Abstract
Anticancer drug design has been reformed by the creation of heterocyclic hybrids. The introduction of a quinoline scaffold affects the activity, toxicity, and bioavailability of new compounds. The aim of this study was to synthesize and evaluate the biological activity of hybrids of 1,4-naphthoquinone with the 8-hydroxyquinoline moiety. The structure of the new compounds was characterized using spectroscopic methods, such as HR-MS, NMR, and IR. The analysis was supplemented by calculated NMR and IR spectra. The physicochemical properties and bioavailability of the compounds were examined using in silico methods. An analysis of reactivity descriptors showed that the compounds are good electron acceptors and exhibit high reactivity. Bioavailability properties confirm that hybrids could be good oral administration drugs. The biological potential of hybrids was examined by designation of the enzymatic conversion rate of the NQO1 protein and in vitro against cancer cell lines with overexpression of the gene encoding the NQO1 protein. The possibility of interaction between the tested ligand and the NQO1 protein was examined by molecular docking methods.
1. Introduction
Compounds with 1,4-naphthoquinone pharmacophore are large groups of natural substances that can be obtained from plants, animals, fungi, and bacteria [1]. One of the best-known 1,4-naphthoquinones is lawsone (Figure 1), which is an extract from Lawsonia inermis. Even though the substance was isolated in the 1950s, the properties of the leaves were well-known in ancient times. People in North Africa, the Middle East, and South Asia use the powdered leaves as a brown dye for hair coloring, body paint, and tattoos. Moreover, the brown powder is used to dye silk, wool, and leather [1,2].
Figure 1.
Chemical structure of natural and semi-synthetic compounds.
Lawsone is characterized by a broad spectrum of pharmacological activities, such as anticancer, antibacterial, antifungal, antiviral, antimalarial, and anti-inflammatory effects [3,4,5,6]. The biological research shows that the compound influences many different enzymes. However, the most important effect seems to be caused by inducing oxidative damage by the production of different types of reactive oxygen species (ROS), which affect DNA mutation [7,8]. This natural compound is also characterized by in vivo genotoxicity, which limits its use in human therapy [2,9].
The most important modification of the lawsone structure is the replacement of the hydroxyl group with an alkoxy group [10]. For example, Durán and co-workers described the series of 2-O-alkyl derivatives, which exhibited high cytotoxicity towards different cancer cell lines, while its activity against non-cancer cell lines was similar to the reference substance, etoposide [11]. The introduction of alkynyloxy or aryloxy substituents at the C2 position of 1,4-naphthoquinone moiety increases the antifungal activity against S. aureus, E. coli, and C. albicans [12,13]. The second possible modification of the lawsone structure is introducing a substituent at the C3 position. This group of compounds includes atovaquone and buparvaquone (Figure 1). Atovaquone is used in the treatment of malaria, toxoplasmosis, and pneumocystis pneumonia, while buparvaquone is used in bovine theileriosis [14]. The analysis of the structure–activity relationship shows that activity depends on the type of substituent at the C2 and C3 positions. The introduction of an ether or thioether group increases antibacterial activity, while substituents with nitrogen atoms, such as amine, alkylamine, arylamine, or N-heterocyclic moieties, increase the anticancer effect [10,11,15,16,17,18].
Alkaloids with a quinoline skeleton are found in many natural products and have been used as a panacea for all ailments. One of the first described compounds with a quinoline moiety was quinine (Figure 1), which was isolated from the bark of a cinchona tree. The antiplasmodial activity of this natural substance led to the development of many analogs exhibiting higher activity and better pharmacokinetic parameters [19,20]. In 1958, camptothecin (Figure 1) was obtained from the break of Camptotheca acuminate. The compound exhibits high anticancer potential, but its uses are limited due to high toxicity and low bioavailability. Its semi-synthetic derivatives show better pharmacological properties and are used in the treatment of small-cell and non-small-cell lung cancer, cervical cancer, and ovarian cancer [21,22]. Currently, quinoline drugs are used as broad-spectrum antibiotics, chemotherapeutics, and antimalarial drugs [23,24,25]. Recently, many novel 8-hydroxyquinoline derivatives were tested as potential anticancer drugs because they contain a nitrogen atom and a hydroxyl group, which could create hydrogen bonds with biological targets and chelate metal ions. The introduction of additional substituents at the C5 and C2 positions increases the activity [26,27,28,29,30,31].
The action of a compound in a live organism depends on its pharmacological and pharmacokinetic properties. For this reason, the modification of a well-known pharmacophore by connecting it with another active compound allows the development of hybrid derivatives with improved biological and physicochemical properties [32,33]. The literature described hybrid molecules with 1,4-naphthoquinone moiety. Connecting 1,4-naphthoquinone with betulin, diosgenin, or nucleoside increases the activity of hybrids compared to natural substances. The biological research shows that hybrids activate the mitochondrial apoptosis pathway by interacting with NAD(P)H quinone dehydrogenase 1 (NQO1) enzyme [16,34,35,36].
Continuing our research on hybrid compounds with a 1,4-quinone moiety, we designed and synthesized hybrids of 1,4-naphthoquinone with an 8-hydroxyquinoline moiety. The bioavailability parameters of the obtained derivatives were determined using in silico methods. The anticancer potential of hybrids was tested in vitro against four human cancer cell lines. The obtained hybrids were also tested as a substrate of quinone oxidoreductase 1 (NQO1), and the interaction between this protein and the compounds was examined using the molecular docking method.
2. Results and Discussion
2.1. Synthesis and Structure Analysis
The literature describes the synthesis of many quinoline compounds that are derivatives of 8-hydroxyquinoline. The reaction occurs in the presence of potassium carbonate and an aprotic solvent such as tetrahydrofuran or dimethylformamide [37,38,39,40]. In this condition, the reaction between 8-hydroxyquinoline derivatives 2–4 and 2-bromo-1,4-naphthoquinone 1 does not occur. Treatment of 2-bromo-1,4-naphthoquinone 1 with the corresponding 8-hydroxyquinoline 2–4 in the presence of potassium tert-butoxide and toluene at reflux allows the obtaining of new derivatives of 1,4-naphthoquinone 5–6 (Figure 2).
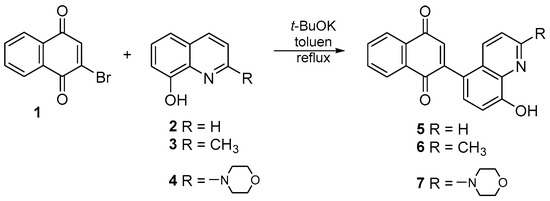
Figure 2.
Synthesis route of compounds 5–7.
After purification by column chromatography, pure compounds 5–7 were obtained with yields of 62–84%. The derivatives 5–7 were the only products of the reaction. The structures of all new compounds were characterized by NMR, FT-IR, Raman, and HR-MS spectroscopy.
2.1.1. HR-MS Spectroscopy
The compounds 5–7 were analyzed by high-resolution mass spectroscopy (HR-MS) by direct infusion. The protonated molecule observed at 302.0801 m/z (C19H12NO3, calculated as 302.0817 m/z) for compound 5, at 316.0999 m/z (C20H14NO3, calculated as 316.0977 m/z) for compound 6, and at 387.1363 m/z (C23H19N2O4, calculated as 387.1345 m/z) for compound 7. For all tested compounds, peaks were observed with m/z values corresponding to the mass of the ion formed by the addition of two protons (Figures S1–S3).
2.1.2. Nuclear Magnetic Resonance Spectroscopy
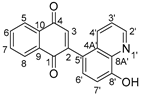
The chemical structures of derivatives 5–7 were analyzed using 1D (1H and 13C NMR) and 2D (ROESY, HSQC, and HMBC) NMR spectroscopy (Figures S4–S18). Table 1 shows the proton–proton and proton–carbon correlations for compound 5.

