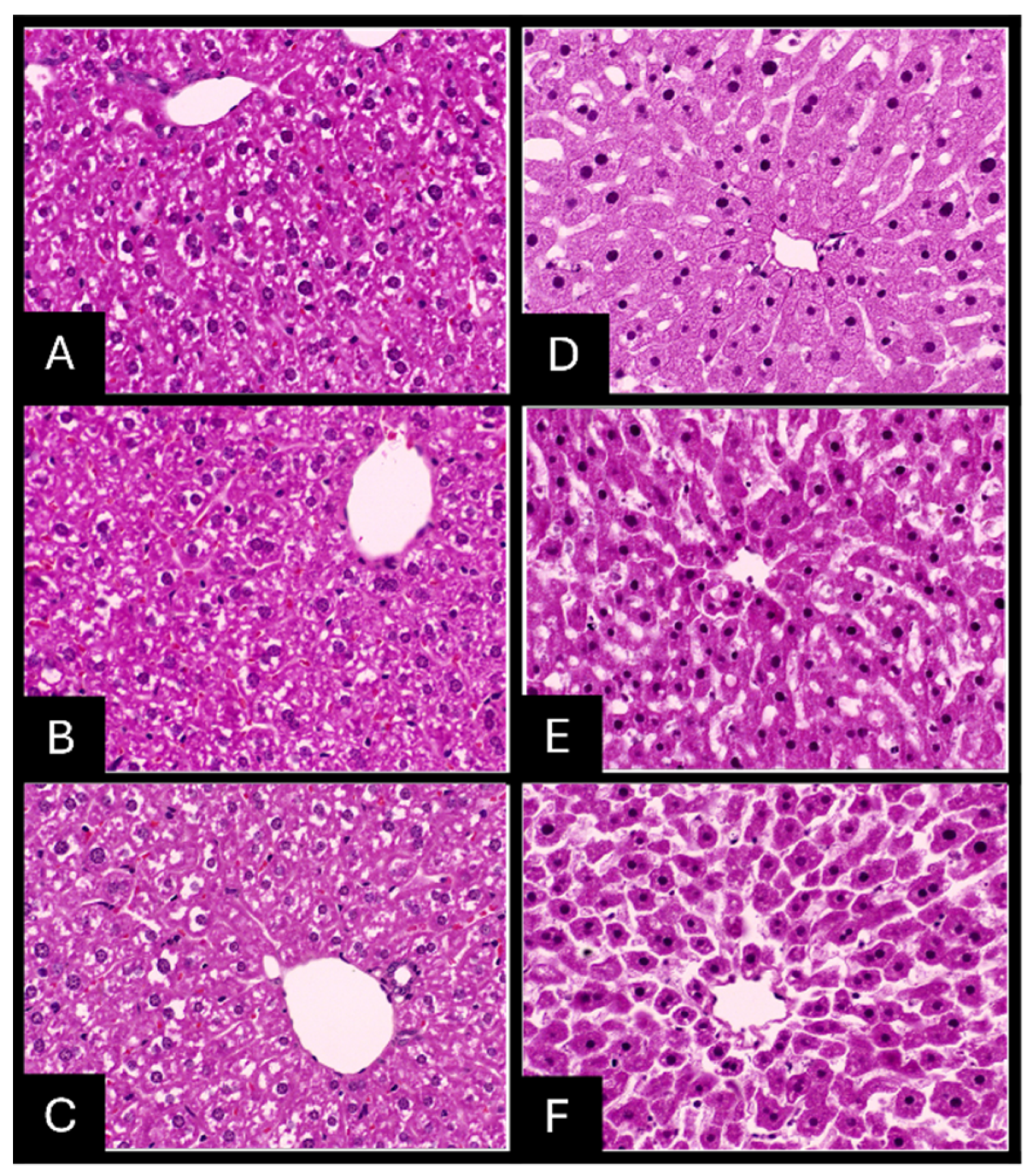
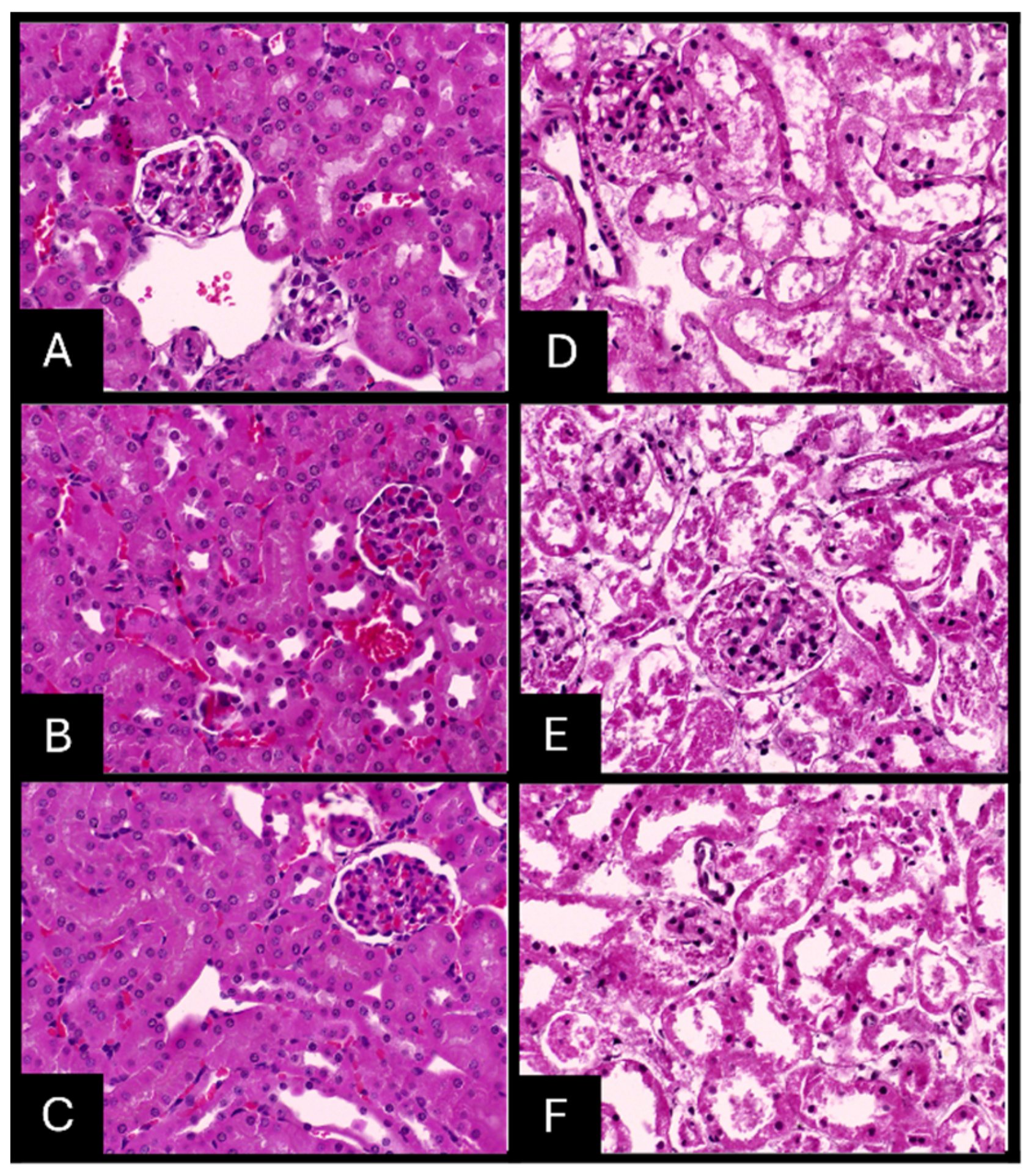
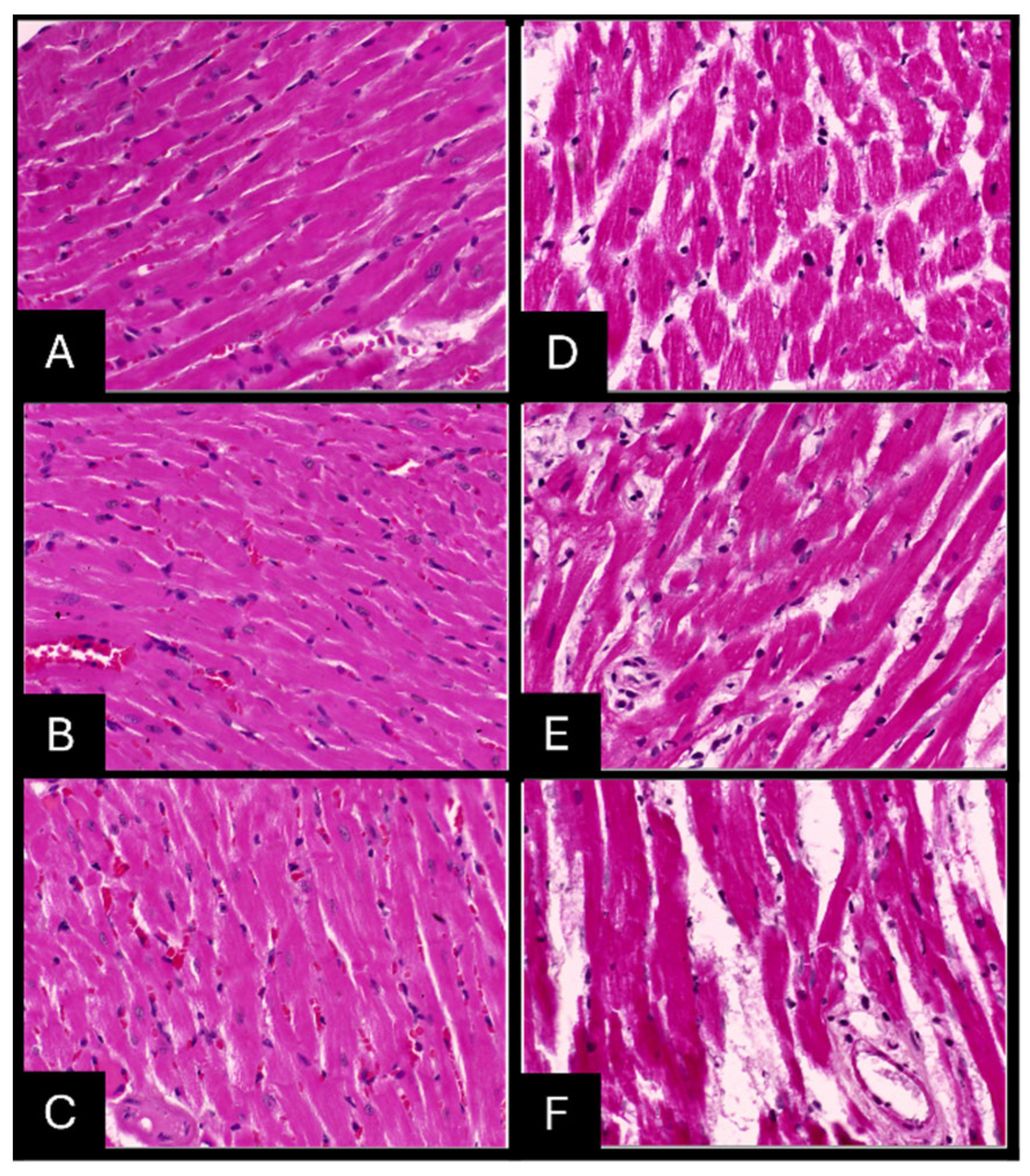
2.7. Histopathologic Evaluation of Tissue Samples
3. Discussion
Limitations Section
4. Materials and Methods
4.1. Synthesis and Characterization of AgNPs
4.2. Preparation of Tissue Samples
4.3. Nucleic Acid Extraction
4.3.1. DNA Extraction
4.3.2. RNA Extraction
4.3.3. Quality Assessment
4.3.4. cDNA Synthesis and PCR
4.4. Protein Extraction and Analysis
SDS-PAGE and Western Blot
4.5. Tissue Processing and Staining
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNPs | Silver nanoparticles |
| NBF | Neutral buffered formalin |
| DLS | Dynamic Light Scattering |
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| SDS-PAGE | Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis |
Appendix A
| Preservative | Organ | Time (Hours) | Nuclear Staining | Cytoplasmic Staining | Tissue Morphology | Clarity of Staining | Uniformity of Staining | Total Score | Interpretation |
| 10% NBF | Liver | 24 | 1 | 1 | 1 | 1 | 1 | 5 | Adequate |
| 48 | 1 | 1 | 1 | 1 | 1 | 5 | Adequate | ||
| 72 | 1 | 1 | 1 | 1 | 1 | 5 | Adequate | ||
| Kidney | 24 | 1 | 1 | 1 | 1 | 1 | 5 | Adequate | |
| 48 | 1 | 1 | 1 | 1 | 1 | 5 | Adequate | ||
| 72 | 1 | 1 | 1 | 1 | 1 | 5 | Adequate | ||
| Heart | 24 | 1 | 1 | 1 | 1 | 1 | 5 | Adequate | |
| 48 | 1 | 1 | 1 | 1 | 1 | 5 | Adequate | ||
| 72 | 1 | 1 | 1 | 1 | 1 | 5 | Adequate | ||
| AgNPs solution | Liver | 24 | 0 | 0 | 0 | 1 | 1 | 2 | Inadequate |
| 48 | 0 | 0 | 0 | 1 | 1 | 2 | Inadequate | ||
| 72 | 0 | 0 | 0 | 1 | 1 | 2 | Inadequate | ||