Table 1.
The chemical shift of compound 5.
The ROESY spectrum of compound 5 shows that the signals at δH 8.89 ppm and δH 8.29 ppm are correlated with the signal at δH 7.53 ppm (Table 1). The signal at δH 7.17 ppm is correlated with the signals at δH 7.53 ppm and δH 7.50 ppm. The results show that these signals describe protons belonging to the quinoline moiety, while the signals at δH 8.08 ppm, δH 7.93 ppm, and δH 7.12 ppm describe protons belonging to the 1,4-naphthoquinone moiety. The HSQC spectrum allows the assignment of the chemical shifts of carbon signals to proton signals (Table 1). The signal at δH 10.24 ppm was assigned to the hydrogen atom in the hydroxyl group because no correlation was observed between this peak and the peak of the carbon atom in the HSQC spectrum. The signals of carbon atoms belonging to the quinoline and 1,4-naphthoquinone moieties were assigned based on the correlation observed in the HMBC spectrum. The analysis of this correlation shows that the signal at δH 8.08 ppm was assigned to protons H5 and H8, while the signal at δH 7.93 ppm was assigned to H6 and H7 atoms. The signal at δH 7.12 ppm was assigned to the proton H3 of the 1,4-naphthoquinone moiety. The signal at δH 8.89 ppm interacts with the signals of carbon atoms at δC 122.4 ppm and δC 135.3 ppm, which were assigned to the C3′ and C4′ carbon atoms, respectively. The carbon atom at the C2 position (δC 148 ppm) was identified based on its correlation with protons at the C6′ and C7′ positions in the quinone moiety. Furthermore, the C5′ carbon (δC 127.8 ppm) was correlated with the H3′ (δH 7.53 ppm), H4′ (δH 8.29 ppm), and H6′ (δH 7.50 ppm) protons, respectively. The signal at δC 155.1 ppm was assigned to the carbon atom at the C8′ position based on its correlation with H3′ (δH 7.53 ppm), H4′ (δH 8.29 ppm), H6′ (δH 7.50 ppm), and H7′ (δH 7.17 ppm).
The analysis was supplemented by computer simulation using the GIAO method [41,42,43]. The 1H NMR spectra of compound 5 show good agreement between the experimental and calculated data, with a correlation coefficient of 0.8634 (Figure 3a).
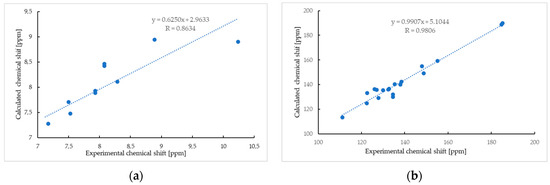
Figure 3.
The linear regression between the experimental and calculated 1H NMR (a) and 13C NMR (b) chemical shifts of compound 5.
The most important differences between the calculated and experimental 1H NMR spectra are observed for the proton of the hydroxyl group (Table 1, Figure 3a). The chemical shifts of the hydroxyl proton in the calculated and experimental spectra are δH 8.90 ppm and δH 10.24 ppm, respectively. This effect could be caused by the formation of a hydrogen bond between the hydrogen of the hydroxyl group and other atoms, like oxygen or nitrogen, which influences the chemical shift of the proton [44,45,46]. As seen in Figure 3b, the chemical shift of carbon atoms in the calculated spectrum reproduces the experimental value well, and the correlation coefficient is 0.9806.
The molecular structures of derivatives 6–7 were confirmed by proton–carbon correlation analysis (Tables S1 and S2).
The analysis of the 2D spectra of derivative 6 shows that the proton at δH 9.82 ppm does not correlate with any carbon or proton, which means that it belongs to the hydroxyl group at the C8′ position of the quinoline moiety (Table S1). The ROESY spectrum shows that the methyl group at the C9′ position of the quinoline ring interacts with the H4′ and H3′ protons. In the HMBC spectrum, the H9′ proton correlates with the carbon atoms at positions C7′ (δC 110.9 ppm), C3′ (δC 123.2 ppm), and C2′ (δC 157.3 ppm) of the quinoline moiety and C3 (δC 137.8 ppm) of the 1,4-naphthoquinone moiety.
The introduction of a morpholine moiety at the C2′ position of the quinoline moiety significantly influences the chemical shifts of carbon and proton signals (Table S2). The introduction of a morpholine moiety influences the chemical shift of protons in the quinoline moiety. Analysis of proton–proton and proton–carbon correlations shows that the signal at δH 7.02 ppm was assigned to the proton at the H7′ position of the quinoline moiety, while for derivative 5, the signal of the proton at H7′ is at δH 7.17 ppm. Comparing the chemical shift of protons H3′ and H6′ in derivatives 7 and 5 shows that these signals are shifted upfield by ΔδH 0.34 ppm and ΔδH 0.36 ppm, respectively. The group at the C2′ position affects the chemical shift of the signal assigned to the proton at the H3 position of the 1,4-naphthoquinone moiety, which is shifted from δH 7.09 ppm to δH 7.02 ppm.
2.1.3. Fourier-Transform Infrared Spectroscopy
The FT-IR spectra were used to confirm the chemical structures of compounds 5–7 by analyzing the bands of hydroxyl and carbonyl groups. The bands were assigned by comparing the experimental and calculated FT-IR spectra (Table 2). As seen in Figure 4, the calculated spectrum reproduces the experimental spectrum well.

Table 2.
Experimental and calculated wavenumber [cm−1] and band assignments for compounds 5–7.
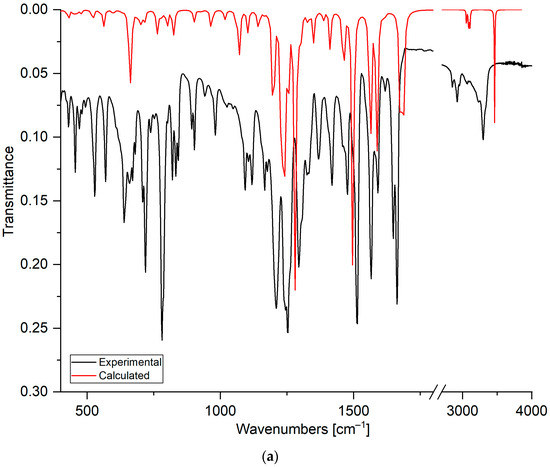
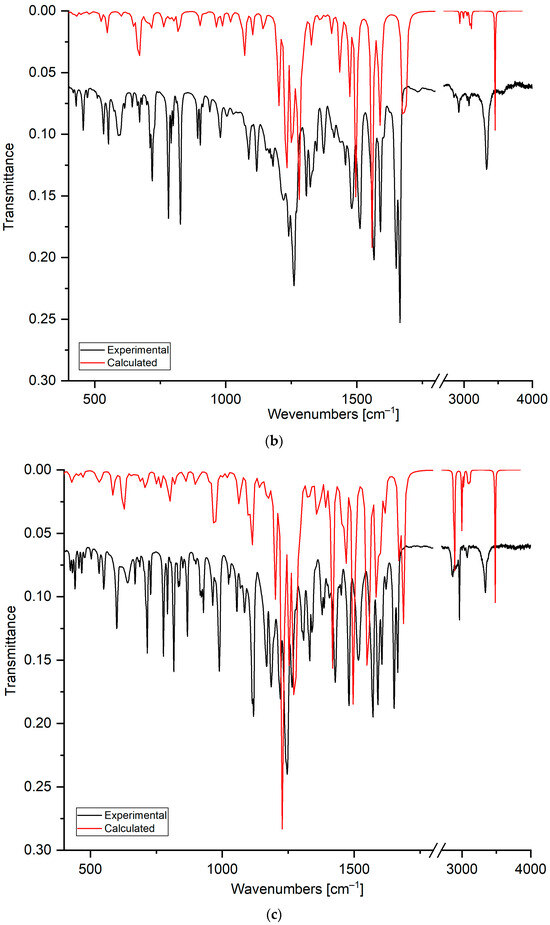
Figure 4.
The FT–IR spectra of compounds: (a) 5, (b) 6, and (c) 7. The experimental spectra are shown as black lines, and the calculated spectra as red lines.
Comparing the experimental and calculated spectra shows that the stretching vibration in the range from 3340 cm−1 to 3298 cm−1 is assigned to the hydroxyl group at the C8′ position of the quinoline moiety. The broadening and shifting of the band towards lower wavenumbers compared to the calculated spectrum shows that the hydroxyl group could form a hydrogen bond [44]. The band deformation of the hydroxyl group was observed at 638 cm−1, 670 cm−1, and 641 cm−1 for compounds 5, 6, and 7, respectively. The band in the range from 3077 cm−1 to 2853 cm−1 is assigned to the stretching vibration of the C-H bond in the quinoline and 1,4-naphthoquinone moieties.
The stretching vibrations of the carbonyl groups are observed in the ranges from 1665 cm−1 to 1649 cm−1 and from 1678 cm−1 to 1673 cm−1 in the experimental and calculated spectra, respectively. For compounds 5–7, in the experimental spectra, the carbonyl bands are split into two separate bands. However, in the calculated spectra, this splitting is observed only for derivative 7. Analysis of the calculated spectra allows the assignment of asymmetric vibrations at higher wavenumbers, mainly to the carbonyl group at the C1 position. Symmetric stretching vibrations are assigned mainly to the carbonyl group at the C4 position. This result suggests that the carbonyl group could create the hydrogen bond [47,48].
The stretching vibrations of carbon–carbon bonds are observed as the wide band in the ranges from 1566 cm−1 to 1565 cm−1 and from 1583 cm−1 to 1588 cm−1 in the experimental and calculated spectra, respectively. In the experimental spectra, in the range from 1515 cm−1 to 1477 cm−1, the bands belong mainly to the deformation vibrations of the C-H group in the naphthoquinone moiety, while the band at 1412–1428 cm−1 is usually assigned to the deformation vibration of the C-H group in the quinoline moiety. As seen in Table 2, the bands below 1400 cm−1 are assigned to the deformation vibrations of C-C and C-H bonds in both moieties of the compound.
2.2. Analysis of Physicochemical Properties
The physicochemical properties influence the fate of a drug in a living organism. The relationship between physicochemical properties and drug absorption after oral administration was first described by Lipinski in 1997. Later studies showed that the bioavailability of a potential drug depends on many properties, such as molecular weight, lipophilicity, topological surface area, and the number of donors and acceptors of hydrogen bonds [49,50]. Table 3 shows the biodistribution parameters of tested compounds 5–7, which were determined using the software available online [51,52].

Table 3.
The ADMET parameters of compounds 5–7.
Compounds 5–7 are characterized by moderate water solubility, which depends on the substituent at the C2′ position of the quinoline moiety, and the order is as follows: morpholine (7) < methyl (6) < hydrogen (5). The same correlation is observed for lipophilicity (logP). Comparing lipophilicity and solubility shows that compounds 5–7 can well penetrate biological barriers through passive transport [50,53]. The tested derivatives 5–7 also meet all Lipinski’s and Veber’s rules, which means that they should be characterized by good oral availability. The in silico oral administration is described by the Caco-2 permeability (logPapp) and human intestinal absorption (HIA). Compounds 5–7 should have good absorption through the intestine because the values of HIA and logPapp are in the ranges from 99% to 97% and from 1.214 to 1.342, respectively. The tested derivatives 5–7 may be transported by the skin because the value of logKp is lower than −2.5. After absorption of the drug, the most important parameter is the volume of distribution (VDss), which describes the distribution of the drug from blood to tissue. The determined in silico values show that the concentration of compounds in tissue should be higher than in plasma. The group at the C2′ position of the quinoline moiety influences the penetration of the compound into tissue. The introduction of a morpholine group (7) increases the concentration of the compound in tissue by almost twofold compared to the unsubstituted compound (5). Compounds are transported to the brain via the bloodstream. The important parameters determining the distribution of a drug in the brain and the central nervous system (CNS) are logBB and logPS. Analysis of these parameters shows that derivatives 5–7 will penetrate weakly across the blood–brain barrier and to the CNS. Therefore, 1,4-quinone derivatives 5–7 exhibit low neurotoxicity. The metabolism of a drug in silico is described through its interaction with cytochrome P450. As seen in Table 4, the 1,4-quinone derivatives 5–7 can interact with cytochrome P450, and they can be transformed into other compounds. The metabolism of the drug depends on its reactivity, which is described by the reactivity descriptors [49,54]. The reactivity properties of the drug are usually designated by the energy of HOMO and LUMO orbitals [55,56]. The molecular orbitals of compounds 5–7 were designated using the calculated structures. As seen in Figure 5, for all ligands, the HOMO orbital is localized near the quinoline moiety and the 1,4-quinone unit, while the LUMO orbital is mainly near the 1,4-naphthoquinone moiety and the benzene ring of the quinoline moiety. The overlap of HOMO and LUMO orbitals influences the mobility of electrons.

Table 4.
The reactivity descriptors of compounds 5–7.
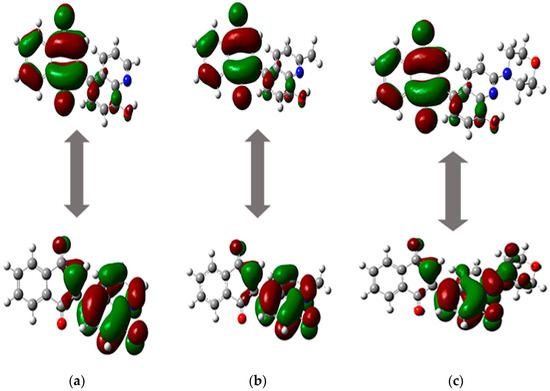
Figure 5.
The HOMO and LUMO orbitals of compounds (a) 5, (b) 6, and (c) 7.
The energy of frontier orbitals depends on the type of substituent at the C2 position of quinoline moiety, and the order is as follows: hydrogen (5) < methyl (6) < morpholine (7) (Table 3). The energy gap (ΔE) between the HOMO and LUMO orbitals is in the range from 2.491 eV to 2.743 eV. A low ΔE value means that compounds 5–7 are highly reactive and can be electron donors or acceptors [57,58]. The energy gap was used to determine the reactivity descriptors of molecules, such as the ionization potential (I), electron affinity (A), hardness (η), chemical potential (µ), electronegativity (χ), and electrophilicity index (ω) (Table 4). Analysis of the ionization potential (I) and electron affinity (A) shows that compounds 5–7 have a stronger tendency to gain electrons than remove them. Molecules 5–7 are good electron acceptors and are characterized by high reactivity because the η and ω values range from 1.246 eV to 1.371 eV and from 7.227 eV to 7.331 eV, respectively (Table 4) [49].
Analysis of molecular electrostatic potential (MEP) maps allows the designation of the fragments of molecules that could interact with nucleophilic and electrophilic compounds (Figure 6).
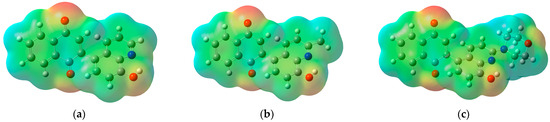
Figure 6.
The molecular electrostatic potential maps of derivatives (a) 5, (b) 6, and (c) 7.