| Kidney | 24 | 0 | 0 | 0 | 0 | 1 | 1 | Inadequate | |
| 48 | 0 | 0 | 0 | 0 | 1 | 1 | Inadequate | ||
| 72 | 0 | 0 | 0 | 0 | 1 | 1 | Inadequate | ||
| Heart | 24 | 0 | 0 | 0 | 1 | 1 | 2 | Inadequate | |
| 48 | 0 | 0 | 0 | 1 | 1 | 2 | Inadequate | ||
| 72 | 0 | 0 | 0 | 1 | 1 | 2 | Inadequate |
References
- Kmiec, Z. J.A. Kiernan. Histological and Histochemical Methods: Theory and Practice. 5th Edition, Scion Publishing, 2015, 571 pp. Folia Histochem. Cytobiol. 2016, 54, 58–59. Available online: https://journals.viamedica.pl/folia_histochemica_cytobiologica/article/view/46908 (accessed on 22 March 2025). [CrossRef]
- Vucevic, D.D.; Seidman, M.A.; Mesaki, K.; Guan, Z.; Juvet, S.; Liu, M.; Keshavjee, S.; Murphy, K. A Novel Tissue Preservation and Transport Solution as a Substitute for Formalin. Lab. Investig. 2023, 103, 100198. [Google Scholar] [CrossRef]
- Bussolati, G.; Chiusa, L.; Cimino, A.; D’armento, G. Tissue transfer to pathology labs: Under vacuum is the safe alternative to formalin. Virchows Arch. 2008, 452, 229–231. [Google Scholar] [CrossRef]
- Shetty, J.K.; Babu, H.F.; Laxminarayana, K.P.H. Histomorphological Assessment of Formalin versus Nonformalin Fixatives in Diagnostic Surgical Pathology. J. Lab. Physicians 2020, 12, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Swami, P.C.; Raval, R.; Kaur, M.; Kaur, J. Accidental intraoral injection of formalin during extraction: Case report. Br. J. Oral Maxillofac. Surg. 2016, 54, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.M.A.; Rahman, M.A.; Zaman, M.K.; Ferdousi, Z.; Abu Reza, M. Toxicological effect of formalin as food preservative on kidney and liver tissues in mice model. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 47–51. [Google Scholar] [CrossRef]
- Islam, M.T.; Akter, S.; Hasan, M.T.; Roy, D.; Sadi, R.S.; Harun, A.B.; Habiba, U.; Khatun, F.; Das, Z.C.; Haider, M.G. Elucidating the effects of formalin as food preservative on gross and histoarchitecture of vital organs of swiss albino mice. Ecol. J. 2024, 6, 17–28. [Google Scholar] [CrossRef]
- Chu, W.-S.; Furusato, B.; Wong, K.; Sesterhenn, I.A.; Mostofi, F.K.; Wei, M.Q.; Zhu, Z.; Abbondanzo, S.L.; Liang, Q. Ultrasound-accelerated formalin fixation of tissue improves morphology, antigen and mRNA preservation. Mod. Pathol. 2005, 18, 850–863. [Google Scholar] [CrossRef]
- Berrino, E.; Annaratone, L.; Detillo, P.; Grassini, D.; Bragoni, A.; Sapino, A.; Bussolati, B.; Bussolati, G.; Marchiò, C. Tissue Fixation with a Formic Acid-Deprived Formalin Better Preserves DNA Integrity over Time. Pathobiology 2022, 90, 155–165. [Google Scholar] [CrossRef]
- Greish, K.; Alqahtani, A.A.; Alotaibi, A.F.; Abdulla, A.M.; Bukelly, A.T.; Alsobyani, F.M.; Alharbi, G.H.; Alkiyumi, I.S.; Aldawish, M.M.; Alshahrani, T.F.; et al. The Effect of Silver Nanoparticles on Learning, Memory and Social Interaction in BALB/C Mice. Int. J. Environ. Res. Public Health 2019, 16, 148. [Google Scholar] [CrossRef]