As seen in Figure 6, the most electrophilic areas (blue) of compounds 5–7 are localized near the hydrogen atoms of the 1,4-naphthoquinone and quinoline moieties. Additionally, in the MEP map of compound 7, an electrophilic area near the hydrogen atom of the morpholine moiety can be observed (Figure 6c). The nucleophilic areas (red) are localized near the oxygen atoms of the carbonyl and hydroxyl groups. The high reactivity of derivatives 5–7 is due to the even arrangement of nucleophilic and electrophilic regions throughout the whole molecule.
Analysis of reactivity descriptors shows that the probability of transformation of the compound into other molecules is high. For this reason, the in silico extraction and toxicity assay parameters do not represent the fate of a drug in a living organism.
2.3. Biological Activity
2.3.1. NQO-1 Activity
The NAD(P)H-quinone oxidoreductase 1 (NQO1) enzyme is a flavoenzyme that catalyzes the two-electron reduction of quinone using NADPH. The products of the redox reaction are hydroquinone and reactive oxygen species (ROS), which damage the DNA, leading to cell death. In normal cells, the enzyme level is hardly detectable, while the overexpression of the gene encoding the NQO1 protein is detected in many types of cancer cells, such as breast, colon, cervix, lung, and pancreas. The interesting targets of cancer therapy are quinone compounds that exhibit high activity against the NQO1 enzyme [59,60].
The 1,4-quinone derivatives 5–7 and 2-bromo-1,4-naphthoquinone 1 were tested as substrates of the NQO1 enzyme using a method described in the literature [61,62]. As a reference substance, β-lapachone (β-Lap) was used, which is an NQO1-directed antitumor agent. The ability of the compounds to be a substrate of the NQO1 protein was examined using the oxidation of NADPH to NADP+. The enzymatic conversion rates of NQO1 for the tested compounds are presented in Table 5.

Table 5.
The enzymatic conversion rate of NQO1 for compounds 1, 5–7, and β-lapachone (β-Lap).
In the series of tested compounds, the lowest value of the enzymatic conversion rate of the NQO1 enzyme was obtained for 2-bromo-1,4-naphthoquinone 1. The introduction of the quinone moiety increases the enzymatic conversion rate. However, the activity of compounds 5–7 depends on the substituent at the C2′ position of quinoline, with the order as follows: methyl group (6) > morpholine group (7) > hydrogen atom (5). Compound 6 with a methyl group at the C2′ position was identified as the NQO1 substrate with the highest efficiency, which is better than β-lapachone.
2.3.2. Anticancer Activity In Vitro
The anticancer potential of compounds 1 and 5–7 was tested against four human cancer cell lines, which show overexpression of the gene encoding the NQO1 protein. As reference substances, β-lapachone and cisplatin were used. The results are presented in Table 6.

Table 6.
The anticancer activity of compounds 1, 5–7, β-lapachone, and cisplatin.
The analysis of the structure–activity relationship shows that the replacement of the bromine atom by the 8-hydroxyquinoline moiety increases the anticancer activity against all tested cell lines (Table 6). In the series of tested hybrids, the observed trend of activity against all tested cell lines is that compound 6, with a methyl group at the C2′ position of the quinoline moiety, shows the highest cytotoxicity, while the introduction of the morpholine moiety reduces the activity compared to derivative 5 with a hydrogen atom at this position.
Comparing the activity of 1,4-naphthoquinone compounds 5–7 and cisplatin shows that hybrids 5–7 exhibit higher cytotoxicity than the reference substance against lung (A549) and breast (MCF-7) cell lines. However, only against A549 cells do they show higher activity than β-lapachone.
As seen in Table 6, the activity of hybrids 5–7 depends on the type of cancer cell line. For this reason, the cytotoxicity of the derivatives was compared with the level of the gene encoding the NQO1 protein and the enzymatic conversion rate of NQO1. According to the Human Protein Atlas, the highest value of the nTPM (transcripts per million) index is determined for lung (A549) and amelanotic melanoma (C-32) cell lines [63,64]. Comparing the IC50 and nTPM values, it can be observed that the activity correlates well with the level of expression of the gene encoding the NQO1 protein, and the order is as follows: A549 > C-32 > MCF-7 > Colo-829. Comparing the activity and enzymatic conversion rate of NQO1 shows that derivative 6 exhibits the highest activity and enzymatic conversion rate.
In our previous study, we obtained hybrids of 1,4-naphthoquinone with quinoline, which are connected by an oxygen linker [18]. Both groups of derivatives exhibit the highest activity against the lung cancer cell line (A549). Compound 5 exhibits slightly lower activity than 2-chloro-3[(quinolin-8-yl)oxy]naphthalene-1,4-dione against A549 cells [18]. Comparing the IC50 value against these cancer cells shows that derivatives 6–7 are characterized by higher activity than the hybrids with an oxygen linker. The obtained results suggest that the hydroxyl group at C8′ and the substituent at the C2′ position of the quinoline moiety influence the anticancer activity.
2.4. Molecular Docking Analysis
The interaction between the NQO1 protein and the tested compounds was analyzed using the molecular docking method. As seen in Table 7, compounds with a quinoline moiety (5–7) show lower binding energy values than 2-bromo-1,4-naphthoquinone 1, which means that they interact more strongly with the active center of the protein. Comparing the values of ΔG and the enzymatic conversion rate of NQO1 shows that molecular docking reproduces the experimental results well.

Table 7.
The scoring values (ΔG) obtained for compounds 1 and 5–7.
The molecular arrangement of the active center and data about the type and length of bindings between the compounds and protein are presented in Figure 7 and Table S3, respectively.
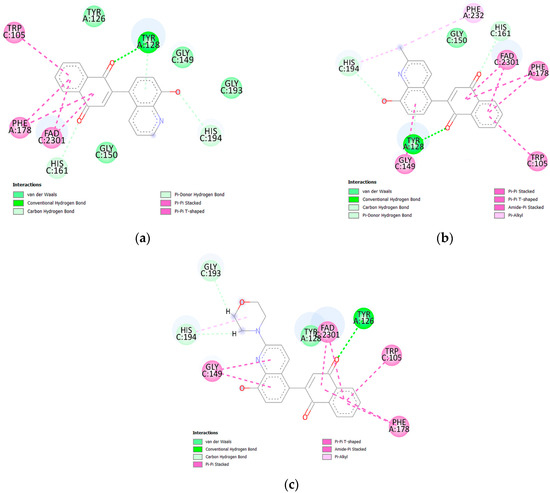
Figure 7.
Docking poses of NQO1 protein complex with compounds (a) 5, (b) 6, and (c) 7.
Ligands 5 and 6 create hydrogen bonds with tyrosine (TYR128) and histidine (HIS161 and HIS194). However, the carbonyl groups at the C1 and C4 positions of the 1,4-naphthoquinone moiety interact with TYR 128 and HIS161, respectively (Figure 7). The hydroxyl group of the quinoline moiety creates a weak H-bond with HIS164. In both ligand–protein complexes, the 1,4-naphthoquinone moiety creates hydrophobic interactions with tryptophan (TRP105), phenylalanine (PHE178), and the FAD cofactor. In the ligand 6–protein complex, hydrophobic interactions are also observed between the quinoline moiety and two amino acids, glycine (GLY149) and phenylalanine (PHE232). The compound with the morpholine group, 7, shows a different arrangement in the active site of the protein. For this ligand, only the carbonyl group at the C4 position of the 1,4-naphthoquinone moiety forms a hydrogen bond with tyrosine (TYR126). Glycine (GLY193) and histidine (HIS194) form H-bonds with the morpholine moiety. In this case, the hydroxyl group does not form a hydrogen bond with any amino acid. Analysis of molecular docking shows that the enzymatic conversion rate depends on the arrangement of the 1,4-quinone moiety in the active site of the NQO1 protein.