- Almatroudi, A. Unlocking the Potential of Silver Nanoparticles: From Synthesis to Versatile Bio-Applications. Pharmaceutics 2024, 16, 1232. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Barber, R.; Harmer, D.; Coleman, R.A.; Clark, B.J. GAPDH as a housekeeping gene: Analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol. Genom. 2005, 21, 389–395. [Google Scholar] [CrossRef]
- Jones, W.; Greytak, S.; Odeh, H.; Guan, P.; Powers, J.; Bavarva, J.; Moore, H.M. Deleterious Effects of Formalin-Fixation and Delays to Fixation on RNA and miRNA-Seq Profiles. Sci. Rep. 2019, 9, 6980. [Google Scholar] [CrossRef]
- Niemantsverdriet, M.; Knol, A.; van der Starre-Gaal, J.; Hoogland, A.M. Formalin-Free Tissue Embedding Is Less Hazardous and Results in Better DNA Quality. PLoS ONE 2024, 19, e0316107. [Google Scholar] [CrossRef]
- Das, D.; Ghosh, D.; Mandal, P. Preservative Potential of Biosynthesized Silver Nanoparticles: Prevention of Xylem Occlusion and Microbial Proliferation at Postharvest Stage of Preservation. Environ. Sci. Pollut. Res. Int. 2021, 28, 22038–22063. [Google Scholar] [CrossRef]
- Morsi, S.; Pittala, V.; Alqudah, M.; Haider, M.; Greish, K. In Vivo Evaluation of Anti-Nociceptive Effects of Silver Nanoparticles. Molecules 2022, 27, 7259. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, G.; Zhang, T.; Yang, Z. Action Potential Changes Associated with the Inhibitory Effects on Voltage-Gated Sodium Current of Hippocampal CA1 Neurons by Silver Nanoparticles. Toxicology 2009, 264, 179–184. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics 2024, 14, 5. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. Available online: https://www.sciencedirect.com/science/article/pii/S0734975008000918 (accessed on 8 May 2025). [CrossRef]