The arrangement of ligand 6 in the active site of the NQO1 protein was compared with the arrangement of 2-chloro-3-[(2-methylquinolin-8-yl)oxy]naphthalene-1,4-dione [18]. In both cases, the ligands form hydrogen bonds with tyrosine (TYR128) and hydrophobic interactions with tryptophan (TRP105), phenylalanine (PHE178), and the cofactor FAD. The analysis shows that the arrangement of these two ligands in the active site of the protein is similar and does not depend on how the two fragments are connected.
3. Materials and Methods
3.1. Physical Characterization
Melting points were measured by the Electrothermal IA 9300 melting point apparatus. The high-resolution mass spectra (HR-MS) were determined using the Bruker Impact II instrument (Brucker Analytische Messtechnik GmbH, Rheinstetten, Germany). The spectra were visualized using the Bruker Compass DataAnalysis 4.3 software. The experimentally determined molecular weight was compared with the theoretical value obtained by the Exact Mass Calculator available online [65].
Nuclear magnetic resonance spectra (NMR) were recorded using a Brucker Avance 600 spectrometer (Bruker, Billerica, MA, USA). The sample was prepared by dissolving 10 mg of the compound in 0.6 mL of hexadeuterodimethyl sulfoxide (DMSO-d6). As a reference substance, we used dimethyl sulfoxide (DMSO). The 1H and 13C NMR spectra were recorded at 600 MHz and 150 MHz frequencies, respectively. Chemical shifts (δ) are reported in ppm and coupling constants (J) in Hz. The multiplicity is marked as singlet (s), broad singlet (bs), doublet (d), or multiplet (m). The space correlations were examined by the ROESY (rotating-frame nuclear Overhauser effect spectroscopy) spectrum. The correlation between proton and carbon was analyzed using HSQC (heteronuclear single quantum coherence) and HMBC (heteronuclear multiple-bond correlation) spectra. The obtained spectra were analyzed using the MestReNova 15.0 software.
Fourier-transform infrared spectra (FT-IR) were determined using the FT-IR spectrometer Nicolet iS50 (Thermo Fisher Scientific, Waltham, USA, USA) equipped with the attenuated total reflection (ATR) diamond accessory MIRacle (PIKE Technology, Fitchburg, WI, USA). The spectra were accumulated with a resolution of 2 cm−1 (digital resolution 0.482 cm−1) in the spectral range of 400–4000 cm−1. The OriginPro 9.1 software was used to analyze the spectra.
3.2. Synthesis of Compounds 5–7
The 2-bromo-1,4-naphthoquinone 1 (0.1 g; 0.422 mmol) was dissolved in 2 mL of toluene and potassium tert-butoxide (1.5 eqv; 0.633 mmol; 0.071 g) was added. After 15 min at room temperature, the corresponding 8-quinolinol 2–4 (1.5 eqv; 0.633 mmol) was added, and the reaction mixture was heated to boiling temperature. The progress of the reaction was controlled using thin-layer chromatography (TLC). After 24 h, the reaction mixture was concentrated under reduced pressure. The crude products 5–7 were purified by column chromatography using chloroform/ethanol (40:1, v/v) as the eluent to give the following:
2-(8-hydroxyquinolin-5-yl)-1,4-naphthalenedione 5 yield 71%; mp. 239–240 °C; 1H NMR (600 MHz, DMSO-d6) δ, ppm: 7.12 (s, 1H, H3), 7.17 (d, J = 7.2 Hz, 1H, H7′), 7.50 (d, J = 7.2 Hz, 1H, H6′), 7.53 (d, J = 7.8 Hz, 1H, H3′), 7.93 (bs, 2H, H6, H7), 8.08 (bs, 2H, H8, H5), 8.29 (d, J = 7.8 Hz, 1H, H4′), 8.89 (s, 1H, H2′), 10.24 (bs, 1H, OH); 13C NMR (150 MHz, DMSO-d6) δ, ppm: 111.1 (C7′), 122.4 (C3′), 122.6 (C4A′), 126.0 (C5), 127.0 (C8), 127.8 (C5′), 130.0 (C6′), 132.4 (C10), 132.7 (C9), 134.6 (C6, C7), 135.3 (C4′), 138.0 (C3), 138.5 (C8A′), 148.0 (C2), 148.7 (C2′), 155.1 (C8′), 184.7 (C5), 185.1 (C8); IR (νmax cm−1, ATR): 3298, 1662, 1649, 1419, 1118, 712; ESI-HRMS m/z [M+1]+ calcd for C19H12NO3+ 302.0817, found 302.0801.
2-(8-hydroxy-2-methylquinolin-5-yl)-1,4-naphthalenedione 6 yield 84%; mp. 221–222 °C; 1H NMR (600 MHz, DMSO-d6) δ, ppm: 2.71 (s, 3H, CH3), 7.09 (s, 1H, H3), 7.17 (d, J = 7.8 Hz, 1H, H7′), 7.40 (s, 1H, H6′), 7.41 (s, 1H, H3′), 7.93 (m, 2H, H6, H7), 8.07 (m, 2H, H8, H5), 8.15 (d, J = 8.4 Hz, 1H, H4′), 9.82 (bs, 1H, OH); 13C NMR (150 MHz, DMSO-d6) δ, ppm: 25.1 (CH3), 110.9 (C7′), 122.6 (C4A′), 123.2 (C3′), 126.0 (C5), 126.1 (C5′), 127.0 (C8), 128.9 (C6′), 132.4 (C10), 132.7 (C9), 134.6 (C6, C7), 135.3 (C4′), 137.8 (C3), 137.8 (C8A′), 148.1 (C2), 154.3 (C8′), 157.3 (C2′), 184.7 (C5), 185.2 (C8); IR (νmax cm−1, ATR): 3330, 1664, 1650, 1412, 1118, 712; ESI-HRMS m/z [M+1]+ calcd for C20H14NO3+ 316.0977, found 316.0999.
2-(8-hydroxy-2-morpholinoquinolin-5-yl)-1,4-naphthalenedione 7 yield 62%; mp. 225–226 °C; 1H NMR (600 MHz, DMSO-d6) δ, ppm: 3.75 (m, 8H, CH2), 7.02 (d, J = 7.8 Hz, 1H, H7′), 7.04 (s, 1H, H3), 7.14 (d, J = 7.8 Hz, 1H, H6′), 7.19 (d, J = 9.0 Hz, 1H, H3′), 7.92 (m, 2H, H6, H7), 7.96 (d, J = 9.0 Hz, 1H, H4′), 8.07 (m, 2H, H8, H5), 9.08 (bs, 1H, OH); 13C NMR (150 MHz, DMSO-d6) δ, ppm: 45.5 (CH2), 66.6 (CH2), 110.7 (C3′), 110.8 (C3), 121.9 (C5′), 122.6 (C4A′), 125.3 (C6′), 126.0 (C5), 127.0 (C8), 132.4 (C10), 132.7 (C9), 134.5 (C6, C7), 136.8 (C4′), 137.2 (C8A′), 137.4 (C7′), 148.3 (C2), 152.7 (C8′), 156.3 (C2′), 184.8 (C5), 185.2 (C8); IR (νmax cm−1, ATR): 3340, 1665, 1652, 1428, 1118, 729; ESI-HRMS m/z [M+1]+ calcd for C23H19N2O4+ 387.1345, found 387.1363.
3.3. Computational Details
The molecular structures of compounds 5–7 were determined using methods described in the literature [16,41,49,62]. The calculated structure of molecules was used to determine the theoretical nuclear magnetic resonance (NMR) and vibrational (IR) spectra [43,66]. The theoretical wavenumber in IR spectra was scaled by a factor of 0.964 [67,68]. The NMR spectra were calculated by the gauge-independent atomic orbital (GIAO) method [41,42]. The optimized ligand structure was used to designate the molecular orbitals and molecular electrostatic potential maps. The visualization of calculated results was performed in the GaussView, Version 5 software [69].