- Ocimum Scientific Publishers Pty Ltd. Formalin Hazards and Health Insurance Coverage Needs among Exposed Personnel. Res. Adv. Biomed. Sci. Technol. 2019, 1, 18–21. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- QIAamp DNA Mini Kit and QIAamp DNA Blood Mini Kit Handbook. Available online: https://www.qiagen.com/br/resources/download.aspx?id=bf602afb-cc1e-4032-b762-6e364adf39ff&lang=en (accessed on 20 March 2025).
- TRIzolTM Reagent. Available online: https://www.thermofisher.cn/order/catalog/product/15596026 (accessed on 20 March 2025).
- Thermo Scientific. T042-TECHNICAL BULLETIN NanoDrop Spectrophotometers. Available online: https://www.scirp.org/reference/referencespapers?referenceid=3666598 (accessed on 20 March 2025).
- Lee, P.Y.; Costumbrado, J.; Hsu, C.Y.; Kim, Y.H. Agarose Gel Electrophoresis for the Separation of DNA Fragments. J. Vis. Exp. 2012, 62, 3923. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4846332/ (accessed on 20 March 2025).
- High-Capacity cDNA Reverse Transcription Kit. Available online: https://www.thermofisher.com/order/catalog/product/4368814 (accessed on 22 March 2025).
- Cell Lysis Buffers-BH. Available online: https://www.thermofisher.com/sa/en/home/life-science/protein-biology/protein-purification-isolation/cell-lysis-fractionation/cell-lysis-total-protein-extraction.html (accessed on 22 March 2025).
- LI COR BIOTECH LLC WesternSure PREMIUM Chemiluminescent Substrate (100 mL Kit). Available online: https://www.fishersci.com/shop/products/westernsure-premium-substrate/50489552 (accessed on 22 March 2025).
- Alwahaibi, N.; Al Dhahli, B.; Al Issaei, H.; Al Wahaibi, L.; Al Sinawi, S. Effectiveness of Neutral Honey as a Tissue Fixative in Histopathology. F1000Research 2022, 11, 1014. [Google Scholar] [CrossRef]

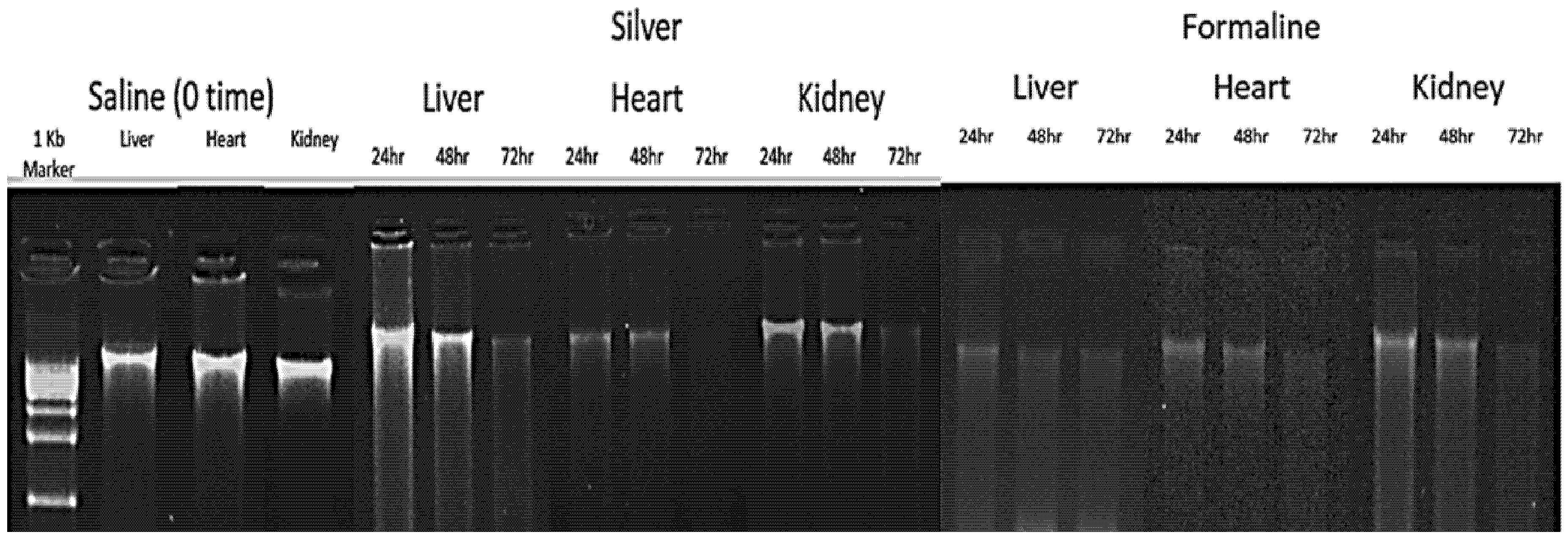

| Gene Name | Product Size | Primers |
| GAPDH | 120 bp | F- AGG TCG GTG TGA ACC GAT TTG R- TGT AGA CCA TGT AGT TGA GGT CA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, S.; Ismaeel, A.; Aljishi, M.; Selvam, S.; Esther, A.; Greish, K. Silver Nanoparticles as a Novel Tissue Preservative: A Comparative Study with 10% Neutral Buffered Formalin. Int. J. Mol. Sci. 2025, 26, 5335. https://doi.org/10.3390/ijms26115335
Taha S, Ismaeel A, Aljishi M, Selvam S, Esther A, Greish K. Silver Nanoparticles as a Novel Tissue Preservative: A Comparative Study with 10% Neutral Buffered Formalin. International Journal of Molecular Sciences. 2025; 26(11):5335. https://doi.org/10.3390/ijms26115335
Chicago/Turabian StyleTaha, Safa, Amina Ismaeel, Muna Aljishi, Samvel Selvam, Angeleena Esther, and Khaled Greish. 2025. "Silver Nanoparticles as a Novel Tissue Preservative: A Comparative Study with 10% Neutral Buffered Formalin" International Journal of Molecular Sciences 26, no. 11: 5335. https://doi.org/10.3390/ijms26115335
APA StyleTaha, S., Ismaeel, A., Aljishi, M., Selvam, S., Esther, A., & Greish, K. (2025). Silver Nanoparticles as a Novel Tissue Preservative: A Comparative Study with 10% Neutral Buffered Formalin. International Journal of Molecular Sciences, 26(11), 5335. https://doi.org/10.3390/ijms26115335