3.4. The ADMET Study
The ADMET parameters were determined using software available online, such as pkCMS and SwissADME [50,51,70,71].
3.5. Enzymatic Study
The enzymatic assay was determined using the previously described methods [62,72,73]. As a reference substance, β-lapachone was used. Briefly, a solution of DT-diaphorase (NQO1, EC 1.6.5.5, human recombinant, Sigma-Aldrich, St. Louis, MO, USA) in potassium phosphate buffer (50 mmol/L) was added to the 96-well plate (Nunc Thermo Fisher Scientific, Waltham, MA, USA). Compounds 5–7 (0.01 µmol) were dissolved in 1 mL of dimethyl sulfoxide (DMSO), and the obtained solution (2 µL) was added to each well. After 2 min of shaking, the 40 µL of water solution of nicotinamide adenine dinucleotide phosphate (NADPH) (400 µmol/L) was added to each well. A BioTek 800TS microplate reader (BioKom, Poland) was used to measure the absorption of NADPH at a wavelength of 340 nm. The data were recorded at 10 s intervals for 10 min at 25 °C. NADPH oxidation rates were compared with reactions lacking compound. Initial velocities were calculated, and data were expressed as µmol NADPH/µmol NQO1/min. For each of compounds 5–7 and the reference substance, the measurements were performed three times.
3.6. Anticancer Study
Compounds 1 and 5–7, β-lapachone, and cisplatin were tested for in vitro anticancer activity against four human cancer cell lines, such as lung (A549, ATCC, Rockville, MD, USA), breast (MCF-7, ATCC, Rockville, MD, USA), and melanoma (C32 and Colo-829, ATCC, Rockville, MD, USA). The cultured cells were kept at 37 °C and 5% CO2. The cells were seeded (5 × 104 cells/well/100 mL DMEM supplemented with 10% FCS and streptomycin/penicillin) using 96-well plates (Nunc Thermo Fisher Scientific, Waltham, MA, USA). The tested compounds with a concentration of 1–100 µg/mL DMSO were incubated with the cancer cells for 72 hrs. The WST-1-formazan (proliferation reagent WST-1 assay, Roche Diagnostics, Mannheim, Germany) was detected using a microplate reader at 450 nm. Results were expressed as a mean value of at least three independent experiments performed in triplicate.
3.7. Molecular Docking Study
The optimized structures of derivatives 5–7 were used as ligands in the molecular docking study. The crystal structure of DT-diaphorase (NQO1), which was identified as 2F1O, was downloaded from the Protein Data Bank (PDB) [74]. The protein was crystallized as a cocrystal structure with the flavin adenine dinucleotide (FAD) molecule, which is a cofactor. The molecular docking analysis was performed using the AutoDock Vina software [75]. The grid center of Vina docking was selected as the center of the reference ligands that accompanied the downloaded protein complexes (x = 11.456160; y = 12.047880; z = −5.676920). The grid size was set to 15 Å × 15 Å × 15 Å, which is large enough to cover the entire target active site. Default values of all other parameters were used, and the complexes were submitted to 8 genetic algorithm runs. All obtained results were visualized using the BIOVIA Discovery Studio software package [76].
4. Conclusions
The new type of quinoline-1,4-naphthoquinone hybrids 5–7 was obtained in the presence of potassium tert-butoxide and toluene. The structures of compounds 5–7 were characterized using different spectroscopic methods, including HSQC and HMBC, which allowed the analysis of the hydroxyl group at the C8′ position of the quinoline moiety. The bioavailability of the compounds 5–7 was determined by in silico methods. Analysis of Lipinski and Veber’s rules showed that these derivatives could be orally administered drugs. Moreover, they could be well absorbed from the gastrointestinal system, and their concentration in tissue should be higher than in blood. Reactivity descriptors were determined based on the energy of HOMO and LUMO orbitals, which showed that the tested hybrids 5–7 are highly reactive and have a stronger tendency to gain electrons than to remove them. The compounds 5–7 are good substrates for the NQO1 protein. The in vitro anticancer activity showed that derivatives 5–7 possessed the highest activity against the lung cancer cell line, which was characterized by the highest nTPM value in the series of tested cell lines. The activity depended on the type of substituent at the C2′ position of the quinoline ring. The same correlation was observed for the scoring value obtained for the ligand–protein complex.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26115331/s1.
Author Contributions
Conceptualization, M.K.-T.; methodology, M.K.-T., R.W., M.L. and A.S.; writing—original draft preparation, M.K.-T.; writing—review and editing, M.K.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Silesia, grant number BNW-2-107/N/4/F.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aminin, D.; Polonik, S. 1,4-Naphthoquinones: Some Biological Properties and Application. Chem. Pharm. Bull. 2020, 68, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.S.; Sekar, M.; Gan, S.H.; Kumarasamy, V.; Subramaniyan, V.; Wu, Y.S.; Mat Rani, N.N.I.; Ravi, S.; Wong, L.S. Lawsone Unleashed: A Comprehensive Review on Chemistry, Biosynthesis, and Therapeutic Potentials. Drug Des. Devel Ther. 2024, 18, 295–3313. [Google Scholar] [CrossRef]
- Lopez, L.; Nary Flores, S.D.; Silva Belmares, S.Y.; Saenz Galindo, A. Naphthoquinones: Biological properties and synthesis of lawsone and derivates—A structured review. Vitae 2014, 21, 248–258. [Google Scholar] [CrossRef]
- Mone, N.; Bhagwat, S.; Sharma, D.; Chaskar, M.; Patil, R.; Zamboni, P.; Nawani, N.S. Satpute, aphthoquinones and Their Derivatives: Emerging Trends in Combating Microbial Pathogens. Coatings 2021, 11, 434. [Google Scholar] [CrossRef]
- Majdi, C.; Duvauchelle, V.; Meffre, P.; Benfodda, Z. An overview on the antibacterial properties of juglone, naphthazarin, plumbagin and lawsone derivatives and their metal complexes. Biomed. Pharmacother. 2023, 162, 114690. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.; Lima, A.; Sousa, J.; Pereira, G.; da Silva, G.; Brandão, G. 8-Methoxy-α-lapachone and lawsone: Antiproliferative effects on bladder tumour cells. Nat. Prod. Res. 2023, 21, 1058–1064. [Google Scholar] [CrossRef]
- Osman, A.M.; van Noort, P.C. Evidence for redox cycling of lawsone (2-hydroxy-1,4-naphthoquinone) in the presence of the hypoxanthine/xanthine oxidase system. J. Appl. Toxicol. 2023, 23, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Sauriasari, R.; Wang, D.H.; Takemura, Y.; Tsutsui, K.; Masuoka, N.; Sano, K.; Horita, M.; Wang, B.L.; Ogino, K. Cytotoxicity of lawsone and cytoprotective activity of antioxidants in catalase mutant Escherichia coli. Toxicology 2007, 235, 103–111. [Google Scholar] [CrossRef]
- Kirkland, D.; Marzin, D. An assessment of the genotoxicity of 2-hydroxy-1,4-naphthoquinone, the natural dye ingredient of Henna. Mutat. Res. 2003, 537, 183–199. [Google Scholar] [CrossRef]
- Kumari, P.; Singh, V.; Kant, V.; Ahuja, M. Current status of 1,4-naphthoquinones and their derivatives for wound healing. EJMCER 2024, 12, 100194. [Google Scholar] [CrossRef]
- Durán, A.; Chinchilla, N.; Simonet, A.; Gutiérrez, M.; Bolívar, J.; Valdivia, M.; Molinillo, J.; Macías, F. Biological Activity of Naphthoquinones Derivatives in the Search of Anticancer Lead Compounds. Toxins 2023, 15, 348. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Carballo, K.; Lamoureux, G.V.; Perez, A.L.; Cruz, A.B.; Filho, V.C. Novel one-pot synthesis of a library of 2-aryloxy-1,4-naphthoquinone derivatives. Determination of antifungal and antibacterial activity. RSC Adv. 2022, 12, 18507. [Google Scholar] [CrossRef] [PubMed]
- Lomlim, L.; Manuschai, J.; Ratti, P.; Kara, J.; Sakunphueak, A.; Panichayupakaranant, P.; Naorungroj, S. Effect of alkynyloxy derivatives of lawsone as an antifungal spray for acrylic denture base: An in vitro study. Heliyon 2023, 9, 13919. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Liang, F.M.; Zhang, W.J.; Zhang, Y.; Cui, J.H.; Dai, Y.Y.; Qiu, X.M.; Wang, W.H.; Zhou, Y.; Chen, D.P.; et al. Novel 2-amino-1,4-naphthoquinone derivatives induce A549 cell death through autophagy. Molecules 2023, 28, 3289. [Google Scholar] [CrossRef]
- Costa Souza, R.M.; Montenegro Pimentel, L.M.L.; Ferreira, L.K.M.; Pereira, V.R.A.; Santos, A.C.D.S.; Dantas, W.M.; Silva, C.J.O.; De Medeiros Brito, R.M.; Andrade, J.L.; De Andrade-Neto, V.F.; et al. Biological activity of 1,2,3-triazole-2-amino-1,4-naphthoquinone derivatives and their evaluation as therapeutic strategy for malaria control. Eur. J. Med. Chem. 2023, 55, 115400. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Krzykawski, K.; Halama, A.; Kubina, R. Hybrids of 1,4-Naphthoquinone with Thymidine Derivatives: Synthesis, Anticancer Activity, and Molecular Docking Study. Molecules 2023, 28, 6644. [Google Scholar] [CrossRef]
- Angulo-Elizari, E.; Henriquez-Figuereo, A.; Morán-Serradilla, C.; Plano, D.; Sanmartín, C. Unlocking the potential of 1,4-naphthoquinones: A comprehensive review of their anticancer properties. Eur. J. Med. Chem. 2024, 268, 116249. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Jastrzębska, M.; Chrobak, E.; Bębenek, E.; Latocha, M. Hybrids of 1,4-Quinone with Quinoline Derivatives: Synthesis, Biological Activity, and Molecular Docking with DT-Diaphorase (NQO1). Molecules 2022, 27, 6206. [Google Scholar] [CrossRef]
- Choudhary, D.; Rani, P.; Rangra, N.K.; Gupta, G.K.; Khokra, S.L.; Bhandare, R.R.; Shaik, A.B. Designing novel anti-plasmodial quinoline-furanone hybrids: Computational insights, synthesis, and biological evaluation targeting Plasmodium falciparum lactate dehydrogenase. RSC Adv. 2024, 14, 18764–18776. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Boechat, N.; Ferreira Mde, L.; Júnior, C.C.; Jesus, A.M.; Leite, M.M.; Souza, N.B.; Krettli, A.U. Anti-Plasmodium falciparum activity of quinoline-sulfonamide hybrids. Bioorg. Med. Chem. 2015, 23, 5979–5984. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C.; Cook, C.E.; Palmer, K.H.; McPhail, A.T.; Sim, G.A. Plant Antitumor Agents. I. The Isolation and Structure of Camptothecin, a Novel Alkaloidal Leukemia and Tumor Inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar] [CrossRef]
- Zhao, C.; Zuo, L.; Ke, X.; Wang, X. Michael addition reaction in C-5 of Camptothecin. J. Saudi Chem. Soc. 2024, 28, 101867. [Google Scholar] [CrossRef]
- Silva, V.L.; Saxena, J.; Nicolini, F.; Hoare, J.I.; Metcalf, S.; Martin, S.A.; Lockley, M. Chloroxine overrides DNA damage tolerance to restore platinum sensitivity in high-grade serous ovarian cancer. Cell Death Dis. 2021, 12, 395. [Google Scholar] [CrossRef]
- Zhang, W.; Shao, W.; Dong, Z.; Zhang, S.; Liu, C.; Chen, S. Cloxiquine, a traditional antituberculosis agent, suppresses the growth and metastasis of melanoma cells through activation of PPARγ. Cell Death Dis. 2019, 10, 404. [Google Scholar] [CrossRef]
- Mohan, I.; Ayyakannu Arumugam, N. Review on recent development of quinoline for anticancer activities. Arab. J. Chem. 2022, 15, 104168. [Google Scholar]
- Parmar, M.C.; Patel, B.Y. Green and traditional one-pot synthesis techniques for bioactive quinoline derivatives: A review. Tetrahedron Green. Chem. 2025, 5, 100062. [Google Scholar] [CrossRef]
- Liang, X.; Lin, W.; Chen, Y.; Yang, W.; Zhou, X.; Ai, S.; Qiu, L.; Cao, R.; Wang, J. Synthesis and in vitro and in vivo evaluation of novel bivalent quinolines as antitumor agents via targeting autophagy in cervical cancer. Eur. J. Med. Chem. 2025, 288, 117421. [Google Scholar] [CrossRef]
- Pakhariya, R.P.; Bhatnagar, A.; Pemawat, G. Quinoline analogs: Multifaceted heterocyclic compounds with varied synthetic strategies and potent antitubercular properties. RSC Adv. 2025, 15, 3646. [Google Scholar] [CrossRef]
- Ribeiro, N.; Bulut, I.; Sergi, B.; Pósa, V.; Spengler, G.; Sciortino, G.; André, V.; Ferreira, L.P.; Biver, T.; Ugone, V.; et al. Promising anticancer agents based on 8-hydroxyquinoline hydrazone copper(II) complexes. Front. Chem. 2023, 11, 1106349. [Google Scholar] [CrossRef]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef]
- Bala, I.A.; Al Sharif, O.F.; Asiri, A.M.; El-Shishtawy, R.M. Quinoline: A versatile bioactive scaffold and its molecular hybridization. Results Chem. 2024, 7, 101529. [Google Scholar] [CrossRef]
- Tietze, L.F.; Bell, H.P.; Chandrasekhar, S. Natural product hybrids as new leads for drug discovery. Angew. Chem. Int. Ed. Engl. 2003, 42, 3996. [Google Scholar] [CrossRef]
- Shaveta-Mishra, S.; Singh, P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016, 124, 500. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Q.; Sun, H.; Li, W. Novel diosgenin-1,4-quinone hybrids: Synthesis, antitumor evaluation, and mechanism studies. J. Steroid Biochem. Mol. Biol. 2021, 214, 105993. [Google Scholar] [CrossRef] [PubMed]
- Kadela-Tomanek, M.; Jastrzębska, M.; Marciniec, K.; Chrobak, E.; Bębenek, E.; Latocha, M.; Kuśmierz, D.; Boryczka, S. Design, synthesis and biological activity of 1,4-quinone moiety attached to betulin derivatives as potent DT-diaphorase substrate. Bioorg. Chem. 2021, 106, 104478. [Google Scholar] [CrossRef]
- Mancini, I.; Vigna, J.; Sighel, D.; Defant, A. Hybrid Molecules Containing Naphthoquinone and Quinolinedione Scaffolds as Antineoplastic Agents. Molecules 2022, 27, 4948. [Google Scholar] [CrossRef]
- Wang, S.B.; Deng, X.Q.; Zheng, Y.; Zhang, H.J.; Quan, Z.S. Synthesis and anticonvulsant activity evaluation of 8-alkoxy-5-(4H-1,2,4-triazol-4-yl)quinoline derivatives. Arch. Pharm. Res. 2013, 36, 32–40. [Google Scholar] [CrossRef]
- Sun, X.Y.; Jin, Y.Z.; Li, F.N.; Li, G.; Chai, K.Y.; Quan, Z.S. Synthesis of 8-alkoxy-4,5-dihydro-[1,2,4]triazole [4,3-a]quinoline-1-ones and evaluation of their anticonvulsant properties. Arch. Pharm. Res. 2006, 29, 1080–1085. [Google Scholar] [CrossRef]
- Vasudevan, A.; Wodka, D.; Verzal, M.K.; Souers, A.J.; Gao, J.; Brodjian, S.; Fry, D.; Dayton, B.; Marsh, K.C.; Hernandez, L.E.; et al. Synthesis and evaluation of 2-amino-8-alkoxy quinolines as MCHr1 antagonists. Part 2. Bioorg. Med. Chem. Lett. 2004, 14, 4879–4882. [Google Scholar] [CrossRef]
- Saadeh, H.A.; Sweidan, K.A.; Mubarak, M.S. Recent Advances in the Synthesis and Biological Activity of 8-Hydroxyquinolines. Molecules 2020, 25, 4321. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision A.03. Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Wolinski, K.; Hinton, J.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, F.; Zhong, A.; Dugu, J.; Li, X. Determination of a new chromone from Aurantii Fructus Immaturus by DFT/GIAO method. Nat. Prod. Res. 2016, 30, 69. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.; Spanget-Larsen, J. NMR and IR Investigations of Strong Intramolecular Hydrogen Bonds. Molecules 2017, 22, 552. [Google Scholar] [CrossRef]
- Novak, U.; Grdadolnik, J. Infrared spectra of hydrogen bond network in lamellar perfluorocarboxylic acid monohydrates. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 253, 119551. [Google Scholar] [CrossRef]
- Novak, U.; Golobič, A.; Klančnik, N.; Mohaček-Grošev, V.; Stare, J.; Grdadolnik, J. Strong Hydrogen Bonds in Acetylenedicarboxylic Acid Dihydrate. Int. J. Mol. Sci. 2022, 23, 6164. [Google Scholar] [CrossRef]
- Socrates, G. Infrared Characteristic Group Frequencies; John Wiley & Sons, Inc.: Hobocken, NJ, USA, 1994. [Google Scholar]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Bitew, M.; Desalegn, T.; Demissie, T.B.; Belayneh, A.; Endale, M.; Eswaramoorthy, R. Pharmacokinetics and drug-likeness of antidiabetic flavonoids: Molecular docking and DFT study. PLoS ONE 2021, 16, 0260853. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, T.; Mieszkowska, A.; Kempińska-Kupczyk, D.; Kot-Wasik, A.; Namieśnik, J.; Mazerska, Z. The impact of lipophilicity on environmental processes, drug delivery and bioavailability of food components. Microchem. J. 2019, 146, 393–406. [Google Scholar] [CrossRef]
- pkCSM. Available online: http://biosig.unimelb.edu.au/pkcsm/prediction# (accessed on 5 April 2025).
- SwissADME. Available online: http://www.swissadme.ch/index.php (accessed on 5 April 2025).
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and its relationship with passive drug permeation. Pharm. Res. 2011, 28, 962–977. [Google Scholar] [CrossRef]
- Rezvan, V.H. Molecular structure, HOMO-LUMO, and NLO studies of some quinoxaline 1,4-dioxide derivatives: Computational (HF and DFT) analysis. Results Chem. 2024, 7, 101437. [Google Scholar] [CrossRef]
- Parr, R.; Pearson, R. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Fukui, K. Role of frontier orbitals in chemical reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Jumabaev, A.; Khudaykulov, B.; Holikulov, U.; Norkulov, A.; Subbiah, J.; Al-Dossary, O.M.; Hushvaktov, H.; Absanov, A.; Issaoui, N. Molecular structure, vibrational spectral assignments, MEP, HOMO-LUMO, AIM, NCI, RDG, ELF, LOL properties of acetophenone and for its solutions based on DFT calculations. Opt. Mater. 2025, 159, 116683. [Google Scholar] [CrossRef]
- Shafieyoon, P.; Khalili, S.; Mehdipour, E.; Khorasani, S.N. Computational analysis and biological investigation of cellulose acetate: PED, HOMO–LUMO, MEP and molecular docking. Results Chem. 2024, 10, 101709. [Google Scholar] [CrossRef]
- Khan, A.; Arutla, V.; Srivenugopal, K. Human NQO1 as a Selective Target for Anticancer Therapeutics and Tumor Imaging. Cells 2024, 13, 1272. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Park, H. Implications of NQO1 in cancer therapy. BMB Rep. 2015, 48, 609. [Google Scholar] [CrossRef]
- Wu, L.Q.; Ma, X.; Zhang, C.; Liu, Z.P. Design, synthesis, and biological evaluation of 4-substituted-3,4-dihydrobenzo[h]quinoline-2,5,6(1H)-triones as NQO1-directed antitumor agents. Eur. J. Med. Chem. 2020, 198, 112396. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M. Design, Synthesis, Physicochemical Properties, and Biological Activity of Thymidine Compounds Attached to 5,8-Quinolinedione Derivatives as Potent DT-Diaphorase Substrates. Int. J. Mol. Sci. 2024, 25, 11211. [Google Scholar] [CrossRef]
- Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 5 April 2025).
- Karlsson, M.; Zhang, C.; Méar, L.; Zhong, W.; Digre, A.; Katona, B.; Sjöstedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 2021, 7, 2169. [Google Scholar] [CrossRef]
- The Exact Mass Calculator, Single Isotope Version. Available online: http://www.sisweb.com/referenc/tools/exactmass.htm (accessed on 5 April 2025).
- Ceylan, Ü.; Hacıyusufoğlu, M.E.; Sönmez, M.; Yalçın, Ş.P.; Özdemir, N. Experimental and theoretical studies of (E)-2-(2-hydroxystyryl)-6-(4-methoxybenzoyl)-5-(4-methoxyphenyl)-1,2,4-triazin-3(2H)-one. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 141, 307. [Google Scholar] [CrossRef]
- Foresman, J.; Frisch, A. Exploring Chemistry with Electronic Structure Methods, 3rd ed.; Gaussian, Inc.: Pittsburg, PA, USA, 2015. [Google Scholar]
- Henschel, H.; Andersson, A.T.; Jespers, W.; Mehdi Ghahremanpour, M.; van der Spoel, D. Theoretical Infrared Spectra: Quantitative Similarity Measures and Force Fields. J. Chem. Theory Comput. 2020, 16, 3307. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.; Millam, J. GaussView Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 4, 42717. [Google Scholar] [CrossRef]
- Pires, D.; Blundell, T.; Ascher, D. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Li, X.; Bian, J.; Wang, N.; Qian, X.; Gu, J.; Mu, T.; Fan, J.; Yang, X.; Li, S.; Yang, T.; et al. Novel naphtho [2,1-d]oxazole-4,5-diones as NQO1 substrates with improved aqueous solubility: Design, synthesis, and in vivo antitumor evaluation. Bioorg. Med. Chem. 2016, 24, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Deng, B.; Xu, L.; Xu, X.; Wang, N.; Hu, T.; Yao, Z.; Du, J.; Yang, L.; Lei, Y.; et al. 2-Substituted 3-methylnaphtho [1,2-b]furan-4,5-diones as novel L-shaped ortho-quinone substrates for NAD(P)H:quinone oxidoreductase (NQO1). Eur. J. Med. Chem. 2014, 82, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Faig, M.; Bianchet, M.A.; Winski, S.; Hargreaves, R.; Moody, C.J.; Hudnott, A.R.; Ross, D.; Amzel, L.M. Structure-based development of anticancer drugs: Complexes of NAD(P)H:quinone oxidoreductase 1 with chemotherapeutic quinones. Structure 2001, 9, 659. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Dessault Systemes BIOVIA. Available online: https://www.3ds.com/products/biovia/biosciences (accessed on 5 April 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).